Novel insecticidal properties of bioactive zoochemicals extracted from sea urchin Salmacis virgulata
-
Karnan Ramachandran
, Ramachandran Vinayagam
and Yi-Hao Lo
Abstract
The present investigation assessed the insecticidal potential of zoo chemicals extracted from the test (skeleton) and spines of the sea urchin Salmacis virgulata against Tribolium castaneum, Aedes aegypti, and the Sf-9 cell line through assays for in vitro acetylcholinesterase (AChE) inhibition, cytotoxicity, repellency, larvicidal activity, and in silico modeling. Gas chromatography–mass spectrometry (GC–MS) analysis of the 50% ethanolic extract identified 40 distinct zoochemicals, including four with known pesticidal properties, from the test and spines of S. virgulata. The zoo extract exhibited promising insecticidal activity, demonstrated by in vitro AChE inhibition with an IC50 of 143.41 µg/ml. Additionally, in vitro cytotoxicity was measured with an EC50 of 194.68 µg/ml, a repellent index (IR) of less than 0.80, and an LC50 for larvicidal toxicity of 153.205 µg/ml. Further statistical and computational techniques confirmed the insecticidal activity of S. virgulata test and spine 50% zoo-extract against T. castaneum and A. aegypti. The identified zoochemicals that are similarly involved in insecticidal activity on all selected insecticide molecular targets have a very strong correlation, with a range of r = 0.977–0.995. This highlights a positive correlation between the insecticide molecular target and strong evidence on zoological insecticides of S. virgulata test and spines against harmful pests through AChE enzyme inhibition, cytotoxicity, repellence, and larvae toxicity. We recommend the utilization of zoo waste from the sea urchin S. virgulata as a promising source of zoological insecticides. These bio-based pesticides offer an eco-friendly approach to pest control through their repellency and toxicity, being readily biodegradable and presenting lower environmental risks compared to synthetic pesticides.
1 Introduction
Insects represent a vast, largely untapped reservoir of bioactive compounds with potential applications in modern medicine. Comprising 80% of all animal species and 99% of invertebrates, they share significant genetic material with all forms of life, including humans, making them an invaluable study subject. Beyond their biological diversity, insects are crucial to ecological systems, performing key functions such as pollination and soil aeration, serving as a nutritional resource through edible species rich in vitamins and minerals, contributing economically through the production of commodities like silk and honey, and regulating pest populations [1,2]. Despite their ecological and economic benefits, insects can also pose significant threats to the environment such as agricultural pests inflict extensive damage on crops fluid extraction, oviposition, and spreading microbial pathogens, leading to severe economic losses and food security challenges [3]. In the realm of public health, mosquitoes are vectors for serious diseases such as Japanese encephalitis, chikungunya, dengue, filariasis, leishmaniasis, malaria, West Nile fever, yellow fever, and Rift Valley fever [4]. Arthropod-borne viruses such as Zika, chikungunya, dengue, and yellow fever are predominantly transmitted in urban settings by Aedes mosquitoes, specifically Aedes albopictus and Aedes aegypti [5]. Globally, a major challenge harmful insects pose is safeguarding crops and livestock [6]. Commercially available pesticides effectively control insects but may also lead to issues such as mammalian toxicity, development of insecticide resistance, and environmental hazards [7]. The urgent need for sustainable and environmentally friendly alternatives has intensified interest in unlocking the bioactive potential of insects themselves. Researchers are exploring their natural defense mechanisms and biochemical compounds to develop innovative solutions for pest control, pharmaceuticals, and antimicrobial agents. By integrating traditional pest management strategies with cutting-edge biotechnological approaches, scientists seek to minimize the detrimental impact of harmful insects while safeguarding the vital ecological functions of beneficial species.
In this pursuit, zoological insecticides have emerged as a promising, cost-effective, and eco-conscious alternative to conventional synthetic pesticides. These bio-insecticides, derived from animal-based secondary metabolites, encompass a diverse array of bioactive compounds, including polyphenols, flavonoids, alkaloids, terpenoids, steroids, glycosides, essential oils, esters, and fatty acids. Their mode of action often mimics natural insect defense strategies, offering potent yet environmentally benign solutions to pest-related challenges. Beyond insects, other animal-derived bioactive compounds present viable substitutes for existing insect control methods. By harnessing the biochemical arsenal found in various organisms, researchers aim to revolutionize pest management, reducing dependency on chemical pesticides while enhancing ecological sustainability. This paradigm shift underscores the potential of nature-inspired innovations in shaping the future of agriculture, public health, and environmental conservation [8].
Molecular targets are essential for agrochemical development, with many insecticides aimed at key proteins in pests. Initial development focuses on ligand-binding sites and active protein regions. Insecticidal efficacy is assessed through in vitro and in vivo assays for acetylcholinesterase (AChE) inhibition, cytotoxicity, repellency, and toxicity. Recent advancements in computational methods and virtual screening have focused on proteins involved in insect biology, including acetylcholinesterase, ecdysone receptor, GlmU, odorant-binding protein (OBP), dopamine receptor, voltage-gated sodium channels, and juvenile hormone-binding protein (JHBP) [9]. AChE is the primary target for insecticides and other chemicals. The extensive use of pesticides aimed at inhibiting AChE activity has led to the development of insecticide resistance [10]. OBPs play a crucial role in detecting chemical signals in insects, making them promising targets for developing attractants and repellents [11]. Additionally, juvenile hormone (JH) regulates insect growth, metamorphosis, and reproduction. JHBP, which transports JH to target tissues, is utilized in pesticide development [12].
The marine ecosystem is a rich source of potential pharmaceuticals, as numerous marine organisms exhibit intriguing bioactive properties that warrant further investigation for applications in biomedicine and pharmaceuticals. Zoological insecticides are derived from animal extracts rich in secondary metabolites, termed zoochemicals. Zoochemicals are functionally analogous to phytochemicals [13,14]. Sea urchins are small and spiny marine invertebrates with a history spanning approximately 500 million years and belong to the phylum Echinodermata and class Echinoidea. Around 800 species of echinoids have been identified worldwide, with 30 species recorded along the Tamil Nadu coast and 105 species found along India's east coast. Notably, the southeast coastal regions are present in large populations of Salmacis virgulata [15]. Sea urchin S. virgulata test containing rich zoochemical with demonstrating nanoparticle synthesis, which confirms the biologically active properties of sea urchin [16]. Sea urchins, particularly the species Diadema setosum, are prized not only for their culinary value but also for the remarkable nutritional properties of their eggs and gonads. A chemical analysis of the gonads from sea urchin D. setosum has revealed a fascinating spectrum of secondary metabolites, including bioactive steroids, alkaloids, and saponins. These compounds are known for various health benefits, with potential roles in immune support, antioxidant activity, and even antimicrobial properties, making D. setosum both a delicacy and a subject of scientific intrigue [17].
The present study focused on the availability of deceased and dried specimens of the sea urchin S. virgulata from the Manora region of Thanjavur, located on the southeast coast of the Bay of Bengal. A laboratory experiment was conducted to investigate these specimens' zoochemical properties and insecticidal activity using various models. The study concludes that extracts from the test and spines of S. virgulata demonstrated insecticidal properties comparable to botanical insecticides in controlling harmful pests. This suggests that zoo-waste materials like sea urchins could serve as an eco-friendly alternative to synthetic pesticides, helping to reduce environmental pollution and offering a sustainable solution.
2 Materials and methods
2.1 Collection and extraction of zoo-extract from the test and spines of S. virgulata
Sea urchin Salmacis virgulata were collected from Manora, Thanjavur (Bay of Bengal, southeast coast), and the collected specimens have been identified the key Venkatraman and Padmanaban [18] and stored at the Zoological Museum of the PG and Research Department of Zoology, RSGC, Thanjavur (Voucher No. RSGC-Z-K01). The sea urchin tests and spines from 20 various-sized sea urchin S. virgulata were washed thoroughly using water and ethanol. Then, the test and spines were separated and mashed together using a mortar and pestle. After 24 h, 10 g powder was extracted with 100 ml of ethanol and water in a 1:1 ratio. Following 24 h, the extract was filtered and refrigerated under 4°C for further use.
2.2 Zoochemical assessment
The zoo extract underwent zoochemical analysis utilizing gas chromatography–mass spectrometry (GC–MS) techniques, specifically the S. virgulata test, and a 50% ethanolic extract was conducted with the Shimadzu 2010 Plus, which includes an AOC-20i autosampler and GC–MS. The Wiley and NIST libraries were utilized for component identification, and their retention indices were compared. The components were identified by comparing them to those in the computer libraries associated with the GC–MS instrument. The results were subsequently aggregated [19].
2.3 Insect
Tribolium castaneum (red flour beetles) was employed to assess the insecticidal efficacy of zoo extract. T. castaneum was cultured in a lab setting free from insecticide exposure. The insect-reared method, as described [20,21], was used to raise the red flour beetles on wheat flour at room temperature.
2.4 In vitro AChE inhibitor assay
The AChE biochemical assay was administered to the tested insects. The extraction method for the AChE enzyme from the T. castaneum insect, as described by Hematpoor et al. [22], and the evaluation of AChE inhibition activity, as conducted [23], with minor modifications.
2.5 In vitro insecticidal efficacy (cytotoxic impact) of the zoo extract on the Sf-9 cell line utilizing the (3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) assay
The cell viability trial adhered to the methodologies outlined [24,25]. The Sf-9 insect ovarian cell line (NCCS) was utilized to cultivate cell suspensions in 100 μl per well of 96-well culture plates. Various concentrations of zoo extract (50, 100, 150, 200, and 250 μg/ml of S. virgulata 50% ethanolic extract) were administered and incubated for 48 h following an initial 24-h incubation period. A control medium was utilized, comprising cells without the experimental drug. Cellular morphological features such as membrane blebbing and cellular shrinkage or swelling were observed using an inverted microscope [25].
2.6 Repellency assays
The repellency assays of S. virgulata 50% ethanolic extract were detailed [26,27], with minor modifications. Test solutions were formulated at concentrations ranging from 50 to 250 µg/ml/cm2 in an aqueous solvent. A micropipette was utilized to apply each concentration of zoo extract to one-half (30 mm²) of Whatman filter paper, which had been divided into two 90 mm² discs. The sole treatment administered to the remaining half of the filter paper was an aqueous solution. The solvent was completely evaporated by drying the halves treated with zoo extract and water. The treated and untreated halves were then placed at the bottom of the petri dish and secured with cellophane tape. Following the introduction of 20 adult T. castaneum into the center of the filter paper disc, the Petri plates were sealed with parafilm and stored in darkness. The quantity of insects in both the treated and untreated sections was documented after 4 h in subdued illumination. Classes were classified on a scale from 0 to 5, and percent repellency (PR) was calculated [28]. The repellency classes are classified as follows: class 0 (PR ≤ 0.1%), class I (PR = 0.1–20%), class II (PR = 20.1–40%), class III (PR = 40.1–60%), class IV (PR = 60.1–80%), and class V (PR = 80.1–100%). Percent repellency (PR) is calculated as (Nc − Nt)/(Nc + Nt) × 100, where Nc represents the number of insects on untreated paper and Nt denotes the number of insects on treated paper.
The index of repellency (IR) was calculated by the following formula: IR = 2 G/G + P. The repellency index was classified by Mazzonetto and Vendramim [29] as: values <1 repellency; 1 neutral; >1 attractant. The percentages of insects present in treated (G) and control/untreated (P) areas were recorded [27].
2.7 Larvicidal efficacy of S. virgulata extracts from test and spines
The larvae of A. aegypti (Linnaeus, 1762) were identified [30] and reared in deionized water enriched with yeast powder and glucose. The standard methodology [31,32] was employed, with slight modifications, to sustain the A. aegypti colony in the laboratory at 27°C, 75 ± 5% relative humidity, and a photoperiod of 14 h. Experiments were performed to evaluate the efficacy of S. virgulata test and spine extract in exterminating fourth-instar A. aegypti larvae. The larvicidal activity was assessed through a bioassay following the standard guidelines established by the WHO [33].
2.8 In silico insecticidal efficacy
2.8.1 Ligand and target selection
The insecticidal ligands classified as zoochemicals 9-octadecenoic acid (CID: 965), geraniol (CID: 637566), n-hexadecanoic acid (CID: 985), and squalene (CID: 638072) – were derived from the 50% ethanolic extract of S. virgulata. Additionally, Aldicarb (CID: 2086), an AChE inhibitor, and diethyltoluamide (CID: 4284), an insect repellent, were obtained from the PubChem database. The molecular targets associated with insecticidal activity, including JHBP (PDB ID: 5V13), OBP (PDB ID: 3K1E), and acetylcholinesterase (PDB ID: 1DX4), were retrieved from the Protein Data Bank (PDB).
2.8.2 Molecular docking
Molecular docking studies were performed using PyRx 0.8, a virtual screening tool known for its user-friendly interface and compatibility across multiple operating systems. This tool integrates several open-source programs, including AutoDock Vina and Open Babel, facilitating docking-based virtual screening techniques. The docking process follows a four-step protocol and enables the simultaneous screening of multiple drug molecules against a specific target site. The molecular docking simulations were conducted using AutoDock Vina, with interactions evaluated through the software. Additionally, semi-flexible protein–ligand docking was performed using AutoDock, serving as a preliminary docking tool. The best conformation was determined based on the root mean square deviation (RMSD) value, where an RMSD of zero indicates the most favorable conformation [34].
PyRx 0.8 employed the AutoDock Vina program for molecular docking, utilizing grid dimensions specific to each target (PDB 1DX4: center x = 24.57, center y = 64.68, center z = 11.56; PDB 3K1E: center x = 8.77, center y = 41.49, center z = 21.83; PDB 5V13: center x = 254.78, center y = 4.65, center z = 363.84). The energy minimization parameters included the Universal Force Field, with optimization performed using the conjugate gradients algorithm. The total number of optimization steps was set to 200, with updates occurring every step, and termination was triggered if the energy difference fell below 0.1 [35]. Docking procedures were conducted following the methodology established by Trott and Olson [36]. The docked complexes were subsequently visualized using PyMOL, BIOVIA Discovery Studio Visualizer, and UCSF Chimera, facilitating structural analysis and interaction interpretation.
2.9 Statistical analysis
Each experiment was conducted thrice, and the statistical methods employed included probit analysis, chi-square test, one-way ANOVA, and post hoc test, all analyzed using IBM SPSS Version 20.0. The data was statistically significant if *p < 0.05, while NS indicated statistical non-significance (p > 0.05).
3 Results and discussion
3.1 Zoochemical profile of 50% ethanolic extract of S. virgulata test and spines
This study investigated the insecticidal properties of zoo-waste extracts from deceased and dried S. virgulata sea urchins, specifically from the test and spines, against T. castaneum, A. aegypti, and the Spodoptera frugiperda cells (sf-9) cell line. The sea urchin samples were extracted using a 50% ethanolic solution over 24 h, yielding a light greenish-brown extract (Figure 1), which was then concentrated for further zoochemical and insecticidal analysis. The zoochemical profile of the S. virgulata extract, detailed in Table 1 and Figure 2, revealed the presence of various zoochemicals. GC–MS analysis identified 40 zoochemical components by comparing them to the NIST and Wiley databases. Notably, Table 2 lists 11 bioactive zoochemicals, including four pesticide compounds: geraniol, 9-octadecenoic acid, n-hexadecanoic acid, and squalene.
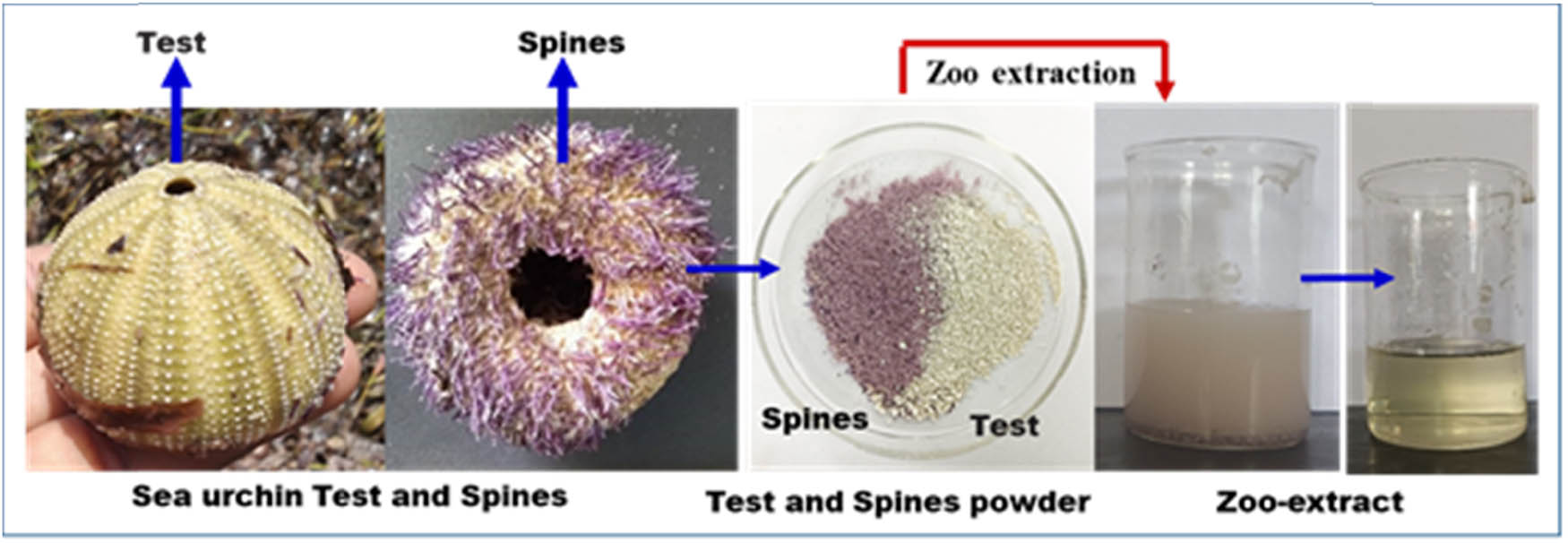
Zoochemical extraction from S. virgulata test and spines.
Identification of zoochemical from S. virgulata test and spines 50% ethanolic extract using GC–MS techniques
| Peak no | R. time | M. weight (g/mol) | M. formula | Compound(s) |
|---|---|---|---|---|
| 1 | 5.283 | 215 | C13H13NO2 | Acrylic acid, 3-[1-hydroxy-N-(3-phenylpropyl)formimidoyl]-, cyclic anhydride |
| 2 | 6.692 | 154 | C10H18O | Geraniol |
| 3 | 8.667 | 178 | C6H7FO5 | Furan-2-one, 3,4-dihydroxy-5-[1-hydroxy-2-fluoroethyl] |
| 4 | 9.344 | 172 | C10H20O2 | Decanoic acid (CAS) capric acid |
| 5 | 9.542 | 224 | C16H32 | 3-Hexadecene |
| 6 | 9.851 | 222 | C12H14O4 | 1,2-Benzenedicarboxylic acid, diethyl ester (CAS) ethyl phthalate |
| 7 | 10.425 | 242 | C15H30O2 | Pentadecanoic acid |
| 8 | 11.383 | 204 | C9H17ClOSi | 1-Chloromethyl-1-(2-propenyloxy)-1-silacyclohexane |
| 9 | 11.639 | 282 | C18H34O2 | 9-Octadecenoic acid |
| 10 | 11.881 | 366 | C19H36Cl2O2 | Dichloroacetic acid, heptadecyl ester |
| 11 | 12.050 | 243 | C12H10FN5 | 1H-Purin-6-amine, [(2-fluorophenyl)methyl] |
| 12 | 12.582 | 618 | C44H90 | Tetratetracontane |
| 13 | 12.917 | 278 | C16H22O4 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester |
| 14 | 13.333 | 186 | C10H18O3 | Nonanoic acid, 9-oxo-, methyl ester |
| 15 | 13.625 | 226 | C15H30O | Z-11-Pentadecenol |
| 16 | 13.774 | 256 | C16H32O2 | n-Hexadecanoic acid |
| 17 | 13.929 | 278 | C16H22O4 | Dibutyl phthalate |
| 18 | 14.042 | 284 | C18H36O2 | Hexadecanoic acid, ethyl ester (CAS) ethyl palmitate |
| 19 | 14.372 | 498 | C35H46O2 | 9(11)-Dehydroergosteryl benzoate |
| 20 | 14.998 | 214 | C14H30O | 1-Tetradecanol |
| 21 | 15.168 | 438 | C21H22FEN2O5 | Iron, monocarbonyl-(1,3-butadiene-1,4-dicarbonic acid, diethyl ester) a,a'-dipyridyl |
| 22 | 15.617 | 254 | C16H30O2 | 9-Hexadecenoic acid |
| 23 | 16.600 | 380 | C27H56 | 2-Methylhexacosane |
| 24 | 17.320 | 604 | C43H88 | Tritetracontane |
| 25 | 17.750 | 210 | C14H26O | 7-Tetradecenal |
| 26 | 17.941 | 157 | C2H7NO3S2 | Thiosulfuric acid (H2S2O3), S-(2-aminoethyl) ester |
| 27 | 18.175 | 346 | C20H39ClO2 | 3-Chloropropionic acid, heptadecyl ester |
| 28 | 18.450 | 228 | C14H28O2 | Tetradecanoic acid |
| 29 | 18.760 | 458 | C14H42 O5SI6 | Tetradecamethylhexasiloxane |
| 30 | 19.004 | 410 | C30H50 | Squalene |
| 31 | 19.620 | 238 | C16H30O | 7-Hexadecenal |
| 32 | 20.350 | 465 | C33H68 | Tritriacontane |
| 33 | 20.573 | 884 | C57H104O6 | 9-Octadecenoic acid, 1,2,3-propanetriyl ester, (E,E,E)- |
| 34 | 21.466 | 296 | C19H36O2 | 9-Octadecenoic acid (Z)-, methyl ester (CAS) methyl oleate |
| 35 | 21.975 | 155 | C7H13N3O | Hydrazinecarboxamide, 2-cyclohexylidene- |
| 36 | 22.282 | 390 | C24H38O4 | 1,2-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester (CAS), bis(2-ethylhexyl) phthalate |
| 37 | 22.470 | 282 | C20H42 | Eicosane |
| 38 | 23.031 | 278 | C18H15OP | Phosphine oxide, triphenyl- (CAS) triphenylphosphine oxide |
| 39 | 23.600 | 354 | C23H46O2 | Dodecanoic acid, undecyl ester |
| 40 | 24.025 | 168 | C11H20O | (2,2,6-Trimethyl-bicyclo[4.1.0]hept-1-yl)-methanol |

GC–MS chromatogram of S. virgulata test and spines 50% ethanolic extract.
Identification of bioactive compounds, from S. virgulata test and spines 50% ethanolic extract using GC–MS techniques with literature review
| Bioactive compound | PubChem structure | Biological activity | References |
|---|---|---|---|
| Geraniol |

|
Antitumour, anti-inflammatory, antioxidant, antimicrobial, hepatoprotective, cardioprotective, neuroprotective effects, insecticidal and repellent properties | [37,38,39, 40] |
| 9-Octadecenoic acid |

|
Anti-inflammatory, antiandrogenic cancer preventive, dermatitigenic hypocholesterolemic, 5-alpha reductase inhibitor, anemia genic, Insectifuge, and flavor | [41] |
| Tetratetracontane |

|
Antioxidant and cytoprotective activities | [42,43] |
| Nonanoic acid, 9-oxo-, methyl ester |

|
Antifungal, antioxidant, and antimicrobial | [44] |
| n-Hexadecanoic acid |

|
Antioxidant, hypocholesterolemic nematicide, pesticide, anti-androgenic flavor, and hemolytic | [45] |
| Dibutyl phthalate |

|
Antimicrobial and antifouling | [46] |
| Tetradecanoic acid |

|
Antioxidant, cancer preventive, nematicide, lubricant, and hypocholesterolemic | [41] |
| Squalene |

|
Antibacterial, antioxidant, pesticide, antitumor, cancer preventive, immunostimulant, chemopreventive, and lipoxygenase-inhibitor | [47] |
| 7-Hexadecenal |

|
Antiviral activity | [48] |
| 9-octadecenoic acid, 1,2,3-propanetriyl ester |

|
Antioxidant, antibacterial and inflammation-suppressing effects | [49,50,51] |
| Eicosane |

|
Antifungal activity | [52] |
Geraniol, a terpene alcohol frequently found in essential oils, can be synthesized via recombinant microorganisms or extracted from natural sources [37]. This compound, valued for its pleasant fragrance, is present in numerous aromatic plants and is noted for its insecticidal and repellent properties, making it a low-toxicity natural pesticide [38]. The present study identified geraniol in sea urchin extracts using GC–MS, suggesting its presence may be linked to the sea urchin's aromatic nature and its repellent effects. Geraniol is also recognized for its hepatoprotective, cardioprotective, antitumor, anti-inflammatory, antioxidant, and neuroprotective activities [37,38,39], in addition to its insecticidal and repellent properties [40]. Additionally, dodecanoic acid, identified as the oviposition pheromone for the sand fly Lutzomyia longipalpis [53], was also found in S. virgulata extracts, along with its undecyl ester.
Recent studies have utilized GC–MS techniques for the screening and identification of zoochemicals from various animal sources (Table 2). Furthermore, chemical analysis of the test and spines of the sea urchin S. virgulata revealed compounds including hexadecanoic acid methyl ester, tetradecanoic acid, and (Z)-9-octadecenoic acid methyl ester. Interestingly, these compounds were also identified in the gonads of the sea urchin D. setosum, highlighting a shared biochemical profile across these species [54]. The findings confirm the presence of valuable zoochemicals in S. virgulata, highlighting their potential contributions to biomedical research and environmental applications and affirming their role as an eco-friendly resource.
3.2 AChE inhibitory and cytotoxicity activity of S. virgulata test and spines
AChE is a molecular insecticide target, and inhibiting by natural or synthetic compounds proves death for the target insects – one of the primary targets of insecticide development. In vitro, T. castaneum AChE enzyme is inhibited by the S. virgulata test and spines 50% ethanolic extract (IC50 = 143.41 µg/ml) to cause toxicity in the target insect T. castaneum. The zoo extract of S. virgulata exhibited strong dose-dependent insecticidal activity (R² = 0.99), as shown in Figure 3a, with effective AChE inhibition that resulted in the mortality of target insect species, indicating its potential as a natural insecticide. Georgiev et al. [55] studied methanolic extracts and essential oils from species of Asteraceae, Lamiaceae, Brassicaceae, and Amaryllidaceae were evaluated in vitro for AChE inhibitory activity using the method by Ellman et al. [23]. Between 45.67 and 58.38 μg/ml, the essential oils of Callicarpa candicans exhibited encouraging activity. IC50 values ranging from 28.71 to 54.69 μg/ml indicated good activity for the essential oils of Callicarpa sinuata, C allicarpa petelotii, Callicarpa nudiflora, Callicarpa erioclona, and Vitex ajugifolia. The IC50 values of 81.34 and 89.38, respectively, indicated modest activity for the essential oils Vitex trifolia subsp. trifolia and Capsella rubella [56]. In the marine environment, seaweed produces a variety of naturally occurring compounds that are both chemically and physiologically active. At 200 μg/ml, ethanolic extracts made from 47 macroalgae species were tested against tyrosinase (TYR), butyrylcholinesterase (BChE), and AChE. Dictyota dichotoma var. intricata was the only algae extract that showed a significant inhibition of BChE (72.0 ± 0.07%). In contrast, the other macroalgae showed no or minimal inhibition of AChE (2.20 ± 0.39%–14.20 ± 2.16%), BChE (0.62 ± 0.07%–41.20 ± 3.07%), and TYR (<10%) [57]. Recently, in vitro insecticidal activity of zoo-extract against AChE enzyme by Karnan et al. [58] used marine sponge Hyattella intestinalis zoo extract (141.10 µg/ml). Hung et al. [56] have reported that the obtained results indicate the potential utility of these essential oils AChE inhibitory activities of essential oils from Vietnamese traditional medicinal plants for the management of mosquito vectors. Mattar et al. [59] reported the essential oil of Schinus areira against Rhipibruchus picturatus and its inhibitory effects on AChE (IC50 0.62 mg/ml). AChE activity tests showed that S. virgulata extract had an excellent inhibitory effect on AChE and was inhibited by zoo insecticides due to the toxicity of T. castaneum. Exposure to organophosphorus compounds (OP) raises ROS levels, which attack proteins, lipids, and DNA. This leads to damage to membranes, inactivation of enzymes, DNA damage, and cell death [60]. Inhibitors of the esterase activity of AChE continued to promote cell death. The mechanism by which extracellular AChE triggers apoptosis is still unknown, even though preventing AChE's cytotoxic effect at low temperatures suggested enzyme-driven processes [61]. The discussions demonstrate the potential use of S. virgulata test and spines with 50% ethanolic extract in the development of new insecticides and further contributors to the fumigant toxicity activity and repellent properties against T. castaneum.

In vitro insecticidal activity of S. virgulata test and spines 50% ethanolic extract: (a) in vitro AChE inhibitory activity and (b) in vitro cytotoxicity activity. *Statistically significant (p < 0.05), compared with control. The dotted line in (a) exhibits the mean correlation coefficient (R 2).
The Sf9 insect cell line from S. frugiperda was used for an in vitro insecticidal model, and its cytotoxic activity was investigated using an MTT assay. The present study revealed that S. virgulata test and spines 50% ethanolic extract were highly toxic based on dose dependence (R 2 = 0.986) against the Sf-9 cell line (EC50 = 194.68 µg/ml), respectively. Figure 3b shows significantly inhibited Sf-9 cell growth at various concentrations between 50 and 250 µg/ml of zoo extract, compared to control (100% cell viability). The morphology of S. virgulata test and spines 50% ethanolic extract treated Sf-9 cells indicate cell alterations, such as cell shrinkage and cell fragmentation, and the control group indicates cell aggression (Figure 4), were observed at 48 h. Present cytotoxicity properties based on the presence of bioactive zoochemicals in the S. virgulata test and spines. Pandya et al. [25] and Saleh et al. [62] recently utilized the S. frugiperda Sf9 insect cell line to evaluate insecticidal efficacy through in vitro assays. According to Pandya et al., toxicity bioassays revealed that ammonia was hazardous for Sf9 (IC50 350 μg/ml) and profenofos was harmful for Sf9 (IC50 400 μg/ml) [25]. Additionally, morphological changes to the cell, such as shrinkage and fragmentation, were detected. Currently available S. virgulata test and spines 50% ethanolic extract is environmentally safe and effective zoological insecticides that destroy Sf-9 cells, using the MTT assay.

The morphology of normal Sf-9 cells and S. virgulata test and spines 50% ethanolic extract treated cells. The red mark indicates cell alterations, such as cell shrinkage and cell fragmentation. (a) control, (b) and (c) S. virgulata test (50–250 µg/ml). (d), (e) and (f) S. virgulata test and spines 50% ethanolic extract (150–250 µg/ml).
The zoochemicals identified in the test and spines of S. virgulata are involved in AChE inhibition, which leads to the overstimulation of AChE receptors, resulting in symptoms of zoochemical poisoning in T. castaneum (Table 3 and Figure 5). Table 3 shows that zoochemical are binding to the insecticidal target AChE (PDB ID: 1DX4), and highest insecticidal activity was observed in squalene (−33.89 kJ/mol), compared with 9-octadecenoic acid (−27.614 kJ/mol), n-hexadecanoic acid (−26.359 kJ/mol), and geraniol (−23.849 kJ/mol). Tyr 71 amino acid residue was involved in all zoo-insecticide binding interactions, which indicates S. virgulata zoochemicals have similar modes and actions of insecticidal activity, as represented in Figure 5. The standard AChE inhibitor Aldicarb commonly interacts with acetylcholinesterase (AChE) (PDB ID: 1DX4) through key amino acid residues, including Tyr370 and Gly150. Similarly, docking analysis revealed that 9-octadecenoic acid, n-hexadecanoic acid, and squalene exhibited comparable interactions with these residues. These findings suggest that the zoochemicals derived from S. virgulata and spines share a mode of inhibitory action similar to that of Aldicarb. Similarly, computational insecticidal development of secondary metabolites against molecular target AChE (PDB ID: 1DX4) was used [63,64,65], whose recommended AChE inhibitions promising potential insecticidal development against the harmful insect. Recently, bioactive compounds against AChE inhibition used molecular modeling approaches, which resulted in the identification of new cholinesterase inhibitors and also insect control [66,67,68].
In silico AChE inhibitory activity of S. virgulata test and spines zoochemicals
| Zoochemicals | Binding affinity | Insecticidal target AChE (PDB ID: 1DX4) binding amino acid residue |
|---|---|---|
| kJ/mol | ||
| Geraniol | −23.849 | Asp 375, Ile 327, Tyr 374, Phe 330, Tyr 324, Tyr 73, Trp 321, Tyr 71, Glu 69 |
| 9-Octadecenoic acid | −27.614 | Leu 159, Tyr 162, Gly 150, Gly 155, Tyr 83, Gly 149, Gly 481, His 480, Tyr 370, Thr 154, Glu 80, Gly 79, Tyr 374, Trp 472, Tyr 71 |
| n-Hexadecanoic acid | −26.359 | His 480, Glu 237, Ser 238, Gly 149, Trp 83, Gly 79, Tyr 370, Gly 150, Glu 80, Tyr 374, Trp 472, Thr 154, Tyr 71 |
| Squalene | −33.89 | Gly 72, Tyr 73, Asp 375, Thr 154, Gly 151, Met 153, Gly 150, Tyr 370, Phe 330, Trp 83, Phe 371, Tyr 71, Tyr 324, Trp 321, Arg 70 |
| Aldicarb (Standard drug) | −22.594 | His 480, Tyr 162, Gly 149, Ile 484, Trp 83, Gly 481, Ala 239, Gly 151, Glu 237, Tyr 370, Phe 330, Phe 371, Ser 238, Gly 150 |

In silico AChE inhibitory activity of S. virgulata test and spines zoochemicals (A: 9-octadecenoic acid; B: geraniol; C: n-hexadecanoic acid; D: squalene; E: Aldicarb; a: 3D docked view and b: 2D view).
3.3 Repellence impact of S. virgulata test and spines
S. virgulata 50% ethanolic extract repellency assays were conducted using 50–250 µg/ml/cm2 in an aqueous solvent. A 90 mm2 Whatman filter paper disc was halved. One-half of the filter paper (30 mm2) was uniformly treated with each S. virgulata zoo extract concentration, while the other half was treated with an aqueous solution. The aqueous-treated halves increased insect populations, while the zoo-extract-treated halves decreased insect populations due to repellent properties on T. castaneum adults (Figure 6a). Zoo extract repellency was dose-dependent (R 2 = 0.976). The repellence effect is statistically significant as determined by the chi-square test (p < 0.05). Each concentration between the zoo-extract-treated and aqueous solvent-treated samples was statistically significant, confirming the reduction of insect populations on zoo-extract-treated filter paper halves, while insect populations increased on aqueous solvent-treated filter paper halves (Table 4). The percentage repellency value varied from 20.00 ± 10.00% to 93.33 ± 5.77% at doses of 50–250 µg/ml/cm2 of S. virgulata test and spines within 4 h of exposure (Figure 6b). It identified repellency classes I, II, IV, and V based on the mean repellency rate. The index of repellency (IR) values range from 0.80 to 0.06, with concentrations below 0.80 (Figure 6c), indicating the repellent efficacy of S. virgulata extract.

Repellence affect of S. virgulata test and spines 50% ethanolic extract against adults T. castaneum: (a) repellency assays; (b) percentage repellency; (c) index of repellency (IR).
Repellence impact of S. virgulata test and spines 50% ethanolic extract
| Concentration (µg/ml/cm2) | Repellency impact of S. virgulata test and spines 50% ethanolic extract | ||||
|---|---|---|---|---|---|
| Chi-square tests | Index of repellency (IR) | Repellency (%) | Repellency class | ||
| χ2 | P value | ||||
| 50 | 4.00 | 0.04* | 0.80 ± 0.10a | 20.00 ± 10.00a | I |
| 100 | 16.00 | 6.33×10−5*** | 0.60 ± 0.10a | 40.00 ± 10.00a | II |
| 150 | 40.14 | 2.33×10−10*** | 0.36 ± 0.05b | 63.33 ± 5.77b | IV |
| 200 | 75.11 | 4.41×10−18*** | 0.13 ± 0.05c | 86.66 ± 5.77c | V |
| 250 | 87.10 | 1.02×10−20*** | 0.06 ± 0.05c | 93.33 ± 5.77c | V |
Values are expressed as Mean ± SD (N = 3). The data was analyzed by statistical techniques of the chi-square test and one-way ANOVA test, using IBM SPSS Version 20.0. Mean values within the column followed by different letters (superscript) are statistically significant (p < 0.05) from each other concentration, and the same letters are statistically non-significant (p > 0.05) and are compared by ANOVA, Duncan’s multiple range test (DMRT). *p < 0.05, ***p < 0.001 the difference was statistically significant and NS p > 0.05 was statistically non-significant. In the present study, treated and untreated populations were statistically significant (p < 0.05) at concentrations of 50–250 (µg/ml/cm2) of zoo extract.
Using S. virgulata zoochemicals against OBP (PDB ID: 3K1E), Table 5 demonstrated the in silico identification of zoo-repellents by ligand-based screening and OBP structure-based molecular docking. Since they transport the chemicals to odorant receptors through the sensillum lymph, the globular proteins known as OBP are crucial to insect olfaction. Because the OBPs and olfactory receptors (ORs) of a species are an integral part of the olfactory system and are a valuable resource for downstream translational research, which eventually aims to improve insect control, accurately identifying them is imperative. The amino acid residues Phe 123, Leu 80, Leu 76, Leu 73, Met 91, Gly 92, and Ala 88 were involved in all zoo-insecticide binding interactions. This indicates that the zoochemicals of S. virgulata exhibit similar modes and mechanisms of insecticidal activity, as illustrated in Figure 7.
In silico repellence impact of S. virgulata test and spines zoochemicals
| Zoochemicals | Binding affinity | Insecticidal target OBP (PDB ID: 3K1E) binding amino acid residue |
|---|---|---|
| kJ/mol | ||
| Geraniol | −26.778 | Phe 123, His 111, Leu 80, Leu 76, Leu 73, Met 91, His 77, Gly 92, Ala 88, Trp 114, Met 84, Tyr 122 |
| 9-Octadecenoic acid | −30.543 | His 77, Glu 74, Leu 73, Gly 92, Trp 114, Phe 123, Met 91, Leu 80, Leu 76, Leu 124, Phe 15, His 111, Ile 125, Phe 59, Met 19, Leu 58, Val 64, Ala 62, Ala 88 |
| n-Hexadecanoic acid | −28.87 | Val 64, Ala 62, Leu 58, Phe 59, Leu 76, Leu 80, Ile 125, His 111, Met 19, Met 91, Met 84, Phe 15, Phe 123, Leu 73, Ala 88, Gly 92, His 77, Leu 89 |
| Squalene | −38.911 | Ala 62, Ala 79, Val 64, Phe 15, Gly 92, Leu 96, Leu 89, Leu 73, Met 91, Trp 114, Ala 88, Tyr 122, Met 84, Leu 80, Leu 76, Leu 124, Phe 123, Ile 125, Phe 59, Leu 58, Met 19 |
| Diethyltoluamide (Standard drug) | −28.87 | Met 91, Ala 88, Phe 123, Trp 114, Phe 15, Leu 124, His 111, Val 64, Ile 125, Leu 58, Phe 59, Ala 62, Met 19, Leu 80, Met 84, Leu 76 |

In silico repellence impact of S. virgulata test and spines zoochemicals (A: 9-octadecenoic acid; B: geraniol; C: n-hexadecanoic acid; D: squalene; E: diethyltoluamide; a: 3D docked view and b: 2D view).
OBP plays a protective role by shielding odorants from degradation by enzymes. Venthur and Zhou [69] have highlighted recent research exploring OBP as potential targets in the insect peripheral nervous system for developing eco-friendly pest control strategies. OBP is believed to facilitate the transport of odorants to ORs, contributing to the signal transduction processes that regulate behaviourally significant odors. A study by Ramsha et al. [70] evaluated the repellent effects of plant extracts from clove, coriander, neem, and mint against the red flour beetle, demonstrating that these natural extracts exhibit significant repellent activity, positioning them as promising sources of biological insect repellents. Murugesan et al. [71] reported a mean repellency of 82% for ethyl acetate leaf extract at a concentration of 1,500 µg/ml/cm2 after 1 h of exposure. The methanol (52%) and hexane (28%) leaf extracts showed lower repellency. In addition, extracts from R. stricta, S. persica, and R. chalepensis at 500 ppm demonstrated repellent effects on adult O. surinamensis, with values of 91.7, 83.3, and 78%, respectively [72]. In the current study, the zoo extract of S. virgulata, comparable to plant extracts, was found to have repellent properties due to its rich composition of zoochemicals, including geraniol, 9-octadecenoic acid, n-hexadecanoic acid, and squalene. Molecular docking studies identified four key zoochemicals with high binding affinities for OBP (PDB ID: 3K1E), showing that S. virgulata triggered significant behavioral responses and potent repellency. The standard insect repellent diethyltoluamide (DEET) commonly interacts with the OBP target (PDB ID: 3K1E) through key amino acid residues, including Met91, Ala88, Phe123, Leu80, and Leu76. Notably, similar interactions were observed in the docking analysis of geraniol, 9-octadecenoic acid, n-hexadecanoic acid, and squalene, suggesting that the zoochemicals derived from S. virgulata and spines exhibit a mode of action comparable to that of DEET. The olfactory system plays a crucial role in regulating insect behaviors like host-seeking, mating, oviposition, toxin avoidance, and negative taxis [73]. Recent molecular docking studies have confirmed the binding interactions of secondary metabolites with OBP (PDB ID: 3K1E), as reported [74,64].
3.4 Larvicidal activity of S. virgulata test and spines
The larvicidal activity of 50% ethanolic crude extracts from the test and spines of S. virgulata was evaluated against A. aegypti fourth instar larvae using concentrations of 50, 100, 150, 200, and 250 ppm over 24 h. The LC50 (lethal concentration for 50% mortality) is 153.205 µg/ml (95% confidence interval: 123.048–196.122 µg/ml), and the LC90 (lethal concentration for 90% mortality) was 414.374 µg/ml (288.595–945.734 µg/ml (Figure 8). The larvicidal efficacy of S. virgulata extract increased in a dose-dependent manner (R² = 0.943), suggesting it to be a highly effective natural insecticide and a promising alternative for mosquito control. This aligns with other research, such as Kamaraj et al. [75], which proposed plant extracts as viable substitutes for synthetic insecticides. Similarly, Bharathi and Suseem reported larvicidal properties in the aqueous extract of Phaseolus vulgaris against A. aegypti mosquitoes [76].

Larvicidal activity of S. virgulata test and spines 50% ethanolic extract against A. aegypti 4th instar larvae.
Further studies by Hasaballah et al. [77] demonstrated the larvicidal potential of zinc oxide nanoparticles (ZnO-NPs) synthesized using marine sponge extract (Spongia officinalis), showing LC50 values of 31.823 µg/ml for Culex pipiens and 12.634 µg/ml for Anopheles pharoensis. These findings indicate that ZnO-NPs derived from marine sources exhibit strong larvicidal properties. Similarly, the larvicidal activity of S. virgulata test and spine extract reinforces its role as an eco-friendly, natural insecticide. Both studies underscore the potential of natural and marine-derived compounds as alternatives for mosquito control, offering environmentally sustainable solutions.
This study aims to investigate the larvicidal activity of pesticide zoochemicals that were identified from S. virgulata tests and spines. Table 6 presents the results of this investigation, and Figure 9 illustrates the interactions between amino acids and their effects on mosquito growth regulator of JH and larvicidal development. In comparison to 9-octadecenoic acid (−33.054 kJ/mol), n-hexadecanoic acid (−31.38 kJ/mol), and geraniol (−22.594 kJ/mol), squalene (−46.861 kJ/mol) exhibited the highest larvicidal activity. The standard drug Aldicarb, an AChE inhibitor, commonly interacts with the JHBP target (PDB ID: 5V13) through key amino acid residues, including Val68, Tyr64, Tyr129, Tyr33, Trp50, Trp53, and Val51. Notably, similar interactions were observed in the docking analysis of 9-octadecenoic acid, n-hexadecanoic acid, and squalene, indicating that the zoochemicals derived from S. virgulata and spines exhibit a mode of inhibitory action comparable to that of Aldicarb. Recently reported JHBP (PDB ID: 5V13), which is a molecular target for larvicidal activity [78,79,80,81]. According to Zalewska et al. [12], JH regulates the development, reproduction, and metamorphosis of insects. Through an in silico model with effective mosquito vector control of sea urchin S. virgulata, the current S. virgulata zoo extract was involved in controlling insect development, such as inhibiting larvae growth of zoochemicals.
In silico larvicidal of S. virgulata test and spines zoochemicals, against JHBP
| Zoochemicals | Binding affinity | Insecticidal target JHBP (PDB ID: 5V13) binding amino acid residue |
|---|---|---|
| kJ/mol | ||
| Geraniol | −22.594 | Phe 169, Gly 199, Arg 198, Glu 27, Glu 205, Gln 28, Thr 204, Arg 73, Glu 71, Arg 201, Ala 32, Tyr 31 |
| 9-Octadecenoic acid | −33.054 | Ser 69, Phe 87, Val 65, Phe 144, Leu 74, Tyr 129, Val 68, Leu 72, Tyr 64, Tyr 33, Trp 50, Trp 53, Val 51, Gly 146, Ala 281, Lys 52, Ala 285, Phe 284 |
| n-Hexadecanoic acid | −31.38 | Leu 72, Ala 281, Val 51, Gly 146, Ala 285, Trp 53, Trp 50, Tyr 33, Tyr 64, Pro 55, Tyr 129, Leu 74, Tyr 133, Ile 140, Val 68, Phe 144 |
| Squalene | −46.861 | Leu 271, Leu 30, Phe 269, Gly 146, Tyr 133, Leu 72, Leu 74, Phe 87, Tyr 129, Phe 144, Ile 140, Val 68, Trp 53, Trp 50, Pro 55, Tyr 33, Val 51, Leu 37, Ala 285, Ala 281, Tyr 64, Tyr 148, Asp 147, His 145, Tyr 155, Asp 150, Pro 26, Ile 151, Thr 154 |
| Aldicarb (Standard drug) | −24.267 | Tyr 133, Val 68, Ser 69, Tyr 64, Pro 55, Tyr 129, Val 65, Tyr 33, Trp 50, Trp 53, Lys 52, Val 51 |

In silico Larvicidal of S. virgulata test and spines zoochemicals, against JHBP (A: 9-octadecenoic acid; B: geraniol; C: n-hexadecanoic acid; D: squalene; E: Aldicarb; a: 3D docked view and b: 2D view).
The geraniol nanoemulsions killed 94% of H. armigera and S. litura larvae, respectively. Thus, the study identified geraniol's possible larvicidal properties, which may be used to formulate biocides for the efficient management of phytopathogenic insects [82]. Rahuman et al. [83] investigated the mosquito-larvicide properties of n-hexadecanoic acid, which was extracted from Feronia limonia leaves using acetone. It was found to be effective against fourth instar larvae of C. quinquefasciatus, A. stephensi, and A. aegypti, with LC50 values of 129.24, 79.58, and 57.23 µg/ml, respectively. In vitro study observed the highest activity of larvicidal (LC50 153.205 µg/ml) compared to cytotoxicity (EC50 194.68 µg/ml); similarly, in silico study observed squalene, n-hexadecanoic acid, and 9-octadecenoic acid, where the highest activity was observed in molecular target on larvicidal (JHBP; PDB ID: 5V13) compared with AChE (PDB ID: 1DX4). One potential mechanism of action of natural insecticides that results in high insect pest mortality is inhibition of AChE activity [84].
Several novel classes of compounds have demonstrated insecticidal, herbicidal, and fungicidal properties, highlighting marine natural products as a promising source for the development of novel agrochemical agents [85]. Ethanolic extracts from marine sponges exhibited insecticidal activity against the fifth instar larvae of Culex quinquefasciatus, Achaea janata, and Pericallia ricini. Among the twelve sponges screened, larval growth inhibition ranged from 9–70% for A. janata and 10–96% for P. ricini. These findings indicate that sponge-derived secondary metabolites serve as effective biopesticides against C. quinquefasciatus larvae and lepidopteran pests [86].
In vivo studies utilizing methanolic extracts of crab shells were conducted on zebrafish (Danio rerio) to evaluate inflammatory responses in a CuSO₄-induced model. The results revealed that crab shell extract significantly increased oxidative stress enzyme mRNA expression and total antioxidant capacity by 1.3–2.15-fold. Furthermore, pro-inflammatory cytokine mRNA levels were downregulated by 1.3–2-fold compared to CuSO₄-induced zebrafish. The shell extract of the Nile crab (Potamonautes niloticus) was found to be rich in antioxidant and anti-inflammatory compounds [87]. Similarly, Galal-Khallaf et al. investigated the shell extract of Charybdis natator, testing both low and high doses for their ability to mitigate copper-induced oxidative stress and pro-inflammatory responses in adult Danio rerio [88]. The current study demonstrated that the zoo extract inhibited the AChE enzyme, which was confirmed by in silico studies and the target insects' death. The sea urchin S. virgulata also contained the bioactive substances geraniol and n-hexadecanoic acid. According to an in-silico study, the 50% ethanolic extract of sea urchin S. virgulata (test and spine) currently has insecticide qualities based on the presence of pesticide compounds.
3.5 Statistical examination of in silico insecticidal efficacy employing correlation methodologies
Table 7 presents the correlation matrix among the insecticidal molecular targets (N = 4 zoochemicals), indicating that the S. virgulata test and spines zoochemicals exhibit comparable involvement in insecticidal activity across all selected molecular targets: AChE (PDB ID: 1DX4), OBP (PDB ID: 3K1E), and JHBP (PDB ID: 5V13). The correlations are exceptionally strong, ranging from r = 0.977 to 0.995, underscoring a positive relationship between the insecticide molecular targets and robust evidence of the insecticidal properties of S. virgulata test and spines against detrimental pests via AChE enzyme inhibition, repellence, and larval toxicity. We advocate for the utilization of zoo-waste material from the sea urchin S. virgulata, specifically its test, and spines, as effective strategies for pest control through repellent and toxic properties.
Correlation matrix of between the insecticidal molecular targets (N = 4 zoochemicals)
| Insecticidal molecular target | AChE (PDB ID: 1DX4) | OBP (PDB ID: 3K1E) | JHBP (PDB ID: 5V13) |
|---|---|---|---|
| AChE (PDB ID: 1DX4) | 1 | ||
| OBP (PDB ID: 3K1E) | 0.995 | 1 | |
| JHBP (PDB ID: 5V13) | 0.992 | 0.977 | 1 |
4 Conclusions
In the sea urchin S. virgulata test and spines 50% ethanol extract, there were 40 zoochemicals. These zoochemicals included four pesticide compounds, such as geraniol, 9-octadecenoic acid, n-hexadecanoic acid, and squalene. The GC–MS techniques were utilized. With models of in vitro AChE inhibitions, cytotoxicity, repellents, larvae toxicity, and in silico, it was determined that the obtained sea urchin zoo extract exhibited insecticidal activity in a dose-dependent manner. Zoochemicals from the S.virgulata zoo-extract were found to have a similar mode of action on insecticidal molecular targets in the computational studies. A correlation statistical model (r = range 0.977–0.995) was used for confirmation. Overall, the zoological insecticides derived from the test and spine extracts of S. virgulata were as effective as botanical insecticides against harmful pests and could serve as viable alternatives to synthetic pesticides. To reduce environmental pollution and provide an eco-friendly alternative to synthetic chemicals, we suggest utilizing naturally occurring zoo-waste materials such as marine sponges, sea urchins, arthropod shells, and Mollusca shells, along with the synthesis of metallic nanoparticles.
Acknowledgments
This research was supported by Zuoying Armed Forces General Hospital (grant MND-MAB-D-114242) and the Ministry of Science and Technology, Taiwan (NSTC-113-2314-B-283-001). The authors extend their appreciation to the Researchers Supporting Project number (RSPD2025R677), King Saud University, Riyadh, Saudi Arabia, for financial support.
-
Funding information: This research was supported by Zuoying Armed Forces General Hospital (grant MND-MAB-D-114242) and the Ministry of Science and Technology, Taiwan (NSTC-113-2314-B-283-001). The authors extend their appreciation to the Researchers Supporting Project number (RSPD2025R677), King Saud University, Riyadh, Saudi Arabia for financial support.
-
Author contributions: Conceptualization, methodology, software K.R. and R.V.; validation, K.R., S.V.R., and U.N.G.; formal analysis, S.B., S.V.R.; investigation, S.M., S.N.G., and Y.H.L.; resources, Z.H.W., A.H.H.; data curation, S.M., Z.H.W.; writing – original draft preparation, K.R., S.B.; writing – review and editing, S.B., R.V., Y.H.L., and S.G.K.; supervision, S.G.K.; funding acquisition, Y.H.L. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: The authors state no conflict of interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: All data used to support the finding of this study are available from the corresponding author upon request.
References
[1] Nowakowski AC, Miller AC, Miller ME, Xiao H, Wu X. Potential health benefits of edible insects. Crit Rev Food Sci Nutr. 2022;62(13):3499–508. 10.1080/10408398.2020.1867053.Search in Google Scholar PubMed
[2] Hung KJ, Kingston JM, Albrecht M, Holway DA, Kohn JR. The worldwide importance of honey bees as pollinators in natural habitats. Proc Biol Sci. 2018;285(1870):20172140. 10.1098/rspb.2017.2140.Search in Google Scholar PubMed PubMed Central
[3] Kumar R, Rathor VS. Nature and types of damage by insect pests. J Entomol Res. 2020;44:639–46. 10.5958/0974-4576.2020.00106.1.Search in Google Scholar
[4] Gangmei K, Bora B, Mandodan S, Abhisubesh V, Aneha K, Manikandan S, et al. A review on vector borne diseases and various strategies to control mosquito vectors. Indian J Entomol. 2024;86(1):329–38. 10.55446/IJE.2023.410.Search in Google Scholar
[5] Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW. Epidemic arboviral diseases: Priorities for research and public health. Lancet Infect Dis. 2017;17(3):e101–6. 10.1016/S1473-3099(16)30518-7.Search in Google Scholar PubMed
[6] Hikal WM, Baeshen RS, Said-Al Ahl HA. Botanical insecticide as simple extractives for pest control. Cogent Biol. 2017;3(1):1404274. 10.1080/23312025.2017.1404274.Search in Google Scholar
[7] Muhammed M, Dugassa S, Belina M, Zohdy S, Irish SR, Gebresilassie A. Insecticidal effects of some selected plant extracts against Anopheles stephensi (Culicidae: Diptera). Malar J. 2022;21(1):295. 10.1186/s12936-022-04320-5.Search in Google Scholar PubMed PubMed Central
[8] Wink M. Production and application of phytochemicals from an agricultural perspective. Phytochem agri. 1993;34:171–213.10.1093/oso/9780198577621.003.0010Search in Google Scholar
[9] Ribeiro MAF, Vieira TF, Fernandes MJG, Pereira RB, Pereira DM, Castanheira EMS, et al. Synthesis and in silico evaluation of potential insecticide activity of benzamides. Chem Proc. 2022;8(1):65. 10.3390/ecsoc-25-11770.Search in Google Scholar
[10] Yousafi Q, Sarfaraz A, Saad Khan M, Saleem S, Shahzad U, Khan AA, et al. In silico annotation of unreviewed acetylcholinesterase (AChE) in some lepidopteran insect pest species reveals the causes of insecticide resistance. Saudi J Biol Sci. 2021;28(4):2197–2209. 10.1016/j.sjbs.2021.01.007.Search in Google Scholar PubMed PubMed Central
[11] Wu D, Wang L, Li W, Li X. Identifying a new target for BtOBP8: Discovery of a small amino ketone molecule containing benzothiazole fragments. J Agric Food Chem. 2023;71(46):17635–45. 10.1021/acs.jafc.3c02594.Search in Google Scholar PubMed
[12] Zalewska M, Kochman A, Estève JP, Lopez F, Chaoui K, Susini C, et al. Juvenile hormone binding protein traffic – Interaction with ATP synthase and lipid transfer proteins. Biochim Biophys Acta. 2009;1788(9):1695–705. 10.1016/j.bbamem.2009.04.022.Search in Google Scholar PubMed
[13] Karnan R, Sukumaran M, Velavan S. Extraction and identification of zoochemicals in marine sponge Hyattella intestinalis (Lamarck, 1814) (Phylum: Porifera) using GC-MS technique. Intern J Zool Invest. 2022;8:113–8. 10.33745/ijzi.2022.v08i0s.01.Search in Google Scholar
[14] Lindshield B. Kansas State University human nutrition (FNDH 400) flexbook. Manhattan: Kansas State University Libraries, New Prairie Press; 2018.Search in Google Scholar
[15] Saravanan R, Jawahar P, Francis T, Ahilan B, Santhakumar R, Gopakumar G. Echinoid landings at Mandapam, South-east Coast of India with a note on gonadal maturity of two species of sea urchins. Indian J Fish. 2017;64:190–3. 10.21077/ijf.2017.64.Search in Google Scholar
[16] Karnan R, Sukumaran M, Mariappan P, Velavan S. Zoo-mediated CuO nanoparticles synthesis using sea urchin Salmacis virgulata (L. Agassiz & Desor, 1846) test as a novel reducing agent and evaluated the in silico antifungal activity. Uttar Pradesh J zool. 2023;44:154–67. 10.56557/upjoz/2023/v44i223730.Search in Google Scholar
[17] Daud NS, Fauziah Y. Identification of sea urchin gonads chemical compounds using thin-layer chromatography from Bokory island, Southeast Sulawesi. J Phys Conf Ser. 2021;1899:012050. 10.1088/1742-6596/1899/1/012050.Search in Google Scholar
[18] Venkatraman C, Padmanaban P. On a collection of shallow-water echinoderms of Gulf of Mannar Biosphere Reserve, southern India. Rec Zool Surv India. 2013;113:95–114. 10.26515/rzsi/v113/i1/2013/121936.Search in Google Scholar
[19] Gomathi D, Kalaiselvi M, Ravikumar G, Devaki K, Uma C. GC-MS analysis of bioactive compounds from the whole plant ethanolic extract of Evolvulus alsinoides (L.) L. J Food Sci Technol. 2015;52(2):1212–7. 10.1007/s13197-013-1105-9.Search in Google Scholar PubMed PubMed Central
[20] Maazoun AM, Hlel TB, Hamdi SH, Belhadj F, Jemâa JMB, Marzouki MN. Screening for insecticidal potential and acetylcholinesterase activity inhibition of Urginea maritima bulbs extract for the control of Sitophilus oryzae (L.). J Asia-Pac Entomol. 2017;20:752–60. 10.1016/j.aspen.2017.04.004.Search in Google Scholar
[21] Chaubey MK. Insecticidal activity of Trachyspermum ammi (Umbelliferae), Anethum graveolens (Umbelliferae) and Nigella sativa (Ranunculaceae) essential oils against stored-product beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Afr J Agric Res. 2007;2:596–600.Search in Google Scholar
[22] Hematpoor A, Liew SY, Azirun MS, Awang K. Insecticidal activity and the mechanism of action of three phenylpropanoids isolated from the roots of Piper sarmentosum Roxb. Sci Rep. 2017;7(1):12576. 10.1038/s41598-017-12898-z.Search in Google Scholar PubMed PubMed Central
[23] Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. 10.1016/0006-2952(61)90145-9.Search in Google Scholar PubMed
[24] Yu X, Zhang Y, Yang M, Guo J, Xu W, Gao J, et al. Cytotoxic effects of tebufenozide in vitro bioassays. Ecotoxicol Env Saf. 2016;129:180–8. 10.1016/j.ecoenv.2016.03.025.Search in Google Scholar PubMed
[25] Pandya N, Thakkar B, Pandya P, Parikh P. Evaluation of insecticidal potential of organochemicals on SF9 cell line. JoBAZ. 2021;82:60. 10.1186/s41936-021-00257-4.Search in Google Scholar
[26] Obeng-Ofori D, Reichmuth CH, Bekele AJ, Hassanali A. Toxicity and protectant potential of camphor, a major component of essential oil of Ocimum kilimandscharicum, against four stored product beetles. Int J Pest Manag. 1998;44:203–9. 10.1080/096708798228112.Search in Google Scholar
[27] Padin SB, Fuse CB, Urrutia MI, Dal Bello G. Toxicity and repellency of nine medicinal plants against Tribolium castaneum in stored wheat. Bull Insectology. 2013;66:45–9.Search in Google Scholar
[28] McDonald LL, Guy RH, Speirs RD. Preliminary evaluation of new candidate materials as toxicants, repellents and attractants against stored product insects. Marketing Research Report no. 882. Washington: Agricultural Research Service, US Department of Agri; 1970. p. 183.Search in Google Scholar
[29] Mazzonetto F, Vendramim JD. Aspectos biológicos de Zabrotes subfasciatus (Boh.) (Coleoptera: Bruchidae) em genótipos de feijoeiro com e sem arcelina. Neotrop Entomol. 2002;31:435–9.10.1590/S1519-566X2002000300013Search in Google Scholar
[30] Pecor D. Mosquito identification guide: Guam. Walter reed biosystematics unit. Meseum support center, Smithsonian Institution, Suitland, USA; 2022. https://vectormap.si.edu/downloads/VHazardReports/Mosquito%20Vector%20Identification%20Guide%20Guam.pdf.Search in Google Scholar
[31] Gerberg EJ, Barnard DR, Ward RA. Manual for mosquito rearing and experimental techniques. Lake Charles, USA: American Mosquito Control Association, Inc.; 1994. p. 98.Search in Google Scholar
[32] Soni N, Dhiman RC. Larvicidal and antibacterial activity of aqueous leaf extract of Peepal (Ficus religiosa) synthesized nanoparticles. Parasite Epidemiol Control. 2020;11:e00166. 10.1016/j.parepi.2020.e00166.Search in Google Scholar PubMed PubMed Central
[33] World Health Organization. Guidelines for laboratory and field testing of Mosquito Larvicides. Geneva, Switzerland: WHO headquarters in Geneva. WHO/CDS/WHOPES/GCDPP/13; 2005. https://iris.who.int/handle/10665/69101.Search in Google Scholar
[34] Kumar P, Alpana A, Dinesh C, Abhinav B. In silico screening and molecular docking of bioactive agents towards human coronavirus receptor. GSC Biol Pharm Sci. 2020;11:132–40. 10.30574/gscbps.2020.11.1.0099.Search in Google Scholar
[35] Ayodele PF, Bamigbade A, Bamigbade OO, Adeniyi IA, Tachin ES, Seweje AJ, et al. Illustrated procedure to perform molecular docking using PyRx and biovia discovery studio visualizer: A case study of 10kt with atropine. Progr Drug Discov Biomed Sci. 2023;6(1):1–33. 10.36877/pddbs.a0000424.Search in Google Scholar
[36] Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput chem. 2010;31:455–61. 10.1002/jcc.21334.Search in Google Scholar PubMed PubMed Central
[37] Chen W, Viljoen AM. Geraniol–A review update. S Afr J Bot. 2022;150:1205–19. 10.1016/j.sajb.2022.09.012.Search in Google Scholar
[38] Kareem D. Antimicrobial activity of cinnamaldehyde or geraniol alone or combined with high pressure processing to destroy Escherichia coli O157: H7 and Salmonella enterica in juices. Doctoral dissertation. Ames, IA, USA: Iowa State University; 2016.Search in Google Scholar
[39] Fajdek-Bieda A, Pawlińska J, Wróblewska A, Łuś A. Evaluation of the antimicrobial activity of geraniol and selected geraniol transformation products against gram-positive bacteria. Molecules. 2024;29:950. 10.3390/molecules29050950.Search in Google Scholar PubMed PubMed Central
[40] Chen W, Viljoen AM. Geraniol-A review of a commercially important fragrance material. S Afr J Bot. 2010;76(4):643–51. 0.1016/j.sajb.2010.05.008.Search in Google Scholar
[41] Natarajan P, Singh S, Balamurugan K. Gas chromatography-mass spectrometry (GC-MS) analysis of bio active compounds presents in Oeophylla smaragdina. Res J Pharm Technol. 2019;12:2736–41. 10.5958/0974-360X.2019.00458.X.Search in Google Scholar
[42] Amudha P, Jayalakshmi M, Pushpabharathi N, Vanitha V. Identification of bioactive components in Enhalus acoroides seagrass extract by gas chromatography-mass spectrometry. Asian J Pharm Clin Res. 2018;11:313–5. 10.22159/ajpcr.2018.v11i10.25577.Search in Google Scholar
[43] Mallick SS, Dighe VV. Detection and estimation of alpha-amyrin, beta-sitosterol, lupeol and n-tricontane in two medicinal plants by high performance thin layer chromatography. Adv Chem. 2014;1:143948. org/10.1155/2014/143948.Search in Google Scholar
[44] Farag SM, Essa EE, Alharbi SA, Alfarraj S, Abu El-Hassan GMM. Agro-waste derived compounds (flax and black seed peels): Toxicological effect against the West Nile virus vector, Culex pipiens L. with special reference to GC-MS analysis. Saudi J Biol Sci. 2021;28(9):5261–7. 10.1016/j.sjbs.2021.05.038.Search in Google Scholar PubMed PubMed Central
[45] Hema R, Kumaravel S, Alagusundaram K. GC/MS determination of bioactive components of Murraya koenigii. J Am Sci. 2011;7(1):80–3.Search in Google Scholar
[46] Kumaravel S, Muthukumaran P, Shanmugapriya K. Chemical composition of Trigonella foenum-graecum through gas chromatography mass spectrometry analysis. J Med Plants Stud. 2017;5(3):1–3.Search in Google Scholar
[47] Sermakkani M, Thangapandian V. GC-MS analysis of Cassia italica leaf methanol extract. Asian J Pharm Clin Res. 2012;5:90–4.Search in Google Scholar
[48] Devakumar J, Keerthana V, Sudha SS. Identification of bioactive compounds by gas chromatography-mass spectrometry analysis of Syzygium jambos (L.) collected from Western Ghats region Coimbatore, Tamil Nadu. Asian J Pharm Clin Res. 2017;10:364–9. 10.22159/ajpcr.2017.v10i1.15508.Search in Google Scholar
[49] Youssef AM, Maaty DA, Al-Saraireh YM. Phytochemical analysis and profiling of antioxidants and anticancer compounds from Tephrosia purpurea (L.) subsp. apollinea family Fabaceae. Molecules. 2023;28(8):3939. 10.3390/molecules28093939.Search in Google Scholar PubMed PubMed Central
[50] Chen YF, Wu KJ, Siao LR, Tsai HY. Trilinolein a natural triacylglycerol, protects cerebral ischemia through inhibition of neuronal apoptosis and ameliorates intimal hyperplasia via attenuation of migration and modulation of matrix metalloproteinase-2 and ras/mek/erk signaling pathway in VSMCs. Int J Mol Sci. 2022;23(21):12820. 10.3390/ijms232112820.Search in Google Scholar PubMed PubMed Central
[51] Ali HAM, Mohammed YH, Imad HH. Determination of metabolites products by Cassia angustifolia and evaluate antimicobial activity. J Pharmacogn Phytother. 2016;8(2):25–48. 10.5897/JPP2015.0367.Search in Google Scholar
[52] Bhat MP, Kumar RS, Chakraborty B, Nagaraja SK, Gireesh Babu K, Nayaka S. Eicosane: An antifungal compound derived from Streptomyces sp. KF15 exhibits inhibitory potential against major phytopathogenic fungi of crops. Env Res. 2024;251(Pt 1):118666. 10.1016/j.envres.2024.118666.Search in Google Scholar PubMed
[53] Dougherty M, Hamilton G. Dodecanoic acid is the oviposition pheromone of Lutzomyia longipalpis. J Chem Ecol. 1997;23:2657–71. 10.1023/A:1022598523803.Search in Google Scholar
[54] Tulandi SM, Tanzil L, Ulfa DM. Analysis of bioactive compounds from methanol extract of Diadema setosum sea urchin gonads using gas Chromatography-mass spectrometry. Res J Pharm Techn. 2021;14(3):1629–34. 10.5958/0974-360X.2021.00289.4.Search in Google Scholar
[55] Georgiev B, Nikolova M, Aneva I, Dzhurmanski A, Sidjimova B, Berkov S. Plant products with acetylcholinesterase inhibitory activity for insect control. BioRisk. 2022;17:309–15. 10.3897/biorisk.17.77052.Search in Google Scholar
[56] Hung NH, Quan PM, Satyal P, Dai DN, Hoa VV, Huy NG, et al. Acetylcholinesterase inhibitory activities of essential oils from Vietnamese traditional medicinal plants. Molecules. 2022;27(20):7092. 10.3390/molecules27207092.Search in Google Scholar PubMed PubMed Central
[57] Kilic M, Orhan IE, Eren G, Okudan ES, Estep AS, Bencel JJ, et al. Insecticidal activity of forty-seven marine algae species from the Mediterranean, Aegean, and Sea of Marmara in connection with their cholinesterase and tyrosinase inhibitory activity. South Afr J Bot. 2021;143:435–42. 10.1016/j.sajb.2021.06.038.Search in Google Scholar
[58] Karnan R, Sukumaran M, Mariappan P, Velavan S. Insecticidal effect of zoochemicals mediated copper oxide nanoparticle using marine sponge Hyattella intestinalis (Lamarck, 1814) and molecular docking. Uttar Pradesh J Zool. 2023;44(15):64–72. 10.56557/upjoz/2023/v44i153570.Search in Google Scholar
[59] Mattar VT, Borioni JL, Hollmann A, Rodriguez SA. Insecticidal activity of the essential oil of Schinus areira against Rhipibruchus picturatus (F.) (Coleoptera: Bruchinae), and its inhibitory effects on acetylcholinesterase. Pestic Biochem Physiol. 2022;185:105134. 10.1016/j.pestbp.2022.105134.Search in Google Scholar PubMed
[60] Colović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr Neuropharmacol. 2013;11(3):315–35. 10.2174/1570159X11311030006.Search in Google Scholar PubMed PubMed Central
[61] Knorr DY, Demirbas D, Heinrich R. Multifaceted promotion of apoptosis by acetylcholinesterase. Front Cell Death. 2023;2:1169966. 10.3389/fceld.2023.1169966.Search in Google Scholar
[62] Saleh M, Ezz-Din D, Al-Masri A. In vitro genotoxicity study of the lambda-cyhalothrin insecticide on Sf9 insect cells line using Comet assay. Jordan. J Biol Sci. 2021;14(2):213–7. 10.54319/jjbs/140203.Search in Google Scholar
[63] de Castro Oliveira JA, Garcia IP, Corrêa EJA, de Lima LHF, de Lima Santos H, de Assis RMA, et al. Larvicidal susceptibility of essential oils from Cinnamodendron dinisii, Callistemon viminalis and Myrcia tomentosa against Culex quinquefasciatus (Say)(Diptera: Culicidae). South Afr J Bot. 2023;63:95–104. 10.1016/j.sajb.2023.10.026.Search in Google Scholar
[64] Benslama O. Bioinsecticidal activity of actinomycete secondary metabolites against the acetylcholinesterase of the legume's insect pest Acyrthosiphon pisum: A computational study. J Genet Eng Biotechnol. 2022;20(1):158. 10.1186/s43141-022-00442-0.Search in Google Scholar PubMed PubMed Central
[65] Loza-Mejía MA, Salazar JR, Sánchez-Tejeda JF. In Silico studies on compounds derived from Calceolaria: Phenylethanoid glycosides as potential multitarget inhibitors for the development of pesticides. Biomolecules. 2018;8(4):121. 10.3390/biom8040121.Search in Google Scholar PubMed PubMed Central
[66] Silva LB, Ferreira EFB, Maryam, Espejo-Román JM, Costa GV, Cruz JV, et al. Galantamine based novel acetylcholinesterase enzyme inhibitors: A molecular modeling design approach. Molecules. 2023;28(3):1035. 10.3390/molecules28031035.Search in Google Scholar PubMed PubMed Central
[67] de Almeida RBM, Barbosa DB, do Bomfim MR, Amparo JAO, Andrade BS, Costa SL, et al. Identification of a novel dual inhibitor of acetylcholinesterase and butyrylcholinesterase: In vitro and in silico studies. Pharm (Basel). 2023;16(1):95. 10.3390/ph16010095.Search in Google Scholar PubMed PubMed Central
[68] da Costa KS, Galúcio JM, da Costa CHS, Santana AR, Dos Santos Carvalho V, do Nascimento LD, et al. Exploring the potentiality of natural products from essential oils as inhibitors of odorant-binding proteins: A structure- and ligand-based virtual screening approach to find novel mosquito repellents. ACS Omega. 2019;4(27):22475–86. 10.1021/acsomega.9b03157.Search in Google Scholar PubMed PubMed Central
[69] Venthur H, Zhou JJ. Odorant receptors and odorant-binding proteins as insect pest control targets: A comparative analysis. Front Physiol. 2018;9:1163. 10.3389/fphys.2018.01163.Search in Google Scholar PubMed PubMed Central
[70] Ramsha A, Saleem KA, Saba B. Repellent activity of certain plant extracts (clove, coriander, neem and mint) against red flour beetle. Am Sci Res J Eng Technol Sci. 2019;55(1):83–91.Search in Google Scholar
[71] Murugesan R, Vasuki K, Kaleeswaran B, Santhanam P, Ravikumar S, Alwahibi MS, et al. Insecticidal and repellent activities of Solanum torvum (Sw.) leaf extract against stored grain Pest, Callosobruchus maculatus (F.)(Coleoptera: Bruchidae). J King Saud Univ-Sci. 2021;33(3):101390. 10.1016/j.jksus.2021.101390.Search in Google Scholar
[72] Asiry KA, Almasoudi NM, Zaitoun AA. Insecticidal and repellent actions of methanolic extracts from five medicinal plants against the saw-toothed grain beetle Oryzaephilus surinamensis (Linn.) (Coleoptera: Silvanidae). Rev de la Soc Entomológica Argentina. 2022;81(3):1–16.10.25085/rsea.810304Search in Google Scholar
[73] Zhou JJ. Odorant-binding proteins in insects. Vitam Horm. 2010;83:241–72. 10.1016/S0083-6729(10)83010-9.Search in Google Scholar PubMed
[74] Neto MFDA, Santos CBRD, Magalhães-Junior JT, Leite FHA. Identification of novel Aedes aegypti odorant-binding protein 1 modulators by ligand and structure-based approaches and bioassays. J Biomol Struct Dyn. 2022;40(1):117–29. 10.1080/07391102.2020.1808074.Search in Google Scholar PubMed
[75] Kamaraj C, Bagavan A, Elango G, Zahir AA, Rajakumar G, Marimuthu S, et al. Larvicidal activity of medicinal plant extracts against Anopheles subpictus & Culex tritaeniorhynchus. Indian J Med Res. 2011;134:101–6.10.1007/s00436-010-1816-zSearch in Google Scholar PubMed
[76] Bharathi JD, Suseem SR. Larvicidal activity of CuO and ZnO nanoparticles against Aedes aegypti and Anopheles stephensi mosquito vectors-a greener approach by Phaseolus vulgaris L. aqueous extract as bio-reductant. Results Chem. 2024;7:101408. 10.1016/j.rechem.2024.101408.Search in Google Scholar
[77] Hasaballah AI, El-Naggar HA, Abdelbary S, Bashar MA, Selim TA. Eco-friendly synthesis of zinc oxide nanoparticles by marine sponge, Spongia officinalis: antimicrobial and insecticidal activities against the mosquito vectors, Culex pipiens and Anopheles pharoensis. Bio Nano Sci. 2022;12(1):1–16. 10.1007/s12668-021-00926-2.Search in Google Scholar
[78] Alawode TT. Identification of Potential Aedes aegypti Juvenile Hormone Inhibitors from Methanol Extract of Leaves of Solanum erianthum: An In Silico Approach. Commun Phys Sci. 2024;11(4):669–79.Search in Google Scholar
[79] Duraisamy R, Al-Shar'i NA, Chandrashekharappa S, Deb PK, Gleiser RM, Tratrat C, et al. Synthesis, biological evaluation, and computational investigation of ethyl 2,4,6-trisubstituted-1,4-dihydropyrimidine-5-carboxylates as potential larvicidal agents against Anopheles arabiensis. J Biomol Struct Dyn. 2024;42(8):4016–28. 10.1080/07391102.2023.2217929.Search in Google Scholar PubMed
[80] de Sousa dos Santos ELV, Cruz JN, da Costa GV, de Sá EMF, da Silva AKP, Fernandes CP, et al. Essential oil of Ocimum basilicum against Aedes aegypti and Culex quinquefasciatus: Larvicidal activity of a nanoemulsion and in silico study. Separations. 2024;11(4):97. 10.3390/separations11040097.Search in Google Scholar
[81] Ribeiro AN, Lopes SQ, Marinho VHS, Araújo IF, Ramos RDS, Souto RN, et al. Valorization of deodorizing distillate palm oil residue for larvicidal activity against Aedes aegypti and synergistic effect of their free fatty acids. Waste Biomass Valor. 2024;15:3367–77. 10.21203/rs.3.rs-3135842/v1.Search in Google Scholar
[82] Kaur G, Ganjewala D, Bist V, Verma PC. Antifungal and larvicidal activities of two acyclic monoterpenes; citral and geraniol against phytopathogenic fungi and insects. Arch phytopathol Pflanzenschutz. 2019;52(5-6):458–69. 10.1080/03235408.2019.1651579.Search in Google Scholar
[83] Rahuman AA, Gopalakrishnan G, Ghouse BS, Arumugam S, Himalayan B. Effect of Feronia limonia on mosquito larvae. Fitoterapia. 2000;71(5):553–5. 10.1016/s0367-326x(00)00164-7.Search in Google Scholar PubMed
[84] López MD, Pascual-Villalobos MJ. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind Crop Prod. 2010;31:284–8. 10.1016/j.indcrop.2009.11.005.Search in Google Scholar
[85] Peng J, Shen X, El Sayed KA, Dunbar DC, Perry TL, Wilkins SP, et al. Marine natural products as prototype agrochemical agents. J Agric Food Chem. 2003;51(8):2246–52. 10.1021/jf0207880.Search in Google Scholar PubMed PubMed Central
[86] Joseph B, Sujatha S, Jeevitha MV. Screening of pesticidal activities of some marine sponge extracts against chosen pests. J Biopest. 2010;3(2):495. 10.57182/jbiopestic.3.2.495-498.Search in Google Scholar
[87] Galal-Khallaf A, Al-Awthan YS, Al-Duais MA, Mohammed-Geba K. Nile crab Potamonautes niloticus shell extract: Chromatographic and molecular elucidation of potent antioxidant and anti-inflammatory capabilities. Bioorg Chem. 2022;127:106023. 10.1016/j.bioorg.2022.106023.Search in Google Scholar PubMed
[88] Galal-Khallaf A, Samir Aboali E, El-Sayed Hassab El-Nabi S, El-Tantawy AI, Schott EJ, Mohammed-Geba K. As healthy as invasive: Charybdis natator shell extract reveals beneficial metabolites with promising antioxidant and anti-inflammatory potentials. Front Mar Sci. 2024;11:1376768. 10.3389/fmars.2024.1376768.Search in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Phytochemical investigation and evaluation of antioxidant and antidiabetic activities in aqueous extracts of Cedrus atlantica
- Influence of B4C addition on the tribological properties of bronze matrix brake pad materials
- Discovery of the bacterial HslV protease activators as lead molecules with novel mode of action
- Characterization of volatile flavor compounds of cigar with different aging conditions by headspace–gas chromatography–ion mobility spectrometry
- Effective remediation of organic pollutant using Musa acuminata peel extract-assisted iron oxide nanoparticles
- Analysis and health risk assessment of toxic elements in traditional herbal tea infusions
- Cadmium exposure in marine crabs from Jiaxing City, China: Insights into health risk assessment
- Green-synthesized silver nanoparticles of Cinnamomum zeylanicum and their biological activities
- Tetraclinis articulata (Vahl) Mast., Mentha pulegium L., and Thymus zygis L. essential oils: Chemical composition, antioxidant and antifungal properties against postharvest fungal diseases of apple, and in vitro, in vivo, and in silico investigation
- Exploration of plant alkaloids as potential inhibitors of HIV–CD4 binding: Insight into comprehensive in silico approaches
- Recovery of phenylethyl alcohol from aqueous solution by batch adsorption
- Electrochemical approach for monitoring the catalytic action of immobilized catalase
- Green synthesis of ZIF-8 for selective adsorption of dyes in water purification
- Optimization of the conditions for the preparation of povidone iodine using the response surface methodology
- A case study on the influence of soil amendment on ginger oil’s physicochemical properties, mineral contents, microbial load, and HPLC determination of its vitamin level
- Removal of antiviral favipiravir from wastewater using biochar produced from hazelnut shells
- Effect of biochar and soil amendment on bacterial community composition in the root soil and fruit of tomato under greenhouse conditions
- Bioremediation of malachite green dye using Sargassum wightii seaweed and its biological and physicochemical characterization
- Evaluation of natural compounds as folate biosynthesis inhibitors in Mycobacterium leprae using docking, ADMET analysis, and molecular dynamics simulation
- Novel insecticidal properties of bioactive zoochemicals extracted from sea urchin Salmacis virgulata
- Elevational gradients shape total phenolic content and bioactive potential of sweet marjoram (Origanum majorana L.): A comparative study across altitudinal zones
- Study on the CO2 absorption performance of deep eutectic solvents formed by superbase DBN and weak acid diethylene glycol
- Preparation and wastewater treatment performance of zeolite-modified ecological concrete
- Multifunctional chitosan nanoparticles: Zn2+ adsorption, antimicrobial activity, and promotion of aquatic health
- Comparative analysis of nutritional composition and bioactive properties of Chlorella vulgaris and Arthrospira platensis: Implications for functional foods and dietary supplements
- Growth kinetics and mechanical characterization of boride layers formed on Ti6Al4V
- Enhancement of water absorption properties of potassium polyacrylate-based hydrogels in CaCl2-rich soils using potassium di- and tri-carboxylate salts
- Electrochemical and microbiological effects of dumpsite leachates on soil and air quality
- Modeling benzene physicochemical properties using Zagreb upsilon indices
- Characterization and ecological risk assessment of toxic metals in mangrove sediments near Langen Village in Tieshan Bay of Beibu Gulf, China
- Protective effect of Helicteres isora, an efficient candidate on hepatorenal toxicity and management of diabetes in animal models
- Valorization of Juglans regia L. (Walnut) green husk from Jordan: Analysis of fatty acids, phenolics, antioxidant, and cytotoxic activities
- Molecular docking and dynamics simulations of bioactive terpenes from Catharanthus roseus essential oil targeting breast cancer
- Selection of a dam site by using AHP and VIKOR: The Sakarya Basin
- Characterization and modeling of kidney bean shell biochar as adsorbent for caffeine removal from aquatic environments
- The effects of short-term and long-term 2100 MHz radiofrequency radiation on adult rat auditory brainstem response
- Biochemical insights into the anthelmintic and anti-inflammatory potential of sea cucumber extract: In vitro and in silico approaches
- Resveratrol-derived MDM2 inhibitors: Synthesis, characterization, and biological evaluation against MDM2 and HCT-116 cells
- Phytochemical constituents, in vitro antibacterial activity, and computational studies of Sudanese Musa acuminate Colla fruit peel hydro-ethanol extract
- Chemical composition of essential oils reviewed from the height of Cajuput (Melaleuca leucadendron) plantations in Buru Island and Seram Island, Maluku, Indonesia
- Phytochemical analysis and antioxidant activity of Azadirachta indica A. Juss from the Republic of Chad: in vitro and in silico studies
- Stability studies of titanium–carboxylate complexes: A multi-method computational approach
- Efficient adsorption performance of an alginate-based dental material for uranium(vi) removal
- Synthesis and characterization of the Co(ii), Ni(ii), and Cu(ii) complexes with a 1,2,4-triazine derivative ligand
- Evaluation of the impact of music on antioxidant mechanisms and survival in salt-stressed goldfish
- Optimization and validation of UPLC method for dapagliflozin and candesartan cilexetil in an on-demand formulation: Analytical quality by design approach
- Biomass-based cellulose hydroxyapatite nanocomposites for the efficient sequestration of dyes: Kinetics, response surface methodology optimization, and reusability
- Multifunctional nitrogen and boron co-doped carbon dots: A fluorescent probe for Hg2+ and biothiol detection with bioimaging and antifungal applications
- Separation of sulphonamides on a C12-diol mixed-mode HPLC column and investigation of their retention mechanism
- Characterization and antioxidant activity of pectin from lemon peels
- Fast PFAS determination in honey by direct probe electrospray ionization tandem mass spectrometry: A health risk assessment insight
- Correlation study between GC–MS analysis of cigarette aroma compounds and sensory evaluation
- Synthesis, biological evaluation, and molecular docking studies of substituted chromone-2-carboxamide derivatives as anti-breast cancer agents
- The influence of feed space velocity and pressure on the cold flow properties of diesel fuel
- Acid etching behavior and mechanism in acid solution of iron components in basalt fibers
- Protective effect of green synthesized nanoceria on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rat
- Evaluation of the antianxiety activity of green zinc nanoparticles mediated by Boswellia thurifera in albino mice by following the plus maze and light and dark exploration tests
- Yeast as an efficient and eco-friendly bifunctional porogen for biomass-derived nitrogen-doped carbon catalysts in the oxygen reduction reaction
- Novel descriptors for the prediction of molecular properties
- Synthesis and characterization of surfactants derived from phenolphthalein: In vivo and in silico studies of their antihyperlipidemic effect
- Turmeric oil-fortified nutraceutical-SNEDDS: An approach to boost therapeutic effectiveness of dapagliflozin during treatment of diabetic patients
- Analysis and study on volatile flavor compounds of three Yunnan cultivated cigars based on headspace-gas chromatography-ion mobility spectrometry
- Near-infrared IR780 dye-loaded poloxamer 407 micelles: Preparation and in vitro assessment of anticancer activity
- Study on the influence of the viscosity reducer solution on percolation capacity of thin oil in ultra-low permeability reservoir
- Special Issue on Advancing Sustainable Chemistry for a Greener Future
- One-pot fabrication of highly porous morphology of ferric oxide-ferric oxychloride/poly-O-chloroaniline nanocomposite seeded on poly-1H pyrrole: Photocathode for green hydrogen generation from natural and artificial seawater
- High-efficiency photocathode for green hydrogen generation from sanitation water using bismuthyl chloride/poly-o-chlorobenzeneamine nanocomposite
- Innovative synthesis of cobalt-based catalysts using ionic liquids and deep eutectic solvents: A minireview on electrocatalytic water splitting
- Special Issue on Phytochemicals, Biological and Toxicological Analysis of Plants
- Comparative analysis of fruit quality parameters and volatile compounds in commercially grown citrus cultivars
- Total phenolic, flavonoid, flavonol, and tannin contents as well as antioxidant and antiparasitic activities of aqueous methanol extract of Alhagi graecorum plant used in traditional medicine: Collected in Riyadh, Saudi Arabia
- Study on the pharmacological effects and active compounds of Apocynum venetum L.
- Chemical profile of Senna italica and Senna velutina seed and their pharmacological properties
- Essential oils from Brazilian plants: A literature analysis of anti-inflammatory and antimalarial properties and in silico validation
- Toxicological effects of green tea catechin extract on rat liver: Delineating safe and harmful doses
- Unlocking the potential of Trigonella foenum-graecum L. plant leaf extracts against diabetes-associated hypertension: A proof of concept by in silico studies
Articles in the same Issue
- Research Articles
- Phytochemical investigation and evaluation of antioxidant and antidiabetic activities in aqueous extracts of Cedrus atlantica
- Influence of B4C addition on the tribological properties of bronze matrix brake pad materials
- Discovery of the bacterial HslV protease activators as lead molecules with novel mode of action
- Characterization of volatile flavor compounds of cigar with different aging conditions by headspace–gas chromatography–ion mobility spectrometry
- Effective remediation of organic pollutant using Musa acuminata peel extract-assisted iron oxide nanoparticles
- Analysis and health risk assessment of toxic elements in traditional herbal tea infusions
- Cadmium exposure in marine crabs from Jiaxing City, China: Insights into health risk assessment
- Green-synthesized silver nanoparticles of Cinnamomum zeylanicum and their biological activities
- Tetraclinis articulata (Vahl) Mast., Mentha pulegium L., and Thymus zygis L. essential oils: Chemical composition, antioxidant and antifungal properties against postharvest fungal diseases of apple, and in vitro, in vivo, and in silico investigation
- Exploration of plant alkaloids as potential inhibitors of HIV–CD4 binding: Insight into comprehensive in silico approaches
- Recovery of phenylethyl alcohol from aqueous solution by batch adsorption
- Electrochemical approach for monitoring the catalytic action of immobilized catalase
- Green synthesis of ZIF-8 for selective adsorption of dyes in water purification
- Optimization of the conditions for the preparation of povidone iodine using the response surface methodology
- A case study on the influence of soil amendment on ginger oil’s physicochemical properties, mineral contents, microbial load, and HPLC determination of its vitamin level
- Removal of antiviral favipiravir from wastewater using biochar produced from hazelnut shells
- Effect of biochar and soil amendment on bacterial community composition in the root soil and fruit of tomato under greenhouse conditions
- Bioremediation of malachite green dye using Sargassum wightii seaweed and its biological and physicochemical characterization
- Evaluation of natural compounds as folate biosynthesis inhibitors in Mycobacterium leprae using docking, ADMET analysis, and molecular dynamics simulation
- Novel insecticidal properties of bioactive zoochemicals extracted from sea urchin Salmacis virgulata
- Elevational gradients shape total phenolic content and bioactive potential of sweet marjoram (Origanum majorana L.): A comparative study across altitudinal zones
- Study on the CO2 absorption performance of deep eutectic solvents formed by superbase DBN and weak acid diethylene glycol
- Preparation and wastewater treatment performance of zeolite-modified ecological concrete
- Multifunctional chitosan nanoparticles: Zn2+ adsorption, antimicrobial activity, and promotion of aquatic health
- Comparative analysis of nutritional composition and bioactive properties of Chlorella vulgaris and Arthrospira platensis: Implications for functional foods and dietary supplements
- Growth kinetics and mechanical characterization of boride layers formed on Ti6Al4V
- Enhancement of water absorption properties of potassium polyacrylate-based hydrogels in CaCl2-rich soils using potassium di- and tri-carboxylate salts
- Electrochemical and microbiological effects of dumpsite leachates on soil and air quality
- Modeling benzene physicochemical properties using Zagreb upsilon indices
- Characterization and ecological risk assessment of toxic metals in mangrove sediments near Langen Village in Tieshan Bay of Beibu Gulf, China
- Protective effect of Helicteres isora, an efficient candidate on hepatorenal toxicity and management of diabetes in animal models
- Valorization of Juglans regia L. (Walnut) green husk from Jordan: Analysis of fatty acids, phenolics, antioxidant, and cytotoxic activities
- Molecular docking and dynamics simulations of bioactive terpenes from Catharanthus roseus essential oil targeting breast cancer
- Selection of a dam site by using AHP and VIKOR: The Sakarya Basin
- Characterization and modeling of kidney bean shell biochar as adsorbent for caffeine removal from aquatic environments
- The effects of short-term and long-term 2100 MHz radiofrequency radiation on adult rat auditory brainstem response
- Biochemical insights into the anthelmintic and anti-inflammatory potential of sea cucumber extract: In vitro and in silico approaches
- Resveratrol-derived MDM2 inhibitors: Synthesis, characterization, and biological evaluation against MDM2 and HCT-116 cells
- Phytochemical constituents, in vitro antibacterial activity, and computational studies of Sudanese Musa acuminate Colla fruit peel hydro-ethanol extract
- Chemical composition of essential oils reviewed from the height of Cajuput (Melaleuca leucadendron) plantations in Buru Island and Seram Island, Maluku, Indonesia
- Phytochemical analysis and antioxidant activity of Azadirachta indica A. Juss from the Republic of Chad: in vitro and in silico studies
- Stability studies of titanium–carboxylate complexes: A multi-method computational approach
- Efficient adsorption performance of an alginate-based dental material for uranium(vi) removal
- Synthesis and characterization of the Co(ii), Ni(ii), and Cu(ii) complexes with a 1,2,4-triazine derivative ligand
- Evaluation of the impact of music on antioxidant mechanisms and survival in salt-stressed goldfish
- Optimization and validation of UPLC method for dapagliflozin and candesartan cilexetil in an on-demand formulation: Analytical quality by design approach
- Biomass-based cellulose hydroxyapatite nanocomposites for the efficient sequestration of dyes: Kinetics, response surface methodology optimization, and reusability
- Multifunctional nitrogen and boron co-doped carbon dots: A fluorescent probe for Hg2+ and biothiol detection with bioimaging and antifungal applications
- Separation of sulphonamides on a C12-diol mixed-mode HPLC column and investigation of their retention mechanism
- Characterization and antioxidant activity of pectin from lemon peels
- Fast PFAS determination in honey by direct probe electrospray ionization tandem mass spectrometry: A health risk assessment insight
- Correlation study between GC–MS analysis of cigarette aroma compounds and sensory evaluation
- Synthesis, biological evaluation, and molecular docking studies of substituted chromone-2-carboxamide derivatives as anti-breast cancer agents
- The influence of feed space velocity and pressure on the cold flow properties of diesel fuel
- Acid etching behavior and mechanism in acid solution of iron components in basalt fibers
- Protective effect of green synthesized nanoceria on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rat
- Evaluation of the antianxiety activity of green zinc nanoparticles mediated by Boswellia thurifera in albino mice by following the plus maze and light and dark exploration tests
- Yeast as an efficient and eco-friendly bifunctional porogen for biomass-derived nitrogen-doped carbon catalysts in the oxygen reduction reaction
- Novel descriptors for the prediction of molecular properties
- Synthesis and characterization of surfactants derived from phenolphthalein: In vivo and in silico studies of their antihyperlipidemic effect
- Turmeric oil-fortified nutraceutical-SNEDDS: An approach to boost therapeutic effectiveness of dapagliflozin during treatment of diabetic patients
- Analysis and study on volatile flavor compounds of three Yunnan cultivated cigars based on headspace-gas chromatography-ion mobility spectrometry
- Near-infrared IR780 dye-loaded poloxamer 407 micelles: Preparation and in vitro assessment of anticancer activity
- Study on the influence of the viscosity reducer solution on percolation capacity of thin oil in ultra-low permeability reservoir
- Special Issue on Advancing Sustainable Chemistry for a Greener Future
- One-pot fabrication of highly porous morphology of ferric oxide-ferric oxychloride/poly-O-chloroaniline nanocomposite seeded on poly-1H pyrrole: Photocathode for green hydrogen generation from natural and artificial seawater
- High-efficiency photocathode for green hydrogen generation from sanitation water using bismuthyl chloride/poly-o-chlorobenzeneamine nanocomposite
- Innovative synthesis of cobalt-based catalysts using ionic liquids and deep eutectic solvents: A minireview on electrocatalytic water splitting
- Special Issue on Phytochemicals, Biological and Toxicological Analysis of Plants
- Comparative analysis of fruit quality parameters and volatile compounds in commercially grown citrus cultivars
- Total phenolic, flavonoid, flavonol, and tannin contents as well as antioxidant and antiparasitic activities of aqueous methanol extract of Alhagi graecorum plant used in traditional medicine: Collected in Riyadh, Saudi Arabia
- Study on the pharmacological effects and active compounds of Apocynum venetum L.
- Chemical profile of Senna italica and Senna velutina seed and their pharmacological properties
- Essential oils from Brazilian plants: A literature analysis of anti-inflammatory and antimalarial properties and in silico validation
- Toxicological effects of green tea catechin extract on rat liver: Delineating safe and harmful doses
- Unlocking the potential of Trigonella foenum-graecum L. plant leaf extracts against diabetes-associated hypertension: A proof of concept by in silico studies

