Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
-
Mohamed S. Othman
, Sofian T. Obeidat
Abstract
In this study, the neuroprotective potential of either Pergularia tomentosa leaf methanolic extract (PtE) alone or in combination with selenium nanoparticles (SeNPs-PtE) was investigated against lead acetate (PbAc)-induced neurotoxicity. Experimental rats were pretreated with PtE (100 mg/kg) or SeNPs-PtE (0.5 mg/kg) and injected intraperitoneally with PbAc (20 mg/kg) for 2 weeks. Notably, SeNPs-PtE decreased brain Pb accumulation and enhanced the level of dopamine and the activity of AChE compared to the control rats. In addition, elevated neural levels of superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, and glutathione along with decreased lipid peroxidation levels were noticed in pretreated groups with SeNPs-PtE. Moreover, SeNPs-PtE significantly suppressed neural inflammation, as indicated by lower levels of interleukin-1 beta, interleukin-6, tumor necrosis factor-alpha, nuclear factor-kappa B p65, and nitric oxide in the examined brain tissue. The molecular results also unveiled significant down-regulation in iNOS gene expression in the brains of SeNPs-PtE-treated rats. In addition, SeNPs-PtE administration counteracted the neural loss by increasing B-cell lymphoma 2 (Bcl-2) and brain-derived neurotrophic factor levels as well as decreasing BCL2-associated X protein and caspase-3 levels. To sum up, our data suggest that P. tomentosa extract alone or in combination with SeNPs has great potential in reversing the neural tissue impairment induced by PbAc via its antioxidant, anti-inflammatory, and anti-apoptotic activities. This study might have therapeutic implications in preventing and treating several lead-induced neurological disorders.
1 Introduction
Lead is a non-biodegradable environmental pollutant that has a wide range of harmful effects, particularly on brain-related disorders [1,2]. It is extensively found in the environment as it is used in pesticides, fertilizers, lead-containing gasoline, lead paints, cosmetics, and metal products as plumbing pipes [3]. The exposure of humans to lead may be occupational or through ingestion of drinking water, and also environmental through traffic pollution, coal burning, or high lead levels in some regions [2]. According to the Institute for Health Metrics and Evaluation, lead exposure was responsible for nearly one million mortalities in 2017 [3]. Lead can also accumulate and cause damage to various organs, such as the brain, kidney, liver, heart, and immune system [4].
The brain is especially vulnerable to lead poisoning. Multiple mechanisms underlie the pathophysiology of lead neurotoxicity, among which are inhibiting neurotransmitters’ release, disrupting intracellular calcium homeostasis, and reducing neural synaptic plasticity [5]. In addition, lead exposure results in excess production of free radicals and disruption of antioxidant system hemostasis with consequent oxidative injury to neuronal cells [5,6]. It was also reported that lead induces a substantial neuroinflammatory response by triggering the transcription of nuclear factor-κB (NF-κB), which upregulates the secretion of inflammatory cytokines, such as tumor necrosis factor-alpha [5,7,8]. Moreover, former reports have documented neural cell death as a result of lead exposure in rats [7,9].
Unfortunately, the ordinarily used chelators for remedying lead toxicity have serious drawbacks, such as kidney and liver damage, gastrointestinal discomfort, hypocalcemia, hypotension, bone marrow damage, and longer bleeding time [6]. Moreover, some of these agents may chelate essential metals, such as calcium and iron. In addition, dimercaptosuccinic acid, a less toxic and most effective chelating agent, cannot pass the cell membrane and the blood–brain barrier [10]. Hence, it is urgent to find alternatives to mitigate the harmful effects of lead poisoning with low or minimal side effects.
Pergularia tomentosa L. (a member of the Asclepiadaceae family) is a medicinal plant native to Saudi Arabia, northern and southern Africa, and the Middle East [11]. It is frequently used in folk medicine to treat bronchitis, skin diseases, and asthma. Furthermore, the plant parts, especially the fruits and leaves, contain several chemicals, including cardiac glycosides, flavonoids, saponins, anthraquinones, alkaloids, and tannins [12]. Bioactivity studies have shown that P. tomentosa exerts antioxidant, antifungal, antibacterial, and cytotoxic activities [13,14]. Lahmar et al. [15] found that the fruits and leaf extracts of P. tomentosa exerted strong antioxidant properties as indicated by high free radical scavenging power and iron-reducing activity. Moreover, it has been shown that the cardenolide-rich fraction of P. tomentosa displayed in vitro and in vivo anti-angiogenic effects by diminishing the migration and viability of endothelial cells [16].
Phyto-manufactured selenium nanoparticles (SeNPs) are regarded as a unique medicinal form of selenium due to their high biocompatibility, low toxicity, and functioning as appropriate mediators for central drug delivery [17,18,19]. Nano-selenium reversed neural oxido-inflammatory stress and cell death in cypermethrin- [20], streptozotocin- [18], and deltamethrin- [21] exposed rats. Moreover, previous studies have demonstrated the neuroprotective efficacy of SeNP therapy against various neurodegenerative disorders, such as Alzheimer’s disease, ischemic cerebral stroke, and epilepsy [17,22,23]. Albrakati et al. [24] reported that prodigiosin loaded with SeNPs decreased the brain oxidative stress, inflammation, and apoptosis induced by chronic unpredictable mild stress in rats.
Increased domestic or occupational exposure to lead has prompted researchers to seek effective therapies with better delivery systems. Hence, this study aimed to elucidate the potential neuroprotective role of P. tomentosa-synthesized SeNPs against lead neurotoxicity. The possible mechanisms underlying this effect are also explained, including oxidative stress, neuroinflammation, apoptosis, and neurotransmission in the brain tissue of intoxicated rats. These findings provide new insights for developing an effective and safe approach to lead detoxification.
2 Materials and methods
2.1 Preparation of P. tomentosa total extracts
P. tomentosa plants were harvested from the valleys east of Hail town, Hail region, KSA, in April 2022. A taxonomist from Ha’il University’s Botany Department verified plant identification. After washing, the dried leaves (500 g) were macerated in methanol for 2 days at room temperature. The extract was concentrated and lyophilized using a rotary evaporator. Distilled water was utilized to achieve a final dilution of 200 mg/mL solution, which was then kept for future use [25].
2.2 Phytochemical studies of Hail P. tomentosa
The plant extract was exposed to qualitative phytochemical analysis using the technique of Mumtaz et al. [26] to evaluate the active compounds in the P. tomentosa plant. In addition, total phenolic compounds were measured by the Folin–Ciocalteu technique. Meanwhile, the total flavonoid compounds were identified utilizing aluminum chloride method and measured spectrophotometry at 430 nm [15].
2.3 Preparation and characterization of nanoparticles
A volume of 5 mL of the extract solution of P. tomentosa (200 mg/mL) was titrated with 5 mL of 5 mmol/mL Na2SeO3 and stirred together for 24 h at 30°C [24]. The average size of SeNPs-PtE was investigated by a Zetasizer (Nano series, ZEN 3600, Malvern, England). Meanwhile, to determine the size range of the freshly generated SeNPs, a TEM investigation was conducted. Fourier transform infrared spectroscopy (FTIR) analysis was utilized for further characterization to identify the functional groups implicated in the production of nanoparticles (PerkinElmer Spectrum 10.5.4, US).
2.4 Animals and treatment protocol
Eight-week-old male Wistar albino rats weighing 175–191 g were obtained from VACSERA animal facility (Cairo, Egypt). The rats were acclimatized for 7 days under standard laboratory conditions of 12 h light/dark cycles at a temperature of 25 ± 2°C and a relative humidity of 50 ± 10% with access to pelleted rodent feed and water ad libitum. The experiment and the procedures and methodologies were designed in compliance with the standards of Helwan University’s Institutional Animal Care and Use Committee (IACUC) (approval no. HU2021/Z/AEO0121-03).
The rats were divided into five groups of eight at random.
The first group (CTL) served as the control. Each rat in this group was given 0.3 mL of distilled water orally, followed by an intraperitoneal (i.p.) injection of 100 μL saline an hour later.
Group II (PtE group) received 100 mg/kg body weight (BW) of P. tomentosa extract orally, and an hour later, rats were injected i.p. with 100 μL saline.
Group III [lead acetate (PbAc)] received i.p. administration of PbAc at 20 mg/kg BW, as previously reported by Abdel Moneim [27].
Group IV (PbAc-PtE group) received 100 mg/kg BW of P. tomentosa extract orally, and an hour later, rats were intraperitoneally injected with PbAc at 20 mg/kg BW.
Group V (PtE-SeNPs) received 0.5 mg/kg BW of (PtE-SeNPs) orally and, after 1 h, rats received an i.p. injection of PbAc at 20 mg/kg BW. All respective doses were administered daily for 14 days.
The PtE dosages used were determined via a preliminary experiment that used 25, 50, and 100 mg/kg BW, respectively. Higher dosages were shown to be more beneficial in treating PbAc-induced neurotoxicity, with no toxic symptoms appearing until 100 mg/kg BW. Euthanasia and scarification of the mice were conducted within 24 h of the last treatment. Blood was instantly sampled, kept at 37°C for 24 min, and centrifuged at 3,300 rpm for 14 min, and serum was kept at −20°C. The rats’ brains were taken out and longitudinally split into two parts for molecular and biochemical analyses. A part of brain tissue was homogenized with 10 mM PBS and centrifuged for 12 min at 3600 rpm. The resulting supernatant was kept at −80°C.
2.5 Assessment of protein and lead concentration
The total protein content of the homogenized brain was determined using the method of Lowry et al. [28]. A Perkin Elmer flame atomic absorption spectrophotometer 3100 was used to measure lead concentrations in the brain tissues at 283.3 nm. The amount of lead in the brain was represented as µg/g wet weigh brain tissue.
2.6 Analysis of markers of oxidative damage
In brain tissues, lipid peroxidation (LPO) concentrations were quantitated according to the method of Yagi [29], while nitric oxide (NO) concentrations were measured based on Green et al. [30] methodology.
2.7 Assessment of antioxidant status
Catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), and glutathione peroxidase (GPx) enzymes activity, as well as glutathione (GSH) levels in brain tissue were evaluated using Aebi [31], Misra and Fridovich [32], Pinto et al. [33], Tappel [34], and Akerboom and Sies [35], respectively.
2.8 Quantitative real-time PCR
To extract total RNA, the RNeasy Plus Minikit was employed (Qiagen, Valencia, CA, USA). The cDNA was produced using Power SYBR® Green Master Mix (Life Technologies, CA, USA) and detected with the Applied Biosystems 7500 equipment. The inducible NO synthase (iNOS) gene expression levels were adjusted to GAPDH. Table 1 lists the primer sequences and gene accession numbers.
Primer sequences
| Name | Accession number | Product length | Sense (5′–3′) | Antisense (5′–3′) |
|---|---|---|---|---|
| iNOS | NM_012611.3 | 241 | GGTGAGGGGACTGGACTTTTAG | TTGTTGGGCTGGGAATAGC |
| GAPDH | NM_017008.4 | 70 | CAGCCGCATCTTCTTGTGC | ATCCGTTCACACCGACCTTC |
2.9 Evaluation of tissue pro‑inflammatory
R&D Systems (MN, USA), ELISA kits were utilized to assess the levels of inflammatory cytokines interleukin-1 beta (IL-1β), interleukin-6 (IL-6), nuclear factor-κB p65 (NFkp65), and tumor necrosis factor-alfa (TNF-α) levels in the brain tissues according to the manufacturer’s instructions using ETI-Max 3000 system (DiaSorin, MN, USA).
2.10 Assessment of neural apoptotic markers
The levels of apoptosis-related components, such as BCL2-associated X protein (Bax), B-cell lymphoma 2 (Bcl-2), and caspase-3, were measured in brain tissues using rat ELISA kits (My BioSource, SD, USA) according to the manufacturer’s instructions. While brain-derived neurotrophic factor (BDNF) was determined by ELISA kits obtained from BD Biosciences (USA), according to the manufacturer’s instructions.
2.11 Biochemical studies
Acetylcholinesterase (AChE) enzyme activity of tissue homogenates was determined as described by Ellman et al. [36]. While dopamine (DA) levels were assessed spectrophotometrically based on DA’s inhibitory action on thionine oxidation by bromate as described by Shishehbore et al. [37].
2.12 Statistical analysis
Statistical Package for the Social Sciences (SPSS) was used for data analysis. The results were expressed as the mean ± standard deviation (SD). One-way analysis of variance followed by Duncan’s test was applied to determine the significance. The acceptable level of significance was established at P < 0.05.
3 Results
3.1 Nanoparticles characterization
SeNPs-PtE had an average diameter of 67.3 nm and an average zeta potential of −14.1 mV (Figure 1). The participation of O–H, N–H, C═O, and C–O functional groups in forming SeNPs, which were connected to bioactive molecules coating their surfaces, was further confirmed by FTIR spectroscopy. A broad peak shows the O–H group at 3325.00 cm−1. The absorption peak at 2121.81 cm−1 indicates C–H stretch alkynes. Carbon compounds cause the band at 1635.19 cm−1 with an asymmetric stretch called C–O. Due to C–X stretching, alkyl halides exhibit a band at 447.81, 431.98, and 415.77 cm−1. Numerous functional groups were discovered in this work, which may be necessary for stabilizing and reducing SeNPs-PtE.

Characteristics of SeNPs coupled with PtE. (a) Hydrodynamic diameter of SeNPs-PtE by Zetasizer; (b) surface charge of SeNPs-PtE by zeta potential; (c) FT-IR spectra of SeNPs-PtE. FT-IR: Fourier transform infrared; SeNPs: selenium nanoparticles.
3.2 Effect of PtE and SeNPs-PtE on lead levels in the brain tissues
In the current study, the effects of different treatments on Pb bioaccumulation in the brain tissue of exposed rats were investigated (Figure 2). Lead levels (µg/g wet of cortical tissue) increased dramatically (P < 0.05) in the PbAc group than in the CTL group. Meanwhile, administration of PtE or SeNPs-PtE to Pb-exposed animals significantly (P < 0.05) reduced the Pb levels compared to the PbAc group, indicating that PtE and its combination with SeNPs were able to reduce Pb levels. SeNPs-PtE, on the other hand, was more successful in lowering Pb accumulation in brain tissues, which may explain why the combined nanoparticles-PtE was more efficient in reducing the neural Pb burden, as observed in the current study.

Effects of orally administered PtE or PtE-SeNPs on lead bioaccumulation in the brain of PbAc-injected rats. ψ,Φ,Σ superscript letters indicate statistical significance at P < 0.05 against control, PbAc, and PtE-PbAc group, respectively. All results are presented as the mean + SD.
3.3 Effect of PtE and SeNPs-PtE on brain oxidative stress markers of PbAc-injected rats
As shown in Figures 3 and 4, PbAc injection considerably (P < 0.05) increased the levels of MDA and NO in the brain homogenates while also considerably (P < 0.05) lowered the levels of antioxidant markers (GR, GSH, GPx, SOD, and CAT) in comparison to the CTL rats. When compared to the PbAc group, PtE and SeNPs-PtE treatment caused significant reductions (P < 0.05) in MDA and NO levels accompanied with observable increases (P < 0.05) in the levels of the evaluated antioxidant indicators (GSH, GPx, GR, SOD, and CAT). Notably, SeNPs-PtE was linked to a significant increase in these antioxidant indices when compared to the PtE-PbAc group, indicating that it possesses a stronger antioxidant activity.

Effects of orally administered PtE or PtE-SeNPs on the antioxidant enzymatic activities of SOD, CAT, GR, and GPx in the brain of PbAc-injected rats. ψ,Φ,Σ superscript letters indicate statistical significance at P < 0.05 against control, PbAc, and PtE-PbAc group, respectively. All results are presented as the mean + SD.
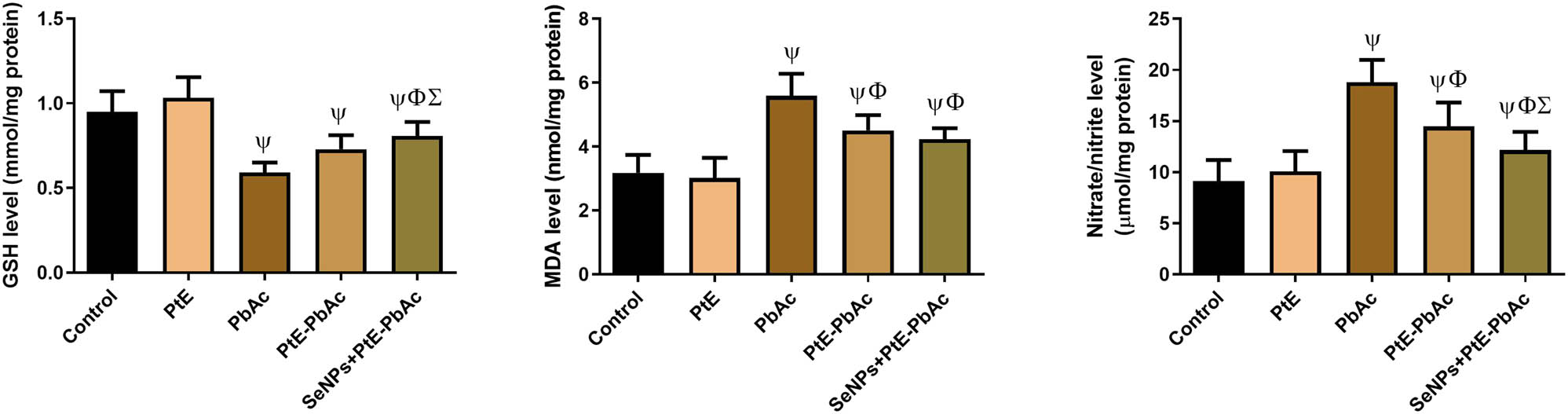
Effects of orally administered PtE or PtE-SeNPs on the levels of non-enzymatic oxidative stress markers (GSH, MDA, and NO) in the brain of PbAc-injected rats. ψ,Φ,Σ superscript letters indicate statistical significance at P < 0.05 against control, PbAc, and PtE-PbAc group, respectively. All results are presented as the mean + SD.
3.4 Effects of PtE and SeNPs-PtE on brain pro-inflammatory markers of PbAc-injected rats
There were no significant changes in the levels of TNF-α, NF-κB p65, IL-1β, IL-6, and iNOS mRNA expression rate in the PtE group compared with those in the CTL group. In contrast, untreated rats exposed to PbAC exhibited a noticeable (P < 0.05) rise in the levels of these aforementioned pro-inflammatory indicators compared to the CTL group. In animals injected with PbAc and pretreated with PtE or SeNPs-PtE, the levels of these pro-inflammatory markers were significantly (P < 0.05) lower than those in the PbAc group, and this reduction was more noticeable in the SeNPs-PtE group (Figure 5).
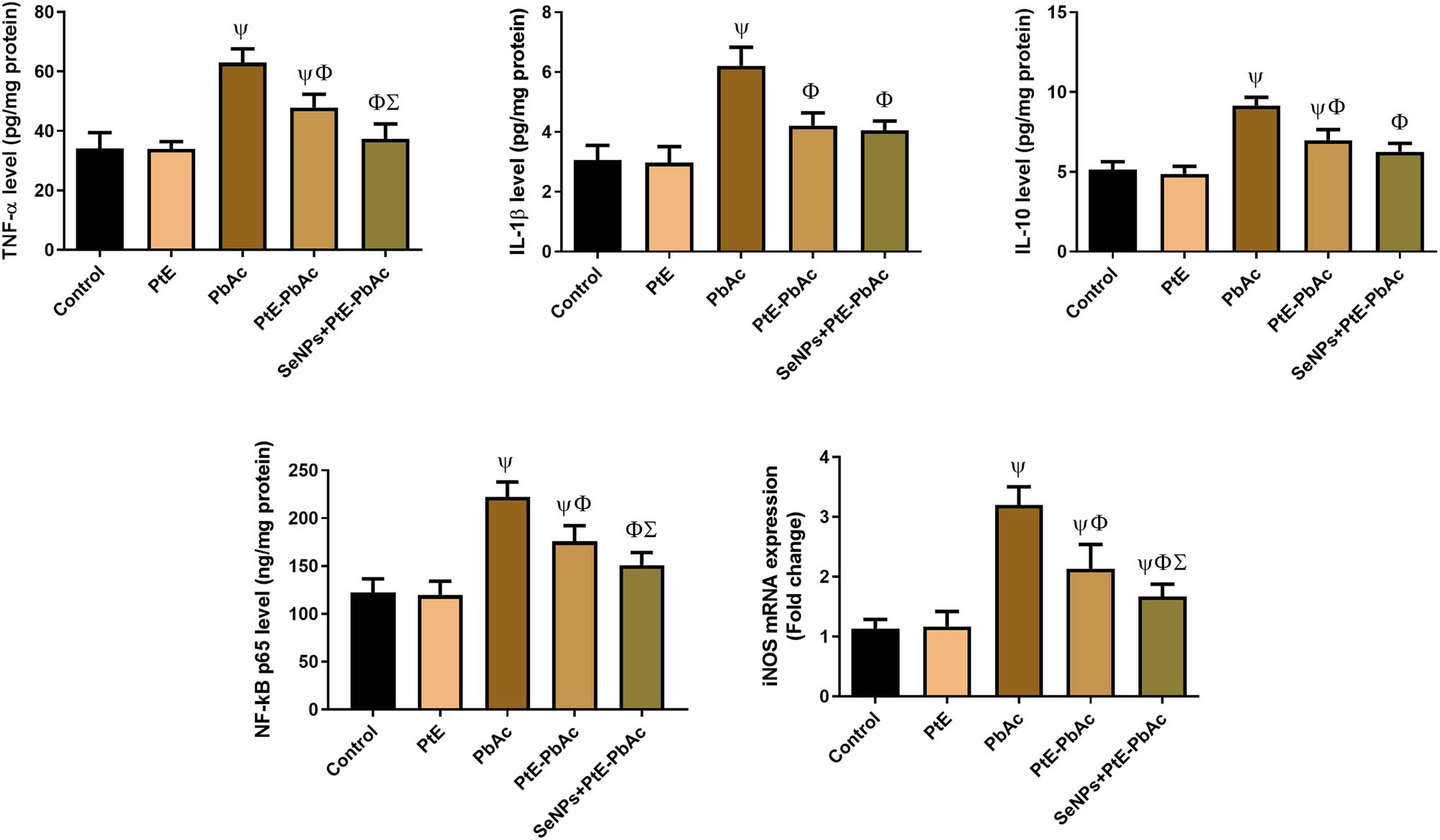
Effects of orally administered PtE or PtE-SeNPs on the levels of neuroinflammatory biomarkers (IL-1β, TNF-α, IL-6, and NF-κB) and the mRNA expression levels of Nos2 in the brain of PbAc-injected rats. ψ,Φ,Σ superscript letters indicate statistical significance at P < 0.05 against control, PbAc, and PtE-PbAc group, respectively. All results are presented as the mean + SD.
3.5 Effects of PtE and SeNPs-PtE on apoptotic markers
As displayed in Figure 6, there was a notable decline (P < 0.05) in the anti-apoptotic factor Bcl-2, accompanied by a considerable increase (P < 0.05) in the levels of pro-apoptotic factors (Bax and caspase-3) in the PbAc group as contrasted to the CTL group. Meanwhile, when compared to the PbAc group, both PtE-PbAc and SeNPs-PtE groups showed a notable rise (P < 0.05) in Bcl-2 levels, as well as a substantial decrease (P < 0.05) in Bax and caspase-3 levels. Furthermore, the current results revealed that SeNPs-PtE has more anti-apoptotic action than PtE alone. However, there were no significant changes in the levels of these apoptosis markers in the PtE group compared with those in the CTL group.
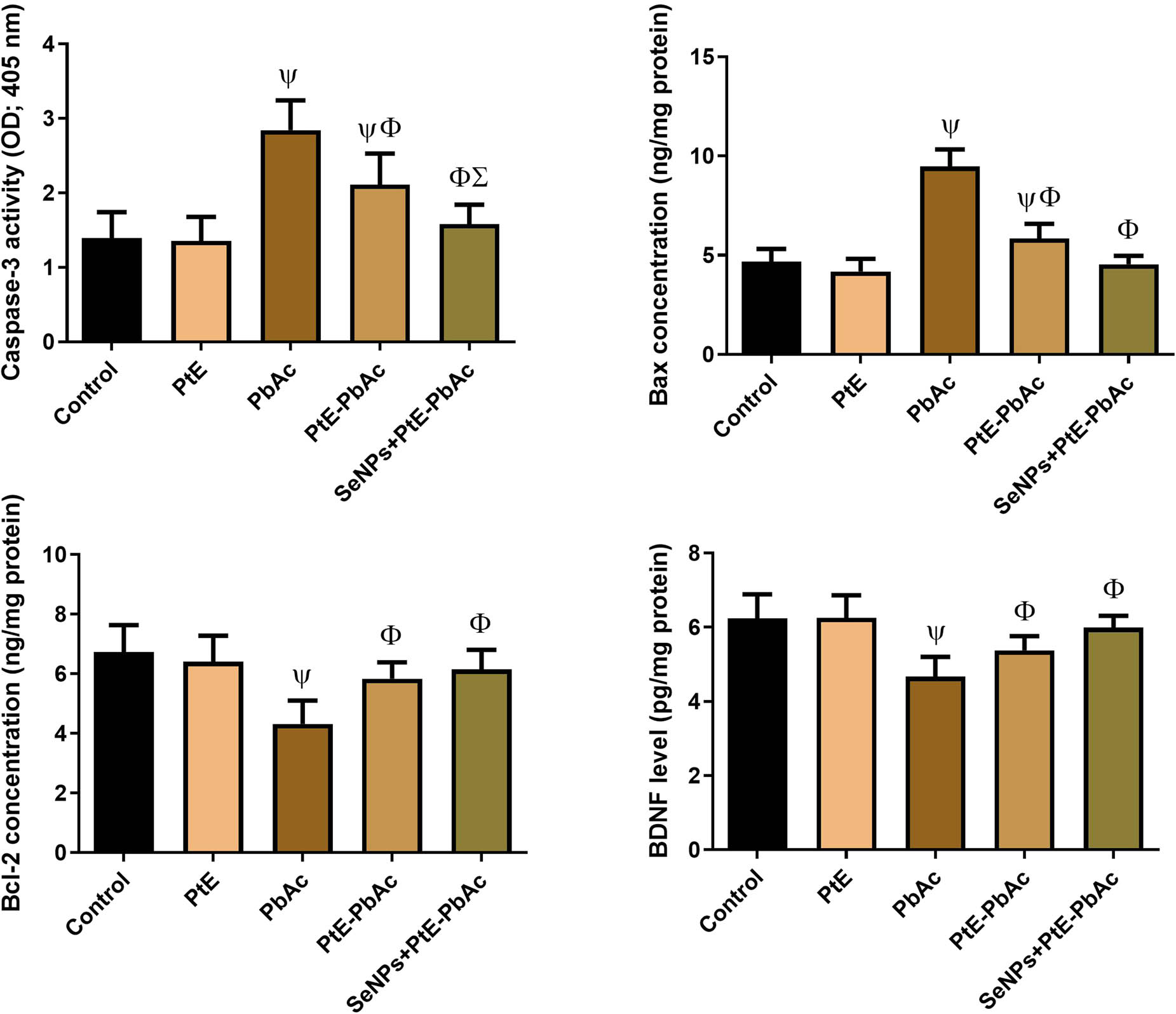
Effects of orally administered PtE or PtE-SeNPs on the levels of neural apoptotic markers (Bax, Cas-3, Bcl-2, and BDNF) in the brain of PbAc-injected rats. ψ,Φ,Σ superscript letters indicate statistical significance at P < 0.05 against control, PbAc, and PtE-PbAc group, respectively. All results are presented as the mean + SD.
In addition, marked decreases (P < 0.05) were detected in the levels of BDNF in the PbAc group in respect to the control ones. However, the pre-administration of SeNPs-PtE or PtE resulted in a notable (P < 0.05) increase in BDNF concentration compared to the PbAc group. Meanwhile, no substantial change was found between the PtE and the CTL group.
3.6 Effect of PtE and SeNPs-PtE on AChE activities and DA concentration
Figure 7 depicts the impact of PtE and SeNPs-PtE on the activity of AChE in rats exposed to PbAc. AChE activity significantly increased (P < 0.05) in the PbAc group as contrasted to the CTL group. When compared to the PbAc group, the AChE activity of SeNPs-PtE and PtE-PbAc treated rats was considerably lower (P < 0.05), and this decline in AChE activity was more noticeable in the SeNPs-PtE group as contrasted to the PtE-PbAc group.

Effects of orally administered PtE or PtE-SeNPs on the levels of neural DA and AChE in the brain of PbAc-injected rats. ψ,Φ,Σ superscript letters indicate statistical significance at P < 0.05 against control, PbAc, and PtE-PbAc group, respectively. All results are presented as the mean + SD.
Furthermore, administration of PbAc significantly reduced (P < 0.05) the DA levels in the PbAc group in respect to the CTL group. In rats injected with PbAc and pretreated with PtE or SeNPs-PtE, DA concentration demonstrated a statistically significant (P < 0.05) increase when compared with the PbAc group; such an increase in DA level was more pronounced in SeNPs-PtE than that observed in the PtE-PbAc group.
4 Discussion
Lead exposure evokes extensive damage to brain morphology and impairs cognition, especially in developing brains, thereby imposing serious health problems in children [38]. It can cross the blood–brain barrier to accumulate in the brain and cause neurotoxicity through various processes, including oxidative stress, apoptosis, and inflammation [39]. In the current study, the injection of animals with PbAc for 14 days caused Pb accumulation in the brain, as evidenced by the higher Pb concentrations in the rat brains. Similar results have been previously reported [40,41]. Meanwhile, treatment with P. tomentosa leaf extract, either alone or in combination with SeNPs, reduced the quantity of lead deposited in the brain tissues. It has been previously shown that P. tomentosa leaf extract is a chelator of heavy metals, including Pb [42]. According to Adhikari et al. [43], the Pb-morin (a flavonoid) complex is more sustainable than the Pb-free morin. In comparison to morin, the metal-morin chelate was also found to be substantially more soluble in aqueous solutions. It is also discovered that the soluble chelate complex’s sustained antioxidant activity and heavy-metal chelation speed up the Pb detoxification (in vitro). El-Fakharany et al. [44] found that SeNPs’ treatment for 12 weeks markedly lowered the lead levels in the blood and testes of PbAc-exposed rats. A notable reduction in lead levels was observed in the serum and thyroid tissues of PbAc-induced hypothyroidism in male rats [45]. These results indicate the chelating power of SeNPs-PtE via binding with Pb in the tissues and creating a compound that can be excreted in bile and urine.
AChE regulates cholinergic neurotransmitters and is crucial for the normal functioning of the cholinergic neural system [46,47]. Our results unveiled a dramatic increase in the brain’s AChE activity in the Pb-treated group. These findings are consistent with previous studies [48]. Lead has been shown to affect cholinergic systems in the hippocampus and septum, impairing cholinergic nervous transmission [48]. Suleman et al. [49] reported that increased oxidative stress and LPO in the brain alter AChE activity, which impairs nervous transmission. Meanwhile, Brini et al. [50] concluded that lead has some similarities with calcium and that calcium ions promote AChE activity, suggesting that lead ions may also significantly influence AChE, affecting the sensitivity and neuromotor activities of the nervous system. In contrast, pretreatment with PtE or SeNPs-PtE considerably decreased AChE activity in brains of PbAc-treated rats, suggesting that P. tomentosa extract, alone or in combination with SeNPs, may protect against PbAc neurotoxicity by alleviating the defects in the AChE activity. The protective abilities of SeNPs and the extract may be due to their powerful antioxidant activities. Many previous reports support the idea that natural antioxidant-rich substances mitigate the abnormalities in AChE activities [50,51].
DA is involved in many cerebral functions, including locomotor activity, cognitive function, emotional stability, and endocrine regulation [52,53]. In harmony with the former study [6], our results showed that PbAc administration declined the DA levels, suggesting that Pb might cause neurotoxicity, at least in part, through the disruption of dopaminergic neurotransmission. Akinyemi et al. [54] reported that Pb administration damages dopaminergic neuron morphology, which may be linked to alterations in DA transporter that might lead to reduced extracellular DA concentration and consequent neurotoxicity. Interestingly, herein supplementation of either PtE or SeNPs-PtE to rats improved the level of brain DA. Similar to our findings, Yuan et al. [17] stated that treating epileptic animals with SeNPs reversed the alterations in the levels of neuromodulators, including DA. In another study, SeNPs conjugated with prodigiosin elevated DA contents in rats subjected to chronic unpredictable mild stress [55]. Selenium administration is associated with increased neurotransmitter levels via monoamine oxidase inhibition [56]. Furthermore, Anusha et al. [57] concluded that the flavonoid apigenin modulates DA neurotransmission by increasing DA biosynthesis and DA D2 receptor expression.
In the current study, exposure of rats to PbAc was accompanied by significant reductions in the antioxidant molecules, including CAT, GPx, SOD, and GR activity, as well as GSH levels, along with a considerable elevation of oxidative stress indices (LPO and NO) in comparison with the control rats. Lead can evoke neurotoxicity through the creation of hazardous complexes with the cellular compounds and the generation of highly ROS [7,8]. Our data support the finding of Abdel Moneim et al. [58], who concluded that PbAc increased LPO and NO generation in the brain while simultaneously lowering GSH and antioxidant enzymes’ activities. Lead can disrupt the cellular antioxidant system by binding to the sulfhydryl groups or metal cofactors of antioxidant molecules with consequent disturbance in their physiologic roles [5]. Nevertheless, Pb not only promotes the production of ROS but also alters the antioxidant defenses, such as GSH interaction and reuse of catalysts, which further reduces the antioxidant activity of GSH enzymes [59].
Our findings demonstrated that pretreated rats with PtE or SeNPs-PtE before PbAc injection demonstrated a statistically significant improvement in the oxidant/antioxidant balance, as evidenced by rises in the antioxidant enzymes coupled with falls in the oxidative stress markers (LPO and NO). Compared with PtE alone, SeNPs-PtE produced much higher levels of antioxidant molecules and lower oxidative stress indicators. Selenium can quench ROS and thus inhibit oxidative stress as it is a component in selenoproteins and selenoenzymes [17]. This is consistent with the findings of the Othman et al. [60] study, which found a significant decrease in LPO and NO concentrations and an increase in GSH concentrations in mice treated with green-synthetized SeNPs with berberine. Moreover, nano-selenium re-established the antioxidant defense in the thyroid gland by decreasing the levels of MDA as well as elevating GSH, CAT, GPx, and SOD contents in PbAc-treated rats [45]. In a previous study, prodigiosin-SeNPs’ administration decreased the hippocampal levels of NO and MDA, together with elevating GSH, GPx, GR, SOD, and CAT in stressed rats [55]. Similar results were reported in previous toxicity studies [21]. SeNPs’ fabrication influences their bioactivity and biological availability. Thus, their surface functionalization using a biologically active compound will increase their bioavailability and boost their therapeutic power [61]. In this regard, P. tomentosa extract contains several bioactive components, such as flavonoids, alkaloids, and polyphenols, with antioxidants and other biologic activities. Our results agreed with Lahmar et al. [15], who reported that P. tomentosa extracts have potent antioxidant activity. Similarly, Yakubu et al. [62] concluded that P. tomentosa’s methanolic and aqueous extracts have antioxidant effects against DPPH, peroxyl, hydroxyl, and hydrogen peroxide radicals. Interestingly, P. tomentosa contains two significant sources of antioxidants: first, the high concentrations of phenolic compounds, which are known to be a good source of potent antioxidants, and second, the hydroxyl groups in flavonoids, which can give hydrogen atoms to free radicals.
Neuroinflammation has been identified as one of the mechanisms underlying lead neurotoxicity [5,7,8]. In the current study, rats exposed to PbAc had notably elevated levels of IL-1β, IL-6, TNF-α, NF-κB P65, and iNOS gene expressions, suggesting that neuroinflammation may be a possible mechanism underlying Pb-induced brain damage. According to earlier research, excess production of free reactive radicals induced by lead exposure boosted the release of inflammatory cytokines and NF-κB, which ultimately led to neuronal death [9]. NF-κB plays a central role during inflammation through the promotion of the release of inflammatory cytokines [63]. Moreover, iNOS enhances NO’s production, contributing to inflammatory and immune responses [7]. Based on a previous study, Pb stimulates iNOS generation in brain endothelial cells [27]. Furthermore, Chen et al. [64] reported that enhanced iNOS expression might be the reason for the increased NO levels found in the frontal cortex and other brain regions of Pb-exposed rats.
In contrast, administration of P. tomentosa extract alone or in combination with SeNPs substantially decreased the levels of IL-1β, IL-6, TNF-α, and NF-κB P65, as well as iNOS gene expression in the brain, indicating their anti-inflammatory action. These outcomes support the findings of Yuan et al. [17] that SeNPs decrease the inflammatory response in the epileptic brain, as indicated by lower TNF-α and IL-1β levels. Furthermore, Albrakati et al. [55] revealed that prodigiosin-SeNPs treatment reduced neuroinflammation in the hippocampus, as evidenced by a decrease in the production of pro-inflammatory markers (TNF-α, IL-1β, and IL-6). Furthermore, Farzadinia et al. [65] showed that P. tomentosa includes several bioactive compounds, including tannins, saponins, and steroid glycosides, which can lessen inflammation and hasten the healing of epidermal wounds in second-degree burns. The phytochemical analysis in this study also confirmed that P. tomentosa extract contains high levels of polyphenols and flavonoids. These compounds were reported to reduce inflammation via decreasing ROS production and downregulating several inflammatory mediators and pathways. Numerous flavonoids, including quercetin, genistein, apigenin, and kaempferol, have been demonstrated in recent research to limit the production of pro-inflammatory molecules, such as NF-κB and TNF-α, as well as to inhibit pro-inflammatory enzymes, such as iNOS and cyclooxygenase-2 [2,66].
Many reports have demonstrated that Pb exposure is associated with cellular apoptosis, resulting in the loss of valuable neurons [9]. The marked reduction in the anti-apoptotic marker Bcl-2 and the marked increase in the apoptotic markers Bax and caspase-3 levels in the PbAc group in the current study indicated that Pb could injure brain tissue through Pb-mediated apoptosis, which was in harmony with the former studies [6,7,9]. Lead is known to enhance the cytochrome release, a hallmark of apoptosis, through increasing ROS production and damaging the mitochondrial membrane [1,2]. Moreover, PbAc-induced activation of the caspase cascade triggers DNA breakage, chromatin condensation, and finally, neuronal apoptosis [67]. The current study also demonstrated that rats exposed to PbAc had considerably lower BDNF levels than rats in the other groups. These findings align with previous studies [4,68]. BDNF maintains the function and survival of neuronal cells. It controls synaptic transmission, plays a central role in cognitive function, and has neuroprotective effects against brain insults [46].
On the contrary, PtE or SeNps-PtE pretreatment counteracted the apoptotic changes induced by PbAc exposure, indicating the powerful anti-apoptotic properties of P. tomentosa plant extract and SeNPs. Such protective benefits have mostly been linked to the antioxidant properties of polyphenols and flavonoids in P. tomentosa [12]. These findings were comparable to the former studies [21,69]. SeNPs also reduced caspase-3 expression and inhibited the neural apoptosis through the mitochondrial pathway [19]. Furthermore, both PtE and SeNPs-PtE improved BDNF levels in the PbAc-treated animals. This could be attributed to the potent antioxidant and anti-inflammatory activities of Se and PtE. Similarly, Albrakati et al. [24] revealed that SeNPs-prodigiosin administration improved BDNF values in the brain tissues of stressed rats. Our data support the previous study by Rendeiro et al. [70], which showed that flavonoids could increase BDNF expression in the hippocampus. In addition, supplementation with flavonoid-rich blueberries enhances cognitive performance in animals by elevating hippocampal BDNF levels [71].
5 Conclusion
Collectively, P. tomentosa extract alone or in combination with SeNPs offered substantial neuroprotection against lead-induced neurotoxicity in rats. The tested extract or the formulated nanoparticles successfully diminished lead levels in the brain tissue of lead-exposed rats. Moreover, SeNPs-PtE restored the neural tissue content of AChE, DA, and antioxidant molecules. Significant anti-inflammatory action was noticed in SeNPs-PtE-treated animals and confirmed by biochemical and molecular analyses. These findings support the therapeutic value of SeNPs-PtE against lead-induced neurotoxicity. This study might have therapeutic implications in preventing and treating several lead-induced neurological disorders.
Acknowledgments
This research was funded by Scientific Research Deanship at University of Ha’il – Saudi Arabia through project number RG-21115.
-
Funding information: This research was funded by Scientific Research Deanship at University of Ha’il – Saudi Arabia through project number RG-21115.
-
Author contributions: Mohamed S. Othman and Mohamed M. Abdel-Daim: conceptualization and methodology. Mohamed S. Othman, Ola A. Habotta, and Ashraf Bakkar: data curation and writing – original draft. Mohamed S. Othman, Ghada M. Aleid, Amal H. Al-Bagawi, and Ashraf Bakkar: visualization, investigations, and practical. Mohamed S. Othman, Sofian T. Obeidat, Mohamed M. and Abdel-Daim: supervision. Mohamed S. Othman, Ghada M. Aleid, and Amal H. Al-Bagawi: resources and validation. Mohamed S. Othman, Laurent Schwartz, and Manal M. Hussein: writing – review and editing. Sofian T. Obeidat: data analysis and software.
-
Conflict of interest: The authors declare no conflict of interest.
-
Ethical approval: The experiment and the procedures and methodologies were designed in compliance with the standards of Helwan University’s Institutional Animal Care and Use Committee (IACUC) (approval no. HU2021/Z/AEO0121-03).
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Albarakati AJA, Baty RS, Aljoudi AM, Habotta OA, Elmahallawy, EK, Kassab RB, et al. Luteolin protects against lead acetate-induced nephrotoxicity through antioxidant, anti-inflammatory, anti-apoptotic, and Nrf2/HO-1 signaling pathways. Mol Biol Rep. 2020;47(4):603–2591.10.1007/s11033-020-05346-1Search in Google Scholar PubMed
[2] Al-Megrin WA, Alkhuriji AF, Yousef AOS, Metwally DM, Habotta OA, Kassab RB, et al. Antagonistic efficacy of luteolin against lead acetate exposure-associated with hepatotoxicity is mediated via antioxidant, anti-inflammatory, and anti-apoptotic activities. Antioxid (Basel, Switz). 2019;9(1):10.10.3390/antiox9010010Search in Google Scholar PubMed PubMed Central
[3] Leão LKR, Bittencourt LO, Oliveira ACA, Nascimento PC, Ferreira MKM, Miranda GHN, et al. Lead-induced motor dysfunction is associated with oxidative stress, proteome modulation, and neurodegeneration in motor cortex of rats. Oxid Med Cell Longev. 2021;2021:5595047.10.1155/2021/5595047Search in Google Scholar PubMed PubMed Central
[4] Long X, Wu H, Zhou Y, Wan Y, Kan X, Gong J, et al. Preventive effect of limosilactobacillus fermentum SCHY34 on lead acetate-induced neurological damage in SD rats. Front Nutr. 2022;854.10.3389/fnut.2022.852012Search in Google Scholar PubMed PubMed Central
[5] Hosseinirad H, Shahrestanaki JK, Moosazadeh Moghaddam M, Mousazadeh A, Yadegari P, Afsharzadeh N. Protective effect of vitamin D3 Against Pb-induced neurotoxicity by regulating the Nrf2 and NF-κB pathways. Neurotox Res. 2021;39(3):687–96.10.1007/s12640-020-00322-wSearch in Google Scholar PubMed
[6] Shaban NZ, Abd El-Kader SE, Mogahed FAK, El-Kersh MAL, Habashy NH. Synergistic protective effect of Beta vulgaris with meso-2,3-dimercaptosuccinic acid against lead-induced neurotoxicity in male rats. Sci Rep. 2021;11(1):252.10.1038/s41598-020-80669-4Search in Google Scholar PubMed PubMed Central
[7] Alqahtani WS, Albasher G. Moringa oleifera Lam. extract rescues lead-induced oxidative stress, inflammation, and apoptosis in the rat cerebral cortex. J Food Biochem. 2021;45(1):e13579.10.1111/jfbc.13579Search in Google Scholar PubMed
[8] Omeiza NA, Abdulrahim HA, Alagbonsi AI, Ezurike PU, Soluoku TK, Isiabor H, et al. Melatonin salvages lead-induced neuro-cognitive shutdown, anxiety, and depressive-like symptoms via oxido-inflammatory and cholinergic mechanisms. Brain Behav. 2021;11(8):e2227.10.1002/brb3.2227Search in Google Scholar PubMed PubMed Central
[9] Yang W, Tian ZK, Yang HX, Feng ZJ, Sun JM, Jiang H, et al. Fisetin improves lead-induced neuroinflammation, apoptosis and synaptic dysfunction in mice associated with the AMPK/SIRT1 and autophagy pathway. Food Chem Toxicol Int J Published Br Ind Biol Res Assoc. 2019;134:110824.10.1016/j.fct.2019.110824Search in Google Scholar PubMed
[10] Miller AL. Dimercaptosuccinic acid (DMSA), a non-toxic, water-soluble treatment for heavy metal toxicity. Alternative Med Rev J Clin Ther. 1998;3(3):199–207.Search in Google Scholar
[11] Abba A, Alzahrani D, Yaradua S, Albokhari EB. Complete plastome genome of Pergularia tomentosa L. (Asclepiadoideae, Apocynaceae). Mitochondrial DNA Part B. 2020;5(1):566–7.10.1080/23802359.2019.1710291Search in Google Scholar PubMed PubMed Central
[12] Martucciello S, Paolella G, Romanelli AM, Sposito S, Meola L, Cerulli A, et al. Pro-apoptotic and pro-autophagic properties of cardenolides from aerial parts of pergularia&nbsp;tomentosa. Molecules. 2022;27(15):4874.10.3390/molecules27154874Search in Google Scholar PubMed PubMed Central
[13] Bekheet SH, Abdel-Motaal FF, Mahalel UA. Antifungal effects of Ficus sycomorus and Pergularia tomentosa aqueous extracts on some organs in Bufo regularis treated with Aspergillus niger. Tissue Cell. 2011;43(6):398–404.10.1016/j.tice.2011.09.002Search in Google Scholar PubMed
[14] Piacente S, Masullo M, De Nève N, Dewelle J, Hamed A, Kiss R, et al. Cardenolides from Pergularia tomentosa display cytotoxic activity resulting from their potent inhibition of Na+/K+-ATPase. J Nat Products. 2009;72(6):1087–91.10.1021/np800810fSearch in Google Scholar PubMed
[15] Lahmar I, Belghith H, Ben Abdallah F, Belghith K. Nutritional composition and phytochemical, antioxidative, and antifungal activities of pergularia tomentosa L. BioMed Res Int. 2017;2017:6903817.10.1155/2017/6903817Search in Google Scholar PubMed PubMed Central
[16] Hosseini M, Ayyari M, Meyfour A, Piacente S, Cerulli A, Crawford A, et al. Cardenolide-rich fraction of Pergularia tomentosa as a novel Antiangiogenic agent mainly targeting endothelial cell migration. Daru J Faculty Pharmacy Tehran Univ Med Sci. 2020;28(2):533–43.10.1007/s40199-020-00356-7Search in Google Scholar PubMed PubMed Central
[17] Yuan X, Fu Z, Ji P, Guo L, Al-Ghamdy AO, Alkandiri A, et al. Selenium nanoparticles pre-treatment reverse behavioral, oxidative damage, neuronal loss and neurochemical alterations in pentylenetetrazole-induced epileptic seizures in mice. Int J Nanomed. 2020;15:6339.10.2147/IJN.S259134Search in Google Scholar PubMed PubMed Central
[18] Ebokaiwe AP, Okori S, Nwankwo JO, Ejike C, Osawe SO. Selenium nanoparticles and metformin ameliorate streptozotocin-instigated brain oxidative-inflammatory stress and neurobehavioral alterations in rats. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(4):591–602.10.1007/s00210-020-02000-2Search in Google Scholar PubMed PubMed Central
[19] Al-Brakati A, Alsharif KF, Alzahrani KJ, Kabrah S, Al-Amer O, Oyouni AA, et al. Using green biosynthesized lycopene-coated selenium nanoparticles to rescue renal damage in glycerol-induced acute kidney injury in rats. Int J Nanomed. 2021;16:4335–49.10.2147/IJN.S306186Search in Google Scholar PubMed PubMed Central
[20] Ali HFH, El-Sayed NM, Khodeer DM, Ahmed AAM, Hanna PA, Moustafa YMA. Nano selenium ameliorates oxidative stress and inflammatory response associated with cypermethrin-induced neurotoxicity in rats. Ecotoxicol Environ Saf. 2020;195:110479.10.1016/j.ecoenv.2020.110479Search in Google Scholar PubMed
[21] Khalil HMA, Azouz RA, Hozyen HF, Aljuaydi SH, AbuBakr HO, Emam SR, et al. Selenium nanoparticles impart robust neuroprotection against deltamethrin-induced neurotoxicity in male rats by reversing behavioral alterations, oxidative damage, apoptosis, and neuronal loss. Neurotoxicology. 2022;91:329–39.10.1016/j.neuro.2022.06.006Search in Google Scholar PubMed
[22] Amani H, Habibey R, Shokri F, Hajmiresmail SJ, Akhavan O, Mashaghi A, et al. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci Rep. 2019;9(1):6044.10.1038/s41598-019-42633-9Search in Google Scholar PubMed PubMed Central
[23] Abozaid OAR, Sallam MW, El-Sonbaty S, Aziza S, Emad B, Ahmed ESA. Resveratrol-selenium nanoparticles alleviate neuroinflammation and neurotoxicity in a rat model of alzheimer’s disease by regulating Sirt1/miRNA-134/GSK3β expression. Biol Trace Elem Res. 2022;200(12):5104–14.10.1007/s12011-021-03073-7Search in Google Scholar PubMed
[24] Albrakati A, Alsharif KF, Al Omairi NE, Alsanie WF, Almalki ASA, Abd Elmageed ZY, et al. Neuroprotective efficiency of prodigiosins conjugated with selenium nanoparticles in rats exposed to chronic unpredictable mild stress is mediated through antioxidative, anti-inflammatory, anti-apoptotic, and neuromodulatory activities. Int J Nanomed. 2021;16:8447–64.10.2147/IJN.S323436Search in Google Scholar PubMed PubMed Central
[25] Ads EN, Abouzied AS, Alshammari MK. Evaluation of cytotoxic effects of methanolic extract of pergularia tomentosa L growing wild in KSA. Asian Pac J Cancer Prev. 2021;22(S1):67–72.10.31557/APJCP.2021.22.S1.67Search in Google Scholar PubMed
[26] Mumtaz F, Raza SM, Ahmad Z, Iftikhar A, Hussain M. Qualitative phytochemical analysis of some selected medicinal plants occurring in local area of Faisalabad. Pak J Pharm Altern Med. 2014;3(3):17–21.Search in Google Scholar
[27] Abdel Moneim AE. Flaxseed oil as a neuroprotective agent on lead acetate-induced monoamineric alterations and neurotoxicity in rats. Biol Trace Elem Res. 2012;148(3):363–70.10.1007/s12011-012-9370-4Search in Google Scholar PubMed
[28] Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–75.10.1016/S0021-9258(19)52451-6Search in Google Scholar
[29] Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol. 1998;108:101–6.10.1385/0-89603-472-0:101Search in Google Scholar PubMed
[30] Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126(1):131–8.10.1016/0003-2697(82)90118-XSearch in Google Scholar PubMed
[31] Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6.10.1016/S0076-6879(84)05016-3Search in Google Scholar
[32] Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–5.10.1016/S0021-9258(19)45228-9Search in Google Scholar
[33] Pinto MC, Mata AM, Lopez-barea J. Reversible inactivation of Saccharomyces cerevisiae glutathione reductase under reducing conditions. Arch Biochem Biophys. 1984;228(1):1–12.10.1016/0003-9861(84)90040-7Search in Google Scholar PubMed
[34] Tappel A. Glutathione peroxidase and hydroperoxides. Methods Enzymol. 1978;52:506–13.10.1016/S0076-6879(78)52055-7Search in Google Scholar
[35] Akerboom TP, Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–82.10.1016/S0076-6879(81)77050-2Search in Google Scholar
[36] Ellman GL, Courtney KD, Andres Jr V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7(2):88–95.10.1016/0006-2952(61)90145-9Search in Google Scholar PubMed
[37] Shishehbore MR, Asgharpoor A, Nasirizadeh N. A novel kinetic spectrophotometric method for the determination of dopamine in biological and pharmaceutical samples. J Chem. 2013;2013:819460.10.1155/2013/819460Search in Google Scholar
[38] Collin MS, Kumar Venkataraman S, Vijayakumar N, Kanimozhi V, Arbaaz SM, Stacey RS, et al. Bioaccumulation of lead (Pb) and its effects on human: A review. J Hazard Mater Adv. 2022;7(3):100094.10.1016/j.hazadv.2022.100094Search in Google Scholar
[39] Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003;126(1):5–19.10.1093/brain/awg014Search in Google Scholar PubMed
[40] Annaç E, Uçkun M, Özkaya A, Yoloğlu E, Pekmez H, Bulmuş Ö, et al. The protective effects of pomegranate juice on lead acetate‐induced neurotoxicity in the male rat: A histomorphometric and biochemical study. J Food Biochem. 2022;46(4):e13881.10.1111/jfbc.13881Search in Google Scholar PubMed
[41] Yamashita R, Takahashi Y, Takashima K, Okano H, Ojiro R, Tang Q, et al. Induction of cellular senescence as a late effect and BDNF-TrkB signaling-mediated ameliorating effect on disruption of hippocampal neurogenesis after developmental exposure to lead acetate in rats. Toxicology. 2021;456:152782.10.1016/j.tox.2021.152782Search in Google Scholar PubMed
[42] Al-Farraj A, Al-Wabel M. Heavy metals accumulation of some plant species grown on mining area at Mahad AD’Dahab, Saudi Arabia. J Appl Sci. 2007;7(8):1170–5.10.3923/jas.2007.1170.1175Search in Google Scholar
[43] Adhikari A, Darbar S, Chatterjee T, Das M, Polley N, Bhattacharyya M, et al. Spectroscopic studies on dual role of natural flavonoids in detoxification of lead poisoning: Bench-to-bedside preclinical trial. ACS Omega. 2018;3(11):15975–87.10.1021/acsomega.8b02046Search in Google Scholar PubMed PubMed Central
[44] El-fakharany YM, Mohamed EM, Etewa RL, Abdel Hamid OI. Selenium nanoparticles alleviate lead acetate-induced toxicological and morphological changes in rat testes through modulation of calmodulin-related genes expression. J Biochem Mol Toxicol. 2022;36(5):e23017.10.1002/jbt.23017Search in Google Scholar PubMed
[45] Atteia HH, Arafa MH, Prabahar K. Selenium nanoparticles prevents lead acetate-induced hypothyroidism and oxidative damage of thyroid tissues in male rats through modulation of selenoenzymes and suppression of miR-224. Biomed Pharmacother. 2018;99:486–91.10.1016/j.biopha.2018.01.083Search in Google Scholar PubMed
[46] Li Z, Liu Y, Wang F, Gao Z, Elhefny MA, Habotta OA, et al. Neuroprotective effects of protocatechuic acid on sodium arsenate induced toxicity in mice: Role of oxidative stress, inflammation, and apoptosis. Chem-Biol Interact. 2021;337:109392.10.1016/j.cbi.2021.109392Search in Google Scholar PubMed
[47] Habotta OA, Elbahnaswy S, Ibrahim I. Neurotoxicity of singular and combined exposure of Oreochromis niloticus to methomyl and copper sulphate at environmentally relevant levels: Assessment of neurotransmitters, neural stress, oxidative injury and histopathological changes. Environ Toxicol Pharmacol. 2022;94:103935.10.1016/j.etap.2022.103935Search in Google Scholar PubMed
[48] Ben-Azu B, Adebayo OG, Wopara I, Aduema W, Onyeleonu I, Umoren EB, et al. Lead acetate induces hippocampal pyramidal neuron degeneration in mice via up-regulation of executioner caspase-3, oxido-inflammatory stress expression and decreased BDNF and cholinergic activity: Reversal effects of Gingko biloba supplement. J Trace Elem Med Biol. 2022;71:126919.10.1016/j.jtemb.2021.126919Search in Google Scholar PubMed
[49] Suleman Z, Engwa GA, Shauli M, Musarurwa HT, Katuruza NA, Sewani-Rusike CR. Neuroprotective effects of Lippia javanica (Burm. F.) Spreng. Herbal tea infusion on Lead-induced oxidative brain damage in Wistar rats. BMC Complemen Med Therapies. 2022;22(1):4.10.1186/s12906-021-03471-3Search in Google Scholar PubMed PubMed Central
[50] Brini M, Calì T, Ottolini D, Carafoli E. Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci. 2014;71(15):2787–814.10.1007/s00018-013-1550-7Search in Google Scholar PubMed
[51] Gazwi HS, Yassien EE, Hassan HM. Mitigation of lead neurotoxicity by the ethanolic extract of Laurus leaf in rats. Ecotoxicol Environ Saf. 2020;192:110297.10.1016/j.ecoenv.2020.110297Search in Google Scholar PubMed
[52] Al-Brakati A, Albarakati AJA, Daabo H, Baty RS, Salem FEH, Habotta OA, et al. Neuromodulatory effects of green coffee bean extract against brain damage in male albino rats with experimentally induced diabetes. Metab Brain Dis. 2020;35(7):1175–87.10.1007/s11011-020-00583-6Search in Google Scholar PubMed
[53] Al Kahtani M. Effect of both selenium and biosynthesized nanoselenium particles on cadmium-induced neurotoxicity in albino rats. Hum Exp Toxicol. 2020;39(2):159–72.10.1177/0960327119880589Search in Google Scholar PubMed
[54] Akinyemi AJ, Miah MR, Ijomone OM, Tsatsakis A, Soares FAA, Tinkov AA, et al. Lead (Pb) exposure induces dopaminergic neurotoxicity in Caenorhabditis elegans: Involvement of the dopamine transporter. Toxicol Rep. 2019;6:833–40.10.1016/j.toxrep.2019.08.001Search in Google Scholar PubMed PubMed Central
[55] Albrakati A, Alsharif KF, Alsanie WF, Almalki AS, Abd Elmageed ZY, Elshopakey GE, et al. Neuroprotective efficiency of prodigiosins conjugated with selenium nanoparticles in rats exposed to chronic unpredictable mild stress is mediated through antioxidative, anti-inflammatory, anti-apoptotic, and neuromodulatory activities. Int J Nanomed. 2021;16:8447.10.2147/IJN.S323436Search in Google Scholar PubMed PubMed Central
[56] Tang Y-L, Wang S-W, Lin S-M. Both inorganic and organic selenium supplements can decrease brain monoamine oxidase B enzyme activity in adult rats. Br J Nutr. 2008;100(3):660–5.10.1017/S0007114508911594Search in Google Scholar PubMed
[57] Anusha C, Sumathi T, Joseph LD. Protective role of apigenin on rotenone induced rat model of Parkinson’s disease: Suppression of neuroinflammation and oxidative stress mediated apoptosis. Chem-Biol Interact. 2017;269:67–79.10.1016/j.cbi.2017.03.016Search in Google Scholar PubMed
[58] Abdel-Moneim AE, Dkhil MA, Al-Quraishy S. The redox status in rats treated with flaxseed oil and lead-induced hepatotoxicity. Biol Trace Elem Res. 2011;143(1):457–67.10.1007/s12011-010-8882-zSearch in Google Scholar PubMed
[59] Flora G, Gupta D, Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip Toxicol. 2012;5(2):47.10.2478/v10102-012-0009-2Search in Google Scholar PubMed PubMed Central
[60] Othman MS, Obeidat ST, Al-Bagawi AH, Fareid MA, Fehaid A, Moneim AEA. Green-synthetized selenium nanoparticles using berberine as a promising anticancer agent. J Integr Med. 2022;20(1):65–72.10.1016/j.joim.2021.11.002Search in Google Scholar PubMed
[61] Chaudhary S, Umar A, Mehta S. Surface functionalized selenium nanoparticles for biomedical applications. J Biomed Nanotechnol. 2014;10(10):3004–42.10.1166/jbn.2014.1985Search in Google Scholar PubMed
[62] Yakubu R, Musa F, Lukman A, Sheikh F. Activity guided fractionation with antimicrobial evaluation of Pergularia tomentosa L.(Asclepiadacea) whole plant. Br Microbiol Res J. 2015;8(5):567–76.10.9734/BMRJ/2015/17027Search in Google Scholar
[63] Kassab RB, Theyab A, Al-Ghamdy AO, Algahtani M, Mufti AH, Alsharif KF, et al. Protocatechuic acid abrogates oxidative insults, inflammation, and apoptosis in liver and kidney associated with monosodium glutamate intoxication in rats. Environ Sci Pollut Res. 2022;29(8):12208–21.10.1007/s11356-021-16578-4Search in Google Scholar PubMed
[64] Chen S, Swilley S, Bell R, Rajanna S, Reddy S, Rajanna B. Lead induced alterations in nitrite and nitrate levels in different regions of the rat brain. Comp Biochem Physiol Part C Pharmacol Toxicol Endocrinol. 2000;125(3):315–23.10.1016/S0742-8413(99)00115-2Search in Google Scholar
[65] Farzadinia P, Mohebbi G, Bargahi A, Akbarzadeh S, Nabipour I, Abdi M, et al. Healing effects of Pergularia tomentosa L., a native medicinal plant in Bushehr province, Iran on burn, in animal model. Pak J Pharm Sci. 2019;32(1):21–8.Search in Google Scholar
[66] Othman MS, Khaled AM, Al-Bagawi AH, Fareid MA, Ghany RA, Habotta OA, et al. Hepatorenal protective efficacy of flavonoids from Ocimum basilicum extract in diabetic albino rats: A focus on hypoglycemic, antioxidant, anti-inflammatory and anti-apoptotic activities. Biomed Pharmacother. 2021;144:112287.10.1016/j.biopha.2021.112287Search in Google Scholar PubMed
[67] Baty R, Hassan K, Alsharif K, El-Hennamy R, Elmahallawy E, Hafez M, et al. Neuroprotective role of luteolin against lead acetate-induced cortical damage in rats. Hum Exp Toxicol. 2020;39(9):1200–12.10.1177/0960327120913094Search in Google Scholar PubMed
[68] Zhou C-C, Gao Z-Y, He Y-Q, Wu M-Q, Chen F, Wang J, et al. Effects of lead, mercury, aluminium and manganese co-exposure on the serum BDNF concentration of pre-school children in Taizhou, China. Chemosphere. 2019;217:158–65.10.1016/j.chemosphere.2018.11.028Search in Google Scholar PubMed
[69] Bashir DW, Rashad MM, Ahmed YH, Drweesh EA, Elzahany EAM, Abou-El-Sherbini KS, et al. The ameliorative effect of nanoselenium on histopathological and biochemical alterations induced by melamine toxicity on the brain of adult male albino rats. Neurotoxicology. 2021;86:37–51.10.1016/j.neuro.2021.06.006Search in Google Scholar PubMed
[70] Rendeiro C, Vauzour D, Rattray M, Waffo-Téguo P, Mérillon JM, Butler LT, et al. Dietary levels of pure flavonoids improve spatial memory performance and increase hippocampal brain-derived neurotrophic factor. PLoS One. 2013;8(5):e63535.10.1371/journal.pone.0063535Search in Google Scholar PubMed PubMed Central
[71] Williams CM, Abd El Mohsen M, Vauzour D, Rendeiro C, Butler LT, Ellis JA, et al. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic Biol Med. 2008;45(3):295–305.10.1016/j.freeradbiomed.2008.04.008Search in Google Scholar PubMed
© 2022 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop
Articles in the same Issue
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop

