Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
-
Fahad A. Alhumaydhi
, Muhammad Nasimullah Qureshi
Abstract
Currently, nanotechnology is gaining massive attention compared to conventional methods as the biosynthesis of plant-based nanoparticles is considered safe, effective, and ecofriendly. Therefore, keeping in view the importance of nanotechnology, the present study was designed to synthesize, characterize, and evaluate the biological effectiveness of saffron stigma-based gold nanoparticles (SS-AuNPs) for their in vitro and in vivo biological properties. These gold nanoparticles were characterized by UV–Vis spectroscopy, Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX), and X-ray diffraction (XRD). The highest antibacterial effect was observed by the saffron extract against Escherichia coli (22 mm). SS-AuNPs significantly inhibited the activity of enzyme urease (54.98%) and CA-II (64.29%). However, the nonsignificant inhibitory effect was observed in the case of α-chymotrypsin. Maximum analgesic (84.98%) and antiinflammatory (88.98%) effects were observed for SS-AuNPs (10 mg/kg). Similarly, SS-AuNPs demonstrated a significant (P < 0.01) sedative effect at all tested doses.
1 Introduction
In the last few decades, nanotechnology has proven to be of significant importance owing to its application in optical, catalytic, electronic, and medicinal fields [1]. The application of nanotechnology in medicinal science is of most importance due to its beneficial impact on human as well as animal and plant health. Control drug delivery, tissue engineering, tumor detection and destruction, electroluminescent, drug and disease sensors, and diagnosis of cancer through MRI are some examples of nanoparticle application in the medical field [2,3,4,5,6,7,8]. Currently, different techniques are applied for the synthesis of nanoparticles including microwave-assisted synthesis, chemical and photochemical synthesis protocols, reduction in solution, and electrochemical synthesis route [9,10,11,12]. Green synthesis of nanoparticles through ecofriendly synthesis methods is gaining attention among the researcher community because it does not require high pressure, temperature, and toxic chemicals but bacteria, fungi, and plant extract are used for the synthesis of green nanoparticles [13,14,15]. These biological systems can synthesize nanoparticles in a safe, easy, and economical way [16]. The formation of green nanoparticles is due to the strong reducing ability of these biological systems, and this reducing ability is attributed to the enzymes and/or biomolecules in plant cells [17,18]. The development of environment-friendly plant extract-based nanoparticles through green synthetic route has vast application in modern science owing to their efficient drug delivery model and less toxicity [19,20]. Green synthesis of nanoparticles developed using algae, plants, fungi, and bacteria is renowned as a safe, efficient, and environmentally friendly approach in drug discovery [21,22].
Around the globe, saffron is most commonly used as a traditional food spice and medicine. Crocus sativus L. (saffron) is a perennial herb that belongs to the family Iridaceae and is also known as red gold. It is known to be the most expensive herb cultivated throughout the world [23]. Flowers of saffron are light purple having reddish thread-like stigmas characterized as spice possessing unique natural colorant (Figure 1). Approximately, 36,000 flowers of saffron yield 1 pound stigmas. To obtain a half kilogram of pure saffron, nearly 200,000 dried stigmas are required [24]. Clinical trials are being conducted to evaluate the effectiveness of saffron against mild depression [25]. Scientists have investigated the antiinflammatory and antinociceptive properties of different parts of stigma flower including petals and stigmas [26]. It is considered a natural spice possessing a strong odor and intense natural yellow color other than its renowned therapeutic properties. Phytochemical characterization has demonstrated that the flavor, color, and bitterness of saffron are associated with safranal, crocin and crocetin, and glucoside picrocrocin, respectively [27].

Saffron (Crocus sativus) flower and stigmas.
The present study is designed to synthesize gold nanoparticles (Au-NPs) by reducing gold ions using alcoholic extract of the saffron flower. Biosynthesized Au-NPs were characterized by UV-Vis spectroscopy, Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX), and X-ray diffraction (XRD). Effects of varying concentrations of salt, pH, and temperature on the stability of synthesized gold nanoparticles were also investigated in this study. Furthermore, SS-AuNPs were screened for antifungal, antibacterial, enzyme inhibitory, antinociceptive, antiinflammatory, and sedative properties.
2 Materials and methods
2.1 Procurement of raw materials and chemicals
Pure dry saffron flower stigmas were obtained from Dubai. Analytical grade reagents and chemicals were procured and used in this study. Hydrogen tetrachloroaurate trihydrate [HAuCl4·3H2O], deionized water, methanol, and sodium chloride were procured from Merck.
2.2 Extraction
Two hundred fifty grams of pure dry saffron flower stigmas was soaked in 250 mL of 50% alcohol for 1 week and then was filtered through Whatman no. 1 filter paper. A bright orange-red filtrate was obtained, which was treated as a source extract for further procedures.
2.3 Synthesis of saffron flower stigma-based gold nanoparticles
For the green synthesis of alcoholic extract of saffron flower stigma-based gold nanoparticles, different ratios (1:1, 10:1, 20:1, 30:1, 40:1, 50:1, and 60:1) of 1 mM (HAuCl4) and extract were used. The obtained extract was added to 1 mM warm gold solution (HAuCl4) already present in a titration flask and was uniformly stirred for a period of 4 h. The different ratios of the extract and the gold solution give different colors, i.e., blue, violet, pink, red, and cherry red as shown in Figure 2.

Different ratio of gold and saffron extract.
2.4 Characterization of SS-AuNPs
The synthesis of AuNPs was detected spectrophotometrically (UV-Vis spectrophotometer: Shimadzu UV-1800 Japan) at a wavelength varying from 200 to 900 nm by placing the sample in the optical path length (10 mm) quartz. For the characterization of the particle size, AuNPs were subjected to atomic force microscopy (AFM) and scanning electron microscopy (SEM) (SM-5910-JEOL, Japan). Energy-dispersive X-ray (EDX) (INCA-200, England) and X-ray diffraction (XRD) studies were carried out for elemental analysis and to determine the crystallinity of the biosynthesized saffron flower stigma-based gold nanoparticles (SS-AuNPs). The nanoparticle solution was subjected to centrifugation for 15 min at 10,000 rpm for the removal of free proteins and other unwanted components. Afterward, the samples were dried under vacuum and ground in a mortar having finely divided potassium bromide (KBr) to form pellets. These KBr pellets were placed in a sample holder of FTIR for further measurement.
2.5 Kinetic and stability studies of SS-AuNPs
To find the time-dependent synthesis of AuNPs, a kinetic study was performed. For this study, samples were drawn from the reaction mixture after a certain interval of time, and the UV data were recorded periodically. The stability of AuNPs was checked through varying the parameter conditions like pH, type of salt, salt concentration, and temperature. Drop-wise addition of either 1 M NaOH or HCl was done for adjustment of pH value of AuNPs solutions between 2 and 14. The effect of sodium chloride (NaCl) on the stability of AuNPs was determined by adding (1 mL) 0.1–1.5 M NaCl to the biosynthesized AuNPs (2 mL). Similarly, the effect of other salts with the same concentration (0.1 M) was also checked. The UV–Vis spectrum was obtained after each treatment. To assess the temperature stability of AuNPs, they were heated in a water bath at 30°C, 50°C, and 100°C for 30 min each.
2.6 Catalytic activity of SS-AuNPs
Reaction studies were performed to check the catalytic activity of saffron stigma-based nanoparticles for the degradation of Rhodamine B (RhB). RhB is one of the most commonly used dyes, which is widely used for industrial purposes, such as printing and dyeing in textile, paper, paints, and leathers. However, the organic dyes will cause serious environmental and biological problems, even capable to induce irritation to the skin, eyes. Thus, the removal of dye from water is a great challenge. The convention methods for removal of RhB include biochemical and physiochemical methods, such as liquid membrane, ozonation, and adsorption, which are expensive and not very effective. Heterogeneous nanocatalyst (metal nanoparticles) present promising application for the organic dye decomposition with superior activity [28].
In the aqueous medium, RhB shows maximum absorption at 552 nm. This reaction was monitored by the UV–Visible spectroscopy in the wavelength between 200 and 700 nm at room temperature. The decrease of absorbance at the maximum wavelength (552 nm) with time was recorded and shown in Figures 17 and 18. The intense bright red color of the RhB solution faded and became colorless during the degradation process. The addition of biosynthesized nanoparticles improved the reduction process (dye degradation up to 95% within 13 min).
2.7 Antibacterial activity of SS-AuNPs
Antibacterial activity of the synthesized SS-AuNPs against Acinetobacter, Providencia, Streptococcus, and Escherichia coli was assessed by using the well diffusion method [28]. Standard antibiotics such as levofloxacin, ciprofloxacin, amoxicillin, and norfloxacin were used for comparison purposes. This assay was performed in triplicate.
2.8 Enzyme inhibition activity of SS-AuNPs
Biosynthesized gold nanoparticles and saffron stigma extract were analyzed for their enzyme inhibitory potential against three different types of enzymes, i.e., xanthine oxidase urease and carbonic anhydrase-II. Xanthine oxidase inhibition of all the experimented samples was evaluated by determining the hydroxylation rate of xanthine (substrate) followed by uric acid formation. This reaction produces a colorless end product, and absorption was noticed at 295 nm [29]. In this assay, allopurinol was sued as a standard. Likewise, carbonic anhydrase-II inhibitory activities of the tested samples were analyzed using 4-nitrophenyl acetate (4-NPA) that is colorless. Hydrolysis of 4-NPA results in the formation of carbon dioxide (CO2) and 4-nitrophenol (yellow). During this experiment, the formation of this yellow-colored compound was monitored. The reaction temperature was maintained between 25°C and 28°C, and acetazolamide was used as a standard inhibitor [30]. Samples were treated with urea for the determination of urease inhibitory activity of respective samples. Urease activity was investigated by measuring ammonia production through the indophenol method. In the urease inhibitory assay, thiourea was used as a standard inhibitor [31]. All the experimented assays were conducted in triplicate.
2.9 In vivo screening
2.9.1 Animals
BALB/c mice of either sex (18–22 g) procured from National Institute of Health (NIH), Islamabad, were used in this experimental work. Before the commencement of experimental procedures, all mice were examined for any physical or behavioral abnormalities. After initial screening, only the healthy animals were selected for the experimental procedure. The selected animals were acclimatized to standard (12 h light/dark cycle at 22 ± 2°C) animal house conditions with strict compliance to laboratory guidelines of NIH for animal care and use. All the experimental work was approved from the ethical commit number UOS/Pharm-234 of the University of Swabi, Pakistan. All mice were classified as the negative control group (n = 6), positive control group, and the tested group.
2.9.2 Sedative activity (open field test)
Apparatus used to assess the sedative activity of gold nanoparticles comprised an area of white wood (150 cm diameter) covered with stainless steel walls. The base of the apparatus was divided into 19 squares with black lines. This open-field apparatus was placed in a sound-attenuated room, and mice were acclimatized in the dim red light for 1 h before the initiation of the experiment. Mice were categorized into negative control group (distilled water; 10 mL/kg), positive control group (diazepam; 0.5 mg/kg), crude saffron stigma extract-treated groups (15, 25, 50, and 100 mg/kg), and saffron flower stigma-based gold nanoparticles-treated groups (2.5, 5, and 10 mg/kg). After 30 min administration of respective treatment, each mouse was placed in the center of the apparatus for 10 min, and the number of lines crossed by each mouse was counted and noted [31].
2.9.3 Acetic acid-induced writhing test
The acetic acid-induced writhing test was conducted to assess the antinociceptive potential of the saffron stigma extract and the biosynthesized gold nanoparticles. Two hours before the commencement of this assay, mice were withdrawn from food. Mice were divided into nine groups. Group-I (negative control) was administrated to distilled water (10 mg/kg). Group-II (positive control) was treated with diclofenac (10 mg/kg). Group-III, IV, V, and VI were subjected to the saffron stigma extract at a dose of 15, 25, 50, and 100 mg/kg, respectively. While group-VII, VIII, and IX received SS-AuNPs at corresponding doses of 2.5, 5, and 10 mg/kg. Acetic acid (1%) was injected (i.p.) to each mouse after 30 min administration of respective treatments. The number of abdominal constrictions (writhing) was counted for 10 min after 5 min administration of acetic acid injections [32]. The percent inhibition was calculated through the following equation:
where N wt is the number of writhing in tested animals and N wc is the number of writhing in negative control animals.
2.9.4 Carrageenan-induced paw edema test
Carrageenan-induced paw edema test was performed to evaluate the antiinflammatory effect of all the tested treatments by following the standard protocols. Animals were categorized and treated as in acetic acid-induced writhing test. Inflammation was induced by administration of carrageenan (1%, 0.05 mL) treatment in the subplantar region of the right hind paw (edema). The volume of paw edema was recorded after 1, 2, 3, 4, and 5 h posttreatment of inflammatory drug. The induced inflammation was quantified using a plethysmometer. The percent inhibition in edema/inflammation was calculated through the following equation [31,32]:
where A is the paw volume of the negative control and B is the paw volume of the other tested group.
2.9.5 Acute toxicity study
For acute toxicological profiling, the animals were treated with the saffron stigma extract (100, 250, 500, and 1,000 mg/kg) and AuNPs (10, 25, 50, and 100 mg/kg). After the aforementioned administration, animals were observed for the first 5 h for any gross changes, and then, mortality was recorded after 24 h by following the standard protocols [28].
2.10 Statistical analysis
Results of this study were stated as mean ± SEM. Significant differences (p ≤ 0.05) among all the experimented groups were assessed using a one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test.
3 Results
3.1 Selection of SS-AuNPs and extract ratio
Figure 3 shows different peaks at the range of 530–580 nm, exhibiting different absorbance values, which indicated different sizes of gold nanoparticles. The sharpness of the peak showed the uniformity of gold nanoparticles. By considering the height of the peak from the baseline of the spectrum, it was observed that the peak for the saffron stigma-based gold nanoparticle (5:1) showed the highest absorbance at 545 nm wavelength. This indicated the presence of a maximum concentration of gold nanoparticles in the solution. However, other peaks showed lower peak height (measured from the baseline of the spectrum) and broadness, which indicates a greater number of nonuniform gold nanoparticles in the solution. Therefore, this ratio (5:1) was used for the preparation of bulk solutions for further investigation.
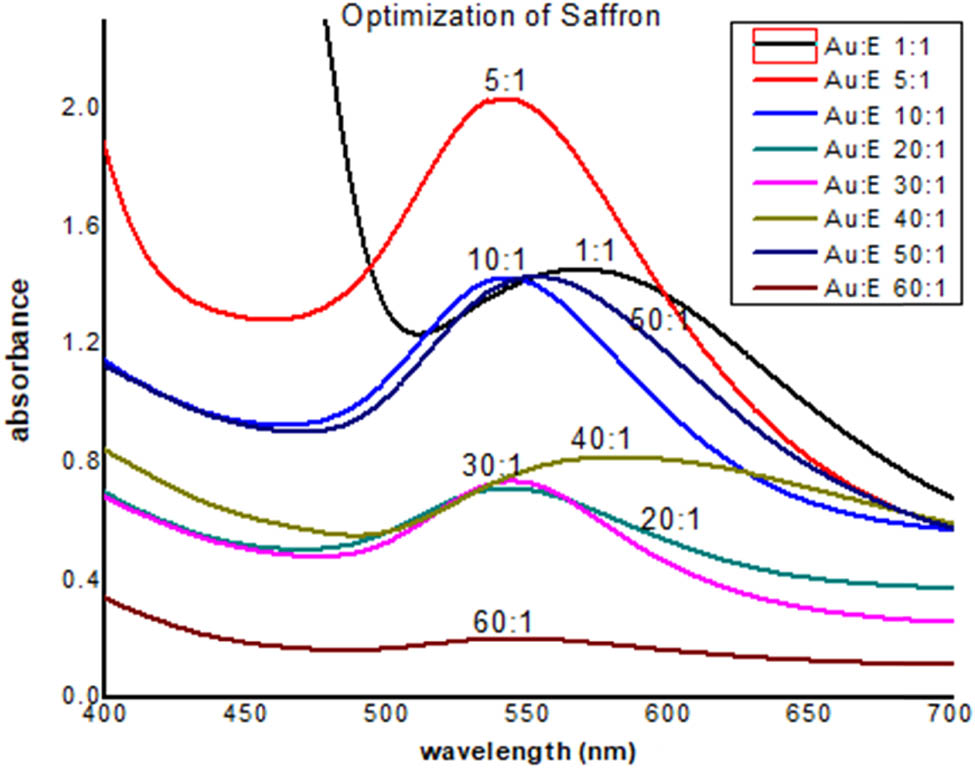
UV-Visible spectra of AuNPs of saffron.
3.2 Kinetic study of SS-AuNPs
The results for kinetic studies of the synthesized SS-AuNPs are shown in Figures 4–6. Outcomes of this study reveal that the number and uniformity of nanoparticles increased with time. The regression analysis showed that R 2 = 0.9895, while the rate of reaction was 2.55 × 10−2.
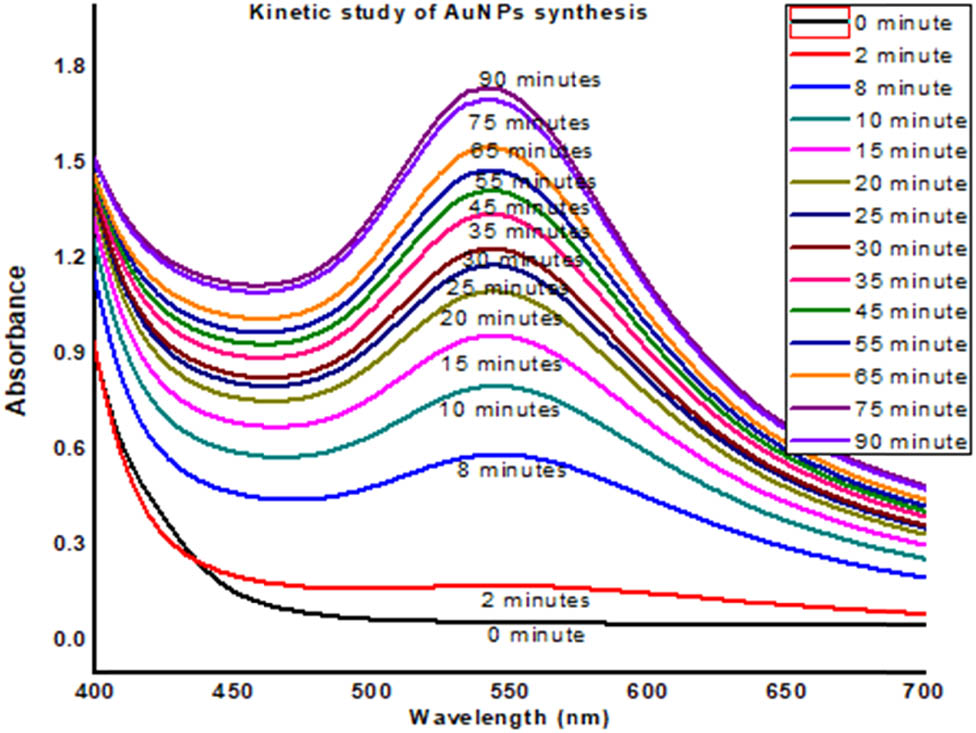
UV-Visible data of kinetic study of AuNPs of saffron stigma (SS-AuNPs).
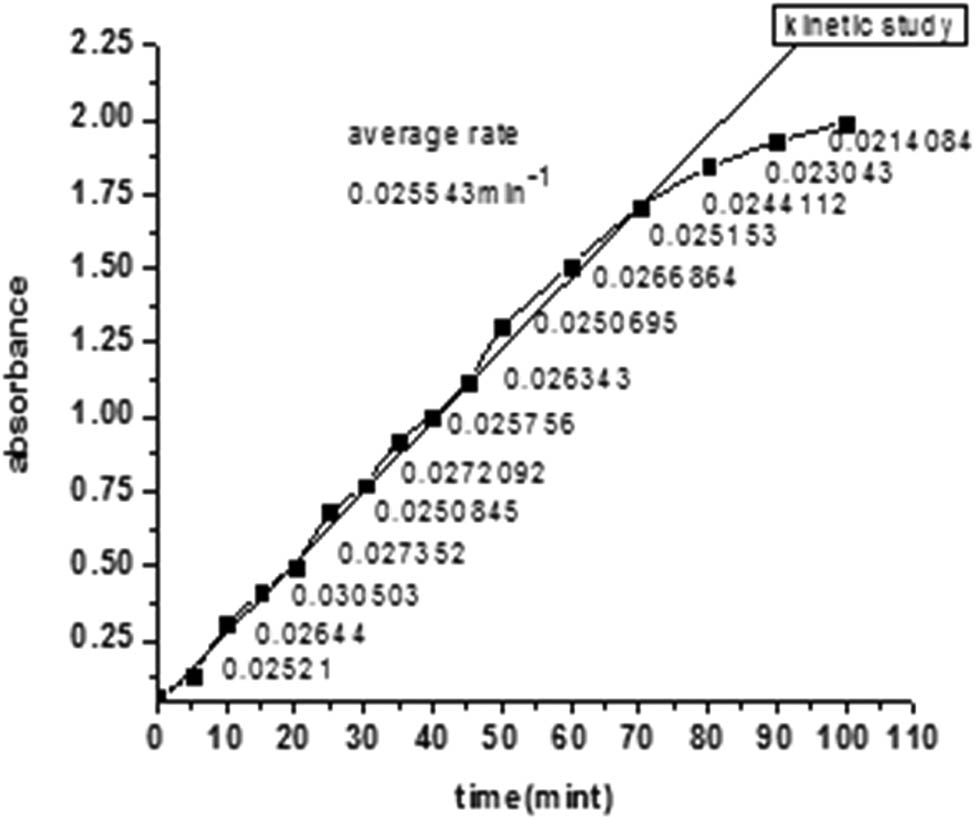
Kinetic study of AuNPs of saffron stigma (SS-AuNPs).
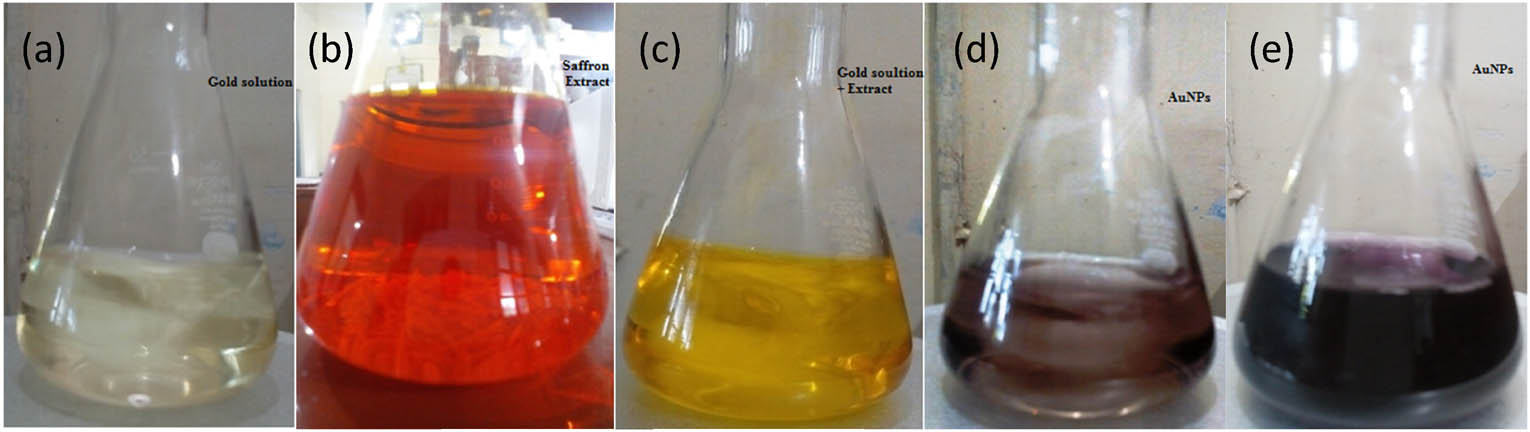
Color change of AuNPs of saffron stigma (SS-AuNPs) formation with time: (a) saffron extract, (b) gold solution, (c) initial mixing of gold solution and extract, (d) after 30 min, and (e) after 1 h.
3.3 Stability of gold nanoparticles
3.3.1 Stability toward pH
To understand the effect of pH on the stability of AuNPs, the pH of AuNPs solution was adjusted between 1 and 14. The solution was kept for 24 h at room temperature, and the effect was obtained by recording UV-Visible spectra. The results showed that the AuNPs were more stable at pH between 3 and 12, while lower stability was observed toward more acidic and basic, i.e., 1–2 and 13–14 (Figure 7). The reason of the instability of AuNPs at lower and higher pH may be due to the removal of stabilizer (plant extract) from the gold surface to destabilize the nanoparticles. Moreover, very low pH caused the re-oxidation of neutral AuNPs [12]. However, maximum stability was noticed at alkaline pH, i.e., 8–10. At the pH range of 3–4, moderate stability of the AuNPs was observed as in this range gold nanoparticles revealed peak broadening [12].
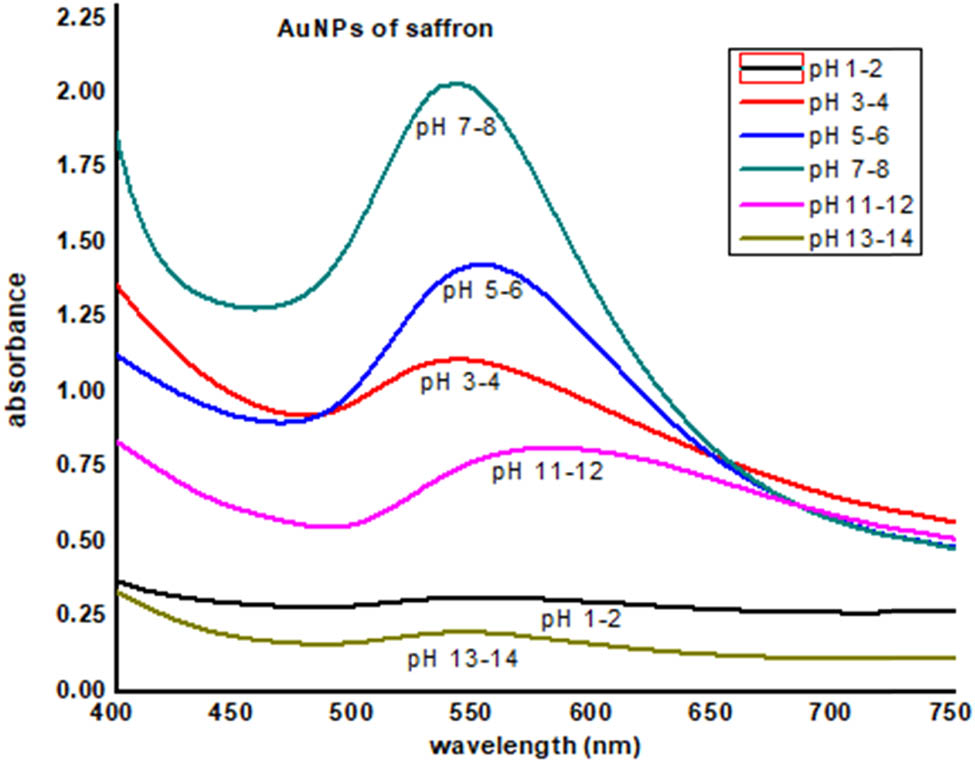
AuNPs of saffron stigma (SS-AuNPs) at different pH values.
3.3.2 Stability toward different salts
To find the stability of AuNPs of saffron in the solution of different salts, 0.1 molar concentration of different salts (NaCl, CaCl2, CuCl2, NiCl2, Hg2Cl2, PbCl2, ZnCl2, and CdCl2) was prepared. Equal volumes of AuNPs were taken in a separate test tube, and 2 mL of each salt solution was added to these test tubes. After 24 h of mixing of the salt solution with AuNPs, UV data were recorded, which show the disruption of these nanoparticles due to salt. This disruption was obvious from their color change and precipitation (Figure 8).
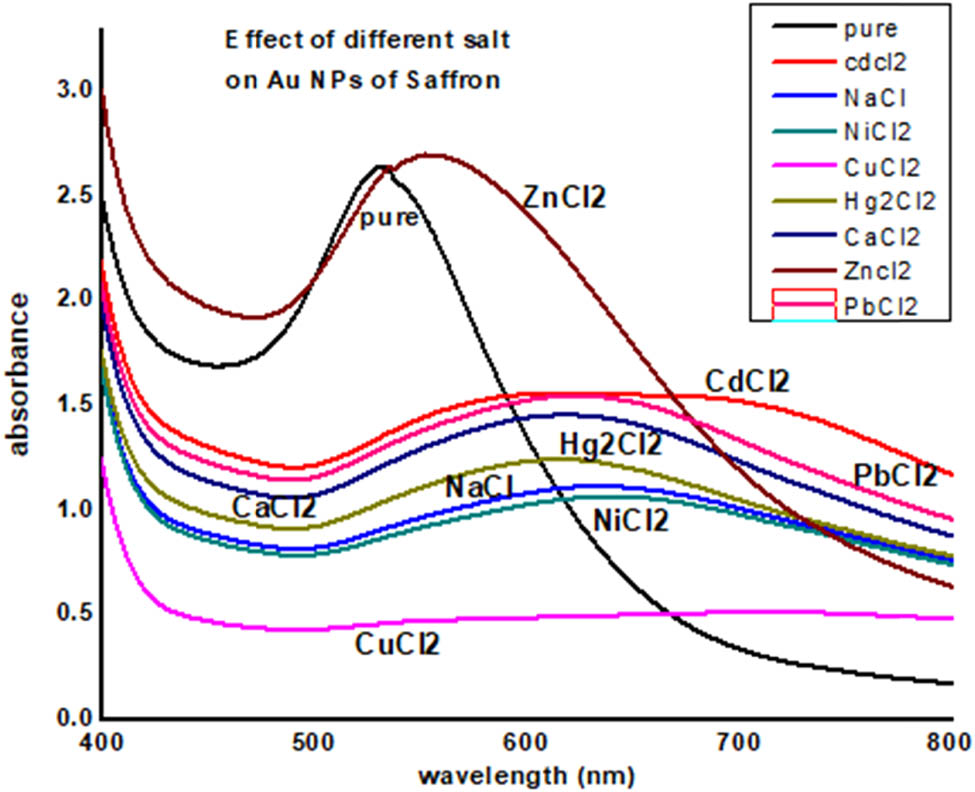
UV-Visible data of effect of different salts on saffron stigma AuNPs (SS-AuNPs).
3.3.3 Stability toward NaCl
The effects of salt (NaCl) on gold nanoparticles of saffron were determined by varying the salt concentration on the synthesized gold nanoparticles. To find the salt effect, 2 mL of the gold nanoparticles were mixed with 1 mL of NaCl solution having a concentration in the range of 0.1–1 M. The intermixed salt and gold nanoparticles solution were kept for 24 h, and then, UV data were recorded. It was observed that by increasing the salt concentration, the gold nanoparticles start precipitating, and the solution become colorless at the high salt concentration (Figures 9 and 10).
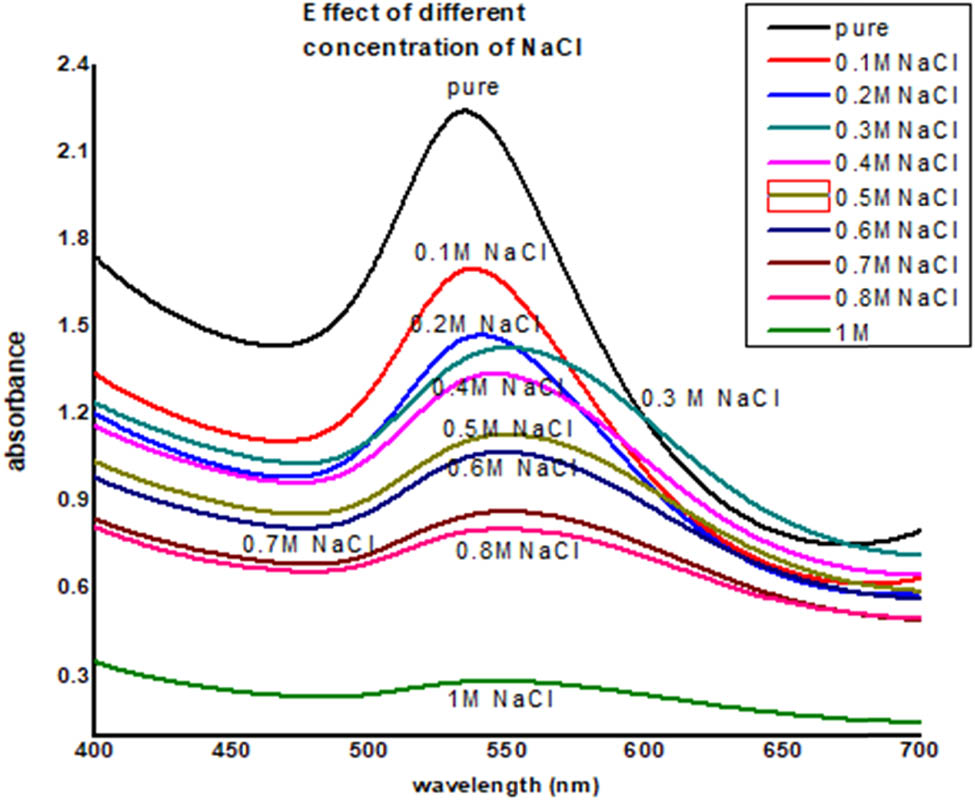
UV-Visible data of effect of NaCl on saffron stigma AuNPs (SS-AuNPs).

Effect of NaCl on saffron stigma AuNPs (SS-AuNPs) (after 24 h).
3.3.4 Stability toward heat
To determine the stability of gold nanoparticles toward heat, the synthesized gold nanoparticles of saffron were heated at different temperature (25°C, 40°C, 60°C, and 80°C) for 30 min. UV data obtained at different temperatures clearly show that the gold nanoparticles destabilized at high temperature, i.e., at 80°C (Figure 11).
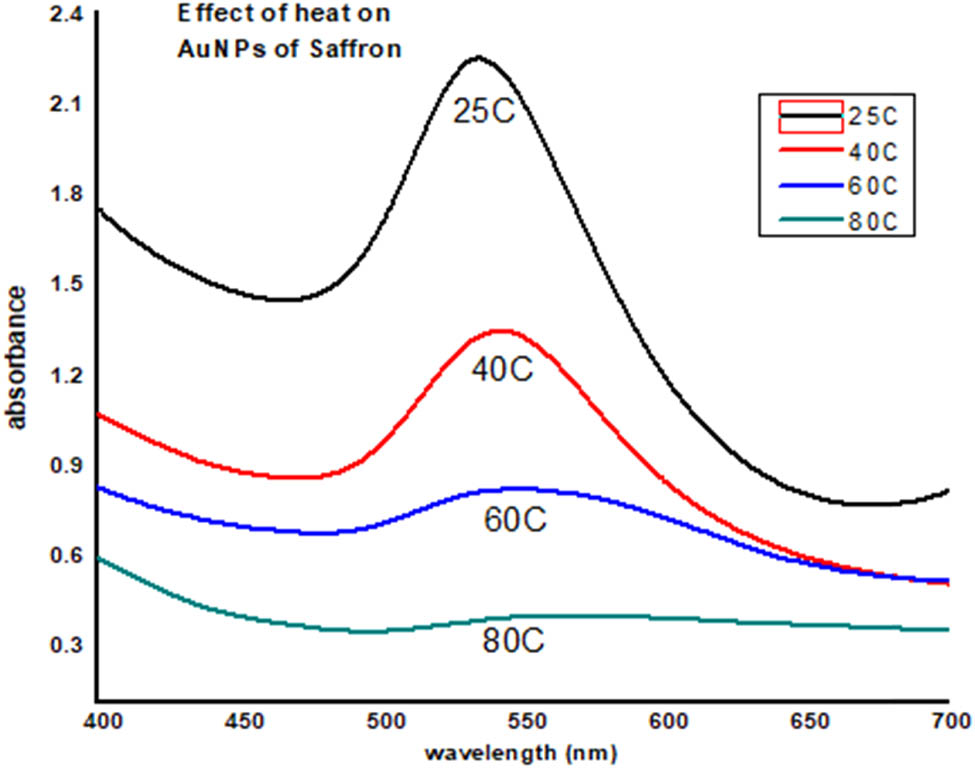
Effect of heat on saffron stigma AuNPs (SS-AuNPs).
3.4 Characterization of gold nanoparticles (SS-AuNPs)
Comparison of FTIR spectra of saffron extract and its biosynthesized gold nanoparticles (SS-AuNPs) showed broadening of certain peaks in the spectrum of nanoparticles. In the FTIR spectra of SS-AuNPs, sharp and broader peak with higher amplitude was noticed at 3,427 cm−1 compared to that in the simple saffron extract (3,462 cm−1), indicating that NH or OH groups present in the saffron extract reduced Au+ ion to Au0 metal and therefore indicating the formation of saffron stigma-based gold nanoparticles SS-AuNPs. Another indication from the figure was the shifting of the peak of the carboxylic ketonic group from 1,776 to 1,664 cm−1 and 1,473 cm−1. Thus, the carboxylate group was involved in the stabilization of gold nanoparticles (Figure 12).
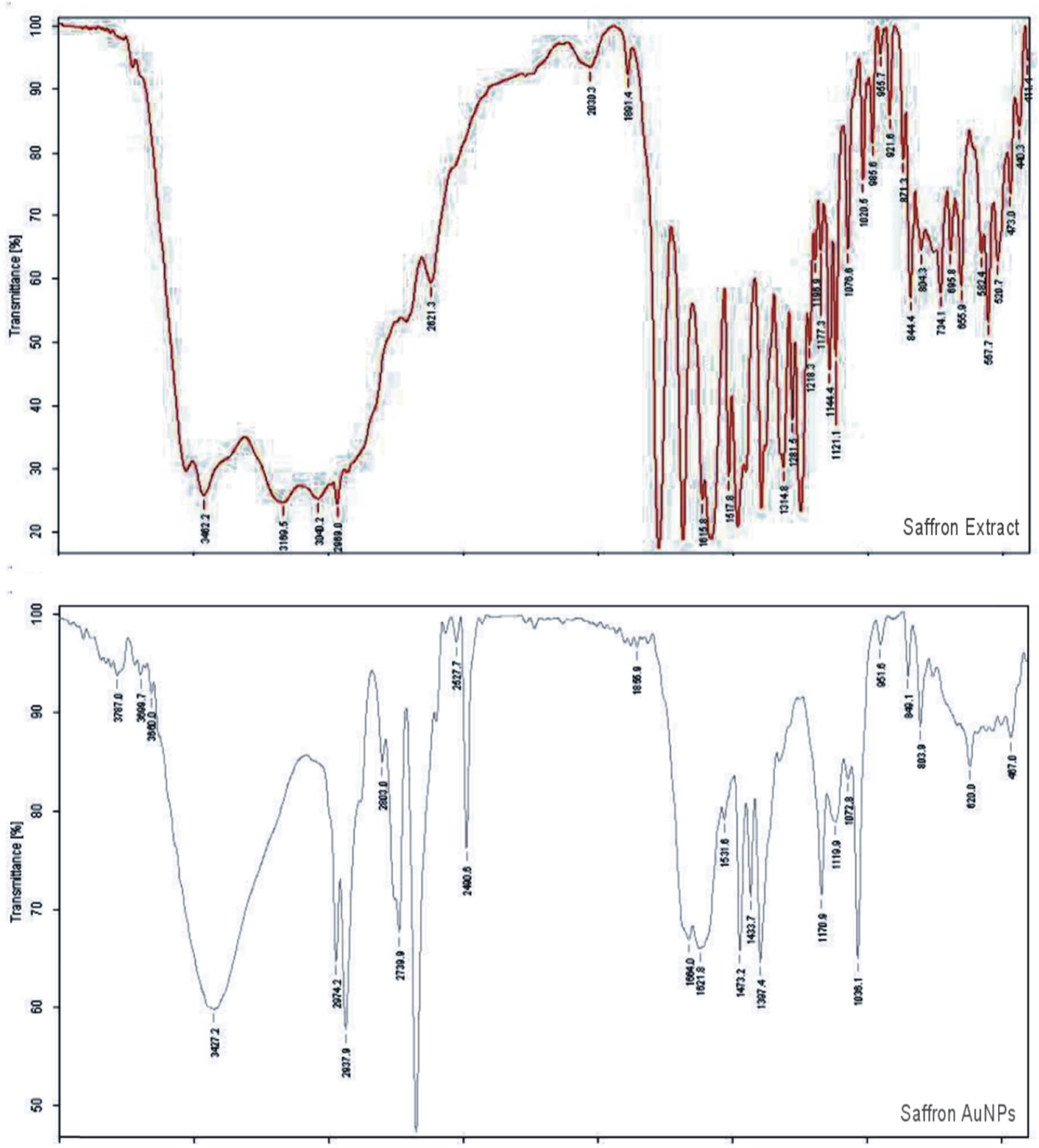
FTIR spectra of saffron extract (upper) and its SS-AuNPs (lower).
Saffron-loaded AuNPs were mostly in the size range of 25–35 nm. Most of the nanoparticles were in spherical shapes; however, a small amount of irregular nanoparticles (nanorods and nanotriangles) was also found (Figure 13). The EDX analysis authenticates the occurrence of the metallic gold (Au) in the synthesized saffron stigma-based AuNPs (SS-AuNPs). In SS-AuNPs, strong signals were observed at 0.5 and 2.3 keV, whereas a weak signal was observed at 9.8 keV. EDX spectra reveal the Si signal due to the use of silicon lattice; however, N signal demonstrated the occurrence of nitrogen-containing organic components in the experimented sample. Furthermore, strong signals for O and C may be owing to the presence of various biomolecules responsible for the capping of AuNPs. Likewise, the presence of Cl in the HAuCl4 molecule was responsible for the chlorine signal in the obtained EDX spectra. In the EDX analysis, the presence of metallic Au supported the reduction of Au3+ (metal cations) to Au0 (elemental form) (Figure 14).
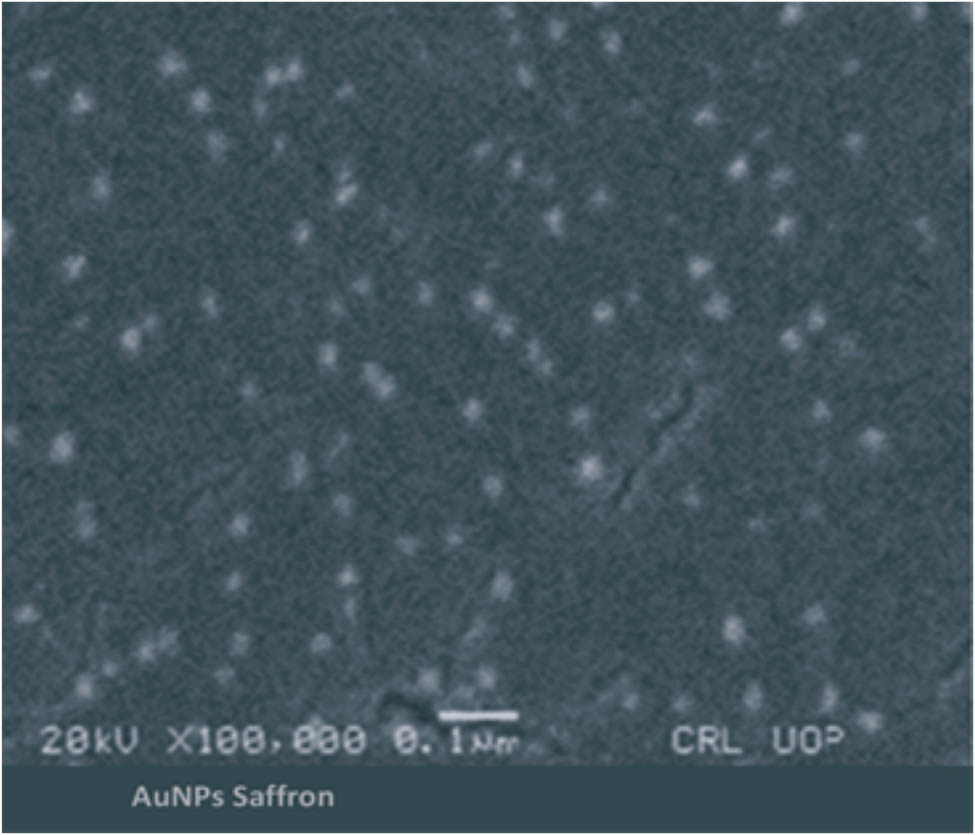
Scanning electron microscopic (SEM) image of saffron-loaded AuNPs (SS-AuNPs).
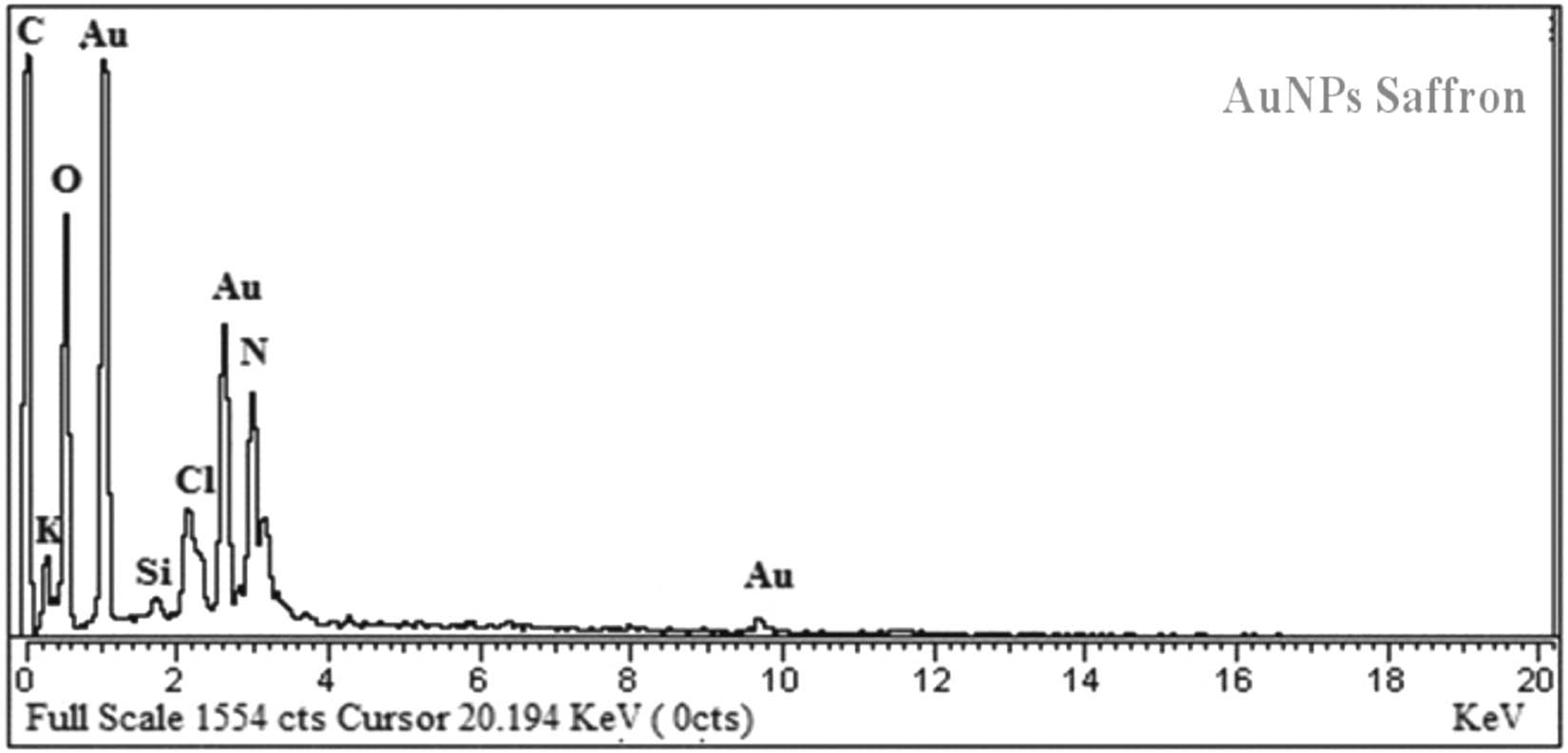
EDX spectra of saffron loaded gold nanoparticles (SS-AuNPs).
The nature of saffron stigma-based gold nanoparticles (SS-AuNPs) was also evaluated through the XRD analysis. The XRD profile of the synthesized nanoparticles revealed that peak positions are consistent with the metallic gold. In the case of gold-containing sample, the typical diffraction peaks of the FCC metallic gold phase were observed at 38.21°, 44.39°, 64.62°, and 77.59°. Like other crystalline phases, no absorption peaks were noticed, highlighting the purity of the products. Among all the peaks of SS-AuNPs, the diffraction peak observed at 38° was relatively intense. The Debye Scherrer equation (D = kλ/β 1/2·cos θ) was used for derivation of mean particle diameters of SS-AuNPs. In this equation, θ, k, β 1/2, and λ are known as peak width at an angle, shape factor, the width of XRD peak at half height, and wavelength, respectively. The average particle size of SS-AuNPS was calculated to be nearly 25 nm (Figure 15). The AFM images showed that the AuNPs of saffron were spherical and oval with a size of about 25 nm (Figure 16). The kinetic study of AuNPs of saffron is shown in Figure 17. The number and the consistency of silver nanoparticles amplified over time. The regression analysis showed R 2 = 0.9891, while the rate of reaction was 2.26 × 10−2 (Figure 18).
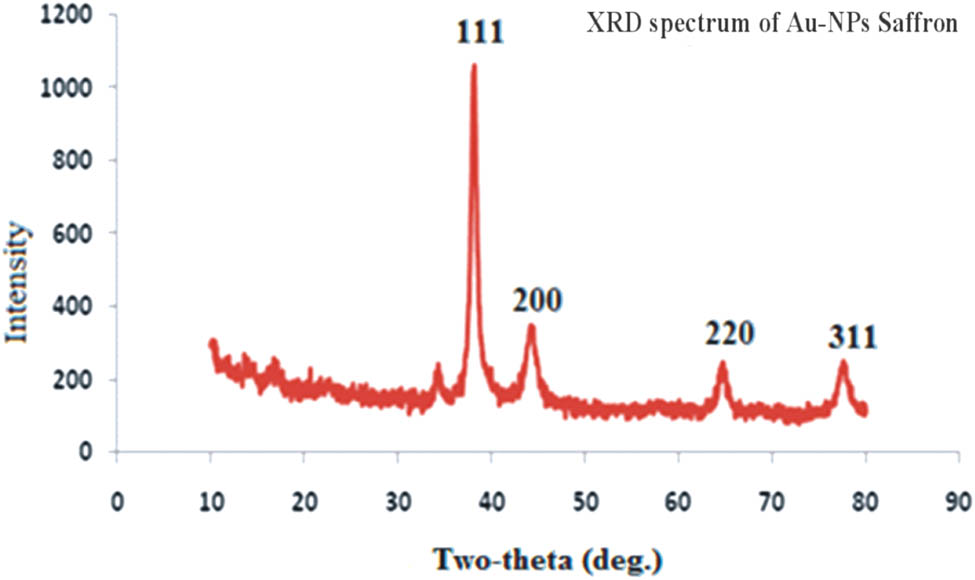
XRD spectra of AuNPs of saffron (SS-AuNPs).
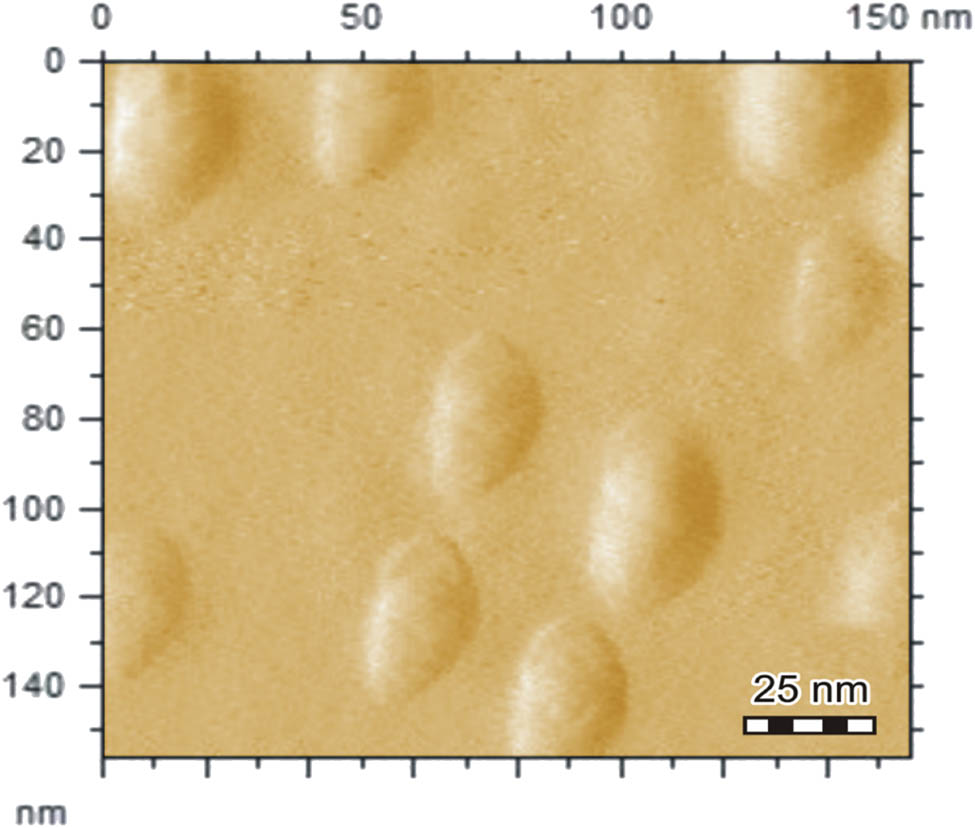
AFM image of AuNPs of saffron (SS-AuNPs).
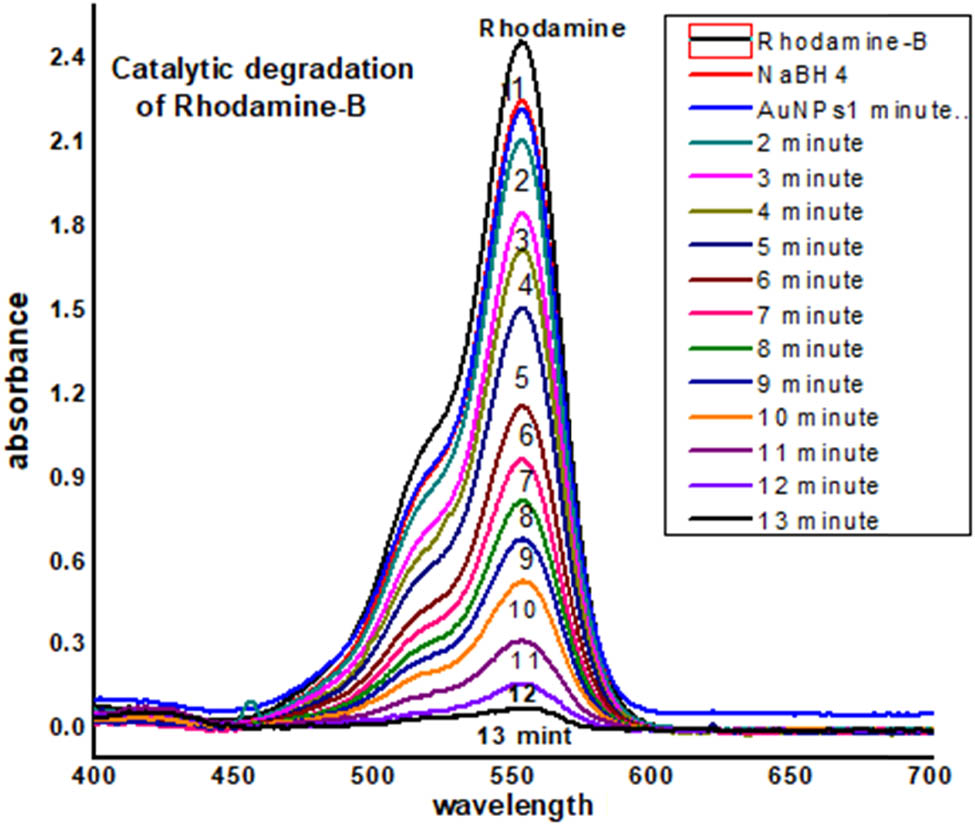
UV-Visible data of catalytic degradation of Rhodamine B.
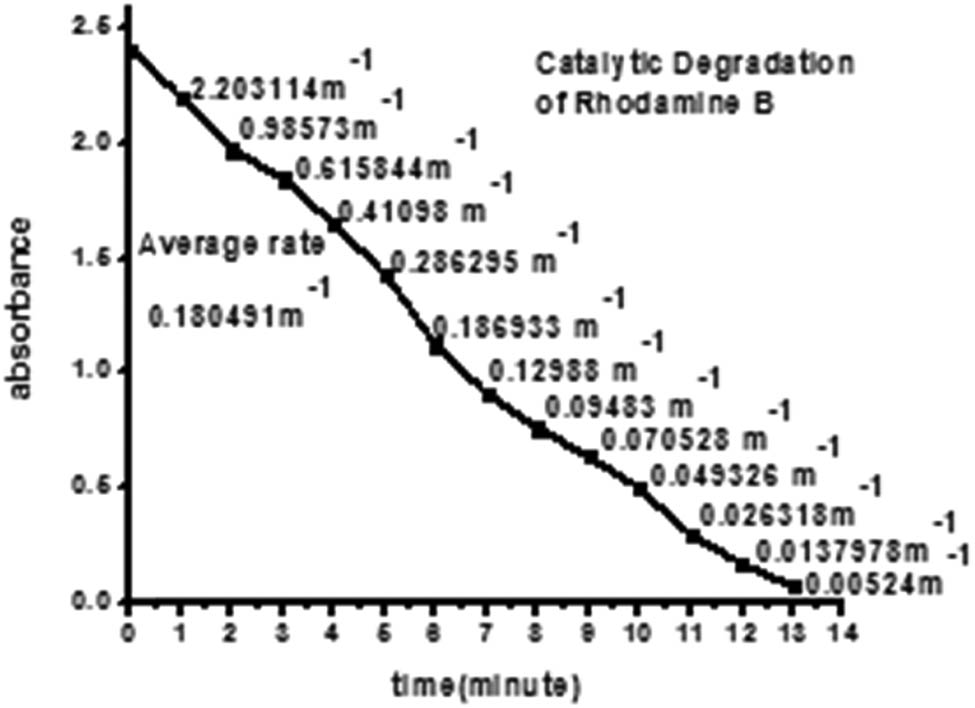
Kinetic study of catalytic degradation of Rhodamine B.
3.5 Antibacterial activities
Saffron extract and synthesized SS-AuNPs were examined against Acinetobacter, Providencia, Streptococcus, and E. coli, respectively. The antibacterial effect of saffron extract and AuNPs of saffron (zone of inhibition in mm) is presented in Table 1. The maximum effect was observed by extract against E. coli (22 mm). The results indicate the mild broad-spectrum antibacterial potential of saffron extract and SS-AuNPs.
Antibacterial effect of saffron extract and AuNPs of saffron (SS-AuNPs) (zone of inhibition in mm)
| Samples | Acinetobacter | Providencia | Streptococcus | Escherichia coli |
|---|---|---|---|---|
| Saffron extract | 10 | 05 | 12 | 22 |
| AuNPs of Saffron (SS-AuNPs) | 08 | 10 | — | 05 |
| Levofloxacin | 30 | 25 | 35 | 22 |
| Norofloxacin | 38 | 27 | 37 | 19 |
| Ciprofloxacin | 50 | 35 | 40 | 19 |
| Amoxicillin | 25 | 16 | 20 | 30 |
3.6 Enzyme inhibition activities
Saffron stabilized the gold nanoparticles, and their extract was screened against enzyme to investigate their enzyme inhibitory property (Tables 2–4). The tested samples (extract and SS-AuNPs) inhibited urease after applying at the same concentration (0.2 µg). The extract demonstrated significant (89.12%) urease inhibitory action compared to AuNPs (54.98%). The IC50 values of the tested samples are presented in Table 2. The CA-II inhibitory potential of the extract was comparatively week than AuNPs in terms of the percent inhibitory effect of 64.29% and 38.74%, respectively, as presented in Table 3. The nonsignificant α-chymotrypsin inhibitory effect was shown in terms of the experimented samples, i.e., saffron extract and SS-AuNPs that are presented in Table 4.
Urease inhibition effect of saffron extract and SS-AuNPs
| Samples/standard | Concentration (mg/mL) | % Inhibition | IC50 (µg/mL) |
|---|---|---|---|
| Saffron extract | 0.2 | 89.12 | 38.16 ± 0.92 |
| AuNPs of saffron (SS-AuNPs) | 0.2 | 54.98 | 177.73 ± 5.20 |
| Standard | 0.2 | 98.21 | 21.24 ± 0.011 |
Carbonic anhydrase-II inhibition effect of saffron extract and SS-AuNPs
| Samples/standard | Concentration (mM) | % Inhibition | IC50 (µg/mL) |
|---|---|---|---|
| Saffron extract | 0.2 | 38.74 | — |
| AuNPs of saffron (SS-AuNPs) | 0.2 | 64.29 | 170.66 ± 1.39 |
| Standard | 0.2 | 89.01 | 0.12 ± 0.03 μM |
α-Chymotrypsin inhibition effect of saffron extract and SS-AuNPs
| Samples/standard | Concentration | % Inhibition | IC50 (µg/mL) |
|---|---|---|---|
| Saffron extract | 0.2 | 22.43 | — |
| AuNPs of saffron (SS-AuNPs) | 0.2 | 26.09 | — |
| Standard | 0.2 | 98.87 | 5.72 ± 0.1 |
3.7 Analgesic effect
The tested samples (saffron extract and SS-AuNPs) inhibited the acetic acid-induced writhing at different experimented doses (Table 5). The maximum analgesic effect was noticed at a higher dose in the case of both saffron extract (100 mg/kg) and SS-AuNPs (10 mg/kg). The percent antihypergesic effect at these higher doses was 66.87% and 84.98%, respectively. Results revealed that SS-AuNPs were more potent than simple extract of saffron.
Analgesic activity of saffron extract and SS-AuNPs
| Treatment | Dose (mg/kg; i.p) | % Inhibition of writhing |
|---|---|---|
| Saline | 10 mL/kg | — |
| Diclofenac sodium | 10 | 83.60 ± 0.64*** |
| Saffron extract | 15 | 32.98 ± 1.00 |
| 25 | 39.70 ± 2.01 | |
| 50 | 51.98 ± 2.45** | |
| 100 | 66.98 ± 2.99** | |
| SS-AuNPs | 2.5 | 64.87 ± 3.01** |
| 5 | 76.23 ± 3.29*** | |
| 10 | 84.98 ± 3.80*** |
**p < 0.01; ***p < 0.001.
3.8 Sedative effect
The sedative potential of saffron extract and SS-AuNPs at different doses is presented in Table 6. The saffron extract demonstrated a significant (P < 0.001) effect at lowest dose (15 mg/kg) compared to examined higher doses (100 mg/kg). The SS-AuNPs also significantly (P < 0.01) hindered the movement of animals at all tested doses as presented in Table 6.
Sedative activity of saffron extract and SS-AuNPs
| Treatment | Dose (mg/kg; i.p) | Number of lines crossed in 10 min |
|---|---|---|
| Saline | 10 mL/kg | 145 ± 3.44 |
| Diclofenac sodium | 0.5 | 8.60 ± 0.64 |
| Saffron extract | 15 | 5.45 ± 0.03*** |
| 25 | 115.55 ± 3.21* | |
| 50 | 110.76 ± 3.88* | |
| 100 | 104.42 ± 3.66* | |
| SS-AuNPs | 2.5 | 80.62 ± 3.01** |
| 5 | 65.67 ± 3.14*** | |
| 10 | 50.98 ± 3.99*** |
*p < 0.05; **p < 0.01; ***p < 0.001.
3.9 Antiinflammatory effect
Both tested samples, i.e., saffron extract and SS-AuNPs, showed the significant antiinflammatory effect. The saffron extract demonstrated 71.22% antiinflammatory effect at the dose of 100 mg/kg, and similarly, the SS-AuNPs showed a high antiinflammatory effect (88.98%) at the dose of 10 mg/kg. The result of antiinflammatory effects at higher doses is depicted in Figure 19.
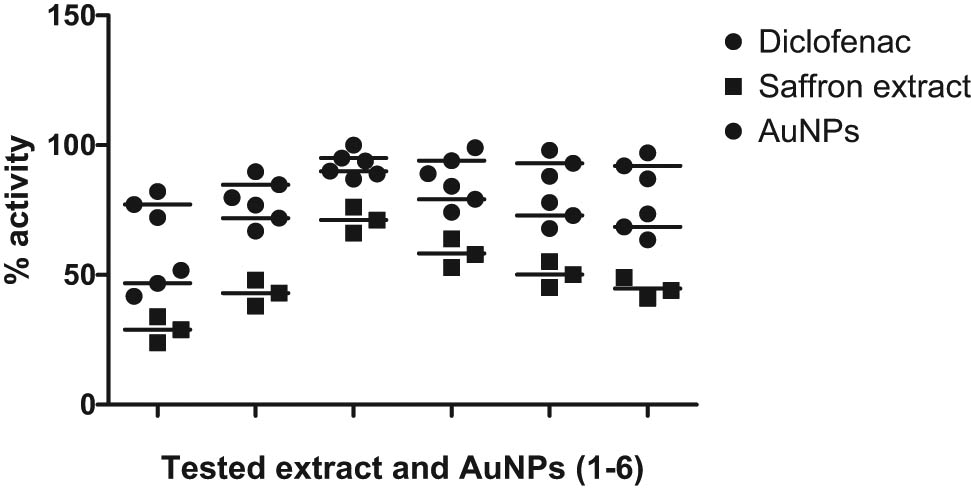
Antiinflammatory activity of extract and AuNPs of Crocus sativus (extract 100 mg/kg; AuNPs 10 mg/kg) on carrageenan paw edema on mice. All values were represented as ±SEM for groups of six animals. The data were analyzed by ANOVA followed by Dunnett’s test.
3.10 Acute toxicity
Results of the acute toxicity study have revealed that both the experimented samples, i.e., saffron extract and SS-AuNPs, proved to be free of mortality and any other unwanted effect as presented in Table 7.
Toxicological study of saffron extract and AuNPs
| Treatment | Dose | Number of died animals/5 | % Mortality | Gross behaviors changes |
|---|---|---|---|---|
| Normal saline | 10 mL/kg | 0/5 | — | — |
| Saffron extract | 100 | 0/5 | — | — |
| 250 | 0/5 | — | — | |
| 500 | 0/5 | — | — | |
| 1,000 | 0/5 | — | — | |
| 10 | 0/5 | — | — | |
| SS-AuNPs | 25 | 0/5 | — | — |
| 50 | 0/5 | — | — | |
| 100 | 0/5 | — | — |
4 Discussion
Medicinal plant-based remedies have a key role in the treatment of various pathological and nonpathological disorders. Synthesis, characterization, and applications of plant-based nanoparticles are novel and trending concepts in sciences and technology [33]. The utilization of plant materials for the biosynthesis of nanoparticles possesses more advantages as it requires less elaborative processes [34]. Gold nanoparticles are being employed in the field of biomedicine, packaging, cosmetics, and electronics efficiently [35]. Extracts from various parts of plants like seed, fruit, and leaf are being significantly used for the synthesis of gold-/silver-based nanoparticles [36]. Keeping in view the importance of plant-based gold nanoparticles, this study was designed to examine the effectiveness of alcoholic saffron stigma extracts and its gold nanoparticles (SS-AuNPs) in different in vitro and in vivo biological assays. The synthesis of gold nanoparticles due to the reduction of gold ions during the reaction with saffron stigma extract was determined by a color change and UV-Vis spectra. UV-Vis spectroscopy is considered one of the most significant analytical tools to determine the stability of the synthesized nanoparticles in the aqueous solution [37]. UV-Vis spectrum revealed that the absorption peak sharpness depends on the volume ratio of the extract as maximum sharpness of peak was noticed for 5:1 ratio. This reaction mixture ratio was further used for the preparation of the bulk solution due to its effectiveness in the formation of gold nanoparticles. The particle size of biosynthesized nanoparticles was in the range of 25–35 nm and 25 nm as shown by SEM and AFM images, respectively. Similar to our study, Sadeghi et al. [38] revealed uniform distribution of gold nanoparticles prepared using the stevia leaf extract, showing the spherical shape of NPs having a particle size between 21 and 45 nm. UV-Vis spectra of all the other experimented ratios (1:1, 10:1, 20:1, 30:1, 40:1, 50:1, and 60:1) of saffron-based gold nanoparticles exhibited broad peaks and lower intensities at the wavelength of 540 nm (plasmon resonance of gold nanoparticles). Aggregation of nanoparticles and/or production of large anisotropic particles may be the reason behind this. Surface plasmon resonance absorbance is quite sensitive to the shape, nature, size, and interparticle distance among formed particles [39]. Particle size and gold nanoparticles content may be ascertained via the UV-Vis spectroscopy [31]. The results of the XRD analysis conducted in this study are in agreement with the results of red algae (Gelidium amansii)-based AuNanocrystals synthesized in the study by Kumar et al. [40]. Similar results of the XRD profile for the synthesized stevia leaf-based nanoparticles were documented by Sadeghi et al. [38]. In this study, energy-dispersive X-ray spectroscopy was conducted to evaluate the elemental composition of the prepared gold nanoparticles. The results of the current study were in accordance with the earlier findings of Abu-Tahon et al. [20]. They also reported a strong peak at nearly 2.2 keV that is typically designated as absorption of metallic AuNPs.
4.1 EDX analysis
The presence of metallic gold Au in saffron-loaded AuNPs was confirmed from the EDX studies. Strong signals were observed from Au atoms in AuNPs at approximately 0.5 and 2.3 keV, while a weak Au signal was observed at 9.8 keV. The appearance of the Si signal corresponded to the use of a silicon lattice in the EDX study, while the signal for N shows the presence of nitrogen-containing organic compounds in the biopolymer. Moreover, the other strong signals for C and O were due to the presence of different organic molecules capping the AuNPs. The appearance of the Cl signal in the EDX spectra of gold nanoparticles was due to the presence of chlorine in the tetra chloro auric acid trihydrate (HAuCl4) molecule. The appearance of the elemental Au in the EDX analysis supports the reduction of metal cations (Au3+) to elemental form (Au0).
The FTIR analysis was conducted to study the possible biomolecules present in saffron stigma extracts, resulting in the capping and effective stabilization of saffron stigma-based gold nanoparticles. Absorption bands observed at 3,462, 2,969, 1,615, and 1,281 cm−1 correspond to –OH/NH2, –CH2, C═C, and C–O stretching, respectively. The FT-IR spectrum of saffron stigma-based nanoparticles showed a shift in –OH stretching in band from 3,462 to 3,427 cm−1, –CH2 band from 2,969 to 2,974 cm−1, and from 1,615 to 1,621 cm−1 (C═O). Another indication from the figure was the shifting of the peak of the carboxylic ketonic group from 1,776 to 1,664 cm−1 and 1,473 cm−1. Thus, the carboxylate group was involved in the stabilization of gold nanoparticles. Results indicated that the synthesized gold nanoparticles using saffron stigma extract are surrounded by metabolites having functional groups of alcohols, carboxylic acid, amines, aldehydes, and ketones. Polyphenols present in the saffron extract are responsible for the reduction and stabilization of the biosynthesized gold nanoparticles [41,42]. Hydroxyl and NH-containing proteins and/or carbohydrates are vital for the synthesis and the capping of the prepared nanoparticles. Furthermore, the band observed in the region 2,969 cm−1 corresponds to CH stretching of aromatic compounds [20].
Stability studies of plant-based nanoparticles at different conditions like varied NaCl (sodium chloride) concentrations, pH, and temperature range are vital for assessing the environmental implications and potent human health risks [43]. The stability of produced nanoparticles could be evaluated by UV-Vis spectroscopy as precipitation, decomposition, and aggregation results characteristics changes in absorption of nanoparticles in the UV-Vis region (UV-Vis spectra). Synthesized nanoparticles were examined at different pH values ranging from 1 to 14. It was noticed that the synthesized nanoparticles were most stable at pH 7–8 followed by pH 5–6, pH 3–4, and pH 11–12. The least stability was observed in a medium having pH 1–2 and pH 13–14. Furthermore, at 0.1 M NaCl concentration, maximum absorbance was revealed, and the least was observed in the case of 1 M NaCl. Extreme peak broadening was noticed in the case of 0.7, 0.8, and 1 M NaCl, indicating the aggregation of SS-AuNPs. Exposure to varied pH values and salt concentrations results in surface and structural changes of nanoparticles to aid drug delivery at the targeted site [44]. Hence, compositional features of gold nanoparticles play a vital role in the stability of synthesized nanoparticles. Likewise, the temperature is another main environmental factor influencing the activity, chemical characteristics, and stability of nanoparticles [31]. The impact of different temperatures (25°C, 40°C, 60°C, and 80°C) on the synthesized SS-AuNPs was examined by heating nanoparticle solutions for 30 min. A decrease in absorption revealed that elevation in the temperature resulted in the aggregation of SS-AuNPs. In any ecosystem, the toxicity, bioavailability, and mobility were determined by the overall stability of nanoparticles [45]. Therefore, the synthesis of stable gold nanoparticles is important as it decreases ion dissolution and helps in retaining its physiochemical properties. The results of this study will be helpful in optimizing the conditions for effective use of saffron stigma-based nanoparticles in different therapies.
Nowadays, scientists are keen and focused in developing safe, efficient, and environment-friendly techniques for the formulation of plant-based therapeutic products [46]. Hence, silver and gold nanoparticles are most commonly being used to biosynthesize stable plant extract-based nanoparticles having enhanced medicinal properties [47]. In this study, saffron stigma-based gold nanoparticles were assessed for their antibacterial and enzyme inhibitory properties. Saffron extract and synthesized SS-AuNPs were examined against Acinetobacter, Providencia, Streptococcus, and E. coli. The highest antibacterial effect was observed by the saffron extract against E. coli (22 mm). The results indicate the mild broad-spectrum antibacterial potential of saffron extract and SS-AuNPs. Saffron extract demonstrated the significant (89.12%) urease inhibitory action compared to SS-AuNPs (54.98%). However, the CA-II inhibitory potential of the saffron extract was comparatively week than SS-AuNPs in terms of the percent inhibitory effect, i.e., 38.74% and 64.29%, respectively. Nonsignificant α-chymotrypsin inhibitory effect was shown in terms of experimented samples, i.e., saffron extract and SS-AuNPs. The gold nanoparticles surrounded by a number of drug moieties now act as a single group against the microbial organisms, thereby increasing the microbial activity [48].
The literature review has highlighted the role of the saffron extract against different diseased experimental subjects owing to its rich polyphenolic content. The acetic acid-induced writhing test was used to assess the antinociceptive properties of any medicinal agents. In this study, saffron extract and synthesized SS-AuNPs inhibited the acetic acid-induced writhing at different experimented doses. The maximum analgesic effect was noticed at a higher dose in the case of both saffron extract (100 mg/kg) and SS-AuNPs (10 mg/kg). The percent antihypergesic effect at these higher doses was 66.87% and 84.98%, respectively. The results revealed that SS-AuNPs were more potent than simple extract of saffron. Similarly, saffron extract demonstrated a significant (P < 0.001) sedative effect at the lowest dose (15 mg/kg) compared to examined higher doses (100 mg/kg). The SS-AuNPs also significantly (P < 0.01) hindered the movement of animals at all tested doses. Both of the tested samples, i.e., saffron extract and SS-AuNPs, resulted in the significant anti-inflammatory effect. The saffron extract demonstrated 71.22% antiinflammatory at the dose of 100 mg/kg, and similarly, the SS-AuNPs also showed a high antiinflammatory effect (88.98%) at the doses of 10 mg/kg, respectively. The results of the acute toxicity study have revealed that both the experimented samples, i.e., saffron extract and SS-AuNPs, proved to be free of mortality and any other unwanted effect. The painkiller and antiinflammatory properties of saffron are well known, and safranal is one of the chemical constitutes responsible for its analgesic effect [26]. This constituent has been reported with a significant analgesic effect [49]. It is further recommended to test the nanoparticles of safranal for the said pharmacological actions. According to the published reports, the saffron is a prostaglandin (PG) blocker, which is a pain, inflammatory, and pyrexia mediator. In addition to the PG blocker, the saffron is considered to be opioids receptor blocker [49]. This finding supports the use of saffron in the treatment of various inflammatory painful conditions. The nanoparticles of saffron with analgesic and antiinflammatory effect are reported first time here. These AuNPs are recommended to further process for mechanistic studies and dosage form development. Saffron is the most commonly used for neurological disorders such as antidepressant, anxiolytic, muscle relaxant, and anticonvulsant [50]. However, we find the significant sedative effect of the saffron extract and SS-AuNPs. This sedative affect is adjuvant for the said effects especially anxiolytic and anticonvulsant. The sedative effect of our tested samples might be attributed to the release in various neurotransmitters. The interactions of extract and AuNPs with GABA receptors resulted in the anxiolytic, muscle relaxant, sedative, and anticonvulsants effects, while the inhibition of serotonin and other bioamines causes antidepressant effects. The application of nanotechnology modifies the chemical and behavioral properties of a substance by enhancing the biological activities of plant extract, promoting the release of bioactive metabolite, and decreasing the side effects [51]. As compared to conventional drug delivery systems, nanotechnology helps in promoting the synthesis of more stable, bioavailable, efficient, and nontoxic plant-based medicinal drugs [52].
5 Conclusion
The biosynthesis of plant-based nanoparticles is gaining importance in the field of nanotechnology as they are considered safe, effective, and ecofriendly. In this study, saffron stigma-based gold nanoparticles were synthesized and evaluated for their biological effectiveness through various in vitro and in vivo assays. These biosynthesized gold nanoparticles were found to have maximum antibacterial activity against E. coli. Furthermore, the results of this study conclude that the prepared saffron stigma-based nanoparticles possessed analgesic, antiinflammatory, and sedative properties.
Acknowledgment
The authors acknowledge Qassim University, Saudi Arabia for providing the instruments facilities, chemicals, and other items used in the study.
-
Funding information: Authors would like to thank the Higher Education Commission of Pakistan for funding this work under Research project No: 7343/KPK/NRPU/R&D/HEC/2017.
-
Author contributions: Fahad A. Alhumaydhi, Abdur Rauf, and Muhammad Nasimullah Qureshi: writing – original draft, writing; Abdullah S. M. Aljohani and Mohamed A El-Esawi: writing – review and editing; Ibrahim Khan: methodology; Shahid Ali Khan and Anees Ahmed Khalil: visualization, project administration; Naveed Muhammad: resources. All authors read and approved the manuscript for submission.
-
Conflict of interest: The corresponding author (Abdur Rauf) is a member of the Editorial Board of Green Processing and Synthesis.
-
Data availability statement: The data associated to this article is given in text.
References
[1] Kasthuri J, Kathiravan K, Rajendiran N. Phyllanthin-assisted biosynthesis of silver and gold nanoparticles: a novel biological approach. J Nanopart Res. 2009;11(5):1075–85.10.1007/s11051-008-9494-9Suche in Google Scholar
[2] Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55(3):329–47.10.1016/S0169-409X(02)00228-4Suche in Google Scholar
[3] Ma J, Wong H, Kong LB, Peng KW. Biomimetic processing of nanocrystallite bioactive apatite coating on titanium. Nanotechnology. 2003;14(6):619.10.1088/0957-4484/14/6/310Suche in Google Scholar
[4] O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209(2):171–6.10.1016/j.canlet.2004.02.004Suche in Google Scholar PubMed
[5] Aslan K, Pérez-Luna VH. Quenched emission of fluorescence by ligand functionalized gold nanoparticles. J Fluoresc. 2004;14(4):401–5.10.1023/B:JOFL.0000031821.74706.eaSuche in Google Scholar
[6] Zhong W. Nanomaterials in fluorescence-based biosensing. Anal Bioanal Chem. 2009;394(1):47–59.10.1007/s00216-009-2643-xSuche in Google Scholar PubMed
[7] Narayanan KB, Sakthivel N. Heterogeneous catalytic reduction of anthropogenic pollutant, 4-nitrophenol by silver-bionanocomposite using Cylindrocladium floridanum. Bioresour Technol. 2011;102(22):10737–40.10.1016/j.biortech.2011.08.103Suche in Google Scholar PubMed
[8] Rivière C, Boudghène FP, Gazeau F, Roger J, Pons JN, Laissy JP, et al. Iron oxide nanoparticle–labeled rat smooth muscle cells: cardiac MR imaging for cell graft monitoring and quantitation. Radiology. 2005;235(3):959–67.10.1148/radiol.2353032057Suche in Google Scholar PubMed
[9] Pastoriza-Santos I, Liz-Marzán LM. Formation of PVP-protected metal nanoparticles in DMF. Langmuir. 2002;18(7):2888–94.10.1021/la015578gSuche in Google Scholar
[10] Taleb A, Petit C, Pileni MP. Synthesis of highly monodisperse silver nanoparticles from AOT reverse micelles: a way to 2D and 3D self-organization. Chem Mater. 1997;9(4):950–9.10.1021/cm960513ySuche in Google Scholar
[11] Andreescu D, Eastman C, Balantrapu K, Goia DV. A simple route for manufacturing highly dispersed silver nanoparticles. J Mater Res. 2007;22(9):2488–96.10.1557/jmr.2007.0308Suche in Google Scholar
[12] Rodriguez-Sanchez L, Blanco MC, Lopez-Quintela MA. Electrochemical synthesis of silver nanoparticles. J Phys Chem B. 2000;104(41):9683–8.10.1021/jp001761rSuche in Google Scholar
[13] Saifuddin N, Wong CW, Yasumira AA. Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. J Chem. 2009;6(1):61–70.10.1155/2009/734264Suche in Google Scholar
[14] Bhainsa KC, D’souza SF. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf B. 2006;47(2):160–4.10.1016/j.colsurfb.2005.11.026Suche in Google Scholar PubMed
[15] Salam HA, Rajiv P, Kamaraj M, Jagadeeswaran P, Gunalan S, Sivaraj R. Plants: green route for nanoparticle synthesis. Int Res. J Biol Sci. 2012;1(5):85–90.Suche in Google Scholar
[16] Ahamed M, Khan MM, Siddiqui MK, AlSalhi MS, Alrokayan SA. Green synthesis, characterization and evaluation of biocompatibility of silver nanoparticles. Phys E Low Dimens Syst Nanostruct. 2011;43(6):1266–71.10.1016/j.physe.2011.02.014Suche in Google Scholar
[17] Sathishkumar G, Gobinath C, Karpagam K, Hemamalini V, Premkumar K, Sivaramakrishnan S. Phyto-synthesis of silver nanoscale particles using Morinda citrifolia L. and its inhibitory activity against human pathogens. Colloids Surf B. 2012;95:235–40.10.1016/j.colsurfb.2012.03.001Suche in Google Scholar PubMed
[18] Dauthal P, Mukhopadhyay M. Biosynthesis of palladium nanoparticles using Delonix regia leaf extract and its catalytic activity for nitro-aromatics hydrogenation. Ind Eng Chem Res. 2013;52(51):18131–9.10.1021/ie403410zSuche in Google Scholar
[19] Gnanavel V, Palanichamy V, Roopan SM. Biosynthesis and characterization of copper oxide nanoparticles and its anticancer activity on human colon cancer cell lines (HCT-116). J Photochem Photobiol B Biol. 2017;171:133–8.10.1016/j.jphotobiol.2017.05.001Suche in Google Scholar PubMed
[20] Abu-Tahon MA, Ghareib M, Abdallah WE. Environmentally benign rapid biosynthesis of extracellular gold nanoparticles using Aspergillus flavus and their cytotoxic and catalytic activities. Process Biochem. 2020;95:1.10.1016/j.procbio.2020.04.015Suche in Google Scholar
[21] Hemalatha K, Madhumitha G, Kajbafvala A, Anupama N, Sompalle R, Mohana, et al. Function of nanocatalyst in chemistry of organic compounds revolution: an overview. J Nanomate. 2013;2013:1–23.10.1155/2013/341015Suche in Google Scholar
[22] Devipriya D, Roopan SM. Cissus quadrangularis mediated ecofriendly synthesis of copper oxide nanoparticles and its antifungal studies against Aspergillus niger, Aspergillus flavus. Mater Sci Eng C. 2017 Nov 1;80:38–44.10.1016/j.msec.2017.05.130Suche in Google Scholar PubMed
[23] Saeidnia S. Future position of Crocus sativus as a valuable medicinal herb in phytotherapy. Pharmacogn J. 2012;27(4):71.10.5530/pj.2012.27.12Suche in Google Scholar
[24] Wani BA, Hamza AK, Mohiddin FA. Saffron: A repository of medicinal properties. J Med Plant Res. 2011;5(11):2131–5.Suche in Google Scholar
[25] Sarris J. Herbal medicines in the treatment of psychiatric disorders: 10-year updated review. Phytother Res. 2018;32(7):1147–62.10.1002/ptr.6055Suche in Google Scholar PubMed
[26] Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2(1):7.10.1186/1471-2210-2-7Suche in Google Scholar PubMed PubMed Central
[27] Gohari AR, Saeidnia S, Mahmoodabadi MK. An overview on saffron, phytochemicals, and medicinal properties. Pharmacogn Rev. 2013;7(13):61.10.4103/0973-7847.112850Suche in Google Scholar PubMed PubMed Central
[28] Rauf A, Abu-Izneid T, Rashid U, Alhumaydhi FA, Bawazeer S, Khalil AA, et al. Anti-inflammatory, antibacterial, toxicological profile, and in silico studies of dimeric naphthoquinones from diospyros lotus. BioMed Res Int. 2020;27:2020.10.1155/2020/7942549Suche in Google Scholar
[29] Lee SK, Mbwambo ZH, Chung H, Luyengi L, Gamez EJ, Mehta RG, et al. Evaluation of the antioxidant potential of natural products. Comb Chem High Throughput Screen. 1998;1(1):35–46.10.2174/138620730101220118151526Suche in Google Scholar
[30] Arslan O. Inhibition of bovine carbonic anhydrase by new sulfonamide compounds. Biochem (Mosc). 2001;66(9):982–3.10.1023/A:1012365424900Suche in Google Scholar
[31] Islam NU, Jalil K, Shahid M, Rauf A, Muhammad N, Khan A, et al. Green synthesis and biological activities of gold nanoparticles functionalized with Salix alba. Arab J Chem. 2019;12(8):2914–25.10.1016/j.arabjc.2015.06.025Suche in Google Scholar
[32] Bawazeer S, Rauf A, Naz S, Khalil AA, Mabkhot YN, Asayari A, et al. In vivo anti-nociceptive potential and cyclooxygenases 1 and 2 selectivity of di-naphthodiospyrols from Diospyros lotus. Revista Brasileira de. Farmacognosia. 2020;21:1–5.10.1007/s43450-020-00057-xSuche in Google Scholar
[33] Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26(18):3995–4021.10.1016/j.biomaterials.2004.10.012Suche in Google Scholar PubMed
[34] Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng. 2009;32(1):79.10.1007/s00449-008-0224-6Suche in Google Scholar PubMed
[35] Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104(1):293–346.10.1021/cr030698+Suche in Google Scholar PubMed
[36] Kumar V, Yadav SK. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol Int Res Process Environ Clean Technol. 2009;84(2):151–7.10.1002/jctb.2023Suche in Google Scholar
[37] Kasthuri J, Veerapandian S, Rajendiran N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf B. 2009;68(1):55–60.10.1016/j.colsurfb.2008.09.021Suche in Google Scholar PubMed
[38] Sadeghi B, Mohammadzadeh M, Babakhani B. Green synthesis of gold nanoparticles using Stevia rebaudiana leaf extracts: characterization and their stability. J Photochem Photobiol B Biol. 2015;148:101–6.10.1016/j.jphotobiol.2015.03.025Suche in Google Scholar PubMed
[39] Sun Y, Xia Y. Increased sensitivity of surface plasmon resonance of gold nanoshells compared to that of gold solid colloids in response to environmental changes. Anal Chem. 2002;74(20):5297–305.10.1021/ac0258352Suche in Google Scholar PubMed
[40] Kumar PS, MubarakAli D, Saratale RG, Saratale GD, Pugazhendhi A, Gopalakrishnan K, et al. Synthesis of nano-cuboidal gold particles for effective antimicrobial property against clinical human pathogens. Microb Pathogen. 2017;113:68–73.10.1016/j.micpath.2017.10.032Suche in Google Scholar PubMed
[41] Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13(10):2638–50.10.1039/c1gc15386bSuche in Google Scholar
[42] Mittal AK, Chisti Y, Banerjee UC. Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. 2013;31(2):346–56.10.1016/j.biotechadv.2013.01.003Suche in Google Scholar PubMed
[43] Levard C, Hotze EM, Lowry GV, Brown GE Jr. Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ Sci Technol. 2012;46(13):6900–14.10.1021/es2037405Suche in Google Scholar PubMed
[44] Gao W, Chan JM, Farokhzad OC. pH-responsive nanoparticles for drug delivery. Mol Pharm. 2010;7(6):1913–20.10.1021/mp100253eSuche in Google Scholar PubMed PubMed Central
[45] Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR. Silver nanoparticles: behaviour and effects in the aquatic environment. Environ Int. 2011;37(2):517–31.10.1016/j.envint.2010.10.012Suche in Google Scholar PubMed
[46] Chauhan RP, Gupta C, Prakash D. Methodological advancements in green nanotechnology and their applications in biological synthesis of herbal nanoparticles. Int J Bioassays (IJB). 2012;1(7):6–10.Suche in Google Scholar
[47] Sadowski Z. Biosynthesis and application of silver and gold nanoparticles. In: David Pozo Perez, editor. Silver nanoparticles. IntechOpen; 2010. p. 257–76.10.5772/8508Suche in Google Scholar
[48] Burygin GL, Khlebtsov BN, Shantrokha AN, Dykman LA, Bogatyrev VA, Khlebtsov NG. On the enhanced antibacterial activity of antibiotics mixed with gold nanoparticles. Nanoscale Res Lett. 2009;4(8):794–801.10.1007/s11671-009-9316-8Suche in Google Scholar PubMed PubMed Central
[49] Hosseinzadeh H, Shariaty VM. Anti-nociceptive effect of safranal, a constituent of Crocus sativus (saffron), in mice. Pharmacologyonline. 2007;2:498–503.Suche in Google Scholar
[50] Siesky B, Harris A, Brizendine E, Marques C, Loh J, Mackey J, et al. Literature review and meta-analysis of topical carbonic anhydrase inhibitors and ocular blood flow. Surv Ophthalmol. 2009;54(1):33–46.10.1016/j.survophthal.2008.06.002Suche in Google Scholar PubMed
[51] Bonifacio BV, da Silva PB, dos Santos Ramos MA, Negri KM, Bauab TM, Chorilli M. Nanotechnology-based drug delivery systems and herbal medicines: a review. Int J Nanomed. 2014;9:1.10.2147/IJN.S52634Suche in Google Scholar
[52] Bhadoriya SS, Mangal A, Madoriya N, Dixit P. Bioavailability and bioactivity enhancement of herbal drugs by “Nanotechnology”: a review. J Curr Pharm Res. 2011;8:1–7.Suche in Google Scholar
© 2021 Fahad A. Alhumaydhi et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis
Artikel in diesem Heft
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis

