Abstract
C30H22N4O18Re2, monoclinic, P21/c (no. 14), a = 10.167(7) Å, b = 17.57(1) Å, c = 19.95(1) Å, β = 98.75(1)°, V = 3522(9) Å3, Z = 4, Rgt(F) = 0.0275, wRref(F2) = 0.0633, T = 100(2) K.
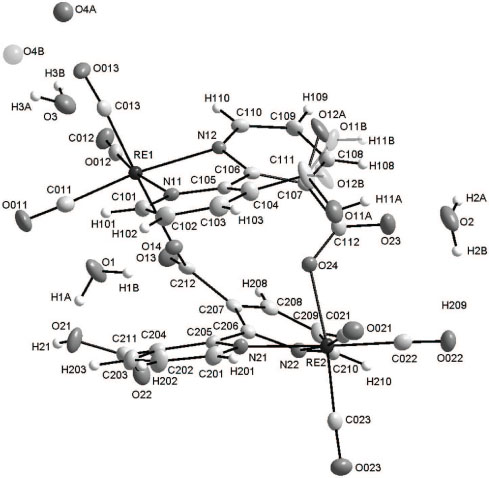
The crystal structure is shown in the figure. Tables 1–3 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow, cuboid, size 0.087×0.126×0.155 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 69.88 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II CCD,, φ and ω scans |
| 2θmax: | 56.74° |
| N(hkl)measured, N(hkl)unique: | 82102, 8763 |
| N(param)refined: | 506 |
| Programs: | Diamond [25], Bruker programs [26], WinGX [27], SHELX [28] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | Site | x | y | z | Uiso |
|---|---|---|---|---|---|
| H(209) | 4e | −0.2656 | 0.2455 | 0.5181 | 0.023 |
| H(110) | 4e | 0.0987 | 0.5331 | 0.4337 | 0.020 |
| H(108) | 4e | −0.2644 | 0.4562 | 0.3591 | 0.022 |
| H(210) | 4e | −0.3167 | 0.1791 | 0.4177 | 0.023 |
| H(201) | 4e | 0.0554 | 0.1083 | 0.2164 | 0.025 |
| H(203) | 4e | 0.3830 | 0.1128 | 0.3541 | 0.028 |
| H(208) | 4e | −0.0575 | 0.3033 | 0.5416 | 0.021 |
| H(101) | 4e | 0.3922 | 0.3137 | 0.2708 | 0.023 |
| H(109) | 4e | −0.1299 | 0.5447 | 0.4258 | 0.021 |
| H(103) | 4e | 0.0919 | 0.2983 | 0.1189 | 0.030 |
| H(102) | 4e | 0.3101 | 0.2685 | 0.1652 | 0.029 |
| H(21) | 4e | 0.4530 | 0.1807 | 0.5019 | 0.050 |
| H(202) | 4e | 0.2824 | 0.0893 | 0.2442 | 0.030 |
| H(11A)a | 4e | −0.2339 | 0.3330 | 0.1155 | 0.039 |
| H(11B)b | 4e | −0.2239 | 0.4330 | 0.1520 | 0.050 |
| H(1A) | 4e | 0.466(6) | 0.366(2) | 0.611(4) | 0.09(3) |
| H(1B) | 4e | 0.363(4) | 0.329(3) | 0.575(3) | 0.06(2) |
adisordered, occupancy factor: 0.706(8); bdisordered, occupancy factor: 0.294(8)
Fractional coordinates and atomic displacement parameters (Å2).
| Atom | Site | x | y | z | U11 | U22 | U33 | U12 | U13 | U23 |
|---|---|---|---|---|---|---|---|---|---|---|
| Re(1) | 4e | 0.30356(2) | 0.410995(9) | 0.389321(8) | 0.01334(8) | 0.01204(8) | 0.01362(8) | −0.00018(6) | 0.00054(6) | −0.00128(6) |
| Re(2) | 4e | −0.18987(2) | 0.16220(1) | 0.281839(8) | 0.01348(8) | 0.01417(9) | 0.01557(9) | −0.00083(6) | 0.00105(6) | −0.00448(6) |
| C(021) | 4e | −0.2131(4) | 0.1521(2) | 0.1852(2) | 0.013(2) | 0.013(2) | 0.027(3) | 0.001(2) | 0.004(2) | −0.001(2) |
| N(12) | 4e | 0.1012(3) | 0.4552(2) | 0.3625(2) | 0.014(2) | 0.012(2) | 0.013(2) | 0.001(1) | 0.003(1) | −0.001(1) |
| N(11) | 4e | 0.2261(3) | 0.3583(2) | 0.2928(2) | 0.017(2) | 0.011(2) | 0.014(2) | −0.002(1) | 0.004(1) | −0.002(1) |
| O(24) | 4e | −0.1328(3) | 0.2792(2) | 0.2857(2) | 0.017(2) | 0.014(2) | 0.020(2) | −0.001(1) | 0.001(1) | −0.004(1) |
| O(021) | 4e | −0.2266(3) | 0.1449(2) | 0.1268(2) | 0.031(2) | 0.023(2) | 0.017(2) | 0.000(1) | 0.004(1) | −0.004(1) |
| O(23) | 4e | −0.3221(3) | 0.3478(2) | 0.2715(2) | 0.017(2) | 0.024(2) | 0.047(2) | 0.001(1) | −0.005(2) | −0.011(2) |
| C(112) | 4e | −0.2012(4) | 0.3397(3) | 0.2878(2) | 0.016(2) | 0.021(2) | 0.018(2) | 0.001(2) | 0.000(2) | −0.005(2) |
| O(011) | 4e | 0.5734(3) | 0.3301(2) | 0.4213(2) | 0.021(2) | 0.032(2) | 0.048(2) | 0.011(2) | 0.001(2) | 0.005(2) |
| C(011) | 4e | 0.4716(5) | 0.3587(3) | 0.4105(2) | 0.024(2) | 0.018(2) | 0.021(2) | −0.002(2) | 0.001(2) | 0.002(2) |
| O(14) | 4e | 0.1891(3) | 0.3180(2) | 0.4193(1) | 0.021(2) | 0.015(2) | 0.015(2) | −0.001(1) | 0.003(1) | −0.001(1) |
| O(013) | 4e | 0.4658(3) | 0.5339(2) | 0.3303(2) | 0.021(2) | 0.023(2) | 0.024(2) | −0.006(1) | −0.001(1) | 0.008(1) |
| N(22) | 4e | −0.1382(3) | 0.1818(2) | 0.3908(2) | 0.016(2) | 0.016(2) | 0.016(2) | 0.000(1) | 0.003(1) | −0.000(1) |
| O(022) | 4e | −0.4924(3) | 0.1865(2) | 0.2659(2) | 0.016(2) | 0.029(2) | 0.031(2) | 0.001(1) | 0.003(1) | −0.005(2) |
| N(21) | 4e | 0.0250(3) | 0.1522(2) | 0.3039(2) | 0.015(2) | 0.015(2) | 0.018(2) | −0.001(1) | 0.002(1) | −0.006(1) |
| C(022) | 4e | −0.3792(5) | 0.1785(2) | 0.2721(2) | 0.023(2) | 0.017(2) | 0.017(2) | −0.002(2) | 0.003(2) | −0.005(2) |
| C(206) | 4e | −0.0103(4) | 0.2052(2) | 0.4099(2) | 0.017(2) | 0.014(2) | 0.014(2) | 0.002(2) | 0.001(2) | 0.002(2) |
| C(205) | 4e | 0.0842(4) | 0.1718(2) | 0.3668(2) | 0.014(2) | 0.012(2) | 0.017(2) | −0.003(2) | 0.001(2) | −0.001(2) |
| C(013) | 4e | 0.3999(4) | 0.4888(2) | 0.3521(2) | 0.017(2) | 0.017(2) | 0.014(2) | 0.001(2) | −0.003(2) | −0.002(2) |
| O(012) | 4e | 0.3624(3) | 0.4945(2) | 0.5259(2) | 0.022(2) | 0.030(2) | 0.019(2) | −0.006(1) | 0.003(1) | −0.006(1) |
| C(106) | 4e | 0.0216(4) | 0.4110(2) | 0.3171(2) | 0.020(2) | 0.011(2) | 0.013(2) | −0.000(2) | 0.000(2) | 0.000(2) |
| C(204) | 4e | 0.2202(4) | 0.1558(2) | 0.3880(2) | 0.018(2) | 0.014(2) | 0.021(2) | −0.002(2) | 0.006(2) | −0.001(2) |
| C(012) | 4e | 0.3425(4) | 0.4618(2) | 0.4755(2) | 0.014(2) | 0.020(2) | 0.020(2) | 0.001(2) | 0.003(2) | 0.000(2) |
| C(105) | 4e | 0.0966(4) | 0.3709(2) | 0.2685(2) | 0.014(2) | 0.011(2) | 0.016(2) | −0.002(2) | 0.002(2) | −0.001(2) |
| C(211) | 4e | 0.2893(4) | 0.1634(3) | 0.4598(2) | 0.019(2) | 0.017(2) | 0.025(2) | 0.003(2) | 0.002(2) | −0.001(2) |
| C(207) | 4e | 0.0200(4) | 0.2560(2) | 0.4640(2) | 0.019(2) | 0.013(2) | 0.010(2) | 0.003(2) | 0.002(2) | 0.002(2) |
| C(209) | 4e | −0.2024(4) | 0.2391(3) | 0.4893(2) | 0.016(2) | 0.030(3) | 0.013(2) | 0.005(2) | 0.005(2) | 0.004(2) |
| C(107) | 4e | −0.1151(4) | 0.4058(2) | 0.3187(2) | 0.018(2) | 0.015(2) | 0.015(2) | 0.001(2) | −0.002(2) | 0.000(2) |
| C(110) | 4e | 0.0439(4) | 0.5027(2) | 0.4030(2) | 0.023(2) | 0.012(2) | 0.015(2) | −0.001(2) | 0.002(2) | −0.003(2) |
| C(108) | 4e | −0.1727(4) | 0.4566(2) | 0.3594(2) | 0.017(2) | 0.016(2) | 0.021(2) | 0.002(2) | 0.006(2) | −0.001(2) |
| C(210) | 4e | −0.2304(4) | 0.1973(3) | 0.4305(2) | 0.015(2) | 0.020(2) | 0.023(2) | 0.002(2) | 0.004(2) | 0.003(2) |
| C(201) | 4e | 0.0977(4) | 0.1213(3) | 0.2596(2) | 0.021(2) | 0.023(2) | 0.020(2) | −0.002(2) | 0.005(2) | −0.006(2) |
| C(203) | 4e | 0.2930(4) | 0.1237(3) | 0.3412(2) | 0.016(2) | 0.027(3) | 0.026(2) | −0.003(2) | 0.002(2) | −0.004(2) |
| C(208) | 4e | −0.0770(4) | 0.2712(2) | 0.5043(2) | 0.024(2) | 0.017(2) | 0.012(2) | 0.005(2) | 0.002(2) | 0.002(2) |
| C(101) | 4e | 0.3029(4) | 0.3219(2) | 0.2536(2) | 0.017(2) | 0.018(2) | 0.023(2) | 0.001(2) | 0.006(2) | −0.001(2) |
| C(109) | 4e | −0.0927(4) | 0.5080(2) | 0.4007(2) | 0.020(2) | 0.014(2) | 0.020(2) | 0.003(2) | 0.006(2) | −0.005(2) |
| C(103) | 4e | 0.1254(5) | 0.3129(3) | 0.1630(2) | 0.027(3) | 0.027(3) | 0.020(2) | −0.002(2) | 0.004(2) | −0.005(2) |
| C(102) | 4e | 0.2559(5) | 0.2963(3) | 0.1899(2) | 0.025(2) | 0.023(2) | 0.027(3) | −0.001(2) | 0.011(2) | −0.007(2) |
| O(21) | 4e | 0.4177(3) | 0.1768(2) | 0.4622(2) | 0.020(2) | 0.056(2) | 0.023(2) | −0.011(2) | −0.002(1) | −0.007(2) |
| C(104) | 4e | 0.0443(5) | 0.3515(3) | 0.2019(2) | 0.023(2) | 0.022(2) | 0.016(2) | 0.003(2) | 0.002(2) | −0.005(2) |
| C(202) | 4e | 0.2332(5) | 0.1082(3) | 0.2762(2) | 0.020(2) | 0.029(3) | 0.026(3) | −0.001(2) | 0.008(2) | −0.013(2) |
| C(111) | 4e | −0.0902(6) | 0.3754(4) | 0.1683(3) | 0.039(3) | 0.077(5) | 0.014(3) | 0.030(3) | −0.007(2) | −0.013(3) |
| O(22) | 4e | 0.2351(3) | 0.1533(2) | 0.5093(2) | 0.023(1) | 0.035(2) | 0.020(1) | 0.004(1) | −0.000(1) | −0.000(1) |
| C(212) | 4e | 0.1433(4) | 0.3056(2) | 0.4744(2) | 0.021(2) | 0.010(2) | 0.015(2) | 0.002(2) | 0.000(2) | −0.001(2) |
| O(13) | 4e | 0.1820(3) | 0.3346(2) | 0.5305(2) | 0.029(2) | 0.025(2) | 0.019(2) | −0.005(1) | −0.001(1) | −0.007(1) |
| O(1) | 4e | 0.4378(5) | 0.3214(3) | 0.5998(2) | 0.058(3) | 0.046(3) | 0.044(3) | −0.022(2) | −0.025(2) | 0.013(2) |
| O(023) | 4e | −0.2483(3) | −0.0074(2) | 0.2979(2) | 0.033(2) | 0.019(2) | 0.032(2) | −0.003(2) | 0.001(2) | −0.001(1) |
| C(023) | 4e | −0.2235(4) | 0.0561(3) | 0.2906(2) | 0.016(2) | 0.021(2) | 0.016(2) | 0.001(2) | −0.003(2) | −0.005(2) |
| O(2) | 4e | 0.5548(4) | 0.1870(3) | 0.5847(2) | 0.039(2) | 0.083(3) | 0.034(2) | −0.027(2) | 0.008(2) | −0.017(2) |
| O(3) | 4e | 0.5327(7) | 0.4381(4) | 0.1737(4) | 0.105(5) | 0.082(4) | 0.111(5) | 0.018(4) | −0.009(4) | 0.030(4) |
| O(12A)a | 4e | −0.1199(8) | 0.4452(6) | 0.1765(6) | 0.048(6) | 0.060(6) | 0.042(3) | 0.034(5) | −0.005(5) | 0.003(4) |
| O(11A)a | 4e | −0.1607(5) | 0.3172(3) | 0.1331(2) | 0.023(1) | 0.035(2) | 0.020(1) | 0.004(1) | −0.000(1) | −0.000(1) |
| O(12B)b | 4e | −0.125(2) | 0.360(1) | 0.1116(8) | 0.048(6) | 0.060(6) | 0.042(3) | 0.034(5) | −0.005(5) | 0.003(4) |
| O(11B)b | 4e | −0.152(2) | 0.434(2) | 0.177(2) | 0.020(2) | 0.056(2) | 0.023(2) | −0.011(2) | −0.002(1) | −0.007(2) |
| O(4B)d | 4e | −0.310(1) | 0.4683(8) | 0.0588(9) | 0.10(1) | 0.09(1) | 0.19(2) | −0.034(8) | −0.08(1) | 0.09(1) |
| O(4A)c | 4e | 0.405(2) | 0.499(2) | 0.072(1) | 0.23(3) | 0.29(3) | 0.13(2) | 0.00(2) | −0.04(2) | −0.08(2) |
adisordered, occupancy factor: 0.706(8); bdisordered, occupancy factor: 0.294(8); cdisordered, occupancy factor: 0.505(14); ddisordered, occupancy factor: 0.495(14)
Source of material
The title compound was prepared as described by Schutte [1] by dissolving fac-[Re(BipyDC)(CO)3(H2O)] (50 mg) in 20 mL methanol. Yellow cuboid crystals were obtained from the mixture within three days.
fac-[NEt4]2[Re(CO)3(Br)3 (ReAA) was prepared as described by Alberto et al. [24]. ReAA (0.1 g, 0.130 mmol) was dissolved in 20 mL water, adjusted to pH 2.2 with HNO3. Silver nitrate (0.066 g, 0.389 mmol) was added to the solution and stirred for 24 h at room temperature. The precipitate (AgBr) was filtered off and 2,2′-bipyridine-3,3′-dicarboxylic acid (0.032 g, 0.131 mmol) was added to the filtrate and stirred for 3 h at room temperature. The product was isolated as a bright yellow precipitate. Yield: 0.127 g, 90%. IR (KBr, cm−1): νCO = 1900, 2025. 1H NMR (CD3COCD3): δ = 7.39 (dd, 1H, J = 5.2 Hz, 8 Hz), 7.70 (dd, 1H, J = 5.2 Hz, 8 Hz), 7.95 (dd, 1H, J = 1.6 Hz, 8 Hz), 8.45 (dd, 1H, J = 1.6 Hz, 8 Hz), 8.72 (dd, 1H, J = 1.6 Hz, 5.2 Hz), 8.87 (dd, 1H, J = 1.6 Hz, 5.2 Hz). 13C NMR (CD3COCD3): δ = 119.4, 120.3, 139.1, 149.2, 152.4, 167.5, 174.1.
Experimental details
Aromatic and hydroxyl H atoms were positioned geometrically and allowed to ride on their parent atoms, with Uiso(H) = 1.2Ueq(parent) and Uiso(H) = 1.5Ueq(parent) of the parent atom with a C—H and O—H distance of 0.93 Å and 0.82 Å respectively. Hydrogen atoms associated with O4A/O4B could not be located from the difference Fourier map. Attempts to include these atoms in calculated positions in order to maximize geometrically feasible hydrogen bonding interactions did not result in sensible structural refinements. Nevertheless, these hydrogen atoms have been included in the sum formula and the name of the compound. The hydroxyl and carbonyl oxygen atoms, O11A/B and O12A/B, are substitutionally disordered over two positions in a 0.706(8):0.294(8) ratio. One of the solvent water molecules also exhibit a disorder over two positions (O4A and O4B) with a 0.505(14):0.495(14) ratio.
Discussion
The title structure crystallizes in the monoclinic P21/c space group with one dinuclear rhenium(I) compound and four water solvent molecules in the asymmetric unit (Figure). The dinuclear compounds are formed by coordination of one of the carboxylate substituents of a coordinated 2,2′-bipyridine-3,3′-dicarboxylic ligand, to a second rhenium(I) atom. Both rhenium(I) metal cores are surrounded by three facial carbonyl ligands and a bidentate 2,2-bipyridine-3,3′-dicarboxylic acid ligand. The sixth position is occupied by the carbonyl oxygen of the carboxylic acid substituent. The rhenium carbonyl bonding distances are all within normal range, varying from 1.895(4) Å to 1.925(5) Å. The distances from the rhenium metal core to the nitrogen atoms of the coordinated bidentate ligands of 2.165(4) Å – 2.183(4) Å are in excellent agreement with the structures obtained by Kemp [2], 2.178(8) Å for fac-[Re(Bipy)(CO)3Br] and 2.172(5) Å and 2.168(5) Å for fac-[Re(Bipy)(CO)3(H2O)]NO3 · H2O with Bipy = 2,2′-bipyridine. It also compares well to a similar 1,10-phenanthroline (Phen) complex, fac-[Re(CO)3(Phen)(H2O)]NO3 · 0.5 Phen [3] with Re—N bond distances of 2.168(4) Å and 2.174(4) Å. The distances from the metal to the carboxyl oxygen atoms (2.138(3) Å (O14) and 2.128(3) Å (O24)) are comparable to that of the rhenium aqua distance of 2.213(5) Å in fac-[Re(Trop)(CO)3(H2O)], with Trop = tropolonato [4]. Slightly distorted octahedral geometries around the Re(I) metal cores are observed which might be due to the small bite angles of 74.55(13)° and 74.28 (13)° for N11—Re1—N12 and N21—Re2—N22 respectively. The twisting effect is possibly occcurring to facilitate the coordination of the carboxyl substituents on the ligand backbones to the rhenium(I) metal centers. Overall, the bond distances and bond angles around the two rhenium cores are similar to related structures and ligand systems ([5–23]). When viewed along the a axis, the rhenium(I) units pack in a head-to-head fashion in column-like structures along the c axis with a slight angle to the neighbouring two units.
Acknowledgements:
Financial assistance from the University of the Free State is gratefully acknowledged. We also express our gratitude towards SASOL, PETLabs Pharmaceuticals, the South African National Research Foundation (SA-NRF/THRIP) and the University of the Free State Strategic Academic Initiative (Advanced Biomolecular Systems) for financial support of this project. This work is based on the research supported in part by the National Research Foundation of South Africa (Grant specific unique reference number (UID) 84913). The Grantholder acknowledges that opinions, findings and conclusions or recommendations expressed in any publication generated by the NRF supported research are that of the authors, and that the NRF accepts no liability whatsoever in this regard. Leo Kirsten is acknowledged for the crystal data collection.
References
1. Schutte, M.: Novel radiopharmaceuticals: Characterization, substitution kinetics and biological evaluation of tricarbonyl complexes of rhenium(I). Ph.D Thesis, University of the Free State, South Africa, 2011.Suche in Google Scholar
2. Kemp, G.: Mechanistic study of rhenium(I) carbonyl complexes as model radiopharmaceuticals. Ph.D Thesis, University of Johannesburg, South Africa, 2006.Suche in Google Scholar
3. Schutte, M.; Kemp, G.; Visser, H. G.; Roodt, A.: Tuning the reactivity in classic low-spin d6 rhenium(I) tricarbonyl radiopharmaceutical synthon by selective bidentate ligand variation (L,L′-Bid; L,L′ = N,N′, N,O, and O,O′ donor atom sets) in fac-[Re(CO)3(L,L′-Bid)(MeOH)]n complexes. Inorg. Chem. 50 (2011) 12486–12498.10.1021/ic2013792Suche in Google Scholar PubMed
4. Schutte, M.; Visser, H. G.; Roodt, A.: Coordinated aqua vs. methanol substitution kinetics in fac-Re(I) tricarbonyl tropolonato complexes. Inorg. Chem. 51 (2012) 11996–12006.10.1021/ic301891uSuche in Google Scholar PubMed
5. Schutte, M.; Visser, H. G.; Steyl, G.: Tetraethylammonium bromidotricar-bonyl -[3,5,7-tribromo-tropolonato(1−)-κ2O,O′]rhenate(I). Acta. Cryst. E63 (2007) 3195–3196.Suche in Google Scholar
6. Schutte, M.; Visser, H. G.: Aquatricarbonyl(4-carboxy-pyridine-2-carboxylato-κ2N,O2)rhenium(I). Acta Crystallogr. E64 (2008) 1226–1227.10.1107/S160053680802761XSuche in Google Scholar
7. Schutte, M.; Visser, H.G.; Roodt, A.: Aqua-tricarbon-yl(3,5,7- tribromo-tropolonato)rhenium(I) methanol solvate. Acta Crystallogr. E64 (2008) 1610–1611.10.1107/S1600536808038737Suche in Google Scholar
8. Schutte, M.; Visser, H. G.; Brink, A.: [N,N-Bis(diphenyl-phosphino) propylamine-κ2P,P]bromidotricarbonyl-rhenium(I). Acta Cryst. E65 (2009) 1575–1576.10.1107/S1600536809047242Suche in Google Scholar
9. Schutte, M.; Visser, H. G.; Roodt, A.: Tetraethyl-ammonium bromidotricarbonyl(tropolonato)rhenate(I). Acta Crystallogr. E66 (2010) 859–860.10.1107/S1600536810024505Suche in Google Scholar PubMed PubMed Central
10. Schutte, M.; Brink, A.; Visser, H. G.; Roodt, A.: Tetra-μ3-hydroxidotetrakis[tricarbonylrhenium(I)] pyridine tetra-solvate. Acta Crystallogr. E68 (2012) 1208–1209.10.1107/S1600536812036033Suche in Google Scholar
11. Schutte, M.; Muller, T. J.; Visser, H. G.; Roodt, A.: [Bis(pyridin-2-ylmeth-yl) amine-κ3N,N′,N′′]tricarbonylrhenium(I) bromide hemihydrate. Acta Crystallogr. E68 (2012) 471–472.10.1107/S1600536812019654Suche in Google Scholar
12. Schutte, M.; Visser, H. G.; Roodt, A.: (μ1-Methanolato-κ1O)-μ1-ethoxo-κ1O-(μ2-2-amino-1-methyl-5H-imidazol-4-one-κ2N:N′)-hexacarbonyldirhenium(I). Acta Crystallogr. E68 (2012) m1359–m1360.10.1107/S1600536812041700Suche in Google Scholar PubMed PubMed Central
13. Schutte-Smith, M.; Muller, T. J.; Visser, H. G.; Roodt, A.: Distorted octa-hedral environments in tricarbonylrhenium(I) complexes of 5-[2-(2,4,6-trimethylphenyl)diazen-1-yl]quinolin-8-olate and 5,7-bis-[2-(2-methylphenyl) diazen-1-yl]quinolin-8-olate. Acta Crystallogr. C69 (2013) 1467–1471.10.1107/S0108270113027947Suche in Google Scholar
14. Manicum, A.; Schutte-Smith, M.; Kemp, G.; Visser, H. G.: Illustration of the electronic influence of coordinated β-diketone type ligands: A kinetic and structural study. Polyhedron 85 (2015) 190–195.10.1016/j.poly.2014.08.005Suche in Google Scholar
15. Twala, T. N.; Schutte-Smith, M.; Roodt, A.; Visser, H. G.: Activation of the manganese(I) tricarbonyl core by selective variation of bidentate ligands (L,L′-Bid = N,N′ and N,O donor atom sets) in fac-[Mn(CO)3(L,L′-Bid)(CH3OH)]n complexes. Dalton Trans. 44 (2015) 3278–3288.10.1039/C4DT03524KSuche in Google Scholar
16. Schutte-Smith, M.; Visser, H. G.: The versatility of pyridine-2,5-dicarboxylic acid in the synthesis of fac-M(CO)3 complexes (M = Re, 99mTc): reactivity towards substitution reactions and derivatization after coordination to a metal. Polyhedron 89 (2015) 122–128.10.1016/j.poly.2015.01.007Suche in Google Scholar
17. Brink, A.; Visser, H. G.; Roodt, A.: Activation of rhenium(I) toward substitution in fac-[Re(N,O′-Bid)(CO)3(HOCH3)] by Schiff-base bidentate ligands (N,O′-Bid). Inorg. Chem. 52 (2013) 8950–8961.10.1021/ic401115jSuche in Google Scholar PubMed
18. Brink, A.; Visser, H. G.; Roodt, A.: Solid state isostructural behavior and quantified limiting substitution kinetics in Schiff-base bidentate ligand complexes fac-[Re(O,N-Bid)(CO)3(MeOH)]n. Inorg. Chem. 53 (2014) 12480–12488.10.1021/ic5019168Suche in Google Scholar PubMed
19. van der Westhuizen, H. J.; Meijboom, R.; Schutte, M.; Roodt, A.: Mechanism for the formation of substituted manganese(V) cyanidonitrido complexes: Crystallographic and kinetic study of the substitution reactions of trans-[MnN(H2O)(CN)4]2− with monodentate pyridine and bidentate pyridine-carboxylate ligands. Inorg. Chem. 49 (2010) 9599–9608.10.1021/ic101274qSuche in Google Scholar PubMed
20. Schutte, M.; Pretorius, C.; Visser, H. G.; Roodt, A.: 5-(Trifluoro-methoxy)isatin. Acta Crystallogr. E68 (2012) o3472.10.1107/S1600536812044297Suche in Google Scholar
21. Brink, A.; Visser, H. G.; Roodt, A.: Tetraethylammonium (acetylacetonato)bromidotricarbonylrhenate(I). Acta Crystallogr. E67 (2011) 34–35.10.1107/S1600536810050105Suche in Google Scholar
22. Schutte, M.; Visser, H. G.; Roodt, A.; Braband, H.: N-Benzyl-isatin. Acta Crystallogr. E68 (2012) o777.10.1107/S1600536812006575Suche in Google Scholar
23. Visser, H. G.; Roodt, A.; Volmink, A.; Kemp, G.: fac-Tricarbonyl(pyridine-κN)(1,1,1-trifluoroacetylacetonato-κ2O,O′)rhenium(I). Acta Crystallogr. E67 (2011) m1631.10.1107/S160053681104476XSuche in Google Scholar
24. Alberto, R.; Schibli, R.; Schubiger, P. A.: Reactions with the technetium and rhenium carbonyl complexes (NEt4)2[MX3(CO)3]. Synthesis and structure of [Tc(CN-But)3(CO)3](NO3) and (NEt4)[Tc2(-SCH2CH2OH)3(CO)6]. Polyhedron 15 (1996) 1079–1089.10.1016/0277-5387(95)00361-4Suche in Google Scholar
25. Brandenburg, K.; Putz, H.: DIAMOND. Crystal Impact GbR, Bonn, Germany, 2005.Suche in Google Scholar
26. Bruker: APEX2 (Version 2.1–4), SAINT (Version 7.34A), SADABS (Version 2007/4), BrukerAXS Inc, Madison, Wisconsin, USA, 2008.Suche in Google Scholar
27. Farrugia, L. J.: WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 32 (1999) 837–838.10.1107/S0021889899006020Suche in Google Scholar
28. Sheldrick, G. M.: A short history of SHELXTL. Acta Cryst. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
©2016 Marietjie Schutte-Smith et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of fac-hexacarbonylbisμ2-(3-carboxy-3′-carboxylato-2,2′-bipyridine)-κ3N,N′:O-dirhenium(I) tetrahydrate, C30H22N4O18Re2
- The crystal structure of bis(4-(2,4-dimethylphenyl)piperazin-1-yl)methane, C25H36N4
- Crystal structure of bis(triphenylphosphine-κP)bis(μ2-1H,1′H-2,2′-biimidazole-κ3N,N′:N′)disilver(I) bis(tetrafluoroborate), C48H42Ag2B2F8N8P2
- The crystal structure of 1,2-bis(2-pyrazinecarboxamido)-benzene, C16H12N6O2
- Redetermination of the crystal structure of 3-bromobenzoic acid, C7H5BrO2
- Crystal structure of bis(ethyltriphenylphos-phonium) tetrabromidocuprate(II), (C20H20P)2[CuBr4]
- Crystal structure of 2-(4-bromophenyl)-1,3-dimethyl-2,3-dihydro-1H-perimidine, C19H17BrN2
- Crystal structure of trans-diaqua-bis(3-(pyrazin-2-yl)-5-(pyridin-4-yl)1,2,4-triazol-1-ido-κ2N,N′)-cobalt(II),C22H18CoN12O2
- Crystal structure of hexaaquamanganese(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MnN6O12S2
- Crystal structure of butyl 2-(3,5-dimethyl-1,1-dioxido-2H-1,2,6-thiadiazin-4-yl)benzoate, C16H20N2O4S
- Crystal structure of hexaaquabis(μ2-3-(6-carboxylatopyridin-2-yl)-5-(pyrazin-2-yl)-1,2,4-triazol-1-ido)bis(μ3-3-(6-carboxylatopyridin-2-yl)-5-(pyrazin-2-yl)-1,2,4-triazol-1-ido)tetra-manganese(II) dihydrate, C48H40Mn4N24O16
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmate(II)] bis(2-aminoisonicotinate) tetrahydrate, C38H50CdN8O10
- Crystal structure of succinic acid — 4-((pyridin-4-ylmethyl)sulfanylpyridine (1/1), C15H16N2O4S
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)bis(2-((pyridin-4-ylmethyl)sulfanyl)pyridine-κN)dicopper(II), C30H32N4O8S2Cu2
- Crystal structure of 1-((2R,3S)-2,3-dimethyl-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)-2-phenoxyethan-1-one, C18H19NO3
- Crystal structure of tetramethylammonium sulfanilate, C10H18N2O3S
- Crystal structure of triethylammonium 2′-carboxy-[1,1′-biphenyl]-2-carboxylate, C20H25NO4
- Crystal structure of 2-(4-acetyl-2,6-dimethylphenyl)-5,6-dichloro-1H-isoindole-1,3(2H)-dione, C18H13Cl2NO3
- Crystal structure of diaqua-bis(μ3-2-methyl-6-oxidopyridinium-4-carboxylato-κ3O:O′:O′′)neodymium(III) chloride, C14H16ClN2O8Nd
- Crystal structure of 5-methoxy-4-methyl-2-(2-methylbenzyl)-2,4-dihydro-3H-1,2,4-triazol-3-one, C12H15N3O2
- The crystal structure of 1-(4-(2-chloroethoxy)phenyl)ethanone
- Crystal structure of (E)-4-(2-(4-(diethylamino)phenyl)diazen-1-ium-1-yl)benzenesulfonate monohydrate
- Crystal structure of 2,2′-[(1E)-prop-1-ene-1,2-diyldisulfanediyl]bis(5-methyl-2,5-dihydro-1,3,4-thiadiazole, C9H10N4S4
- Crystal structure of poly [μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)cerium(III)] monohydrate (C10H13O9Ce)
- Crystal structure of tris((2-(2,2-dicyanovinyl)phenoxy)ethyl)amine, C36H27N7O3
- Crystal structure of catena[diaqua-bis(μ2-1,3-bis((1H-tetrazol-1-yl)methyl)benzene-κ2N:N′)copper(II)] dinitrate, C20H24CuN18O8
- Crystal structure of (4-(1H-imidazol-5-yl)benzoic acid-κN) (4-(1H-imidazol-5-yl)benzoato-κN)silver(I), C20H15N4O4Ag
- Crystal structure of 2-amino-3-cyano-7,7-dimethyl-5-oxo-4-(3,4,5-trifluorophenyl)-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C18H15F3N2O2
- Crystal structure of (2-(2-chlorophenyl)-5-ethyl-1,3-dioxan-5-yl)methanol hemihydrate, C13H17ClO3 · 0.5 H2O
- Crystal structure of catena-poly[diaqua-bis(benzene-1,2,4,5-tetracarboxylato-κN)(m2-2-(1H-1,2,4-trizol-1-ylmethyl)-1H-3,1-benzimidazol-3-ium-κ2O:O′)zinc(II)] dihydrate, C30H30N10O12Zn
- Crystal structure of [2,2′-((((ethane-1,2-diylbis(oxy-κ2O,O′))bis(2,1-phenylene))bis(azanylylidene-κ2N,N′))bis(methanylylidene))diphenolato-κ2O′′,O′′′]zinc(II), C28H22N2O4Zn
- Crystal structure of (E)-1-(3,4-dimethoxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C13H17NO3
- Crystal structure of bis(2-fluoro-4-nitrophenyl) terephthalate C20H10F2N2O8
- Crystal structure of catena-[aqua((4-carboxyphenyl)acetato-κO)(μ2-(4-carboxyphenyl)acetato-κ2O:O′)bis(4,4′-ethene-1,2-diyldipyridine-κN)manganese(II)], C42H36N4O9Mn
- Crystal structure of N′-(2-hydroxybenzylidene)-3,4-dimethyl-1H-pyrrole-2-carbohydrazide, C14H15N3O2
- Crystal structure of bis(8-ethyl-5-oxo-2-(piperazin-4-ium-1-yl)-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylato-κ2O,O′)copper(II) benzene-1,4-dicarboxylate dihydrate, C36H42CuN10O12
- Crystal structure of diaquabis(μ2-biphenyl-2,2′-dicarboxylato-κ2O:O′)bis(1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylato)zinc(II), C60H56N6Zn2O16F2
- Crystal structure of 2-amino-4-(4-fluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13FN2O2
- The crystal structure of hexaqua(μ2-3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-1κ2O,O′;2κO′)(3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-κO)(3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-κ2O,O′)digadolinium(III) octahydrate, C60H76Gd2N18O32
- Crystal structure of hexacarbonyl bis(μ2-2-methoxybenzenethiolato-κ2S)pyridine(triphenylphosphane)dirhenium(I), C43H34NO8PS2Re2
- Crystal structure of 14-((1-(benzyloxycarbonyl-amino)-2-methylpropan-2-yl)sulfanyl)acetate Mutilin, C34H49NO6S
- Crystal structure of 2-methoxy-6-(((2-(1-methyl-1H-benzo[d] imidazol-2-yl)phenyl)imino)methyl)phenol — ethanol (1/1), C24H25N3O3
- Crystal structure of 2-(bis(methylthio)methylene)-1-phenylbutane-1,3-dione, C13H14O2S2
- Crystal structure of (E)-2-(bis(methylthio)methylene)-1-phenyl-3-(2-phenylhydrazono)butan-1-one, C19H20N2OS2
- Crystal structure of dichlorido(2-(1-methyl-1H-benzo[d]imidazol-2-yl)aniline-κ2N,N′)zinc(II)
- Crystal structure of bis(1-methyl-1H-tetrazole-5-thiolato)mercury(II)
- Crystal structure of (E)-2-styryl-1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C24H19FeN3O2S
- Crystal structure of aquabis(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-4-ium-1-yl)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)copper(II) thiophene-2,5-dicarboxylate trihydrate, [Cu(C17H18N3FO3)2(H2O)](C6H2SO4)·3(H2O)
- Crystal structure of 2-amino-4-(2,6-dichlorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16Cl2N2O2
- Crystal structure of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazatetracyclo[5·5·0·05·9·03·11]dodecane 1/3 hydrate, C6H8N12O13
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ2O,O′)(triphenylarsine-κAs)rhodium(I), C25H20AsN2O3Rh
- The crystal structure of 1-(4-(4-chlorophenoxy)-2-chlorophenyl)ethanone, C14H10Cl2O2
- Crystal structure of N,N-diethyl-2-(2-(6-(4-methoxybenzyl)-7-oxo-7H-thiazolo[3,3-b][1,2,4]triazin-3-yl)phenoxy)acetamide, C25H26N4O4S
- Crystal structure of tetraqua((E)-4,4′-(diazene-1,2-diyl)bis(5-oxo-4,5-dihydro-1,2,4-triazol-1-ide)-κ2N:O)barium(II), C4H10N8O6Ba
- Crystal structure of 2-amino-4-(3-phenoxyphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C22H18N2O3
- Crystal structure of diethylammonium 5-((4-fluorophenyl)(6-hydroxy-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate, C23H30FN5O6
- Crystal structure of 2-amino-4-(3,5-difluoro-phenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12F2N2O2
- Crystal structure of tris(N-nitroso-N-oxyanilino-κ2O, O′) oxidoniobium(V), C18H15N6O7Nb
- Crystal structure of 1-(5-benzoyl-4-methyl-2-(phenylamino)thiophen-3-yl)ethan-1-one, a structure with Z′ = 6, C20H17NO2S
- Crystal structure of diethyl-3-methyl-4-phenylthieno[2,3-b]thiophene-2,5-dicarboxylate, C19H18O4S2
- Crystal structure of 3,5-dicarboxybenzoate — benzene-1,3,5-tricarboxylic acid (1/1), C24H22N2O12
- The crystal structure of 2-chloro-1,3-bis(2,4,6-trimethylphenyl)-4,4-dimethyl-1,3,2λ3,4-diazaphosphasiletidine
- Crystal structure of hexaquamanganese(II) bis(hexaborato-κ3O,O′,O′′)manganese(II) dihydrate, B12H28Mn2O34
- Crystal structure of 1-propyl-3-methylimidazolium pentaborate, [C7H13N2][B5O6(OH)4]
- Crystal structure of 13-(4-fluorophenyl)-11,13-dihydro-1H-benzo[h]indazolo[6,7-b] [1, 6]naphthyridin-12(6H)-one — dimethylformamide — water (1/2/1), C29H31FN6O4
- Crystal structure of 1-(2-chlorophenyl)-2-(2-nitrophenyl)ethan-1-ol, C14H12ClNO3
- Crystal structure of (Z)-2-((2-bromo-1-phenylvinyl)oxy)benzonitrile, C15H10BrNO
- Crystal structure of tetrachlorido(1E,1′E)-N,N′-((1,4-phenylenebis(propane-2,2-diyl))bis(4,1-phenylene))bis(1-(pyridin-2-yl-κN)methanimine-κN)dizinc(II), C36H34N4Zn2Cl4
- Crystal structure of 2,6-bis(3-methylpyridinyl)hexahydro-4,8-ethenopyrrolo-[3,4-f]isoindole-1,3,5,7(2H,6H)-tetrone, C24H20N4O4
- Crystal structure of trans-bis(2-methylmaleato-κ2O,O′) bis(piperazinium-κN) cobalt(II) trihydrate, C18H36CoN4O11
- Crystal structure of (E)-4-chloro-N′-(4-(diethylamino)benzylidene)benzohydrazide, C18H20ClN3O
- Crystal structure of 3,6-di(1H-imidazol-1-yl)-9H-carbazole, C18H13N5
- Crystal structure of 4-(4-pyridinyl)-1-naphthoic acid, C16H11NO2
- Crystal structure of 1,1′-diformyl-4,4′-(6H,12H-5,11-methano-dibenzo[b,f][11,5]diazocine-2,8-diyl)dibenzene, C29H22N2O2
- Crystal structure of N′-(adamantan-2-ylidene)pyridine-3-carbohydrazide, C16H19N3O
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(5-methyl-1H-pyrazole-3-carbaldehyde), C14H18N4O2
- Crystal structure of methyl 8-hydroxy-3-isopropyl-5a,8-dimethyl-2,3,4,5,5a,6,7,8,10a,10b-decahydrocyclohepta[e]indene-3a(1H)-carboxylate, C21H34O3
- Crystal structure of 6-oxo-4-propyl-2-(propylthio)-1,6-dihydropyrimidine-5-carbonitrile, C11H15N3OS
- Crystal structure of poly[diacetato(μ2-1,4-bis(1H-imidazol-1-yl)benzene-κ2N:N′)nickel(II)], C26H22N8NiO4
- Crystal structure of bis(2,4-dibromo-6-{(E)[(4-fluorobenzyl)imino]methyl}phenolato-κ2N,O) copper(II), C28H18Br4F2N2O2Cu
- Crystal structure of 1-(adamantan-1-yl)-3-phenylthiourea, C17H22N2S
- Crystal structure of 3-(6-(5-amino-1-phenyl-1H-pyrazol-3-yl)pyridin-2-yl)-1-phenyl-1H-pyrazol-5-amine – dioxan (2/1), C25H23N7O
- Crystal structure of 5-ethyl-6-[(3-methylphenyl)sulfanyl]pyrimidine-2,4(1H,3H)-dione, C13H14N2O2S
- Crystal structure of (((1E,1′E)-(cyclohexane-1,2-diylbis(azanylylidene-κ2N,N′))bis(methanylylidene))bis(2,1-phenylene))bis((2,6-diisopropylphenyl)amide-κ2N′′,N′′′)manganese(II), C44H54N4Mn
- Crystal structure of prop-2-en-1-yl 2-oxo-2H-1-benzopyran-3-carboxylate, C13H10O4
- Crystal structure of bis(μ2-2-((3-methylphenyl)imino)methylphenolato-κ2N,O:O)hexacarbonyldimanganese(I), C34H24Mn2N2O8
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxybut-2-en-1-one C9H12N2O2
- Crystal structure of 2,2′-[pentane-1,5-diylbis(oxy)]dibenzaldehyde, C19H20O4
- Crystal structure of 2-phenyl-5,6,7,8-tetrahydro-4H-benzo[4,5]thieno[2,3-d][1,3]oxazin-4-one, C16H13NO2S
- Crystal structure of (E)-1-(2-chlorophenyl)-N-(4-chlorophenyl)methanimine, C13H9Cl2N
- Crystal structure of ethyl 2-amino-5-bromothiazole-4-carboxylate, C6H7BrN2O2S
- Crystal structure of 2-benzylisothiouronium tetraphenylborate, C32H31BN2S
- Crystal structure of poly[(μ2-biphenyl-2,2′-dicarboxylato-κ4O,O′:O′′,O′′′)(μ2-4,4′-bipyridine-κ2N:N′)copper(II)], C24H16CuN2O4
- Crystal structure of (η5-pentamethylcyclopentadienyl)titanium(III)dichloride (THF), C14H23Cl2OTi
- Crystal structure of 3-ferrocenylsulfonyl-2-(4-methoxyphenyl)-3H-imidazo[4,5-b]pyridine, C23H19FeN3O3S
- Crystal structure of 2-benzoyl-3-(4-fluorophenyl)cyclopropane-1,1-dicarbonitrile, C18H11FN2O
- Crystal structure of 1,6-ditosyl-1,6-diazecane, C22H30N2O4S2
- Crystal structure of N-phenyl-2-(pyridin-4-ylcarbonyl)hydrazinecarboxamide with Z′ = 4, C13H12N4O2
- Crystal structure of N,N-bis(diphenylphosphanyl)cyclohexylamine, C30H31NP2
- Crystal structure of 3-(4-hydroxy-3-methoxyphenyl)-N-phenylpropanamide, C16H17NO3
- Crystal structure of 6-(2-fluorophenyl)-3-phenyl-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazole, C15H9FN4S
- Crystal structure of catena-poly[aqua(dicyanoazanido-2κN-μ2-dicyanoazanido-1κN:2κN′)(μ2-2-methoxy-6-(((2-((3-methoxy-2-oxidobenzylidene)amino)ethyl)imino)methyl)phenolato-1κ2N,N′,2κ2O,O′,1κ2O′′,O′′′:2κ2O′′,O′′′)cadmium(II)copper(II)], C22H20CdCuN8O5
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of fac-hexacarbonylbisμ2-(3-carboxy-3′-carboxylato-2,2′-bipyridine)-κ3N,N′:O-dirhenium(I) tetrahydrate, C30H22N4O18Re2
- The crystal structure of bis(4-(2,4-dimethylphenyl)piperazin-1-yl)methane, C25H36N4
- Crystal structure of bis(triphenylphosphine-κP)bis(μ2-1H,1′H-2,2′-biimidazole-κ3N,N′:N′)disilver(I) bis(tetrafluoroborate), C48H42Ag2B2F8N8P2
- The crystal structure of 1,2-bis(2-pyrazinecarboxamido)-benzene, C16H12N6O2
- Redetermination of the crystal structure of 3-bromobenzoic acid, C7H5BrO2
- Crystal structure of bis(ethyltriphenylphos-phonium) tetrabromidocuprate(II), (C20H20P)2[CuBr4]
- Crystal structure of 2-(4-bromophenyl)-1,3-dimethyl-2,3-dihydro-1H-perimidine, C19H17BrN2
- Crystal structure of trans-diaqua-bis(3-(pyrazin-2-yl)-5-(pyridin-4-yl)1,2,4-triazol-1-ido-κ2N,N′)-cobalt(II),C22H18CoN12O2
- Crystal structure of hexaaquamanganese(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MnN6O12S2
- Crystal structure of butyl 2-(3,5-dimethyl-1,1-dioxido-2H-1,2,6-thiadiazin-4-yl)benzoate, C16H20N2O4S
- Crystal structure of hexaaquabis(μ2-3-(6-carboxylatopyridin-2-yl)-5-(pyrazin-2-yl)-1,2,4-triazol-1-ido)bis(μ3-3-(6-carboxylatopyridin-2-yl)-5-(pyrazin-2-yl)-1,2,4-triazol-1-ido)tetra-manganese(II) dihydrate, C48H40Mn4N24O16
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmate(II)] bis(2-aminoisonicotinate) tetrahydrate, C38H50CdN8O10
- Crystal structure of succinic acid — 4-((pyridin-4-ylmethyl)sulfanylpyridine (1/1), C15H16N2O4S
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)bis(2-((pyridin-4-ylmethyl)sulfanyl)pyridine-κN)dicopper(II), C30H32N4O8S2Cu2
- Crystal structure of 1-((2R,3S)-2,3-dimethyl-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)-2-phenoxyethan-1-one, C18H19NO3
- Crystal structure of tetramethylammonium sulfanilate, C10H18N2O3S
- Crystal structure of triethylammonium 2′-carboxy-[1,1′-biphenyl]-2-carboxylate, C20H25NO4
- Crystal structure of 2-(4-acetyl-2,6-dimethylphenyl)-5,6-dichloro-1H-isoindole-1,3(2H)-dione, C18H13Cl2NO3
- Crystal structure of diaqua-bis(μ3-2-methyl-6-oxidopyridinium-4-carboxylato-κ3O:O′:O′′)neodymium(III) chloride, C14H16ClN2O8Nd
- Crystal structure of 5-methoxy-4-methyl-2-(2-methylbenzyl)-2,4-dihydro-3H-1,2,4-triazol-3-one, C12H15N3O2
- The crystal structure of 1-(4-(2-chloroethoxy)phenyl)ethanone
- Crystal structure of (E)-4-(2-(4-(diethylamino)phenyl)diazen-1-ium-1-yl)benzenesulfonate monohydrate
- Crystal structure of 2,2′-[(1E)-prop-1-ene-1,2-diyldisulfanediyl]bis(5-methyl-2,5-dihydro-1,3,4-thiadiazole, C9H10N4S4
- Crystal structure of poly [μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)cerium(III)] monohydrate (C10H13O9Ce)
- Crystal structure of tris((2-(2,2-dicyanovinyl)phenoxy)ethyl)amine, C36H27N7O3
- Crystal structure of catena[diaqua-bis(μ2-1,3-bis((1H-tetrazol-1-yl)methyl)benzene-κ2N:N′)copper(II)] dinitrate, C20H24CuN18O8
- Crystal structure of (4-(1H-imidazol-5-yl)benzoic acid-κN) (4-(1H-imidazol-5-yl)benzoato-κN)silver(I), C20H15N4O4Ag
- Crystal structure of 2-amino-3-cyano-7,7-dimethyl-5-oxo-4-(3,4,5-trifluorophenyl)-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C18H15F3N2O2
- Crystal structure of (2-(2-chlorophenyl)-5-ethyl-1,3-dioxan-5-yl)methanol hemihydrate, C13H17ClO3 · 0.5 H2O
- Crystal structure of catena-poly[diaqua-bis(benzene-1,2,4,5-tetracarboxylato-κN)(m2-2-(1H-1,2,4-trizol-1-ylmethyl)-1H-3,1-benzimidazol-3-ium-κ2O:O′)zinc(II)] dihydrate, C30H30N10O12Zn
- Crystal structure of [2,2′-((((ethane-1,2-diylbis(oxy-κ2O,O′))bis(2,1-phenylene))bis(azanylylidene-κ2N,N′))bis(methanylylidene))diphenolato-κ2O′′,O′′′]zinc(II), C28H22N2O4Zn
- Crystal structure of (E)-1-(3,4-dimethoxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C13H17NO3
- Crystal structure of bis(2-fluoro-4-nitrophenyl) terephthalate C20H10F2N2O8
- Crystal structure of catena-[aqua((4-carboxyphenyl)acetato-κO)(μ2-(4-carboxyphenyl)acetato-κ2O:O′)bis(4,4′-ethene-1,2-diyldipyridine-κN)manganese(II)], C42H36N4O9Mn
- Crystal structure of N′-(2-hydroxybenzylidene)-3,4-dimethyl-1H-pyrrole-2-carbohydrazide, C14H15N3O2
- Crystal structure of bis(8-ethyl-5-oxo-2-(piperazin-4-ium-1-yl)-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylato-κ2O,O′)copper(II) benzene-1,4-dicarboxylate dihydrate, C36H42CuN10O12
- Crystal structure of diaquabis(μ2-biphenyl-2,2′-dicarboxylato-κ2O:O′)bis(1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylato)zinc(II), C60H56N6Zn2O16F2
- Crystal structure of 2-amino-4-(4-fluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13FN2O2
- The crystal structure of hexaqua(μ2-3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-1κ2O,O′;2κO′)(3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-κO)(3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-κ2O,O′)digadolinium(III) octahydrate, C60H76Gd2N18O32
- Crystal structure of hexacarbonyl bis(μ2-2-methoxybenzenethiolato-κ2S)pyridine(triphenylphosphane)dirhenium(I), C43H34NO8PS2Re2
- Crystal structure of 14-((1-(benzyloxycarbonyl-amino)-2-methylpropan-2-yl)sulfanyl)acetate Mutilin, C34H49NO6S
- Crystal structure of 2-methoxy-6-(((2-(1-methyl-1H-benzo[d] imidazol-2-yl)phenyl)imino)methyl)phenol — ethanol (1/1), C24H25N3O3
- Crystal structure of 2-(bis(methylthio)methylene)-1-phenylbutane-1,3-dione, C13H14O2S2
- Crystal structure of (E)-2-(bis(methylthio)methylene)-1-phenyl-3-(2-phenylhydrazono)butan-1-one, C19H20N2OS2
- Crystal structure of dichlorido(2-(1-methyl-1H-benzo[d]imidazol-2-yl)aniline-κ2N,N′)zinc(II)
- Crystal structure of bis(1-methyl-1H-tetrazole-5-thiolato)mercury(II)
- Crystal structure of (E)-2-styryl-1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C24H19FeN3O2S
- Crystal structure of aquabis(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-4-ium-1-yl)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)copper(II) thiophene-2,5-dicarboxylate trihydrate, [Cu(C17H18N3FO3)2(H2O)](C6H2SO4)·3(H2O)
- Crystal structure of 2-amino-4-(2,6-dichlorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16Cl2N2O2
- Crystal structure of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazatetracyclo[5·5·0·05·9·03·11]dodecane 1/3 hydrate, C6H8N12O13
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ2O,O′)(triphenylarsine-κAs)rhodium(I), C25H20AsN2O3Rh
- The crystal structure of 1-(4-(4-chlorophenoxy)-2-chlorophenyl)ethanone, C14H10Cl2O2
- Crystal structure of N,N-diethyl-2-(2-(6-(4-methoxybenzyl)-7-oxo-7H-thiazolo[3,3-b][1,2,4]triazin-3-yl)phenoxy)acetamide, C25H26N4O4S
- Crystal structure of tetraqua((E)-4,4′-(diazene-1,2-diyl)bis(5-oxo-4,5-dihydro-1,2,4-triazol-1-ide)-κ2N:O)barium(II), C4H10N8O6Ba
- Crystal structure of 2-amino-4-(3-phenoxyphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C22H18N2O3
- Crystal structure of diethylammonium 5-((4-fluorophenyl)(6-hydroxy-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate, C23H30FN5O6
- Crystal structure of 2-amino-4-(3,5-difluoro-phenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12F2N2O2
- Crystal structure of tris(N-nitroso-N-oxyanilino-κ2O, O′) oxidoniobium(V), C18H15N6O7Nb
- Crystal structure of 1-(5-benzoyl-4-methyl-2-(phenylamino)thiophen-3-yl)ethan-1-one, a structure with Z′ = 6, C20H17NO2S
- Crystal structure of diethyl-3-methyl-4-phenylthieno[2,3-b]thiophene-2,5-dicarboxylate, C19H18O4S2
- Crystal structure of 3,5-dicarboxybenzoate — benzene-1,3,5-tricarboxylic acid (1/1), C24H22N2O12
- The crystal structure of 2-chloro-1,3-bis(2,4,6-trimethylphenyl)-4,4-dimethyl-1,3,2λ3,4-diazaphosphasiletidine
- Crystal structure of hexaquamanganese(II) bis(hexaborato-κ3O,O′,O′′)manganese(II) dihydrate, B12H28Mn2O34
- Crystal structure of 1-propyl-3-methylimidazolium pentaborate, [C7H13N2][B5O6(OH)4]
- Crystal structure of 13-(4-fluorophenyl)-11,13-dihydro-1H-benzo[h]indazolo[6,7-b] [1, 6]naphthyridin-12(6H)-one — dimethylformamide — water (1/2/1), C29H31FN6O4
- Crystal structure of 1-(2-chlorophenyl)-2-(2-nitrophenyl)ethan-1-ol, C14H12ClNO3
- Crystal structure of (Z)-2-((2-bromo-1-phenylvinyl)oxy)benzonitrile, C15H10BrNO
- Crystal structure of tetrachlorido(1E,1′E)-N,N′-((1,4-phenylenebis(propane-2,2-diyl))bis(4,1-phenylene))bis(1-(pyridin-2-yl-κN)methanimine-κN)dizinc(II), C36H34N4Zn2Cl4
- Crystal structure of 2,6-bis(3-methylpyridinyl)hexahydro-4,8-ethenopyrrolo-[3,4-f]isoindole-1,3,5,7(2H,6H)-tetrone, C24H20N4O4
- Crystal structure of trans-bis(2-methylmaleato-κ2O,O′) bis(piperazinium-κN) cobalt(II) trihydrate, C18H36CoN4O11
- Crystal structure of (E)-4-chloro-N′-(4-(diethylamino)benzylidene)benzohydrazide, C18H20ClN3O
- Crystal structure of 3,6-di(1H-imidazol-1-yl)-9H-carbazole, C18H13N5
- Crystal structure of 4-(4-pyridinyl)-1-naphthoic acid, C16H11NO2
- Crystal structure of 1,1′-diformyl-4,4′-(6H,12H-5,11-methano-dibenzo[b,f][11,5]diazocine-2,8-diyl)dibenzene, C29H22N2O2
- Crystal structure of N′-(adamantan-2-ylidene)pyridine-3-carbohydrazide, C16H19N3O
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(5-methyl-1H-pyrazole-3-carbaldehyde), C14H18N4O2
- Crystal structure of methyl 8-hydroxy-3-isopropyl-5a,8-dimethyl-2,3,4,5,5a,6,7,8,10a,10b-decahydrocyclohepta[e]indene-3a(1H)-carboxylate, C21H34O3
- Crystal structure of 6-oxo-4-propyl-2-(propylthio)-1,6-dihydropyrimidine-5-carbonitrile, C11H15N3OS
- Crystal structure of poly[diacetato(μ2-1,4-bis(1H-imidazol-1-yl)benzene-κ2N:N′)nickel(II)], C26H22N8NiO4
- Crystal structure of bis(2,4-dibromo-6-{(E)[(4-fluorobenzyl)imino]methyl}phenolato-κ2N,O) copper(II), C28H18Br4F2N2O2Cu
- Crystal structure of 1-(adamantan-1-yl)-3-phenylthiourea, C17H22N2S
- Crystal structure of 3-(6-(5-amino-1-phenyl-1H-pyrazol-3-yl)pyridin-2-yl)-1-phenyl-1H-pyrazol-5-amine – dioxan (2/1), C25H23N7O
- Crystal structure of 5-ethyl-6-[(3-methylphenyl)sulfanyl]pyrimidine-2,4(1H,3H)-dione, C13H14N2O2S
- Crystal structure of (((1E,1′E)-(cyclohexane-1,2-diylbis(azanylylidene-κ2N,N′))bis(methanylylidene))bis(2,1-phenylene))bis((2,6-diisopropylphenyl)amide-κ2N′′,N′′′)manganese(II), C44H54N4Mn
- Crystal structure of prop-2-en-1-yl 2-oxo-2H-1-benzopyran-3-carboxylate, C13H10O4
- Crystal structure of bis(μ2-2-((3-methylphenyl)imino)methylphenolato-κ2N,O:O)hexacarbonyldimanganese(I), C34H24Mn2N2O8
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxybut-2-en-1-one C9H12N2O2
- Crystal structure of 2,2′-[pentane-1,5-diylbis(oxy)]dibenzaldehyde, C19H20O4
- Crystal structure of 2-phenyl-5,6,7,8-tetrahydro-4H-benzo[4,5]thieno[2,3-d][1,3]oxazin-4-one, C16H13NO2S
- Crystal structure of (E)-1-(2-chlorophenyl)-N-(4-chlorophenyl)methanimine, C13H9Cl2N
- Crystal structure of ethyl 2-amino-5-bromothiazole-4-carboxylate, C6H7BrN2O2S
- Crystal structure of 2-benzylisothiouronium tetraphenylborate, C32H31BN2S
- Crystal structure of poly[(μ2-biphenyl-2,2′-dicarboxylato-κ4O,O′:O′′,O′′′)(μ2-4,4′-bipyridine-κ2N:N′)copper(II)], C24H16CuN2O4
- Crystal structure of (η5-pentamethylcyclopentadienyl)titanium(III)dichloride (THF), C14H23Cl2OTi
- Crystal structure of 3-ferrocenylsulfonyl-2-(4-methoxyphenyl)-3H-imidazo[4,5-b]pyridine, C23H19FeN3O3S
- Crystal structure of 2-benzoyl-3-(4-fluorophenyl)cyclopropane-1,1-dicarbonitrile, C18H11FN2O
- Crystal structure of 1,6-ditosyl-1,6-diazecane, C22H30N2O4S2
- Crystal structure of N-phenyl-2-(pyridin-4-ylcarbonyl)hydrazinecarboxamide with Z′ = 4, C13H12N4O2
- Crystal structure of N,N-bis(diphenylphosphanyl)cyclohexylamine, C30H31NP2
- Crystal structure of 3-(4-hydroxy-3-methoxyphenyl)-N-phenylpropanamide, C16H17NO3
- Crystal structure of 6-(2-fluorophenyl)-3-phenyl-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazole, C15H9FN4S
- Crystal structure of catena-poly[aqua(dicyanoazanido-2κN-μ2-dicyanoazanido-1κN:2κN′)(μ2-2-methoxy-6-(((2-((3-methoxy-2-oxidobenzylidene)amino)ethyl)imino)methyl)phenolato-1κ2N,N′,2κ2O,O′,1κ2O′′,O′′′:2κ2O′′,O′′′)cadmium(II)copper(II)], C22H20CdCuN8O5

