CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
-
Alexey Pechenkin
, Dmitry Potemkin
Abstract
This work presents results on CO2 hydrogenation to dimethyl ether (DME) over bifunctional catalysts consisting of In2O3, supported on natural clay halloysite nanotubes (HNT), and HNT modified with Al-MCM-41 silica arrays. The catalysts were characterized by TEM, STEM, EDX-mapping, NH3-TPD, XRD, low-temperature nitrogen adsorption, TPO, and H2-TPR techniques. Catalytic properties of In2O3/HNT and In2O3/Al-MCM-41/HNT in the CO2 hydrogenation to DME were investigated in a fixed-bed continuous flow stainless steel reactor at 10–40 atm, in the temperature range of 200–300°C, at GHSV = 12,000 h−1 and molar ratio of H2:CO2 = 3:1. The best catalyst for CO2 hydrogenation was In2O3/Al-MCM-41/HNT that provided DME production rate 0.15 gDME·(gcat·h)−1 with DME selectivity 53% and at 40 bar, GHSV = 12,000 h−1, and T = 250°C. It was shown that In2O3/Al-MCM-41/HNT exhibited stable operation for at least 40 h on stream.
1 Introduction
Currently, many efforts of various researchers around the world are being made to solve environmental problems. Air pollution is considered one of the main such problems. Various options are offered – the use of alternative energy sources, such as solar [1], wind [2], and biofuel [3]. However, it is also necessary to pay great attention to the utilization of CO2. The ever-growing production capabilities of different countries lead to an increase in carbon dioxide emissions into the atmosphere. This is the reason for the increase in the so-called “greenhouse effect,” which leads to an increase in the global temperature of the planet and, accordingly, climate change. This, as well as the fact that CO2 is an inexpensive, readily available compound, requires the search for new technologies, methods, and ways of processing carbon dioxide. Recently, more and more attention of researchers from all over the world has been attracting the study of the reaction of CO2 hydrogenation into various compounds such as methane [4], methanol [5,6,7,8], dimethyl ether (DME) [9,10,11], or hydrocarbons [12]. Among these compounds, DME attracts attention as a multipurpose product – it is used in the synthesis of methyl acetate, dimethyl sulfate, various petrochemical compounds, as a feedstock for powering fuel cells [13,14,15]. Due to its properties – high cetane number (55–60), low autoignition temperature, and high oxygen content (∼35%), DME is considered as an alternative to diesel fuel or LPG. In terms of its physicochemical properties, DME is close to LPG, which allows its simple storage and transportation.
Commonly, DME is synthesized according to a two-stage scheme through the synthesis of methanol (MeOH) from synthesis gas on Cu/ZnO/Al2O3 (CZA) catalyst and its subsequent conversion into DME on a solid acid catalyst. However, the direct synthesis of DME is thermodynamically more favorable than the synthesis of methanol [16], which attracts more attention to the study of this process. Catalysts for the direct synthesis of DME by hydrogenation of CO2 are divided into two types: first, a mechanical mixture of catalysts for the synthesis of methanol and a catalyst for its dehydration; second, a catalyst called “bifunctional” which contains both types of necessary catalytic sites on its surface. Typically, first type systems are prepared by mixing or grinding of methanol synthesis and acidic components. Such method has some disadvantages like disintegration of its components during the reaction, mass, and heat transfer limitations [17]. So, recently, bifunctional catalysts have attracted much attention of scientists [10]. Another challenge is to perform direct DME synthesis with high selectivity without formation of CO. Industrial Cu/ZnO/Al2O3 methanol synthesis catalyst is also known to be active in reverse water gas shift (RWGS) reaction causing hydrogen losses due to formation of CO.
According to the recent density functional theory calculations [18], it is possible to obtain methanol with high selectivity via the hydrogenation of CO2 on indium oxide. The reaction proceeds by the cyclic mechanism of the formation of oxygen vacancies and subsequent activation of CO2 on them. Later, these calculations were experimentally confirmed. It was shown that methanol with ∼100% selectivity is achieved on bulk In2O3 at low CO2 conversions [19]. These works gave an impetus to further extensive studying of catalysts on indium oxide in the hydrogenation of CO2 – the effect of various supports, preparation methods, the structure of indium oxide, and various additives on the catalytic activity [20–24]. Thus, indium oxide as a catalyst for methanol synthesis looks promising. An acid component is required for the design of a bifunctional DME direct synthesis catalyst. Usually, γ-Al2O3 or various zeolites – H-ZSM-5, Y, MOR, FER – are used as an acid catalyst in a two-stage process [17,25–33].
In this work for the first time, halloysite aluminosilicate nanotubes (HNT) were used as an acid support for the direct synthesis of DME catalysts. Halloysite nanotubes (HNT) have a rolled tubular structure (length ∼1–2 μm, inner diameter 10–30 nm) [34–37]. In particular, halloysite was successfully applied as a support for catalysts of various applications, including aromatics hydrogenation [38–42], DME conversion to olefins [43], hydrogen production [44], Fischer–Tropsch synthesis [45], xylene isomerization [46,47], catalytic cracking [48], photocatalysis [49,50], etc. Its feature is that HNT contains two different types of active centers – functional groups of SiO2 are present on the surface of nanotubes, while Al2O3 groups are located inside. This surface chemistry allows the metal component to be applied to both the external and internal surfaces, depending on the desired properties.
Nowadays, core-shell structure catalysts, such as Cu–ZnO–Al2O3@HZSM-5 [51] or CuO–ZnO–Al2O3@SiO2–Al2O3 [52], are intensively studying in direct DME synthesis from CO2. These systems prevent metal particles from sintering [53] and deactivation due coke formation by side reactions [54]. So, in the literature there are works on the modification surface of halloysite with MCM-41 [36] to make core-shell structure. This core-shell halloysite – based aluminosilicate composite is promising for catalytic applications due to high specific surface area and enhanced thermal and mechanical properties [39]. However, pure MCM-41 doesn’t have the required acid sites on its surface. Therefore, before using it as a support, the surface was modified with aluminum in order to increase acid sites. The resulting Al-MCM-41 has a large amount of acid sites, which are necessary to produce DME by CO2 hydrogenation.
In this work, we present novel bifunctional In2O3 catalysts supported on natural clay nanotubes (10 wt% In2O3/HNT) and composite with structured mesoporous silica (10 wt% In2O3/MCM-41/HNT) for CO2 hydrogenation to DME.
2 Experimental section
2.1 Synthesis and characterization of catalysts
As a support for catalysts, HNT (≥98%, Sigma-Aldrich, St. Louis, MO, USA) and ordered mesoporous composite Al-MCM-41/HNT were used. This modified support was prepared by the template synthesis method as described in literature [43]. Cetyltrimethylammonium bromide (≥98%, Sigma-Aldrich, St. Louis, MO, USA) was used for the formation of MCM-41. Aluminum isopropoxide (≥98%, Sigma-Aldrich, St. Louis, MO, USA) was used as an aluminum source. The weight ratio between Al-MCM-41 and HNT in the synthesized support was 60:40%.
In2O3/HNT and In2O3/Al-MCM-41/HNT catalysts were synthesized by the incipient wetness impregnation method of HNT and Al-MCM-41/HNT with aqueous solutions of indium nitrate(iii) (Reakhim, Moscow, Russia, purity 99.99%) taken at desired ratio, respectively. The samples were dried at 80°C in air for 4 h and after that calcined at 400°C (heating rate 1°C·min−1) for 3 h in air.
Actual In2O3 loadings in the catalysts were determined by inductively coupled plasma atomic emission spectrometry (Optima instrument; Perkin-Elmer).
Transmission electron microscope (TEM) JEOL JEM-2100 (UHR) operated at 200 kV (the lattice resolution of 0.19 nm) and equipped with LaB6 gun was employed to investigate structure, morphology, and chemical composition of the obtained samples. The samples for the TEM analysis were prepared by the dispersing in ethanol. The as-prepared dispersed solution was dropped onto carbon-coated formvar TEM Cu grid (300 mesh, Ted Pella, Inc.). The acquisition of TEM/HRTEM images was performed in TEM mode using Olympus Quemesa 11 megapixel CCD camera. The collection of each EDX map was performed in STEM mode with help of EX-24065JGT energy dispersive X-ray (EDX) analyser.
The specific BET surface areas (S BET) and pore volume (V p) of the support and the catalysts were determined using the low-temperature N2-adsorption method using a TriStar3000 apparatus. Before experiment, all samples were outgassed in vacuum at 300°C, then nitrogen adsorption/desorption isotherms were recorded at −196°C. The specific surface area of the samples was calculated by the Brunauer–Emmett–Teller (BET) equation. The pore volume was evaluated in accordance with Barrett–Joyner–Halenda model.
NH3 temperature-programmed desorption (NH3-TPD) was used to evaluate the acid properties of the samples. The catalyst was saturated by mixture of NH3 and N2 at 100°C for 30 min. After that, the sample was purged with a stream of nitrogen to remove physisorbed ammonia at same conditions. Then NH3-TPD curve was recorded up to 700°C with a rate of 10° per minute.
Temperature-programmed reduction (H2-TPR) and temperature-programmed oxidation (TPO) experiments were carried out using a STA 409 PC Luxx derivatograph fitted with a QMS-200 mass spectrometer. For H2-TPR, the samples (∼50 mg) were heated from room temperature to 500°C (5°C·min−1) in a 10 vol% H2–Ar mixture flowing at 100 mL·min−1. For TPO, samples were heated from 25°C to 800°C in a 10 vol% O2–Ar mixture flowing at 100 mL·min−1.
X-ray structural analysis (XRD) of the samples was recorded on a Bruker D8 Advance (Bruker, Germany) diffractometer (CuKα) in the 2θ range of 8°–63° with a step 0.05° per 4 s. Analysis of the obtained diffraction data was carried out using the PowderCell 2.4 programme using the JCPDS international diffraction database as a reference.
2.2 Catalyst testing
Catalytic experiments on CO2 hydrogenation were studied in a fixed-bed continuous-flow stainless steel reactor (inner diameter 8 mm) at a 10–40 atm pressure in the temperature interval 200–300°C, at GHSV = 12,000 h−1 and molar ratio H2:CO2 = 3:1. Prior to the reaction, all the catalysts (V cat = 2 cm3, m cat = ∼1.4 g for In2O3/HNT and ∼0.5 g for In2O3/Al-MCM-41/HNT, particle size of 0.5–1 mm) were pretreated at 300°C for 1 h in helium flow. The temperature was measured using a chromel-alumel thermocouple, which was placed in the middle of the catalytic bed. The results were obtained after multiple catalytic experiments. The catalysts were tested in several temperature increasing/decreasing cycles. At each temperature, the catalyst was kept for 1–2 h. Thus, total time onstream under CO2 hydrogenation conditions was not less than 10 h. The catalytic performance during this period remained stable. The compositions of the inlet and outlet gas mixtures were analyzed by a gas chromatograph (Chromos-1000) equipped with TCD and FID detectors and molecular sieve (5A) and Carbowax columns. Argon was used as a carrier gas. The detection limits for CO, CO2, CH4, DME, and methanol were 5 × 10−3 vol%. The carbon imbalance in all catalytic experiments was ±5%.
CO2 conversion (
where
3 Results and discussion
3.1 Characterization of catalysts
The catalysts were characterized by TEM, STEM, EDX-mapping, NH3-TPD, XRD, low-temperature nitrogen adsorption, TPO, and H2-TPR techniques. The In2O3 loading, textural parameters, and structural data obtained from XRD patterns of fresh and used In2O3, In2O3/HNT, and In2O3/Al-MCM-41/HNT are presented in Table 1. We can see that for the In2O3/HNT and In2O3/Al-MCM-41/HNT, real loadings of In2O3 are less than calculated. This is due to the fact that during impregnation supports absorbed lower volume of the indium nitrate water solution. The BET surface (Figure A1 in Appendix) areas of In2O3 and HNT are quite similar and equal 68 and 71 m2·g−1, respectively. After the impregnation of In2O3 on the HNTs’ surface, morphological characteristics are practically unchanged: S BET and pore volume slightly decreased to 62 m2·g−1 and 0.13 cm3·g−1, respectively. The most likely reason is blocking of some pores by the indium oxide particles.
In2O3 loading, S BET, pore volume, and coherent scattering region
| Textural characteristics | In2O3 | ||||
|---|---|---|---|---|---|
| Catalyst | S BET (m2·g−1) | V p (cm3·g−1) | wt% | CSRa (nm) | |
| HNT | 71 | 0.16 | — | — | |
| MCM-41/HNT | 514 | 0.42 | — | — | |
| In2O3 | Fresh | 68 | 0.41 | 100 | 13 |
| 10% In2O3/HNT | Fresh | 62 | 0.13 | 9.12 | 16.5 |
| Used | 61 | 0.13 | 9.12 | 16.4 | |
| 10% In2O3/Al-MCM-41/HNT | Fresh | 412 | 0.31 | 8.71 | 10.1 |
| Used | 410 | 0.3 | 8.71 | 10.1 | |
- a
CSR – coherent scattering region.
Formation of MCM-41 phase on HNT leads to significant increase of surface area due to ordered structure of silica arrays. After deposition of indium oxide, specific surface area decreased by 100 m2·g−1, for the same reason as on the In2O3/HNT catalyst. Acidity parameters of the catalysts and supports calculated from NH3-TPD method are listed in Table 2 (Figure A2). Based on the desorption spectra, the acidity was classified as weak and medium (amount of ammonia (μmol·g−1) desorbed below 300°C) and strong sites (amount of ammonia (μmol·g−1) desorbed above 300°C).
Acidity properties of catalysts and supports
| Sample | Acidity parameters | ||
|---|---|---|---|
| Weak and medium acid sites (μmol·g−1) | Strong acid sites (μmol·g−1) | Total acidity (μmol·g−1) | |
| HNT | 22 | 122 | 144 |
| Al-MCM-41/HNT | 35 | 495 | 530 |
| 10% In2O3/HNT | 17 | 98 | 115 |
| 10% In2O3/Al-MCM-41/HNT | 31 | 451 | 482 |
As shown in Table 2, the acidity of unmodified halloysite is seriously lower than that of HNT/Al-MCM-41 due to the fact that modified with aluminum MCM-41 has strong acid sites on the surface [43]. The total amount of acid sites on the surface of In2O3 supported catalysts is reduced in comparison with supports. This fact can be explained by partial blocking of the pores by the indium oxide particles. For the catalysts after the experiment, we can say that total amount of acid sites remained the same.
Figure 1 shows the XRD patterns for fresh and used catalysts. According to XRD data, we can say that indium oxide on the surface 10% In2O3/HNT has cubic crystal phase structure [21] with crystallite size of 13 nm. In case of 10% In2O3/Al-MCM-41/HNT, the crystallite size of the indium oxide is slightly smaller – 10 nm. We assumed that this is due to the higher dispersion of indium oxide particles. As we can see from the diffraction patterns of the used catalysts (curve 3 and 5 on Figure 1), there are no significant changes in the number and composition of the peaks. We only note that for the used catalysts, the peaks related to indium oxide are slightly smaller compared to fresh catalysts. Both used catalysts don’t have any considerable changes in the crystal structure – pore volume and CSR remained almost the same. This tells us that indium oxide particles are stable on the catalysts surface.

XRD patterns of catalysts. (1) halloysite, (2) In2O3/HNT fresh, (3) In2O3/HNT used, (4) In2O3/Al-MCM-41/HNT fresh, and (5) In2O3/MCM-41/HNT used.
The In2O3/HNT and In2O3/Al-MCM-41/HNT catalysts were studied by TEM, STEM, and EDX techniques. Figure 2 shows the TEM images of the fresh and used catalyst In2O3/Al-MCM-41/HNT, which are obviously similar.

TEM images of fresh (a and b) and used (c and d) In2O3/Al-MCM-41/HNT catalyst.
HNT were observed in both samples, and the images show that the nanotubes remained stable under reaction conditions. The same results were obtained for the In2O3/HNT catalyst. Also, in Figure 2b and d, we can see the structure of the mesoporous MCM-41 type silica deposited on the outer surface of HNTs. Some agglomerates of fresh and used In2O3/Al-MCM-41/HNT catalysts were studied by STEM and EDX-mapping. Results are shown in Figure 3. It is seen that the STEM images (Figure 3c and d) of both catalysts are similar. It can be noted that the indium particles are located mainly in the same place as the silicon particles in the case of both catalysts. Thus, it can be concluded that In2O3/Al-MCM-41/HNT catalyst contains two types of active sites on the surface – indium oxide particles, supported on silica, and alumina oxide particles.

TEM images (a,c), STEM images (b,d) and the corresponding Al, Si and In mapping of fresh (a,c) and used (b,d) In2O3/Al-MCM-41/HNT catalyst.
Catalysts were examined by H2-TPR (Figure 4) to study reducibility of catalysts. We can see that pure indium oxide isn’t reduced in the investigated temperature range. For the In2O3/Al-MCM-41-HNT, hydrogen consumption occurs only at ∼170°C, indicating that the indium oxide nanoparticles are mainly localized on mesoporous silica of Al-MCM-41 type. This peak can be correlated to reduction of In2O3 surface and can also be attributed as indirect evidence of the formation of oxygen vacancies on the surface of indium oxide [57,58]. For the In2O3/HNT, there is also a peak at ∼170, which could be assigned to reduction of In2O3 surface particles on the outer (SiO2) tubes surface as for the In2O3/Al-MCM-41/HNT. Calculation of H2-consumption over In2O3/Al-MCM-41/HNT shows us that 20% of In2O3 loading was reduced. In the case of In2O3/HNT, there are 16% In2O3 on 170°C peak. So, in In2O3/Al-MCM-41/HNT, a higher amount of surface active indium oxide is observed.

H2-TPR profiles of fresh In2O3/HNT and In2O3/Al-MCM-41/HNT catalysts.
Based on the data obtained, it can be concluded that the 10% In2O3/HNT and 10% In2O3/Al-MCM-41/HNT catalysts contain two types of sites on their surface – In2O3 particles as a metal component for the synthesis of methanol and acidic sites of HNT or Al-MCM-41/HNT for its further dehydration to DME.
3.2 Catalytic results
The catalytic properties of the In2O3, 10% In2O3/HNT, and 10% In2O3/Al-MCM-41/HNT in CO2 hydrogenation samples were measured at T = 200–300°C, P = 10–40 atm, and GHSV = 12,000 h−1, respectively. Figure 5 shows temperature dependencies of CO2 conversion and selectivity to MeOH (Figure 5a) and DME (Figure 5b) for In2O3, In2O3/HNT, and In2O3/Al-MCM-41/HNT catalysts. Only CO, H2O, CH3OH, and DME were detected as products; no hydrocarbons were identified.

(a) Effect of temperature on CO2 conversion and methanol selectivity over In2O3, In2O3/HNT, and In2O3/Al-MCM-41/HNT catalysts in CO2 hydrogenation. (b) Effect of temperature on CO2 conversion and dimethyl ether selectivity over In2O3/HNT and In2O3/Al-MCM-41/HNT catalysts in CO2 hydrogenation. Reaction conditions: P = 40 atm, GHSV = 12,000 h−1; inlet composition (vol%): H2:CO2 = 3:1.
Among the tested catalysts, In2O3 catalyst exhibited the lowest CO2 conversion, but the highest methanol selectivity over the entire temperature range. This can be explained by the fact that no DME was observed in reaction products. It seems to be quite obvious, since this catalyst does not have required acid sites on its surface. We can see that CO2 conversion increases with increasing temperature from 1% at 200°C up to 4% at 300°C. The selectivity for methanol, on the contrary, decreases with increasing temperature from 99% at 200°C to 65% at 300°C due to the CO formation by RWGS reaction.
In contrast to bulk In2O3 for the supported In2O3/HNT and In2O3/Al-MCM-41/HNT catalysts, DME appears in reaction products, due to the presence of acid sites. Temperature dependence for DME selectivity is similar to methanol in case of bulk In2O3 – the curve decreases with increasing temperature. There are some reasons for that. First, methanol dehydration is exothermic reaction, so increasing temperature leads to decrease of DME/MeOH equilibrium ratio. Second, at high temperatures, RWGS reaction (which is endothermic) contributes more to product distribution. So, it should be an optimal temperature, where the combination of formation rate of DME and CO2 conversion will be maximum. Over the temperature range, we can see that on In2O3/Al-MCM-41/HNT there are higher values of CO2 conversion and DME selectivity than on In2O3/HNT. Most likely, it is connected with higher surface area and more acid sites on In2O3/Al-MCM-41/HNT catalyst. Figure 6 shows temperature dependencies of DME production rate on In2O3, In2O3/HNT, and In2O3/Al-MCM-41/HNT catalysts.

Effect of temperature on DME production rate over In2O3, In2O3/HNT, and In2O3/Al-MCM-41/HNT catalysts. Reaction conditions: P = 40 atm, GHSV = 12,000 h−1; inlet composition (vol%):H2:CO2 = 3:1.
The highest DME formation rate of 0.15 gDME·(gcat·h)−1 was observed at 250°C on In2O3/Al-MCM-41/HNT. Further, the performance of the most active and selective catalyst In2O3/Al-MCM-41/HNT was studied in more detail.
It is well-known that with increasing pressure, the equilibrium of the CO2 hydrogenation reaction shifts towards the products according to the Le Chatelier principle. So, we studied the pressure influence on catalytic activity in DME direct synthesis from CO2 and H2. The results are shown in the Figure 7. The experiments were carried out at T = 250°C, GHSV = 12,000 h−1.
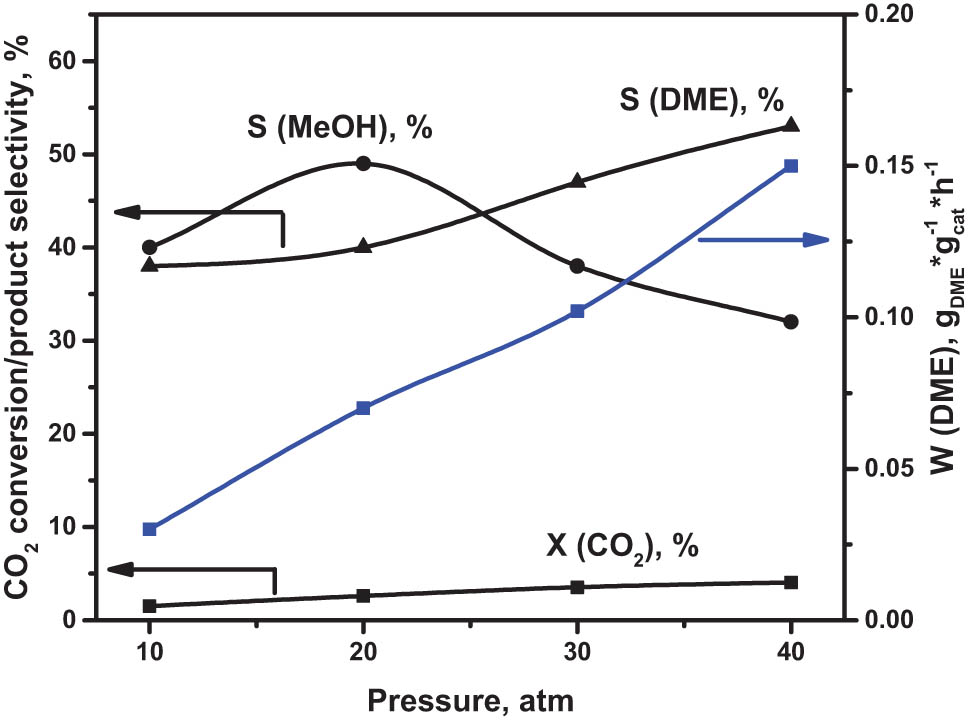
Effect of pressure on CO2 conversion, methanol, and DME selectivity and DME production rate over In2O3/Al-MCM-41/HNT catalyst. Reaction conditions: T = 250°C, GHSV = 12,000 h−1; inlet composition (vol%): H2:CO2 = 3:1.
As expected, with increasing pressure, CO2 conversion and DME selectivity also increase, while MeOH passes through the maximum at 20 atm and then decreases. This is in accordance with thermodynamic equations for this system [9]. The highest value of the DME formation rate is observed at 4 MPa. Note that at temperatures higher than 250°C, W DME decreased, despite an increase in the CO2 conversion due to a significant drop of DME selectivity.
One of the key properties of the catalyst, in addition to activity, is the stability under reaction conditions. A series of experiments were carried out to investigate this aspect. Figure 8 shows the effect of time-on-stream on the outlet product concentrations and CO2 conversion. The In2O3/Al-MCM-41/HNT catalyst was tested at 250°C, the inlet mixture H2:CO2 = 3:1, and GHSV = 12,000 h−1. Under these conditions, only CO, MeOH, and DME were detected as reaction products; methane appeared only in trace amounts. During 10 h on stream, no significant changes were observed either in the conversion of CO2 or in the MeOH and DME selectivity. No significant changes in the selectivity of DME were observed after 8 h of the experiment. After that, catalyst remained in operation conditions for 24 h, and after that catalyst activity was recorded. All the parameters, such as methanol, DME selectivity, and CO2 conversion, remained the same. In our view, these results mean that indium oxide particles remained stable as well as acid sites of modified HNT. In addition, the spent catalyst was tested by TPO. No carbon deposition was observed. This means that acidic properties of HNT (strength and number of acidic sites) are optimal for DME synthesis reaction and do not induce condensation reactions.

Effect of time on stream on CO2 conversion, methanol, and DME selectivity, DME production rate over In2O3/Al-MCM-41/HNT catalyst. Reaction conditions: T = 250°C, P = 40 atm, GHSV = 12,000 h−1; inlet composition (vol%): H2:CO2 = 3:1.
Up to now, almost no works of using indium oxide for direct synthesis of DME can be found in literature; only one work devoted to the study Cu–In–Zr–O catalyst mixed with SAPO-34 zeolite for direct DME synthesis is available [59]. Basically, all works on the direct synthesis of DME from CO2 and H2 are devoted to the study of copper catalysts mixed with zeolites. So, we compared the best In2O3/Al-MCM-41/HNT catalyst with literature data. Table 3 shows comparative data, in particular, experimental conditions (temperature, pressure, flow), CO2 conversion, and DME selectivity. Since a fairly large number of works devoted to the hydrogenation of CO2 to DME are currently presented in the literature, the table shows those with similar experimental conditions with this work, in particular – pressure of 10–50 atm, temperature of 200–300°C, and inlet composition H2:CO2 = 3:1.
Comparison of catalyst activities in CO2 hydrogenation to dimethyl ether
| Catalyst | T (°C) | Pressure (atm) | GHSV (mL·(gcat·h)−1) | W (DME) (gDME·(gcat·h)−1) | Reference |
|---|---|---|---|---|---|
| In2O3/Al-MCM-41/HNT | 250 | 40 | 12,000 | 0.15 | This work |
| CIZO1/SAPO-34 | 250 | 30 | 6,000 | 0.08 | [59] |
| CZA2/HZSM-5 | 260 | 30 | 1,500 | 0.12 | [28] |
| CZZ3/ferrierite | 260 | 50 | 8,800 | 0.435 | [29] |
| CZA/HZSM-5 | 260 | 50 | 3,000 | 0.29 | [30] |
| CZZ/MFI | 240 | 50 | 10,000 | 0.251 | [17] |
| CZZ/BEA | 260 | 30 | 8,800 | 0.3 | [31] |
| CZZ/WO x −ZrO2 | 260 | 30 | 4,333 | 0.27 | [32] |
| PdZn/TiO2-H-ZSM-5 | 270 | 20 | 3,500 | 0.025 | [33] |
- 1
CuO-In2O3-ZrO2;
- 2
CuO-ZnO-Al2O3;
- 3
CuO-ZnO-ZrO2.
Catalytic activity of In2O3/Al-MCM-41/HNT is lower than literature data, but study of these systems is at the very beginning. Such systems look very promising due to the following factors: the possibility of a significant increase in CO2 conversion and selectivity for DME after optimization of the catalyst composition, its dispersion, the method of preparation, and adding of promoters. According to the literature data [60], catalysts based on indium oxide make it possible to obtain methanol with a selectivity of about 100%, and with the appropriate selection of the acid component, high DME yields can be achieved. It is also important that In2O3/MCM-41/HNT catalyst shows good stability, due to the fact that indium oxide particles do not sinter during the reaction, and acid sites remain stable in presence of water. There is a wide field for further catalyst improvement, including optimization of In2O3 morphology and interaction with the support, tuning acidic properties, doping by metals active in CO hydrogenation, such as Cu, Pd, Ga, and even Ni and Co. These points will be the subject of our further studies.
4 Conclusion
Indium oxide catalysts, bulk and supported on aluminosilicate HNTs and modified HNTs with ordered Al-MCM-41 silica arrays, were studied in CO2 hydrogenation to DME. Based on data from physicochemical methods, such as XRD, S BET, FTIR, and H2-TPR, we can suggest that these catalysts have two types of active sites – indium oxide particles, which are responsible for methanol formation, and acid sites of HNT, which are responsible for methanol dehydration to DME. The influence of temperature and pressure was studied. The best catalyst for CO2 hydrogenation was In2O3/Al-MCM-41/HNT that provides 4% CO2 conversion with DME selectivity 53% and DME production rate 0.15 gDME·(gcat·h)−1 at 40 bar, GHSV = 12,000 h−1, and T = 250°C. It was shown that this catalyst didn’t lose activity after 40 h of experiment. So, it is very promising systems, based on new material for direct hydrogenation of CO2 to DME.
-
Funding information: This research was funded by RFBR project 19-33-60056 and as a part of the state task of Gubkin University (synthesis of MCM-41/HNT, textural properties evaluation, TEM), project number FSZE-2020-0007 (0768-2020-0007, A.G., V.V., K.Ch.).
-
Author contributions: Alexey Pechenkin: conceptualization, writing – original draft, writing – review and editing, methodology, formal analysis, investigation; Dmitry Potemkin: writing – review and editing, investigation, conceptualization, formal analysis; Sukhe Badmaev: resources, investigation; Ekaterina Smirnova: methodology, visualization; Kirill Cherednichenko: methodology, visualization, resources; Vladimir Vinokurov: writing – review and editing, project administration; Aleksandr Glotov: writing – review and editing, supervision.
-
Conflict of interest: Authors state no conflict of interest.
Appendix

Low-temperature nitrogen adsorption isotherms for the HNT, Al-MCM-41/HNT, In2O3/HNT, and In2O3/Al–MCM-41/HNT samples.

NH3-TPD curves for HNT, In2O3/HNT, Al-MCM-41/HNT, and In2O3/Al-MCM-41/HNT catalysts.
References
[1] Strielkowski W, Volkova E, Pushkareva L, Streimikiene D. Innovative policies for energy efficiency and the use of renewables in households. Energies. 2019;12(7):1392.10.3390/en12071392Suche in Google Scholar
[2] Munters W, Meyers J. Dynamic strategies for yaw and induction control of wind farms based on large-eddy simulation and optimization. Energies. 2018;11(1):177.10.3390/en11010177Suche in Google Scholar
[3] Gonçalves A, Puna JF, Guerra L, Campos Rodrigues J, Gomes JF, Santos MT, et al. Towards the development of syngas/biomethane electrolytic production, using liquefied biomass and heterogeneous catalyst. Energies. 2019;12(19):3787.10.3390/en12193787Suche in Google Scholar
[4] Frontera P, Macario A, Ferraro M, Antonucci P. Supported catalysts for CO2 methanation: a review. Catalysts. 2017;7:59.10.3390/catal7020059Suche in Google Scholar
[5] Graciani J, Mudiyanselage K, Xu F, Baber AE, Evans J, Senanayake SD, et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science. 2014;345:546–50.10.1126/science.1253057Suche in Google Scholar PubMed
[6] Kattel S, Ramírez PJ, Chen JG, Rodriguez JA, Liu P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science. 2017;355:1296–9.10.1126/science.aal3573Suche in Google Scholar PubMed
[7] Liu XM, Lu GQ, Yan ZF, Beltramini J. Recent advances in catalysts for methanol synthesis via hydrogenation of CO and CO2. Ind Eng Chem Res. 2003;42:6518–30.10.1021/ie020979sSuche in Google Scholar
[8] Dang S, Yang H, Gao P, Wang H, Li X, Wei W, et al. A review of research progress on heterogeneous catalysts for methanol synthesis from carbon dioxide hydrogenation. Catal Today. 2019;330:61–75.10.1016/j.cattod.2018.04.021Suche in Google Scholar
[9] Catizzone E, Bonura G, Migliori M, Frusteri F, Giordano G. CO2 recycling to dimethyl ether: state-of-the-art and perspectives. Molecules. 2018;23:31.10.3390/molecules23010031Suche in Google Scholar PubMed PubMed Central
[10] Saravanan K, Ham H, Tsubaki N, Bae JW. Recent progress for direct synthesis of dimethyl ether from syngas on the heterogeneous bifunctional hybrid catalysts. Appl Catal B: Environ. 2017;217:494–522.10.1016/j.apcatb.2017.05.085Suche in Google Scholar
[11] Bonura G, Migliori M, Frusteri L, Cannilla C, Catizzone E, Giordano G, et al. Acidity control of zeolite functionality on activity and stability of hybrid catalysts during DME production via CO2 hydrogenation. J CO2 Util. 2018;24:398–406.10.1016/j.jcou.2018.01.028Suche in Google Scholar
[12] Tan L, Zhang P, Cui Y, Suzuki Y, Li H, Guo L, et al. Direct CO2 hydrogenation to light olefins by suppressing CO by-product formation. Fuel Process Technol. 2019;196:106174.10.1016/j.fuproc.2019.106174Suche in Google Scholar
[13] Volkova G, Badmaev S, Belyaev V, Plyasova L, Budneva A, Paukshtis E, et al. Bifunctional catalysts for hydrogen production from dimethyl ether. Stud Surf Sci Catal. 2007;167:445–50.10.1016/S0167-2991(07)80172-8Suche in Google Scholar
[14] Badmaev SD, Akhmetov NO, Pechenkin AA, Sobyanin VA, Parmon VN. Low-temperature partial oxidation of dimethyl ether to hydrogen-rich gas over CuO–CeO2/γ-Al2O3 catalysts for fuel cell supply. Doklady Phys Chem. 2019;487:95–8.10.1134/S0012501619080013Suche in Google Scholar
[15] Badmaev SD, Akhmetov NO, Belyaev VD, Kulikov AV, Pechenkin AA, Potemkin DI, et al. Syngas production via partial oxidation of dimethyl ether over Rh/Ce0.75Zr0.25O2 catalyst and its application for SOFC feeding. Int J Hydrog Energy. 2020;45(49):26188–96. 10.1016/j.ijhydene.2020.01.101.Suche in Google Scholar
[16] Shikada T, Ohno Y, Ogawa T, Ono M, Mizuguchi M. Direct synthesis of dimethyl ether form synthesis gas. Stud Surf Sci Catal. 1998;119:515–20.10.1016/S0167-2991(98)80483-7Suche in Google Scholar
[17] Frusteri F, Bonura G, Cannilla C, Ferrante GD, Aloise A, Catizzone E, et al. Stepwise tuning of metal-oxide and acid sites of CuZnZr-MFI hybrid catalysts for the direct DME synthesis by CO2 hydrogenation. Appl Catal B: Environ. 2015;176:522–31.10.1016/j.apcatb.2015.04.032Suche in Google Scholar
[18] Ye J, Liu C, Mei D, Ge Q. Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3 (110): a DFT study. ACS Catal. 2013;3:1296–306.10.1021/cs400132aSuche in Google Scholar
[19] Sun K, Fan Z, Ye J, Yan J, Ge Q, Li Y, et al. Hydrogenation of CO2 to methanol over In2O3 catalyst. J CO2 Util. 2015;12:1–6.10.1016/j.jcou.2015.09.002Suche in Google Scholar
[20] Martin O, Martín AJ, Mondelli C, Mitchell S, Segawa TF, Hauert R, et al. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation. Angew Chem Int Ed. 2016;55:6109.10.1002/anie.201603172Suche in Google Scholar
[21] Chen TY, Cao C, Chen TB, Ding X, Huang H, Shen L, et al. Unraveling highly tunable selectivity in CO2 hydrogenation over bimetallic In–Zr oxide catalysts. ACS Catal. 2019;9:8785–97.10.1021/acscatal.9b01869Suche in Google Scholar
[22] Chou CY, Lobo RF. Direct conversion of CO2 into methanol over promoted indium oxide-based catalysts. Appl Catal A: Gen. 2019;583:117144.10.1016/j.apcata.2019.117144Suche in Google Scholar
[23] Rui N, Wang Z, Sun K, Ye J, Ge Q, Liu CJ. CO2 hydrogenation to methanol over Pd/In2O3: effects of Pd and oxygen vacancy. Appl Catal B: Environ. 2017;218:488–97.10.1016/j.apcatb.2017.06.069Suche in Google Scholar
[24] Snider JL, Streibel V, Hubert MA, Choksi TS, Valle E, Upham DC, et al. Revealing the synergy between oxide and alloy phases on the performance of bimetallic In–Pd catalysts for CO2 hydrogenation to methanol. ACS Catal. 2019;9:3399–3412.10.1021/acscatal.8b04848Suche in Google Scholar
[25] Ham H, Baek SW, Shin CH, Bae JW. Roles of structural promoters for direct CO2 hydrogenation to dimethyl ether over ordered mesoporous bifunctional Cu/M–Al2O3 (M = Ga or Zn). ACS Catal. 2018;9(1):679–90.10.1021/acscatal.8b04060Suche in Google Scholar
[26] Ham H, Kim J, Cho SJ, Choi JH, Moon DJ, Bae JW. Enhanced stability of spatially confined copper nanoparticles in an ordered mesoporous alumina for dimethyl ether synthesis from syngas. ACS Catal. 2016;6(9):5629–40.10.1021/acscatal.6b00882Suche in Google Scholar
[27] Tan L, Zhang P, Suzuki Y, Li H, Guo L, Yoneyama Y, et al. Bifunctional capsule catalyst of Al2O3@Cu with strengthened dehydration reaction field for direct synthesis of dimethyl ether from syngas. Ind Eng Chem Res. 2019;58(51):22905–11.10.1021/acs.iecr.9b04864Suche in Google Scholar
[28] Hu Y, Zhang Y, Du J, Li C, Wang K, Liu L, et al. The influence of composition on the functionality of hybrid CuO–ZnO–Al2O3/HZSM-5 for the synthesis of DME from CO2 hydrogenation. RSC Adv. 2018;8(53):30387–95.10.1039/C8RA04814BSuche in Google Scholar
[29] Bonura G, Cannilla C, Frusteri L, Mezzapica A, Frusteri F. DME production by CO2 hydrogenation: key factors affecting the behaviour of CuZnZr/ferrierite catalysts. Catal Today. 2017;281:337–44.10.1016/j.cattod.2016.05.057Suche in Google Scholar
[30] Naik SP, Ryu T, Bui V, Miller JD, Drinnan NB, Zmierczak W. Synthesis of DME from CO2/H2 gas mixture. Chem Eng J. 2011;167:362–8.10.1016/j.cej.2010.12.087Suche in Google Scholar
[31] Bonura G, Cannilla C, Frusteri L, Catizzone E, Todaro S, Migliori M, et al. Interaction effects between CuO–ZnO–ZrO methanol phase and zeolite surface affecting stability of hybrid systems during one-step CO2 hydrogenation to DME. Catal Today. 2020;345:175–82.10.1016/j.cattod.2019.08.014Suche in Google Scholar
[32] Witoon T, Kidkhunthod P, Chareonpanich M, Limtrakul J. Direct synthesis of dimethyl ether from CO2 and H2 over novel bifunctional catalysts containing CuO–ZnO–ZrO2 catalyst admixed with WOx/ZrO2 catalysts. Chem Eng J. 2018;348:713–22.10.1016/j.cej.2018.05.057Suche in Google Scholar
[33] Bahruji H, Armstrong RD, Ruiz Esquius J, Jones W, Bowker M, Hutchings GJ. Hydrogenation of CO2 to dimethyl ether over Brønsted acidic PdZn catalysts. Ind Eng Chem Res. 2018;57:6821–9.10.1021/acs.iecr.8b00230Suche in Google Scholar
[34] Glotov A, Vutolkina A, Pimerzin A, Vinokurov V, Lvov Y. Clay nanotube-metal core/shell catalysts for hydroprocesses. Chem Soc Rev. 2021;50:9240–77. 10.1039/D1CS00502B.Suche in Google Scholar
[35] Lvov Y, Wang W, Zhang L, Fakhrullin R. Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv Mater. 2016;28:1227–50.10.1002/adma.201502341Suche in Google Scholar PubMed
[36] Lvov Y, Panchal A, Fu Y, Fakhrullin R, Kryuchkova M, Batasheva S, et al. Interfacial self-assembly in halloysite nanotube composites. Langmuir. 2019;35:8646–57.10.1021/acs.langmuir.8b04313Suche in Google Scholar PubMed
[37] Vinokurov VA, Stavitskaya AV, Glotov AP, Novikov AA, Zolotukhina AV, Kotelev MS, et al. Nanoparticles formed onto/into halloysite clay tubules: architectural synthesis and applications. Chem Rec. 2018;18:858–67.10.1002/tcr.201700089Suche in Google Scholar PubMed
[38] Stavitskaya A, Glotov A, Mazurova K, Nedolivko V, Gushchin P, Huang W, et al. Formation of ruthenium nanoparticles inside aluminosilicate nanotubes and their catalytic activity in aromatics hydrogenation: the impact of complexing agents and reduction procedure. Pure Appl Chem. 2020;92:909–18.10.1515/pac-2019-1113Suche in Google Scholar
[39] Glotov A, Vutolkina A, Pimerzin A, Nedolivko V, Zasypalov G, Stytsenko V, et al. Ruthenium catalysts templated on mesoporous MCM-41 type silica and natural clay nanotubes for hydrogenation of benzene to cyclohexane. Catalysts. 2020;10:537.10.3390/catal10050537Suche in Google Scholar
[40] Glotov A, Stavitskaya A, Chudakov Y, Ivanov E, Huang W, Vinokurov V, et al. Mesoporous metal catalysts templated on clay nanotubes. Bull Chem Soc Jpn. 2019;92:61–9.10.1246/bcsj.20180207Suche in Google Scholar
[41] Glotov AP, Stavitskaya AV, Chudakov YA, Artemova MI, Smirnova EM, Demikhova NR, et al. Nanostructured ruthenium catalysts in hydrogenation of aromatic compounds. Pet Chem. 2018;58(14):1221–6.10.1134/S0965544118140013Suche in Google Scholar
[42] Vinokurov V, Glotov A, Chudakov Y, Stavitskaya A, Ivanov E, Gushchin P, et al. Core/shell Ruthenium–halloysite nanocatalysts for hydrogenation of phenol. Ind Eng Chem Res. 2017;56:14043–52.10.1021/acs.iecr.7b03282Suche in Google Scholar
[43] Afokin MI, Smirnova EM, Starozhitskaya AV, Gushchin PA, Glotov AP, Maksimov AL. Halloysite as a zeolite catalyst component for converting dimethyl ether into hydrocarbons. Chem Technol Fuels Oils. 2020;55:1–7.10.1007/s10553-020-01082-1Suche in Google Scholar
[44] Vinokurov V, Stavitskaya A, Glotov A, Ostudin A, Sosna M, Gushchin P, et al. Halloysite nanotube-based cobalt mesocatalysts for hydrogen production from sodium borohydride. J Solid State Chem. 2018;268:182–9.10.1016/j.jssc.2018.08.042Suche in Google Scholar
[45] Stavitskaya A, Mazurova K, Kotelev M, Eliseev O, Gushchin P, Glotov A, et al. Ruthenium-loaded halloysite nanotubes as mesocatalysts for fischer–tropsch synthesis. Molecules. 2020;25:1764.10.3390/molecules25081764Suche in Google Scholar PubMed PubMed Central
[46] Glotov AP, Roldugina EA, Artemova MI, Smirnova EM, Demikhova NR, Stytsenko VD, et al. Isomerization of xylenes in the presence of pt-containing catalysts based on halloysite aluminosilicate nanotubes. Russ J Appl Chem. 2018;91(8):1353–62.10.1134/S1070427218080141Suche in Google Scholar
[47] Glotov AP, Artemova MI, Demikhova NR, Smirnova EM, Ivanov EV, Gushchin PA, et al. A study of platinum catalysts based on ordered Al–MCM-41 aluminosilicate and natural halloysite nanotubes in xylene isomerization. Pet Chem. 2019;2019(59):1226–34.10.1134/S0965544119110033Suche in Google Scholar
[48] Abbasov V, Mammadova T, Andrushenko N, Hasankhanova N, Lvov Y, Abdullayev E. Halloysite–Y-zeolite blends as novel mesoporous catalysts for the cracking of waste vegetable oils with vacuum gasoil. Fuel. 2014;117:552–5.10.1016/j.fuel.2013.09.013Suche in Google Scholar
[49] Stavitskaya AV, Kozlova EA, Kurenkova AY, Glotov AP, Selischev DS, Ivanov EV, et al. Ru/CdS quantum dots templated on clay nanotubes as visible light active photocatalysts: optimization of S/Cd ratio and Ru content. Chem–A Eur J. 2020;26(57):13085–92. 10.1002/chem.202002192.Suche in Google Scholar PubMed
[50] Vinokurov VA, Stavitskaya AV, Ivanov EV, Gushchin PA, Kozlov DV, Kurenkova AY, et al. Halloysite nanoclay based CdS formulations with high catalytic activity in hydrogen evolution reaction under visible light irradiation. ACS Sustain Chem Eng. 2017;5:11316–23.10.1021/acssuschemeng.7b02272Suche in Google Scholar
[51] Liu R, Tian H, Yang A, Zha F, Ding J, Chang Y. Preparation of HZSM-5 membrane packed CuO–ZnO–Al2O3 nanoparticles for catalysing carbon dioxide hydrogenation to dimethyl ether. Appl Surf Sci. 2015;345:1–9.10.1016/j.apsusc.2015.03.125Suche in Google Scholar
[52] Wang Y, Wang W, Chen Y, Ma J, Li R. Synthesis of dimethyl ether from syngas over core–shell structure catalyst CuO–ZnO–Al2O3@SiO2–Al2O3. Chem Eng J. 2014;250:248–56.10.1016/j.cej.2014.04.018Suche in Google Scholar
[53] Baktash E, Littlewood P, Schomäcker R, Thomas A, Stair PC. Alumina coated nickel nanoparticles as a highly active catalyst for dry reforming of methane. Appl Catal B: Environ. 2015;179:122–7.10.1016/j.apcatb.2015.05.018Suche in Google Scholar
[54] Abdalla A, Arudra P, Al-Khattaf SS. Catalytic cracking of 1-butene to propylene using modified H-ZSM-5 catalyst: a comparative study of surface modification and core-shell synthesis. Appl Catal A: Gen. 2017;533:109–20.10.1016/j.apcata.2017.01.003Suche in Google Scholar
[55] Glotov A, Levshakov N, Stavitskaya A, Artemova M, Gushchin P, Ivanov E, et al. Templated self-assembly of ordered mesoporous silica on clay nanotubes. Chem Commun. 2019;55:5507–10.10.1039/C9CC01935ASuche in Google Scholar PubMed
[56] Paukshtis EA. IR spectroscopy for heterogeneous acid-base catalysis. Novosibirsk: Nauka; 1992.Suche in Google Scholar
[57] Bielz T, Lorenz H, Jochum W, Kaindl R, Klauser F, Klötzer B, et al. Hydrogen on In2O3: reducibility, bonding, defect formation, and reactivity. J Phys Chem C. 2010;114(19):9022–9.10.1021/jp1017423Suche in Google Scholar
[58] Martin O, Martín AJ, Mondelli C, Mitchell S, Segawa TF, Hauert R, et al. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation. Angew Chem Int Ed. 2016;55(21):6261–5.10.1002/anie.201600943Suche in Google Scholar PubMed
[59] Yao L, Shen X, Pan Y, Peng Z. Unravelling proximity-driven synergetic effect within CIZO–SAPO bifunctional catalyst for CO2 hydrogenation to DME. Energy Fuels. 2020;34(7):8635–43.10.1021/acs.energyfuels.0c01256Suche in Google Scholar
[60] Pustovarenko A, Dikhtiarenko A, Bavykina A, Gevers L, Ramírez A, Russkikh A, et al. Metal–organic framework-derived synthesis of cobalt indium catalysts for the hydrogenation of CO2 to methanol. ACS Catal. 2020;10(9):5064–76.10.1021/acscatal.0c00449Suche in Google Scholar
© 2021 Alexey Pechenkin et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis
Artikel in diesem Heft
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis

