Abstract
Various means have been proposed to solve problems such as high ash content and complex composition in the recycling of nickel-containing residue produced by battery manufacturing enterprises. Microwave roasting pretreatment is proposed to improve the nickel leaching rate from the residue. The effect of different experimental conditions like microwave roasting temperatures, roasting times, and microwave powers on the nickel leaching rate was studied. It was found that the effect of roasting temperature on the nickel leaching rate was more significant than those of roasting time and microwave power. Meanwhile, after microwave roasting pretreatment, the rate of nickel leaching from the residue could be increased by 20.43%, and weight of the material could also be reduced by more than 21%. After microwave roasting at 450°C, there was no significant change in the main phases of the material, but the surface of the particles exhibited an apparent stratified dissociation phenomenon. Response surface methodology (RSM) was used to optimize the parameters of microwave roasting with nickel leaching rate as the response value. The results showed that the nickel leaching rate could reach 93.11% for roasting at the microwave power of 962 W for 6.2 min under the temperature of 452°C.
1 Introduction
During nickel electroplating, chemical precipitation from nickel-containing wastewater produces nickel-containing residues which are harmful solid waste containing Ni, Cd, Fe, Cu, Ca, and other major components [1,2]. In the absence of proper management, these residues cause secondary pollution, greatly impacting the environment and human health. But, these residues containing precious metals can be used as a cheap secondary resource of nickel. The commonly used treatment methods at present include the separation and recovery of heavy metals [3,4] and stabilization treatment [5,6]. However, the nickel-containing residue has an extremely complex composition. In addition to a large amount of water, it also contains ash, inorganic particles, colloids, and other harmful substances [7]. In the case of proposed direct ammonium leaching [8] or sulfuric acid leaching [9], there will be wastage of large quantity of chemical reagents as well as numerous equipments. Therefore, in order to concentrate the valuable metal elements and reduce residue, proper roasting pretreatment should be conducted prior to metal recovery. Therefore, it is imperative to choose an efficient pretreatment method for comprehensive utilization of nickel-containing residue.
Under the action of a high-frequency electromagnetic field of the microwaves, the polar molecules in a medium change their orientation due to the changing external electric field. In this process, the rotation of the molecules takes place at high speeds which consequently results in collision of the molecules among each other. Subsequently, the electromagnetic energy is converted into heat energy in the medium, raising the temperature of the material [10]. Thus, microwave heating is the result of electromagnetic energy loss by a dielectric material [11]. As a source of clean energy, microwave is widely used in the comprehensive treatment of multicomponent and multiphase solid waste [12,13].
During microwave heating of multicomponent and multiphase complex materials, thermal stress develops on the embedded surface due to the differences between strong and weak absorbing components. This results in the formation of cracks and promotes the segregation of inclusions, which thereby increases the specific surface area of the material. This ultimately strengthens the reaction process and improves the reaction efficiency by playing an activation role. Togari et al. [14] found that the degradation and biogas production during medium-temperature anaerobic digestion from highly concentrated, dehydrated sludge from the oxidation ditch process were improved due to the application of microwave pretreatment. They found an increase of biogas production by 42% due to the pretreatment of dehydrated sludge employing a new microwave continuous irradiation device. Nag-Choul et al. [15] studied the effect of microwave pretreatment on improving the efficiency of gold leaching from gold concentrates. They found that thiourea leaching from the untreated gold concentrate could recover 80% of the gold, but quantitative recovery of gold was possible from the microwave-pretreated gold concentrate. They also observed improvement in the leaching efficiency of gold by increasing the microwave irradiation time as well as increasing thiourea concentration. Zhang et al. [16] proposed microwave pretreatment of waste zinc catalyst prior to HCl leaching and measured the temperature change profile of the waste catalyst under microwave irradiation. The results indicated that for microwave pretreatment temperature of 950°C and roasting time of 12 min, the zinc leaching rate reached ∼96.5%. During microwave pretreatment, the contact area between the leaching agent and zinc was increased due to opening up of the pores blocked by the spent catalyst. Lambert et al. [17] improved the efficiency of leaching rare earth elements from phosphogypsum by microwave irradiation. The obtained results indicate that microwaves can produce cracks and pores in the particles and thus enhance the penetration of the impregnant. As per the reported results, there was an enhancement of the rare earth elements leaching rate by more than 20% due to microwave pretreatment.
In this study, microwave heating was used to study the nickel leaching rate employing nickel-containing residue produced by battery manufacturing enterprises as the raw material. High microwave heating efficiency and selective heating were used to comprehensively utilize the nickel-containing residue. During microwave heating, local thermal stress is generated between the nickel-containing phase and the gangue which promotes their separation and reduction of residue, while enrichment of valuable metals takes place due to difference in dielectric loss for each phase. The effects of various parameters like roasting temperature, roasting time, and microwave power on the nickel leaching rate were studied. Response surface methodology (RSM) was adopted to optimize the microwave roasting with the nickel leaching rate as the response value.
2 Materials and methods
2.1 Raw materials
The nickel-containing residue was obtained from a battery manufacturer in Henan Province, China. The samples were dried in a constant-temperature drying oven for 48 h. The elemental composition of the residue was measured by inductively coupled plasma optical emission spectrometry (ICP-OES, Agilent5100, Agilent Technologies, Palo Alto, USA). Changes of the microstructure and phase after roasting were analyzed by SEM (SPM-S3400N, Hitachi, Tokyo, Japan) and X-ray powder diffraction (XRD) (XRD-7000S/L, Shimadzu, Kyoto, Japan), respectively. The chemical composition of the nickel-containing residue was determined by X-ray fluorescence (XRF, Epslion 1, Panalytical, Netherlands).
Table 1 shows the chemical composition of nickel-containing residue determined by ICP-OES and XRF. As shown in Table 1, the metal element and nickel contents of the residue are about 8%. The residue mainly contained 44.06% CaO, 15.32% MgO, 11.37% NiO, 9.36% Fe2O3, 4.52% SiO2, 1.04% CdO, 0.90% Al2O3, and 0.52% ZnO.
Chemical composition of the nickel-containing residue
| Composition | Element composition (measured by ICP) | |||||||
|---|---|---|---|---|---|---|---|---|
| Ca | Mg | Ni | Fe | Si | Cd | Al | Zn | |
| Content (wt%) | 14.09 | 8.67 | 8.02 | 4.64 | 1.57 | 0.71 | 0.24 | 0.42 |
| Composition | Components (measured by XRF) | |||||||
|---|---|---|---|---|---|---|---|---|
| CaO | MgO | NiO | Fe2O3 | SiO2 | CdO | Al2O3 | ZnO | |
| Content (wt%) | 44.06 | 15.32 | 11.37 | 9.47 | 4.52 | 1.04 | 0.90 | 0.52 |
The XRD patterns of the dried sample are shown in Figure 1, which shows that the material composition of the residue is relatively complex. CaCO3 is found to be the major component. Magnesium mainly exists as MgSiO3, and Ni primarily exists as NiO, with the existence of small amounts of NiCO3 and Ni(OH)2·2(H2O), whereas Fe mainly exists as FeSiO3.
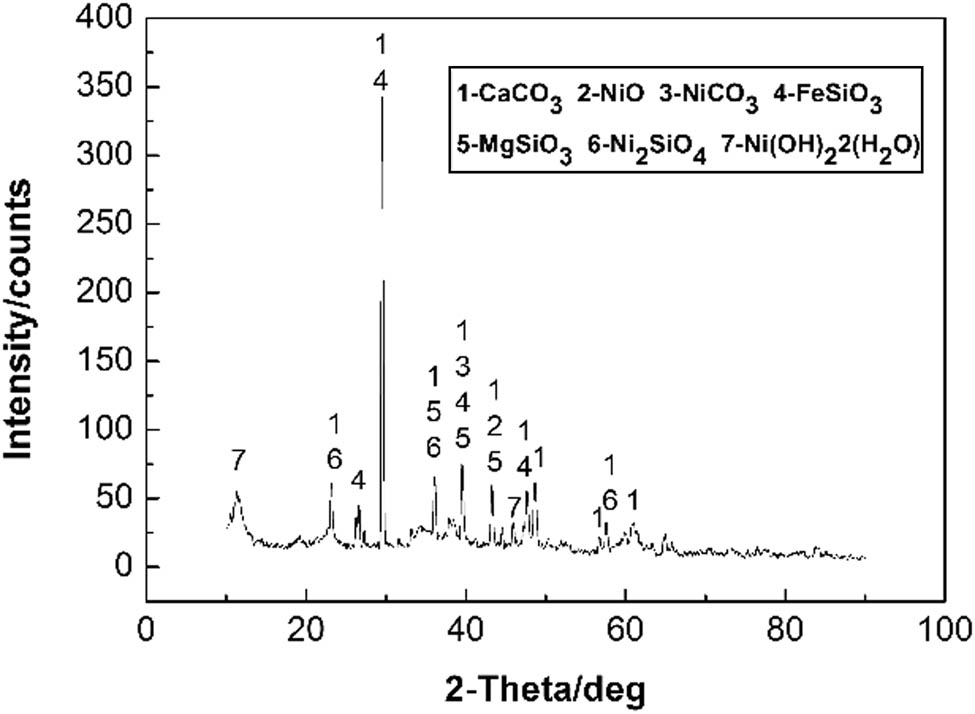
XRD pattern of the raw material.
2.2 Experimental device
A microwave reactor with a power of 3 kW and a frequency of 2.450 GHz, developed by the Key Laboratory of Unconventional Metallurgy, Ministry of Education, Kunming University of Science and Technology (Figure 2), was used in this study. The microwave heating system consists of a temperature control unit, two magnetrons, a multimode cavity, a quartz glass container with glass fiber wool, and a data acquisition computer. The temperature control unit consists of a high-temperature thermocouple with a metal shielding layer and a temperature regulator. The thermocouple was inserted into the material center to control the microwave roasting temperature. The systematic error in this experiment as obtained from the infrared temperature measurements was found to be ±3°C. After microwave heating for a period of time, the system measured temperature was 386°C and after turning off the microwave power, the system temperature was found to be 384°C, while the surface temperature of the material measured by the infrared thermometer was 383°C. Therefore, the microwave heating system used in this experiment was found to be reliable.

Schematic of the microwave roasting system.
During the microwave roasting experiment, each time 100 g of the residue was weighed and loaded into the microwave cavity as a 15 mm thin layer under a certain microwave power (600, 800, 1,000, 1,200, and 1,400 W). The temperature was set to different predetermined values (150°C, 300°C, 450°C, 600°C, and 800°C) for different time intervals (2, 3, 4, 5, 6, and 7 min). Then, the effects of temperature, microwave power, and roasting time on the nickel leaching rate were investigated using these factors as independent variables.
The pretreated samples were subjected to conventional sulphuric acid leaching experiments. A certain amount (10 g) of the pretreated nickel-containing residue was weighed, and a sulfuric acid solution with a mass fraction of 15% was mixed in beaker keeping a liquid-to-solid ratio of 7:1. The beaker was placed into a water bath maintained under a preset temperature, and magnetic stirring was started (rotation speed: 150 rpm). After the leaching reaction, the leached products were filtered and separated, and the slag samples were finally taken out and dried for testing.
3 Results and discussion
3.1 Single-factor experiment
3.1.1 Influence of roasting temperature
The effect of microwave roasting temperature on the nickel leaching rate was investigated maintaining 1,000 W as the microwave power and heating time of 5 min. Figure 3 shows the influence of the microwave roasting temperature on the nickel leaching rate and weight change.
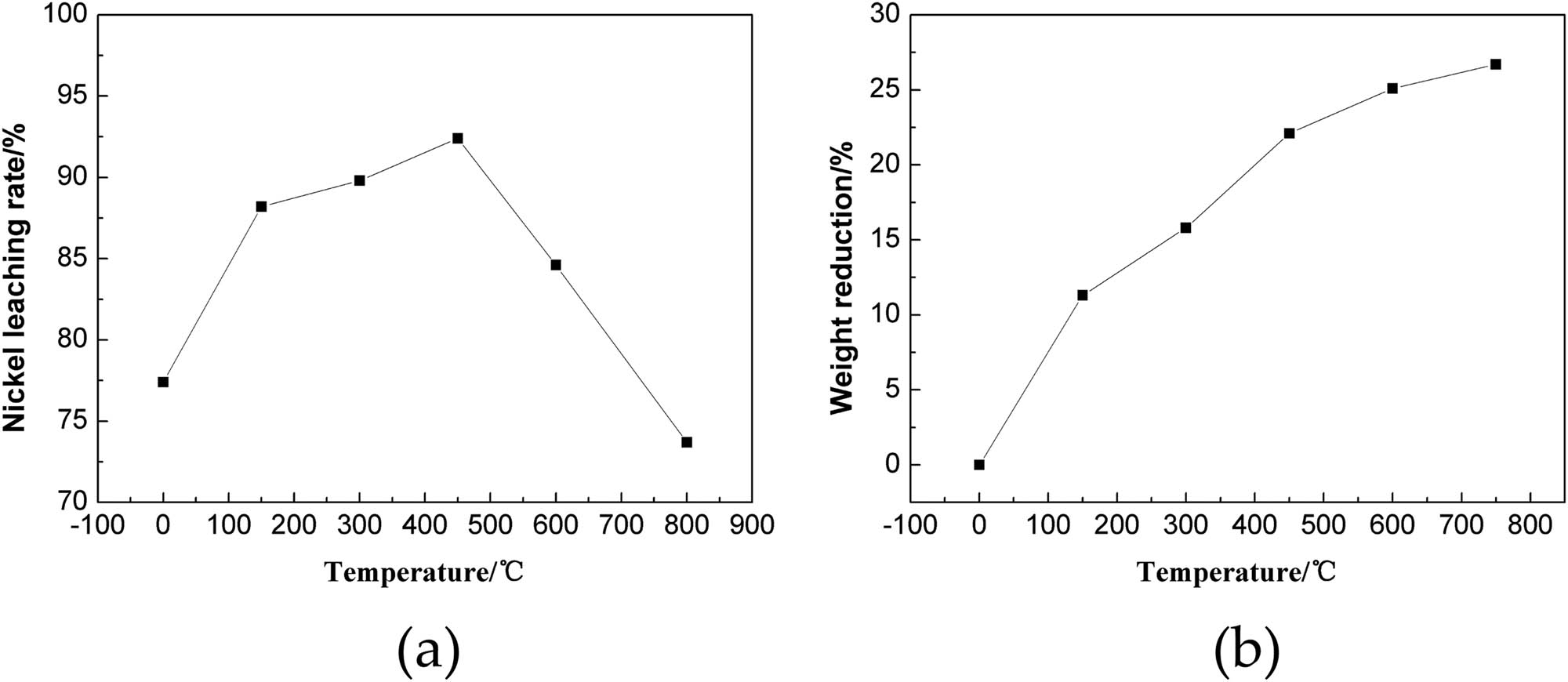
Influence of temperature on the nickel leaching rate and weight change. (a) Nickel leaching rate and (b) weight change.
As shown in Figure 3a, the microwave roasting temperature had significant influence on the nickel leaching rate, which showed an initial increase followed by subsequent decrease. Without pretreatment by microwave roasting, 77.42% was found to be the nickel leaching rate from the residue. Gradual increase was observed for the nickel leaching rate with increasing microwave roasting temperature. Maximum leaching rate of 93.24% was observed for the roasting temperature of 450°C. However, the nickel leaching rate was found to decrease to 75.32% when the temperature exceeded 750°C. Selective heating of the microwave results in the uneven temperature distribution inside the particles causing thermal stress. The thermal stress inside the material causes the formation of cracks and holes on the microstructure of the material, exposing some originally wrapped nickel and thereby improving the nickel leaching rate [18]. However, with further increase in temperature, the material obviously sintered, leading to the formation of a dense material wrapping a part of the original material preventing the penetration of the leachant. This led to a decrease in the nickel leaching rate.
As shown in Figure 3b, roasting temperature has a significant influence on the material reduction also. There was gradual decrease of the material weight with increasing roasting temperature. The major chemical reactions taking place during the roasting process have been studied by Guo et al., using the thermol-analytical instrument (NETZSCH, SAT 449F5, Selb, Germany) [19]. The reduction of material weight in the temperature range of 20–200°C mainly corresponds to the volatilization of organic matter and crystal water. At 200–600°C, the decrease in material weight is mainly caused by the decomposition of NiCO3 and Ni(OH)2 into NiO. With further increase of temperature, decomposition reaction of calcium carbonate will constitute the main phase change reaction. After roasting at 800°C for 5 min, there was a decrease in the material weight by more than 26.71%, but it was followed by the decrease of nickel leaching rate to 75.13%. But roasting at 450°C for 5 min showed maximum leaching rate of nickel, while there was a decrease in the material weight by 21.6%. Therefore, in the subsequent experiments, the microwave roasting temperature was set to 450°C.
3.1.2 Influence of roasting time
The effect of microwave roasting time on the nickel leaching rate was investigated maintaining the microwave power of 1,000 W and 450°C as the roasting temperature.
Figure 4 shows the plot of nickel leaching rate against the microwave roasting time. There was gradual increase in the leaching rate of nickel with increasing time of microwave roasting. At the fixed microwave roasting temperature of 450°C, the nickel leaching rate is 84.43% when the microwave power is on for 1 min, which reaches a maximum of 93.74% for microwave roasting time of 5 min. On one hand, during microwave roasting, parts of the nickel compounds are oxidized and transformed into easily leachable nickel oxides, and thus improving the nickel leaching rate. On the other hand, because of the different microwave absorption capacities of various components in the material, a certain temperature gradient is generated between each component during the microwave heating, resulting in large thermal stress inside the lattice. This results in the subsequent formation of cracks inside the particles, thus providing a new reaction channel for the leachant resulting in improved nickel leaching [20,21]. Shorter the microwave heating time, greater is the temperature gradient inside the material, which results in greater thermal stress and thus greater is the possibility of material dissociation. However, with further increase of roasting time, the nickel leaching rate could not be improved mainly because longer time duration of roasting weakened the temperature gradient inside the material and also caused partial sintering of the material. These led to a decrease in the nickel leaching rate. Therefore, 5 min was chosen as the optimal microwave roasting time.
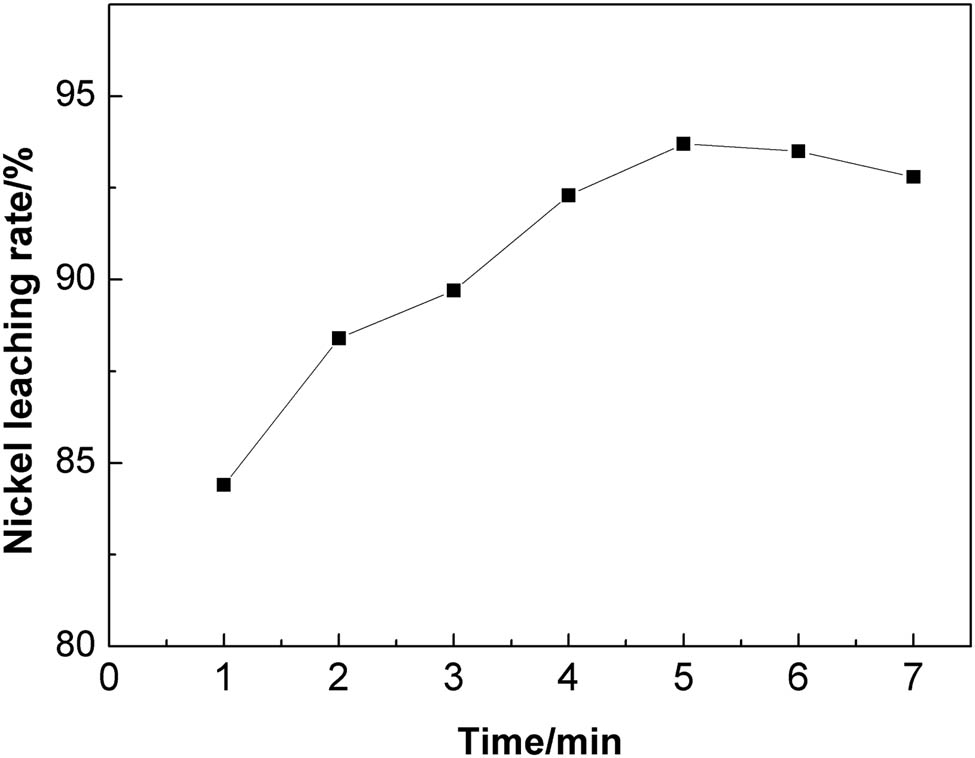
The influence of microwave roasting time on the nickel leaching rate.
3.1.3 Influence of microwave power
The influence of microwave power on the nickel leaching rate was investigated under a roasting temperature of 450°C and a holding time of 5 min. Figure 5 shows the results.
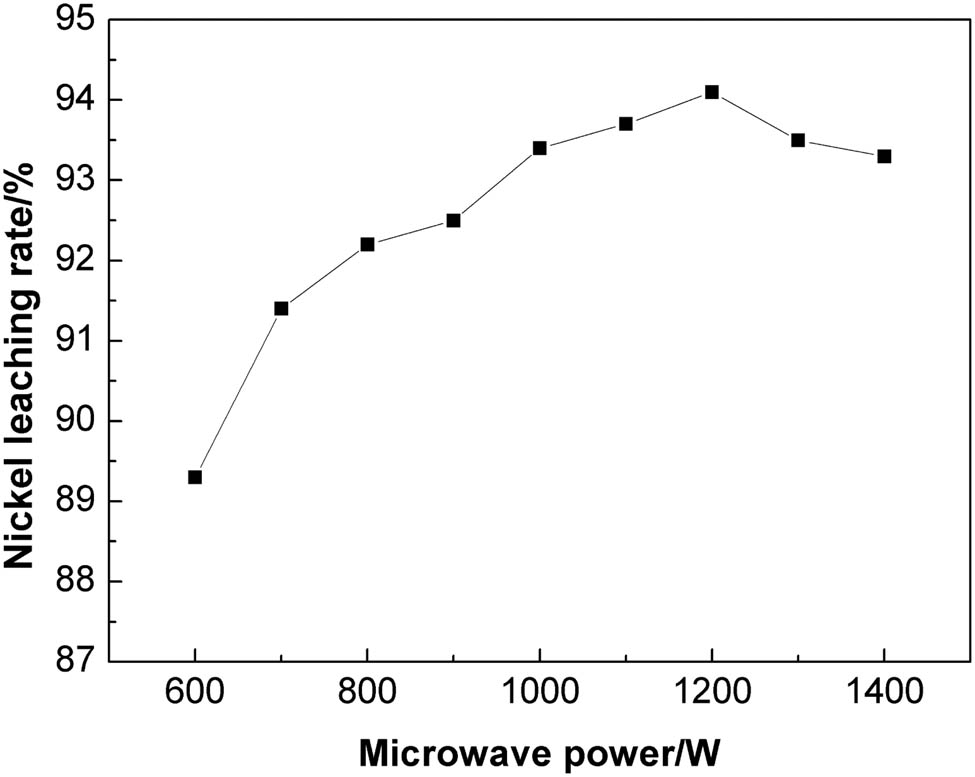
The influence of microwave power on the nickel leaching rate.
Figure 5 shows that increasing microwave power results in the increase of nickel leaching rate. For low microwave power, the nickel leaching rate was found to be low. When the microwave roasting power was 600 W, the nickel leaching rate was 89.33% which rose to 91.21% for the microwave power of 800 W. With further increase of the microwave power, the nickel leaching rate rose to 94.32%. Low energy absorption by the residue for low microwave power makes destruction of the structure of various components of the residue difficult, thus affecting the nickel leaching rate. With increasing microwave power, different absorbability of microwave for each component results in rapid increase of the temperature gradient among the components, thus enabling dissociation inside the material. But excessive microwave power is also not conducive for the nickel recovery. When the microwave power is over 1,200 W, because of the fast heating rate, some phases with strong wave absorption ability melt and wrap the nickel components to form sintered blocks, thereby reducing the subsequent nickel leaching.
3.2 Changes in physical properties of the residue after microwave roasting
Figure 6 shows the microstructures of the residue before and after microwave roasting obtained from SEM analysis. As shown in Figure 6a, after drying, the surface of the nickel-containing residue particles is rough, with good dispersion between the particles. Figure 6b shows that the surface of the material particles exhibited a stratified dissociation phenomenon after microwave roasting at 450°C. As shown in Figure 6c, roasting at 600°C resulted in the disappearance of surface delamination of particles with the appearance of an agglomeration trend. Figure 6d shows the formation of dense and large particles by the material particles and thus seriously affecting the nickel leaching after roasting at 800°C.

Microstructure analysis before and after microwave roasting. (a) Raw material, (b) 450°C, (c) 600°C, and (d) 800°C.
As shown in Figure 7, there was certain change in the major phases of the nickel-containing waste residue with increasing microwave roasting temperature. Under 600°C, there was slight change in the main diffraction peaks, except Ni(OH)2·2H2O in the raw material. When the temperature rose to 800°C, there was an increase in the number of diffraction peaks and the peak pattern became more complex. The main phase changed from CaCO3 to CaO along with the production of small amounts of Ca2SiO4 and MgO. In the meantime, there was complete conversion of nickel into NiO. However, the material exhibited apparent agglomeration at high temperatures, affecting the nickel leaching rate.
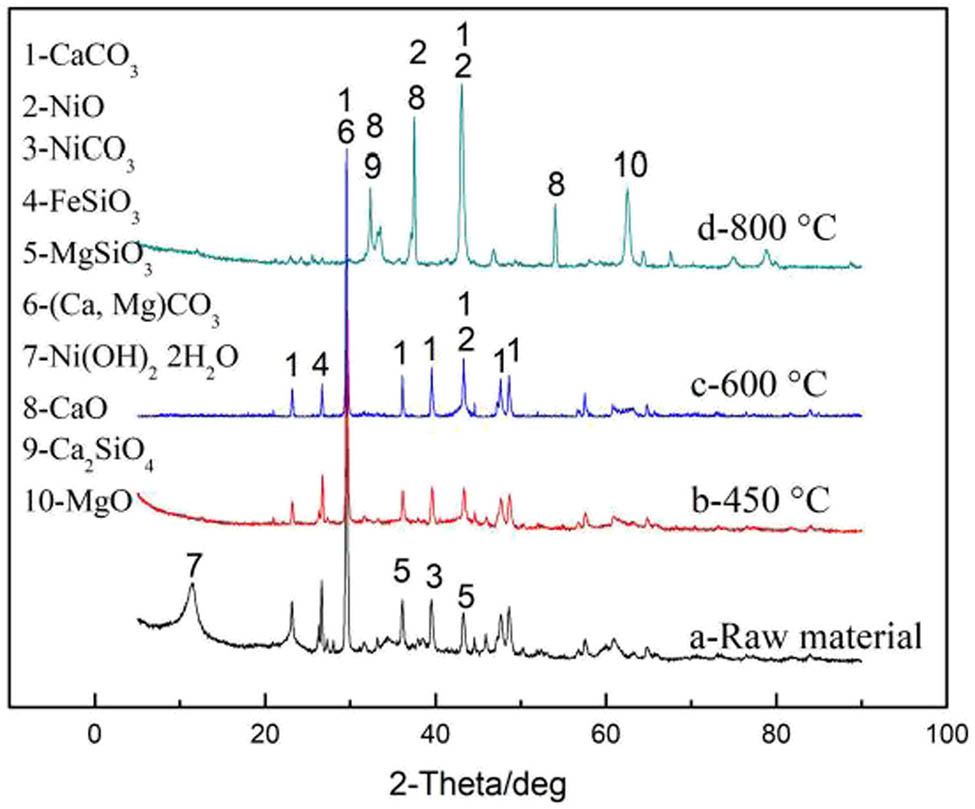
XRD analysis before and after microwave roasting.
3.3 Process optimization
The experimental data were analyzed using RSM. The nickel leaching rate (Y) was chosen as the dependent variable, while roasting temperature (χ 1), duration of roasting (χ 2), and microwave power (χ 3) were chosen as the three independent variables. The three independent variables and their levels in coded and actual values are listed in Table 2, while the exact experimental conditions are shown in Table 3. The ranges of these variables were selected based on the results from the preliminary experiments.
The levels and codes of factors for central composite design
| Variables | Coded variable level | ||||
|---|---|---|---|---|---|
| −1.682 | −1 | 0 | 1 | 1.682 | |
| Temperature (°C) | 197.731 | 300 | 450 | 600 | 702.269 |
| Time (min) | 1.636 | 3 | 5 | 7 | 8.364 |
| Power (W) | 663.641 | 800 | 1,000 | 1,200 | 1336.36 |
Experimental design scheme and experimental results
| Run | Temperature (°C) | Time (min) | Power (W) | Leaching rate (%) |
|---|---|---|---|---|
| 1 | 300 | 3 | 800 | 87.51 |
| 2 | 600 | 3 | 800 | 83.65 |
| 3 | 300 | 7 | 800 | 89.44 |
| 4 | 600 | 7 | 800 | 83.72 |
| 5 | 300 | 3 | 1,200 | 88.45 |
| 6 | 600 | 3 | 1,200 | 84.11 |
| 7 | 300 | 7 | 1,200 | 92.23 |
| 8 | 600 | 7 | 1,200 | 83.54 |
| 9 | 197.73 | 5 | 1,000 | 86.43 |
| 10 | 702.27 | 5 | 1,000 | 75.45 |
| 11 | 450 | 1.64 | 1,000 | 84.87 |
| 12 | 450 | 8.36 | 1,000 | 90.36 |
| 13 | 450 | 5 | 663.64 | 88.58 |
| 14 | 450 | 5 | 1336.36 | 93.15 |
| 15 | 450 | 5 | 1,000 | 92.86 |
| 16 | 450 | 5 | 1,000 | 92.35 |
| 17 | 450 | 5 | 1,000 | 93.64 |
| 18 | 450 | 5 | 1,000 | 93.48 |
| 19 | 450 | 5 | 1,000 | 93.73 |
| 20 | 450 | 5 | 1,000 | 93.44 |
Note: Considering the operability of the experiment in the actual process, the actual temperatures of experiments 9 and 10 were 200°C and 700°C, respectively. The holding times of experiments 11 and 12 were 1.5 and 8.5 min, respectively. The microwave powers of experiments 13 and 14 were 650 and 1,300 W, respectively.
3.3.1 Model selection and accuracy analysis
Table 3 shows the design scheme and experimental results for the microwave roasting pretreatment of nickel-containing residue based on a central composite design.
During the experiment, the effects of roasting temperature, roasting duration, and microwave power on the nickel leaching rate were investigated. Under these conditions, the nickel leaching rate of the nickel-containing residue increased from 75.45% to 93.73%.
The experimental data were fitted with different models, such as linear, 2FI, quadratic, and cubic models in order to obtain the most accurate model. The adequacy and significance of each model could be verified through the sequential model sum of square (see in Table 4) and the model summary statistics (see in Table 5). As can be seen from Table 4, the minimum P value of the quadratic model is <0.001, and the maximum F value is 103.96. Therefore, the quadratic model is adopted to describe the influence of microwave roasting pretreatment on nickel leaching. As shown in Table 5, the correlation coefficient R 2 for the quadratic model is 0.9795, indicating that this model is able to explain 97.95% of the data in the experiment. Therefore, the quadratic model can be assumed to be highly reliable in this experiment. In addition, for this model, the adj. R 2 = 0.9611, pred. R 2 = 0.8612, std. dev = 0.97, and the calibration difference std. Dev is small, adj. R 2 is large, while the difference between adj. R 2 and pred. R 2 is relatively small. It thus further proves that the quadratic model can well reflect the influence of microwave roasting conditions on the subsequent sulfuric acid leaching.
Sequential model sum of squares
| Source | Sum of squares | df | Mean squares | F-value | P-value | |
|---|---|---|---|---|---|---|
| Mean | 1.57 × 105 | 1 | 1.57 × 105 | |||
| Linear | 148.84 | 3 | 49.61 | 2.59 | 0.0891 | |
| 2FI | 6.49 | 3 | 2.16 | 0.094 | 0.9622 | |
| Quadratic | 290.98 | 3 | 96.99 | 103.96 | <0.0001 | Suggested |
| Cubic | 7.04 | 4 | 1.76 | 4.6 | 0.0485 | |
| Residual | 2.29 | 6 | 0.38 | |||
| Total | 1.57 × 105 | 20 | 7863.8 |
Model summary statistics
| Source | Std. dev. | R-squared | Adjusted R-squared | Predicted R-squared | PRESS | |
|---|---|---|---|---|---|---|
| Linear | 4.38 | 0.3267 | 0.2004 | −0.0187 | 464.15 | |
| 2FI | 4.81 | 0.3409 | 0.0367 | −0.4479 | 659.7 | |
| Quadratic | 0.97 | 0.9795 | 0.9611 | 0.8612 | 63.22 | Suggested |
| Cubic | 0.62 | 0.995 | 0.9841 | 0.5793 | 191.69 |
Table 6 gives the analysis of variance (ANOVA) for the quadratic model. The Adeq Precision signal-to-noise ratio (the ratio of trusted and un-trusted data) is 24.97 (greater than 4), indicating that the model has sufficient applicability. CV is the coefficient of variation for the response value Y, and lower the CV, better is the stability of the experiment. In this experiment, the CV is 1.09%, indicating the stable and reliable nature of the experiment.
Analysis of variance of the model
| Source | Coefficient | Standard error | Sum of squares | df | Mean square | F-value | P-value Prob >F |
|---|---|---|---|---|---|---|---|
| Model | 93.23 | 0.39 | 446.31 | 9 | 49.59 | 53.15 | <0.0001 |
| χ 1 | −3.01 | 0.26 | 123.55 | 1 | 123.55 | 132.42 | <0.0001 |
| χ 2 | 1.06 | 0.26 | 15.27 | 1 | 15.27 | 16.37 | 0.0023 |
| χ 3 | 0.86 | 0.26 | 10.02 | 1 | 10.02 | 10.74 | 0.0083 |
| χ 1 χ 2 | −0.78 | 0.34 | 4.82 | 1 | 4.82 | 5.17 | 0.0463 |
| χ 1 χ 3 | −0.43 | 0.34 | 1.49 | 1 | 1.49 | 1.59 | 0.2353 |
| χ 2 χ 3 | 0.15 | 0.34 | 0.18 | 1 | 0.18 | 0.2 | 0.6673 |
|
|
−4.24 | 0.25 | 259.41 | 1 | 259.41 | 278.05 | <0.0001 |
|
|
−1.88 | 0.25 | 51.08 | 1 | 51.08 | 54.76 | <0.0001 |
|
|
−0.73 | 0.25 | 7.76 | 1 | 7.76 | 8.32 | 0.0163 |
R 2 = 0.9795; adj.R 2 = 0.9611; CV = 1.09%; Adeq Precision = 24.97.
The F-value of χ
1 as can be seen in Table 6 is 132.42, indicating that, in comparison to the microwave power and roasting time, microwave roasting temperature is the most significant parameter responsible for the nickel leaching rate from the nickel-containing waste residue. In addition, P-values are also important in illustrating the significance of the impact factor. It is generally believed that the influence factor is considered to have a significant impact on the model when the P-values of the variable are <0.05 [22]. The results shown in Table 6 indicate that χ
1, χ
2, and χ
3 in the first order;
Based on the above analysis, the quadratic model can be represented by the following equation after ignoring the insignificant terms:
where χ 1, χ 2, χ 3, and Y represent the roasting temperature (°C), roasting time (min), microwave power (W), and nickel leaching rate (%), respectively.
Figure 8 shows the comparative nickel leaching rate between the predicted and experimental values of the nickel leaching rate after the pretreatment of the nickel-containing residue by microwave roasting. The figure shows that the software predicted values are close to the experimentally obtained actual value, indicating that the model can accurately reflect the relationship between the impact factors and the response value.
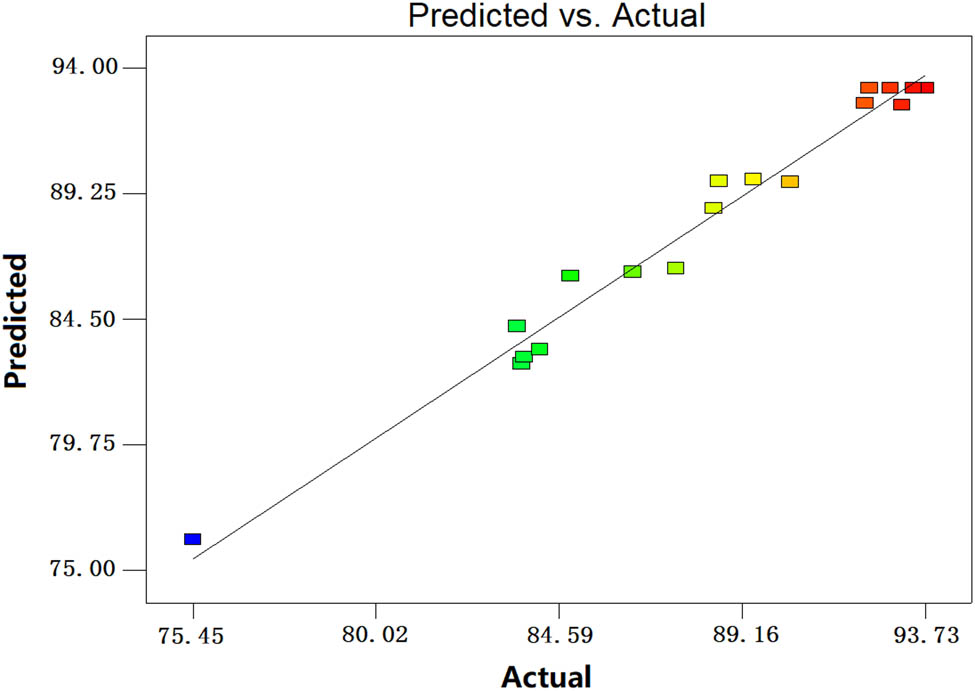
Comparison of predicted and actual values.
3.3.2 Interaction of the factors
A three-dimensional (3D) mathematical model was used to study the influence of independent factors along with their interaction on the nickel leaching rate.
Figure 9 shows the 3D diagrams representing the effects of roasting temperature, roasting time, and microwave power on the nickel leaching rate. Similar trends in the 3D images as shown in Figure 9a and b indicate that the impact of roasting temperature on the nickel leaching rate is more significant than that of roasting time and microwave power, and it is consistent with the F-value results obtained for each impact factor from the model. As shown in Figure 9a and b, within the experimental range, there was an initial increase of the nickel leaching rate of the nickel-containing waste residue with increasing roasting temperature which was followed by a subsequent decrease. The influence of microwave power and roasting time was found to be relatively mild. During the initial stage of pretreatment, the nickel leaching rate obviously increased, but after reaching a value of more than 92%, the leaching rate started to decrease with further increase of temperature. The main reason is that the melting point of heavy metals being generally high, the other materials with lower melting points melted first to surround and protect the heavy metal elements, thereby reducing their leaching rates.
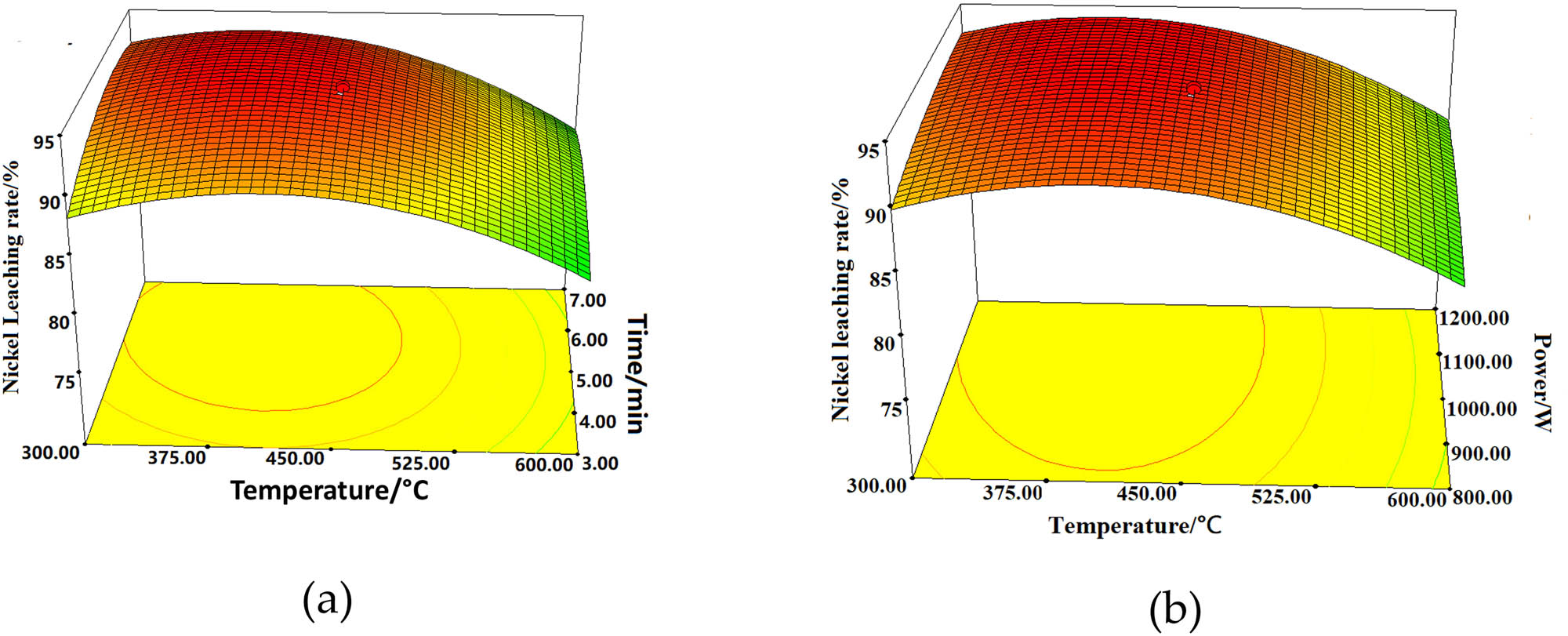
Effect of roasting temperature, time, and microwave power on the nickel leaching rate. (a) Interaction of roasting temperature and roasting time (microwave power = 1,000 W) and (b) interaction of roasting temperature and microwave power (holding time = 5 min).
Figure 10 shows the 3D diagram of the interaction between microwave power and roasting time. As shown in Figure 10, the response surface is gentle, and the nickel leaching rate is over 87% in the measured range. Thus, the interaction of microwave roasting time and microwave power has no significant influence on the nickel leaching rate, which is consistent with the ANOVA results. Therefore, microwave power and roasting time can be appropriately reduced in practical applications to improve the overall microwave pretreatment efficiency.

Influence of the interaction of microwave power and roasting time on the nickel leaching rate (roasting temperature = 450°C).
3.3.3 Process optimization
Through the optimization function of the response surface software, the predicted values of nickel leaching rate after microwave pretreatment employing different roasting temperatures (322–590°C), roasting time (3.8–6.2 min), and microwave power (811–1,190 W) were selected. The actual values of nickel leaching rate under the corresponding parameters were obtained from three parallel experiments, as shown in Table 7. It can be seen from Table 7 that deviation between the predicted value and the actual value is small, signifying that the quadratic model can well-describe the influence of microwave roasting pretreatment conditions on the nickel leaching rate. Under the first set of experimental conditions, smallest deviation was observed between the predicted value and actual value. At the same time, temperature being the most significant parameter affecting the nickel leaching rate, conditions from the first experiment were selected as the optimal process conditions in this experiment with temperature being the main selection basis, under the premise of ensuring as high as possible nickel leaching rate, while taking into account the factors such as energy consumption and efficiency. Under the condition of 450°C as the roasting temperature and 962 W as the microwave power for a roasting time of 6.2 min, nickel leaching rate could reach 93.11% after process optimization.
Optimal process parameters and model validation
| Number | Temperature (°C) | Time (min) | Power (W) | Leaching rate (%) | Deviation (%) | ||
|---|---|---|---|---|---|---|---|
| Predicted | Actual | ||||||
| 1 | 452 | 6.2 | 962 | 92.92 | 93.11 | 0.21 | Suggested |
| 2 | 322 | 3.8 | 849 | 89.67 | 88.54 | 1.26 | |
| 3 | 344 | 4.1 | 811 | 90.41 | 89.43 | 1.08 | |
| 4 | 502 | 3.6 | 938 | 90.02 | 89.37 | 0.72 | |
| 5 | 542 | 5.8 | 1,190 | 89.68 | 89.26 | 0.47 | |
| 6 | 560 | 4.5 | 994 | 88.42 | 89.34 | 1.04 | |
| 7 | 590 | 6.1 | 904 | 85.89 | 84.57 | 1.54 | |
4 Conclusions
In the single-factor experiment for the microwave roasting pretreatment of the nickel-containing residue, under a roasting temperature of 450°C and microwave power of 1,000 W for 5 min, the nickel leaching rate was increased from 77.42% to 93.24%, thereby increasing the leaching efficiency by 20.43%, while the material weight was reduced by more than 21%.
RSM was used to optimize the microwave pretreatment process; the quadratic model and optimal process parameters for microwave roasting pretreatment of the nickel-containing waste residue were obtained.
The process was optimized by response surface method; it was found that, under a roasting temperature of 452°C, roasting time of 6.2 min, and microwave power of 962 W, the nickel leaching rate could reach 93.11%.
-
Funding information: This research was funded by the National Natural Science Foundation of China (Grant No. 51864042 and 51804220), the Youth Foundation of Natural Science Foundation of Henan Province (Grant No. 202300410100 and 212300410130), Key Scientific and Technological Project of Henan Province (Grant No. 192102310499, 212102310521, and 152102210306), and the High-level Talents Start-up Fund of Henan Institute of Technology (Grant No. KY1706 and KQ1820).
-
Author contributions: Guang Su: writing – original draft, writing – review and editing, methodology, formal analysis; Zhanyong Guo: formal analysis, visualization, project administration; Ping Guo: resources; Ping Guo: resources; Fachaung Li: resources; Qian Zhang: resources; Huilin Zhou: resources; Jun Chang: resources.
-
Conflict of interest: Authors state no conflict of interest.
References
[1] Yan X, Li Q, Chai L, Wang Q. Formation of abiological granular sludge-A facile and bioinspired proposal for improving sludge settling performance during heavy metal wastewater treatment. Chemosphere. 2014;113(10):36–41. 10.1016/j.chemosphere.2014.04.038.Suche in Google Scholar PubMed
[2] Hsieh CH, Shih K, Hu CY, Lo SL, Li NH, Cheng YT. The effects of salinity and temperature on phase transformation of copper-laden sludge. J Hazard Mater. 2013;244–5:501–6. 10.1016/j.jhazmat.2012.10.066.Suche in Google Scholar PubMed
[3] Zhang P, Ma Y, Xie F. Impacts of ultrasound on selective leaching recovery of heavy metals from metal-containing waste sludge. J Mater Cycles Waste. 2013;15(4):530–8. 10.1007/s10163-013-0131-z.Suche in Google Scholar
[4] Silva JE, Paiva AP, Soares D, Labrincha A, Castro F. Solvent extraction applied to the recovery of heavy metals from galvanic sludge. J Hazard Mater. 2005;120(1–3):113–8. 10.1016/j.jhazmat.2004.12.008.Suche in Google Scholar PubMed
[5] Liang YJ, Chai LY, Min XB, Tang CJ, Zhang HJ, Ke Y, et al. Hydrothermal sulfidation and floatation treatment of heavy-metal-containing sludge for recovery and stabilization. J Hazard Mater. 2012;(217–8):307–14. 10.1016/j.jhazmat.2012.03.025.Suche in Google Scholar PubMed
[6] Shih K, White T, Leckie JO. Nickel stabilization efficiency of aluminate and ferrite spinels and their leaching behavior. Environ Sci Technol. 2006;40(17):5520–6. 10.1021/es0601033.Suche in Google Scholar PubMed
[7] Li CT, Lee WJ, Huang KL, Fu SF, Lait YC. Vitrification of chromium electroplating sludge. Environ Sci Technol. 2007;41(8):2950–6. 10.1021/es062803d.Suche in Google Scholar PubMed
[8] Yi Z, Wang ZK, Xia X, Chen YQ, Qi T. Recovery of heavy metals from electroplating sludge and stainless steel pickle waste liquid by ammonia leaching method. J Environ Sci. 1999;11(3):381–4. 10.3321/j.issn:1001-0742.1999.03.023.Suche in Google Scholar
[9] Silva JE, Soares D, Paiva AP, Labrincha JA, Castro F. Leaching behaviour of a galvanic sludge in sulphuric acid and ammoniacal media. J Hazard Mater. 2005;121(1-3):195–202. 10.1016/j.jhazmat.2005.02.008.Suche in Google Scholar PubMed
[10] Raveendran A, Sebastian MT, Raman S. Applications of microwave materials: a review. J Electron Mater. 2019;48:2601–34. 10.1007/s11664-019-07049-1.Suche in Google Scholar
[11] Yang G, Park SJ. Conventional and microwave hydrothermal synthesis and application of functional materials: a review. Materials. 2019;12(7):1177. 10.3390/ma12071177.Suche in Google Scholar PubMed PubMed Central
[12] Cui K, Liao T, Qiu C, Zhou J. Microwave-induced heating behavior of Y-TZP ceramics under multiphysics system. Green Process Synth. 2020;9(1):119–30. 10.1515/gps-2020-0013.Suche in Google Scholar
[13] Arpia AA, Chen WH, Su SL, Rousset P, Luna MDGD. Sustainable biofuel and bioenergy production from biomass waste residues using microwave-assisted heating: a comprehensive review. Chem Eng J. 2021;403:126233. 10.1016/j.cej.2020.126233.Suche in Google Scholar
[14] Togari T, Yamamoto-Ikemoto R, Ono H, Takashima K, Tanaka K. Effects of microwave pretreatment of dewatered sludge from an oxidation-ditch process on the biogas yield in mesophilic anaerobic digestion. J Water Environ Technol. 2016;14(3):158–65. 10.2965/jwet.15-048.Suche in Google Scholar
[15] Nag-Choul C, Bong-Ju K, Kanghee C, Soonjae L, Cheon-Young P. Microwave pretreatment for thiourea leaching for gold concentrate. Metals. 2017;7(10):404. 10.3390/met7100404.Suche in Google Scholar
[16] Zhang ZB, Zhang ZY, Niu H, Peng JH, Zhangm LB, Qu W, et al. Effects of microwave pretreatment on zinc extraction from spent catalyst saturated with zinc acetate. T Nonferr Met Soc. 2010;20(S1):182–6. 10.1016/S1003-6326(10)60036-2.Suche in Google Scholar
[17] Lambert A, Anawati J, Walawalkar M, Tam J, Azimi G. Innovative application of microwave treatment for recovering of rare earth elements from phosphogypsum. ACS Sustain Chem Eng. 2018;6(12):16471–81. 10.1021/acssuschemeng.8b03588.Suche in Google Scholar
[18] Gholami H, Rezai B, Hassanzadeh A, Mehdilo A, Yarahmadi M. Effect of microwave pretreatment on grinding and flotation kinetics of copper complex ore. Int J Min Met Mater. 2020. 10.1007/s12613-020-2106-0.Suche in Google Scholar
[19] Guo Z, Guo P, Su G, Zhai D, Cheng F, Li C. High temperature permittivity and microwave pretreatment characteristics of nickel-containing sludge from battery production. Processes. 2019;7(5):257. 10.3390/pr7050257.Suche in Google Scholar
[20] Meng Y, Yan Y, Jiang P, Zhang M, Pang CH. Investigation on breakage behaviour of oil shale with high grinding resistance: a comparison between microwave and conventional thermal processing. Chem Eng Process. 2020;151:107909. 10.1016/j.cep.2020.107909.Suche in Google Scholar
[21] Wang JP, Jiang T, Liu YJ, Xue XX. Influence of microwave treatment on grinding and dissociation characteristics of vanadium titano-magnetite. Int J Met Mater. 2019;26(2):160–7. CNKI:SUN:BJKY.0.2019-02-003.Suche in Google Scholar
[22] Mh A, Vp A, Hn B, Alihosseini A. Application of response surface methodology to optimize high active Cu-Zn-Al mixed metal oxide fabricated via microwave-assisted solution combustion method. Adv Powder Technol. 2020;31(4):1470–9. 10.1016/j.apt.2020.01.010.Suche in Google Scholar
© 2021 Guang Su et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis
Artikel in diesem Heft
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis

