Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
Abstract
Caesalpinia sappan L. (C. sappan L.), a traditional Chinese medicine, has been widely used to treat bruises and dysmenorrhea, performing pharmacological activities such as anti-inflammatory and anti-tumor. C. sappan L. has been reported to contain compounds such as protosappanins, brazilins, and homoisoflavones. In the pre-experiments, we discovered that there were many unstable components in the characteristic chromatogram of C. sappan L. Here, silica-gel column chromatography, high-speed counter-current chromatography, and preparative high-performance liquid chromatography were combined and applied to isolate the unstable components from alcohol extract of C. sappan L. The results showed that four unstable compounds were collected with the purity higher than 95.0%, characterized as episapponal, brazilin, sapponal, and 4-O-methylsapponal by hydrogen-1 and carbon-13 nuclear magnetic resonance. Based on the above results, the characteristic chromatogram of C. sappan L. was established, and the characteristic peaks were identified. These results provided a theoretical basis for the quality assessment of C. sappan L.
1 Introduction
Caesalpinia sappan L. (C. sappan L.) is a species of the legume family and cultivated widely in Southeast Asia, especially in Guangxi Province, China, where a complete industry chain has been formed [1,2]. As a valuable traditional Chinese medicine (TCM), C. sappan L. has a wealth of pharmacological activities, including anti-bacterial [3,4], hypoglycemic [5], anti-inflammatory [6], and vasodilator [7]. Various types of compounds have been isolated and identified from the heartwood of C. sappan L., such as protosappanins, brazilins, and homoisoflavones by previous research [8]. In the study of Mitani et al. [9], six active compounds were isolated and characterized from C. sappan L. extract. And a new compound, (6aS,11bR)-7,11b-dihydro-6H-indeno[2,1-c]chromene-3,6a,10,11-tetrol, was identified for the first time. Brazilin and protosappanin B were isolated from 70% (v/v) ethanol extract of C. sappan L. and they exhibited effects on inhibiting the formation of melanin in vivo [10].
Separation methods for isolating polar compounds of C. sappan L. included silica gel column chromatography (SGCC), preparative column, high-speed counter-current chromatography (HSCCC), and gel column chromatography (GCC) [11]. Notably, preparative high-performance liquid chromatography (prep-HPLC) was a powerful method developed by a high-loading and high-separation preparative column, which had been commonly employed in separating natural products and was suitable for preparative-scale separation of target compounds with high purity and recovery rate [12]. Furthermore, HSCCC represented a liquid–liquid chromatographic separation technique whose stationary and mobile phases were liquids without irreversible adsorption during separation [13–15]. The method applying HSCCC provided significant advantages over other traditional separation methods, including non-contamination, higher separation efficiency, low sample loss, high recovery, and high separation volume [16–18]. Previously, some researchers had achieved the separation of homoisoflavonoids from C. sappan L. by liquid–liquid partition chromatography. Uddin et al. [19] used centrifugal partition chromatography to separate sappanol and brazilin from the ethyl acetate-soluble ingredient (350 mg) of C. sappan L. As described by Xu et al. [20], 3′-deoxysappanol (5 mg), 3-deoxysappanone B (8 mg), 4-O-methylsappanol (20 mg), and brazilin (18 mg) were isolated from an ethyl acetate extract (120 mg) of C. sappan L. by HSCCC.
Currently, the quality control (QC) of C. sappan L. in the Chinese Pharmacopoeia (2020) was limited to thin layer chromatography (TLC), water content determination and extractive determination [1], and lack of quantitative indicators and fingerprint. There has been no adequate research on its fingerprint, and identification of the characteristic peaks in the fingerprint has been restricted to protosappanin B and brazilin. In therapies of TCM, C. sappan L. was typically used in decoction. Several characteristic peaks were discovered to be substantially reduced after the adjustment of the decoction time, while information on characteristic peaks was still not clear. As a result, it was required and meaningful to isolate and identify the characteristic peaks in the fingerprint of C. sappan L. The principal aim of this study was to isolate and identify unstable components of C. sappan L. by column chromatography, counter-current chromatography, and prep-HPLC, so as to establish a characteristic chromatogram for comprehensively controlling the quality of C. sappan L.
2 Materials and methods
2.1 Reagents and materials
The heartwood of C. sappan L. was collected from Luchuan, Guangxi, China. Methanol, n-hexane, n-butanol, ethyl alcohol, petroleum ether, chloroform, and ethyl acetate were purchased from Huipu chemical store, Hangzhou, China. The above reagents were all of analytical grade. Acetonitrile (chromatographic grade) and methanol (chromatographic grade) were obtained from Tedia (Fairfield, USA). Protosappanin B (purity >98.0%) was purchased from Chengdu Pusi Biotechnology Company Inc. The silica gel (200–300 mesh) and TLC plates were purchased from Yantai Jiangyou Silica Gel Development Co., Ltd.
2.2 Instruments
A TBE-200V HSCCC separation instrument (Shanghai Tauto Biotechnology, Shanghai, China) was employed, outfitted with a constant flow pump model MP-0106 (Sanwei Science and Technology Co., Ltd, Shanghai, China), a thermostatic controller model SDC-6 (Nanjing Xinchen Biotechnology Co., Ltd, Nanjing, China), a constant flow pump Model TBP 5002 (Shanghai Tauto Biotechnology Co., Ltd, Shanghai, China), and a UV detector Model HD-2 (Husi Analytical Instruments, Shanghai, China). The separation column was constructed of 1.6 mm polytetrafluoroethylene tubes with a total volume of 190 mL. The coils were simultaneously moved in a clockwise or counterclockwise planetary motion around the central axis. The maximum coil rotation was 1,000 rpm, and a reasonable value of 800 rpm was adopted in this study. HPLC was carried out using an Agilent-1290 system (Agilent, USA) outfitted with an Agilent Eclipse C18 column (4.6 mm × 250 mm, 5 μm). Separation was carried out using instrument of Shimadzu LC-20AP (Shimadzu, Japan) with preparative column model of Shimadzu shim-pack C18 column (20 mm × 250 mm, 15 μm). The structures of the compounds were identified on a Bruker AVANCE III 400 MHz nuclear magnetic resonance (NMR) spectrometer with tetramethylsilicane as an internal standard (Bruker Company, Switzerland).
2.3 Preparation of ethyl acetate extract sample
The heartwood of C. sappan L. (1 kg, dry weight) was extracted with 5 L of 75% (v/v) ethanol for 2 h, then cooled and filtered. The herbal residues were extracted with 5 L of 75% (v/v) ethanol for 1 h and successively extracted twice. Then, the crude extracts were combined and concentrated to dry in a vacuum rotary evaporator at a water bath temperature of 50°C to obtain the alcoholic extract (97 g). After being redissolved with water (2 L), the aqueous phase was originally extracted with an equal volume of petroleum ether three times to remove small polar parts. Moreover, the lower phase was extracted with 2 L of ethyl acetate twice and concentrated under reduced pressure using a vacuum rotary evaporator at a water bath temperature of 50°C. The combined crude sample (21 g) was stored in a desiccator and was used for the next step of column chromatography and HSCCC enrichment.
2.4 SGCC combined with prep-HPLC separation procedure
Silica gel of 140 g was packed into a glass column (4 cm × 40 cm), and the crude sample (12 g) was taken and thoroughly mixed with an equal quantity of silica gel. Afterward, the crude sample (Section 2.3) was dried and loaded onto the silica gel column with the elution system of petroleum ether and ethyl acetate (5:1–1:2, v/v). Each fraction was analyzed via TLC on silica gel GF254 plate with chloroform/acetone/formic acid (8:4:1, v/v/v) as the unfolding solvent, and the ultraviolet light at 254 nm was observed. Eventually, a total of fractions (1–4) were obtained, and fraction 4 was collected from the eluting solvent of petroleum ether and ethyl acetate (1:2, v/v).
Fraction 4 was further separated by prep-HPLC to obtain compounds 1, 2, and 4 (Figure 1). Prep-HPLC separations were performed on a Shimadzu shim-pack C18 column (20 mm × 250 mm, 15 μm) using an isocratic elution program at a flow rate of 10 mL/min. The mobile phase, a solution of acetonitrile (A)/water (B) (12:88, v/v), was monitored at 285 nm. In addition, the sample concentration and the injection volume were 200 mg/mL and 300 μL, respectively. The peak fractions were collected according to the chromatogram, and the purity of compounds was analyzed by HPLC.
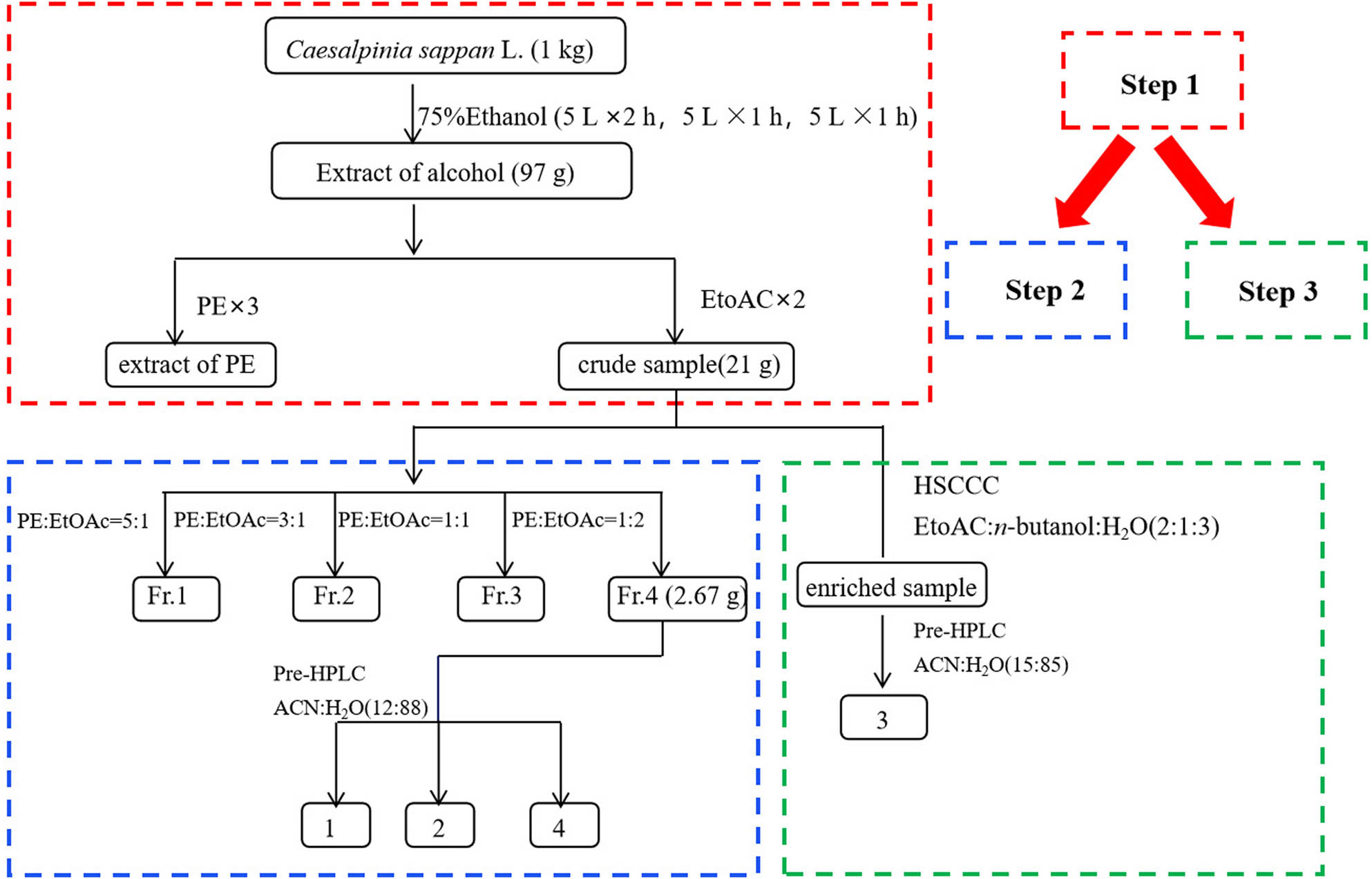
Flow chart of the separation of C. sappan L.
2.5 HSCCC combined with prep-HPLC separation procedure
The crude sample (1 g) from ethyl acetate extraction was dissolved in the mixture of mobile phase and stationary phase (ethyl acetate/n-butanol/water = 2:1:3, v/v, 10 mL) for the HSCCC separation. The entire column was filled with the stationary phase at a flow rate of 10 mL/min, and the mobile phase was pumped at a flow rate of 2.0 mL/min. The temperature of the thermostat was set to 25°C. When the upper and lower phase systems reached equilibrium, the eluate was monitored at 214 nm. At last, the mixtures of the stationary phase were pushed out of the column by compressed air, and the upper layer of the mixed solution was collected and evaporated under reduced pressure to afford the enriched sample (0.45 g).
Subsequently, the enriched sample was also further separated by prep-HPLC to obtain compound 3. The elution gradient was as follows: 15–15% A (v/v) in 0–30 min and 15–30% A (v/v) in 30–60 min. The graphical flow chart is shown in Figure 1.
2.6 HPLC fingerprint analysis
Chromatograms were captured on an Agilent 1290 series HPLC system. The mobile phase consisted of phase A (acetonitrile) and phase B (water). A gradient program was used according to the following profile: 0–15 min, 15–15% A (v/v), 15–30 min, 15–30% A (v/v), and 30–40 min, 30–100% A (v/v) with the flow rate of 1.0 mL/min, and the detection wavelength was set at 285 nm. In addition, the injection volume was 10 μL.
3 Results and discussion
3.1 Discovery of the unstable components from C. sappan L.
The traditional usage of C. sappan L. was commonly using water extraction. During the preparation of the aqueous extract of C. sappan L., a noticeable difference in the relative content of characteristic peaks was observed in the fingerprint after decocting for different times. Five characteristic peaks were selected from the fingerprint, and four characteristic peaks were found to be unstable, as illustrated in Figure 2.

HPLC fingerprint of C. sappan L. at different decoction time.
The relative content of characteristic peaks at various decoction times (20, 40, 60, 80, 120, 140, 160, and 180 min) is shown in Figure 3. The temperature of the decoction process was 120–150°C. It should be noted that the relative content was calculated as a percentage of the total chromatographic peaks from the target peaks. The results indicated that the relative content of peak 1 and peak 3 decreased from 11.05 to 4.91% (0–180 min) and 12.00 to 4.03%, respectively, while the relative content of peak 2 increased from 19.45 to 24.89%. The pH of the extracting solution was also measured at various decoction times, and its value was 5 before heating, while its value decreased to 4 after heating. These findings might indicate a potential transformation relationship between peaks 1–3 with extended heating time in acidic solutions. The obtained findings should nevertheless be helpful when C. sappan L. was used as herbal medicine, and we would like to investigate these unstable components further. As a result, isolation and identification of unstable components from C. sappan L. were essential for controlling the preparation process and ensuring the integrity of the QC assessment system of C. sappan L.
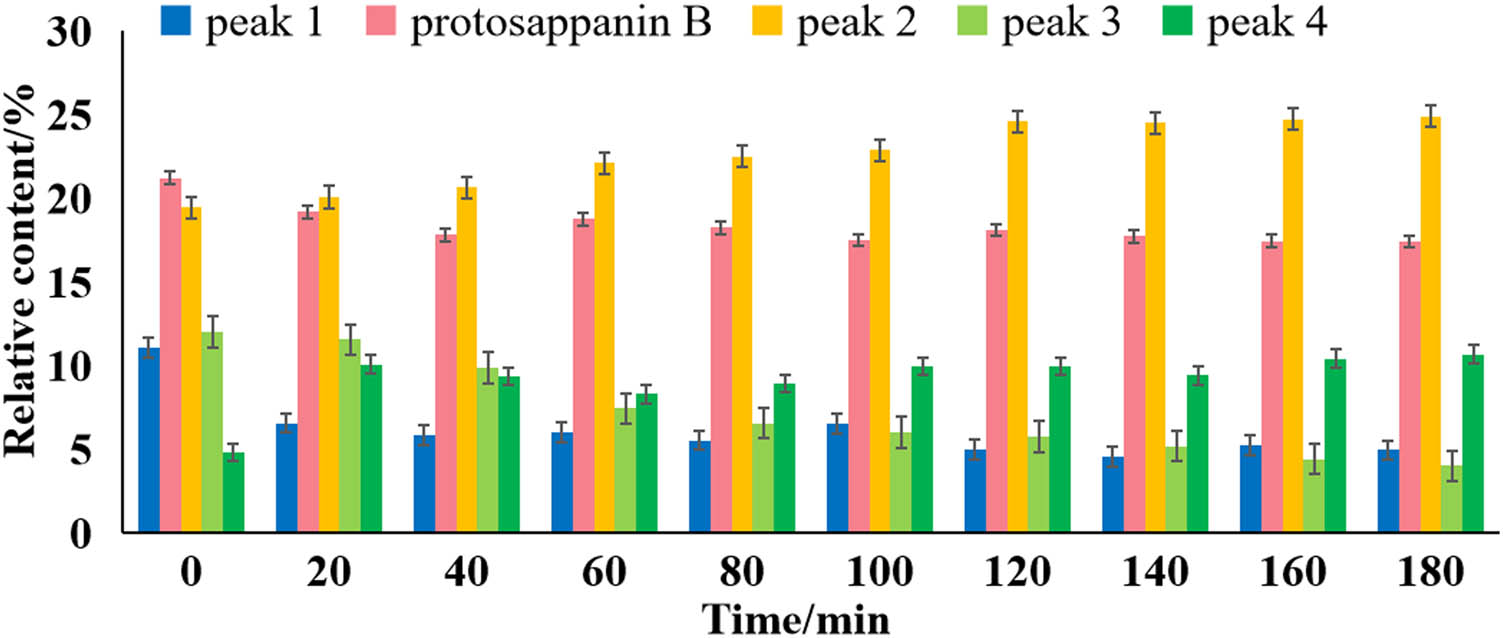
The relative content of characteristic peaks at different decoction time.
3.2 Separation of the characteristic compounds by SGCC combined with prep-HPLC
In order to separate unstable components of C. sappan L., the SGCC combined with prep-HPLC was used. The binary solvent system of petroleum ether and ethyl acetate was finally chosen by optimizing the elution condition. One column volume was eluted with petroleum ether and ethyl acetate (5:1, v/v) and a gradient of petroleum ether and ethyl acetate (3:1, v/v). The column was successively eluted by increasing the polarity of mixtures of petroleum ether/ethyl acetate, and each fraction was checked using TLC. Fractions 1–4 were obtained by SGCC. Nevertheless, spots on the TLC plate were observed when the gradient was changed to ether and ethyl acetate (1:2, v/v) and washed at 250 mL. Fractions with similar TLC patterns were combined to give fraction 4 (2.67 g) and identified by HPLC.
3.3 Separation of the unstable compounds by HSCCC
Peak 3 could not be obtained by column chromatography due to significant loss during the silica gel column separation. Therefore, we used HSCCC as a sample pretreatment methodology to improve peak 3 enrichment. In addition, some experiments were performed to optimize the biphasic solvent system for HSCCC. In the chosen solvent system, the solubility of the crude sample in the stationary and mobile phases differed significantly. The number of peaks in the two phases differed considerably, thus favoring the enrichment of peak 3. K-values that were not in the range of 0.5–2.0 were chosen, and the enrichment of peak 3 was achieved. Finally, the biphasic solvent system of ethyl acetate/n-butanol/water (2:1:3, v/v) was chosen as the elution system.
In addition, a single injection volume up to the gram level was used in this experiment, eliminating the need to repeat collection of fractions. At first, 200 mg of crude sample were dissolved in a 10 mL two-phase solvent system by HSCCC analysis for 300 min. It was found that the upper phase of the nitrogen-blown fraction under this system contained peak 3 of 16%, which was suitable for further separation by prep-HPLC. So we considered the nitrogen-blown fraction as the next step of separation sample, and 1 g was finally chosen as the sample size. The upper solution of the nitrogen-blown push-out fraction (57 mL) was collected after the crude sample was analyzed for 350 min by HSCCC, and the mixture of peak 2 and peak 3 was obtained with a relatively large proportion of both components after enrichment. From the HPLC chromatogram shown in Figure 4a and b, we knew this method effectively increased the percentage content of peak 2 from 18 to 34% and peak 3 from 7 to 15% (area normalization method). An enriched sample (0.45 g) was also obtained in a single pass. Eventually, ethyl acetate/n-butanol/water (2:1:3, v/v) was chosen as a solvent system for enriching peak 3. The stationary phase retention of ethyl acetate/n-butanol/water (2:1:3, v/v) was 30%.

HPLC analysis of C. sappan L. HPLC conditions are presented in Section 2.6. Peak 1 is episappanol, peak 2 is brazilin, peak 3 is sappanol, and peak 4 is sappanol 4-O-methylsappanol. (a) Ethyl acetate extract sample of C. sappan L.; (b) enriched sample of HSCCC (the same below); (c) episappanol separated from SGCC (the same below) combined with prep-HPLC (the same below); (d) brazilin separated from SGCC combined with prep-HPLC; (e) sappanol separated from HSCCC combined with prep-HPLC; and (f) 4-O-methylsappanol separated from SGCC combined with prep-HPLC.
3.4 Identification of the unstable compounds
Ethyl acetate extract of C. sappan L. was subjected to column chromatography and HSCCC for enrichment. And then, fraction 4 and enriched sample were further separated by prep-HPLC to obtain characteristic peaks 1–4. The results showed that four unstable compounds were collected with the purity higher than 95.0% (Figure 4c–f). The structures of the unstable compounds were identified as episapponal, brazilin, sapponal, and 4-O-methylsapponal by comparing the 1H NMR and 13C NMR data with literature values, as reflected in Figure S1.
Episappanol (1): 1 H NMR (400 MHz, DMSO-d 6): δ 6.97 (1H, d, J = 8.3 Hz, H-5′), 6.72 (1H, s, H-2′), 6.61 (1H, d, J = 8.3 Hz, H-5′), 2 6.53 (1H, d, J = 8.3 Hz, H-6′), 6.28 (1H, d, J = 8.3 Hz, H-6), 6.13 (1H, s, H-8), 3.98 (1H, s, H-4), 3.88 (1H, d, J = 10.4 Hz, Hb-2), 3.60 (1H, d, J = 10.4 Hz, Ha-2), 2.69 (1H, d, J = 13.7 Hz, Hb-9), and 2.49 (1H, d, J = 13.7 Hz, Ha-9). 13 C NMR (100 MHz, DMSO-d 6): δ 29.5 (C-9), 68.3 (C-2), 68.8 (C-3), 70.4 (C-4), 102.2 (C-8), 108.2 (C-6), 115.3 (C-5′), 116.6 (C-2′), 118.9 (C-4a), 122.1 (C-6′), 128.1 (C-5), 130.1 (C-1′), 143.8 (C-4′), 144.8 (C-3′), 154.7 (C-7), and 158.0 (C-8a). Peak 1 was identified as episappanol [21] based on the reported data.
Brazilin (2): 1 H NMR (400 MHz, DMSO-d 6): δ 7.14 (1H, d, J = 8.3 Hz, H-5), 6.62 (1H, s, H-5′), 6.53 (1H, s, H-2′), 6.40 (1H, d, J = 8.3 Hz, H-6), 6.20 (1H, br s, H-8), 3.84 (1H, s, H-4), 3.84 (1H, d, J = 11.3 Hz, Hb-2), 3.56 (1H, d, J = 11.3 Hz, Ha-2), 2.87 (1H, d, J = 15.6 Hz, Hb-9), and 2.69 (1H, d, J = 15.6 Hz, Ha-9). 13 C NMR (101 MHz, DMSO-d 6): δ 156.96 (C-7), 154.51 (C-8a), 144.75 (C-3′), 144.41 (C-4′), 136.01 (C-6′), 131.38 (C-5), 130.21 (C-1′), 114.79 (C-4a), 112.51 (C-2′), 112.12 (C-5′), 109.17 (C-6), 103.23 (C-8), 76.78 (C-3), 70.06 (C-2), 49.8 (C-4), and 42.40 (C-9). Peak 2 was identified as brazilin [21] based on the reported data.
Sappanol (3): 1 H NMR (400 MHz, DMSO-d 6): δ 10.87 (s, 1H, H-7), 10.18 (s, 1H, H-4′), 10.12 (s, 1H, OH), 8.48 (d, J = 8.3 Hz, 1H, H-5), 8.11-8.02 (m, 2H, H-6, H-8), 8.03 (s, 1H, H-2′), 7.80 (dd, J = 14.8, 2.1 Hz, 1H, H-5′), 7.80 (s, 1H, H-6′), 7.62 (d, J = 2.3 Hz, 1H, OH), 5.77 (s, 1H, H-2), 5.53 (d, J = 5.2 Hz, 1H, H-4), 5.21 (d, J = 10.5 Hz, 1H, OH), and 3.91 (s, 2H, H-9). 13 C NMR (101 MHz, DMSO-d 6): δ 158.1 (C-7), 154.2 (C-8a), 154.2 (C-8a), 143.6 (C-4′), 131.5 (C-5), 127.6 (C-1′), 121.4 (C-6′), 118.1 (C-5′), 115.7 (C-2′), 114.3 (C-4a), 108.4 (C-6), 102.0 (C-8), 69.1 (C-4), 67.8 (C-3), and 67.1 (C-2) (C-9 was hidden by solvent [22]). Peak 3 was identified as sappanol [22] based on the reported data.
4-O-Methysapponal (4): 1 H NMR (400 MHz, DMSO-d 6): δ 8.66 (s, 2H, OH, OH), 7.04 (d, J = 8.3 Hz, 1H, H-5), 6.60 (d, J = 7.5 Hz, 3H, H-6, H-8, H-2′), 6.36 (t, J = 7.7 Hz, 2H, H-5, H-6′), 6.17 (s, 1H, OH), 5.20 (s, 1H, OH), 4.29-4.08 (s, 2H, H-2), 3.76 (d, J = 10.5 Hz, 1H, H-4), 3.54-3.17 (s, 2H, H-9), and 2.53-2.47 (m, 3H, OMe). 13 C NMR (100 MHz, DMSO-d 6): δ 24.1 (C-9), 49.1 (OMe), 67.4 (C-2), 68.2 (C-3), 69.5 (C-4), 102.4 (C-8), 108.8 (C-6), 115.4 (C-5′), 116.0 (C-2′), 118.5 (C-4a), 121.7 (C-6′), 127.9 (C-5), 131.9 (C-1′), 144.1 (C-4′), 144.9 (C-3′), 154.3 (C-7), and 158.4 (C-8a). Peak 4 was identified as 4-O-methylsapponal [22] based on the reported data.
3.5 Interconversion between unstable components
According to the structural properties of the isolated compounds, it was determined that all four characteristic peaks were homoisoflavones. Peaks 1 and 3 belonged to the homoisoflavones(iv), peak 2 was homoisoflavones(viii), and peak 4 was homoisoflavones [11].
Based on the similarity of their structures, there were transformation relationships between peaks 1–4. The possible conversion pathways are shown in Figure S2, which demonstrated that episapponal (peak 1), sapponal (peak 3), and 4-O-methylsapponal (peak 4) would convert to brazilin (peak 2) [23]. We discovered that episapponal (peak 1) and sapponal (peak 3) were unstable in acidic circumstances, according to Mueller et al. [2]. As a result, we hypothesized that the reduction in pH after prolonged heating would enhance the conversion of these two compounds to brazilin. Our findings perhaps explained the phenomenon observed in Section 3.1. Furthermore, due to the structural properties of the chemical components involved, the heating time in the preparation of C. sappan L. should be controlled within 1 h. The analysis of unstable components by HPLC fingerprint demonstrated that content was not the only indicator for assessing the preparation process. Furthermore, the pharmacological actions of TCM were thought to result from the synergistic effects of multiple ingredients rather than a single component. And the characteristic compounds obtained in isolation were the material basis of the pharmacological effects of C. sappan L., among which episappanol and sappanol have been shown to have anti-inflammatory effects [2]. 4-O-Methylsappanol has been reported to possess multiple biological activities, such as antifungal activity [24], intense melanin inhibitory activity [9], and neuroprotective effects [25]. Brazilin also performed abundant pharmacological activities, including anti-inflammatory [26] and anti-bacterial [26]. In summary, these characteristic peaks made different contributions to the pharmacological activities of C. sappan L. Based on the above discussions, an appropriate analytical method was developed. The HPLC fingerprint of C. sappan L. is shown in Figure S3, and five characteristic peaks were identified for HPLC fingerprint of C. sappan L.
4 Conclusion
During the optimization of the decoction time of C. sappan L., four peaks were discovered to be unstable. Therefore, four characteristic peaks were separated and identified as episapponal, brazilin, sapponal, and 4-O-methylsapponal by NMR. Episapponal, brazilin, and 4-O-methylsapponal were obtained from column chromatography combined with prep-HPLC, and sappanol was obtained from HSCCC combined with prep-HPLC. Moreover, episapponal, sappanol, and 4-O-methylsapponal might transform to brazilin after prolonged heating. These results were consistent with the phenomenon mentioned above. These findings provided a useful theoretical foundation for improving the quality of C. sappan L., and specifically for targeting control of production processes of C. sappan L.
-
Funding information: This research was supported by the Basic Public Welfare Research Project of Zhejiang Province (Grant No. LBY22H280003) and Zhejiang Provincial Department of Science and Technology Project (Grant No. 2022C03062).
-
Author contributions: Yameng Wu – conceptualization, methodology, writing – original draft; Jianhui Xie – investigation, methodology; Jielin Zeng – data curation, validation; Rui Bai – data curation, supervision; Hui Zhang – writing – review and editing; and Jizhong Yan – writing – review and editing.
-
Conflict of interest: The authors declare no conflicts of interest.
-
Ethical approval: This conducted research is not related to either human or animal use.
-
Data availability statement: This work contains all of the data that were collected or analyzed throughout the research.
References
[1] Chinese Pharmacopoeia Committee. Pharmacopeia of the people’s Republic of China. Beijing: China Medical Science and Technology Press; 2020; p. 171–2.Suche in Google Scholar
[2] Mueller M, Weinmann D, Toegel S, Holzer W, Unger FM, Viernstein H. Compounds from Caesalpinia sappan with anti-inflammatory properties in macrophages and chondrocytes [J]. Food Funct. 2016;7(3):24–32. 10.1039/C5FO01256B.Suche in Google Scholar
[3] Hemthanon T, Ungcharoenwiwat P. Antibacterial activity, stability, and hemolytic activity of heartwood extract from Caesalpinia sappan for application on nonwoven fabric. Electron J Biotechn. 2022;55:9–17. 10.1016/J.EJBT.2021.10.002.Suche in Google Scholar
[4] Batubara I, Mitsunaga T, Ohashi H. Brazilin from Caesalpinia sappan wood as an antiacne agent. J Wood Sci. 2010;56(1):77–81. 10.1007/s10086-009-1046-0.Suche in Google Scholar
[5] Won HS, Lee J, Khil LY, Chae SH, Ahn MY, Lee BH, et al. Mechanism of action of brazilin on gluconeogenesis in isolated rat hepatocytes. Planta Med. 2004;70(8):740–4. 10.1055/s-2004-827205.Suche in Google Scholar
[6] Bae IK, Min HY, Han AR, Seo EK. Suppression of lipopolysaccharide induced expression of inducible nitric oxide synthase by brazilin in RAW 264.7 macrophage cells. Eur J Pharmacol. 2005;513(3):237–42. 10.1016/j.ejphar.2005.03.011.Suche in Google Scholar
[7] Chien MH, Jaw JK, Chen CL, Ching HL, Jiunn WL, Yu WC. Induction of vasorelaxation through activation of nitric oxide synthase in endothelial cells by brazilin. Eur J Pharmacol. 2003;468(1):37–45. 10.1016/S0014-2999(03)01639-X.Suche in Google Scholar
[8] Nguyen VB, Duong Vu B, Pham GK, Quang Le B, Nguyen VC, Men CV, et al. Phenolic compounds from Caesalpinia sappan. Pharmcogn Mag. 2020;12(2):410–4. 10.5530/pj.2020.12.63.Suche in Google Scholar
[9] Mitani K, Takano F, Kawabata T, Allam AE, Ota M, Takahashi T, et al. Suppression of melanin synthesis by the phenolic constituents of sappanwood (Caesalpinia sappan). Planta Med. 2013;79(1):37–44. 10.1055/s-0032-1327897.Suche in Google Scholar PubMed
[10] Niu Y, Wang S, Li C, Wang J, Kang W. Effective compounds from Caesalpinia sappan L. on the tyrosinase in vitro and in vivo. Nat Prod Commun. 2020;15(4):1–8. 10.1177/1934578X20920055.Suche in Google Scholar
[11] Mottaghipisheh J, Stuppner H. A comprehensive review on chemotaxonomic and phytochemical aspects of homoisoflavonoids, as rare flavonoid derivatives. Int J Mol Sci. 2021;22(5):2735–54. 10.3390/ijms22052735.Suche in Google Scholar PubMed PubMed Central
[12] Latif Z, Sarker SD. Isolation of natural products by preparative high performance liquid chromatography (prep-HPLC). Methods Mol. 2012;864:255–74. 1007/978-1-61779-624-1_10.Suche in Google Scholar
[13] Surana K, Bhattacharya B, Majumder S. Extraction of yellow fluorescent Caesalpinia sappan L. dye for photovoltaic application. Opt Mater. 2021;119:111347–53. 10.1016/J.OPTMAT.2021.111347.Suche in Google Scholar
[14] Zhang GY, Chi XF. A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water. Open Chem. 2020;18(1):702–10. 10.1515/chem-2020-0079.Suche in Google Scholar
[15] Zhu H, Chen L, Yu J, Cui L, Ali I, Song X, et al. Flavonoid epimers from custard apple leaves, a rapid screening and separation by HSCCC and their antioxidant and hypoglycaemic activities evaluation. Sci Rep. 2020;10(1):8819–29. 10.1038/s41598-020-65769-5.Suche in Google Scholar PubMed PubMed Central
[16] Cai X, Xiao M, Zou X, Tang J, Xue H. Extraction and separation of flavonoids from Malus hupehensis using high-speed countercurrent chromatography based on deep eutectic solvent. J Chromatogr A. 2021;1641(2021):461998–2005. 10.1016/j.chroma.2021.461998.Suche in Google Scholar PubMed
[17] Peng ST, Liu ZL, Song ZQ, Wang C, Liang DR, Wan XY, et al. Application of high-speed counter-current chromatography in traditional Chinese medicine and natural products research. J Tradit Chin Med. 2021;27:821–9. 10.19945/j.cnki.issn.1006-3250.2021.05.028.Suche in Google Scholar
[18] Yao S, Cao Y, Jia CM, Wang Y, Song H. Developments of instruments and methods related with high-speed countercurrent chromatography and their applications in research of natural medicines. Open Chem. 2011;10(3):417–32. 10.2478/s11532-011-0141-4.Suche in Google Scholar
[19] Uddin GM, Kim CY, Chung D, Kim KA, Jung SH. One-step isolation of sappanol and brazilin from Caesalpinia sappan and their effects on oxidative stress-induced retinal death. BMB Rep. 2015;48(5):289–94. 10.5483/BMBRep.2015.48.5.189.Suche in Google Scholar PubMed PubMed Central
[20] Xu PP, Guan SH, Feng RH, Tang RD, Guo D. Separation of four homoisoflavonoids from Caesalpinia sappan by high-speed counter-current chromatography. Phytochem Anal. 2012;23(3):228–31. 10.1002/pca.1347.Suche in Google Scholar PubMed
[21] Fu LC, Huang XA, Lai ZY, Hu YJ, Liu HJ, Cai XL. A new 3-benzylchroman derivative from Sappan Lignum (Caesalpinia sappan). Molecules. 2008;13(8):1923–30. 10.3390/molecules13081923.Suche in Google Scholar PubMed PubMed Central
[22] Michio N, Hiroyuki N, Marko N, Takashi O, Tamotsu S. Homoisoflavonoids and related compounds. III. Phenolic constituents of Caesalpinia japonica Sieb. et Zucc. Chem Pharm Bull. 1987;35(9):3568–75. 10.1248/cpb.35.3568.Suche in Google Scholar
[23] Tamotsu S, Shigemi S, Hiroyuki N, Takashi S, Jun-Ei K, Johji Y, et al. 3-Benzylchroman derivatives related to brazilin from Sappan Lignum. Chem Pharm Bull. 1986;34(6):2506–11. 10.1248/cpb.34.2506.Suche in Google Scholar
[24] Reddy V, Ravikanth V, Lakshmi V, Murty US, Venkateswarlu Y. Inhibitory activity of homoisoflavonoids from Caesalpinia sappan against Beauveria bassiana. Fitoterapia. 2003;74(6):600–2. 10.1016/S0367-326X(03)00153-9.Suche in Google Scholar
[25] Gil ST, Dong SL, Tae OK, Hye SL, Ren BA, Youn CK. Cytoprotective constituents of the heartwood of Caesalpinia sappan on glutamate-induced oxidative damage in HT22 cells. Biol Pharm Bull. 2009;32(5):945–9. 10.1248/bpb.32.945.Suche in Google Scholar PubMed
[26] Nilesh P, Nirmal P. Antioxidant, antibacterial, and anti-inflammatory activities of standardized brazilin-rich Caesalpinia sappan extract. Pharm Biol. 2015;53(9):1339–43. 10.3109/13880209.2014.982295.Suche in Google Scholar PubMed
© 2022 Yameng Wu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop
Artikel in diesem Heft
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop

