Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
Abstract
To improve the biogas potential in anaerobic digestion of cattle manure in cold regions, we investigated, in this study, the potential of the anaerobic digestion of cattle manure through sequencing batch anaerobic digestion experiments at sub-mesophilic temperatures (15°C, 20°C, 25°C, and 37°C) for 50 days. Specifically, the changes in the biogas yield, pH, sCOD concentration, enzyme activity, and microbial community structure were examined. The maximum daily yield of biogas was 19.18 mL·gVS−1, which was recorded on day 6 at 37°C, and the final biogas accumulation yield at this temperature was 403.64 mL·gTS−1. The concentration of sCOD gradually increased as enzyme activity increased. The maximum activities of cellulase, hemicellulase, protease, and amylase were recorded in the 37°C experimental group. The decrease in temperature severely limited the activity of different types of enzymes, resulting in a decrease in the activity of microorganisms, which greatly influenced the methanogenic reaction. The dominant bacteria at the phylum level were Bacteroidetes and Proteobacteria, and the dominant methanogen at the genus level was Methanosaeta.
1 Introduction
With the strategic adjustment and industrialization promotion of China’s agricultural structure, the livestock industry has changed from small scale and scattered management to large scale and intensive production, which results in an increase in livestock manure production year by year and distribution is getting more concentrated [1]. In 2016, China produced 2,802 million tons of livestock manure, of which about 633 million tons was cattle manure [2]. Untreated or poorly treated cattle manure not only pollutes soil, water, and air but also adversely affects the animals themselves. But, cattle manure is also a readily available and highly accessible source of biomass. Against the backdrop of China’s increasing energy demand and greater emphasis on environmental protection, there is an urgent need to improve the treatment of livestock manure to reduce its negative impacts and further explore its potential as a source of renewable energy. It is also imperative to promote the sustainable and healthy development of the renewable energy industry.
Anaerobic digestion technology, which is applied to the treatment of biodegradable organic waste, has low energy consumption and produces clean energy [3,4]. Biogas production is completed by a combination of various microorganisms. These are mainly divided into hydrolyzed, acidified bacteria and methanogenic archaea, which exist in a co-nutrient form [5].
Biogas engineering requires a suitable temperature environment. It is difficult for the microorganisms inside the digestive slurry to adapt to environments with low temperatures, and this leads to a slow matrix decomposition, a long fermentation cycle, and a low gas production rate [6]. As such, it is necessary to supply additional energy to the anaerobic digestion reactor to maintain a suitable temperature, especially in cold regions with long winters. However, the additional energy consumption will reduce the economic benefits, resulting in the actual biogas engineering maintaining its operation by relying on government subsidies barely. Therefore, to sustainably develop the economy and environment, it is critical to determine how to increase the biogas yield in the anaerobic digestion of cattle manure at low temperatures. Numerous studies have focused on this subject. For instance, Mcdermott et al. [7] used ultrasonic to pretreat aquaculture wastewater under low-temperature conditions to increase anaerobic digestion of biogas production. The results showed that ultrasonic pretreatment can enhance the chemical oxygen demand removal rate by 10%, as well as the biogas production increased from 0.20 to 0.45 L·day−1, and the biogas concentration increased by 10% on average. Lu et al. [8] studied the effect of adding iron oxide-zeolite to the anaerobic digestion of cattle manure and corn stover using a batch anaerobic digestion reactor at 35 ± 2°C. The results showed that adding iron oxide-zeolite can increase the biogas yield. The biogas yield reached 394.38 mL·gVS–1, which was nearly 372.85% higher than the biogas yield of the control group. Besides, Liu et al. [9] examined the effects of temperature (35°C, 38°C, 41°C, and 44°C) on the biogas yield and microbial community structure characteristics in the anaerobic digestion of corn stover using a continuous stirred tank reactor (CSTR). The results showed that the biogas yield was the highest in the 44°C set. Also, the relative abundance of the Firmicutes, which degrade cellulose and hemicelluloses, was 66.58%. At present, studies on the anaerobic digestion of cattle manure at low temperatures mainly focus on how to increase the reactor temperature and heat preservation, improve pretreatment to increase the biogas yield, and establish a low-temperature kinetic model of anaerobic digestion. However, a few studies have explored the relationship between the biogas yield and enzyme (cellulase, hemicellulase, protease, and amylase) activity, as well as the microbial community structure in the anaerobic digestion of cattle manure at low temperatures.
To respond to this research gap from a microbiological perspective, this study investigated the biogas potential of the digesters in the anaerobic digestion of cattle manure through sequencing batch anaerobic digestion experiments at sub-mesophilic temperatures (15°C, 20°C, 25°C, and 37°C). Regular changes in gas production efficiency, pH, and concentrations of volatile fatty acids (VFAs), soluble chemical oxygen demand (sCOD), and ammonia nitrogen were monitored. In addition, changes in different enzyme activities (cellulase, hemicellulase, protease, and amylase) and microbial community structure were mainly examined. This study aims to improve the gas production performance of biogas projects in cold regions and provide a biological basis for system operating stability.
2 Materials and methods
2.1 Experimental materials
Fresh cattle manure was used as feedstock for the anaerobic digestion experiments in this study. The cattle manure was obtained from a cattle farm in Shenbei New District, Shenyang City, Liaoning Province, China. Before the experiments, the fresh cattle manure samples were stored in a bag in a refrigerator at −20°C. They were thawed at 5°C for 12 h before use; 5°C can ensure the stability of the substrate composition and will not affect the start of the experiment due to the low temperature. The anaerobic digestion sludge as inoculum, which was obtained from the anaerobic digestion tank of the sewage treatment plant in northern Shenyang, was used after a week of cultivation. Table 1 shows the initial characteristics of the cattle manure and inoculation sludge.
Basic properties of cattle manure and inoculated sludge
| Species | TS (%) | VS (%) | pH |
|---|---|---|---|
| Cattle manure | 26.43 | 18.27 | 5.53 |
| Inoculation sludge | 18.12 | 8.36 | 7.12 |
2.2 Materials and methods
The experiments were conducted in a 1.2 L sequencing batch anaerobic digestion bioreactor under different temperature conditions (15°C, 20°C, 25°C, and 37°C) for 50 days. The anaerobic digestion bioreactor used in this study mainly consisted of two jars (1.2 L), one conical flask (1.5 L), and a constant temperature water bath pot (Figure 1), which were used as matrix fermentation flasks, biogas collection bottles, a drainage collection bottle, and a thermostat. The sequencing batch anaerobic digestion bioreactor was connected through anti-aging rubber hoses to form a gas connection device, which had to be airtight when connected. To ensure an absolute anaerobic environment, each of the matrix fermenters was purged with nitrogen for 2 min to remove air. The matrix digestion bottle was placed in a constant-temperature water bath at different temperatures. The heating temperature of the water bath was displayed by a temperature sensor and regulated by a temperature control box. The constant-temperature water bath pot was placed in a 15°C air-conditioning thermostatic chamber to achieve a more precise temperature.
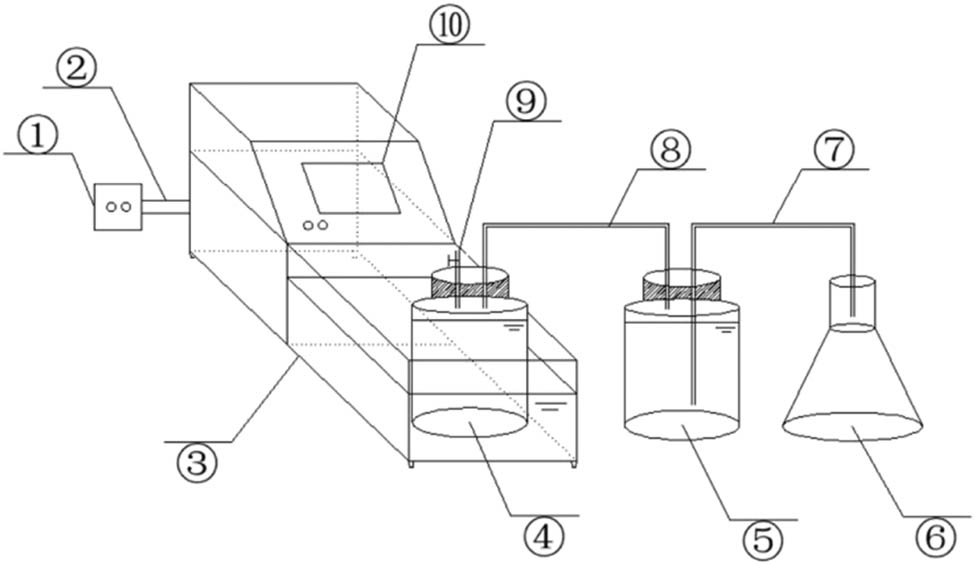
Anaerobic digestion reaction device for cattle manure in sub-mesophilic temperatures. (1) Controller, (2) heating rod, (3) water bath, (4) anaerobic digestion reactor, (5) collecting gas bottle, (6) collecting water bottle, (7) outlet pipe, (8) airway, (9) sampling place, and (10) temperature monitor.
A total of 16 sets of anaerobic digestion experiments were set up in this study (R1–R16). The temperatures of the R1–R4 experimental group were 15°C, 20°C, 25°C, and 37°C. The R5–R8 was composed of parallel experiments. Eight sets of inoculum sludge anaerobic digestion experiments were set as the blank control group (R9–R16) under different temperatures, and R13–R16 were parallel experiments. The final net gas production was the biogas production of the experimental group minus the biogas production of the blank control group. Table 2 shows the specific sample feeding information. R1–R8 used 1 L of deionized water. This experiment uses manual mixing, with stirring done twice a day for 0.5–1 min during each round. From the first day of the experiment, gas production and pH were measured at the same time every day; samples were taken every 3 days to determine the concentration of sCOD and VFAs and taken every 5 days to detect enzyme activity and microbial diversity. The experimental results are taken as the average values of two groups of parallel experiments.
Experimental sample feeding information in the anaerobic digestion of cattle manure at sub-mesophilic temperatures
| Sample information | R1–R8 | R9–R12 |
|---|---|---|
| Cattle manure (g) | 100 | 0 |
| Inoculation sludge (g) | 300 | 300 |
2.3 Analytical methods
2.3.1 Conventional parameter
Moisture, total solid (TS), and volatile solid (VS) were measured according to standard methods reported by APHA [10]. The Leici precision pH meter (PHB-4) was used to analyze pH. The concentrations of sCOD and ammonia nitrogen in the digestive supernatant were determined. The digestive slurry involved centrifuging the experimental sample at 5,000 rpm for 15 min and centrifuging the supernatant through a 0.45 μm water filtration membrane to be detected. The sCOD concentration was determined using the potassium dichromate method [11], and the ammonia nitrogen concentration was determined using Nessler’s reagent spectrophotometry [12]. The content of VFAs was determined using acetic acid as a base, and the measurement method was colorimetric [13] with a 754N ultraviolet-visible spectrophotometer (INESA, Shanghai). Cellulase, hemicellulase, protease, and amylase activities were detected using an RT-6100 microplate reader (Rayto, USA) [14].
2.3.2 DNA extraction and PCR
Total genome DNA from samples were extracted using the hexadecyl trimethyl ammonium bromide/sodium dodecyl sulfate (CTAB/SDS) method. DNA concentration and purity were monitored on 2% agarose gels. According to the concentration, DNA was diluted to 1 ng·μL−1 using sterile water. All polymerase chain reactions (PCRs) were carried out with Phusion® High-Fidelity PCR Master Mix (New England Biolabs). The primer sets 515 F (5′-GTGCCAGCAGCCGCGGTAA-3′) and 806 R (5′-GGACTACHVGGGTWTCTAAT-3′) were used in amplifying the V4 region of the bacterial 16S rRNA gene. The primer set 1106F (TTWAGTCAGGCAACGAGC) and 1378R (TGTGCAAGGAGGGAC) was used for the archaeal 16S rRNA gene. The same volume of buffer (containing SYB green) with PCR products was subjected to electrophoresis on 2% agarose gel for detection. PCR products were mixed in equidensity ratios. Sequencing libraries were generated using Ion Plus Fragment Library Kit 48 rxns (Thermo Scientific) following the manufacturer’s recommendations. The library quality was assessed on the Qubit 2.0 Fluorometer (Thermo Scientific). At last, the library was sequenced on an Ion S5TM XL platform, and 400 bp/600 bp single-end reads were generated.
3 Results and discussion
3.1 Changes in the gas production characteristics
To improve the biogas yield in the anaerobic digestion of cattle manure in cold regions and the stability of the anaerobic digestion process, in this study, we examined the variation of daily and cumulative biogas yields in the anaerobic digestion of cattle manure at sub-mesophilic temperatures. Figure 2a shows the changes in daily biogas yield. At different temperatures (15°C, 20°C, 25°C, and 37°C), the yield initially increased and then decreased. However, the peak value of daily gas production and occurrence time at different temperatures were different. The anaerobic digestion of cattle manure started more rapidly at 37°C without any lag phase, and the biogas yield reached 10.21 mL·gVS−1 on day 1. In the early stage of the anaerobic digestion experiment, the microbial activity was lower in the 15°C, 20°C, and 25°C experiment group, in which the experiment started slowly and the daily biogas yield was generally low. In the initial stage of the experiments, the hydrolyzed acidified bacteria rapidly converted the organic matter dissolved in the digestive juices to VFAs, which were continuously converted into biogas through the methanogenic Achaea. These nutrients were converted to biogas, such that the daily biogas production increased. The highest maximum daily biogas yield was 19.18 mL·gVS−1, which was recorded on day 6 at 37°C. At 15°C, 20°C, and 25°C, the first gas production peak was reached on day 7, day 13, and day 10, respectively. Biogas yield was 4.11, 9.36, and 13.55 mL·gVS−1, respectively. It can be concluded that the lower the temperature, the slower the starting-up speed in the anaerobic digestion of cattle manure, and the smaller the daily yield peak value of biogas. Due to temperature differences, the microbial activity in the digestive juices was inconsistent, and the hydrolysis acidification bacteria and the biogas archaea had different rates of matrix utilization, resulting in different peaks in the biogas yield [15].

Analysis of physical and chemical properties of fermentation broth in the anaerobic digestion of cattle manure at sub-mesophilic temperatures. Daily production of biogas (a), cumulative production of biogas (b), and pH (c).
As the reaction continued, the part of the nonsoluble cellulose, hemicellulose, and other macromolecular polymers in the digestive juices was decomposed into small molecular organic substances by the extracellular enzymes (cellulase, hemicellulase, protease, and lipase) secreted through the hydrolyzed microorganisms. The hydrogen-producing and acid-producing bacteria in the digestive juices continuously converted this part of the organic matter to hydrogen and VFAs. The methanogenic archaea quickly used this part of hydrogen and VFAs to produce biogas. Therefore, as the reaction continued, the daily biogas yield increased, and the daily biogas yield appeared as the second gas production peak at different temperatures. By comparison, it was found that the daily yield of biogas showed that the second gas production peak was reached on day 12 at 37°C, day 18 at 25°C, and day 25 at 20°C, and the biogas yield was 27.56, 9.96, and 14.40 mL·gVS−1, respectively. The daily biogas yield was lower during the whole anaerobic digestion at 15°C, and the daily biogas yield showed that the second gas production peak was reached on day 37 but it was not clear; the daily biogas yield was 6.92 mL·gVS−1. When the temperature was lower, the activities of bacteria and archaea were significantly inhibited, which reduced the biogas yield. There were two gas production peaks in the anaerobic digestion of cattle manure at different temperatures. The main reason is that the degradation of complex organic matter varied, making the anaerobic digestion of cattle manure a multistage process. The different peaks showed differences in anaerobic digestion. The first peak is mainly caused by converted methanation of the soluble small molecule organic matter, the second peak is caused by biodegradable, or a difficult biodegradable compound is further decomposed and methanation. After the microbe entered the starvation stage, the daily biogas yields gradually decreased [16]. As the reaction continued, the different temperatures anaerobic digestion experiments entered the final stage, and the biodegradable organic matter in the digestive juices was gradually utilized, the daily biogas yield decreased, and the gas production finally stopped.
Figure 2b shows the changes in the cumulative biogas yield at different temperatures. The variation trend of the biogas accumulation yield of R1–R4 in each experimental group was the same. It initially increased and then gradually stabilized. In the case of an equal mass of feed solids, the cumulative biogas yield in the descending order anaerobic digestion of cattle manure at different temperatures was R1 < R2 < R3 < R4. The cumulative biogas production was 176.66, 234.95, 270.31, and 403.64 mL·gTS−1, respectively. It can be seen that the efficiency of gas production at 37°C is obviously better than that of the other three temperatures. As the temperature increased, the starting-up speed of the anaerobic digestion experiments gradually accelerated, and the cumulative biogas production gradually increased. Many studies [17,18,19] have found that a decrease in temperature inhibits the activity of microorganisms and the production of biogas in the anaerobic digestion system. The metabolism of microorganisms is significantly affected, which reduces the rate of hydrolysis of microorganisms to biodegradable organic matter and the rate of methanogenesis, and ultimately reduces the cumulative biogas yield. Therefore, a suitable increase in temperature can speed up the start of anaerobic digestion and increase biogas production. However, increasing the temperature requires additional energy consumption. This study considers the economic benefits of the actual biogas project, so it is very important to obtain considerable biogas production at a suitable low temperature.
3.2 Relationship between changes in pH and the concentration of VFAs
pH is an important monitoring indicator and control parameter in anaerobic digestion because it can reflect the acid–base environment of the anaerobic digestion system directly. The pH of the anaerobic digestion system is directly related to the concentration of VFAs in the digestive juices. A very high or low pH inhibits not only the activity of methanogens but also the growth of acid-producing bacteria by increasing the nonionic organic acid, which also affects the formation of VFAs. Such inhibition will reduce the biogas yield [20]. Therefore, it is necessary to study the changes in pH and concentration of VFAs in the anaerobic digestion of cattle manure at different temperatures. Figure 2c shows the relationship between pH and VFAs in the anaerobic digestion of cattle manure at sub-mesophilic temperatures. The figure illustrates that the pH changes initially decreased, increased, and then stabilized. However, the rate of pH decline in R4 (37°C) was significantly faster than that in other experimental groups, indicating that the activity of the hydrolyzed acidified bacteria was higher at 37°C and the hydrolysis acidification rate was faster.
In the initial stage of the experiment, macromolecular organic substances, such as cellulose and hemicellulose, in the digestive juices were hydrolyzed into soluble small molecules, such as monosaccharides, amino acids, and fatty acids, by the extracellular enzymes secreted by the hydrolytic bacteria. Acidified bacteria use these small molecular organics that constantly convert to VFAs. At this point, the methanogenic archaea in the digestive juices have not fully adapted to the environment, and the production rate and activity of the methanogen are not high. Also, the rate of the methanogens’ use of VFAs is lower than the rate of various acidified bacteria to produce VFAs, and VFAs in the digestive juices remain [21]. Therefore, in the early stage of the experiment, the concentration of VFAs gradually increased, and the pH in the digestive juices gradually decreased. As the anaerobic digestion reaction continued, the concentrations of VFAs in the R1, R2, R3, and R4 experimental groups reached maximum values on day 30, day 25, day 15, and day 15, respectively. The maximum concentrations of VFAs were 1,497, 1,638, 1,600, and 1,806 mg·L−1, respectively. The concentration of VFAs peaked the earliest in the R4 experimental group. As the temperature continued to decrease, the peak of the volatile fatty acid concentration appeared far later. This is consistent with the findings of the previous study [22]. Hydrolysis and acidification reaction are processes in which macromolecular organic compounds are converted to small molecular organic compounds. Methanogenic archaea use these small molecular organic compounds to produce biogas. The effect of changes in temperature is in line with the general biological reaction law. Therefore, in the appropriate temperature range, the higher the temperature, the higher the activity of the hydrolyzed and acidified bacteria, resulting in the peak of VFAs earlier [23]. As the reaction continued, the pH of the digestive juices was stable between 6.5 and 7.0. The methanogens in the digestive juices gradually proliferated, and the relative abundance and diversity of methanogens gradually increased. Methanogenic archaea used VFAs at a faster rate than the rate various acidified bacteria produced VFAs. The concentration of VFAs in the digestive juices gradually decreased and then stabilized. In addition, the pH at 37°C was more stable and remained between 6.8 and 7.0 during the stable period, which was more suitable for the growth and reproduction of methanogens.
3.3 Analysis of enzyme activity and the sCOD concentration
The biological enzymes in microorganisms are extremely sensitive to temperature. They significantly influence the growth and metabolism of microorganisms, such that they participate in most of the chemical reactions in the microorganisms so that the metabolism of the microorganisms can proceed in an orderly manner [24]. Figure 3 shows the relationship between the sCOD concentration and different enzyme activities. The figure illustrates that the changes in different enzyme activities initially increased and then decreased. In a certain temperature range, the activity of the same enzyme gradually decreased as the temperature decreased. In the early stage of the experiments, the sCOD concentration in the digestive juices gradually increased as the temperature increased. In the late stage of the experiments, the sCOD concentration in the digestive juices decreased as the temperature increased. In the initial stage of the experiment, the organic matter dissolved in the digestive juices was directly consumed by anaerobic microorganisms as nutrients, resulting in a slight decrease in the sCOD concentration. As the anaerobic digestion reaction continued, the relative abundance and diversity of different kinds of hydrolytic bacteria gradually became larger, so the activities of cellulase, hemicellulase, and amylase gradually increased. Cellulase and hemicellulase are proteins secreted by all microorganisms that can utilize crystalline lignocellulose, which can improve the hydrolysis of cellulose and hemicellulose; it also can promote the dissolution of plant cell walls, release more internal dissolved matter in plant cells, and improve the utilization efficiency of the anaerobic digestion raw materials [25]. Protease and amylase can catalyze the decomposition of organic substances, such as proteins and starch, into small molecular organic compounds such as amino acids, peptides, and monosaccharides. As the temperature gradually increased, the activity of enzymes gradually increased. The maximum activity of cellulase (for other three-enzyme data, see Table A1 in Appendix) during different temperature conditions were 187.75 U·mL−1 (15°C), 226.05 U·mL−1 (20°C), 232.59 U·mL−1 (25°C), and 242.8 U·mL−1 (37°C). In the hydrolysis stage in the anaerobic digestion of cattle manure, macromolecular organic substances, such as cellulose, hemicellulase, and protein, were converted into soluble monosaccharides and disaccharides, such as glucose, amino acids, and fatty acids, through extracellular enzymes (cellulase, hemicellulase, protease, and lipase). The concentration of organic matter dissolved in the digestive juices gradually increased. Therefore, the concentration of sCOD in the digestive juices gradually increased. The maximum sCOD concentrations in the R1–R4 experimental group were 14073.29, 12835.31, 13917.66, and 14016.70 mg·L−1, respectively. As the anaerobic digestion reaction continued, the activities of cellulase and hemicellulase in the digestive juices gradually decreased and hen stabilized. Then, the biodegradable organic matter in the digestive juices was decomposed and gradually utilized. At the same time, the sCOD concentration gradually decreased.
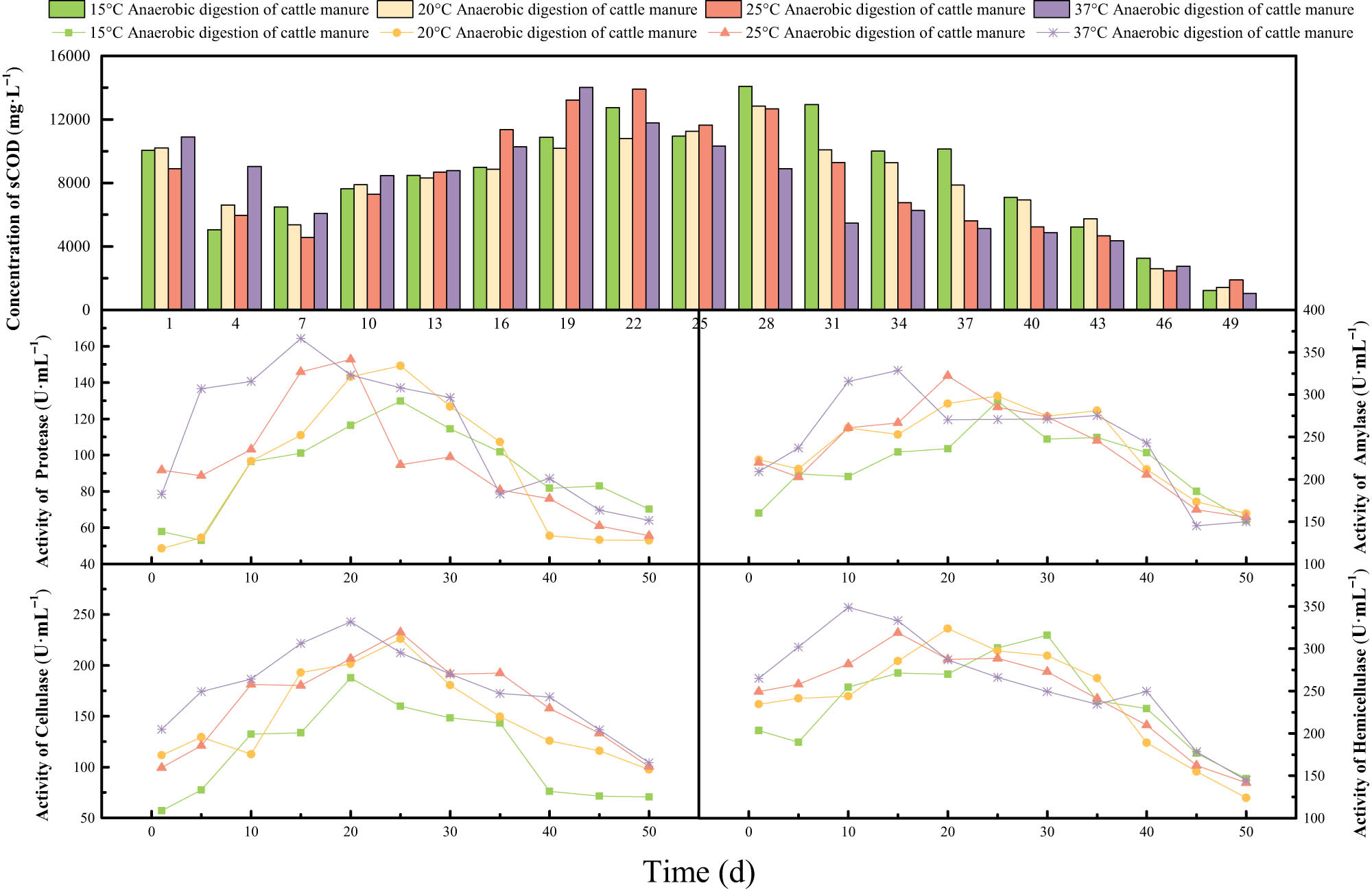
Relationship between the sCOD concentration and different enzyme activities in the anaerobic digestion of cattle manure at sub-mesophilic temperatures.
On the whole, different enzyme activities gradually increased as the temperature increased. Different enzyme activities in the digestive juices play an important role in the hydrolysis of macromolecular organic substances such as cellulose, hemicellulose, and protein. The activities of various hydrolytic bacteria are low at low temperatures. The addition of cellulase, hemicellulase, protease, and amylase can synergistically promote the hydrolysis reaction, accelerate reactor startup, and increase the biogas yield.
3.4 Analysis of the microbial community structure
The anaerobic digestion system of cattle manure is mainly composed of hydrolyzed bacteria, acidified bacteria, and methanogenic archaea. Hydrolyzed bacteria and acidified bacteria provide nutrients for growth reproduction and suitable redox potential for methanogenic archaea. Methanogenic archaea can relieve feedback inhibition, which comes from hydrogen and acid accumulation. Therefore, the microorganisms in anaerobic digestion are interrelated and mutually restricted, and a variety of microorganisms cooperate to complete the anaerobic digestion of cattle manure at sub-mesophilic temperatures. Based on 16S-rRNA sequencing technology, in this study, we analyzed the microbial community structure of sub-mesophilic temperature anaerobic digestion system in cattle manure and explained the anaerobic digestion mechanism of sub-mesophilic temperature cattle manure from the perspective of microbiology. The evaluation of microbial alpha diversity was based on microbial community richness (number of species detected and Chao1 value) and evenness index (Shannon and Simpson index). Tables 3 and 4 show the changes in the microbial diversity index in the anaerobic digestion process of cattle manure under the conditions of sub-mesophilic temperature. As can be seen from Tables 3 and 4, as the temperature increased, the number of detected species, Chao1 index, Shannon and Simpson index, increased gradually. The maximum bacterial and archaea diversity index appeared in the medium temperature 37°C experimental group (R4). The bacteria diversity indexes were 2546.726, 1,924, 0.993, and 9.526, and archaea diversity indexes were 277.294, 216, 0.894, and 4.479. It can be concluded that 37°C is conducive to the growth and reproduction of microorganisms and has good methanogenesis performance. Many studies have found [26,27] that temperature is an important control parameter in anaerobic digestion and strongly influences the microbial community structure and diversity of anaerobic digestion systems.
Changes in the bacterial diversity index in the anaerobic digestion system of cattle manure at sub-mesophilic temperatures
| Temperature (°C) | Number of species | Chao1 index | Shannon index | Simpson index |
|---|---|---|---|---|
| 15 | 2061.218 | 1,631 | 0.914 | 7.081 |
| 20 | 2179.493 | 1,706 | 0.978 | 7.901 |
| 25 | 2372.745 | 1,877 | 0.991 | 8.432 |
| 37 | 2546.726 | 1,924 | 0.993 | 9.526 |
Changes in the archaea diversity index in the anaerobic digestion system of cattle manure at sub-mesophilic temperatures
| Temperature (°C) | Number of species | Chao1 index | Shannon index | Simpson index |
|---|---|---|---|---|
| 15 | 207.404 | 201 | 0.883 | 4.399 |
| 20 | 207.721 | 201 | 0.883 | 4.468 |
| 25 | 223.450 | 214 | 0.893 | 4.478 |
| 37 | 227.294 | 216 | 0.894 | 4.479 |
3.4.1 Analysis of the microbial community abundance with evolutionary relationship
Figure 4a shows the relative abundance change of microbial communities at the phylum levels under different periods of the anaerobic digestion of cattle manure at sub-mesophilic temperatures. As illustrated in Figure 4a, the dominant bacteria at the phylum level during the initial stage of the reaction were Bacteroidetes, Proteobacteria, and Firmicutes. Bacteroidetes, Proteobacteria, and Firmicutes in anaerobic digestion systems can hydrolyze macromolecular organics such as cellulose, hemicellulose, and protein. In Figure A1, the dominant bacteria at the genus level were VadinBC27_wastewater-sludge_group. VadinBC27_wastewater-sludge_group can ferment amino acids (cysteine, leucine, serine, and tryptophan) to produce small molecule fatty acids. These bacteria are involved in the hydrolysis acidification reaction to provide usable nutrients for methanogenic archaea [28]. In the early stage of the anaerobic digestion experiments in this study, hydrolytic microorganisms, such as VadinBC27_wastewater-sludge_group and Anaerocella, were relative of high abundance. Generally, these microorganisms can degrade macromolecular organic substances such as cellulose. As the reaction continued, the relative abundances of Bacteroidetes, Firmicutes, and VadinBC27_wastewater-sludge_group decreased gradually, while the relative abundance of Proteobacteria, Chloroflexi, Cloacimonetes and Euryarchaeota gradually increased. In anaerobic digestion, Chloroflexi can decompose glucose and soluble small molecule organic matter produced by Bacteroides, Proteobacteria, and Firmicutes to produce acetic acid and hydrogen [29]. Toward the end of the anaerobic digestion experiments, the relative abundance and diversity of microorganisms in the digestive juices were gradually reduced.
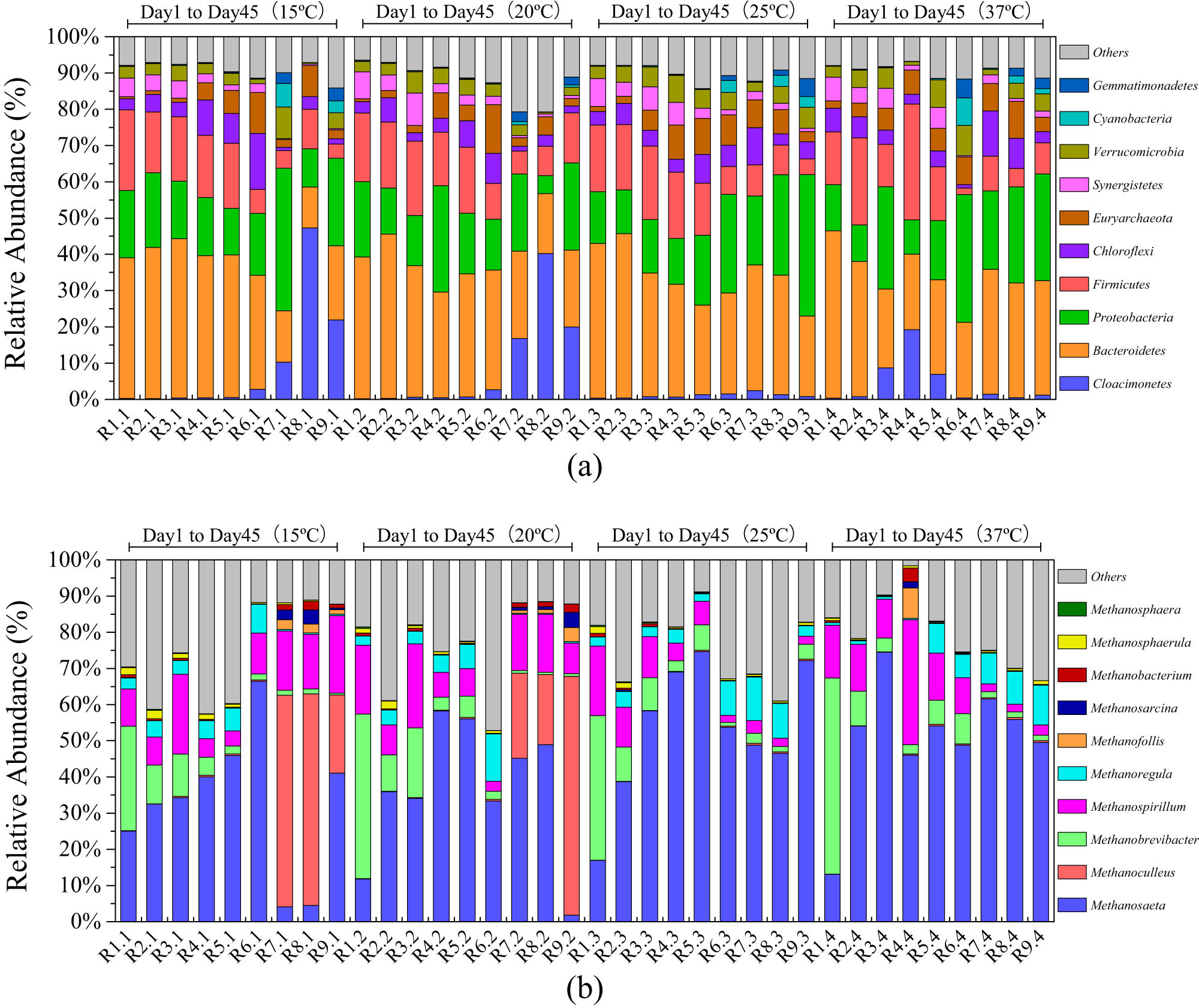
Relative abundance changes of the microbial community at phylum levels (a) and the methanogenic microbial community at the genus level (b) in the anaerobic digestion of cattle manure at sub-mesophilic temperatures.
Methanogenic archaea, belonging to Euryarchaeota, Methanomicrobia, Methanosarcinales, and Methanosaetaceae, can degrade acetic acid to produce CH4 and CO2 but cannot use alcohol, methylamine, and H2/CO2 [30]. Related studies have shown that temperature has a very significant effect on the relative abundance and diversity of methanogens [31]. Figure 4b shows the relative abundance change of methanogenic archaea at the genus level of the cattle manure anaerobic digestion at different temperatures. The dominant bacterial group in the digestion fluids of anaerobic digestion of cattle manure at different temperatures was Methanosateta. Other methanogens were rare and had low abundances such as Methanosphaera, Methanosphaerula, and Methanobacterium, but it is good for improving the stability of the anaerobic digestion system. The relative abundance of Methanosateta gradually increased in the 15°C experimental group, but its relative abundance suddenly decreased on day 35, and Methanoculleus gradually adapted to the low-temperature environment to become the dominant flora, which may help increase the rate of methanogenesis under low-temperature conditions. The relative abundance of Methanosateta gradually increased in the 20°C experimental group. However, the relative abundance of Methanosateta gradually decreased in the late anaerobic digestion experiment (days 30–50) but the diversity of methanogenic archaea increased. Methanoculleus in the biogas phase of R1 and R2 became the dominant methanogen of the methanogenic phase in the late anaerobic digestion experiment. As the temperature increases, the maximum relative abundance of different types of methanogenic archaea gradually increases, and the corresponding peak of daily biogas yield also gradually increases. The relative abundance of Methanoregula increased at the late stage (days 30–50) of the 25°C and 37°C anaerobic digestion experiment group. It can directly use CO2 and H2 and play an important role in promoting the smooth progress of the anaerobic digestion experiment.
3.4.2 Analysis of relative abundance of microbial communities in the peak gas production
Figure 5a shows the relative abundance of microbial communities at the phylum levels in the peak gas production. As illustrated in Figure 5a, the dominant bacteria in the period at the phylum level were mainly Bacteroidetes, Firmicutes, and Proteobacteria at different temperatures. By comparison, the relative abundances of Bacteroidetes, Proteobacteria, and Firmicutes were higher at different temperatures. All of them in the anaerobic digestion process usually degrade lignocellulose, such as cellulose and hemicellulose [32,33,34]. In addition, Firmicutes contains bacteria that produce acetic acid, which can convert long-chain fatty acids to acetic acid [35]. The relative abundance of the dominant microbial community in the anaerobic digestion of cattle manure significantly varied at different temperatures. Compared with different temperature experimental groups, the relative abundances of Firmicutes, Verrucomicrobia, and Euryarchaeota increased with an increase in temperature: Firmicutes abundance increased from 16.73% to 24.00%, Verrucomicrobia abundance increased from 3.13% to 4.82%, and Euryarchaeota relative abundance increased from 1.07% to 3.80%. It shows that as the temperature gradually increases, the above microorganisms become more adaptable to the environment and become dominant bacteria groups, and their relative abundance gradually increases. However, the relative abundance of Proteobacteria gradually decreased from 20.59% to 10.13%, indicating that Proteobacteria is more adaptable to low-temperature environments and has a higher relative abundance at lower temperatures. Bacteroidetes, Chloroflexi, Synergistetes, and other microorganisms have a high ability to adapt to temperature changes, and the relative abundance changes are not obvious. Synergistetes ferments amino acids and plays an important role in removing intermediate metabolites from anaerobic digestion systems [36].
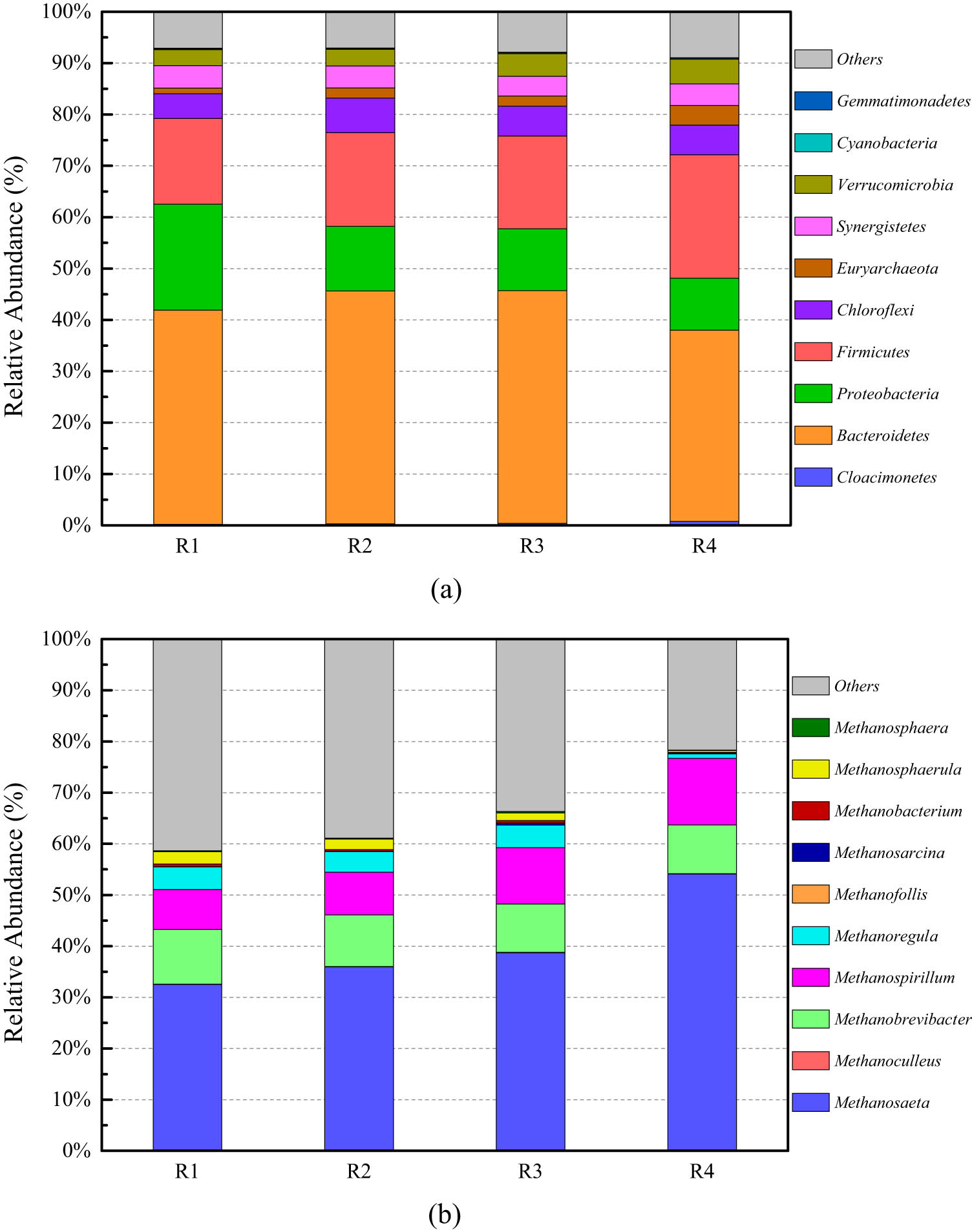
The relative abundance of microbial communities at the phylum levels (a) and the methanogenic microbial community at the genus level (b) in the peak gas production of anaerobic digestion system of cattle manure under conditions of the sub-mesophilic temperatures.
In Figure A2, dominant bacteria in the peak gas production at the genus level were mainly VadinBC27_wastewater-sludge_group, Sulfurovum, and Arcobacter. The relative abundance of the dominant microbial community in the anaerobic digestion of cattle manure significantly varied at different temperatures. As the temperature increases, the relative abundance of Methanosaeta gradually increases from 0.16% to 1.41%. When the temperature is low, the proportion of other bacteria and archaea was not high. The methanogenic archaea were significantly reduced, which directly led to the reduction of biogas production in the anaerobic digestion of cattle manure at sub-mesophilic temperatures.
Figure 5b shows the relative abundance changes of the methanogenic phase at the peak gas production of the anaerobic digestion experiment of sub-mesophilic temperature. The dominant methanogen was Methanosaeta. Through comparative analysis, it is found that the Methanosaeta have a higher abundance under four different temperature conditions, which has more strong adaptability. As the temperature continues to increase, the relative abundance of Methanosaeta gradually increases, which was an obligate acetic acid type methanogenic archaea. This type of methanogenic archaea uses acetic acid in the anaerobic digestive fluid as the reaction substrate. It can convert 98–99% of the methyl group in acetic acid into biogas, which is an important and specific acetic acid type methanogen in an anaerobic environment. When the temperature is higher, the concentration of acetic acid in the digestive juice was higher than can be consumed by Methanosaeta. Therefore, the relative abundance of Methanosaeta in the anaerobic digestion juice of the 37°C experimental group was higher. In addition, the relative abundance of Methanospirillum increased with the increase of temperature and Methanoregula dropped significantly at 37°C. According to the relationship between the relative abundance of microorganisms in the anaerobic digestion system and the temperature, there was a certain response relationship between the composition of the anaerobic microbial community structure and temperature.
3.4.3 Results of microbial operational taxonomic units cluster analysis
Figure 6 shows a Venn diagram based on the results of microbial OTUs (operational taxonomic units) cluster analysis during the anaerobic digestion of sub-mesophilic temperature cattle manure, where Figure 6a represents bacteria and Figure 6b is the archaea. Each circle in the figure represents a sample amount (group), while the number of circles and circle overlaps represent the number of OTUs shared between the samples (groups). The numbers without overlapping parts represent the number of unique OTUs of the sample (group), and the different colors represent the temperatures of different experiment groups. As can be seen from Figure 6a, the total number of bacterial OTUs shared during the anaerobic digestion of cattle manure at different temperatures are 5,254. The number of OTUs peculiar to the experimental group of 15°C, 20°C, 25°C, and 37°C were 130, 111, 147, and 125, respectively, and the number of the same OTUs at the four temperature experimental groups was 3,227. As can be seen from Figure 6b, the total number of OTUs of archaea during the anaerobic digestion of cattle manure at different temperatures are 306. The number of OTUs peculiar to the experimental groups of 15°C, 20°C, 25°C, and 37°C were 6, 5, 6, and 3, respectively, and the number of the same OTUs at the four temperature experimental groups was 259. It can be concluded that there is a common part of the microbial community structure during the anaerobic digestion of cattle manure at sub-mesophilic temperatures, and at the same time, there are also significant differences.
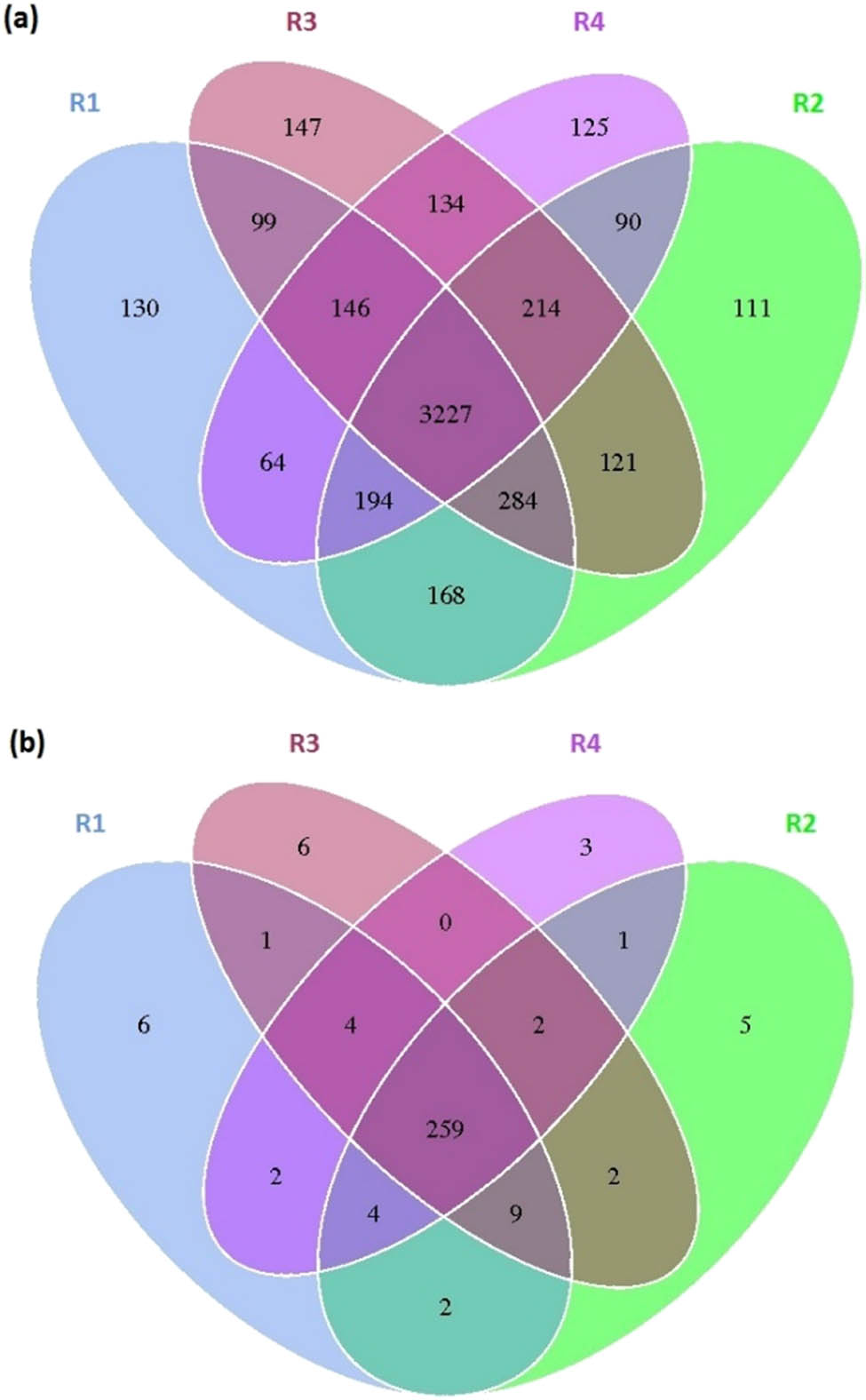
The Venn diagram based on the cluster analysis results of bacterial (a) and archaeal (b) OTUs in the stable phase in the anaerobic digestion of cattle manure at sub-mesophilic temperatures.
4 Conclusion
This study investigated the biogas potential of the digesters in the anaerobic digestion of cattle manure in cold regions, particularly by comparing the changes in gas production characteristics. It was found that the anaerobic digestion of cattle manure at 37°C had better biogas performance; the start-up time of the anaerobic digestion experiment increased as the temperature decreased. The highest maximum daily biogas yield (19.18 mL·gVS−1) and the final biogas accumulation yield (403.64 mL·gTS−1) were significantly higher than 15°C, 20°C, and 25°C. According to the changes of sCOD and VFAs in each group, the hydrolytic acidification rate of the medium-temperature anaerobic digestion is faster than that of low-temperature anaerobic digestion due to the increase in the activity of hydrolase. The microbial richness and diversity indices of the R4 (37°C) set were the highest. The dominant bacteria at the phylum level were Bacteroidetes and Proteobacteria. The dominant methanogenic archaea at the genus level were Methanosaeta. With the decrease of temperature, the richness and diversity of hydrolytic acidified bacteria and methanogenic archaea decreased and the abundance of Methanosaeta decreased accordingly.
-
Funding information: This work was funded by the Gansu Youth Science and Technology Fund Project (20JR10RA258) and Supported by the Open Research Subject of Sichuan Provincial Engineering Research Center of Hydroelectric Energy Power Equipment Technology (grant number SDNY2021-001), Tianyou Youth Talent Lift Program of Lanzhou Jiaotong University, the Youth Scholars Science Foundation of Lanzhou Jiaotong University (2020018), Wuwei City Science and Technology Plan Project (WW2002029).
-
Author contributions: Xiaofei Zhen contributed to the conception of the study and performed the data analyses and wrote the manuscript; Miao Luo contributed significantly to analysis and manuscript preparation; Haiying Dong helped perform the analysis with constructive discussions; Lei Fang performed the experiment; Weiwei Wang helped perform the analysis with constructive discussions; Lei Feng performed the experiment; and Qin Yu contributed significantly to analysis and manuscript preparation.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The data used to support the findings of this study are available from the corresponding author upon request.
Appendix
Maximum activities of different enzymes in the anaerobic digestion of cattle manure at medium to low temperatures
| Temperature (°C) | Cellulase (U·mL−1) | Hemicellulase (U·mL−1) | Protease (U·mL−1) | Amylase (U·mL−1) |
|---|---|---|---|---|
| 15 | 187.75 | 315.94 | 129.69 | 292.23 |
| 20 | 226.05 | 323.80 | 149.28 | 298.50 |
| 25 | 232.59 | 318.90 | 152.78 | 322.14 |
| 37 | 242.81 | 348.85 | 164.31 | 328.74 |

Relative abundance changes of the microbial community at the genus level in the anaerobic digestion of cattle manure at medium to low temperatures.
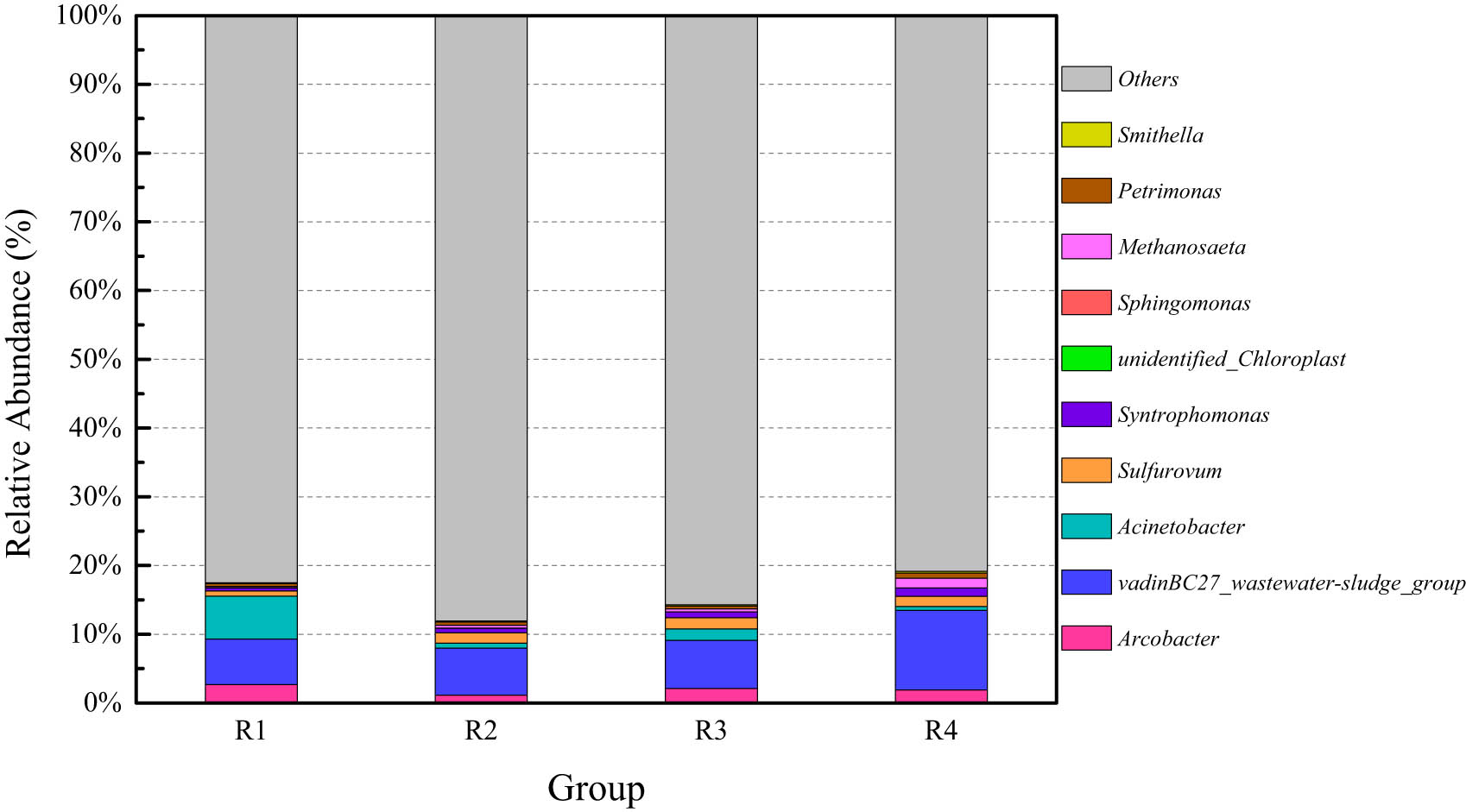
Relative abundance of microbial communities at the genus levels in the peak gas production of anaerobic digestion system of cattle manure at medium to low temperatures.
References
[1] Zhan XM, Xiao LW. LivestockWaste 2016-International conference on recent advances in pollution controland resource recovery for the livestock sector. Front Environ Sci Eng. 2017;11:16.10.1007/s11783-017-0958-ySuche in Google Scholar
[2] Zhu BN, Gikas P, Zhang RH, Lord J, Jenkins B, Li XJ. Characteristics and biogas production potential of municipal solid wastes pretreated with a rotary drum reactor. Bioresour Technol. 2009;100:1122–9.10.1016/j.biortech.2008.08.024Suche in Google Scholar PubMed
[3] Vedrenne F, Béline F, Dabert P, Bernet N. The effect of incubation conditions on the laboratory measurement of the biogas producing capacity of livestock wastes. Bioresour Technol. 2008;99:146–55.10.1016/j.biortech.2006.11.043Suche in Google Scholar PubMed
[4] Prajapati SK, Malik A, Vijay VK. Comparative evaluation of biomass production and bioenergy generation potential of Chlorella spp. through anaerobic digestion. Appl Energy. 2014;114:790–7.10.1016/j.apenergy.2013.08.021Suche in Google Scholar
[5] Gou CL, Yang ZH, Huang J, Wang HL, Xu HY, Wang L. Effects of temperature and organic loading rate on the performance and microbial community of anaerobic co-digestion of waste activated sludge and food waste. Chemosphere. 2014;105:146–51.10.1016/j.chemosphere.2014.01.018Suche in Google Scholar PubMed
[6] Wang YJ, Zhang C, Gao CY, Wang HY, Wang L, Sun N, et al. Research progress and prospect on heat insulation and temperature increasing technology for biogas project in winter of northern China. China Biogas. 2017;35:93–9.Suche in Google Scholar
[7] McDermott BL, Chalmers AD, Goodwin JAS. Ultrasonication as a pre-treatment method for the enhancement of the psychrophilic anaerobic digestion of aquaculture effluents. Environ Technol. 2001;22:823–30.10.1080/095933322086180317Suche in Google Scholar PubMed
[8] Lu XF, Wang HD, Ma F, Zhao G, Wang SW. Enhanced anaerobic digestion of cow manure and rice straw by the supplementation of an iron oxide-zeolite system. Energy Fuels. 2016;31:599–606.10.1021/acs.energyfuels.6b02244Suche in Google Scholar
[9] Liu CM, Wachemo AC, Tong H, Shi SH, Zhang L, Yuan HR, et al. Biogas production and microbial community properties during anaerobic digestion of corn stover at different temperatures. Bioresour Technol. 2018;261:93–103.10.1016/j.biortech.2017.12.076Suche in Google Scholar PubMed
[10] APHA. Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association; 2005.Suche in Google Scholar
[11] National Environmental Protection Agency. GB/T 11914-1989 water quality-determination of the chemical oxygen demand-dichromate method. Beijing: Standards Press of China; 1989.Suche in Google Scholar
[12] National Environmental Protection Agency. GB/T 7479-1987 water quality-determination of ammonium-Nessler’s reagent colorimetric method. Beijing: Standards Press of China; 1987.Suche in Google Scholar
[13] Ren NQ, Wang AJ. Principles and applications of anaerobic biotechnology. China: Environmental Science and Engineering Publishing Center; 2004.Suche in Google Scholar
[14] Wang S, Zhang HY, Wang JP. Enzyme linked immunosorbent assay: basic principles and application in the detection of food chemical pollutants. Beijing: Science Press; 2011.Suche in Google Scholar
[15] Massé DI, Masse L, Xia Y, Gilbert Y. Potential of low-temperature anaerobic digestion to address current environmental concerns on swine production. J Anim Sci. 2010;88:E112–20.10.2527/jas.2009-2432Suche in Google Scholar PubMed
[16] Zhen GY, Lu XQ, Kobayashi T, Kumar G, Xu KQ. Anaerobic co-digestion on improving biogas production from mixed microalgae (Scenedesmus sp., Chlorella sp.) and food waste: kinetic modeling and synergistic impact evaluation. Chem Eng J. 2016;299:332–41.10.1016/j.cej.2016.04.118Suche in Google Scholar
[17] Chae KJ, Jang A, Yim SK, Kim IS. The effects of digestion temperature and temperature shock on the biogas yields from the mesophilic anaerobic digestion of swine manure. Bioresour Technol. 2008;99:1–6.10.1016/j.biortech.2006.11.063Suche in Google Scholar PubMed
[18] Zamanzadeh M, Hagen LH, Svensson K, Linjordet R, Svensson K, Horn SJ. Anaerobic digestion of food waste-Effect of recirculation and temperature on performance and microbiology. Water Res. 2016;96:246–54.10.1016/j.watres.2016.03.058Suche in Google Scholar PubMed
[19] Song YC, Kwon SJ, Woo JH. Mesophilic and thermophilic temperature co-phase anaerobic digestion compared with single-stage mesophilic- and thermophilic digestion of sewage sludge. Water Res. 2004;38:1653–62.10.1016/j.watres.2003.12.019Suche in Google Scholar PubMed
[20] Elefsiniotis P, Wareham DG. Utilization patterns of volatile fatty acids in the denitrification reaction. Enzyme Microb Technol. 2007;41:92–7.10.1016/j.enzmictec.2006.12.006Suche in Google Scholar
[21] Zhou MM, Yan BH, Wong JWC, Zhang Y. Enhanced VFAs production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour Technol. 2018;248:68–78.10.1016/j.biortech.2017.06.121Suche in Google Scholar PubMed
[22] Peces M, Astals S, Clarke WP, Jensen PD. Semi-aerobic fermentation as a novel pre-treatment to obtain VFA and increase biogas yield from primary sludge. Bioresour Technol. 2016;200:631–8.10.1016/j.biortech.2015.10.085Suche in Google Scholar PubMed
[23] Parawira W, Murto M, Read JS, Mattiasson B. Profile of hydrolases and biogas production during two-stage mesophilic anaerobic digestion of solid potato waste. Process Biochem. 2005;40:2945–52.10.1016/j.procbio.2005.01.010Suche in Google Scholar
[24] Sun QH, Wu D, Zhang ZC, Zhao Y, Xie XY, Wu JQ, et al. Effect of cold-adapted microbial agent inoculation on enzyme activities during composting start-up at low temperature. Bioresour Technol. 2017;244:635–40.10.1016/j.biortech.2017.08.010Suche in Google Scholar PubMed
[25] Qing Q, Wyman CE. Hydrolysis of different chain length xylooliogmers by cellulase and hemicellulase. Bioresour Technol. 2011;102:1359–66.10.1016/j.biortech.2010.09.001Suche in Google Scholar PubMed
[26] Treu L, Kougias PG, Campanaro S, Bassani I, Angelidaki I. Deeper insight into the structure of the anaerobic digestion microbial community; the biogas microbiome database is expanded with 157 new genomes. Bioresour Technol. 2016;216:260–6.10.1016/j.biortech.2016.05.081Suche in Google Scholar PubMed
[27] Pervin HM, Dennis PG, Lim HJ, Tyson GW, Batstone DJ, Bond PL. Drivers of microbial community composition in mesophilic and thermophilic temperature-phased anaerobic digestion pre-treatment reactors. Water Res. 2013;47:7098–108.10.1016/j.watres.2013.07.053Suche in Google Scholar
[28] Xie ZF, Wang ZW, Wang QY, Zhu CW, Wu ZC. An anaerobic dynamic membrane bioreactor (AnDMBR) for landfill leachate treatment: Performance and microbial community identification. Bioresour Technol. 2014;161:29–39.10.1016/j.biortech.2014.03.014Suche in Google Scholar
[29] Yi J, Dong B, Jin JW, Dai XH. Effect of increasing total solids contents on anaerobic digestion of food waste under mesophilic conditions: performance and microbial characteristics analysis. PLoS One. 2014;9:e102548.10.1201/b18872-4Suche in Google Scholar
[30] Schattauer A, Abdoun E, Weiland P, Plöchl M, Heiermann M. Abundance of trace elements in demonstration biogas plants. Biosyst Eng. 2011;108:57–65.10.1016/j.biosystemseng.2010.10.010Suche in Google Scholar
[31] Ke X, Wang CY, Li RD, Zhang Y. Effects of oxytetracycline on biogas production and the microbial communities during anaerobic digestion of cow manure. J Integr Agric. 2014;13:1373–81.10.1016/S2095-3119(13)60683-8Suche in Google Scholar
[32] Wang M, Zhou J, Yuan YX, Dai YM, Li D, Li ZD, et al. Biogas production characteristics and microbial community dynamics of mono-digestion and co-digestion using corn stalk and pig manure. Int J Hydrog Energy. 2017;42:4893–901.10.1016/j.ijhydene.2016.10.144Suche in Google Scholar
[33] Ye NF, Lü F, Shao LM, Godon JJ, He PJ. Bacterial community dynamics and product distribution during pH-adjusted fermentation of vegetable wastes. J Appl Microbiol. 2007;103:1055–65.10.1111/j.1365-2672.2007.03321.xSuche in Google Scholar PubMed
[34] Roest K, Heilig HGHJ, Smidt H, de Vos WM, Stams AJM, Akkermans ADL. Community analysis of a full-scale anaerobic bioreactor treating paper mill wastewater. Syst Appl Microbiol. 2005;28:175–85.10.1016/j.syapm.2004.10.006Suche in Google Scholar PubMed
[35] Singh J, Suhag M, Dhaka A. Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: a review. Carbohydr Polym. 2015;117:624–31.10.1016/j.carbpol.2014.10.012Suche in Google Scholar PubMed
[36] Baena S, Fardeau ML, Labat M, Ollivier B, Garcia JL, Patel B. Aminobacterium mobile sp. nov., a new anaerobic amino-acid-degrading bacterium. Int J Syst Evol Microbiol. 2000;50:259–64.10.1099/00207713-50-1-259Suche in Google Scholar PubMed
© 2021 Xiaofei Zhen et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis
Artikel in diesem Heft
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis

