Abstract
Ferrate salt is a powerful oxidant for dye degradation. This work demonstrates a new method for degrading dyes containing Fe(vi) by synthesizing NaOCl from the electrolysis of table salt. NaOCl is then reacted with Fe(OH)3 in an alkaline condition to form ferrate. Electrolysis of table salt was successfully carried out using platinum as an anode and zinc as a cathode. The obtained ferrate was characterized by using Fourier transform infrared, UV-Vis, and X-ray diffraction spectroscopy. The ferrate solution has a maximum wavelength of 505 nm with a characteristic purple color. Furthermore, the ferrate produced was utilized to remove methylene blue (MB), remazol black blue (RBB), and methyl orange (MO) dyes with varying contact times. The degraded dyes were then analyzed using LC/MS. The results showed that ferrate was effective to remove dyes with an optimum contact time of 60 min that follows an order one reaction. In this study, MB showed a percent degradation close to 100% with the fastest decolorization rate compared with MO and RBB. This research provides new insights into the benefits of table salt as a base material for NaOCl through electrolysis for synthesizing ferrate, used in dye removal applications.
1 Introduction
The availability of clean water is a crucial need at this time. This is due to reduced water sources because many lands are used for settlement. In addition, the increasing population makes clean water supply techniques an unavoidable need. One strategy to get clean water is to treat polluted water through physical, chemical, and microbiological methods. Some studies also use membranes to purify water [1,2,3,4]. Oxidizing agents are one of the most often used chemical treatments for water purification.
Ferrate (
Under highly alkaline circumstances, iron(iii) oxide (such as Fe2O3) or its salts (Fe(NO3)3, FeCl3) are reacted with sodium hypochlorite to produce wet ferrate [11,15]. People often utilize commercial sodium hypochlorite. However, in this study, NaOCl was obtained from electrolysis of the table salt solution, and then it was reacted with Fe(OH)3 in the alkaline condition to form ferrate solution. Then, liquid ferrate was used to remove dyes, which are a substantial source of nonaesthetic pollution. Remazol black blue (RBB), MB, and MO dye wastes are often used as the selected dyes and also used as model compounds in this study. Subsequently, the ability of potassium ferrate to remove dye was investigated. Through this research, researchers will be more aware of promoting the use of more environmentally friendly materials and green chemistry to support efforts to prevent sustainable environmental problems. In addition, the analysis presented in this study provides information that can be used in future research to explore various materials for dye removal.
2 Materials and methods
2.1 Materials
HCl (37%, Merck), NaOH (≥99%, Merck), KOH (≥85%, Merck), H2SO4 (95–97%, Merck), FeCl3 (99.99%), Remazol Black B (Sigma Aldrich), MB (Merck), MO (Merck), Na2S2O3 (≥97%, Merck), Starch (Merck), KI (≥99.5%, Merck), and table salt was obtained from the market.
2.2 Instrumentations
An electrochemical cell for ferrate electrolysis was prepared with platinum wire (≥99%, Osilla) as anode and zinc plate with an area of 3.4 cm × 3 cm (≥99%) as cathode. The electrodes were then attached to a DC power (Aditeg APS 3005) to carry out the electrolysis process. The instruments used were magnetic stirrer (Thermo Scientific), Ohaus analytical balance (Pioneer), thermometer, and AVOmeter. UV-Vis Spectrophotometer (Genesys 10 S UV-Vis) was used for dye analysis before and after the treatment, while solid Fe(OH)3 and ferrate were examined using instruments of Fourier transform infrared (FTIR) (Shimadzu IRPRESTIGE 21), SEM-EDX (JEOL JSM-6510LA), XRF (PANalytical XRF), and X-ray diffraction (XRD) (Shimadzu XRD-7000).
2.3 Synthesis of NaOCl by electrolysis of NaCl solution
Before use, platinum and zinc plates were washed with soap, aquadest, and alcohol to eliminate any contaminants adhered to the plate’s surface. Then the electrodes were attached to a DC power with platinum and zinc at anode and cathode, respectively, and they were immersed in 250 mL NaCl (table salt content 97% NaCl) solution with concentrations ranging from 0.25 to 5 M and electrolyzed at 3.5 V for 60 min. Previously, variations in the voltage and time of electrolysis were also carried out to see the ratio of the resulting sodium hypochlorite.
2.4 NaOCl determination using iodometric titration
The hypochlorite solution obtained through electrolysis was determined using the iodometric method, which included 25 mL of hypochlorite sample solution added with 15 mL distilled water, 20 mL KI 10%, 20 mL HCl 2 M, and 2 mL of starch solution as indicators and then titrated with 0.26 M Na2S2O3 until the solution was clear. The changing of the starch indicator from blue to clear (colorless) indicates the endpoint of the titration (colorless).
2.5 Synthesis of iron hydroxide (Fe(OH)3)
Sodium hydroxide 12 g and FeCl3 16 g were dissolved with 25 mL distilled water in separate two beakers. The NaOH solution was then added to the FeCl3 solution and reddish-brown precipitate was obtained. After 10 min the precipitate was filtered using filter paper, and dried in oven at 108oC and stored in desiccator. Part of the precipitate was characterized using FTIR and XRD and another part was used for ferrate synthesis.
2.6 Alkaline ferrate synthesis using Fe(iii) and NaOCl
A wet oxidation process was used to synthesize ferrate by reacting NaOH 28 g with 120 mL NaOCl solution (as a result of the electrolysis of NaCl 3 M for 6 h). Then the liquid was stirred until the NaOH was completely dissolved. After addition of Fe(OH)3 2 g to the solution, the solution was stirred for 30 min until the solution became dark purple. The solution was sealed and left for one day, then filtered through glass wool. The maximum wavelength of ferrate was measured at wavelength from 400 to 800 nm using a UV-Vis spectrophotometer.
2.7 Characterization
The presence of functional groups and bonds in the samples was determined using FTIR characterization. The crystalline phase of the sample was determined by XRD using Cu Kα radiation at an angle of 10–90°. SEM-EDX analysis and XRF were used to identify the elemental chemical composition. The absorption spectrum of the electrolysis solution was studied with UV-Vis to determine the ferrate produced and dyes. Absorbance measurements were done between 200 and 800 nm. After reaction with ferrate the concentration of dyes were measured with a UV-Vis spectrophotometer.
2.8 Ferrate application for dye removal
The dyes used in this study were MB, MO, and RBB. Each dye was prepared in a volume of 10 mL with concentration of 10 mg/L, then it was added with 3 drops of 170 mg/L ferrate solution (equal to 170 mg/L × 0.15 mL = 2.55 μg). The solution was stirred for varied times from 5 to 60 min. UV-Vis Spectrophotometer was used to determine the concentration of dye (before and after treated with ferrate).
3 Results and discussion
3.1 Synthesis of NaOCl through electrolysis of NaCl solution
Sodium hypochlorite was synthesized by electrolyzing 0.5, 1, 2, 3 and 5 M NaCl (from table salt) solutions using a platinum electrode as an anode and a zinc electrode as a cathode. In the electrolytic process, the voltage was scanned from 0 to 8 V. Currents were then recorded and displayed as in Figure 1(a).
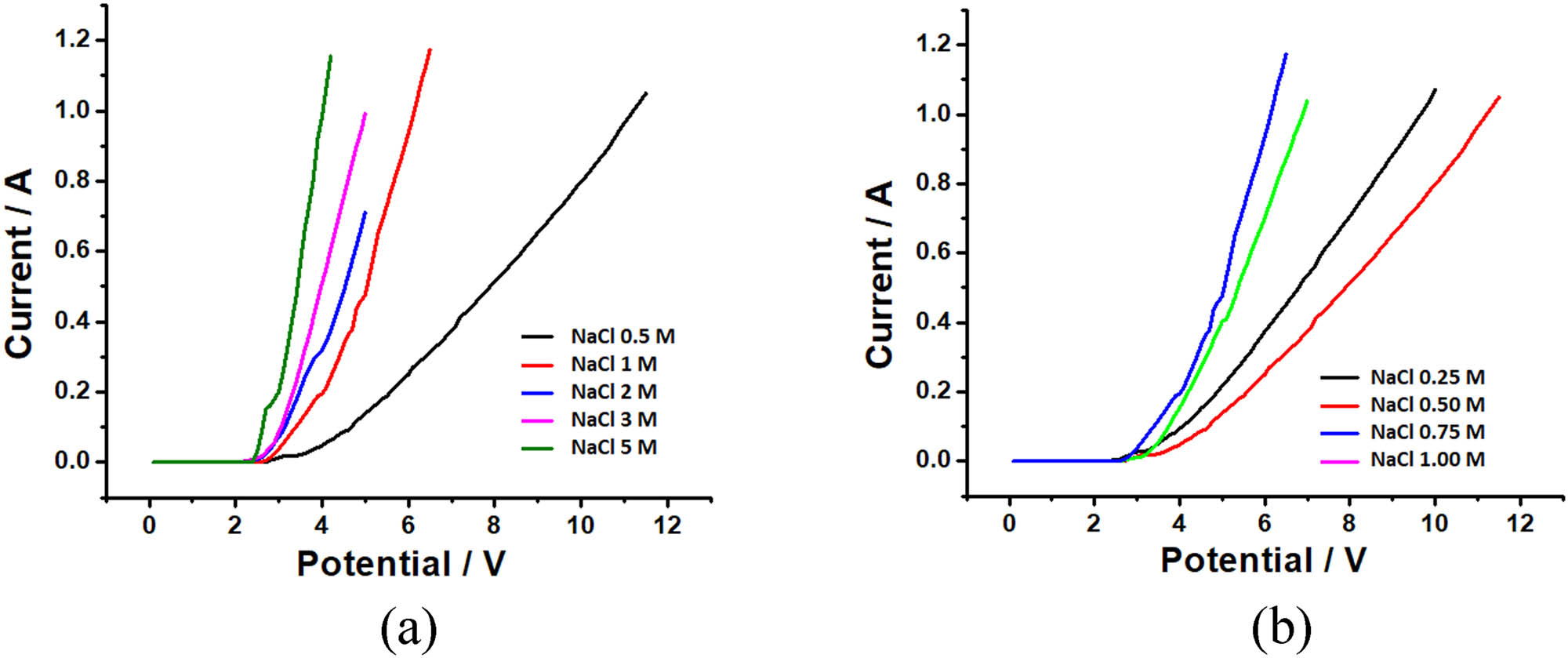
The current–voltage curve for electrolysis of (a) 0.5; 1; 2, 3 and 5 M Nacl and (b) 0.25; 0.50; 0.75 and 1.0 M NaCl concentration used for the NaOCl synthesis.
Figure 1(a) shows that the greater the concentration of NaCl, the lower the potential for the generation of a positive current (E d). As the NaCl concentration decreases, the E d value will be even more significant, allowing a competitive reaction to form chlorine gas with oxygen gas. To avoid the formation of oxygen gas, the potential used is not too high, which is around 3–4 V. Figure 1(b) shows the electrolysis of NaOCl synthesis using a smaller NaCl concentration of 0.25, 0.5, 0.75, and 1 M. From the figure, it can be seen that the smaller the concentration of NaCl, the greater the potential for decomposition that occurs. This allows the formation of oxygen gas more significant in addition to the primary purpose of producing NaOCl.
Electrolysis during the NaOCl synthesis process forms chlorine at the anode and NaOH at the cathode. NaOCl formation due to chemical interaction between chlorine gas and NaOH solution [16].
At cathode:
At anode:
In the beaker glass:
3.2 NaOCl measurement study using iodometric titration
The iodometric titration method was used, namely the titration of NaOCl with sodium thiosulfate after addition of KI solution. The changing of the starch indicator from blue to colorless indicates the endpoint of the titration (colorless) [17]. Figure 2 depicts the concentration of NaOCl produced by electrolysis using NaCl with varied times measured using iodometric method.
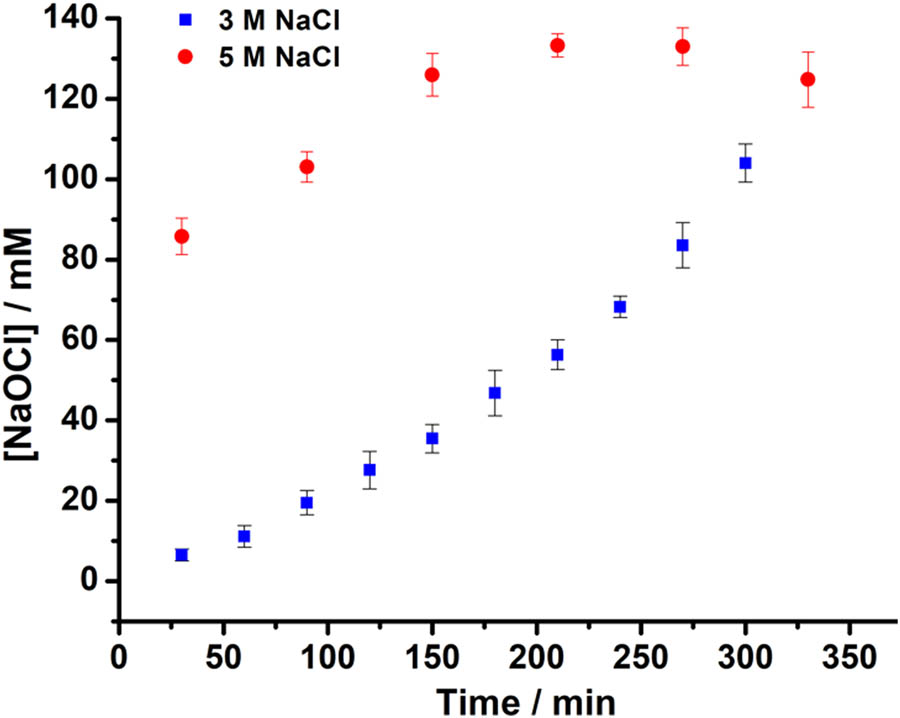
Concentration of NaOCl electrolyzed using 3 and 5 M NaCl at varied times.
Figure 2 shows that the greater the concentration of NaCl used, the greater the NaOCl produced. It can be seen that NaCl 5 M gives a NaOCl yield of about 130 mM by electrolysis for 240 min. Meanwhile, for NaCl 3 M with the same electrolysis time, the result is about 65 mM NaOCl. For electrolysis with 5 M NaCl, the maximum yield of NaOCl is about 130 mM, and then NaOCl begins to decrease. Meanwhile, for electrolysis with 3 M NaCl, the NaOCl concentration continued to increase until 300 min.
Figure 3 shows that at the same electrolysis time, the higher the NaCl concentration, the higher the NaOCl produced [18]. At the electrolysis time of 60 min with a NaCl concentration of 5 M was able to produce NaOCl close to 90 mM. But the result is still small compared to the pro analysis and technical NaOCl concentrations, namely 738.3, and 280.8 mM, respectively. However, the NaOCl obtained can be used for ferrate synthesis. For the synthesis of ferrate, NaOCl was used as a result of electrolysis with 3 M NaCl solution for 300 min. The ferrate is produced from the reaction of iron hydroxide precipitate with NaOCl under alkaline conditions.

The influence of NaCl concentration on the resulting NaOCl concentration. Electrolysis time is 60 min.
3.3 Synthesis of iron hydroxide and Characterization
After mixing the ferric chloride solution with the NaOH solution, Fe(OH)3 was formed. When OH− of NaOH are substituted with chloride ions (Cl) in the solution, the following reaction occurs, resulting in the creation of iron(iii) hydroxide (Fe(OH)3) with no additional secondary phase.
This iron hydroxide precipitate is gelatin which is very insoluble in water. By adding excess NaOH, there will be a common ion effect, and the chemical equilibrium shifts towards the formation of more perfect and insoluble precipitates. The yield of Fe(OH)3 obtained was 86.14%. The resulting iron hydroxide precipitate is brownish.
Characterization was carried out for Fe(OH)3 and compared with FeCl3 as the starting material for preparing Fe(OH)3. Figure 4 depicts the pattern of the FTIR and XRD spectra.
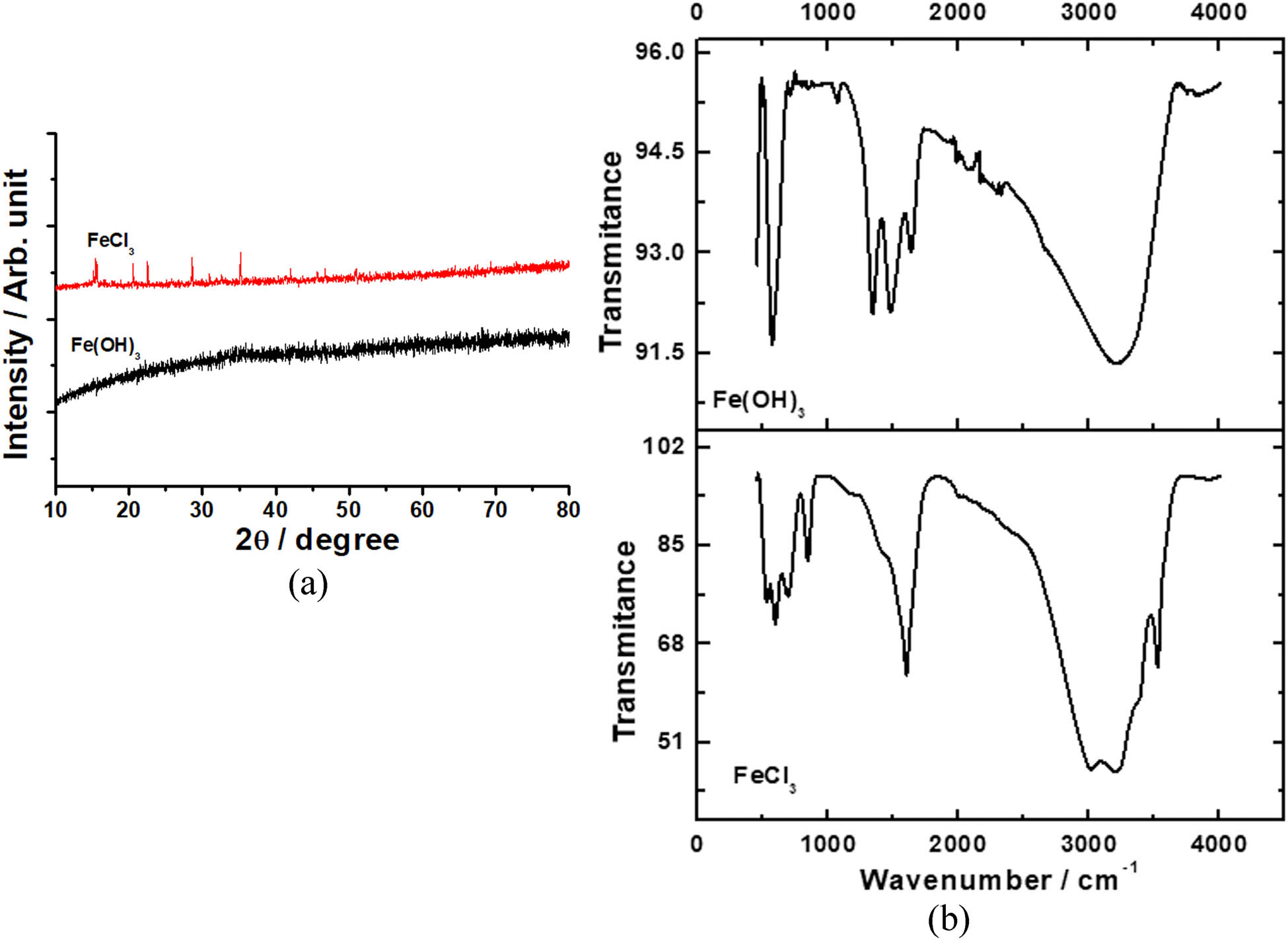
XRD (a) and FTIR spectra (b) of FeCl3 and Fe(OH)3.
The Fe–O bond was connected with the absorption at 578 cm−1, confirming the production of iron hydroxide. Absorption peak of –OH appeared at 3,400 cm−1 [19,20]. In sample FeCl3, there was a peak observed at 586 cm−1, which proved the existence of iron. The graphs show the diffraction peaks at 2θ = 15.47, 20.55, and 50.82°, which is in agreement with the standard FeCl3 diffraction peaks [CAS No. 10025-77-1] [21]. The peaks at 2θ = 35.00 matches well with standard of Fe(OH)3 (JCPDS number 22-0346) [19]. The resulted product, according to XRD and FTIR measurements, is iron hydroxide Fe(OH)3.
Table 1 shows the EDX of Fe(OH)3. The presence of FeO components in EDX indicates the presence of the target substance, Fe(OH)3. Carbon peaks in the EDX spectrum are caused by the carbon bands employed during the EDX testing.
EDX of Fe(OH)3
| No | Sample name | Component | Unit | Analysis result value | Method |
|---|---|---|---|---|---|
| 1. | Fe(OH)3 | FeO | % Weight | 86.01 | EDX |
| C | 10.16 | ||||
| ZrO2 | 1.93 | ||||
| Na2O | 1.49 | ||||
| Al2O3 | 0.41 |
3.4 Alkaline ferrate synthesis using Fe(iii) and NaOCl
Using wet chemical techniques, a purple ferrate solution was obtained by mixing NaOCl as an oxidizing agent with Fe(OH)3 as a source of Fe(iii) in a strongly alkaline state (NaOH) [22,23,24]. The reaction for ferrate production is shown below:
The wavelength and concentration of the resulting ferrate solution (sodium ferrate) were measured with UV-Vis. The maximum detection wavelength was 505 nm with an absorbance of 0.300 (with a dilution factor of 4×). Other investigations [7,15,25,26,27] have validated the absorption spectra of ferrate at 505 nm. By using the formula c = 166000A/1170 and by entering the absorbance at 505 nm and multiplying by the dilution factor, the ferrate concentration obtained was 170 mg/L (Figure 5).
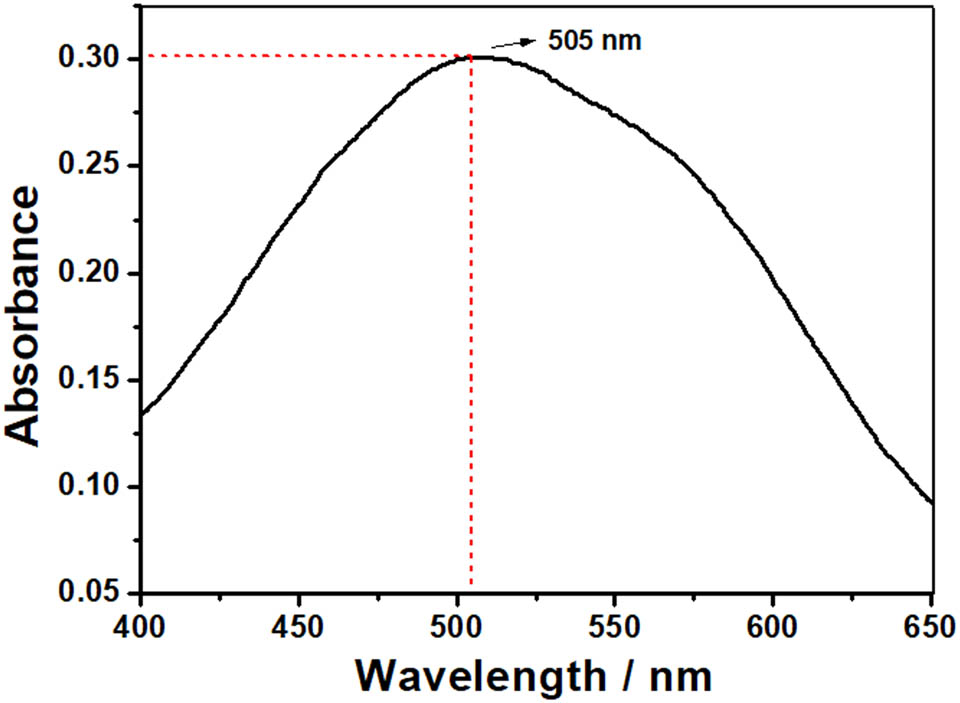
Visible spectrum of ferrate solution.
3.5 Ferrate application for dye removal
Sodium ferrate is the ferrate formed (Na2FeO4). Fe(iii) is an effective coagulant capable of removing suspended particles from wastewater [28]. Sodium ferrate is gradually converted into oxygen and (Fe(OH)3) according to equation (8). In wastewater treatment, oxygen is released, causing the concentration of dissolved oxygen (DO) in the wastewater to rise. A higher DO concentration in the wastewater can avoid anaerobic conditions and prevent odor formation in other parts of the wastewater treatment plant [29].
3.5.1 The influence of contact time on dye degradation by ferrate
Contact time is a crucial element influencing material adsorption capability. Figure 6 depicts the influence of contact time on dye removal utilizing the ferrate oxidation procedure. Contact time is an essential factor affecting the adsorption capacity of materials. Figure 6 depicts the effect of contact time on removal dyes using the ferrate oxidation procedure.
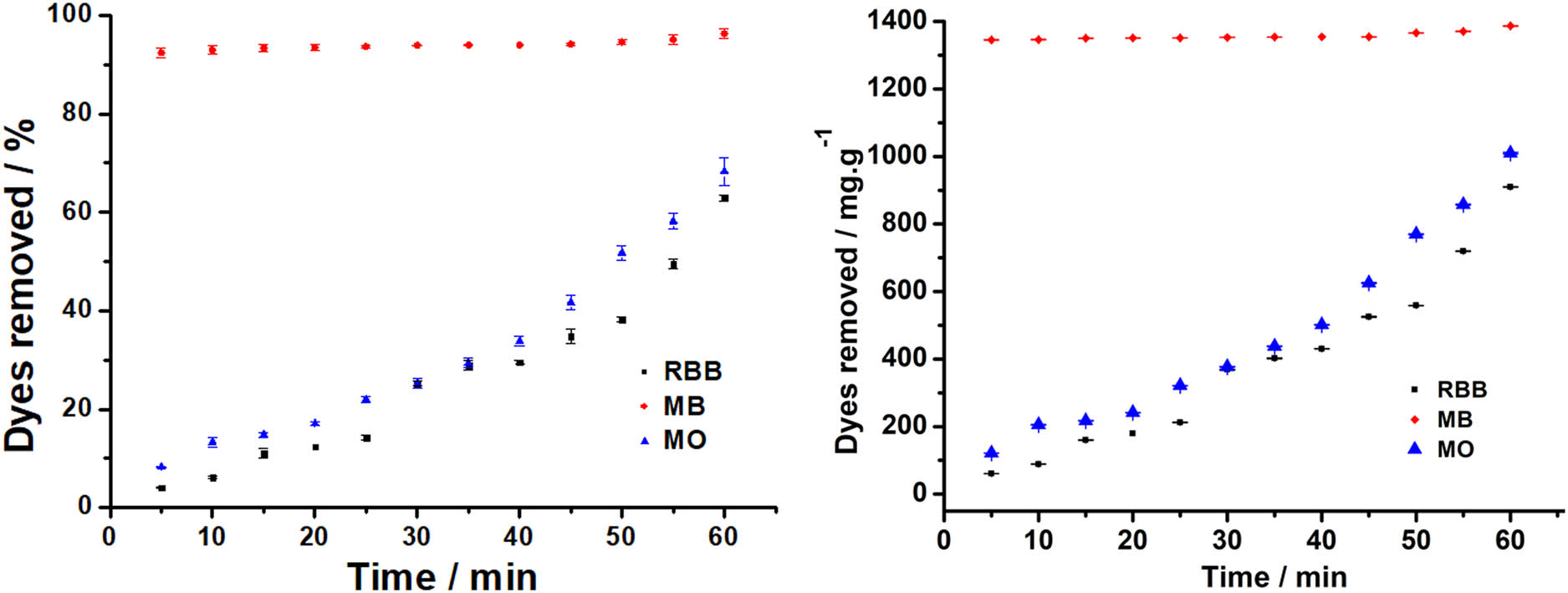
Degradation of dyes by ferrate with the effect of contact time.
Figure 6 demonstrates that the destruction of MB produces the highest yield (almost 100 %), followed by MO and RBB, and the reaction time is classified as fast. After entering the reactor, ferrate needs times to diffuse in dye solution [30]. For MB, from the first 5 min, the removal percentage was already high, and with increasing contact time, there was an increase but not too significant in removal percentage. In such cases, the active sites are saturated and the dye molecules in the adsorbent (ferrate) have achieved equilibrium [31]. When it was considered by the amount of dye taken against the weight of ferrate as a oxidator, it can be seen that MB gives a large yield of about 1,400 mg/g of ferrate used. For MO and RBB, there was a significant increase in destruction with contact time. The more complicated the structure of the dyestuff, the more it will be destroyed by ferrate. The results revealed that the dye clearance % rose with contact duration, with 60 min producing the greatest outcomes. The contact duration of 60 min, according to Talaiekhozani et al., is the optimum for eliminating COD and hydrogen sulfide using ferrate(vi) from household wastewater [32].
3.5.2 Reaction kinetics of dyes removal
Figure 7 shows the reaction kinetics of dye degradation (MB, MO, and RBB). Degradation of dyes by ferrate was the first order reaction indicated by the value of R 2 which was close to 1. The corresponding first-order kinetic plots shown in Figure 8 indicate that the initial rate of decolorization was much faster for MB than for MO and RBB.
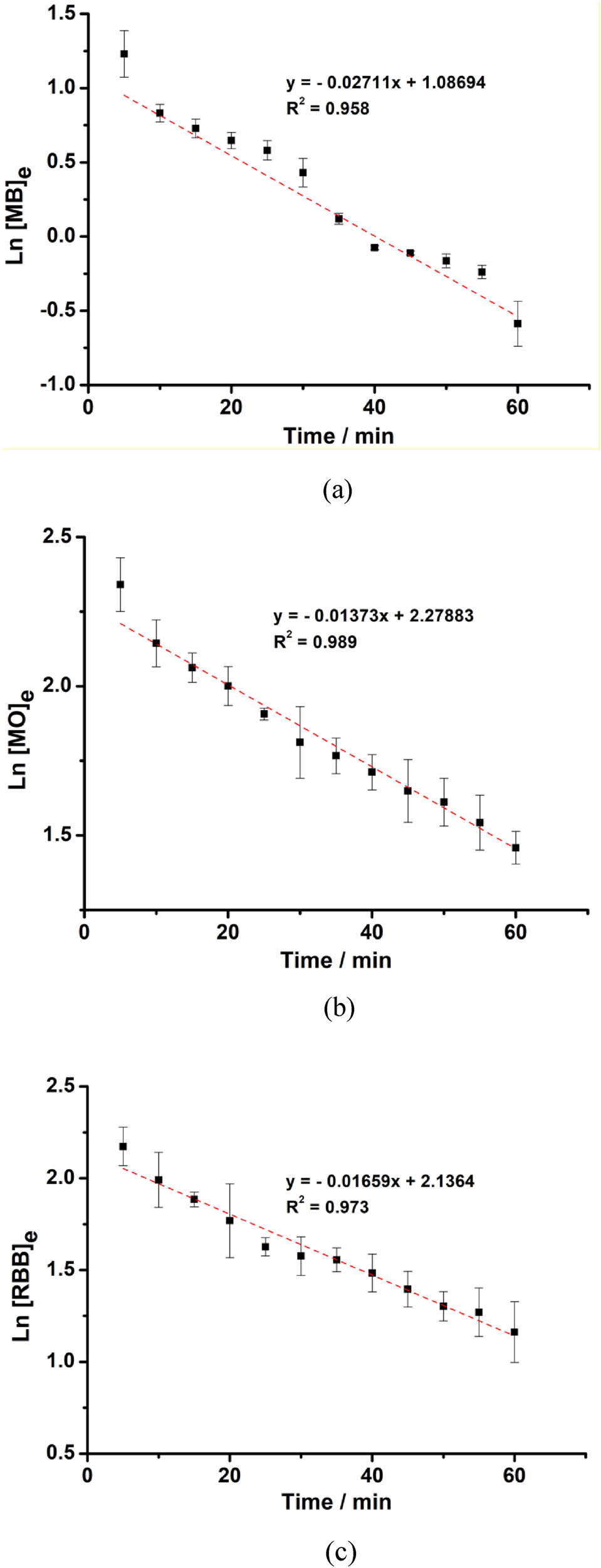
Kinetics curves of the dyes (MB (a), MO (b), and RBB (c)) destruction by ferrate.
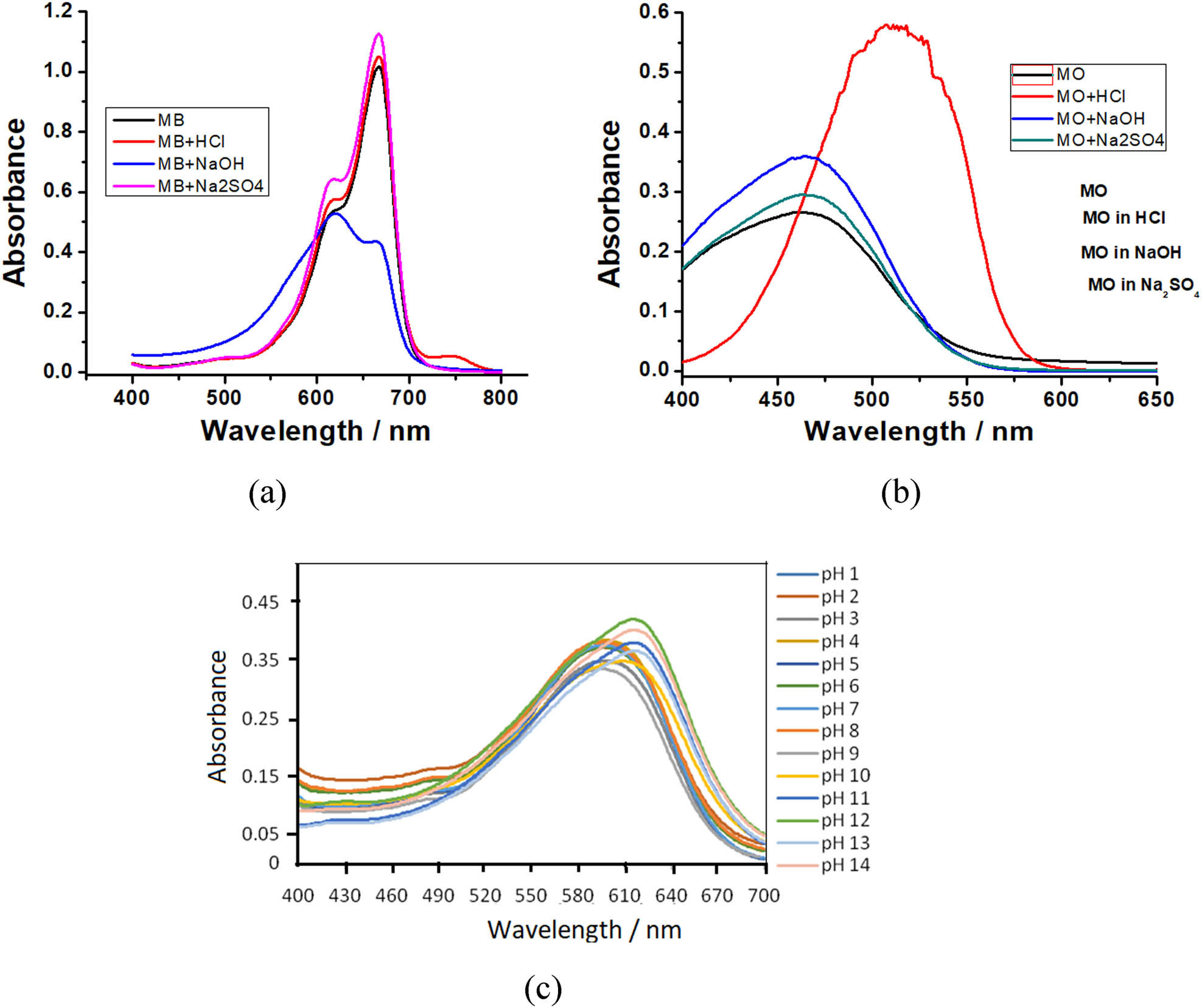
The maximum wavelength of (a) MB, (b) MO, and (c) RBB at various pH.
3.5.3 Study of dyes on pH
To evaluate the influence of pH on the shift of the maximum wavelength peak used for concentration measurements before and after ferrate treatment was done by measurement the dye UV-Vis spectrum with different pH. Figure 8, especially for MB, there is a drastic color change when the pH is high from blue to purple, and it occurs because the ferrate used is derived from extreme base synthesis. So that measurements at alkaline pH must be carried out at the maximum peak of the dye under alkaline conditions.
3.6 Characterization
3.6.1 Ferrate characterization
The Fe–O bond stretching vibration peaks in ferrate is detected at roughly 700, 766, and 877 cm−1, demonstrating the presence of Fe–O bonds in the crystal, namely sodium ferrate salt [27,33]. The H–O bond from water is responsible for the peaks at 1,651 cm−1 and between 2,492 and 3,500 cm−1 [34,35]. The strength of the ferrate characteristic vibrational peak is strong in Figure 9a, suggesting that the purity of the produced ferrate is greater. The graph shows the diffraction peaks at 2θ = 27.68, 30.16, 32.33, 34.53, 35.28, 40, 45.41, and 56.75° which is in agreement with the reference (Na2FeO4). Figure 9b shows a significant resemblance and proves the crystal structure of ferrates, as discovered by El Maghraoui et al. [27]. Therefore, the obtained product is sodium ferrate (Na2FeO4), according to the XRD and FTIR measurements.
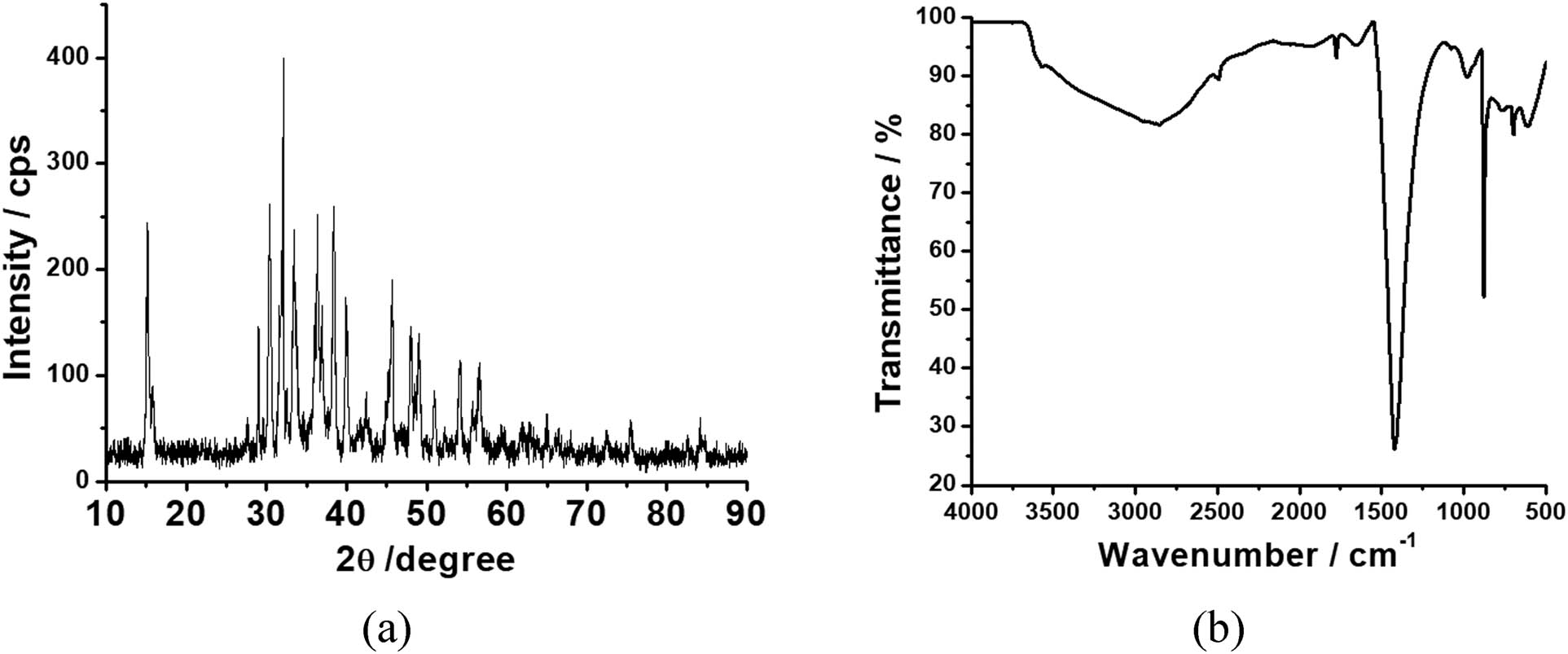
XRD (a) and FTIR spectra (b) of ferrate.
The elemental composition of ferrate is shown in Table 2. From the table, we know that the sample contains Na, Fe, and most of the other Fe was found in its oxide form with the value 93.774%. The existence of the components in XRF demonstrates that the target substance, Na2FeO4, was formed.
XRF Analysis of Ferrate
| Component | Unit | Analysis result value |
|---|---|---|
| Na | % Weight | 5.1802 |
| Si | 0.2461 | |
| Cl | 0.8394 | |
| K | 0.0204 | |
| Ca | 0.0099 | |
| Fe | 0.0237 | |
| Zn | 0.0519 | |
| Ag | 0.0545 | |
| Balance | 93.574 |
3.6.2 Characterization of dye destruction by ferrate
Tests with LC/MS gave the spectral results as shown in Figure 10 for MB, MO, and RBB.
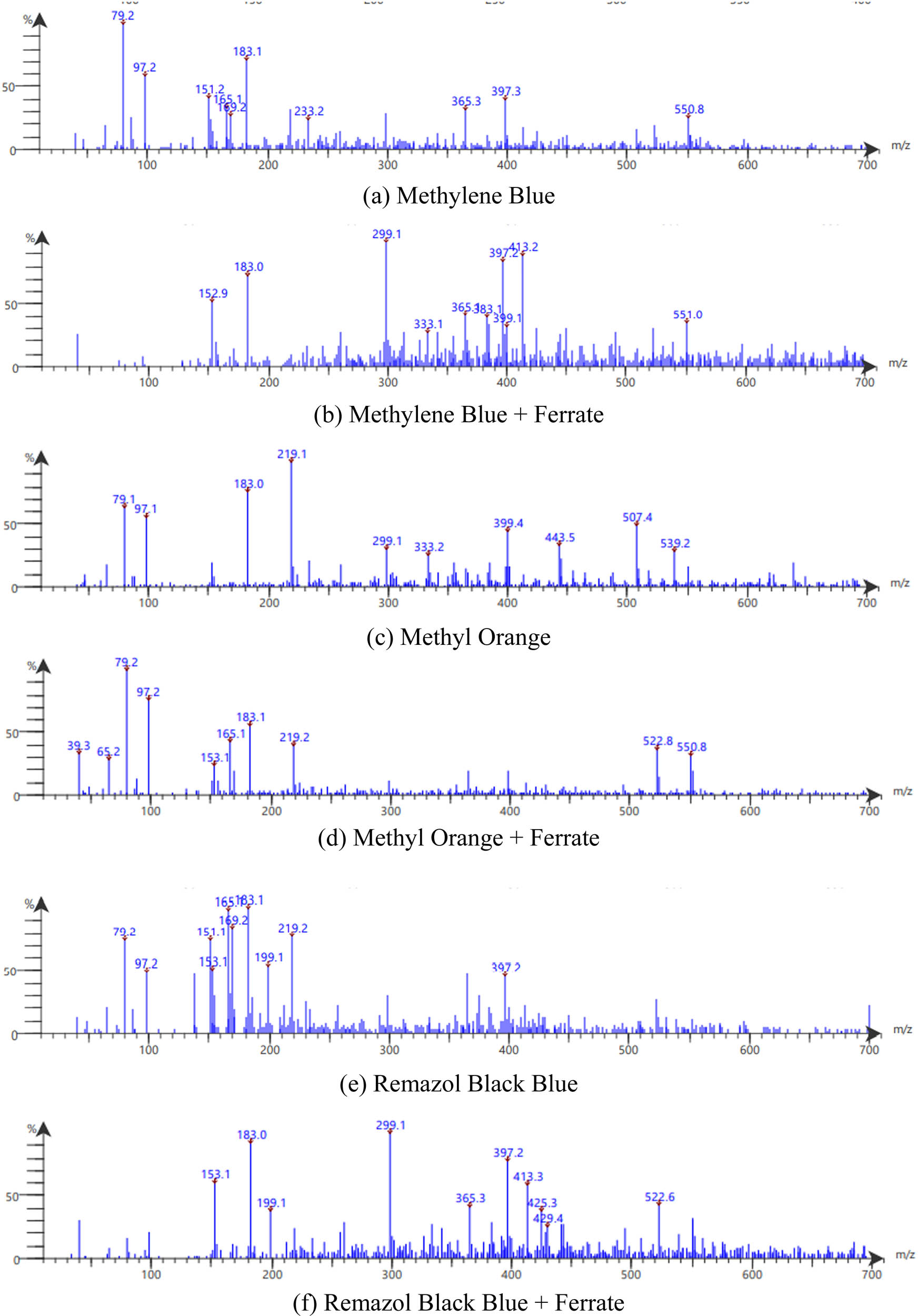
LC/MS spectra of MB (a) & (b), MO (c) & (d), and RBB (e) & (f) before and after treated with ferrate.
MB degradation begins with the breakdown of the C–S═C bond, followed by the dissociation of the two benzene rings. The aromatic ring is subsequently hydroxylated, yielding phenolic metabolites. The last aromatic component discovered before the ring opened was hydroxyhydroquinone. The amino group can either produce an ammonium ion, which is slowly reduced to nitrate, or it can be immediately converted to hydroxylamine, which is then oxidized to nitrate. The existence of the MB molecule was established by the signal at m/z 284. Scheme 1 depicts the sequential hydroxylation processes of the MB benzene ring. The addition of hydroxyl promotes ring-opening and the formation of mineralized products. The intermediate will decompose and mineralize into CO2, H2O,
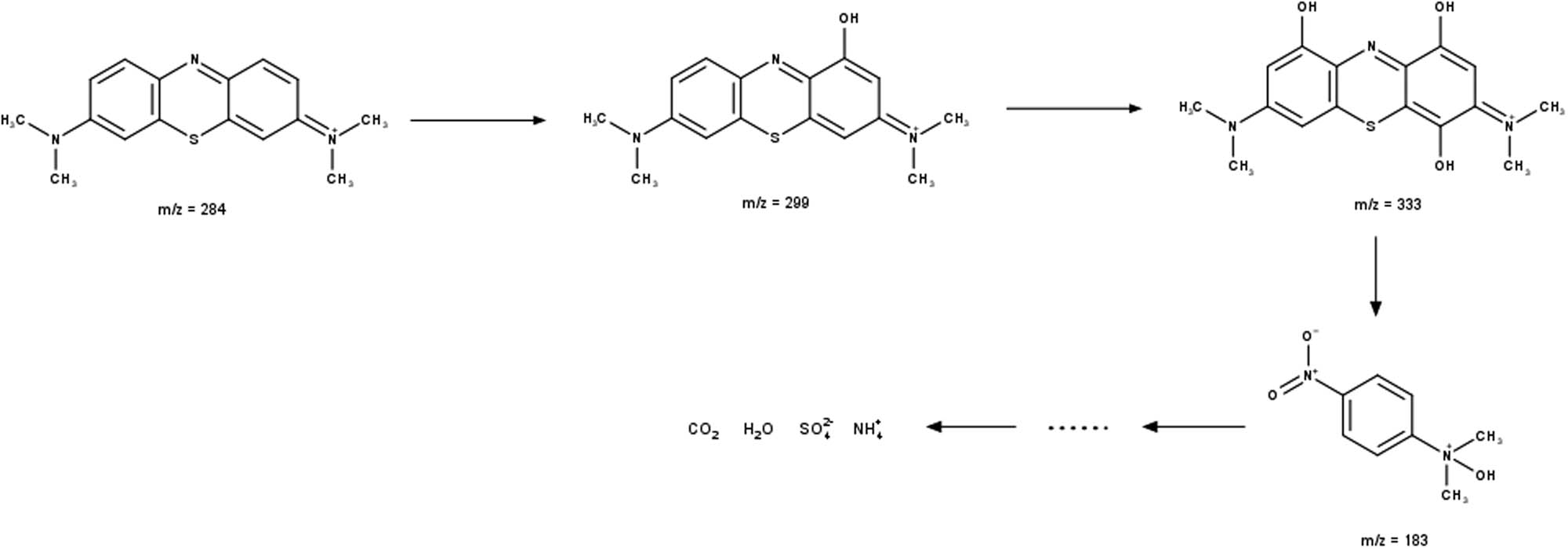
Degradation of MB by ferrate.
It can be observed that the MO spectra show a peak at m/z 306, which indicates MO characteristics as shown in Scheme 2. Meanwhile, the MO chromatograms degraded by ferrate showed a total loss of MO peaks and new m/z peaks at 165, 153, and 97, corresponding to the intermediate product of MO degradation. The product formed at m/z 165 is dimethyl-(4-nitro-phenyl)-amine, followed by further degradation of the intermediate product. MO breakdown products can be further degraded to inorganic ions (ammonium, nitrate, and sulfate), H2O, and CO2 [39,40,41].
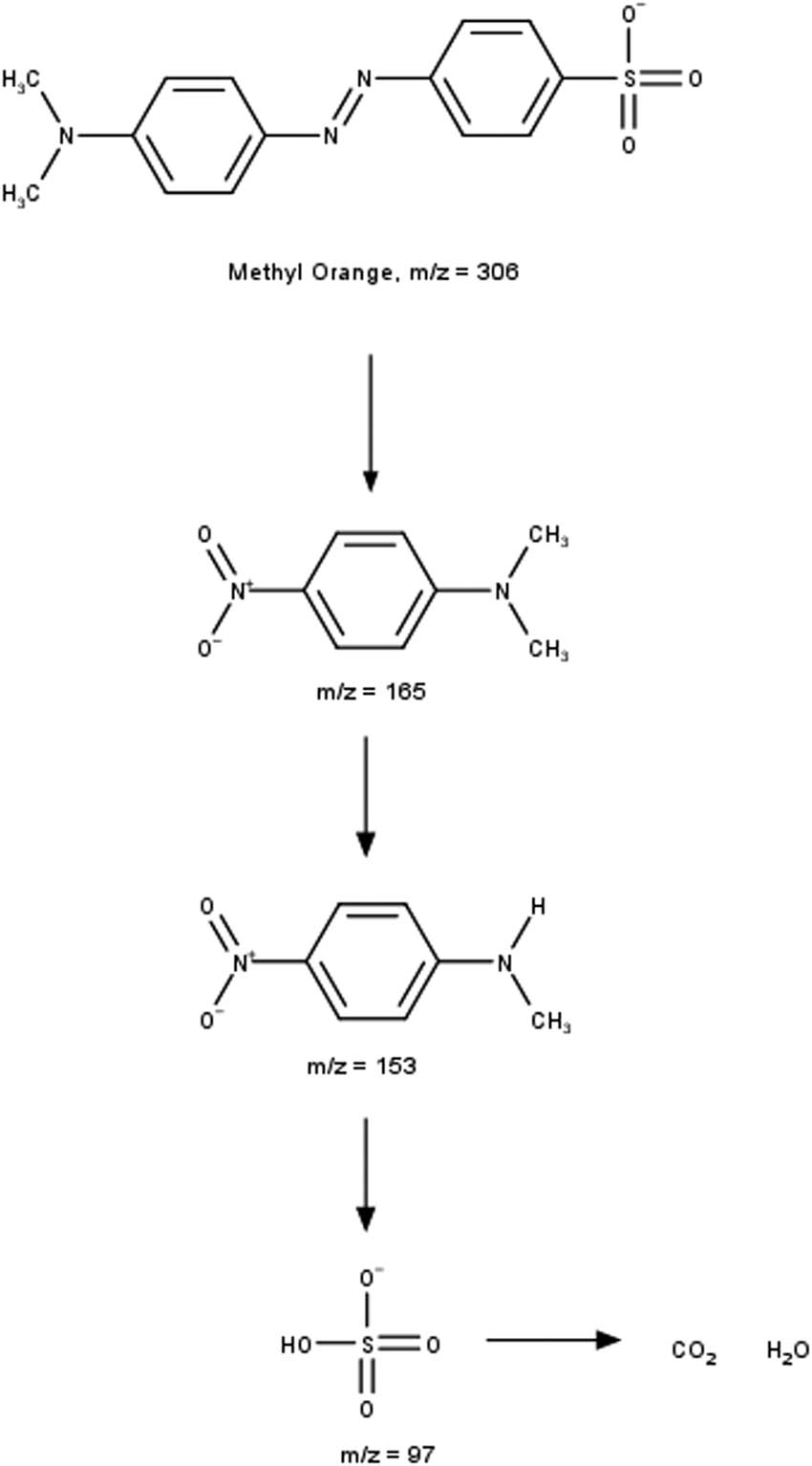
Degradation of MO by ferrate.
Based on the intermediates identified by LC-MS, possible degradation pathways of RBB dyes have been suggested and are shown in Scheme 3. The schematic shows that RBB dyes are degraded through the breakdown of azo bonds (N═N) into intermediate products. The breakdown of dyestuffs via the breaking of azo linkages results in the creation of aromatic amines. Fragmentation continues until CO2 and H2O are formed as the end result of the ferrate degradation of remazol black B [42,43].
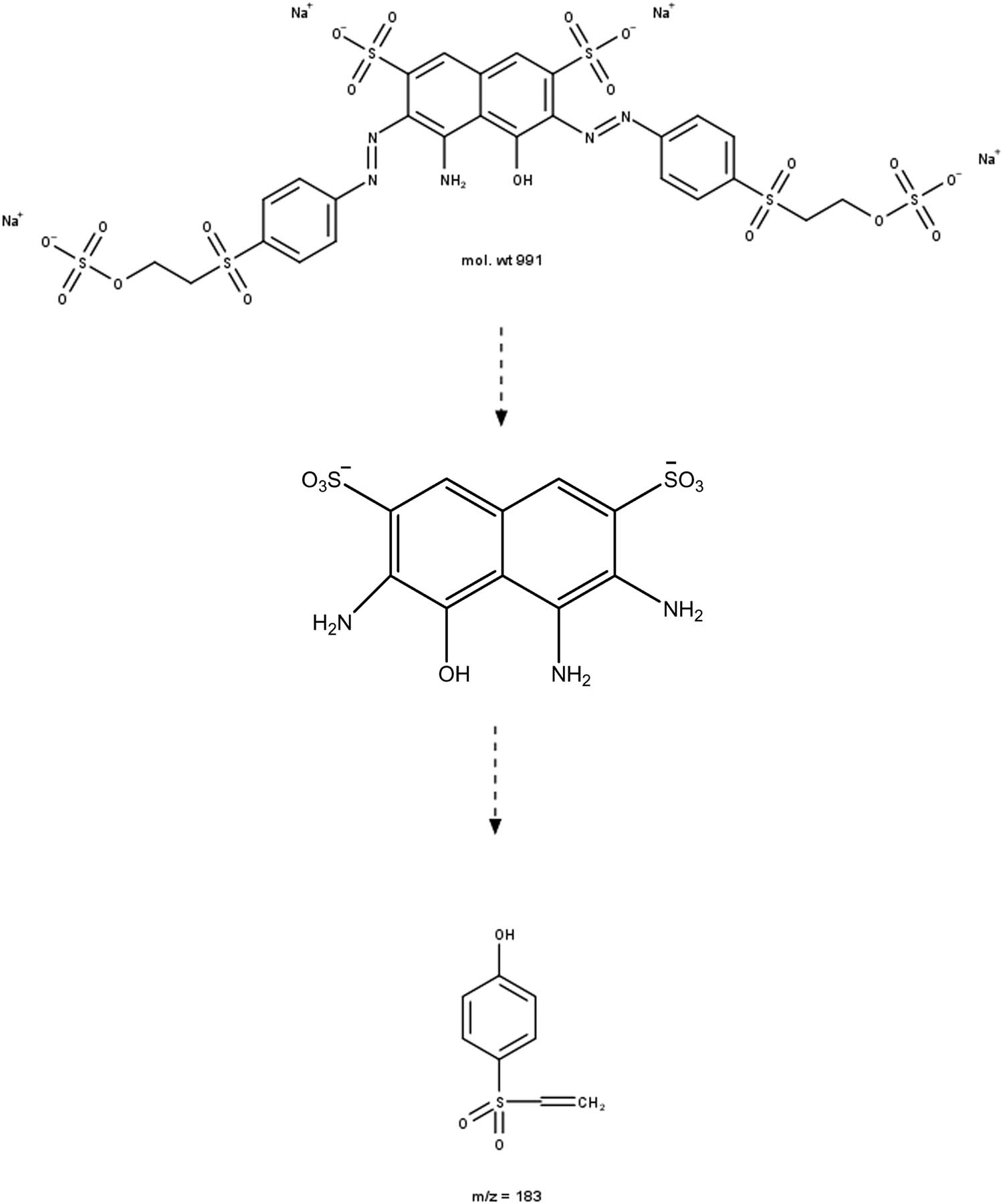
Degradation of remazol black B by ferrate.
4 Conclusion
The synthesis of NaOCl by electrolysis of table salt has been successfully obtained. NaOCl reacted with iron(iii) ions in alkaline condition to produce purple ferrate. The resulted product is sodium ferrate (Na2FeO4) according to the XRD and FTIR measurements. The ferrates were used to remove MB, MO, and RBB dyes with contact times of 5–60 min. The ferrate dyes can be removed through destruction process, resulting in almost 100% removal of the MB sample without any harmful by-products.
Acknowledgments
The authors are thankful to the DIKTI (PDUPT scheme) for funding this work.
-
Funding information: The Ministry of Research and Technology/National Research and Innovation Agency financed this work through the Directorate of Research and Community Service, Deputy for Strengthening Research and Development (PDUPT research grant with contract number 225-91/UN7.6.1/PP/2021).
-
Author contributions: The published version of the work has been reviewed and approved by all authors.
-
Conflict of interest: The authors state that they have no conflict of interest.
-
Ethical approval: The research was not undertaken for human or animal use.
-
Data availability statement: All the data is available within the manuscript.
References
[1] Chen W, Mo J, Du X, Zhang Z, Zhang W. Biomimetic dynamic membrane for aquatic dye removal. Water Res. 2019;151:243–51. 10.1016/j.watres.2018.11.078.Suche in Google Scholar PubMed
[2] Zhang W, Jiang F. Membrane fouling in aerobic granular sludge (AGS)-membrane bioreactor (MBR): Effect of AGS size. Water Res. 2019;157:445–53. 10.1016/j.watres.2018.07.069.Suche in Google Scholar PubMed
[3] Meng S, Meng X, Fan W, Liang D, Wang L, Zhang W, et al. The role of transparent exopolymer particles (TEP) in membrane fouling: A critical review. Water Res. 2020;181:115930. 10.1016/j.watres.2020.115930.Suche in Google Scholar PubMed
[4] Zhang W, Liang W, Zhang Z, Hao T. Aerobic granular sludge (AGS) scouring to mitigate membrane fouling: Performance, hydrodynamic mechanism and contribution quantification model. Water Res. 2021;188:116518. 10.1016/j.watres.2020.116518.Suche in Google Scholar PubMed
[5] Malik SN, Ghosh PC, Vaidya AN, Waindeskar V, Das S, Mudliar SN, et al. Comparison of coagulation, ozone and ferrate treatment processes for color, COD and toxicity removal from complex textile wastewater. Water Sci Technol. 2017 ;76(5):1001–10. 10.2166/wst.2017.062.Suche in Google Scholar PubMed
[6] Sharma VK, Chen L, Zboril R. Review on high valent FeVI (Ferrate): A sustainable green oxidant in organic chemistry and transformation of pharmaceuticals. ACS Sustain Chem Eng. 2015;4(1):18–34. 10.1021/acssuschemeng.5b01202.Suche in Google Scholar
[7] Munyengabe A, Zvinowanda C. Production, characterization and application of Ferrate(VI) in water and wastewater treatments. Braz J Anal Chem. 2019;6(25):40–57. 10.30744/brjac.2179-3425.RV-19-2019.Suche in Google Scholar
[8] Barışçı S. The disinfection and natural organic matter removal performance of electro-synthesized ferrate(VI). J Water Process Eng. 2017;20:84–9. 10.1016/j.jwpe.2017.10.005.Suche in Google Scholar
[9] McBeath ST, Wilkinson DP, Graham NJD. Exploiting water contaminants: In-situ electrochemical generation of ferrates using ambient raw water iron (Fe2+). J Env Chem Eng. 2020;8(4):103834. 10.1016/j.jece.2020.103834.Suche in Google Scholar
[10] Majid D, Kim I-K. Degradation of toluene by liquid ferrate(VI) and solid ferrate(VI) in aqueous phase. J Env Eng. 2018;144(9):04018093. 10.1061/%28ASCE%29EE.1943-7870.0001440.Suche in Google Scholar
[11] Karelius K, Nopriawan BA. Synthesis of ferrate (FeO42−) using Fe(NO3)3 and NaOCl and its application to methylene blue degradation. J Ilm Berk Sains dan Terap Kimia. 2016;10(1):1–7. 10.20527/jstk.v10i1.3156.Suche in Google Scholar
[12] Soni BD, Patel UD, Agrawal A, Ruparelia JP. Application of BDD and DSA electrodes for the removal of RB 5 in batch and continuous operation. J Water Process Eng. 2017;17:11–21. 10.1016/j.jwpe.2017.01.009.Suche in Google Scholar
[13] Song Y, Men B, Wang D, Ma J. On-line batch production of ferrate with an chemical method and its potential application for greywater recycling with Al(iii) salt. J Env Sci. 2017;52:1–7. 10.1016/j.jes.2016.05.002.Suche in Google Scholar PubMed
[14] Dwiasi DW, Kurniasih M. Studi degradasi zat pewarna azo, metil oranye menggunakan ferrat (FeO42−). Molekul. 2008;3(1):15. 10.20884/1.jm.2008.3.1.42.Suche in Google Scholar
[15] Luo Z, Strouse M, Jiang J-Q, Sharma VK. Methodologies for the analytical determination of ferrate(VI): A review. J Env Sci Heal Part A. 2011;46(5):453–60. 10.1080/10934529.2011.551723.Suche in Google Scholar PubMed
[16] Asokan K, Subramanian K. Design of a Tank Electrolyser for In-situ Generation of NaClO. Proceedings of the World Congress on Engineering and Computer Science; 2009 October 20–22; San Francisco, USA: WCECS. p. 139–42.Suche in Google Scholar
[17] Wright PP, Cooper C, Kahler B, Walsh LJ. From an assessment of multiple chelators, clodronate has potential for use in continuous chelation. Int Endod J. 2020;53(1):122–34. 10.1111/iej.13213.Suche in Google Scholar PubMed
[18] Ghalwa NA, Tamos H, Askalni ME, Agha AR, Ghalwa NA, Tamos H, et al. Generation of sodium hypochlorite (NaOCl) from sodium chloride solution using C/PbO2 and Pb/PbO2 electrodes. Int J Min Metall Mater. 2012;19(6):561–6. 10.1007/s12613-012-0596-0.Suche in Google Scholar
[19] Yan F, Zhang S, Zhang X, Li C, Zhu C, Zhang X, et al. Growth of CoFe2O4 hollow nanoparticles on graphene sheets for high-performance electromagnetic wave absorbers. J Mater Chem C. 2018;6(47):12781–7. 10.1039/C8TC04222E.Suche in Google Scholar
[20] Zhu BS, Jia Y, Jin Z, Sun B, Luo T, Kong LT, et al. A facile precipitation synthesis of mesoporous 2-line ferrihydrite with good fluoride removal properties. RSC Adv. 2015;5(103):84389–97. 10.1039/C5RA15619J.Suche in Google Scholar
[21] Ghosh A, Chatterjee A. Iron making and steel making: Theory and practice. New Delhi: Prentice-Hall of India; 2008.Suche in Google Scholar
[22] Jiang JQ. Advances in the development and application of ferrate(VI) for water and wastewater treatment. J Chem Technol Biotechnol. 2014;89(2):165–77. 10.1002/jctb.4214.Suche in Google Scholar
[23] Prasetya NB, Haris A, Febriliani F. Synthesis of ferrate using NaOCl and Fe(OH)3 from electrolysis of used iron, and its application for metanil yellow degradation. J Phys Conf Ser. 2021;1943(1):012181. 10.1088/1742-6596/1943/1/012181.Suche in Google Scholar
[24] Karim AV, Krishnan S, Pisharody L, Malhotra M, et al. Application of Ferrate for Advanced Water and Wastewater Treatment. Adv Oxid Process – Appl Trends, Prospect. 2020;79:1–20. 10.5772/intechopen.90231.Suche in Google Scholar
[25] Castañeda JM, Martínez MV, Almazán SPT, Linares HI, Vázquez MG, Castañeda JM, et al. Electrosynthesis of sodium and potassium ferrate for the treatment of indigo blue aqueous solutions and denim wastewater. Rev Int Contam Ambient. 2020;36(3):607–22. 10.20937/rica.53381.Suche in Google Scholar
[26] Cheung PCW, Williams DR, Barrett J, Barker J, Kirk DW, Barrett DR, et al. On the origins of some spectroscopic properties of “Purple Iron” (the Tetraoxoferrate(VI) Ion) and its pourbaix safe-space. Mol 2021;26(17):5266. 10.3390/molecules26175266.Suche in Google Scholar
[27] El Maghraoui A, Zerouale A, Ijjaali M, Benbrahim KF. The role of ferrates(VI) as a disinfectant: quantitative and qualitative evaluation for the inactivation of pathogenic bacteria. Afr J Microbiology Res. 2013;7(28):3690–7. 10.5897/AJMR2012.2402.Suche in Google Scholar
[28] Kobya M, Can OT, Bayramoglu M. Treatment of textile wastewaters by electrocoagulation using iron and aluminum electrodes. J Hazard Mater. 2003;100(1–3):163–78. 10.1016/S0304-3894(03)00102-X.Suche in Google Scholar
[29] Talaiekhozani A, Talaei MR, Rezania S. An overview on production and application of ferrate(VI) for chemical oxidation, coagulation and disinfection of water and wastewater. J Env Chem Eng. 2017;5(2):1828–42. 10.1016/j.jece.2017.03.025.Suche in Google Scholar
[30] Talaiekhozani A, Torkan N, Banisharif F, Eskandari Z, Rezania S, Park J, et al. Comparison of reactive blue 203 dye removal using ultraviolet irradiation, ferrate(VI) oxidation process and MgO nanoparticles. Avicenna J Env Heal Eng. 2018;5(2):78–90. 10.15171/ajehe.2018.11.Suche in Google Scholar
[31] Labiebah G, Gunawan G, Djunaidi MC, Haris A, Widodo DS. Removal of methylene blue using used paper powder. J Kim Sains dan Apl. 2019;22(1):23–8. 10.14710/jksa.22.1.23-28.Suche in Google Scholar
[32] Talaiekhozani A, Eskandari Z, Bagheri M, Talaie MR. Removal of H2S and COD using UV, ferrate and UV/ferrate from municipal wastewater. J Human, Env Heal Promot. 2016;2(1):1–8. 10.29252/jhehp.2.1.1.Suche in Google Scholar
[33] Munyengabe A, Zvinowanda C, Ramontja J, Zvimba JN, et al. Effective desalination of acid mine drainage using an advanced oxidation process: Sodium ferrate(VI) salt. Water. 2021;13(19):2619. 10.3390/w13192619.Suche in Google Scholar
[34] Lei B, Zhou G, Cheng T, Du J. Synthesis of potassium ferrate by chemical dry oxidation and its properties in degradation of methyl orange. Asian J Chem. 2013;25(1):27–31. 10.14233/ajchem.2013.11685.Suche in Google Scholar
[35] Nikolić BL, Čekerevac M, Simičić M, Tomić M. Encapsulation of micro-sized barium ferrate(VI) and its effectiveness in removing clomazone pesticide from water. J Mater Sci. 2020;55(17):7295–303. 10.1007/s10853-020-04519-4.Suche in Google Scholar
[36] Wu L, Xie Q, Lv Y, Wu Z, Liang X, Lu M, et al. Degradation of methylene blue via dielectric barrier discharge plasma treatment. Water. 2019;11(9):1818. 10.3390/w11091818.Suche in Google Scholar
[37] de Brito Benetoli LO, Cadorin BM, Baldissarelli VZ, Geremias R, de Souza IG, Debacher NA, et al. Pyrite-enhanced methylene blue degradation in non-thermal plasma water treatment reactor. J Hazard Mater. 2012;237–238:55–62. 10.1016/j.jhazmat.2012.07.067.Suche in Google Scholar PubMed
[38] Oliveira LCA, Gonçalves M, Guerreiro MC, Ramalho TC, Fabris JD, Pereira MC, et al. A new catalyst material based on niobia/iron oxide composite on the oxidation of organic contaminants in water via heterogeneous Fenton mechanisms. Appl Catal A Gen. 2007;316(1):117–24 10.1016/j.apcata.2006.09.027.Suche in Google Scholar
[39] Sharma B, Deswal R. Single pot synthesized gold nanoparticles using Hippophae rhamnoides leaf and berry extract showed shape-dependent differential nanobiotechnological applications. Artif Cells Nanomed Biotechnol. 2018;46(2):408–18. 10.1080/21691401.2018.1458034.Suche in Google Scholar PubMed
[40] Bahrudin NN, Nawi MA, Nawawi WI. Enhanced photocatalytic decolorization of methyl orange dye and its mineralization pathway by immobilized TiO2/polyaniline. Res Chem Intermed. 2019;45(5):2771–95. 10.1007/s11164-019-03762-y.Suche in Google Scholar
[41] Kgatle M, Sikhwivhilu K, Ndlovu G, Moloto N. Degradation kinetics of methyl orange dye in water using trimetallic Fe/Cu/Ag nanoparticles. Catalysts. 2021;11(4):428. 10.3390/catal11040428.Suche in Google Scholar
[42] Elizalde-González MP, Arroyo-Abad U, García-Díaz E, Brillas E, Sirés I, Dávila- Jiménez MM, et al. Formation of sulfonyl aromatic alcohols by electrolysis of a bisazo reactive dye. Molecules. 2012;17(12):14377–92. 10.3390/molecules171214377.Suche in Google Scholar PubMed PubMed Central
[43] Hisaindee S, Meetani MA, Rauf MA. Application of LC-MS to the analysis of advanced oxidation process (AOP) degradation of dye products and reaction mechanisms. TrAC - Trends Anal Chem. 2013;49:31–44. 10.1016/j.trac.2013.03.011.Suche in Google Scholar
© 2022 Gunawan Gunawan et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop
Artikel in diesem Heft
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop

