Abstract
Novel clivia biochar adsorbing daptomycin (DAP) was prepared by microwave digestion–anaerobic carbonization in this work. Fe/Ag submicron particles were introduced to the biochar surface based on the reducibility of biochar to enhance its adsorption capacity. Characterization confirmed that modified biochar (AF-biochar) had a higher particle size (126 μm), larger specific surface area (521.692 m2 g−1), richer pore structure, and higher thermal stability. The effects of the main variables (e.g., the solution pH, contact time, initial DAP concentration, and temperature) were investigated during adsorption. The results showed that AF-biochar could reach the adsorption equilibrium at pH 4.8 for 85 min. Besides, the adsorption capacity was 48.25 mg g−1, and the adsorption efficiency was 96.50% when the concentration of DAP was 25 mg L−1. The pseudo-second-order kinetics (R 2 = 0.9997), Langmuir equation (R 2 = 0.9999), and thermodynamics (R 2 = 0.9631) of AF-biochar fit well, indicating that the main adsorption process of AF-biochar was spontaneous, exothermic, and monolayer. Their adsorption was analyzed by physical and chemical adsorption. The main adsorption mechanisms included the electron donor–acceptor interaction, electrostatic force interaction, Lewis acid–base interaction, and H-bond interaction.
Graphical abstract
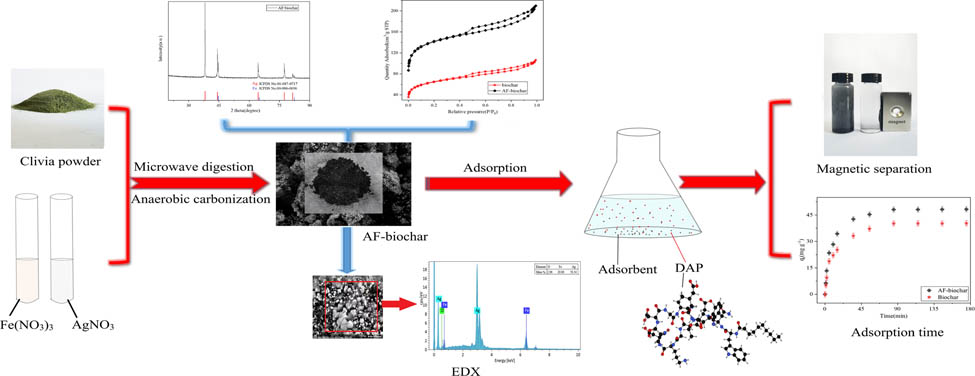
1 Introduction
Over the past decade, pharmaceutical compounds have received increasing attention for their potential environmental effects [1]. Antibiotics are widely used in preventing and treating bacterial infections of humans, animals, and plants [2]. However, some antibiotics cannot be completely metabolized and excreted out of the body, thus becoming one of the sources of antibiotics in the aquatic environment [3]. With the widespread use of antibiotics, one can detect antibiotics in surface water, groundwater, hospital wastewater, and even in drinking water [4]. Therefore, antibiotics are considered a new worldwide water-borne pollutant [5], and developing an effective strategy for removing antibiotics in water is essential [6].
Daptomycin (DAP) is a new cyclic lipopeptide antibiotic, which is acidic, highly water-soluble, and active against Gram-positive organisms [7,8,9]. It is extracted from the fermentation of streptomyces used for treating complex skin and skin structure infections as well as right-heart infective endocarditis caused by Staphylococcus aureus [10]. Although the concentration of DAP in aquatic environments is low, its continued use poses a potential threat to aquatic and terrestrial organisms [11].
Recently, various techniques and methods for removing antibiotics based on physical, chemical, and biological principles have been reported [12], including adsorption, nanofiltration membrane, biodegradation, reverse osmosis, and catalytic degradation [13,14,15]. However, compared to the existing treatment technologies, using biochar adsorption is more practical and environmentally friendly [16]. Biochar is a carbonaceous biomass-derived material, namely negative carbon material [17,18]. It can be used to adsorb some hydrophilic organic compounds and inorganic pollutants because of its aromatics and hydrophobicity [19]. Biochar can be produced by the thermal degradation of wood wastes, crop residues, or animal wastes, such as pine sawdust, corn stalks, and poultry wastes [17]. The use of biochar as a low-cost adsorbent is well documented in ref. [11,16,17,24]
Anshan clivia is a specialty of Anshan, Liaoning Province, China. In 2016, more than 50,000 people planted Anshan clivia, and an industrial base of clivia covering an area of more than 120 acres has been applied. Anshan clivia is an excellent new variety of clivia, successfully bred by crossing Japan clivia as the female parent and clivia “round head short leaf monk” in Anshan as the male parent. It has an elegant and solemn plant type, with wide and thick leaves and beautiful flowers. There are 40–50 μm onion-shaped stomas on the far-axis leaves surface of Anshan clivia, and the developed internal structure and excellent specific surface area have become one of the important reasons as experimental objects. Clivia leaf is a cheap and easy-to-obtain biological residue, but there are few studies on the biochar of clivia leaves, so it is of great significance to generate low-cost biochar from the biological residue by thermal degradation.
The adsorption capacity of most carbon-based materials mainly depends on the specific surface area, and the materials have high affinity and high selectivity to pollutants [4,12]. Iron-based magnetic nanocomposites are widely used in environments, energy, industry, and medicine. Fatimah et al. reviewed various methods for synthesizing iron oxide nanocomposites. The magnetic nanocomposites synthesized by microwave digestion have good electrochemical properties and cyclic stability [20]. Moreover, in their subsequent research on magnetic nanocomposites, magnetic silicon nanocomposites have been well applied in catalysts, photocatalyst drug delivery, and sensors [21]. It is also believed that magnetic nanomaterials have the prospect of sustainable development in easy separation, recyclability, chemical stability combined with adsorption, catalysts, and photocatalysts [21]. An appropriate amount of metals (such as Au, Ag, Pb, Cu, and Al) on the catalyst surface can improve their catalytic activities [22]. The Ag/ZnO/C photocatalyst was synthesized by Xue et al. using calcination and photodeposition. Ag has the visible light response ability of Ag–ZnO composites, which can separate and transfer photoelectrons and increase the reactive sites. The photocatalyst has an excellent photocatalytic activity for degrading tetracycline hydrochloride [23].
Previously, our research group used two magnetic ultra-fine wood-based biochars for the adsorption and magnetic separation of DAP [24]. On this basis, AgNO3 and Fe(NO3)3 were loaded onto clivia leaves by microwave digestions to obtain magnetically separable biochar. Then unmodified biochar (Biochar) was used as blank control to analyze the adsorption mechanism of modified biochar (AF-biochar), with element composition, structure, surface morphology, functional groups, and magnetic properties of characterized biochar. The pH, contact time, initial DAP concentrations, and temperatures of solutions were experimentally studied. Moreover, the adsorption mechanism was described by the adsorption isotherm, kinetic model, and thermodynamics. The retrievability of biochar was studied by five repeated experiments.
2 Materials and methods
2.1 Chemicals
Anshan clivia leaves were purchased from the flower market (Anshan, China). They were repeatedly rinsed with distilled water to remove impurities and then dried with blotting papers. The blades were cut into pieces with scissors and then placed on the filter paper. They were then placed in a dryer at 70°C for 240 min. Samples were ground using a lapping machine mill for 3 min. Finally, the obtained particles were sieved.
DAP (purity > 98%) was purchased from Guangzhou Kafen Biotech Co., Ltd (Guangzhou, China). Table S1 (Supplementary Information) lists the chemical structure and basic physicochemical properties of DAP. All the other reagents were above the analytical grade.
2.2 Synthesis of raw materials
Magnetic clivia biochar (AF-biochar) was prepared by the microwave digestion–anaerobic carbonization method. Then 0.5 g clivia leaf powders, 2 mL of 0.5 M Fe(NO3)3 solution, 2 mL of 0.5 M AgNO3 solution, and 6 mL of deionized water were added into the digestion tube. The pH was adjusted between 10 and 11 with NaOH solution. They were digested at 150, 180, and 210°C for 10, 10, and 15 min in a microwave digestion instrument, respectively, and then carbonized at 850°C for 240 min in the carbonization furnace in the N2 environment. Under the same conditions, clivia biochar was prepared by anaerobic carbonization with Anshan clivia as a blank control group.
2.3 Characterization of the synthesized adsorbents
Main substances and functional groups on the biochar surface were detected by X-ray diffraction (XRD) (Bruker Discover D8, Germany) and Fourier transform infrared (FTIR) spectroscopy (FT/IR-410, JASCO, Japan), respectively. A laser particle size analyzer (BT-9300S, Battersize, China) was used to measure the particle-size distribution of the microparticles. Measurement was repeated to determine the mean value. The adsorption–desorption of nitrogen on the biochar surface was measured using a surface area and porosity analyzer (Micromeritics Tristar II 3020, USA) at 77 K. Also, the specific surface area and pore-size distribution of sediments were estimated by Brunner-Emmet-Teller (BET) and Barret-Joyner-Halenda. Elements C, H, S, and N in biochar were determined using an elemental analyzer (Elemental model Vario MICRO Cube, Germany). The room-temperature Raman spectra were obtained using a confocal Raman microscope (Renishaw RM2000, UK) with a laser wavelength of 785 nm.
The scanning electron microscope (SEM) (SU8020, Japan) was used for the morphological survey of biochar and energy dispersive X-ray (EDX) (Zeiss model Sigma 500, Germany). The contact angle of biochar was measured by a contact angle meter (Dataphysics OCA50, Germany) to test the hydrophilicity of biochar before and after modification. Ash contents were measured by heating the biochar samples at 800°C for 240 min in the muffle furnace. Thermal gravimetric analysis (TG-DTA) of samples was carried out using a thermogravimetric analyzer (Perkin Elmer model Diamond 6300, USA) at a heating rate of 10°C min−1 from the room temperature to 850°C in the N2 atmosphere. Inductively coupled plasma optical emission spectrometry (Jena Model PQ9000, Germany) was used to qualitatively and quantitatively analyze Fe and Ag in the samples. The point of zero charge (PZC) was measured with potentiometric mass titration using an automatic titrator (Rex Electric Chemical model PHS-3C, China). The vibrating sample magnetometer (VSM) (model WSM-01; Changchun Great Wall Teaching Instrument Co., Ltd, China) was used to measure the magnetic properties of the samples. DAP concentration was measured using an ultraviolet spectrophotometer (model UV-1700SPC; Shanghai Meishan Instrument Co., Ltd, China). All drawings were completed using Origin2019 software.
2.4 Analysis of DAP
Prepare a 25 mg L−1 solution of DAP in a brown conical flask with a stopper. Add 25 mg adsorbent to the aqueous solution, and then put it into the thermostatic water-bath oscillator at 25°C in a dark place for 240 min. Filter with a 0.45 μm membrane before measurement. The concentration of DAP in the filtrate was analyzed by an ultraviolet spectrophotometer. The maximum wavelength used in the detection was 221 nm. Based on these results, the adsorption capacity of the adsorbent to DAP was calculated.
where q e (mg g−1) is the adsorption amount of DAP adsorbed at equilibrium; q t (mg g−1) the adsorption amount of DAP adsorbed at time t; C 0 (mg L−1) and C 1 (mg L−1) are the initial and equilibrium concentrations of DAP; C t (mg L−1) the concentration of DAP at time t; V (L) the volume of the solution; and M (g) is the mass of the adsorbent.
2.5 Recyclability tests of adsorbent
Prepare 14 groups of 50 mL DAP solutions with 25 mg L−1 concentration. Then 25 mg AF-biochar was added to seven groups, and the remaining seven groups were added with 25 mg Biochar. Adsorption experiments were carried out under the same conditions. When the concentration of the solution reached equilibrium, a small amount of the solution was taken to measure its concentration. The modified biochar was sucked out of the remaining solution by magnets, and unmodified biochar was filtered out by the vacuum pump. Then rinsed and filtered four times, and dried at 50°C for 300 min. After weighing the adsorbents, 25 mg was added into the conical flask, to keep the solid–liquid ratio constant. Finally, repeat this process five times.
2.6 Data analysis
The adsorption of DAP was fitted on biochar by the pseudo-first-order (3), pseudo-second-order (4) and particle internal diffusion models (5). The theory behind each model is given in Text S1 (Supplementary Information).
Langmuir (6), Freundlich (7), Tempkin (8), and D–R (9) models were used to analyze the adsorption isotherm model. The detailed procedure for calculating adsorption is explained in Text S2.
The thermodynamic parameters of the standard Gibbs free energy (ΔG°), the enthalpy change (ΔH°), and the entropy change (ΔS°) (10–13) were calculated to evaluate the thermodynamic behaviors for adsorbing DAP (Text S3 for detailed calculation).
3 Results and discussion
3.1 Characterization of biochar adsorbents
Figure 1 and Table 1 show the preparation of sample particles. The mean particle sizes of AF-biochar and Biochar were observed to be 126.0 and 55.1 μm, 37.6 and 10.6 μm for D10 (particle diameter corresponding to 10%-cumulation [from 0% to 100%] undersize-particle-size distribution), and 279 and 226 μm for D90 (particle diameters corresponding to 90%-cumulation (from 0 to 100%), undersize-particle-size distribution), respectively. The average particle size of modified biochar decreases because the introduced AF material has a strong squeezing effect on biochar.
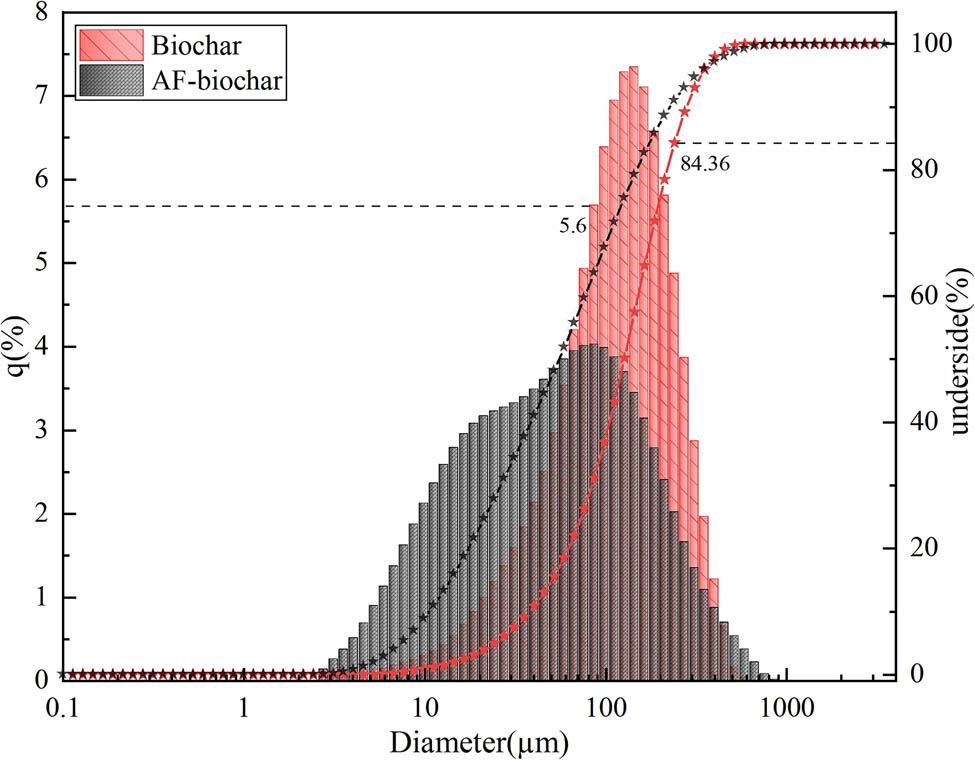
Particle size distribution of AF-biochar and biochar.
Physicochemical properties of AF-biochar and biochar
| Adsorbent | AF-biochar | Biochar | |
|---|---|---|---|
| pHpzc | 4.80 | 4.80 | |
| Ash (%) | 65.54 | 24.86 | |
| Elemental composition (%, mass based) | C | 17.490 | 55.100 |
| H | 0.179 | 1.737 | |
| O | 81.874 | 38.198 | |
| N | 0.150 | 2.560 | |
| S | 0.307 | 2.405 | |
| Ag | 15.150 | — | |
| Fe | 8.360 | — | |
| H/C | 0.0102 | 0.0315 | |
| O/C | 4.6812 | 0.6932 | |
| BET surface area (m2 g−1) | 521.692 | 232.196 | |
| Average pore diameter (nm) | 2.488 | 2.834 | |
| Total pore volume (cm3 g−1) | 0.325 | 0.165 | |
| The largest pore (nm) | 210.4 | 185.9 | |
| D[3,2] (μm) | 75.7 | 27 | |
| D[4,3] (μm) | 145 | 94 | |
| Dx(10) (μm) | 37.6 | 10.6 | |
| Dx(50) (μm) | 126 | 55.1 | |
| Dx(90) (μm) | 279 | 226 | |
Table 1 shows the main element composition and content of AF-biochar and Biochar. After introducing Ag and Fe, C, H, N, and S in biochar decrease significantly, and the ash content increases significantly after combustion at 800°C. The ratios of H/C and O/C can reflect the aromaticity and polarity of biochar [25]. After the contact-angle test (Figure S1), the water contact angle before and after modification was 153.6° and 132.7°, respectively, indicating that the biochar before and after modification was hydrophobic. However, the water contact angle of modified biochar is significantly reduced, indicating that the addition of elemental Fe and Ag promotes biochar toward hydrophilicity, and the increased O/C value can also demonstrate this view. The H/C value of AF-biochar was 0.0102 lower than that of unmodified biochar (0.0315), indicating that modified biochar contains fewer organic plant residues than modified biochar.
The magnetic field-related behavior is measured using VSM to determine the magnetism of AF-biochar. The hysteresis loop in Figure 2 shows typical ferromagnetic characteristics. Magnetization increases sharply with the increased external field, and then gradually approaches saturation. The low magnetization of biochar is because submicron Ag particles reduce the coercivity of AF-biochar [26], so the saturation magnetization of AF-biochar is relatively weak (9.6 Am2 kg−1). Besides, solid–liquid separation can be completed under the action of magnets.
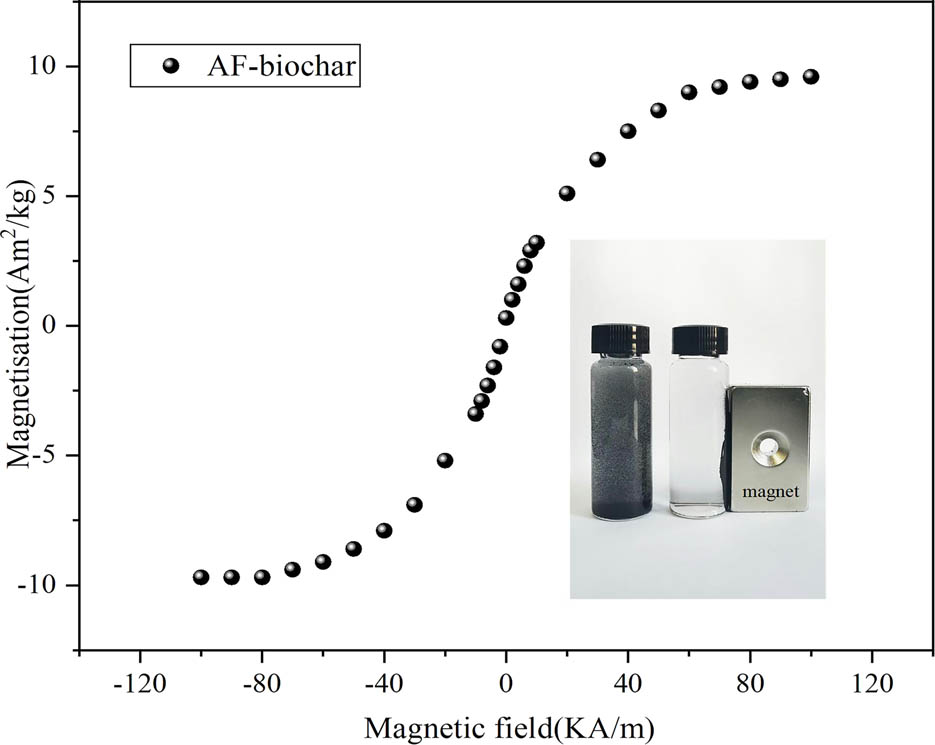
Magnetic hysteresis cycles of AF-biochar (inset plots show the magnetic-separation AF-biochar after DAP adsorption).
TG-derivative thermogravimetry (TG-DTG) was used to study the thermal stability of biochar. TG image in Figure S2(b) shows that the weight losses of biochar before and after modification are 75.5 and 10.2%, respectively. AF materials can greatly improve the thermal stability of carbon materials. DTG image in Figure S2(a) shows that the weight losses mainly have four stages. The decomposition peaks at 40 and 58°C are likely attributed to the free water loss, and the weight losses at 467 and 500°C are due to the pyrolyzed carboxyl and carbonyl functional groups of hemicellulose, cellulose, and lignin. The decomposition peaks at 699 and 698°C are because part of the carbon skeleton is pyrolyzed, which causes weight loss. Weight loss at 886°C may be due to the pyrolysis of a more heat-resistant structure in biochar.
The specific surface area and pore volume of AF-biochar increased significantly through detecting the biochar structure (Table 1) compared with those before modification. It indicates that some Ag and Fe particles were pressed into biochar, resulting in more pores. However, in contrast, the average pore size of modified biochar decreased slightly due to the introduced submicron Ag and Fe particles blocking the pores of some biochars and preventing the entry of N2. N2 adsorption isotherm in Figure S3(a) conforms to the type-I adsorption isotherm, belonging to monolayer adsorption [27]. Under relatively low pressure, the rapid adsorption and high adsorption of N2 may be caused by the adsorbing micropores. Under the relative medium pressure, N2 adsorption is relatively stable and forms hysteresis loops under medium and high pressures, indicating macropores in biochar [28]. When P/P 0 approaches 1.0, the adsorption capacity of macropores increases rapidly.
XRD analysis of AF-biochar was carried out to study the crystallization behaviors of biochar. In Figure 3(a), there are mainly eight group peaks, and the peaks at 2θ = 38.11°, 44.30°, 64.43°, 77.39°, and 81.52° are considered for submicron element Ag (JCPDS No. 01-087-0717). Besides, the peaks at 2θ = 44.67°, 65.03°, and 82.34° are consistent with element Fe (JCPDS No. 00-006-0696). Therefore, AF-biochar materials are considered to be mainly loaded with Fe and Ag. Raman spectroscopy (Figure 3(b)) was performed to further study the structure of AF-biochar materials. In Raman spectroscopy, the D peak represents the defects of the carbon atomic lattice, and the G peak represents the in-plane stretching vibration of sp2 hybrid carbon atoms [29]. The I D/I G (the ratios of integral regions of D and G bands) value of 2.86 indicates that the number of crystal defects in AF-biochar structure is large. The main reason may be that Ag and Fe nanoparticles are uniformly dispersed in the crystal structure of biochar, which indicates that AF materials are successfully embedded in biochar [30].
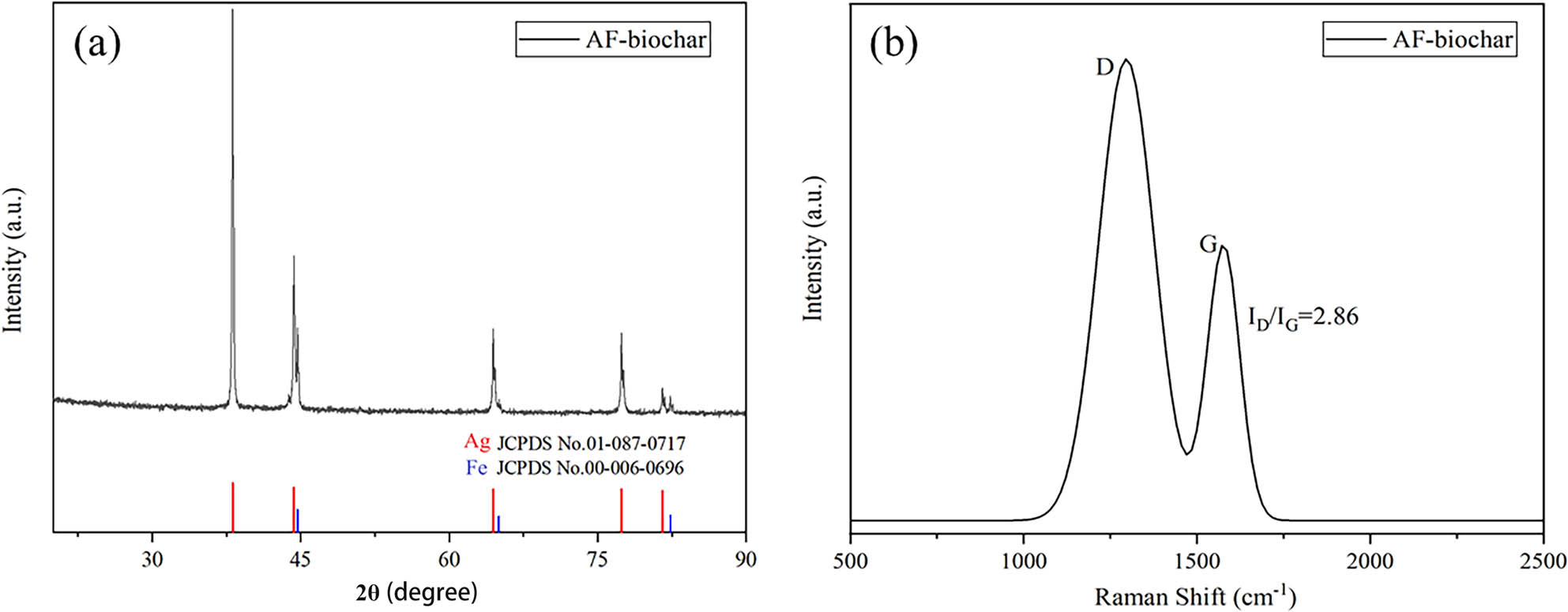
(a) XRD pattern of AF-biochar; (b) Raman spectra of AF-biochar.
SEM was used to compare the microcosms before and after biochar modification (Figure 4). The pore distribution of biochar before modification (Figure 4(a) and (b)) is extremely uneven with porous layers. Irregular holes of different sizes and shapes can be observed, indicating that the unmodified biochar mechanism is in heterogeneous structures. After modification (Figure 4(c) and (d)), different spherical particles and pores are on the biochar surface. Compared with the unmodified biochar, the AF-biochar surface becomes rougher with the increased pores due to the successful grafting of biochar with small particles formed after adding AF materials.

(a and b) SEM of Biochar; (c and d) SEM of AF-biochar.
EDX spectrometry was performed for AF-biochar (Figure 5). Particles of different sizes can be found, with diameters distributed between submicrons. The proportions of Ag, Fe, and O are 76.34, 20.80, and 2.86%, respectively. Therefore, these particles may be submicron Ag and Fe particles, and solids and liquids are separated under the action of magnets due to submicron Fe. In addition, C was not detected in the measured area, indicating that Ag and Fe were thickly distributed and covered the biochar surface.
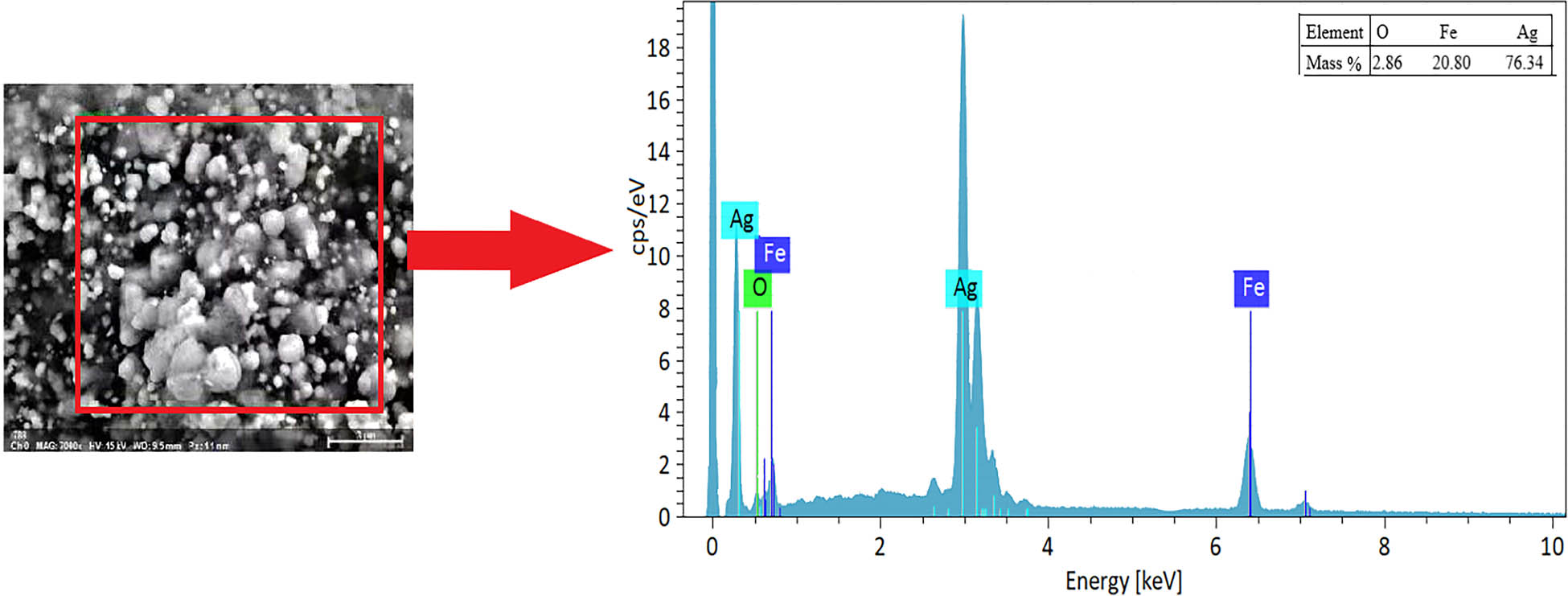
EDX spectrometry of AF-biochar.
3.2 Effect of solution pH on DAP adsorption
The pH values of the key factors affect the adsorption capacity. In this experiment, the pH value of the solution is mainly adjusted by different concentrations of HCl and NaOH solutions (all drops were less than three drops). Figure S4 shows that the general trend of AF-biochar is similar to that of Biochar, but AF-biochar has better adsorption. The adsorption capacity increases with the increased initial solution at pH 2–4.8; when the pH further increases, the adsorption capacity decreases sharply and tends to be stable at pH 10–12. For further analysis, see Section 3.7.
3.3 Adsorption kinetics
The relationship between adsorption capacity and time is measured at pH 4.8 and 25°C (Figure 6(a)). The results show that AF-biochar and Biochar reach the adsorption equilibrium in 85 min. The adsorption rate of AF-biochar is faster than that of most adsorbents in Table S3, compared with the equilibrium time reached by other adsorbents in references. The initial adsorption capacity of AF-biochar changes more obviously than that of Biochar with time. This excellent performance can be ascribed to the abundant availability of active sites and the high driving force in mass transfer. Pseudo-first-order (Figure 6(b)), pseudo-second-order (Figure 6(c)), and intramolecular diffusion (Figure 6(d)) models are used to fit experimental data (Table 2) to study the adsorption kinetics. The pseudo-second-order model (for AF-biochar, R 2 = 0.9997; for Biochar, R 2 = 0.9993) well fits the adsorption of DAP on two adsorbents. The maximum adsorption values are 47.96 (AF-biochar) and 40.03 mg g−1 (Biochar) estimated by pseudo-second-order kinetics, close to the experimental results of 48.25 mg g−1 (AF-biochar) and 40.35 mg g−1 (Biochar). It indicates that chemisorption dominates the experiment and controls the adsorption process. It can be seen from Figure 6(b) that the pseudo-first-order kinetic models of AF-biochar and Biochar have poor overall fitting effect and are not suitable for discussing their adsorption kinetics, so this study is no longer discussed. However, it can be seen from Figure 6(a) that the adsorption rate of the adsorbent was the highest in the first 15 min, indicating that AF-biochar had good hydrophobicity in the early stage of adsorption [31].
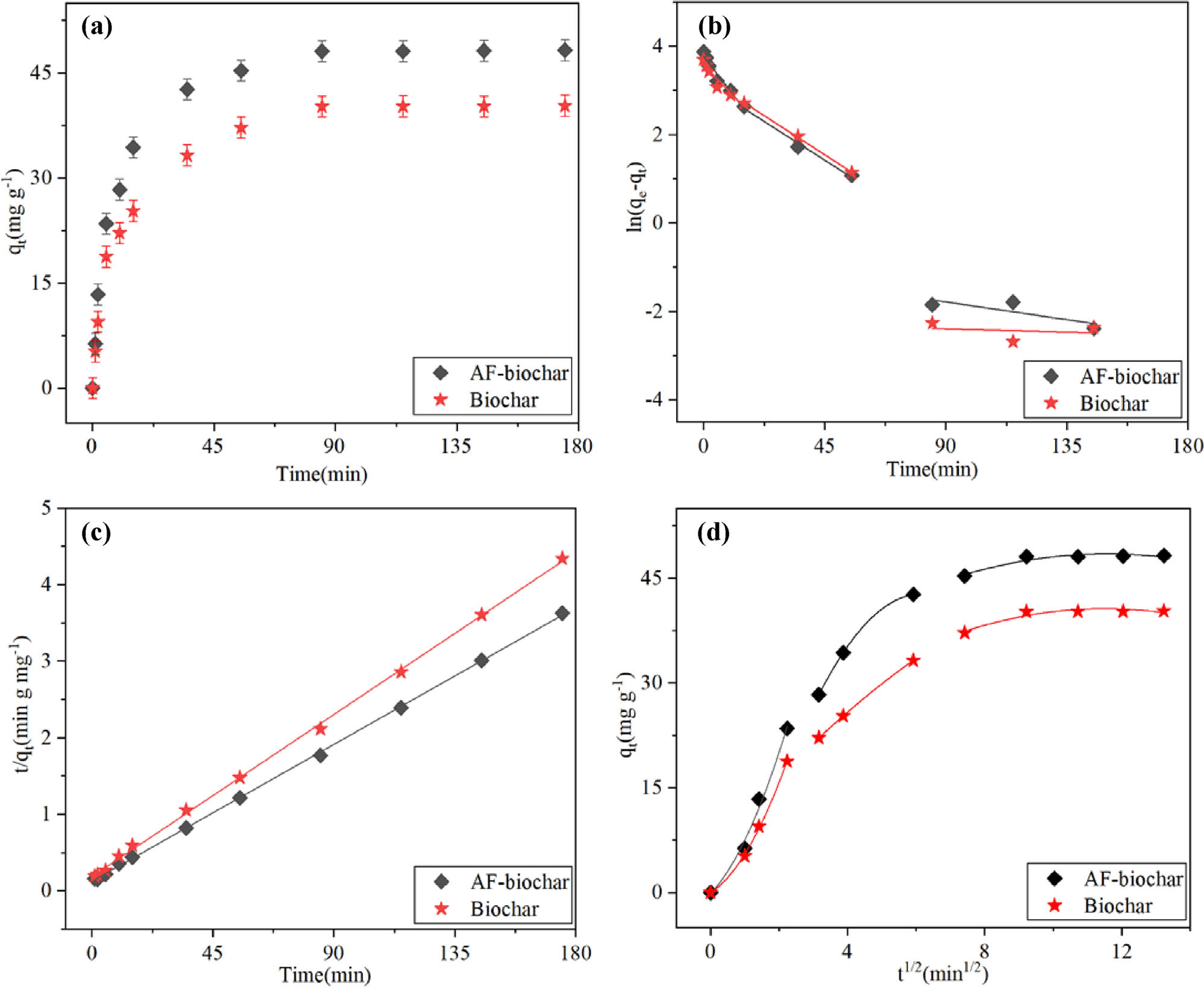
(a) Effect of contact time on adsorption of DAP by AF-biochar and Biochar (experimental conditions: pH 4.8, initial concentration 25 mg L−1, and temperature 298 K); (b) pseudo-first order model plot; (c) pseudo-second order model plot; and (d) intraparticle diffusion kinetics.
Parameters of pseudo-first-order, pseudo-second-order, and intraparticle diffusion kinetic models for adsorbing DAP on AF-biochar and Biochar (conditions: T = 25°C, pH 4.6, and initial concentration of 25 mg L−1)
| Kinetic model | Parameters | Value of the parameters | |
|---|---|---|---|
| AF-biochar | Biochar | ||
| Pseudo-first-order | k f (min−1) | 8.5680 | 8.0720 |
| R 2 | 0.9488 | 0.9296 | |
| Pseudo-second-order |
|
0.0199 | 0.0235 |
|
|
47.96 | 40.03 | |
|
|
48.25 | 40.32 | |
| R 2 | 0.9997 | 0.9993 | |
| Intraparticle diffusion |
|
14.6709 | 9.6670 |
|
|
−9.0997 | −3.5062 | |
|
|
0.9900 | 0.9991 | |
|
|
5.8840 | 4.0057 | |
|
|
10.6579 | 9.6973 | |
|
|
1.00 | 1.00 | |
|
|
0.6210 | 0.6974 | |
|
|
41.2844 | 32.7127 | |
|
|
0.9114 | 0.9126 | |
The model of intraparticle diffusion can be fitted at three stages (Table 2). In the first stage, the straight line is relatively steep due to the large specific surface area and large pore distribution on the surface, which increases the absorption rate. The gradual flattening in the second and third stages may be because the pores in the macropores are occupied and reduced, and DAP is mainly adsorbed by the macropores, leading to the decreased absorption rate.
3.4 Adsorption isotherm
The relationship between concentration and adsorption capacity was determined at pH 4.8 and 25°C to study the adsorption mechanism of biochar (Figure S5). The lower the concentration of DAP, the better the adsorption capacity of biochar. The adsorption capacity gradually declines with the increased concentration when the adsorption of biochar reaches saturation.
When the system reaches equilibrium, the adsorption isotherm is generally used to describe the distribution of adsorbed molecules between liquids and solids. In this experiment, Langmuir (Figure S6(a)), Freundlich (Figure S6(b)), Tempkin (Figure S6(c)), and D–R (Figure S6(d)) were used for analysis, and Table 3 shows the main parameters. In terms of the fitting effect of all models, the fitting degree of the Langmuir model (both R 2 = 0.9999) is in complete agreement with experimental data and an optimal fitting effect can be obtained, indicating that the adsorption process was homogeneous and monolayer within the experimental concentration range [32].
Parameters of Langmuir, Freundlich, Tempkin, and D–R (conditions: T = 25°C; pH 4.6; duration = 85 min)
| Isotherm model | Parameters | Value of the parameters | |
|---|---|---|---|
| AF-biochar | Biochar | ||
| Langmuir |
|
81.530 | 44.807 |
| K L (L mg−1) | 88.976 | 227.960 | |
|
|
0.9999 | 0.9999 | |
| Freundlich |
|
3.360 | 7.668 |
|
|
1.135 | 2.246 | |
|
|
0.9731 | 0.9842 | |
| Tempkin |
|
29.728 | 11.807 |
|
|
0.144 | 0.679 | |
|
|
0.9593 | 0.9648 | |
| D–R |
|
66.714 | 44.961 |
|
|
0.169 | 0.220 | |
|
|
0.9227 | 0.9832 | |
In Table 3, the maximum adsorption capacity of AF-biochar is 81.53 mg g−1, and that of Biochar is 44.81 mg g−1. The former is 1.82 times better than that of the latter. The adsorption effect of modified biochar is far better than that of unmodified biochar because submicron Ag and Fe on the surface of AF-biochar can be complexed with DAP. Besides, submicron Ag can promote the degradation of DAP into small molecular organics, thus accelerating DAP into a smaller hole. Therefore, the adsorption capacity of DAP is accelerated. This is also one of the reasons for introducing submicron Fe and Ag particles in this experiment. Table S3 compares the maximum adsorption capacity or removal efficiency of macrolide antibiotics with other adsorbents in references. The maximum adsorption capacity of AF-biochar is not particularly prominent, but it can be considered an efficient adsorbent for removing macrolide antibiotics.
In the Freundlich model, the values of 1/n are 0.760 (AF-biochar) and 0.455 (Biochar), indicating that the physical adsorption effect is favorable [33]. The K T of the Tempkin model is related to the thermodynamic properties of the adsorption process. The K T of two adsorbents are 29.728 (AF-biochar) and 11.807 (Biochar), respectively, which are greater than 1. It indicates that the adsorption of the two adsorbents to DAP is exothermic within the range of concentration studied [34].
E (average adsorption energy) in the D–R model can be used to distinguish whether the adsorption process is physical or chemical adsorption [35]. The E values of two adsorbents in this experiment were 0.169 kJ mol−1 (AF-biochar) and 0.220 kJ mol−1(Biochar), both less than 8 kJ mol−1. It indicates that the adsorption process may be physical adsorption [36], consistent with Freundlich’s judgment.
3.5 Adsorption thermodynamics
Thermodynamic-energy adsorption reflected the internal changes in the adsorption process. Adsorption experiments were carried out between 285 and 320 K at a certain temperature. Figure S7 shows that the adsorption capacity varies with the increased temperature, indicating that the adsorption capacity depends on the temperature [37].
According to the reports, the free energy of chemical adsorption is at −400 to 80 kJ mol−1, and the free energy of physical adsorption is at −80 to 0 kJ mol−1. Table S2 shows the main thermodynamic parameters. The adsorption process of this experiment is mainly physical adsorption, indicating that adsorption occurs spontaneously. ΔH° > 0 of Biochar indicates that the adsorption of unmodified biochar has endothermic properties. ΔH° < 0 of AF-biochar indicates that the adsorption of modified biochar has exothermic properties because the introduced AF materials change the absorption and exothermic properties in the adsorption process. ΔS° of the adsorption process of two adsorbents is greater than 0, indicating that the adsorption process is entropy-increasing and irreversible. It is conducive to stable adsorption.
3.6 Regeneration and reusability
Recyclability of adsorbents is essential in reducing process costs and in environmental protection. Five cycles of recycling experiments were carried out under the same conditions (Figure S8 for the results). The adsorption capacity of AF-biochar was stronger than that of Biochar in the first three cycle experiments. However, the adsorbability of two adsorbents gradually decreased with more experiments. In the third-cycle experiment, the adsorption capacity of AF-biochar was 46.35% of the first adsorption, while that of unmodified biochar was 51.85%. In the fifth-cycle experiment, the adsorption capacity of AF-biochar was only 14.01% of the first adsorption, and that of biochar was 32.31%. The recoveries of AF-biochar and biochar were more than 94 and 70%, respectively. It is because parts of Ag and Fe on the biochar surface were gradually precipitated out, and parts of the micropores were occupied by the previously adsorbed DAP with the increased experiments. Therefore, it is easy to be separated from the solution by magnets, but with weak reusability.
3.7 Mechanism of adsorption
The morphology and properties of some functional groups of adsorbents may be significantly affected by variable pH. In general, the ionization, solubility, and hydrophilicity of organic chemicals increase with increased pH, leading to the reduced adsorption capacity of adsorbents [38]. However, when the pH increases, the interaction between ionized amino groups and electron donor–acceptor (EDA) is strengthened, and the adsorption capacity increases [39].
The acidity coefficient (pK a) of DAP is 4.1 [40]. When pH < 4.1, the solution is dominated by cations; when pH > 4.1, the solution is dominated by anions. That is, anions in the solution increase with increased pH, so they are dominant.
In Table 1, the points of zero charge (pHpzc) of biochar and AF-biochar are around 4.8. When pH < 4.8 for AF-biochar, the biochar surface has mainly positive charge; when pH > 4.8, the surface of biochar has mainly negative charge.
Figure 7 shows the strong electrostatic repulsion between DAP cations and the positive charges on the AF-biochar surface seriously reduce adsorption when pH < 4.1. The strong interaction between them gradually weakens with the increased pH, so the adsorption capacity gradually increases. DAP mainly exists in the form of DAP−, and a strong electrostatic attraction exists between DAP and biochar when 4.1 < pH < 4.8. Also, DAP contains carboxyl and amino groups. The interaction between the Lewis acid and adsorbability is enhanced when pK a < pH [41].
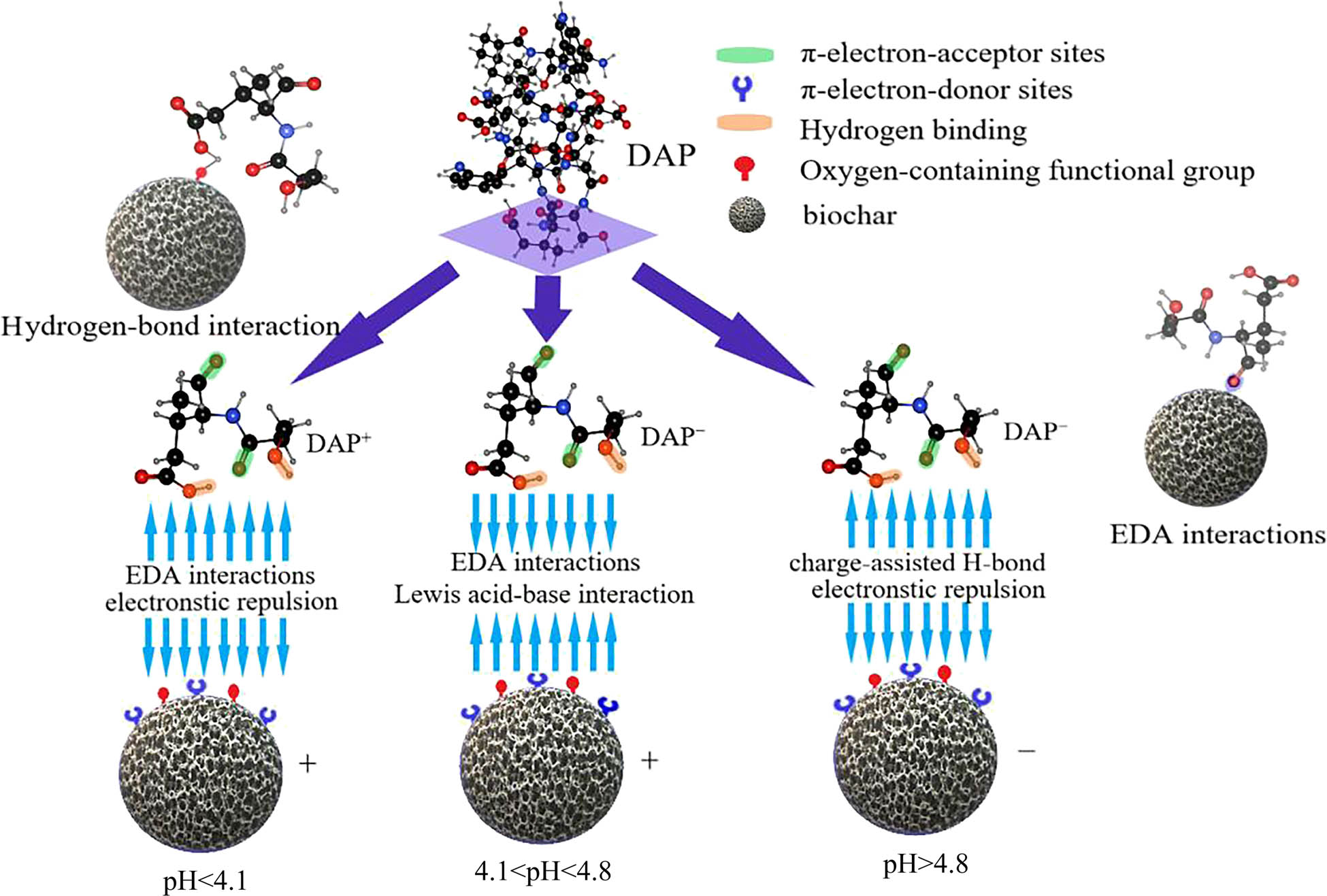
Mechanism of AF-biochar adsorbing DAP.
When pH is about 4.8, the adsorption capacity reaches the maximum of 47.71 mg g−1, consistent with the experimental results. Its hydrophilicity also increases with increasing pH, which is offset by the electrostatic force; therefore, the adsorption capacity gradually decreases.
When pH > 4.8, the biochar surface has negative charges, and there is electrostatic repulsion between biochar and DAP. Electrostatic repulsion and hydrophilicity increase with the increased pH, so the adsorption capacity gradually decreases.
The interaction between the π hydrogen bond and hydrogen bond affects non-ionization adsorption [42]. Hydrogen bond receptors on biochar at a lower pH are more likely to bind to H in an aqueous solution, thus reducing the hydrogen bond between DAP and biochar. At a higher pH, the hydrogen bond donor on the biochar surface is ionized and interacts with H in an aqueous solution. Besides, the hydrogen bond donor on the DAP surface interacts with the hydrogen bond acceptor and π donor on the biochar surface, which weakens the influence of hydrophilicity and electrostatic repulsion on adsorption. Therefore, the adsorption capacity gradually flattens at a higher pH, consistent with the experimental results.
FTIR spectra before and after modification and adsorption are made to study the adsorption mechanism of biochar (Figure 8). Compared with the biochar before modification, two groups of new characteristic absorption peaks appear at 1,698 and 467 cm−1 after modification, caused by the C═O bond stretching of the aldehyde group and metal–oxygen stretching, respectively. Besides, the obvious displacement of some peaks may be caused by electronic attractions and transfers between metal ions and functional groups, indicating that submicron Fe and Ag are grafted with biochar.
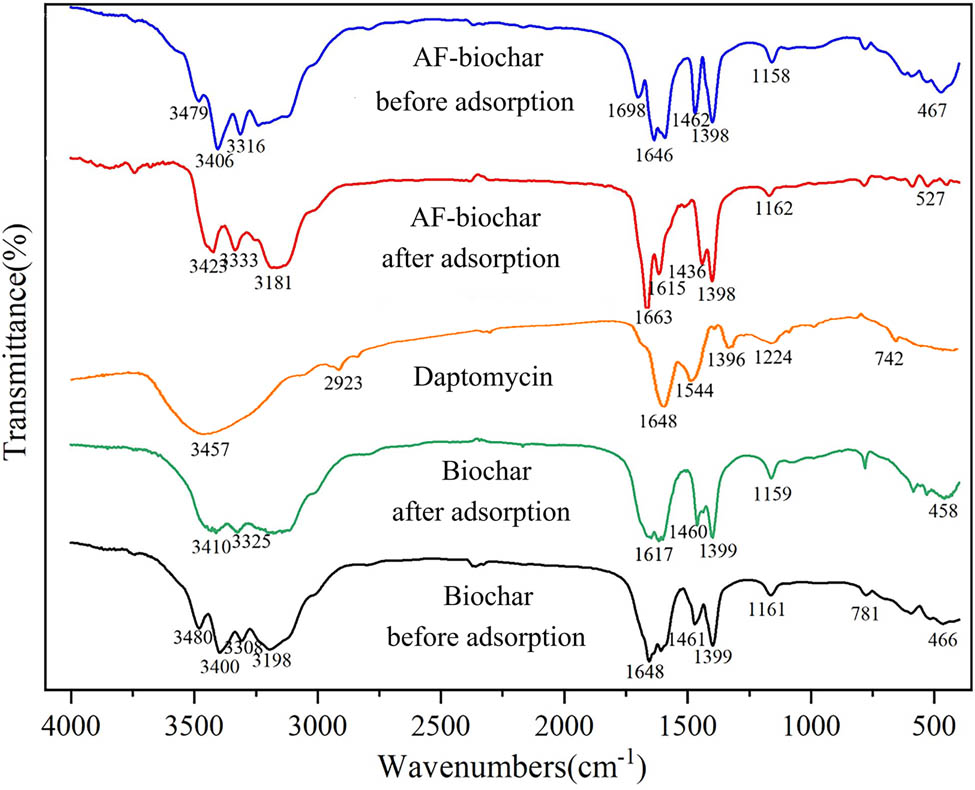
FTIR spectra of AF-biochar and Biochar before and after adsorbing DAP.
Submicron Ag can be complexed with biochar to decompose DAP into small molecular organic matters. Then DAP easily enters the pores and can be easily absorbed, which accelerates the adsorption of biochar. Moreover, submicron Fe loaded on biochar can magnetize biochar, which separates solids and liquids and recycles biochar. This is the main reason for introducing Fe and Ag in this experiment.
The work studied FTIR spectra before and after adsorbing DAP by AF-biochar. Peaks at 3,479–3,316 cm−1 were due to the –NH stretching of amino groups or the O–H stretching of alcohol and carboxyl groups [43]. Double peaks near 1,646 cm−1 were caused by the C═C double-bond stretching of olefin, and the absorption peak at 1,462 cm−1 was attributed to the skeleton vibration in the benzene ring (υC═C) [44]. The peak at 1,398 cm−1 was mainly caused by the O–H inward bending of a primary alcohol, while that at 1,158 cm−1 was probably caused by the C–N bond stretching in the amino group.
The modified biochar contained the amino group, carbon–carbon double bond, benzene ring, hydroxyl group, aldehyde group, and other functional groups. However, the absorption band appeared near 3,181 cm−1 after adsorbing DAP, caused by the H-bond extension in the carboxyl group. The oxygen-containing functional group in AF-biochar formed charge with DAP−, confirming the connection between the carboxylic acid group in DAP and the oxygen-containing group in biochar.
The peak at 3,479 cm−1 disappeared and decreased from 3,406 to 3,316 cm−1, indicating that the N–H bond on biochar was grafted to the oxygen-containing group on DAP. The increased double-peak value at 1,646 cm−1 and the obvious shift were caused by the electron-induced effect, indicating that the π–π interaction adsorption mechanism existed between C═C and DAP [44]. The disappearance of the C═O bond stretching at 1,698 cm−1 was due to the interaction with the Lewis acid on DAP.
4 Conclusion
AF-biochar was prepared by microwave digestion–anaerobic carbonization in a furnace. According to the pseudo-second-order kinetics and adsorption thermodynamics, both chemical and physical adsorption played an important role in adsorbing DAP. The average pore size of modified biochar was 126.0 μm, with a specific surface area of 521.692 m2 g−1 and a total void volume of 0.325 cm3 g−1.
The Langmuir function fits the adsorption isotherm data, showing that the adsorption process was homogeneous and monolayer with more adsorption sites. The analysis of the FTIR spectrum, pH value, and pHpzc showed that EDA interaction, electrostatic force interaction, Lewis acid–base interaction, H-bond interaction, and submicron silver could be complexed with biochar and decompose DAP into small molecular organics in the adsorption process. Thus, chemical adsorption had a good advantage in the adsorption process. In conclusion, AF-biochar has a more efficient adsorption capacity than unmodified biochar in the adsorption process of DAP and can be separated by the magnetic field.
Acknowledgments
We acknowledge Dr Pengcheng Li and Professor Zhao Li of the School of Mining Engineering, University of Science and Technology Liaoning for the support and data analysis of XRD, SEM, EDX, and FTIR instruments in this study.
-
Funding information: This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 51874168, 51574146, and 52174254), the Innovation and Entrepreneurship Program of University of Science and Technology Liaoning (Grant No. X202010146234), and Xingliao Yingcai Science and Technology Innovation Leading Talent Project (Grant No. XLYC2002028).
-
Author contributions: Conceptualization: T.A., S.D.; data curation: L.Z., T.A.; formal analysis: T.A., L.Z.; funding acquisition: T.A.; investigation: L.Z., C.X., Y.W., Z.Y.; project administration: T.A., X.T.; resources: T.A., S.D.; software: L.Z.; validation: L.Z., C.X., Y.W., Z.Y.; writing – original draft: L.Z.; writing – review and editing: T.A., S.D., L.Z.; methodology: T.A., S.D. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Rosset M, Sfreddo LW, Hidalgo GEN, Perez-Lopez OW, Feris LA. Adsorbents derived from hydrotalcites for the removal of diclofenac in wastewater. Appl Clay Sci. 2019;175:150–8.10.1016/j.clay.2019.04.014Suche in Google Scholar
[2] Martinez JL. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ Pollut. 2009;157(11):2893–902.10.1016/j.envpol.2009.05.051Suche in Google Scholar PubMed
[3] Song Q, Fang Y, Liu Z, Li L, Wang Y, Liang J, et al.The performance of porous hexagonal BN in high adsorption capacity towards antibiotics pollutants from aqueous solution. Chem Eng J. 2017;325:71–9.10.1016/j.cej.2017.05.057Suche in Google Scholar
[4] Zhang XX, Tong Z, Fang HHP. Antibiotic resistance genes in water environment. Appl Microbiol Biotechnol. 2009;82(3):397–414.10.1007/s00253-008-1829-zSuche in Google Scholar PubMed
[5] Petrie B, Barden R, Kasprzyk-Hordern B. A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015;72:3–27.10.1016/j.watres.2014.08.053Suche in Google Scholar PubMed
[6] Liao P, Zhan ZY, Dai J, Wu X, Zhang W, Wang K, et al. Adsorption of tetracycline and chloramphenicol in aqueous solutions by bamboo charcoal: a batch and fixed-bed column study. Chem Eng J. 2013;228:496–505.10.1016/j.cej.2013.04.118Suche in Google Scholar
[7] Pfaller MA, Sader HS, Shortridge D, Castanheira M, Mendes RE. Activity of tedizolid against gram-positive clinical isolates causing infections in Europe and surrounding areas (2014–2015). J Chemother. 2019;31(4):188–94.10.1080/1120009X.2019.1609740Suche in Google Scholar PubMed
[8] Tally FP, Zeckel M, Wasilewski MM, Carini C, Berman CL, Drusano GL, et al. Daptomycin: a novel agent for Gram-positive infections. Expert Opin Invest Drugs. 1999;8(8):1223–38.10.1517/13543784.8.8.1223Suche in Google Scholar PubMed
[9] Baltz RH, Miao V, Wrigley SK. Natural products to drugs: daptomycin and related lipopeptide antibiotics. Nat Prod Rep. 2005;22:717–41.10.1039/b416648pSuche in Google Scholar PubMed
[10] Enoch DA, Bygott JM, Daly ML, Karas JA. Daptomycin. J Infect. 2007;55(3):205–13.10.1016/j.jinf.2007.05.180Suche in Google Scholar PubMed
[11] Ahmed MJ, Theydan SK. Microwave assisted preparation of microporous activated carbon from Sins seed pods for adsorption of metronidazole antibiotic. Chem Eng J. 2013;214:310–8.10.1016/j.cej.2012.10.101Suche in Google Scholar
[12] Li S, Zhang X, Huang Y. Zeolitic imidazolate framework-8 derived nanoporous carbon as an effective and recyclable adsorbent for removal of ciprofloxacin antibiotics from water. J Hazard Mater. 2017;321:711–9.10.1016/j.jhazmat.2016.09.065Suche in Google Scholar PubMed
[13] Liang Z, Gao F, Li W, Hon S. In-situ monitoring of polyelectrolytes adsorption kinetics by electrochemical impedance spectroscopy: application in fabricating nanofiltration membranes via layer-by-layer deposition. J Membr Sci. 2021;619:118747–55.10.1016/j.memsci.2020.118747Suche in Google Scholar
[14] Yang L, Sheng M, Li Y, Xue W, Li K, Cao G. A hybrid process of Fe-based catalytic ozonation and biodegradation for the treatment of industrial wastewater reverse osmosis concentrate. Chemosphere. 2020;238:124639–47.10.1016/j.chemosphere.2019.124639Suche in Google Scholar PubMed
[15] Wang W, Chen M, Wang D, Yan M, Liu Z. Different activation methods in sulfate radical-based oxidation for organic pollutants degradation: catalytic mechanism and toxicity assessment of degradation intermediates. Sci Total Environ. 2021;772:145522–40.10.1016/j.scitotenv.2021.145522Suche in Google Scholar PubMed
[16] Tan XF, Liu SB, Liu YG, Gu YL, Zeng GM, Hu XJ, et al. Biochar as potential sustainable precursors for activated carbon production: multiple applications in environmental protection and energy storage. Bioresour Technol. 2017;227:359–72.10.1016/j.biortech.2016.12.083Suche in Google Scholar PubMed
[17] Reguyal F, Ajit KS, Gao W. Synthesis of magnetic biochar from pine sawdust via oxidative hydrolysis of FeCl2 for the removal sulfamethoxazole from aqueous solution. J Hazard Mater. 2017;321:868–78.10.1016/j.jhazmat.2016.10.006Suche in Google Scholar PubMed
[18] Jing XR, Wang YY, Liu WJ, Wang YK, Jiang H. Enhanced adsorption performance of tetracycline in aqueous solutions by methanol-modified biochar. Chem Eng J. 2014;248:168–74.10.1016/j.cej.2014.03.006Suche in Google Scholar
[19] Lonappan L, Rouissi T, Brar SK, Verma M, Rao YS. An insight into the adsorption of diclofenac on different biochars: mechanisms, surface chemistry, and thermodynamics. Bioresour Technol. 2018;249:386–94.10.1016/j.biortech.2017.10.039Suche in Google Scholar PubMed
[20] Fatimah I, Fadillah G, Yudha SP. Synthesis of iron-based magnetic nanocomposites: a review. Arab J Chem. 2021;14(8):103301–23.10.1016/j.arabjc.2021.103301Suche in Google Scholar
[21] Fatimah I, Fadillah G, Purwiandono G, Sahroni I, Purwaningsih D, Riantana H, et al. Magnetic-silica nanocomposites and the functionalized forms for environment and medical applications: a review. Inoeg Chem Commun. 2022;137:109213–30.10.1016/j.inoche.2022.109213Suche in Google Scholar
[22] Liu Y, Zhou S, Yang F. Degradation of phenol in industrial wastewater over the F–Fe/TiO2 photocatalysts under visible light illumination. Chin J Chem Eng. 2016;24(12):1712–8.10.1016/j.cjche.2016.05.024Suche in Google Scholar
[23] Xue J, Ma S, Zhou Y, Zhang Z, Jiang P. Synthesis of Ag/ZnO/C plasmonic photocatalyst with enhanced adsorption capacity and photocatalytic activity to antibiotics. RSC Adv. 2015;5:18832–40.10.1039/C5RA00217FSuche in Google Scholar
[24] Ai T, Jiang X, Liu Q, Lv L, Wu H. Streptomycin adsorption on magnetic ultra-fine wood-based biochars from water: kinetics, isotherms, and mechanism studies. Bioresour Technol. 2019;273:8–15.10.1016/j.biortech.2018.10.039Suche in Google Scholar PubMed
[25] Leng L, Huang H, Li H, Li J, Zhou W. Biochar stability assessment methods: a review. Sci Total Environ. 2019;647:210–22.10.1016/j.scitotenv.2018.07.402Suche in Google Scholar PubMed
[26] Wang B, Song J, Rui G, Zhao X. Preparation and wave-absorbing property of Ag coated barium ferrite hollow microspheres. New Chem Mater. 2016;45:94–6.Suche in Google Scholar
[27] Yan L, Liu Y, Zhang Y, Liu S, Wang C, Chen W, et al. ZnCl2 modified biochar derived from aerobic granular sludge for developed microporosity and enhanced adsorption to tetracycline. Bioresour Technol. 2020;297:122381–91.10.1016/j.biortech.2019.122381Suche in Google Scholar PubMed
[28] Tian W, Sun H, Duan X, Zhang H, Ren Y, Wang S. Biomass-derived functional porous carbons for adsorption and catalytic degradation of binary micropollutants in water. J Hazard Mater. 2020;389:121881–903.10.1016/j.jhazmat.2019.121881Suche in Google Scholar PubMed
[29] Kang J, Duan K, Zhou L, Sun H, Tadé MO, Wang S. Carbocatalytic activation of persulfate for removal of antibiotics in water solutions. Chem Eng J. 2016;288:399–405.10.1016/j.cej.2015.12.040Suche in Google Scholar
[30] Gan L, Wang L, Xu L, Fang X, Pei C, Wu Y, et al.Fe3C-porous carbon derived from Fe2O3 loaded MOF-74(Zn) for the removal of high concentration BPA: the integrations of adsorptive/catalytic synergies and radical/non-radical mechanisms. J Hazard Mater. 2021;413:125305–20.10.1016/j.jhazmat.2021.125305Suche in Google Scholar PubMed
[31] Li R, Liu L, Zhang Y, Yang F. Preparation of a nano-MnO2 surface-modified reduced graphene oxide/PVDF flat sheet membrane for adsorptive removal of aqueous Ni(II). RSC Adv. 2016;6:20542–50.10.1039/C5RA20776BSuche in Google Scholar
[32] Shen JC, Zeng HY, Gohi BFCA, Chen CR. Adsorption behaviour of Cr(VI) from ternary mesoporous Mg/Fe/Al oxide using glucose as a soft template. J Nanosci Nanotechnol. 2020;20(9):5555–62.10.1166/jnn.2020.17875Suche in Google Scholar PubMed
[33] Salary N, Tehrani RMA, Motamedi M. Zeolite modification with cellulose nanofiber/magnetic nanoparticles for the elimination of reactive red 198. Int J Biol Macromol. 2021;176:342–51.10.1016/j.ijbiomac.2021.01.219Suche in Google Scholar PubMed
[34] Sayn M, Can M, Mamolu M. Adsorption of Pd(II) and Au(III) ions by commercial tris (2-aminoethyl) amine polystyrene polymer beads. J Chem Eng Data. 2021;66:1132–43.10.1021/acs.jced.0c00920Suche in Google Scholar
[35] Molu ZB, Yurdako K. Preparation and characterization of aluminum pillared K10 and KSF for adsorption of trimethoprim. Microporous Mesoporous Mater. 2010;127(1–2):50–60.10.1016/j.micromeso.2009.06.027Suche in Google Scholar
[36] Basaleh AA, Al-Malack MH, Saleh TA. Poly (acrylamide acrylic acid) grafted on steel slag as an efficient magnetic adsorbent for cationic and anionic dyes. J Environ Chem Eng. 2021;9(2):105126–40.10.1016/j.jece.2021.105126Suche in Google Scholar
[37] Sevda PF, Jamaleddin PS, Rauf F, Nasser A, Bahman R. Crystal violet dye sorption over acrylamide/graphene oxide bonded sodium alginate nanocomposite hydrogel. Chemosphere. 2021;270:129419–30.10.1016/j.chemosphere.2020.129419Suche in Google Scholar PubMed
[38] Hoon H, Jae-Hong K. Natural organic matter (NOM) adsorption to multi-walled carbon nanotubes: effect of NOM characteristics and water quality parameters. Environ Sci Technol. 2008;42(12):4416–21.10.1021/es702916hSuche in Google Scholar PubMed
[39] Yang W, Lu Y, Zheng F, Xue X, Na L, Liu D. Adsorption behavior and mechanisms of norfloxacin onto porous resins and carbon nanotube. Chem Eng J. 2012;179:112–8.10.1016/j.cej.2011.10.068Suche in Google Scholar
[40] Jiang Q, Yu L, Kirsch LE. Estimated pKa values for specific amino acid residues in daptomycin. J Pharm Sci. 2011;100(10):4225–33.10.1002/jps.22608Suche in Google Scholar PubMed
[41] Wang L, Zhu D, Duan L, Chen W. Adsorption of single-ringed N- and S-heterocyclic aromatics on carbon nanotubes. Carbon. 2010;48(13):3906–15.10.1016/j.carbon.2010.06.057Suche in Google Scholar
[42] Teixidó M, Pignatello JJ, Beltrán JL, Granados M, Peccia J. Speciation of the ionizable antibiotic sulfamethazine on black carbon (biochar). Environ Sci Technol. 2011;45(23):10020–7.10.1021/es202487hSuche in Google Scholar PubMed
[43] Mallampati R, Valiyaveettil S. Apple peels – a versatile biomass for water purification? ACS Appl Mater Interfaces. 2013;5(10):4443–9.10.1021/am400901eSuche in Google Scholar PubMed
[44] Jia M, Fang W, Bian Y, Xin J. Effects of pH and metal ions on oxytetracycline sorption to maize-straw-derived biochar. Bioresour Technol. 2013;136:87–93.10.1016/j.biortech.2013.02.098Suche in Google Scholar PubMed
© 2022 Lei Zhang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop
Artikel in diesem Heft
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop

