Abstract
C16H13NO2S, P1̅ (no. 2), a = 8.0873(4) Å, b = 12.7820(7) Å, c = 13.5574(7) Å, α = 74.880(2)°, β = 75.421(2)°, γ = 76.391(2)°, V = 1287.61(12) Å3, Z = 4, Rgt(F) = 0.0413, wRref(F2) = 0.1075, T = 100 K.
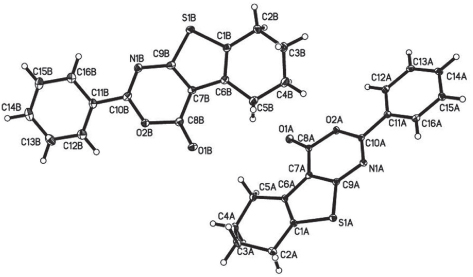
The crystal structure is shown in the figure. Tables 1–3 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow, needle, size 0.050×0.125×0.425 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.51 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II CCD, φ and ω scans |
| 2θmax: | 50° |
| N(hkl)measured, N(hkl)unique: | 28481, 4534 |
| N(param)refined: | 361 |
| Programs: | Bruker programs [16], SHELX [17] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | Site | x | y | z | Uiso |
|---|---|---|---|---|---|
| H(2AA) | 2i | 0.2537 | 0.7054 | 0.1062 | 0.019 |
| H(2AB) | 2i | 0.0492 | 0.7041 | 0.1331 | 0.019 |
| H(3AA) | 2i | 0.1659 | 0.6081 | −0.0001 | 0.021 |
| H(3AB) | 2i | 0.3208 | 0.5371 | 0.0565 | 0.021 |
| H(4AA) | 2i | 0.1043 | 0.4303 | 0.0764 | 0.020 |
| H(4AB) | 2i | −0.0376 | 0.5167 | 0.1345 | 0.020 |
| H(5AA) | 2i | 0.0382 | 0.3781 | 0.2688 | 0.019 |
| H(5AB) | 2i | 0.2400 | 0.3620 | 0.2145 | 0.019 |
| H(12A) | 2i | 0.2641 | 0.2634 | 0.7382 | 0.017 |
| H(13A) | 2i | 0.3316 | 0.2352 | 0.9016 | 0.016 |
| H(14A) | 2i | 0.4142 | 0.3752 | 0.9490 | 0.018 |
| H(15A) | 2i | 0.4292 | 0.5455 | 0.8319 | 0.017 |
| H(16A) | 2i | 0.3621 | 0.5742 | 0.6686 | 0.016 |
| H(2BA) | 2i | 0.8921 | −0.0281 | 0.4850 | 0.024 |
| H(2BB) | 2i | 0.6862 | −0.0209 | 0.5260 | 0.024 |
| H(3BA) | 2i | 0.7593 | 0.1342 | 0.5580 | 0.023 |
| H(3BB) | 2i | 0.8602 | 0.1659 | 0.4393 | 0.023 |
| H(4BA) | 2i | 0.5753 | 0.2773 | 0.4686 | 0.025 |
| H(4BB) | 2i | 0.4922 | 0.1684 | 0.4984 | 0.025 |
| H(5BA) | 2i | 0.4712 | 0.2351 | 0.3283 | 0.026 |
| H(5BB) | 2i | 0.6566 | 0.2724 | 0.2963 | 0.026 |
| H(12B) | 2i | 0.5987 | 0.1016 | −0.1372 | 0.025 |
| H(13B) | 2i | 0.6188 | 0.0361 | −0.2858 | 0.029 |
| H(14B) | 2i | 0.8066 | −0.1281 | −0.3122 | 0.029 |
| H(15B) | 2i | 0.9762 | −0.2268 | −0.1893 | 0.028 |
| H(16B) | 2i | 0.9488 | −0.1649 | −0.0375 | 0.023 |
Fractional coordinates and atomic displacement parameters (Å2).
| Atom | Site | x | y | z | U11 | U22 | U33 | U12 | U13 | U23 |
|---|---|---|---|---|---|---|---|---|---|---|
| S(1A) | 2i | 0.22167(7) | 0.66481(4) | 0.33083(4) | 0.0156(3) | 0.0126(3) | 0.0127(3) | −0.0030(2) | −0.0034(2) | −0.0024(2) |
| O(1A) | 2i | 0.1546(2) | 0.2726(1) | 0.4557(1) | 0.0239(9) | 0.0135(8) | 0.0194(9) | −0.0058(7) | −0.0066(7) | −0.0054(7) |
| O(2A) | 2i | 0.2217(2) | 0.3553(1) | 0.5625(1) | 0.0180(8) | 0.0115(8) | 0.0134(8) | −0.0043(6) | −0.0044(6) | −0.0037(7) |
| N(1A) | 2i | 0.2618(2) | 0.5394(1) | 0.5213(1) | 0.0119(9) | 0.014(1) | 0.013(1) | −0.0012(7) | −0.0026(7) | −0.0041(8) |
| C(1A) | 2i | 0.1767(3) | 0.6001(2) | 0.2453(2) | 0.007(1) | 0.019(1) | 0.010(1) | −0.0001(9) | −0.0010(9) | −0.006(1) |
| C(2A) | 2i | 0.1654(3) | 0.6574(2) | 0.1349(2) | 0.014(1) | 0.019(1) | 0.014(1) | −0.0029(9) | −0.0026(9) | −0.001(1) |
| C(3A) | 2i | 0.1963(3) | 0.5714(2) | 0.0686(2) | 0.015(1) | 0.028(1) | 0.011(1) | −0.003(1) | −0.0021(9) | −0.006(1) |
| C(4A) | 2i | 0.0868(3) | 0.4820(2) | 0.1227(2) | 0.015(1) | 0.022(1) | 0.014(1) | −0.0021(9) | −0.0026(9) | −0.008(1) |
| C(5A) | 2i | 0.1333(3) | 0.4176(2) | 0.2273(2) | 0.014(1) | 0.018(1) | 0.017(1) | −0.0016(9) | −0.0032(9) | −0.007(1) |
| C(6A) | 2i | 0.1623(3) | 0.4922(2) | 0.2885(2) | 0.007(1) | 0.016(1) | 0.012(1) | 0.0000(9) | −0.0002(9) | −0.006(1) |
| C(7A) | 2i | 0.1889(2) | 0.4609(2) | 0.3935(2) | 0.007(1) | 0.014(1) | 0.014(1) | −0.0002(8) | 0.0009(9) | −0.005(1) |
| C(8A) | 2i | 0.1838(3) | 0.3574(2) | 0.4652(2) | 0.009(1) | 0.016(1) | 0.013(1) | 0.0007(9) | −0.0022(9) | −0.006(1) |
| C(9A) | 2i | 0.2243(3) | 0.5462(2) | 0.4260(2) | 0.008(1) | 0.014(1) | 0.013(1) | 0.0005(9) | −0.0005(9) | −0.0049(9) |
| C(10A) | 2i | 0.2605(3) | 0.4448(2) | 0.5838(2) | 0.008(1) | 0.010(1) | 0.015(1) | −0.0009(8) | −0.0003(9) | −0.006(1) |
| C(11A) | 2i | 0.3042(2) | 0.4216(2) | 0.6875(2) | 0.007(1) | 0.015(1) | 0.013(1) | 0.0002(8) | 0.0004(9) | −0.0055(9) |
| C(12A) | 2i | 0.2970(3) | 0.3210(2) | 0.7571(2) | 0.013(1) | 0.011(1) | 0.019(1) | −0.0024(9) | −0.0008(9) | −0.005(1) |
| C(13A) | 2i | 0.3375(3) | 0.3042(2) | 0.8541(2) | 0.012(1) | 0.013(1) | 0.014(1) | 0.0004(9) | −0.0018(9) | −0.0005(9) |
| C(14A) | 2i | 0.3866(3) | 0.3872(2) | 0.8824(2) | 0.010(1) | 0.021(1) | 0.012(1) | 0.0008(9) | −0.0021(9) | −0.004(1) |
| C(15A) | 2i | 0.3955(3) | 0.4883(2) | 0.8129(2) | 0.011(1) | 0.016(1) | 0.015(1) | −0.0015(9) | −0.0031(9) | −0.006(1) |
| C(16A) | 2i | 0.3552(3) | 0.5053(2) | 0.7161(2) | 0.012(1) | 0.011(1) | 0.015(1) | −0.0010(9) | −0.0018(9) | −0.0023(9) |
| S(1B) | 2i | 0.85336(7) | −0.08916(5) | 0.30267(5) | 0.0236(3) | 0.0111(3) | 0.0224(3) | 0.0012(2) | −0.0103(2) | −0.0052(2) |
| O(1B) | 2i | 0.5437(2) | 0.2531(1) | 0.1065(1) | 0.031(1) | 0.0138(9) | 0.0199(9) | 0.0047(7) | −0.0053(7) | −0.0028(7) |
| O(2B) | 2i | 0.6602(2) | 0.1123(1) | 0.0272(1) | 0.0210(9) | 0.0143(8) | 0.0146(9) | −0.0006(7) | −0.0021(7) | −0.0034(7) |
| N(1B) | 2i | 0.8150(2) | −0.0570(2) | 0.1032(1) | 0.019(1) | 0.014(1) | 0.017(1) | −0.0043(8) | −0.0037(8) | −0.0047(9) |
| C(1B) | 2i | 0.7572(3) | 0.0187(2) | 0.3661(2) | 0.015(1) | 0.013(1) | 0.020(1) | −0.0045(9) | −0.0046(9) | −0.005(1) |
| C(2B) | 2i | 0.7770(3) | 0.0150(2) | 0.4736(2) | 0.024(1) | 0.018(1) | 0.021(1) | −0.003(1) | −0.010(1) | −0.006(1) |
| C(3B) | 2i | 0.7598(3) | 0.1339(2) | 0.4849(2) | 0.024(1) | 0.018(1) | 0.018(1) | −0.005(1) | −0.004(1) | −0.007(1) |
| C(4B) | 2i | 0.5914(3) | 0.2030(2) | 0.4547(2) | 0.025(1) | 0.016(1) | 0.021(1) | −0.003(1) | 0.000(1) | −0.006(1) |
| C(5B) | 2i | 0.5922(3) | 0.2136(2) | 0.3393(2) | 0.029(1) | 0.015(1) | 0.021(1) | −0.001(1) | −0.005(1) | −0.005(1) |
| C(6B) | 2i | 0.6749(3) | 0.1072(2) | 0.3045(2) | 0.013(1) | 0.014(1) | 0.016(1) | −0.0045(9) | −0.0002(9) | −0.003(1) |
| C(7B) | 2i | 0.6890(3) | 0.0880(2) | 0.2029(2) | 0.014(1) | 0.012(1) | 0.016(1) | −0.0050(9) | −0.0022(9) | −0.001(1) |
| C(8B) | 2i | 0.6224(3) | 0.1603(2) | 0.1162(2) | 0.015(1) | 0.016(1) | 0.017(1) | −0.004(1) | 0.0002(9) | −0.003(1) |
| C(9B) | 2i | 0.7823(3) | −0.0147(2) | 0.1916(2) | 0.014(1) | 0.013(1) | 0.021(1) | −0.0041(9) | −0.0036(9) | −0.003(1) |
| C(10B) | 2i | 0.7513(3) | 0.0080(2) | 0.0267(2) | 0.013(1) | 0.012(1) | 0.021(1) | −0.0051(9) | 0.001(1) | −0.002(1) |
| C(11B) | 2i | 0.7690(3) | −0.0260(2) | −0.0721(2) | 0.015(1) | 0.019(1) | 0.014(1) | −0.008(1) | 0.0007(9) | −0.004(1) |
| C(12B) | 2i | 0.6734(3) | 0.0340(2) | −0.1469(2) | 0.018(1) | 0.022(1) | 0.019(1) | −0.007(1) | 0.001(1) | −0.002(1) |
| C(13B) | 2i | 0.6865(3) | −0.0044(2) | −0.2355(2) | 0.021(1) | 0.034(2) | 0.017(1) | −0.012(1) | −0.002(1) | −0.000(1) |
| C(14B) | 2i | 0.7982(3) | −0.1019(2) | −0.2514(2) | 0.028(1) | 0.033(2) | 0.016(1) | −0.017(1) | 0.002(1) | −0.008(1) |
| C(15B) | 2i | 0.8977(3) | −0.1610(2) | −0.1779(2) | 0.026(1) | 0.022(1) | 0.021(1) | −0.009(1) | 0.003(1) | −0.009(1) |
| C(16B) | 2i | 0.8823(3) | −0.1238(2) | −0.0882(2) | 0.021(1) | 0.018(1) | 0.019(1) | −0.008(1) | −0.002(1) | −0.003(1) |
Source of material
A mixture of 4,5,6,7-tetrahydrobenzo[b]thiophene-2-amino-3-carboxylic acid (0.01 mol) in dry pyridine (30 mL) and benzoyl chloride (0.02 mol) was stirred for 1 h in an ice bath. After cooling, the reaction mixture was acidified with ice-cold hydrochloric acid. The solid formed after complete acidification was collected and recrystallized to give 4,5,6,7-tetrahydrobenzothiophene-2-benzoylamino-3-carboxylic acid. A mixture of 4,5,6,7-tetrahydrobenzothiophene-2-benzoylamino-3-carboxylic acid (0.01 mol) and acetic anhydride (10 mL) was heated on a water bath for 5 h. The solid formed after removal of excess acetic anhydride was triturated with petroleum ether (40–60°C) and recrystallized from petroleum ether (80–100°C) to give 2-phenyl-5,6,7,8-tetrahydro-4H-benzo[4,5]thieno[2,3-d][1,3]oxazin-4-one as yellow crystals; mp 137–140°C [1].
Experimental details
Cell refinement and data reduction were carried out by Bruker SAINT and APEX2 [16]. The hydrogen atoms were placed on calculated positions using a riding model (AFIX 23 or 43 option of the SHELX program [17]).
Discussion
Tetrahydrobenzothiophene derivatives have many biological activities, such as antivirus, antibacterial, insecticides, acaricides, and antifungal [2, 3] as well as anticancer, antitumor, antihistaminic and anti-inflammatory activities [4–14]. Furthermore, tetrahydrobenzothiophene derivatives were reported to inhibit hepatitis C virus NS5B polymerase [15].
The asymmetric unit of the crystal structure contains two independent molecules with little difference in bond lengths and angles. In molecule A, the dihedral angle between the phenyl ring (C12—C16) and the thieno[2,3-d][1,3]oxazin-4-one moiety (S1/C1-C10/O2/N1) is 4.54(2)°, whereas this value in molecule B slightly is larger (12.44°). The molecules in the crystal structure at least interact via two non-classical intermolecular hydrogen bonds, of which O1B and S1B work as hydrogen bond acceptors and C14A and C12A work as hydrogen bond donors. The distance of the interactions between C14A—H14A⋯O1Bii and C12A—H12A⋯S1Bi are 2.59 and 2.84 Å, respectively and the angles are 153 and 175°, respectively. Symmetry codes: (i) −x+1, −y, −z+1; (ii) x, y, z+1.
The almost flat molecules are stacked along the a direction with distances suggesting attractive interactions.
Acknowledgements:
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project NO (RGP-1436–038).
References
1. Abdalha, A. A.; Abou El-Regal, M. K.; El-Kassaby, M. A.; Ali, A. T.: Synthesis of some new tetrahydrobenzo[b]thiophene derivatives and tetrahydrobenzothieno pyrimidine derivatives under microwave irradiation. Synth. Commun. 41 (2011) 2811–2821.10.1080/00397911.2010.501479Search in Google Scholar
2. Tserng, K. Y.; Bauer, L.: Synthesis of 3-hydroxythieno pyrimidine-2,4-(1H,3H)-diones from 2,3 and 3,4 thiophene dicarboxylic acids. J. Org. Chem. 40 (1975) 172–175.10.1021/jo00890a004Search in Google Scholar
3. Nielsen, K. E.; Pederson, E. B.: Phosphoramides, VII: Phenyl N,N'-dimethyl phosphordiamidate as a reagent for synthesis of 3-methylthieno[2,3-d]pyrimidine derivatives. Acta Chem. Scand. B32 (1978) 302.Search in Google Scholar
4. Bhuiyan, M. H.; Rahman, M.; Mizanur, M.; Hossain, K.; Rahim, A.; Hossain, M. I.; Abu Naser, M.: Synthesis and antimicrobial evaluation of some new thienopyrimidine derivatives. Acta Pharm. 56 (2006) 441–450.Search in Google Scholar
5. Bedair, A. H.; El-Hady, N. A.; Abdel-Latif, M. S.; Fakery, A. H.; El-Agrody, A. M.: 4-Hydroxycoumarin in heterocyclic synthesis, part II: Synthesis of some new pyrano[2,3-d]pyrimidine, 2-substituted[1,2,4]triazolo[1,5-c]pyrimidine, and pyrimido[1,6-b][1,2,4]triazine derivatives. Farmaco 55 (2000) 708–714..10.1016/S0014-827X(00)00097-5Search in Google Scholar
6. Ghorab, M. M.; Abdel-Gawad, S. M.; El-Gaby, M. S. A.: Synthesis and evaluation of some new fluorinated hydroquinazoline derivatives as antifungal agents. Farmaco 55 (2000) 249–255.10.1016/S0014-827X(00)00029-XSearch in Google Scholar
7. El-Gaby, M. S. A.; Abdel-Hamide, S. G.; Ghorab, M. M.; El-Sayed, S. M.: Synthesis and anticancer activity in vitro of some new pyrimidines. Acta Pharm. 49 (1999) 149–158.Search in Google Scholar
8. Nasr, M. N., Gineinah, M. M.: Pyrido[2,3-d]pyrimidines and pyrimido[5′,4′:5,6] pyrido[2,3-d]pyrimidines as new antiviral agents: Synthesis and biological activity. Arch. Pharm. 335 (2002) 289–295.10.1002/chin.200250118Search in Google Scholar
9. Baraldi, P. G.; Pavani, M. G.; Nunez, M.; Brigidi, P.; Vitali, B.; Gambari, R.; Romagnoli, R.: Antimicrobial and antitumor activity of n-hetero-immine-1,2,3-dithiazoles and their transformation in triazolo-, imidazo-, and pyrazolo pyrimidines. Bioorg. Med. Chem. 10 (2002) 449–456.10.1016/S0968-0896(01)00294-2Search in Google Scholar
10. Sondhi, S. M.; Johar, M.; Rajvanshi, S.; Dastidar, S. G.; Shukla, R.; Raghubir, R.; Lown, J. W.: Anticancer, anti-inflammatory, and analgesic activity evaluation of heterocyclic compounds synthesized by reaction of 4-isothiocyanato-4-methylpentan-2-one with substituted phenylenediamines, o-diamino pyridine and (un)substituted. Aust. J. Chem. 54 (2001) 69–74.10.1071/CH00141Search in Google Scholar
11. Shishoo, C. J.; Shirsath, V. S.; Rathod, I. S.; Patil, M. J.; Bhargava, S. S.: Design, synthesis and antihistaminic (H1) activity of some condensed 2-(substituted) aryl aminoethylpyrimidin-4(3H)-ones. Arzneimittelforsch. 51 (2001) 221–231.10.1055/s-0031-1300028Search in Google Scholar
12. Bruno, O.; Brullo, C.; Schenone, S.; Ranise, A.; Bondavalli, F.; Barocelli, E.; Tognolini, M.; Magnanini, F.; Bollabeni, V.: Progress in 5H[1]benzopyrano[4,3-d]pyrimidin-5-amine series: 2-methoxy derivatives effective as antiplatet agents with analgesic activity. Farmaco 57 (2002) 753–758.10.1016/S0014-827X(02)01269-7Search in Google Scholar
13. Aluagarsamy, V.; Pathak, U. S.; Rajasolomon, V.; Meena, S.; Ramseshu, K. V.; Rajesh R.: Anticancer, antibacterial and antifungal activities of some 2-substituted-1,3,4-thiazolo(2,3-b)tetrahydrobenzo[b]thieno(3,2-e)pyrimidines. Indian J. Heterocycl. Chem. 13 (2004) 347–350.Search in Google Scholar
14. Gouda, M. A.; Berghot, M. A.; Abd El-Ghani, G. E.; Khalil, A. M.: Synthesis and antimicrobial activities of some new thiazole and pyrazole derivatives based on 4,5,6,7-tetrahydrobenzothiophene moiety. Eur. J. Med. Chem. (2010) 1–8.Search in Google Scholar
15. Laporte, M. G.; Lessen, T. A.; Leister, L.; Cebzanov, D.; Amparo, E.; Faust, C.; Ortlip, D.; Bailey, T. R.; Nitz, T. J.; Chunduru, S. K.; Young, D. C.; Burns, C. J.: Tetrahydrothiophene inhibitors of hepatitis C virus NS5B polymerase. Bioorg. & Med. Chem. Lett. 16 (2006) 100–103.10.1016/j.bmcl.2005.09.047Search in Google Scholar PubMed
16. Brucker: APEX2, SAINT and SADABS. Brucker AXS Inc., Madison, Wisconsin, USA, 2009.Search in Google Scholar
17. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
©2016 Hazem A. Ghabbour, published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of fac-hexacarbonylbisμ2-(3-carboxy-3′-carboxylato-2,2′-bipyridine)-κ3N,N′:O-dirhenium(I) tetrahydrate, C30H22N4O18Re2
- The crystal structure of bis(4-(2,4-dimethylphenyl)piperazin-1-yl)methane, C25H36N4
- Crystal structure of bis(triphenylphosphine-κP)bis(μ2-1H,1′H-2,2′-biimidazole-κ3N,N′:N′)disilver(I) bis(tetrafluoroborate), C48H42Ag2B2F8N8P2
- The crystal structure of 1,2-bis(2-pyrazinecarboxamido)-benzene, C16H12N6O2
- Redetermination of the crystal structure of 3-bromobenzoic acid, C7H5BrO2
- Crystal structure of bis(ethyltriphenylphos-phonium) tetrabromidocuprate(II), (C20H20P)2[CuBr4]
- Crystal structure of 2-(4-bromophenyl)-1,3-dimethyl-2,3-dihydro-1H-perimidine, C19H17BrN2
- Crystal structure of trans-diaqua-bis(3-(pyrazin-2-yl)-5-(pyridin-4-yl)1,2,4-triazol-1-ido-κ2N,N′)-cobalt(II),C22H18CoN12O2
- Crystal structure of hexaaquamanganese(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MnN6O12S2
- Crystal structure of butyl 2-(3,5-dimethyl-1,1-dioxido-2H-1,2,6-thiadiazin-4-yl)benzoate, C16H20N2O4S
- Crystal structure of hexaaquabis(μ2-3-(6-carboxylatopyridin-2-yl)-5-(pyrazin-2-yl)-1,2,4-triazol-1-ido)bis(μ3-3-(6-carboxylatopyridin-2-yl)-5-(pyrazin-2-yl)-1,2,4-triazol-1-ido)tetra-manganese(II) dihydrate, C48H40Mn4N24O16
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmate(II)] bis(2-aminoisonicotinate) tetrahydrate, C38H50CdN8O10
- Crystal structure of succinic acid — 4-((pyridin-4-ylmethyl)sulfanylpyridine (1/1), C15H16N2O4S
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)bis(2-((pyridin-4-ylmethyl)sulfanyl)pyridine-κN)dicopper(II), C30H32N4O8S2Cu2
- Crystal structure of 1-((2R,3S)-2,3-dimethyl-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)-2-phenoxyethan-1-one, C18H19NO3
- Crystal structure of tetramethylammonium sulfanilate, C10H18N2O3S
- Crystal structure of triethylammonium 2′-carboxy-[1,1′-biphenyl]-2-carboxylate, C20H25NO4
- Crystal structure of 2-(4-acetyl-2,6-dimethylphenyl)-5,6-dichloro-1H-isoindole-1,3(2H)-dione, C18H13Cl2NO3
- Crystal structure of diaqua-bis(μ3-2-methyl-6-oxidopyridinium-4-carboxylato-κ3O:O′:O′′)neodymium(III) chloride, C14H16ClN2O8Nd
- Crystal structure of 5-methoxy-4-methyl-2-(2-methylbenzyl)-2,4-dihydro-3H-1,2,4-triazol-3-one, C12H15N3O2
- The crystal structure of 1-(4-(2-chloroethoxy)phenyl)ethanone
- Crystal structure of (E)-4-(2-(4-(diethylamino)phenyl)diazen-1-ium-1-yl)benzenesulfonate monohydrate
- Crystal structure of 2,2′-[(1E)-prop-1-ene-1,2-diyldisulfanediyl]bis(5-methyl-2,5-dihydro-1,3,4-thiadiazole, C9H10N4S4
- Crystal structure of poly [μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)cerium(III)] monohydrate (C10H13O9Ce)
- Crystal structure of tris((2-(2,2-dicyanovinyl)phenoxy)ethyl)amine, C36H27N7O3
- Crystal structure of catena[diaqua-bis(μ2-1,3-bis((1H-tetrazol-1-yl)methyl)benzene-κ2N:N′)copper(II)] dinitrate, C20H24CuN18O8
- Crystal structure of (4-(1H-imidazol-5-yl)benzoic acid-κN) (4-(1H-imidazol-5-yl)benzoato-κN)silver(I), C20H15N4O4Ag
- Crystal structure of 2-amino-3-cyano-7,7-dimethyl-5-oxo-4-(3,4,5-trifluorophenyl)-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C18H15F3N2O2
- Crystal structure of (2-(2-chlorophenyl)-5-ethyl-1,3-dioxan-5-yl)methanol hemihydrate, C13H17ClO3 · 0.5 H2O
- Crystal structure of catena-poly[diaqua-bis(benzene-1,2,4,5-tetracarboxylato-κN)(m2-2-(1H-1,2,4-trizol-1-ylmethyl)-1H-3,1-benzimidazol-3-ium-κ2O:O′)zinc(II)] dihydrate, C30H30N10O12Zn
- Crystal structure of [2,2′-((((ethane-1,2-diylbis(oxy-κ2O,O′))bis(2,1-phenylene))bis(azanylylidene-κ2N,N′))bis(methanylylidene))diphenolato-κ2O′′,O′′′]zinc(II), C28H22N2O4Zn
- Crystal structure of (E)-1-(3,4-dimethoxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C13H17NO3
- Crystal structure of bis(2-fluoro-4-nitrophenyl) terephthalate C20H10F2N2O8
- Crystal structure of catena-[aqua((4-carboxyphenyl)acetato-κO)(μ2-(4-carboxyphenyl)acetato-κ2O:O′)bis(4,4′-ethene-1,2-diyldipyridine-κN)manganese(II)], C42H36N4O9Mn
- Crystal structure of N′-(2-hydroxybenzylidene)-3,4-dimethyl-1H-pyrrole-2-carbohydrazide, C14H15N3O2
- Crystal structure of bis(8-ethyl-5-oxo-2-(piperazin-4-ium-1-yl)-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylato-κ2O,O′)copper(II) benzene-1,4-dicarboxylate dihydrate, C36H42CuN10O12
- Crystal structure of diaquabis(μ2-biphenyl-2,2′-dicarboxylato-κ2O:O′)bis(1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylato)zinc(II), C60H56N6Zn2O16F2
- Crystal structure of 2-amino-4-(4-fluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13FN2O2
- The crystal structure of hexaqua(μ2-3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-1κ2O,O′;2κO′)(3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-κO)(3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-κ2O,O′)digadolinium(III) octahydrate, C60H76Gd2N18O32
- Crystal structure of hexacarbonyl bis(μ2-2-methoxybenzenethiolato-κ2S)pyridine(triphenylphosphane)dirhenium(I), C43H34NO8PS2Re2
- Crystal structure of 14-((1-(benzyloxycarbonyl-amino)-2-methylpropan-2-yl)sulfanyl)acetate Mutilin, C34H49NO6S
- Crystal structure of 2-methoxy-6-(((2-(1-methyl-1H-benzo[d] imidazol-2-yl)phenyl)imino)methyl)phenol — ethanol (1/1), C24H25N3O3
- Crystal structure of 2-(bis(methylthio)methylene)-1-phenylbutane-1,3-dione, C13H14O2S2
- Crystal structure of (E)-2-(bis(methylthio)methylene)-1-phenyl-3-(2-phenylhydrazono)butan-1-one, C19H20N2OS2
- Crystal structure of dichlorido(2-(1-methyl-1H-benzo[d]imidazol-2-yl)aniline-κ2N,N′)zinc(II)
- Crystal structure of bis(1-methyl-1H-tetrazole-5-thiolato)mercury(II)
- Crystal structure of (E)-2-styryl-1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C24H19FeN3O2S
- Crystal structure of aquabis(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-4-ium-1-yl)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)copper(II) thiophene-2,5-dicarboxylate trihydrate, [Cu(C17H18N3FO3)2(H2O)](C6H2SO4)·3(H2O)
- Crystal structure of 2-amino-4-(2,6-dichlorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16Cl2N2O2
- Crystal structure of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazatetracyclo[5·5·0·05·9·03·11]dodecane 1/3 hydrate, C6H8N12O13
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ2O,O′)(triphenylarsine-κAs)rhodium(I), C25H20AsN2O3Rh
- The crystal structure of 1-(4-(4-chlorophenoxy)-2-chlorophenyl)ethanone, C14H10Cl2O2
- Crystal structure of N,N-diethyl-2-(2-(6-(4-methoxybenzyl)-7-oxo-7H-thiazolo[3,3-b][1,2,4]triazin-3-yl)phenoxy)acetamide, C25H26N4O4S
- Crystal structure of tetraqua((E)-4,4′-(diazene-1,2-diyl)bis(5-oxo-4,5-dihydro-1,2,4-triazol-1-ide)-κ2N:O)barium(II), C4H10N8O6Ba
- Crystal structure of 2-amino-4-(3-phenoxyphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C22H18N2O3
- Crystal structure of diethylammonium 5-((4-fluorophenyl)(6-hydroxy-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate, C23H30FN5O6
- Crystal structure of 2-amino-4-(3,5-difluoro-phenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12F2N2O2
- Crystal structure of tris(N-nitroso-N-oxyanilino-κ2O, O′) oxidoniobium(V), C18H15N6O7Nb
- Crystal structure of 1-(5-benzoyl-4-methyl-2-(phenylamino)thiophen-3-yl)ethan-1-one, a structure with Z′ = 6, C20H17NO2S
- Crystal structure of diethyl-3-methyl-4-phenylthieno[2,3-b]thiophene-2,5-dicarboxylate, C19H18O4S2
- Crystal structure of 3,5-dicarboxybenzoate — benzene-1,3,5-tricarboxylic acid (1/1), C24H22N2O12
- The crystal structure of 2-chloro-1,3-bis(2,4,6-trimethylphenyl)-4,4-dimethyl-1,3,2λ3,4-diazaphosphasiletidine
- Crystal structure of hexaquamanganese(II) bis(hexaborato-κ3O,O′,O′′)manganese(II) dihydrate, B12H28Mn2O34
- Crystal structure of 1-propyl-3-methylimidazolium pentaborate, [C7H13N2][B5O6(OH)4]
- Crystal structure of 13-(4-fluorophenyl)-11,13-dihydro-1H-benzo[h]indazolo[6,7-b] [1, 6]naphthyridin-12(6H)-one — dimethylformamide — water (1/2/1), C29H31FN6O4
- Crystal structure of 1-(2-chlorophenyl)-2-(2-nitrophenyl)ethan-1-ol, C14H12ClNO3
- Crystal structure of (Z)-2-((2-bromo-1-phenylvinyl)oxy)benzonitrile, C15H10BrNO
- Crystal structure of tetrachlorido(1E,1′E)-N,N′-((1,4-phenylenebis(propane-2,2-diyl))bis(4,1-phenylene))bis(1-(pyridin-2-yl-κN)methanimine-κN)dizinc(II), C36H34N4Zn2Cl4
- Crystal structure of 2,6-bis(3-methylpyridinyl)hexahydro-4,8-ethenopyrrolo-[3,4-f]isoindole-1,3,5,7(2H,6H)-tetrone, C24H20N4O4
- Crystal structure of trans-bis(2-methylmaleato-κ2O,O′) bis(piperazinium-κN) cobalt(II) trihydrate, C18H36CoN4O11
- Crystal structure of (E)-4-chloro-N′-(4-(diethylamino)benzylidene)benzohydrazide, C18H20ClN3O
- Crystal structure of 3,6-di(1H-imidazol-1-yl)-9H-carbazole, C18H13N5
- Crystal structure of 4-(4-pyridinyl)-1-naphthoic acid, C16H11NO2
- Crystal structure of 1,1′-diformyl-4,4′-(6H,12H-5,11-methano-dibenzo[b,f][11,5]diazocine-2,8-diyl)dibenzene, C29H22N2O2
- Crystal structure of N′-(adamantan-2-ylidene)pyridine-3-carbohydrazide, C16H19N3O
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(5-methyl-1H-pyrazole-3-carbaldehyde), C14H18N4O2
- Crystal structure of methyl 8-hydroxy-3-isopropyl-5a,8-dimethyl-2,3,4,5,5a,6,7,8,10a,10b-decahydrocyclohepta[e]indene-3a(1H)-carboxylate, C21H34O3
- Crystal structure of 6-oxo-4-propyl-2-(propylthio)-1,6-dihydropyrimidine-5-carbonitrile, C11H15N3OS
- Crystal structure of poly[diacetato(μ2-1,4-bis(1H-imidazol-1-yl)benzene-κ2N:N′)nickel(II)], C26H22N8NiO4
- Crystal structure of bis(2,4-dibromo-6-{(E)[(4-fluorobenzyl)imino]methyl}phenolato-κ2N,O) copper(II), C28H18Br4F2N2O2Cu
- Crystal structure of 1-(adamantan-1-yl)-3-phenylthiourea, C17H22N2S
- Crystal structure of 3-(6-(5-amino-1-phenyl-1H-pyrazol-3-yl)pyridin-2-yl)-1-phenyl-1H-pyrazol-5-amine – dioxan (2/1), C25H23N7O
- Crystal structure of 5-ethyl-6-[(3-methylphenyl)sulfanyl]pyrimidine-2,4(1H,3H)-dione, C13H14N2O2S
- Crystal structure of (((1E,1′E)-(cyclohexane-1,2-diylbis(azanylylidene-κ2N,N′))bis(methanylylidene))bis(2,1-phenylene))bis((2,6-diisopropylphenyl)amide-κ2N′′,N′′′)manganese(II), C44H54N4Mn
- Crystal structure of prop-2-en-1-yl 2-oxo-2H-1-benzopyran-3-carboxylate, C13H10O4
- Crystal structure of bis(μ2-2-((3-methylphenyl)imino)methylphenolato-κ2N,O:O)hexacarbonyldimanganese(I), C34H24Mn2N2O8
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxybut-2-en-1-one C9H12N2O2
- Crystal structure of 2,2′-[pentane-1,5-diylbis(oxy)]dibenzaldehyde, C19H20O4
- Crystal structure of 2-phenyl-5,6,7,8-tetrahydro-4H-benzo[4,5]thieno[2,3-d][1,3]oxazin-4-one, C16H13NO2S
- Crystal structure of (E)-1-(2-chlorophenyl)-N-(4-chlorophenyl)methanimine, C13H9Cl2N
- Crystal structure of ethyl 2-amino-5-bromothiazole-4-carboxylate, C6H7BrN2O2S
- Crystal structure of 2-benzylisothiouronium tetraphenylborate, C32H31BN2S
- Crystal structure of poly[(μ2-biphenyl-2,2′-dicarboxylato-κ4O,O′:O′′,O′′′)(μ2-4,4′-bipyridine-κ2N:N′)copper(II)], C24H16CuN2O4
- Crystal structure of (η5-pentamethylcyclopentadienyl)titanium(III)dichloride (THF), C14H23Cl2OTi
- Crystal structure of 3-ferrocenylsulfonyl-2-(4-methoxyphenyl)-3H-imidazo[4,5-b]pyridine, C23H19FeN3O3S
- Crystal structure of 2-benzoyl-3-(4-fluorophenyl)cyclopropane-1,1-dicarbonitrile, C18H11FN2O
- Crystal structure of 1,6-ditosyl-1,6-diazecane, C22H30N2O4S2
- Crystal structure of N-phenyl-2-(pyridin-4-ylcarbonyl)hydrazinecarboxamide with Z′ = 4, C13H12N4O2
- Crystal structure of N,N-bis(diphenylphosphanyl)cyclohexylamine, C30H31NP2
- Crystal structure of 3-(4-hydroxy-3-methoxyphenyl)-N-phenylpropanamide, C16H17NO3
- Crystal structure of 6-(2-fluorophenyl)-3-phenyl-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazole, C15H9FN4S
- Crystal structure of catena-poly[aqua(dicyanoazanido-2κN-μ2-dicyanoazanido-1κN:2κN′)(μ2-2-methoxy-6-(((2-((3-methoxy-2-oxidobenzylidene)amino)ethyl)imino)methyl)phenolato-1κ2N,N′,2κ2O,O′,1κ2O′′,O′′′:2κ2O′′,O′′′)cadmium(II)copper(II)], C22H20CdCuN8O5
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of fac-hexacarbonylbisμ2-(3-carboxy-3′-carboxylato-2,2′-bipyridine)-κ3N,N′:O-dirhenium(I) tetrahydrate, C30H22N4O18Re2
- The crystal structure of bis(4-(2,4-dimethylphenyl)piperazin-1-yl)methane, C25H36N4
- Crystal structure of bis(triphenylphosphine-κP)bis(μ2-1H,1′H-2,2′-biimidazole-κ3N,N′:N′)disilver(I) bis(tetrafluoroborate), C48H42Ag2B2F8N8P2
- The crystal structure of 1,2-bis(2-pyrazinecarboxamido)-benzene, C16H12N6O2
- Redetermination of the crystal structure of 3-bromobenzoic acid, C7H5BrO2
- Crystal structure of bis(ethyltriphenylphos-phonium) tetrabromidocuprate(II), (C20H20P)2[CuBr4]
- Crystal structure of 2-(4-bromophenyl)-1,3-dimethyl-2,3-dihydro-1H-perimidine, C19H17BrN2
- Crystal structure of trans-diaqua-bis(3-(pyrazin-2-yl)-5-(pyridin-4-yl)1,2,4-triazol-1-ido-κ2N,N′)-cobalt(II),C22H18CoN12O2
- Crystal structure of hexaaquamanganese(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MnN6O12S2
- Crystal structure of butyl 2-(3,5-dimethyl-1,1-dioxido-2H-1,2,6-thiadiazin-4-yl)benzoate, C16H20N2O4S
- Crystal structure of hexaaquabis(μ2-3-(6-carboxylatopyridin-2-yl)-5-(pyrazin-2-yl)-1,2,4-triazol-1-ido)bis(μ3-3-(6-carboxylatopyridin-2-yl)-5-(pyrazin-2-yl)-1,2,4-triazol-1-ido)tetra-manganese(II) dihydrate, C48H40Mn4N24O16
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmate(II)] bis(2-aminoisonicotinate) tetrahydrate, C38H50CdN8O10
- Crystal structure of succinic acid — 4-((pyridin-4-ylmethyl)sulfanylpyridine (1/1), C15H16N2O4S
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)bis(2-((pyridin-4-ylmethyl)sulfanyl)pyridine-κN)dicopper(II), C30H32N4O8S2Cu2
- Crystal structure of 1-((2R,3S)-2,3-dimethyl-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)-2-phenoxyethan-1-one, C18H19NO3
- Crystal structure of tetramethylammonium sulfanilate, C10H18N2O3S
- Crystal structure of triethylammonium 2′-carboxy-[1,1′-biphenyl]-2-carboxylate, C20H25NO4
- Crystal structure of 2-(4-acetyl-2,6-dimethylphenyl)-5,6-dichloro-1H-isoindole-1,3(2H)-dione, C18H13Cl2NO3
- Crystal structure of diaqua-bis(μ3-2-methyl-6-oxidopyridinium-4-carboxylato-κ3O:O′:O′′)neodymium(III) chloride, C14H16ClN2O8Nd
- Crystal structure of 5-methoxy-4-methyl-2-(2-methylbenzyl)-2,4-dihydro-3H-1,2,4-triazol-3-one, C12H15N3O2
- The crystal structure of 1-(4-(2-chloroethoxy)phenyl)ethanone
- Crystal structure of (E)-4-(2-(4-(diethylamino)phenyl)diazen-1-ium-1-yl)benzenesulfonate monohydrate
- Crystal structure of 2,2′-[(1E)-prop-1-ene-1,2-diyldisulfanediyl]bis(5-methyl-2,5-dihydro-1,3,4-thiadiazole, C9H10N4S4
- Crystal structure of poly [μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)cerium(III)] monohydrate (C10H13O9Ce)
- Crystal structure of tris((2-(2,2-dicyanovinyl)phenoxy)ethyl)amine, C36H27N7O3
- Crystal structure of catena[diaqua-bis(μ2-1,3-bis((1H-tetrazol-1-yl)methyl)benzene-κ2N:N′)copper(II)] dinitrate, C20H24CuN18O8
- Crystal structure of (4-(1H-imidazol-5-yl)benzoic acid-κN) (4-(1H-imidazol-5-yl)benzoato-κN)silver(I), C20H15N4O4Ag
- Crystal structure of 2-amino-3-cyano-7,7-dimethyl-5-oxo-4-(3,4,5-trifluorophenyl)-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C18H15F3N2O2
- Crystal structure of (2-(2-chlorophenyl)-5-ethyl-1,3-dioxan-5-yl)methanol hemihydrate, C13H17ClO3 · 0.5 H2O
- Crystal structure of catena-poly[diaqua-bis(benzene-1,2,4,5-tetracarboxylato-κN)(m2-2-(1H-1,2,4-trizol-1-ylmethyl)-1H-3,1-benzimidazol-3-ium-κ2O:O′)zinc(II)] dihydrate, C30H30N10O12Zn
- Crystal structure of [2,2′-((((ethane-1,2-diylbis(oxy-κ2O,O′))bis(2,1-phenylene))bis(azanylylidene-κ2N,N′))bis(methanylylidene))diphenolato-κ2O′′,O′′′]zinc(II), C28H22N2O4Zn
- Crystal structure of (E)-1-(3,4-dimethoxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C13H17NO3
- Crystal structure of bis(2-fluoro-4-nitrophenyl) terephthalate C20H10F2N2O8
- Crystal structure of catena-[aqua((4-carboxyphenyl)acetato-κO)(μ2-(4-carboxyphenyl)acetato-κ2O:O′)bis(4,4′-ethene-1,2-diyldipyridine-κN)manganese(II)], C42H36N4O9Mn
- Crystal structure of N′-(2-hydroxybenzylidene)-3,4-dimethyl-1H-pyrrole-2-carbohydrazide, C14H15N3O2
- Crystal structure of bis(8-ethyl-5-oxo-2-(piperazin-4-ium-1-yl)-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylato-κ2O,O′)copper(II) benzene-1,4-dicarboxylate dihydrate, C36H42CuN10O12
- Crystal structure of diaquabis(μ2-biphenyl-2,2′-dicarboxylato-κ2O:O′)bis(1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylato)zinc(II), C60H56N6Zn2O16F2
- Crystal structure of 2-amino-4-(4-fluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13FN2O2
- The crystal structure of hexaqua(μ2-3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-1κ2O,O′;2κO′)(3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-κO)(3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-κ2O,O′)digadolinium(III) octahydrate, C60H76Gd2N18O32
- Crystal structure of hexacarbonyl bis(μ2-2-methoxybenzenethiolato-κ2S)pyridine(triphenylphosphane)dirhenium(I), C43H34NO8PS2Re2
- Crystal structure of 14-((1-(benzyloxycarbonyl-amino)-2-methylpropan-2-yl)sulfanyl)acetate Mutilin, C34H49NO6S
- Crystal structure of 2-methoxy-6-(((2-(1-methyl-1H-benzo[d] imidazol-2-yl)phenyl)imino)methyl)phenol — ethanol (1/1), C24H25N3O3
- Crystal structure of 2-(bis(methylthio)methylene)-1-phenylbutane-1,3-dione, C13H14O2S2
- Crystal structure of (E)-2-(bis(methylthio)methylene)-1-phenyl-3-(2-phenylhydrazono)butan-1-one, C19H20N2OS2
- Crystal structure of dichlorido(2-(1-methyl-1H-benzo[d]imidazol-2-yl)aniline-κ2N,N′)zinc(II)
- Crystal structure of bis(1-methyl-1H-tetrazole-5-thiolato)mercury(II)
- Crystal structure of (E)-2-styryl-1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C24H19FeN3O2S
- Crystal structure of aquabis(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-4-ium-1-yl)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)copper(II) thiophene-2,5-dicarboxylate trihydrate, [Cu(C17H18N3FO3)2(H2O)](C6H2SO4)·3(H2O)
- Crystal structure of 2-amino-4-(2,6-dichlorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16Cl2N2O2
- Crystal structure of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazatetracyclo[5·5·0·05·9·03·11]dodecane 1/3 hydrate, C6H8N12O13
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ2O,O′)(triphenylarsine-κAs)rhodium(I), C25H20AsN2O3Rh
- The crystal structure of 1-(4-(4-chlorophenoxy)-2-chlorophenyl)ethanone, C14H10Cl2O2
- Crystal structure of N,N-diethyl-2-(2-(6-(4-methoxybenzyl)-7-oxo-7H-thiazolo[3,3-b][1,2,4]triazin-3-yl)phenoxy)acetamide, C25H26N4O4S
- Crystal structure of tetraqua((E)-4,4′-(diazene-1,2-diyl)bis(5-oxo-4,5-dihydro-1,2,4-triazol-1-ide)-κ2N:O)barium(II), C4H10N8O6Ba
- Crystal structure of 2-amino-4-(3-phenoxyphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C22H18N2O3
- Crystal structure of diethylammonium 5-((4-fluorophenyl)(6-hydroxy-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate, C23H30FN5O6
- Crystal structure of 2-amino-4-(3,5-difluoro-phenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12F2N2O2
- Crystal structure of tris(N-nitroso-N-oxyanilino-κ2O, O′) oxidoniobium(V), C18H15N6O7Nb
- Crystal structure of 1-(5-benzoyl-4-methyl-2-(phenylamino)thiophen-3-yl)ethan-1-one, a structure with Z′ = 6, C20H17NO2S
- Crystal structure of diethyl-3-methyl-4-phenylthieno[2,3-b]thiophene-2,5-dicarboxylate, C19H18O4S2
- Crystal structure of 3,5-dicarboxybenzoate — benzene-1,3,5-tricarboxylic acid (1/1), C24H22N2O12
- The crystal structure of 2-chloro-1,3-bis(2,4,6-trimethylphenyl)-4,4-dimethyl-1,3,2λ3,4-diazaphosphasiletidine
- Crystal structure of hexaquamanganese(II) bis(hexaborato-κ3O,O′,O′′)manganese(II) dihydrate, B12H28Mn2O34
- Crystal structure of 1-propyl-3-methylimidazolium pentaborate, [C7H13N2][B5O6(OH)4]
- Crystal structure of 13-(4-fluorophenyl)-11,13-dihydro-1H-benzo[h]indazolo[6,7-b] [1, 6]naphthyridin-12(6H)-one — dimethylformamide — water (1/2/1), C29H31FN6O4
- Crystal structure of 1-(2-chlorophenyl)-2-(2-nitrophenyl)ethan-1-ol, C14H12ClNO3
- Crystal structure of (Z)-2-((2-bromo-1-phenylvinyl)oxy)benzonitrile, C15H10BrNO
- Crystal structure of tetrachlorido(1E,1′E)-N,N′-((1,4-phenylenebis(propane-2,2-diyl))bis(4,1-phenylene))bis(1-(pyridin-2-yl-κN)methanimine-κN)dizinc(II), C36H34N4Zn2Cl4
- Crystal structure of 2,6-bis(3-methylpyridinyl)hexahydro-4,8-ethenopyrrolo-[3,4-f]isoindole-1,3,5,7(2H,6H)-tetrone, C24H20N4O4
- Crystal structure of trans-bis(2-methylmaleato-κ2O,O′) bis(piperazinium-κN) cobalt(II) trihydrate, C18H36CoN4O11
- Crystal structure of (E)-4-chloro-N′-(4-(diethylamino)benzylidene)benzohydrazide, C18H20ClN3O
- Crystal structure of 3,6-di(1H-imidazol-1-yl)-9H-carbazole, C18H13N5
- Crystal structure of 4-(4-pyridinyl)-1-naphthoic acid, C16H11NO2
- Crystal structure of 1,1′-diformyl-4,4′-(6H,12H-5,11-methano-dibenzo[b,f][11,5]diazocine-2,8-diyl)dibenzene, C29H22N2O2
- Crystal structure of N′-(adamantan-2-ylidene)pyridine-3-carbohydrazide, C16H19N3O
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(5-methyl-1H-pyrazole-3-carbaldehyde), C14H18N4O2
- Crystal structure of methyl 8-hydroxy-3-isopropyl-5a,8-dimethyl-2,3,4,5,5a,6,7,8,10a,10b-decahydrocyclohepta[e]indene-3a(1H)-carboxylate, C21H34O3
- Crystal structure of 6-oxo-4-propyl-2-(propylthio)-1,6-dihydropyrimidine-5-carbonitrile, C11H15N3OS
- Crystal structure of poly[diacetato(μ2-1,4-bis(1H-imidazol-1-yl)benzene-κ2N:N′)nickel(II)], C26H22N8NiO4
- Crystal structure of bis(2,4-dibromo-6-{(E)[(4-fluorobenzyl)imino]methyl}phenolato-κ2N,O) copper(II), C28H18Br4F2N2O2Cu
- Crystal structure of 1-(adamantan-1-yl)-3-phenylthiourea, C17H22N2S
- Crystal structure of 3-(6-(5-amino-1-phenyl-1H-pyrazol-3-yl)pyridin-2-yl)-1-phenyl-1H-pyrazol-5-amine – dioxan (2/1), C25H23N7O
- Crystal structure of 5-ethyl-6-[(3-methylphenyl)sulfanyl]pyrimidine-2,4(1H,3H)-dione, C13H14N2O2S
- Crystal structure of (((1E,1′E)-(cyclohexane-1,2-diylbis(azanylylidene-κ2N,N′))bis(methanylylidene))bis(2,1-phenylene))bis((2,6-diisopropylphenyl)amide-κ2N′′,N′′′)manganese(II), C44H54N4Mn
- Crystal structure of prop-2-en-1-yl 2-oxo-2H-1-benzopyran-3-carboxylate, C13H10O4
- Crystal structure of bis(μ2-2-((3-methylphenyl)imino)methylphenolato-κ2N,O:O)hexacarbonyldimanganese(I), C34H24Mn2N2O8
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxybut-2-en-1-one C9H12N2O2
- Crystal structure of 2,2′-[pentane-1,5-diylbis(oxy)]dibenzaldehyde, C19H20O4
- Crystal structure of 2-phenyl-5,6,7,8-tetrahydro-4H-benzo[4,5]thieno[2,3-d][1,3]oxazin-4-one, C16H13NO2S
- Crystal structure of (E)-1-(2-chlorophenyl)-N-(4-chlorophenyl)methanimine, C13H9Cl2N
- Crystal structure of ethyl 2-amino-5-bromothiazole-4-carboxylate, C6H7BrN2O2S
- Crystal structure of 2-benzylisothiouronium tetraphenylborate, C32H31BN2S
- Crystal structure of poly[(μ2-biphenyl-2,2′-dicarboxylato-κ4O,O′:O′′,O′′′)(μ2-4,4′-bipyridine-κ2N:N′)copper(II)], C24H16CuN2O4
- Crystal structure of (η5-pentamethylcyclopentadienyl)titanium(III)dichloride (THF), C14H23Cl2OTi
- Crystal structure of 3-ferrocenylsulfonyl-2-(4-methoxyphenyl)-3H-imidazo[4,5-b]pyridine, C23H19FeN3O3S
- Crystal structure of 2-benzoyl-3-(4-fluorophenyl)cyclopropane-1,1-dicarbonitrile, C18H11FN2O
- Crystal structure of 1,6-ditosyl-1,6-diazecane, C22H30N2O4S2
- Crystal structure of N-phenyl-2-(pyridin-4-ylcarbonyl)hydrazinecarboxamide with Z′ = 4, C13H12N4O2
- Crystal structure of N,N-bis(diphenylphosphanyl)cyclohexylamine, C30H31NP2
- Crystal structure of 3-(4-hydroxy-3-methoxyphenyl)-N-phenylpropanamide, C16H17NO3
- Crystal structure of 6-(2-fluorophenyl)-3-phenyl-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazole, C15H9FN4S
- Crystal structure of catena-poly[aqua(dicyanoazanido-2κN-μ2-dicyanoazanido-1κN:2κN′)(μ2-2-methoxy-6-(((2-((3-methoxy-2-oxidobenzylidene)amino)ethyl)imino)methyl)phenolato-1κ2N,N′,2κ2O,O′,1κ2O′′,O′′′:2κ2O′′,O′′′)cadmium(II)copper(II)], C22H20CdCuN8O5


