Abstract
Glutamine transporters transport different amino acids for cell growth and metabolism. In tumor cells, glutamine transporters are often highly expressed and play a crucial role in their growth. By inhibiting the amino acid transport of these transporters, the growth of cancer cells can be inhibited. In recent years, more and more attention has been paid to the study of glutamine transporter. In this article, the differences between the ASC system amino acid transporter 2 (ASCT2), L-type amino acid transporter 1 (LAT1), and the cystine–glutamate exchange (xCT) transporters research progress on the mechanism of action and corresponding small molecule inhibitors are summarized. This article introduces 62 related small molecule inhibitors of different transporters of ASCT2, LAT1, and xCT. These novel chemical structures provide ideas for the research and design of targeted inhibitors of glutamine transporters, as well as important references and clues for the design of new anti-tumor drugs.
1 Introduction
At present, the global cancer mortality rate is increasing year by year. The incidence of breast cancer and lung cancer is high in the world (Figure 1a) [1]. According to the national cancer statistics in 2020, lung cancer possessed the highest incidence, accounting for about 17.9% of the country (Figure 1b), followed by colorectal cancer, gastric cancer, breast cancer, and liver cancer [2]. Therefore, combining global and national data on different cancer rates, it is an urgent need to design novel small molecule inhibitors that can inhibit the growth of malignant tumor cells.

The incidence of cancers in the world (a) and China (b).
One of the important features of tumor cells is metabolic abnormalities. In the process of in-depth research on tumor metabolism, the focus of research has gradually shifted from the original glucose metabolism to amino acid metabolism, especially the glutamine-dependent transport pathway. Compared with normal cellular metabolism, tumor cells need to consume far more nutrients than their own metabolism. The metabolic needs of tumor cells are met by providing nutrients through the collective action of angiogenesis [3]. The amino acid transporters are expressed at different levels in many tissues of ordinary humans and cancer patients, and amino acid-related transporters have very important effect on cancer cell metabolism. The abundant glutamine in the body provides the necessary carbon and nitrogen sources for the growth and proliferation of tumor cells [4,5], and ultimately synthesizes energy substances such as nucleotides, glutathione, amino sugars, and proteins to achieve the energy metabolism in the body [6]. Glutamine metabolism relies on amino acid transporters to pass through the cell membrane and enter the mitochondria through cell membranes to exert metabolic effects [7]. Related transporters consist of amino acid transporters, monocarboxylic acid transporters, and glucose transporters [8]. Due to the differences in biochemical and genetic factors, the genesis mechanisms of different cancers are different [9]. So, we think it is worthy to study further glutamine transporter target among the different cancer targets.
2 Glutamine transporter mechanism of action
2.1 Classification of glutamine transporters
Since most amino acids are hydrophilic, and the major site of glutamine metabolism is mitochondria, energy in the human body must cross the cell membrane via selective transporters [10,11]. Amino acid transporters in human cells can be classified into four classes according to whether they are dependent on Na+ transport: Na+-dependent transporter A, transporter ASC (alanine–serine–cysteine) (ASCT1 and ASCT2), transporter N (SN1 and SN2)E, and the Na+-independent transporter L (LAT1, LAT2, LAT3, and LAT4) [12]. Na+-dependent transporters mainly rely on Na+/K+-ATP enzyme for energy supply, whereas Na+-independent transporter LAT (large neutral amino acid transporter) does not depend on Na+ to supply energy [13,14] (Table 1). Na+-dependent transporter A mainly transports non-essential amino acids such as glutamine and alanine. The transporter ASC and transporter L are responsible for exchanging glutamine and alanine in the cell membrane and essential amino acids outside the membrane. Due to the affinity between glutamine transporter and the non-essential amino acid glutamine, this affinity of ASCT2 is involved in the uptake of glutamine [15]. The transporter N is responsible for regulating the concentration of glutamine in the cell membrane [16,17].
Classification of ASCT, LAT, and cystine–glutamate exchange (xCT) transporters
| Transporter | Transporter system | Na+-dependence | Substrate |
|---|---|---|---|
| ASCT1 | System ASC | Na+-dependence | Basic and small neutral amino acids |
| ASCT2 | System ASC | Na+-dependence | Basic and small neutral amino acids |
| LAT1 | System y | Na+-independence | Neutral amino acids/basic amino acids |
| LAT2 | System y | Na+-independence | Neutral amino acids |
| LAT3 | System L | Na+-independence | Large neutral amino acids |
| LAT4 | System L | Na+-independence | Large neutral amino acids |
| xCT | System xc- | Na+-independence | Acidic amino acids |
2.2 Mechanism of action of glutamine transporter
Now the most frequently reported glutamine transporters in the literature are ASCT2, LAT1, and xCT [18,19]. The expression of ASCT2 protein is associated with poor prognosis in patients with colorectal cancer and prostate cancer [20]. The xCT transporter is responsible for the exchange of cystine–glutamate and is synthesized by the light chain (xCT) and the heavy chain (4F2hc). Human sample tissue analysis studies have shown that xCT is highly expressed in both lung and breast cancer patients. The LAT1 transporter is mainly responsible for the transport of macromolecular amino acids. The relationship between glutamine transporters LAT1, ASCT2, and xCT has not been reported so far. Therefore, we sort out the relationship between the three kinds of transporters and is shown in Figure 2 [21].

Mechanism of action of glutamine transporter. NEAA: non-essential amino acids; GLUD1: glutamate dehydrogenase 1; LAT2: alanine aminotransferase 2 recombinant protein; GPT2: glutamate pyruvic transaminase 2; TCA: cycle tricarboxylic acid cycle; and OAA: oxaloacetic acid.
ASCT2, encoded by SLC1A5 gene, is a neutral amino acid, which is mainly responsible for transporting serine, threonine, valine, and other amino acids into the body. L-type amino acid transporter is a neutral amino acid transporter, which is independent of Na+ when transporting amino acids, including LAT1, LAT2, LAT3, and LAT4. The L-type amino acid transporter (LAT1) transports neutral amino acids, branched-chain amino acids, aromatic amino acids, and some essential amino acids. Amino acids are transported into cells. The different amino acids transported into the cell by ASCT2 and LAT1 are catalyzed by glutaminase (GLS) to produce glutamate and NH4+, which are then converted to α-ketoglutaric acid (α-kG) by glutamate dehydrogenase; α-KG enters the tricarboxylic acid (TCA) cycle [22,23,24], where oxalacetic acid produce acetic acid, acetyl-coa, and citric acid, providing a carbon source for the synthesis of fat [25]. Glutamine contains two amino groups, and amino nitrogen provides nitrogen source for the synthesis of other non-essential amino acids [26,27,28]. Glutamine also increases the production of glutathione to resist oxidative stress against cell death [29,30,31].
xCT (SLC7A11) is a cystine/glutamate antiporter that imports cystine into cells and exports glutamate [32,33]. It transports cystine into the cells, where it binds with glutamate and glycine in the body, and finally forms glutathione to resist redox stress and cell death [34,35].
Glutamine increases the production of glutathione to resist redox stress against cell death. Glutamate-ammonia ligase (GLUL) also exists in the cells, and glutamate synthesizes glutamine under the action of GLUL [36,37,38]. In tumor cells, the cellular utilization of glucose is carried out in a wasteful manner, regardless of the availability of oxygen, it utilizes glucose in aerobic glycolysis to produce adenosine triphosphate (ATP) (Warburg effect) [39,40,41]. Due to rapid proliferation, tumor cells must use an alternative energy supply method – through glutamine-driven oxidative phosphorylation and use glutamine to produce ATP by the glycolytic pathway [42]. Since the TCA cycle constantly consumes citric acid, it is necessary to supplement the TCA cycle intermediates, which significantly increases the consumption of glutamine [43]. The role of glutamine in tumor cells is mainly to supply the TCA cycle to provide nitrogen source for the synthesis of non-essential amino acids and nucleotides, and to resist redox stress by promoting the synthesis of glutathione.
3 Glutamine transporter inhibitors
3.1 ASCT2 inhibitors
3.1.1 ASCT2 positive control inhibitors
According to previous studies, high expression of glutamine transporter can lead to the large uptake of glutamine by lung cancer cells. Inhibition of glutamine transport is another more effective method to limit glutamine metabolism [44]. In the study of lung cancer patients, the inhibitor of ASCT2 receptor, l-γ-glutamine-p-nitroaniline (GPNA) (Figure 3a), only has a certain inhibitory effect on the glutamine uptake of ASCT2 cells. So, we used two experimental methods for comparison: the positron emission tomography technique and the glutamine analog labeling technique to study the uptake of glutamine by ASCT2. Evaluation analysis showed that glutamine transporter small molecule inhibitor GPNA had a certain inhibitory effect on lung cancer patients. Preclinical pharmacology studies have shown that V-9302 is another novel inhibitor of transmembrane transport glutamine (Figure 3b). The therapeutic effect of V-9302 not only showed significant inhibitory effect on tumor cell growth and proliferation, but also induced tumor cell death and oxidative stress, ultimately leading to tumor cell apoptosis. Since glutamine is transported from outside the cell membrane to the cell membrane, it has a certain limiting effect, which will not only affect the glutamine metabolism process but also reduce the mTORC1 signal transduction effect, and eventually reduce the proliferation of lung cancer cells. Through experimental studies, the comparative study of inhibitor V-9302 and GPNA inhibitor shows that V-9302 has a stronger inhibitory effect on tumor cells and a more effective therapeutic effect [45].

Structure of GPNA (a) and V-9302 (b).
Current ASCT2 inhibitors are mainly non-selective small molecule compounds, such as serine biphenyl-4-carboxylate, benzylserine, and glutamine structure-related inhibitors [46]. GPNA has a weak inhibitory effect on ASCT2 (IC50 = 1,000 μM) [47]. Competitive inhibitors of ASCT2 have been developed, including 2-amino-4-bis (aryloxybenzyl) aminobutyric acid and benzylproline, if hydroxyl or amide group is introduced into the structure of these inhibitors, increasing the hydrophilicity of the compound will significantly increase the affinity of the inhibitor for ASCT2 [48]. Schulte’s team reported that competitive antagonist V-9302 (IC50 = 9.6 μM), which strongly inhibits ASCT2, slows the proliferation and growth of cancer cells, and increases the number of cancer cells. The number of cell death and the incidence of oxidative stress increased [49].
3.1.2 2-Substituted Nc-glutamyl anilide series derivatives
Manning’s research group discussed the structure–activity relationship of a series of derivatives containing glutamine structure, designed and synthesized 20 new small molecule inhibitors, and about 12 compounds were inactive. Compound a first obtains b under the conditions of CbzCl, NEt3, and MeOH, and b under the conditions of SOCI2 and CH2CI2 obtains compound c, and c under the conditions of lithium hydroxide and hydrogen synthesize 2-substituted Nc-glutamyl anilides (Figure 4).

Synthetic route towards 2-substituted Nc-glutamyl anilides (Compound a as initial material, b and c as synthetic intermediates).
It mainly includes compounds 3–9 (Figure 5), among which compound 7 has better activity. Biological experiments found that N-(2-(morpholino methyl) phenyl)-l-glutamine had the best effect on inhibiting glutamine absorption [50]. The introduction of a morpholine ring group significantly enhanced the inhibitory effect on glutamine uptake. This level of inhibition is three times higher than the previous levels of GPNA inhibition. In addition, the chemical properties of other compounds in this series need to be further studied and explored. This series of compounds provides a useful reference for the design of ASCT2 small molecule inhibitors.

Structures of compounds 1, 3–9.
3.1.3 Amino acid and l-glutamine nitroaniline inhibitors
Singh’s team designed and synthesized a series of derivatives and reported the derivatives, and modified the structure of l-alanine inhibitor, and l-proline and l-glutamyl nitroaniline inhibitors including serine derivatives (Figure 6). The structure of benzylproline was analyzed and studied. Finally, the structure of benzylproline was modified at the position of 2,3,4 substitution on the benzene ring of proline structure [51]. The study showed that the substituents on the benzene ring had little effect on the inhibitory effect, but the more hydrophobic groups in the structure, the stronger the binding ability to the ASCT2 transporter [52]. Benzylproline skeleton further enhances the binding capacity of ASCT2 inhibitors
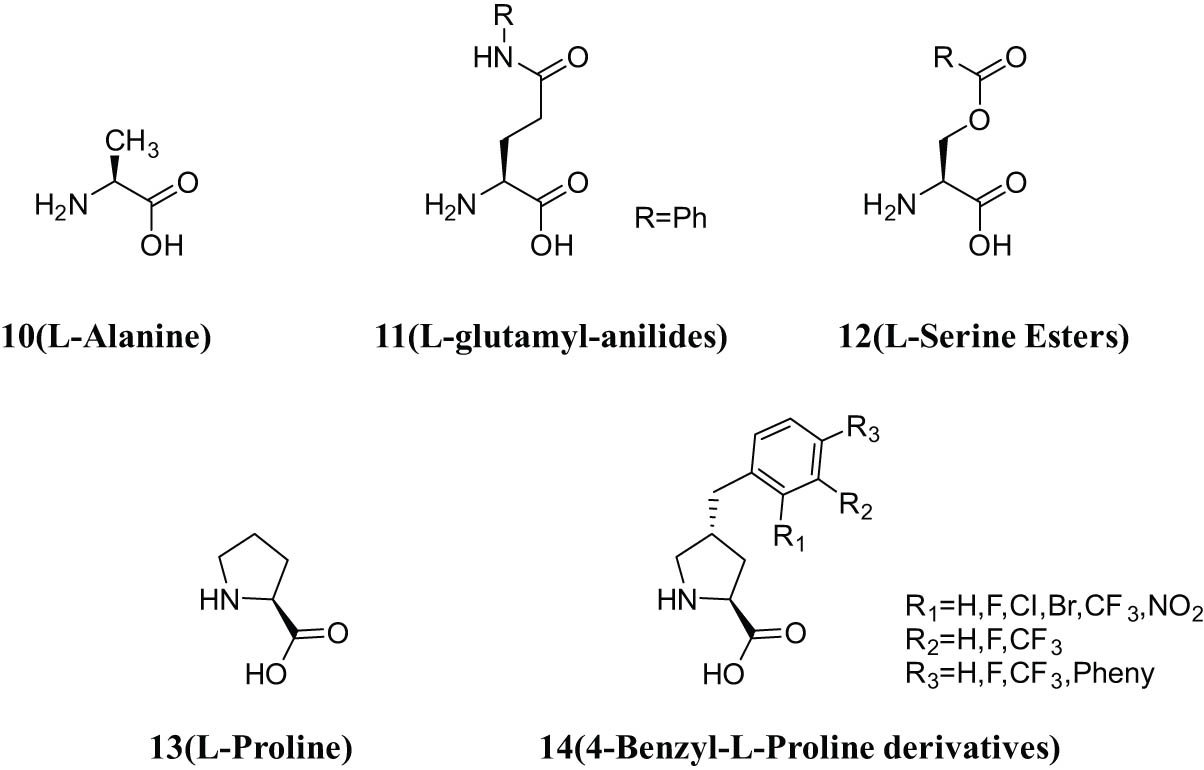
Structures of compounds 10–14.
3.2 LAT1 inhibitors
3.2.1 LAT1 positive control inhibitors
Inhibitors of LAT1 transporters are known to block their protein synthesis and cell proliferation processes. Leucine stimulates protein synthesis and cell growth through mammalian target of rapamycin (mTOR). LAT1 transporter inhibitors continue to inhibit mTOR signaling, thereby inhibiting tumor growth. However, the detailed mechanism by which leucine activates the mTOR signaling pathway in this nutrient is still unclear. At present, inhibitors of LAT1 are relatively few. 2-Aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH) (Figure 7a) and (S)-2-amino-3-(4-((5-amino-2-phenylbenzo[d]oxazole-7-yl) methoxy)-3,5-dichlorophenyl)-propionic acid 2 (JPH-203 or KYT-0353) JPH-203 are the main inhibitors of LAT1 transporters [53,54,55].
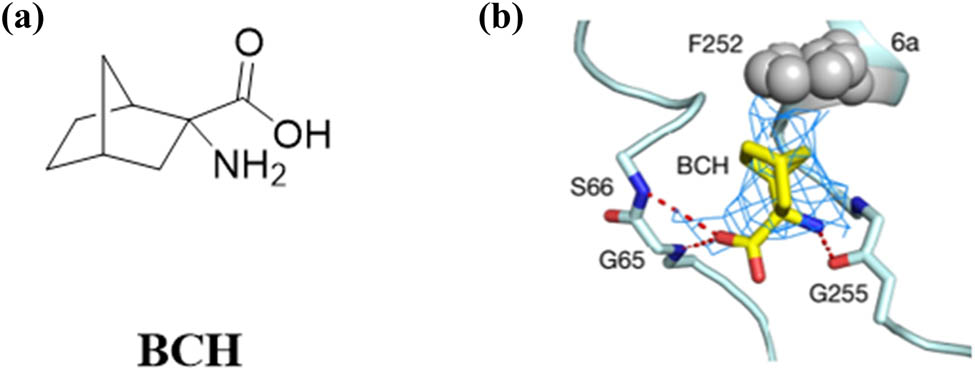
(a) Structure of BCH and (b) schematic diagram of BCH binding sites.
BCH is an L-system inhibitor that inhibits the growth and apoptosis of cancer cells, including breast, prostate, and lung cancer [56]. A schematic diagram of the BCH inhibitor binding site is shown in Figure 7b (BCH density and isoline as shown in the blue grid), where the amino and carboxyl groups in the parent nucleus bind to G255, G65, and S66 (BCH, pdb: 6IRT).
In 2010, Oda’s team published another inhibitor JPH-203 (Figure 8), which is based on the structure of the thyroid hormone triiodothyronine (T3). T3 is a substrate of LAT1 and LAT2. As a result, JPH-203 inhibited the proliferation of human colon cancer-derived HT-29 cells and human oral cancer cells with IC50 values of 4.1 and 69 μM, significantly reducing tumor growth in vivo [57,58].
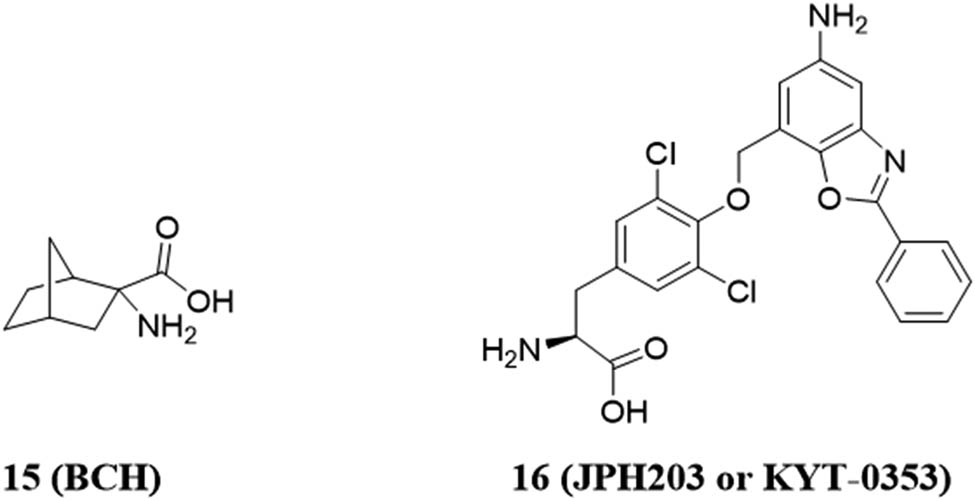
Chemical structures of inhibitor 15 (BCH) and inhibitor 16 (JPH 203).
The researchers then tested JPH-203 in other cancers and the results showed that JPH-203 reduced survival in brain, stomach, head and neck cancer, leukemia, prostate cancer, kidney cancer, thymic cancer, and thyroid cancer cell lines. Furthermore, it was observed that the inhibitory effect of JPH-203 on tumor cells in vitro was highly dependent on LAT1 substrate concentration [59]. In order to make the inhibitory effect of JPH-203 on tumor cells more obvious, it is necessary to solve the problem whether the concentration of LAT1 substrate in tumor cells affects the in vivo efficacy of JPH-203.
JPH-203 first entered phase I clinical trials in humans, primarily for the treatment of patients with advanced solid tumors. For 17 patients diagnosed with advanced solid tumors, the performance of some patients was relatively stable. Of the six samples tested, four were diagnosed with biliary cancer, and plasma levels of the LAT1 substrate remained high. JPH-203 was found to be effective in biliary tract cancer [60]. It is currently undergoing a clinical trial to evaluate phase II clinical trial in patients with advanced biliary tract cancer (UMIN Clinical Trial Registry, UMIN000034080) [61]. By comparing the chemical structures of BCH and JPH-203, it can be speculated that phenylalanine in the structure of JPH-203 may have a stronger inhibitory effect on cancer cells.
3.2.2 Phenylalanine series inhibitors
MF Wempe’s group designed and synthesized a series of derivatives according to the structure of JPH-203. As shown in Figure 9, compound 17 (IC50 = 0.42 µM) is currently undergoing clinical studies and experiments. They also introduced the characteristics of the inhibitor; its biological activity is not as good as JPH-203. Compound 18 (IC50 = 14 µM) is currently under the biological test stage and is a large neutral amino acid transporter small subunit 1 (SLC7A5; LAT1) inhibitor. Published by MF Wempe’s group and colleagues, it is mainly used to study the treatment, diagnosis, or monitoring of some cancer diseases. Compound 19 (IC50 = 11.5 µM) is a large neutral amino acid transporter small subunit 1 (LAT1) inhibitor. It is used to treat some cancer diseases and has been ineffective in biological trials. At present, the clinical research is in the stage of biological test. Compound 20 (IC50 = 9.20 µM) is currently under biological testing. The introduction of trifluoromethyl and chlorine atom groups on the benzene ring significantly improves the inhibitory effect on glutamine uptake. The MF Wempe’s team described the properties of these inhibitors, which target their respective cancer diseases [62].

Chemical structures of compounds 17–20.
3.2.3 Aryloxyalkylamines, butanediol acid derivatives
Zhang’s group reported compound 21, which is currently in the biological trial stage (Figure 10). Compound 21 targeted epidermal growth factor receptor (EGFR) and used monoclonal antibodies and EGFR-mediated tumor cell endocytosis to improve PAMAM Vector response. Compound 22 (IC50 = 3.00 ± 0.020 µM) is currently undergoing biological testing. Compound 23 (IC50 = 0.339 µM) inhibitor is currently under the biological trial stage, and its biological activity data is worse. Compound 24 is currently under the preclinical research stage, and other related studies have not been reported in detail. Compared with the arrangement of other positions, the three-dimensional structure of the benzene ring from the outside to the inside significantly weakens the inhibitory effect on glutamine uptake. Compound 25 is currently under biological trials, which is another molecular structure of the inhibitor [63].

Chemical structures of compounds 21–25.
3.2.4 Indole alanine derivatives
Based on the l-tryptophan structural backbone, Julien Graff’s group evaluated a series of new l-tryptophan derivatives indole ring backbone benzene moiety substituents as potential inhibitory groups of L-type amino acid transporter LAT1. The structural skeleton of compound 26 was structurally modified and transformed (Figure 11), compounds 27, 28, and 29 were designed, and the degree of inhibition of 3H-l-Leu entering HT-29 cells was tested by a biological activity test. The results showed that the IC50 value of compound 26 was 18.8 μM and the IC50 values of compounds 27, 28, and 29 were all greater than 100 μM. The experimental data showed that compound 26 had the best inhibitory effect on l-glutamine uptake, and the modification of amino groups in the structure of compound 26 did not improve the inhibitory effect on glutamine uptake [64]. The indole alanine group increases the hydrophilicity of the inhibitor, making it more effective in inhibiting glutamine uptake.

Chemical structures of compounds 26–29.
3.2.5 N,N dichloroethyl-phenylbutanine derivatives
Jandeleit’s group reported the bioactivity data of compounds 30–36. Compound 30 (IC50 < 0.100 mM) is currently under the stage of biological testing and is a large inhibitor of amino acid transporters (Figure 12). Compounds 31 (IC50 < 0.100 mM), 32, 33, 34, and 35 (IC50 < 0.100 mM) are currently under the biological test stage and are large neutral amino acid transporter inhibitors. Jandeleit’s group reported compound 36 (IC50 < 0.100 mM) and is currently in the stage of biological testing. In summary, most of the different LAT1 inhibitors are currently in the stage of biological testing, and their biological activity data are not very good [65]. A related inhibitor of JPH-203 (IC50 = 0.12 µM) is currently in phase II clinical development by J-Pharma for the treatment of ending biliary tract cancer disease. This inhibitor has a more potent effect than other inhibitors.

Chemical structures of compounds 30–36.
3.2.6 Phenylalanine derivatives
Compound 37 is currently in the biological test stage and is a large neutral amino acid transporter inhibitor. No other data on this structural type of inhibitor has been reported, and further studies are needed. The introduction of m-2 phenol and cyano groups improves the hydrophilicity of the inhibitor, making it more effective in inhibiting glutamine uptake. The structure of Dopa-CBT is shown in Figure 13. Compound 38 (Dopa-CBT) (IC50 = 27.7 ± 1.04 µM) is currently in biological test, and Thiele’s group has presented the structure of the inhibitor [66]. Compound 39 (IC50 = 18.2 ± 1.20 µM) is currently in preclinical testing stage, and it is an inhibitor of a large neutral amino acid transporter.

Chemical structures of compounds 37–39.
3.2.7 Benzocyclohexane derivatives
Yoshikatsu Kanai’s group screened out candidate compound 40 through computer virtual screening and docking, they modified the structure of compound 40, and designed and synthesized compounds 41 and 42 (Figure 14). These compounds were tested for biological activity, and the IC50 values of compounds 40, 41, and 42 were 0.121, 0.165, and 0.234 μM, respectively. Comparing the biological activity data, compound 40 had the strongest inhibitory effect. It shows that after the introduction of biphenyl ring, compound 40 was better than naphthalene ring and benzoxazole ring [67].
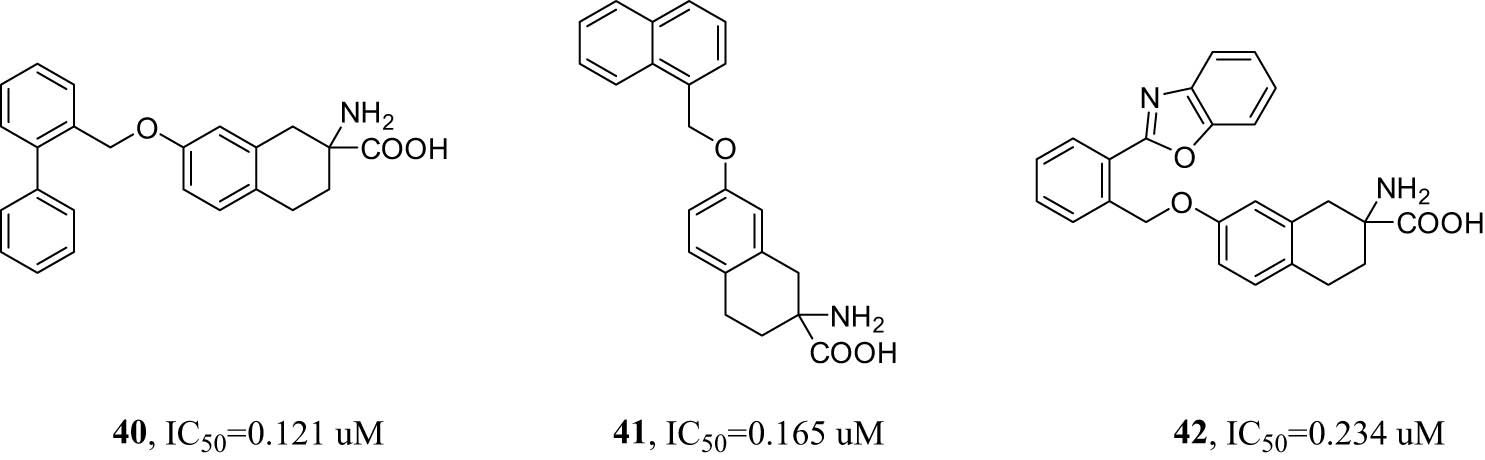
Chemical structures of compounds 40–42.
In addition, several research groups have demonstrated that LAT1-targeting nanoparticles are highly effective against tumors that overexpress LAT1. In conclusion, currently available LAT1 inhibitors and LAT1-targeting nanoparticles have made some progress in different preclinical and clinical research experiments. Future research questions will predict which type of cancer patients are most sensitive to LAT1 inhibition. This is important for competitive LAT1 inhibitors, as it is unclear whether the level of LAT1 substrate in the tumor microenvironment will be affected.
3.3 xCT (SLC7A11) inhibitor
At present, the inhibitors of xCT transporters are rare. The inhibitors sulfasalazine (SAS) and Erastin are effective inhibitors of xCT transporters. As a small molecule inhibitor, SAS is widely used in the treatment of arthritis and enteritis. It induces cytotoxic effects by inhibiting cystine or glutamate anti-transporters in cancer cells [68,69,70].
Nuclear factor kappa B (NFKB) is a nuclear transcription factor of all cell types and plays an important role in regulating the immune response to infection. SAS is an inhibitor of NFKB activation. It is speculated that the metabolites of SAS, 5-aminosalicylic acid (5-ASA) and sulfadiazine can inhibit the activation of NFKB. The experimental results of SAS in the treatment of enteritis and rheumatism showed that the number of proliferating cells gradually decreased significantly in several cell lines and mice, suggesting that SAS had a certain inhibitory effect on tumor cells. These results provide reference for the study of cystine–glutamate transporters [71,72].
Erastin mainly acts on mitochondrial voltage-dependent anion channel (VDAC) to induce iron death in tumor cells. Erastin has the same effect as glutamate, which inhibits cystine uptake by the cystine/glutamate transporter. Finally, Erastin disrupts the cellular oxidative defense system and induces iron-dependent oxidative death.
3.3.1 SAS derivatives
Davide Cirillo’s team screened some xCT anti-transporter inhibitors in the molecular library. Using SAS (compound 43) as lead compound, compounds 44–46 were synthesized (Figure 15). The bioactivity of compound 43 in U87MG cells was IC50 = 30 μM. The EC50 value of compound 44 is 18 μM in HT1080 cells. The toxicity test of compounds 45 and 46 in normal human astrocytes showed that the bioactivity data of compound 45 was IC50 = 74.6 μM and compound 46 was IC50 = 110.30 μM.

Chemical structures of compounds 43–46.
Compound 45 had a strong inhibitory effect on glutamine uptake and less cytotoxicity. At present, it is in progress and can be used as a candidate compound for further biological studies in vitro and in vivo testing and evaluation. The introduction of hydroxyl and carboxylic acid groups improved the hydrophilicity of the inhibitor, which made the inhibition of glutamine uptake more obvious. It is hoped that compound 45 will have a better inhibitory effect on tumor cells than compound 43 in future clinical trials [73].
3.3.2 4-Ketobenzopyrimidine derivatives
Brents’s group designed the synthetic routes of compound g. Compound g is synthesized through a three-step reaction. The process is as follows: compound e is obtained by reducing compound d with iron powder, then compound e is synthesized under the action of N,N-diisopropylethylamine and PCI3, and f is synthesized under the action of 4-dimethylaminopyridine and dichloromethane. The parent nucleus of the target structure g (Figure 16).

Structure g synthetic route map.
Compounds 47–52 were all obtained by structural modification of the target structure g (Figure 17). In this series of compounds, most of the small molecule inhibitors are currently in the stage of biological activity testing. Nicholas’s teams have researched and launched a series of small molecule inhibitors, and their bioactivity data vary widely. By summing and comparing, the introduction of imidazole ring or piperazine ring will improve its biological activity, it was found that the biological activity of compound 47 was superior to other similar compounds.

Chemical structures of compounds 47–52.
3.3.3 4-Ketobenzopyrimidopiperazine derivatives
Dixon’s group selected benzopyrimidine 4-ketone from compound 53 as the basic parent nucleus and designed compounds 54–58 through structural modification, and tested the inhibitory effect of different compounds on glutamate uptake in CCF-STTG1 cells (Figure 18). The IC50 values of compounds 53–58 were 0.09, 0.14, 0.54, 0.042, 0.074, and 0.15 μM. Compounds 54, 57, and 58 were found to have stronger inhibitory effects on glutamate uptake in CCF-STTG1 cells. Introducing chlorine atoms into the benzene ring or keeping the structure of the benzene ring unchanged could inhibit the uptake of glutamate in CCF-STTG1 l-glutamine. These candidate compounds provide more reference for the subsequent compound design and screening [74].

Chemical structures of compounds 53–58.
3.3.4 Erastin derivatives
Erastin (compound 59) is the most active small molecule inhibitor of all xCT inhibitors studied till date (Figure 19). The IC50 value of compound 59 is 625 nM. It is a ferroptosis activator that acts on mitochondrial VDACs and selectively acts on tumor cells containing the oncogene RAS [75]. It selectively kills and causes oncogenic Ras mutant cell lines and triggers a unique Fe-dependent non-apoptotic cell death called ferroptosis.
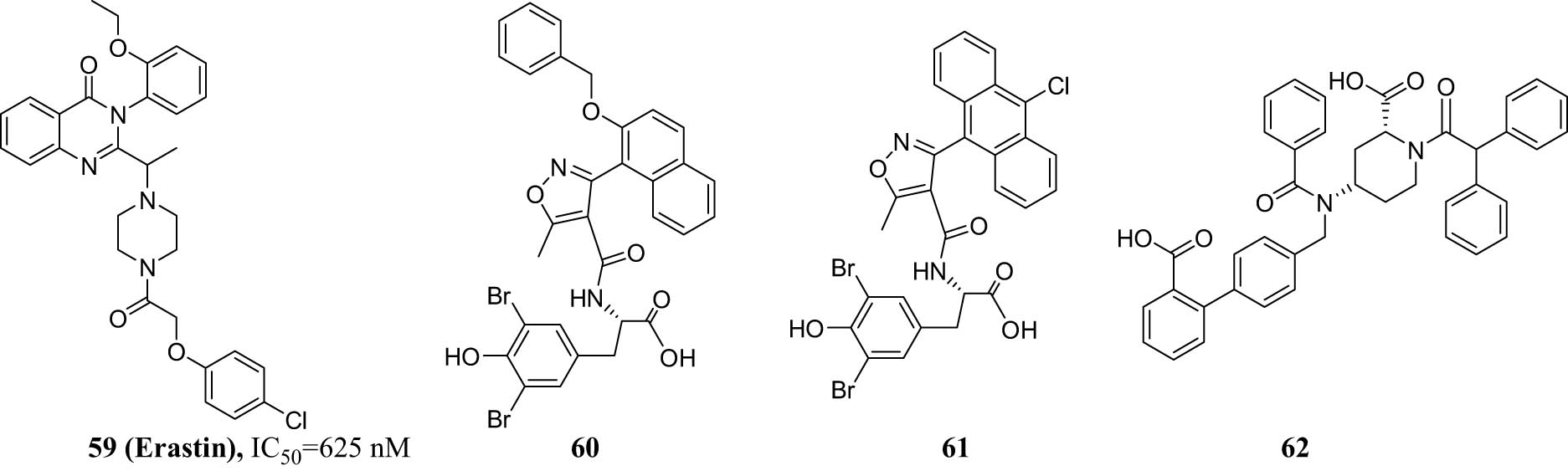
Chemical structures of compounds 59–62.
Erastin binds directly to VDAC2 and causes mitochondrial damage by reactive oxygen species (ROS) production in a nicotinamide adenine dinucleotide-dependent manner. Furthermore, Erastin strongly enhanced the effect of wild-type EGFR cells by inducing caspase-independent cell death mediated by ROS. Compounds 60, 61, and 62 are currently in biological testing. For the same series of compounds, the biological activity data varied according to their structure–activity relationship [76].
4 Conclusion
At present, the death rate caused by cancer is increasing year by year. With the deepening of understanding of tumor metabolism, the focus of tumor metabolism research has gradually shifted from glucose metabolism to amino acid metabolism [77,78,79], especially the glutamine pathway [80,81,82,83]. Changes in cellular metabolism are one of the important features of tumors [84,85,86,87]. The glutamine transport pathway mainly includes three transport modes: ASCT2, LAT1, and xCT [88,89,90,91]. Therefore, altering the metabolism of tumor cells by inhibiting the l-glutamine pathway is relatively new [92,93]. This article reviews the different types of l-glutamine transporters and their mechanisms of action, as well as the characteristics of reported small molecule inhibitors (Table 2).
Different inhibitors of transporters
| Target | Mechanism | Inhibitor | Remarks |
|---|---|---|---|
| ASCT2 | Inhibition of glutamine transport | GPNA | Animal experiments and lung cancer cell lines |
| (SCL1A5) | V-9302 | ||
| LAT1 | Inhibition of glutamine–EAA transport | BCH | Lung cancer cell lines |
| (SCL7A5) | |||
| xCT | Inhibition of cysteine–glutamate transport | SAS | Animal experiments and lung cancer cell lines |
| (SCL7A11) |
In addition, by understanding and summarizing the information from the currently reported literature, some practical design ideas for glutamine transporter inhibitors were proposed, which involved the structural characteristics of the reported inhibitors. By detailed introduction and analysis of the small molecule inhibitors reported so far, it is believed that they will play an important role in future drug development and overcome the difficulties better. Thus, the future of designing better inhibitors can be helped by combining the different strengths and weaknesses of the existing small molecule inhibitors. It is hoped that this review can provide reference information for other studies to further understand and provide more ideas to develop new glutamine transporter inhibitors.
Acknowledgment
The authors are grateful to the Fundamental Research Funds for the Central Universities (2632022ZD01).
-
Funding information: This work was supported by the Fundamental Research Funds for the Central Universities (2632022ZD01).
-
Author contributions: Jiye Zhao: methodology, writing, and editing; Jiayi Lv: revised and checked for writing and grammar; Yang Chen: revised and checked the concept; Dong Qile: conceptualization and supervision; Dong Hao: software and validation.
-
Conflict of interest: All the authors have no conflicts of interest and agree to the publication of the article.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
[1] Deo SV, Sharma J, Kumar S. GLOBOCAN 2020 report on global cancer burden: challenges and opportunities for surgical oncologists. Ann Surg Oncol. 2022(11):6497–500.10.1245/s10434-022-12151-6Search in Google Scholar PubMed
[2] Zhang S, Sun K, Zheng R, Zeng H, Wang S, Chen R, et al. Cancer incidence and mortality in China, 2015. J Natl Cancer Cent. 2021;1(1):2–11.10.1016/j.jncc.2020.12.001Search in Google Scholar
[3] Jin L, Alesi GN, Kang S. Glutaminolysis as a target for cancer therapy. Oncogene. 2016 Jul;35(28):3619–25.10.1038/onc.2015.447Search in Google Scholar PubMed PubMed Central
[4] Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol. 2005 Aug;15(4):254–66.10.1016/j.semcancer.2005.04.005Search in Google Scholar PubMed
[5] Quek LE, van Geldermalsen M, Guan YF, Wahi K, Mayoh C, Balaban S, et al. Glutamine addiction promotes glucose oxidation in triple-negative breast cancer. Oncogene. 2022 Aug;41(34):4066–78.10.1038/s41388-022-02408-5Search in Google Scholar PubMed PubMed Central
[6] Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015 Jun;93:52–79.10.1016/j.ejpb.2015.03.018Search in Google Scholar PubMed
[7] Kanai Y, Hediger MA. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflugers Arch. 2004 Feb;447(5):469–79.10.1007/s00424-003-1146-4Search in Google Scholar PubMed
[8] Scopelliti AJ, Font J, Vandenberg RJ, Boudker O, Ryan RM. Structural characterisation reveals insights into substrate recognition by the glutamine transporter ASCT2/SLC1A5. Nat Commun. 2018 Jan;9(1):38.10.1038/s41467-017-02444-wSearch in Google Scholar PubMed PubMed Central
[9] Pejin B, Iodice C, Tommonaro G, Bogdanovic G, Kojic V, De Rosa S. Further in vitro evaluation of cytotoxicity of the marine natural product derivative 4′-leucine-avarone. Nat Prod Res. 2014;28(5):347–50.10.1080/14786419.2013.863201Search in Google Scholar PubMed
[10] Quirico L, Orso F, Cucinelli S, Paradzik M, Natalini D, Centonze G, et al. miRNA-guided reprogramming of glucose and glutamine metabolism and its impact on cell adhesion/migration during solid tumor progression. Cell Mol Life Sci. 2022 Mar;79(4):216.10.1007/s00018-022-04228-ySearch in Google Scholar PubMed PubMed Central
[11] Silva C, Andrade N, Guimarães JT, Patrício E, Martel F. The in vitro effect of the diabetes-associated markers insulin, leptin and oxidative stress on cellular characteristics promoting breast cancer progression is GLUT1-dependent. Eur J Pharmacol. 2021 May;898:173980.10.1016/j.ejphar.2021.173980Search in Google Scholar PubMed
[12] Suzuki M, Toki H, Furuya A, Ando H. Establishment of monoclonal antibodies against cell surface domains of ASCT2/SLC1A5 and their inhibition of glutamine-dependent tumor cell growth. Biochem Biophys Res Commun. 2017 Jan;482(4):651–7.10.1016/j.bbrc.2016.11.089Search in Google Scholar PubMed
[13] Vaupel P, Multhoff G. Revisiting the Warburg effect: historical dogma versus current understanding. J Physiol. 2021 Mar;599(6):1745–57.10.1113/JP278810Search in Google Scholar PubMed
[14] Pascale RM, Calvisi DF, Simile MM, Feo CF, Feo F. The Warburg effect 97 years after its discovery. Cancers (Basel). 2020 Sep;12(10):12.10.3390/cancers12102819Search in Google Scholar PubMed PubMed Central
[15] Garaeva AA, Guskov A, Slotboom DJ, Paulino C. A one-gate elevator mechanism for the human neutral amino acid transporter ASCT2. Nat Commun. 2019 Jul;10(1):3427.10.1038/s41467-019-11363-xSearch in Google Scholar PubMed PubMed Central
[16] Bröer S. Adaptation of plasma membrane amino acid transport mechanisms to physiological demands. Pflugers Arch. 2002 Jul;444(4):457–66.10.1007/s00424-002-0840-ySearch in Google Scholar PubMed
[17] Zander CB, Albers T, Grewer C. Voltage-dependent processes in the electroneutral amino acid exchanger ASCT2. J Gen Physiol. 2013 Jun;141(6):659–72.10.1085/jgp.201210948Search in Google Scholar PubMed PubMed Central
[18] Bhutia YD, Babu E, Ramachandran S, Ganapathy V. Amino acid transporters in cancer and their relevance to glutamine addiction: novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015 May;75(9):1782–8.10.1158/0008-5472.CAN-14-3745Search in Google Scholar PubMed
[19] Pouyssegur J, Marchiq I, Parks SK, Durivault J, Ždralević M, Vucetic M, et al. Vucetic, ‘Warburg effect’ controls tumor growth, bacterial, viral infections and immunity – genetic deconstruction and therapeutic perspectives. Semin Cancer Biol. 2022;86:334–46.10.1016/j.semcancer.2022.07.004Search in Google Scholar PubMed
[20] Cederkvist H, Kolan SS, Wik JA, Sener Z, Skålhegg BS. Identification and characterization of a novel glutaminase inhibitor. FEBS Open Bio. 2022 Jan;12(1):163–74.10.1002/2211-5463.13319Search in Google Scholar PubMed PubMed Central
[21] Liu Y, Zhao T, Li Z, Wang L, Yuan S, Sun L. The role of ASCT2 in cancer: a review. Eur J Pharmacol. 2018 Oct;837:81–7.10.1016/j.ejphar.2018.07.007Search in Google Scholar PubMed
[22] Bothwell PJ, Kron CD, Wittke EF, Czerniak BN, Bode BP. Targeted suppression and knockout of ASCT2 or LAT1 in epithelial and mesenchymal human liver cancer cells fail to inhibit growth. Int J Mol Sci. 2018 Jul;19(7):19.10.3390/ijms19072093Search in Google Scholar PubMed PubMed Central
[23] A. Halama, Suhre K. Advancing cancer treatment by targeting glutamine metabolism – a roadmap. Cancers (Basel). 2022;14(3):553.10.3390/cancers14030553Search in Google Scholar PubMed PubMed Central
[24] Zhao X, Sakamoto S, Maimaiti M, Anzai N, Ichikawa T. Contribution of LAT1-4F2hc in urological cancers via toll-like receptor and other vital pathways. Cancers (Basel). 2022 Jan;14(1):14.10.3390/cancers14010229Search in Google Scholar PubMed PubMed Central
[25] Scalise M, Pochini L, Galluccio M, Console L, Indiveri C. Glutamine transport and mitochondrial metabolism in cancer cell growth. Front Oncol. 2017 Dec;7:306.10.3389/fonc.2017.00306Search in Google Scholar PubMed PubMed Central
[26] Garibsingh RA, Ndaru E, Garaeva AA, Shi Y, Zielewicz L, Zakrepine P, et al. Rational design of ASCT2 inhibitors using an integrated experimental-computational approach. Proc Natl Acad Sci USA. 2021 Sep;118(37):118.10.1073/pnas.2104093118Search in Google Scholar PubMed PubMed Central
[27] Wang J, Dong Y, Grewer C. Functional and kinetic comparison of alanine cysteine serine transporters ASCT1 and ASCT2. Biomolecules. 2022 Jan;12(1):12.10.3390/biom12010113Search in Google Scholar PubMed PubMed Central
[28] Ibrahiem AT 2nd, Fawzy MS, Abdulhakim JA, Toraih EA. GLUT1 and ASCT2 Protein expression in papillary thyroid carcinoma patients and relation to hepatitis C virus: a propensity-score matched analysis. Int J Gen Med. 2022 Mar;15:2929–44.10.2147/IJGM.S354108Search in Google Scholar PubMed PubMed Central
[29] Zhou Q, Lin W, Wang C, Sun F, Ju S, Chen Q, et al. Neddylation inhibition induces glutamine uptake and metabolism by targeting CRL3SPOP E3 ligase in cancer cells. Nat Commun. 2022 May;13(1):3034.10.1038/s41467-022-30559-2Search in Google Scholar PubMed PubMed Central
[30] Freidman NJ, Briot C, Ryan RM. Characterizing unexpected interactions of a glutamine transporter inhibitor with members of the SLC1A transporter family. J Biol Chem. 2022 Aug;298(8):102178.10.1016/j.jbc.2022.102178Search in Google Scholar PubMed PubMed Central
[31] Scalise M, Pappacoda G, Mazza T, Console L, Pochini L, Indiveri C. Cysteine 467 of the ASCT2 amino acid transporter is a molecular determinant of the antiport mechanism. Int J Mol Sci. 2022 Jan;23(3):23.10.3390/ijms23031127Search in Google Scholar PubMed PubMed Central
[32] Ji X, Qian J, Rahman SM, Siska PJ, Zou Y, Harris BK, et al. xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small cell lung cancer progression. Oncogene. 2018 Sep;37(36):5007–19.10.1038/s41388-018-0307-zSearch in Google Scholar PubMed PubMed Central
[33] Lee N, Carlisle AE, Peppers A, Park SJ, Doshi MB, Spears ME, et al. xCT-driven expression of GPX4 determines sensitivity of breast cancer cells to ferroptosis inducers. Antioxidants. 2021 Feb;10(2):10.10.3390/antiox10020317Search in Google Scholar PubMed PubMed Central
[34] Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021 Aug;12(8):599–620.10.1007/s13238-020-00789-5Search in Google Scholar PubMed PubMed Central
[35] Zou L, Gao Z, Zeng F, Xiao J, Chen J, Feng X, et al. Sulfasalazine suppresses thyroid cancer cell proliferation and metastasis through T-cell originated protein kinase. Oncol Lett. 2019 Oct;18(4):3517–26.10.3892/ol.2019.10721Search in Google Scholar PubMed PubMed Central
[36] Tang Y, Wang S, Li Y, Yuan C, Zhang J, Xu Z, et al. Simultaneous glutamine metabolism and PD-L1 inhibition to enhance suppression of triple-negative breast cancer. J Nanobiotechnol. 2022 May;20(1):216.10.1186/s12951-022-01424-7Search in Google Scholar PubMed PubMed Central
[37] Yu W, Huang J, Dong Q, Li W, Jiang L, Zhang Q, et al. Ag120-mediated inhibition of ASCT2-dependent glutamine transport has an anti-tumor effect on colorectal cancer cells. Front Pharmacol. 2022 Mar;13:871392.10.3389/fphar.2022.871392Search in Google Scholar PubMed PubMed Central
[38] Seo H, Kramer AC, McLendon BA, Cain JW, Burghardt RC, Wu G, et al. Elongating porcine conceptuses can utilize glutaminolysis as an anaplerotic pathway to maintain the TCA cycle. Biol Reprod. 2022 Sep;107(3):823–33.10.1093/biolre/ioac097Search in Google Scholar PubMed
[39] DeBerardinis RJ, Chandel NS. We need to talk about the Warburg effect. Nat Metab. 2020 Feb;2(2):127–9.10.1038/s42255-020-0172-2Search in Google Scholar PubMed
[40] Nakagawa T, Lanaspa MA, Millan IS, Fini M, Rivard CJ, Sanchez-Lozada LG, et al. Fructose contributes to the Warburg effect for cancer growth. Cancer Metab. 2020 Jul;8(1):16.10.1186/s40170-020-00222-9Search in Google Scholar PubMed PubMed Central
[41] Lemasters JJ. Metabolic implications of non-electrogenic ATP/ADP exchange in cancer cells: a mechanistic basis for the Warburg effect. Biochim Biophys Acta Bioenerg. 2021 Jul;1862(7):148410.10.1016/j.bbabio.2021.148410Search in Google Scholar PubMed PubMed Central
[42] Gao G, Li C, Fan W, Zhang M, Li X, Chen W, et al. Brilliant glycans and glycosylation: seq and ye shall find. Int J Biol Macromol. 2021 Oct;189:279–91.10.1016/j.ijbiomac.2021.08.054Search in Google Scholar PubMed
[43] Hayashi K, Anzai N. L-type amino acid transporter 1 as a target for inflammatory disease and cancer immunotherapy. J Pharmacol Sci. 2022 Jan;148(1):31–40.10.1016/j.jphs.2021.09.006Search in Google Scholar PubMed
[44] Ndaru E, Garibsingh RA, Shi Y, Wallace E, Zakrepine P, Wang J, et al. Novel alanine serine cysteine transporter 2 (ASCT2) inhibitors based on sulfonamide and sulfonic acid ester scaffolds. J Gen Physiol. 2019 Mar;151(3):357–68.10.1085/jgp.201812276Search in Google Scholar PubMed PubMed Central
[45] Yang WH, Qiu Y, Stamatatos O, Janowitz T, Lukey MJ. Enhancing the efficacy of glutamine metabolism inhibitors in cancer therapy. Trends Cancer. 2021 Aug;7(8):790–804.10.1016/j.trecan.2021.04.003Search in Google Scholar PubMed PubMed Central
[46] Wang W, Pan H, Ren F, Chen H, Ren P. Targeting ASCT2-mediated glutamine metabolism inhibits proliferation and promotes apoptosis of pancreatic cancer cells. Biosci Rep. 2022 Mar;42(3):42.10.1042/BSR20212171Search in Google Scholar PubMed PubMed Central
[47] Esslinger CS, Cybulski KA, Rhoderick JF. Ngamma-aryl glutamine analogues as probes of the ASCT2 neutral amino acid transporter binding site. Bioorg Med Chem. 2005 Feb;13(4):1111–8.10.1016/j.bmc.2004.11.028Search in Google Scholar PubMed
[48] Grewer C, Grabsch E. New inhibitors for the neutral amino acid transporter ASCT2 reveal its Na+-dependent anion leak. J Physiol. 2004 Jun;557(Pt 3):747–59.10.1113/jphysiol.2004.062521Search in Google Scholar PubMed PubMed Central
[49] Schulte ML, Fu A, Zhao P, Li J, Geng L, Smith ST, et al. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat Med. 2018 Feb;24(2):194–202.10.1038/nm.4464Search in Google Scholar PubMed PubMed Central
[50] Jiang H, Zhang N, Tang T, Feng F, Sun H, Qu W. Target the human alanine/serine/cysteine transporter 2(ASCT2): achievement and future for novel cancer therapy. Pharmacol Res. 2020 Aug;158:104844.10.1016/j.phrs.2020.104844Search in Google Scholar PubMed
[51] Hochegger P, Faist J, Seebacher W, Saf R, Mäser P, Kaiser M, et al. Synthesis and structure–activity relationships for new 6-fluoroquinoline derivatives with antiplasmodial activity. Bioorg Med Chem. 2019 May;27(10):2052–65.10.1016/j.bmc.2019.03.061Search in Google Scholar PubMed
[52] Seo Y, Kang J, Kim TI, Joo CG. MRI assessment of glutamine uptake correlates with the distribution of glutamine transporters and cancer stem cell markers. Sci Rep. 2022;12(1):5511.10.1038/s41598-022-09529-7Search in Google Scholar PubMed PubMed Central
[53] Prejanò M, Romeo I, La Serra MA, Russo N, Marino T. Computational study reveals the role of water molecules in the inhibition mechanism of LAT1 by 1,2,3-dithiazoles. J Chem Inf Model. 2021 Dec;61(12):5883–92.10.1021/acs.jcim.1c01012Search in Google Scholar PubMed PubMed Central
[54] Teixeira E, Silva C, Martel F. The role of the glutamine transporter ASCT2 in antineoplastic therapy. Cancer Chemother Pharmacol. 2021 Apr;87(4):447–64.10.1007/s00280-020-04218-6Search in Google Scholar PubMed
[55] Li Q, Zhong X, Yao W, Yu J, Wang C, Li Z, et al. Inhibitor of glutamine metabolism V9302 promotes ROS-induced autophagic degradation of B7H3 to enhance antitumor immunity. J Biol Chem. 2022 Apr;298(4):101753.10.1016/j.jbc.2022.101753Search in Google Scholar PubMed PubMed Central
[56] Ottestad-Hansen S, Hu QX, Follin-Arbelet VV, Bentea E, Sato H, Massie A, et al. The cystine–glutamate exchanger (xCT, Slc7a11) is expressed in significant concentrations in a subpopulation of astrocytes in the mouse brain. Glia. 2018 May;66(5):951–70.10.1002/glia.23294Search in Google Scholar PubMed
[57] Oda K, Hosoda N, Endo H, Saito K, Tsujihara K, Yamamura M, et al. L-type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Sci. 2010 Jan;101(1):173–9.10.1111/j.1349-7006.2009.01386.xSearch in Google Scholar PubMed
[58] Yamaga T, Suehiro J, Wada Y, Sakurai H. Induction of CTH expression in response to amino acid starvation confers resistance to anti-LAT1 therapy in MDA-MB-231 cells. Sci Rep. 2022 Jan;12(1):1021.10.1038/s41598-022-04987-5Search in Google Scholar PubMed PubMed Central
[59] Häfliger P, Graff J, Rubin M, Stooss A, Dettmer MS, Altmann KH, et al. The LAT1 inhibitor JPH203 reduces growth of thyroid carcinoma in a fully immunocompetent mouse model. J Exp Clin Cancer Res. 2018 Sep;37(1):234.10.1186/s13046-018-0907-zSearch in Google Scholar PubMed PubMed Central
[60] Okano N, Naruge D, Kawai K, Kobayashi T, Nagashima F, Endou H, et al. First-in-human phase I study of JPH203, an L-type amino acid transporter 1 inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2020 Oct;38(5):1495–506.10.1007/s10637-020-00924-3Search in Google Scholar PubMed
[61] Okano N, Hana K, Naruge D, Kawai K, Kobayashi T, Nagashima F, et al. Biomarker analyses in patients with advanced solid tumors treated with the LAT1 inhibitor JPH203. In Vivo. 2020 Sep–Oct;34(5):2595–606.10.21873/invivo.12077Search in Google Scholar PubMed PubMed Central
[62] Cormerais Y, Massard PA, Vucetic M, Giuliano S, Tambutté E, Durivault J, et al. The glutamine transporter ASCT2 (SLC1A5) promotes tumor growth independently of the amino acid transporter LAT1 (SLC7A5). J Biol Chem. 2018 Feb;293(8):2877–87.10.1074/jbc.RA117.001342Search in Google Scholar PubMed PubMed Central
[63] Li R, Zhang J, Guo J, Xu Y, Duan K, Zheng J, et al. Application of nitroimidazole-carbobane-modified phenylalanine derivatives as dual-target boron carriers in boron neutron capture therapy. Mol Pharm. 2020 Jan;17(1):202–11.10.1021/acs.molpharmaceut.9b00898Search in Google Scholar PubMed
[64] Graff J, Müller J, Sadurní A, Rubin M, Canivete Cuissa IA, Keller C, et al. The evaluation of l-tryptophan derivatives as inhibitors of the l-type amino acid transporter LAT1 (SLC7A5). ChemMedChem. 2022 Sep;17(17):e202200308.10.1002/cmdc.202200308Search in Google Scholar PubMed PubMed Central
[65] Botas A, Eitel M, Schwarz PN, Buchmann A, Costales P, Núñez LE, et al. Genetic engineering in combination with semi-synthesis leads to a new route for gram-scale production of the immunosuppressive natural product Brasilicardin A. Angew Chem Int Ed Engl. 2021 Jun;60(24):13536–41.10.1002/anie.202015852Search in Google Scholar PubMed PubMed Central
[66] Thiele NA, Kärkkäinen J, Sloan KB, Rautio J, Huttunen KM. Secondary carbamate linker can facilitate the sustained release of dopamine from brain-targeted prodrug. Bioorg Med Chem Lett. 2018 Sep;28(17):2856–60.10.1016/j.bmcl.2018.07.030Search in Google Scholar PubMed
[67] Kanai Y. Amino acid transporter LAT1 (SLC7A5) as a molecular target for cancer diagnosis and therapeutics. Pharmacol Ther. 2022 Feb;230:107964.10.1016/j.pharmthera.2021.107964Search in Google Scholar PubMed
[68] Yan R, Xie E, Li Y, Li J, Zhang Y, Chi X, et al. The structure of erastin-bound xCT-4F2hc complex reveals molecular mechanisms underlying erastin-induced ferroptosis. Cell Res. 2022 Jul;32(7):687–90.10.1038/s41422-022-00642-wSearch in Google Scholar PubMed PubMed Central
[69] Rabinowitz J, Sharifi HJ, Martin H, Marchese A, Robek M, Shi B, et al. xCT/SLC7A11 antiporter function inhibits HIV-1 infection. Virology. 2021 Apr;556:149–60.10.1016/j.virol.2021.01.008Search in Google Scholar PubMed PubMed Central
[70] Liu T, Cui Y, Dong S, Kong X, Xu X, Wang Y, et al. Treadmill training reduces cerebral ischemia-reperfusion injury by inhibiting ferroptosis through activation of SLC7A11/GPX4. Oxid Med Cell Longev. 2022 Jun;2022:8693664.10.1155/2022/8693664Search in Google Scholar PubMed PubMed Central
[71] Wang Q, Hardie RA, Hoy AJ, van Geldermalsen M, Gao D, Fazli L, et al. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J Pathol. 2015;236(3):278–89.10.1002/path.4518Search in Google Scholar PubMed PubMed Central
[72] Roy G, Bhattacharya A, Leprohon P, Ouellette M. Decreased glutamate transport in acivicin resistant Leishmania tarentolae. PLoS Negl Trop Dis. 2021 Dec;15(12):e0010046.10.1371/journal.pntd.0010046Search in Google Scholar PubMed PubMed Central
[73] Cirillo D, Sarowar S, Øyvind Enger P, Bjørsvik HR. Structure–activity-relationship-aided design and synthesis of xCT antiporter inhibitors. ChemMedChem. 2021 Sep;16(17):2650–68.10.1002/cmdc.202100204Search in Google Scholar PubMed PubMed Central
[74] Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, et al. Pharmacological inhibition of cystine–glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife. 2014 May;3:e02523.10.7554/eLife.02523Search in Google Scholar PubMed PubMed Central
[75] Yamaguchi I, Yoshimura SH, Katoh H. High cell density increases glioblastoma cell viability under glucose deprivation via degradation of the cystine/glutamate transporter xCT (SLC7A11). J Biol Chem. 2020 May;295(20):6936–45.10.1074/jbc.RA119.012213Search in Google Scholar PubMed PubMed Central
[76] Okazaki F, Matsunaga N, Hamamura K, Suzuki K, Nakao T, Okazaki H, et al. Administering xCT inhibitors based on circadian clock improves antitumor effects. Cancer Res. 2017 Dec;77(23):6603–13.10.1158/0008-5472.CAN-17-0720Search in Google Scholar PubMed
[77] Koppula P, Zhang Y, Shi J, Li W, Gan B. The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. J Biol Chem. 2017 Aug;292(34):14240–9.10.1074/jbc.M117.798405Search in Google Scholar PubMed PubMed Central
[78] Scalise M, Pochini L, Cosco J, Aloe E, Mazza T, Console L, et al. Interaction of cholesterol with the human SLC1A5 (ASCT2): insights into structure/function relationships. Front Mol Biosci. 2019 Oct;6:110.10.3389/fmolb.2019.00110Search in Google Scholar PubMed PubMed Central
[79] Katt WP, Cerione RA. Glutaminase regulation in cancer cells: a druggable chain of events. Drug Discov Today. 2014 Apr;19(4):450–7.10.1016/j.drudis.2013.10.008Search in Google Scholar PubMed PubMed Central
[80] Garaeva AA, Oostergetel GT, Gati C, Guskov A, Paulino C, Slotboom DJ. Cryo-EM structure of the human neutral amino acid transporter ASCT2. Nat Struct Mol Biol. 2018 Jun;25(6):515–21.10.1038/s41594-018-0076-ySearch in Google Scholar PubMed
[81] Ren P, Yue M, Xiao D, Xiu R, Gan L, Liu H, et al. ATF4 and N-Myc coordinate glutamine metabolism in MYCN-amplified neuroblastoma cells through ASCT2 activation. J Pathol. 2015 Jan;235(1):90–100.10.1002/path.4429Search in Google Scholar PubMed
[82] Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009 Apr;458(7239):762–5.10.1038/nature07823Search in Google Scholar PubMed PubMed Central
[83] Yuneva MO, Fan TW, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T, et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012 Feb;15(2):157–70.10.1016/j.cmet.2011.12.015Search in Google Scholar PubMed PubMed Central
[84] Felsher DW. MYC inactivation elicits oncogene addiction through both tumor cell-intrinsic and host-dependent mechanisms. Genes Cancer. 2010 Jun;1(6):597–604.10.1177/1947601910377798Search in Google Scholar PubMed PubMed Central
[85] Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013 Mar;4(3):e532.10.1038/cddis.2013.60Search in Google Scholar PubMed PubMed Central
[86] Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, et al. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med. 2008 Jun;14(6):629–32.10.1038/nm1772Search in Google Scholar PubMed
[87] Yin Z, Bai L, Li W, Zeng T, Tian H, Cui J. Targeting T cell metabolism in the tumor microenvironment: an anti-cancer therapeutic strategy. J Exp Clin Cancer Res. 2019 Sep;38(1):403.10.1186/s13046-019-1409-3Search in Google Scholar PubMed PubMed Central
[88] Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015 Apr;520(7545):57–62.10.1038/nature14344Search in Google Scholar PubMed PubMed Central
[89] Lo M, Wang YZ, Gout PW. The x(c)-cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol. 2008 Jun;215(3):593–602.10.1002/jcp.21366Search in Google Scholar PubMed
[90] Scalise M, Galluccio M, Console L, Pochini L, Indiveri C. The human SLC7A5 (LAT1): the intriguing histidine/large neutral amino acid transporter and its relevance to human health. Front Chem. 2018 Jun;6:243.10.3389/fchem.2018.00243Search in Google Scholar PubMed PubMed Central
[91] Gaglio D, Metallo CM, Gameiro PA, Hiller K, Danna LS, Balestrieri C, et al. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol. 2011 Aug;7(1):523.10.1038/msb.2011.56Search in Google Scholar PubMed PubMed Central
[92] White E. Exploiting the bad eating habits of Ras-driven cancers. Genes Dev. 2013 Oct;27(19):2065–71.10.1101/gad.228122.113Search in Google Scholar PubMed PubMed Central
[93] Nguyen TL, Durán RV. Glutamine metabolism in cancer therapy. Cancer Drug Resist. 2018;1:126–38.10.20517/cdr.2018.08Search in Google Scholar
© 2022 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop
Articles in the same Issue
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop

