Abstract
Though ionic liquids (ILs) are considered potential materials for CO2 capture because of their unique properties, it is time-consuming and costly to choose task-specific and suitable IL using the traditional “try-and-error” method. From the point of molecular design view, 25 cations and 20 anions are combined and screened using COSMOtherm to predict CO2 solubility at 298 K and 100 kPa. The prediction result showed that ILs with bFAP(tris(nonafluorobutyl)trifluorophosphate) anion could dissolve more CO2 than any others. To further understand the absorption performance of CO2 in ILs, molecular dynamic simulations are carried out to explore the interactions between CO2 and the four selected ILs, namely, [EMPyr][bFAP](1-ethyl-2-methylpyrazolium tris(nonafluorobutyl)trifluorophosphate), [B(Hex)3P][bFAP](butyl-trihexyl-phosphonium tris(nonafluorobutyl) trif-luorobutyl trifluorophosphate), [(Me)5isobuGua][bFAP](n,n,n,n,n-pentamethyl-n-isopropylguanidinium tris(nona-fluorobutyl)-trifluorophosphate), and [BEIM][bFAP] (1-butyl-3-ethyl-imidazolium tris(nonafluorobutyl)trifluo-rophosphate), at the atomic and molecular levels.
1 Introduction
Human emissions of carbon dioxide are continuously increasing and have been a primary driver of global climate change [1,2]. The Intergovernmental Panel on Climate Change (IPCC) predicted that the global surface temperature change at the end of the twenty-first century would likely exceed 1.5°C [3]. It is a vast and urgent challenge for human beings to cope with global warming [4]. Consequently, CO2 capture and storage/utilization (CCS/CCU) have been widely accepted as an effective way to reduce greenhouse gas emissions. New materials such as ionic liquids (ILs) have been researched and developed for cost-effective CO2 capture and utilization. Except for its own unique physical characteristics, the properties of ILs can also be adjusted and designed for specific applications. Since Blanchard et al. [5] first reported that CO2 has high solubility in [BMIM][BF6] in 1999, a quite large number of studies have been focused on the application of the possibility of ILs in CO2 absorption processes [6,7,8,9,10]. Aki et al. studied the effects of the alkyl chain length from butyl to octyl on CO2 solubility [11]. The result showed that the longer the chain length was, the more soluble it was. Pankaj et al. [12,13] studied the role of anions in ILs and found ILs containing different anions had different CO2 solubility. The mechanism of CO2 solubility in ILs is generally categorized into physical absorption and chemical absorption. Some functionalized ILs are capable of chemically binding to CO2 where covalent bonds are formed during CO2 absorption in ILs [14,15]. The two types of absorptions have their own advantages and disadvantages. Compared with chemical absorption, physical absorption has the advantage of less energy required for desorption [16]. Whether ILs are classified into physical or chemical absorption, as a potential alternative for CO2 capture, the possible number of ILs was theoretically huge up to 1018. Obviously, it was time-consuming and costly to develop novel ILs used for specific applications from the vast sea of ILs by the traditional “try and error” method. The Conductor-like Screening Model for Real Solvents (COSMO-RS) used to predict the thermodynamic properties of ILs has been successfully proven to be effective in many literature [17,18,19,20,21]. It combined statistical thermodynamics and quantum chemistry and can be used as a valuable tool to screen molecules for a specific application based on the molecular structure.
The aim of this work is to systematically develop a way by using COSMOtherm to quickly screen 500 different ILs, which are combined by 25 cations and 20 anions, and get CO2 solubility in ILs. Molecular dynamic (MD) simulation had been proved to be a powerful tool in studying and predicting the physical properties of IL-related systems [22,23,24]. It can provide a fundamental relationship between microscopic and macroscopic properties. To further have a deep understanding of the structural properties and the interactions of ILs and CO2, simulation was also carried out in this work. To achieve the goal, we investigate the solvation energy and transport properties of CO2 in four different ILs which shared a common anion based on the screened results. It is hoped that our study could help provide a better understanding of the CO2 absorption mechanism by ILs and facilitate the novel ILs design for CO2 absorption.
2 Methods
2.1 Screened ILs and COSMO-RS calculations
ILs can theoretically be designed by combining different anions and cations; 25 cations and 20 anions are selected to combine 500 IL solvents. Their structures and names are listed in Tables S1 and S2 of the supporting information. The cations include pyrazolium, phosphonium, ammonium, imidazolium, pyridinium, pyrrolidinium, guanidinium, and piperidinium. The anions include acetate, phosphate, sulfate, and dicyanamide. COSMOtherm was used to predict the solubility of CO2 in the above-mentioned ILs. The standard operating procedure of COSMOtherm contains two steps. First, quantum calculation of the involved molecule species is performed by Gaussian or Turbmole suit program. Second, statistical thermodynamics calculation is carried out within COSMOtherm. All screened IL structures could be seen as the combination of ion pairs, namely, cation and anion. The structures of ion pairs, which had been early optimized using four different theoretical levels and incorporated into the program, were directly loaded from the database. As suggested in the study by ref. [25], a molecular model of counter ions was applied in COSMOtherm calculations. ILs are treated as equimolar mixtures of cations and anions. The parameterization (BP_TZVPD_FINE_20.ctd) contains the intrinsic parameters for the calculation and is used. The σ-profile, which contains the screening charges on the molecular surface, was used as an input in the COSMOtherm to calculate the solubility of CO2 in ILs at near ambient pressure and temperature.
2.2 Molecular dynamics simulations
MD simulations were carried out using GROMACS 2018.2 program package [26]. The force field, General AMBER Force Field (GAFF), was used in the MD simulations. Each anion or cation in ILs was geometrically optimized at B3LYP/6-311 + G(d,p) in Gaussian 16 package [27]. The atom RESP charges of ILs were calculated using Multiwfn 3.7 program [28,29]. The bonded and non-bonded parameters used in the simulation are obtained by ACPYPE online server [30]. A leap-frog algorithm was used to integrate Newton’s equations of motion, and the time step of 2fs was adopted in all the simulations. The temperature was controlled by a velocity rescaling thermostat with a coupling constant of 0.4 ps [31]. The pressure was maintained by Parrinello–Rahman barostat with a coupling constant of 2.0 ps [32]. The periodic boundary conditions were applied in all directions. The bonds were constrained with the LINCS algorithm [33]. The cut-off for the short-range electrostatics and Lennard–Jones interactions were both set to 1.0 nm, and the particle mesh Ewald algorithm [34] was used to deal with the long-range electrostatic interactions. For each system, energy minimization of the simulation system was first performed with the steepest descent algorithm to generate an appropriate starting configuration and then equilibrated for 1 ns at isothermal and fixed volume (NVT) ensemble to reach the reference temperature of 298.15 K and the reference pressure of 1 bar, respectively. Then, 20 ns trajectory was produced at the isothermal and isobaric (NPT) ensemble, and the last 10 ns trajectory was used for statistical analysis.
Free energy of CO2 in pure water and ILs was calculated using the BAR (Bennett Acceptance Ratio) method with 21 equidistant sets of λ values from λ = 0 to λ = 1. TIP4P water model was used. Coordinates, velocities, and δH/δλ were saved to disk at every ten steps for the post-processing of BAR. Each node was subjected to energy minimization with 20,000 steps of the steepest descent method. The simulation was then pre-equilibrated at NVT ensemble with no position restrains. After NVT equilibration, the process was continued with 2 ns simulations at NPT at 298 K for all systems. Data collection was done from the last 1 ns.
3 Solubility data and simulation models
3.1 CO2 solubility in ILs
The solubility of CO2 in 500 ILs is calculated at 298 K and 100 kPa using the method mentioned above, and the results are tabulated in Table S3. Figure 1 shows the trend and difference of CO2 solubility in the screened ILs.
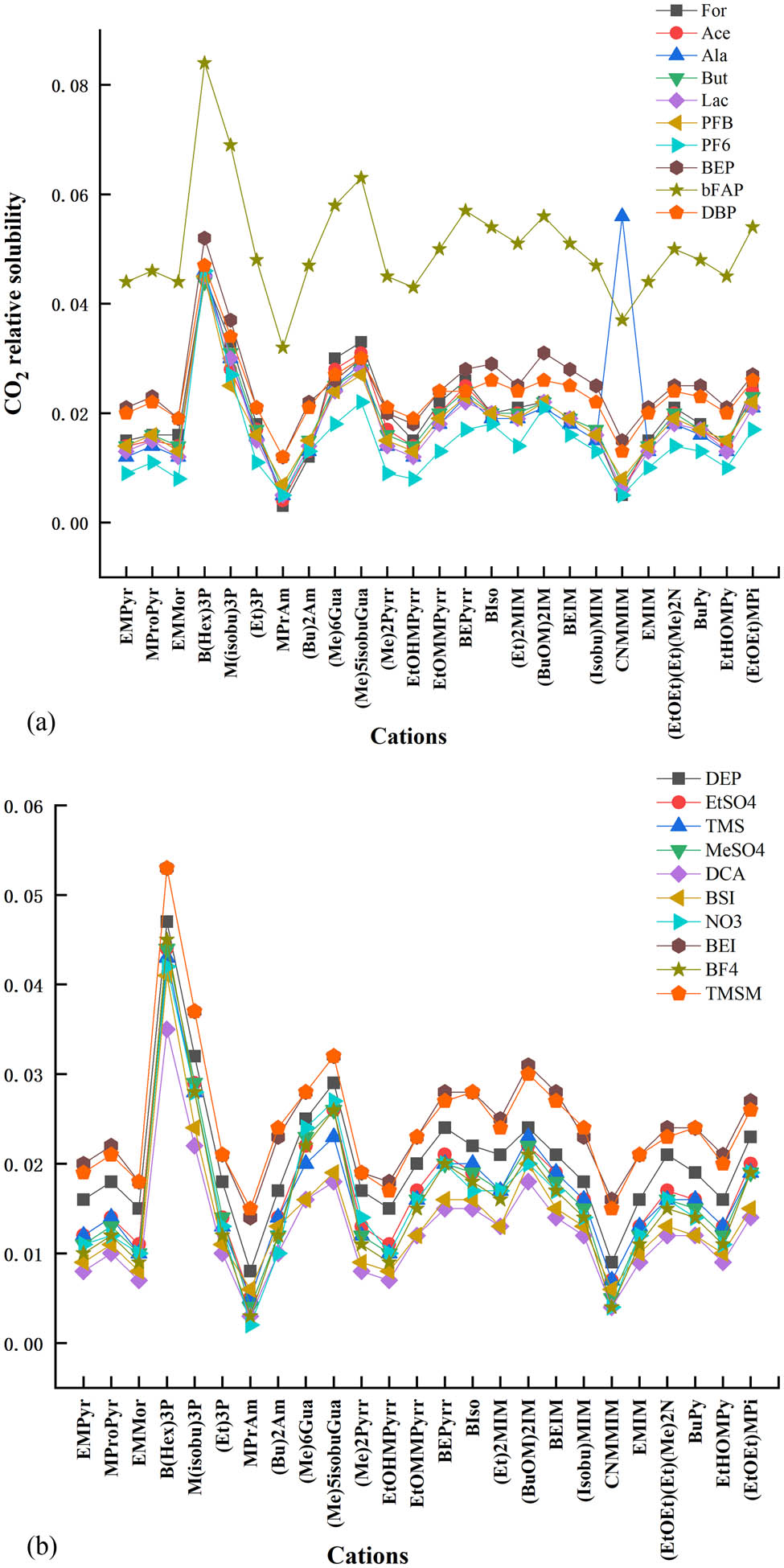
CO2 relative solubility in ILs calculated by COSMOtherm.
The previous studies have investigated cation and anion effects on CO2 solubility [35,36,37]. The combination of cation and anion can affect CO2 solubility. Generally, CO2 solubility in ILs strongly depends on the choice of the anion. Aki et al. also studied the influence of the anion with the same 1-butyl-3-methylimidazolium cation [11]. They proved that the fluorination of the anion can improve CO2 solubility. The trend of CO2 solubility in ILs predicted by COSMOtherm is consistent with the previous work. It was concluded from Figure 1 that ILs with B(Hex)3P cation have much higher CO2 solubility than any other ILs, and CO2 solubility in ILs with bFAP anion was also much higher than any other ILs. CO2 solubility in various ILs is much different. Among the 500 screened ILs, the highest CO2 solubility is 0.084 mole fraction in [B(Hex)3P][bFAP], while the lowest CO2 solubility is 0.002 mole fraction in [MPrAm][NO3] at 298 K and 100 kPa.
The previous experiments explored ILs containing nitrile anion that had a higher CO2 solubility [38,39]. Their work showed that CO2 solubility in [emim][B(CN)4] (1-ethyl-3-methyl imidazolium tetracyanoborate) is 0.027 mole fraction. Compared with that, ILs containing [bFAP] anion have much higher CO2 solubility.
3.2 MD simulations of ILs and ILs/CO2
A few of ILs were selected according to the CO2 solubility data. Specifically, bFAP anion was paired with four different cations, namely, [EMPyr](1-ethyl-2-methylpyrazolium), [B(Hex)3P](butyl-trihexyl-phosphonium), [(Me)5isobuGua](n,n,n,n,n-pentamethyl-n-isopropyl-guanidinium), and [BEIM](1-butyl-3-ethyl-imidazolium). Two kinds of MD simulations were performed. The first set was to examine CO2 solvation free energy in ILs. The second was to mimic the equilibrium and dynamic properties of pure ILs and the IL/CO2 system. For the calculation of CO2 solvation free energy in IL, 1 CO2 molecule was put into the center of the cubic box. For the pure IL simulation system, a cubic box of 8 nm × 8 nm × 8 nm was created with 256 pairs of cation and anion. For IL/CO2 system, the simulation box was constructed by 10 CO2 molecules and 256 ion pairs. The initial configurations of the MD simulations were built by the Packmol package [40].
4 Results and discussions
4.1 Henry constant and solvation free energy of CO2 in ILs
Henry constant, as another measure of CO2 solubility in ILs, could also be computed using COSMOtherm. The computed Henry constants of CO2 in pure water and the four selected ILs are listed in Table 1. Additionally, to understand and compare the CO2 absorption ability in ILs and water from the point of solvation free energy, the CO2 solvation free energy in pure water was also computed by the BAR method and COSMOtherm. The values of the solvation free energy of CO2 (abbreviated as ΔG) in pure water and four different ILs are listed in Table 1. The calculated results using the BAR method are 3.80 kJ mol−1 in pure water, −10.18 kJ mol−1 in [EMPyr][bFAP], −11.37 kJ mol−1 in [BEIM][bFAP], −12.21 kJ mol−1 in [(Me)5isobuGua][bFAP], and −10.43 kJ mol−1 in [B(Hex)3P][bFAP], respectively. Compared with the solvation free energy of CO2 in pure water, the negative values clearly showed that the CO2 molecule is more easily dissolved in IL than in water.
Henry constant and solvation free energy of CO2 in ILs
| Entry | ILs |
|
ΔG (kJ mol−1) | |
|---|---|---|---|---|
| BAR method | COSMOtherm | |||
| 1 | H2O | 342.6 | 3.80 ± 0.10 | 0.54 |
| 2 | [EMPyr][bFAP] | 1.74 | −10.18 ± 0.37 | −4.26 |
| 3 | [BEIM][bFAP] | 1.46 | −10.43 ± 0.34 | −4.44 |
| 4 | [(Me)5isobuGua][bFAP] | 1.16 | −11.37 ± 0.75 | −4.84 |
| 5 | [B(Hex)3P][bFAP] | 0.86 | −12.21 ± 0.08 | −4.86 |
Though the four ILs could dissolve CO2 more easily than water, it was concluded that there were different CO2 solubility capabilities among the four selected ILs. Though the values of ΔG obtained by COSMOtherm were much smaller than that obtained by the BAR method, they all showed the same trend. The solvation free energy of CO2 in studied ILs followed the increasing order: [EMPyr][bFAP] < [BEIM][bFAP] < [(Me)5isobuGua][bFAP] < [B(Hex)3P][bFAP], which agrees with the relative CO2 solubility predicted by COSMOtherm.
4.2 Structural properties
Some important structural properties of the simulated systems were investigated through MD simulations. The density, solvent-accessible area (SAS), and free volume (FV) of all simulated systems are summarized in Table 2.
Calculated density and other properties for the simulated systems
| System | Density (g cm−3) | SAS (m2 cm−3) | FV (%) | V (nm3 mol−1) |
|---|---|---|---|---|
| IL | ||||
| [BEIM][bFAP] | 1.752 | 45 | 35.99 | 109 |
| [B(Hex)3P][bFAP] | 1.431 | 81 | 38.03 | 162 |
| [EMPyr][bFAP] | 1.844 | 62 | 36.05 | 99 |
| [(Me)5isobuGua][bFAP] | 1.706 | 67 | 36.01 | 114 |
| IL/CO2 | ||||
| [BEIM][bFAP]/CO2 | 1.697 | 113 | ||
| [B(Hex)3P][bFAP]/CO2 | 1.357 | 165 | ||
| [EMPyr][bFAP]/CO2 | 1.774 | 103 | ||
| [(Me)5isobuGua][bFAP]/CO2 | 1.660 | 119 | ||
The maximum density corresponds to [EMPyr][bFAP] (1.844 g cm−3), and the minimum density corresponds to [B(Hex)3P][bFAP] (1.431 g cm−3). It was noticed that the density of the IL/CO2 binary system was a little smaller than that of the corresponding pure IL. FV within ILs was correlated to the gas solubility. CO2 molecules were preferentially solvated within the vicinity of the anions [41]. Figure 2 shows the representative vicinity within ILs. It was noticed that the four ILs with bFAP anion all had a larger FV of more than 35%. The larger the FV was, the more CO2 molecules were solvated. It was consistence with the CO2 solubility in the four ILs.
![Figure 2
Representative snapshot of the FV space (green) within bFAP-based ILs: (a) [BEIM][bFAP], (b) [B(Hex)3P][bFAP], (c) [EMPyr][bFAP], and (d)[(Me)5isobuGua][bFAP].](/document/doi/10.1515/chem-2022-0154/asset/graphic/j_chem-2022-0154_fig_002.jpg)
Representative snapshot of the FV space (green) within bFAP-based ILs: (a) [BEIM][bFAP], (b) [B(Hex)3P][bFAP], (c) [EMPyr][bFAP], and (d)[(Me)5isobuGua][bFAP].
4.3 Radial distribution function of ILs and ILs/CO2 system
To study the influence of CO2 absorption on the overall IL structure, the equilibrium structures of ILs and CO2 are presented by the radial distribution function g(r). The g(r) of the constituent compounds are calculated to study cation–anion, cation–cation, and anion–anion interactions in IL and shown from a to c in Figure 3. The g(r) of cation, anion, and CO2 in the ILs/CO2 system is also calculated and shown from (d) to (i) in Figure 3.
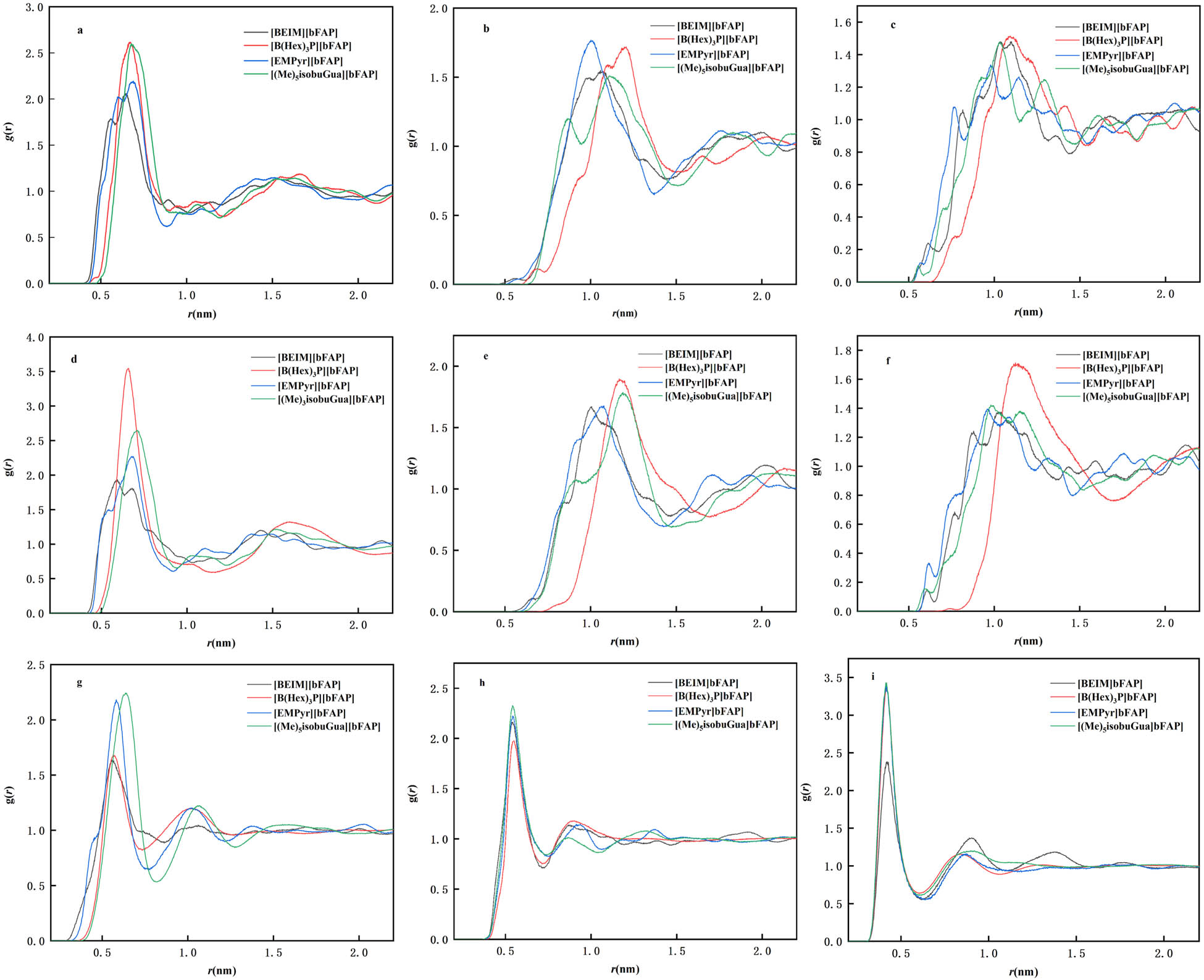
Radial distribution functions in ILs and IL/CO2 systems at 298 K and 100 kPa: (a) cation–anion in IL, (b) cation–cation in IL, (c) anion–anion in IL, (d) cation–anion in IL/CO2, (e) cation–cation in IL/CO2, (f) anion–anion in IL/CO2, (g) CO2–cation in IL/CO2, (h) CO2–anion in IL/CO2, and (i) CO2–CO2 in IL/CO2.
The g(r) provided deep insights into the interactions that occurred in the studied system. It was revealed from Figure 3(a–c) that cation–anion interaction of both ILs is stronger than those of cation–cation or anion–anion interactions. Figure 3a shows the interaction between the cation and anion in ILs. Similar observations were found that the weaker interaction between the cation and the anion affected the CO2 solubility in ILs [42]. Taking [B(hex)3][bFAP] as an example, the intense cation–anion g(r) peak appears at about 0.67 nm. The g(r) peak of cation–anion in [BEIM][bFAP] was about 0.56 nm, while in Figure 3b and c, the cation–cation and anion–anion g(r) of [B(hex)3][bFAP] peaks appear at 1.20 and 1.10 nm, respectively. It was clear in Figure 3d that the cation–anion g(r) shape in IL/CO2 systems did not change obviously compared with pure IL systems. It was consistent with the previous simulation studies which showed that the addition of CO2 molecules does not change the structures of ILs [36,43]. The interactions between CO2 and ILs ions are shown in Figure 3(g and h). The CO2–anion interactions in the four IL/systems all display g(r) peaks at 0.54 nm. It is obvious that CO2 molecules are interacting stronger with the anions as compared with the cations since the CO2–anion peak is located at a lower distance than that of the CO2–cation peak.
The electrostatic and the van der Waals energies between ions and CO2 interactions were also computed from the MD simulation systems and are listed in Table 3.
Electrostatic and the van der Waals interaction energies computed from MD simulation (kJ mol−1)
| System | Electrostatic interaction (Coulomb) | Van der Waals interaction (LJ) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [c]…[c] | [c]…[a] | [a]…[a] | [c]…CO2 | [a]…CO2 | [c]…[c] | [c]…[a] | [a]…[a] | [c]…CO2 | [a]…CO2 | |
| [BEIM][bFAP]/CO2 | 43651.3 | −95239.5 | 188,402 | −120.28 | −6.47 | −4389.09 | −29561.8 | 9993.66 | −105.28 | −266.25 |
| [B(Hex)3P][bFAP]/CO2 | 288,630 | −537,241 | 408,878 | −64.85 | −6.33 | −18377.8 | −43958.1 | 18228.7 | −171.65 | −189.98 |
| [EMPyr][bFAP]/CO2 | 292,123 | −614,795 | 449,418 | −125.76 | 9.85 | −1764.45 | −23068.1 | 7274.88 | −78.19 | −282.65 |
| [(Me)5isobuGua][bFAP]/CO2 | 346,539 | −761,467 | 521,422 | −81.92 | −3.97 | −6261.04 | −30233 | 10860.8 | −117.08 | −244.85 |
For IL and IL/CO2 systems, the total interaction energies of cation–cation and anion–anion mainly showed unfavorable interactions. As for cation–anion, it was favorable interaction and electrostatic interaction made more contributions to the total interaction than Van der Waals interaction. Electrostatic interaction of cation and CO2 was larger than that of anion and CO2. Van der Waal’s interaction of anion and CO2 was larger than that of cation and CO2. It showed that the interaction of cation and CO2 was mainly electrostatic interaction, while the interaction of anion and CO2 was mainly Van der Waals interaction.
4.4 Mean-squared displacement (MSD) and Diffusion coefficients
The dynamic properties of ILs and IL/CO2 systems are quantified using MSD and diffusivity. Figure 4(a–e) show the MSDs of cation, anion, and CO2 in ILs and ILs/CO2 at 298 K.
![Figure 4
Comparison of the mean square displacement of of cations, anions, and CO2 in IL and IL/CO2 at 298 K: (a) [BEIM] and [bFAP] in pure [BEIM][bFAP], (b) [B(Hex)3P] and [bFAP] in pure [B(Hex)3P][bFAP], (c) [EMPyr] and [bFAP] in pure [EMPyr][bFAP], (d) [(Me)5isobuGua] and [bFAP] in pure [(Me)5isobuGua][bFAP], and (e) CO2 in the four ILs.](/document/doi/10.1515/chem-2022-0154/asset/graphic/j_chem-2022-0154_fig_004.jpg)
Comparison of the mean square displacement of of cations, anions, and CO2 in IL and IL/CO2 at 298 K: (a) [BEIM] and [bFAP] in pure [BEIM][bFAP], (b) [B(Hex)3P] and [bFAP] in pure [B(Hex)3P][bFAP], (c) [EMPyr] and [bFAP] in pure [EMPyr][bFAP], (d) [(Me)5isobuGua] and [bFAP] in pure [(Me)5isobuGua][bFAP], and (e) CO2 in the four ILs.
The initial rapid increase in MSD within 1 ns showed that a longer simulation time was required to evaluate the self-diffusion coefficients of ions or CO2 in both IL and IL/CO2 systems. The slopes of the MSDs for the cations are steeper than those for the anions in IL and IL/CO2 systems. It was possibly due to the fact that a larger anion size resulted in a slower movement than the cation.
The diffusion of cation, anion, and CO2 in ILs and ILs/CO2 systems has also been determined. The diffusion coefficients of the constituent compounds were obtained by the linear fitting of the slope of MSD. Table 4 reports the diffusivities of cations, anions, and CO2 in the system.
Diffusion coefficient of cation, anion, and CO2 at 298 K and 100 kPa
| System | Diffusion coefficient (10−8cm2 s−1) | ||
|---|---|---|---|
| D cation | D anion |
|
|
| IL | |||
| [BEIM][bFAP] | 0.55 | 0.41 | |
| [B(Hex)3P][bFAP] | 0.68 | 0.57 | |
| [EMPyr][bFAP] | 0.56 | 0.15 | |
| [(Me)5isobuGua][bFAP] | 1.19 | 0.46 | |
| IL/CO2 | |||
| [BEIM][bFAP]/CO2 | 0.60 | 0.27 | 9.93 |
| [B(Hex)3P][bFAP]/CO2 | 0.41 | 0.31 | 13.9 |
| [EMPyr][bFAP]/CO2 | 1.06 | 0.39 | 7.16 |
| [(Me)5isobuGua][bFAP]/CO2 | 1.15 | 0.21 | 9.65 |
The diffusion of cation, anion, and CO2 in ILs and ILs/CO2 systems have also been determined. The diffusion coefficients of the constituent compounds were obtained by the linear fitting of the slope of MSD. Table 4 reports the diffusivities of cations, anions, and CO2 in the system. When CO2 molecules were dissolved in the IL, it was synergistic competition. It is clear from Table 4 that the diffusion coefficient of CO2 was much higher than those of cations and anions. It was mainly due to the small size of CO2 molecules. For CO2 molecule in IL/CO2 system, the self-diffusion coefficient followed the decreasing order: [B(Hex)3P][bFAP]/CO2 > [(Me)5isobuGua][bFAP]/CO2 > [BEIM][bFAP]/CO2 > [EMPyr][bFAP]/CO2, which agreed with the predicted CO2 solubility in ILs.
5 Conclusion
Using COSMOtherm, 500 ILs were selected and screened to predict the CO2 solubility at 298 K and 100 kPa. CO2 solubility in various ILs is much different. Among the 500 screened ILs, CO2 solubility is in the range of 0.002 and 0.084 mole fraction. Anions have much more effect on the solubility of CO2 than cations. ILs with bFAP anion could dissolve more CO2 than any other ILs. The molecular understanding of structural properties of ILs and ILs/CO2 system was further studied using MD simulation. The calculated results of CO2 solvation free energy were −10.18 kJ mol−1 in [EMPyr][bFAP], −11.37 kJ mol−1 in [BEIM][bFAP], −12.21 kJ mol−1 in [(Me)5isobuGua][bFAP], and −10.43 kJ mol−1 in [B(Hex)3P][bFAP], respectively. In IL/CO2 system, CO2 molecules are interacting stronger with the anions as compared with the cations according to the analysis of g(r). The interaction of anion and CO2 is mainly from Van der Waals interaction. The more dissolved in ILs CO2 molecules are, the greater the self-diffusion coefficient is. Basically, the self-diffusion coefficient of cation is higher than that of anion in the IL and IL/CO2 system.
-
Funding information: This research was supported by the National Natural Science Foundation of China (No. 52073228 and No. 22002117). The authors also appreciate the modern analysis and testing center of Xi’an Shiyou University for its hyper computation service.
-
Author contributions: Conceptualization, Xingang Jia; methodology, Xiaoling Hu; software, Xingang Jia; validation, Kehe Su; formal analysis, Xingang Jia; investigation, Xiaoling Hu; re-sources, Wenzhen Wang; data curation, Chunbao Du; writing – original draft preparation, Xingang Jia; writing – review and editing, Kehe Su; visualization, Xingang Jia; supervision, Xiaoling Hu; project administration, Xiaoling Hu and Wenzhen Wang; funding acquisition, Wenzheng Wang and Chunbao Du. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: Authors declare there is no conflict of interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: All CO2 solubility in 500 ILs predicted by COSMOtherm and the structures of cations and anions are included in supplementary information files.
References
[1] Dong Q, Wang W, Kunkel KE, Shao Q, Xing W, Wei J. Heterogeneous response of global precipitation concentration to global warming. Int J Climatol. 2021;41:2347–12.10.1002/joc.6851Suche in Google Scholar
[2] Rogelj J, Geden O, Cowie A, Reisinger A. Three ways to improve net-zero emissions targets. Nature. 2021;591(7850):365–3.10.1038/d41586-021-00662-3Suche in Google Scholar PubMed
[3] IPCC. Climate change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. In: Core writing team, RK Pachauri, LA Meyer, editors. Geneva, Switzerland: IPCC; 2014. p. 151.Suche in Google Scholar
[4] Yun L, Hui T, Buda S, Zbigniew WK, Tong J. Impacts of 1.5 and 2°C global warming on winter snow depth in Central Asia. Sci Total Environ. 2019;651(2):2866–7.10.1016/j.scitotenv.2018.10.126Suche in Google Scholar PubMed
[5] Blanchard LA, Hancu D, Beckman EJ, Brennecke JF. Green processing using ionic liquids and CO2. Nature. 1999;399:28–31.10.1038/19887Suche in Google Scholar
[6] Junhua H, Thomas R. Why are ionic liquids attractive for CO2 absorption? An overview. Aust J Chem. 2009;62(4):298–10.10.1071/CH08559Suche in Google Scholar
[7] Gurkan BE, de la Fuente JC, Mindrup EM, Ficke LE, Goodrich BF, Price EA, et al. Equimolar CO2 absorption by anion-functionalized ionic liquids. J Am Chem Soc. 2010;132(7):2116–7.10.1021/ja909305tSuche in Google Scholar PubMed
[8] Gonzalez-Miquel M, Bedia J, Abrusci C, Palomar J, Rodriguez F. Anion effects on kinetics and thermodynamics of CO2 absorption in ionic liquids. J Phys Chem B. 2013;117(12):3398–8.10.1021/jp4007679Suche in Google Scholar PubMed
[9] Qiwei Y, Zhiping W, Zongbi B, Zhiguo Z, Yiwen Y, Qilong R, et al. New insights into CO2 absorption mechanisms with amino‐acid ionic liquids. ChemSusChem. 2016;9(8):806–6.10.1002/cssc.201501691Suche in Google Scholar PubMed
[10] Yuan S, ChenYJi, X, Yang Z, Lu X. Experimental study of CO2 absorption in aqueous cholinium-based ionic liquids. Fluid Phase Equilibria. 2017;445:14–10.10.1016/j.fluid.2017.04.001Suche in Google Scholar
[11] Aki SNK, Mellein BR, Saurer EM, Brennecke JF. High-pressure phase behavior of carbon dioxide with imidazolium-based ionic liquids. J Phys Chem B. 2004;108(52):20355–10.10.1021/jp046895+Suche in Google Scholar
[12] Pankaj S, Soo-Hyun C, Sang-Do P, Il-Hyun B, Gil-Sun L. Selective chemical separation of carbondioxide by ether functionalized imidazolium cation based ionic liquids. Chem Eng J. 2012;181–182:834–7.10.1016/j.cej.2011.12.024Suche in Google Scholar
[13] Pankaj S, Sang Do PKTP, Sung CN, Soon KJ, Yeo IY, Baek IH. Solubility of carbon dioxide in amine-functionalized ionic liquids: role of the anions. Chem Eng J. 2012;193–194:267–8.10.1016/j.cej.2012.04.015Suche in Google Scholar
[14] Huang J, Rüther T. Why are ionic liquids attractive for CO2 absorption? An overview. Aust J Chem. 2009;62(4):298–10.10.1071/CH08559Suche in Google Scholar
[15] Yokozeki A, Shiflett MB, Junk CP, Grieco LM, Foo T. Physical and chemical absorptions of carbon dioxide in room temperature ionic liquids. J Phys Chem B. 2008;112(51):16654–9.10.1021/jp805784uSuche in Google Scholar PubMed
[16] Earle MJ, Seddon KR. Ionic liquids. Green solvents for the future. Pure Appl Chem. 2000;72(7):1391–7.10.1021/bk-2002-0819.ch002Suche in Google Scholar
[17] Klamt A. COSMO-RS: from quantum chemistry to fluid phase thermodynamics and drug design. Amsterdam, The Netherlands: Elsevier; 2005.Suche in Google Scholar
[18] Palomar J, Torrecilla JS, Ferro VR, Rodríguez F. Development of a priori ionic liquid design tool. 1. Integration of a novel COSMO-RS molecular descriptor on neural networks. Ind Eng Chem Res. 2008;47:4523–9.10.1021/ie800056qSuche in Google Scholar
[19] Palomar J, Ferro VL, Torrecilla JS, Rodriguez F. Density and molar volume predictions using COSMO-RS for ionic liquids. An map proach to solvent design. Ind Eng Chem Res. 2007;46(18):6041–7.10.1021/ie070445xSuche in Google Scholar
[20] Diedenhofen M, Klamt A, Marsh K, Schafer A. Prediction of the vapor pressure and vaporization enthalpy of 1-n-alkyl-3-methylimidazolium-bis-(trifluoromethanesulfonyl) amide ionic liquids. Phys Chem Chem Phys. 2007;9(33):4653–3.10.1039/b706728cSuche in Google Scholar PubMed
[21] Diedenhofen M, Klamt A. COSMO-RS as a tool for property prediction of IL mixtures – a review. Fluid Phase Equilibria. 2010;294(1–2):31–7.10.1016/j.fluid.2010.02.002Suche in Google Scholar
[22] Liu H, Dai S, Jiang DE. Molecular dynamics simulation of anion effect on solubility, diffusivity, and permeability of carbon dioxide in ionic liquids. Ind Eng Chem Res. 2014;53(25):10485–90.10.1021/ie501501kSuche in Google Scholar
[23] Stevanovic S, Podgorsek A, Moura L, Santini CC, Padua AA, Gomes MC. Absorption of carbon dioxide by ionic liquids with carboxylate anions. Int J Green Gas Con. 2013;17:78–10.10.1016/j.ijggc.2013.04.017Suche in Google Scholar
[24] Yang Q, Achenie LEK. Exploration of bulk and interface behavior of gas molecules and 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid using equilibrium and nonequilibrium molecular dynamics simulation and quantum chemical calculation. Phys Chem Chem Phys. 2018;20(15):10121–10.10.1039/C7CP07714ASuche in Google Scholar
[25] Palomar J, Gonzalez-Miquel M, Polo A, Rodriguez F. Understanding the physical absorption of CO2 in ionic liquids using the COSMO-RS method. Ind Eng Chem Res. 2011;50(6):3452–11.10.1021/ie101572mSuche in Google Scholar
[26] Abraham MJ, Murtola T, Schulz R, Pall S, Smith JC, Hess B, et al. GROMACS:high performance molecular simulations through multi-level parallelism from laptops to supercomputers. Software X. 2015;1–2:19–25.10.1016/j.softx.2015.06.001Suche in Google Scholar
[27] Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. Gaussian 16, Revision B.01. Wallingford CT: Gaussian, Inc; 2016.Suche in Google Scholar
[28] Bayly CI, Cieplak P, Cornell W, Kollman PA. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J Chem Phys. 1993;97(40):10269–11.10.1021/j100142a004Suche in Google Scholar
[29] Tian L, Feiwu C. Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem. 2012;33(5):580–12.10.1002/jcc.22885Suche in Google Scholar
[30] Sousda D, Silva AW, Vranken WF. ACPYPE – Antechamber python parser interface. BMC Res Notes. 2012;5:367.10.1186/1756-0500-5-367Suche in Google Scholar
[31] Parrinello M, Rahman A. Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys. 1981;52(12):7182–8.10.1063/1.328693Suche in Google Scholar
[32] Bussi G, Donadio D, Parrinello M. Canonical sampling through velocity rescaling. J Chem Phys. 2007;126(1):014101.10.1063/1.2408420Suche in Google Scholar
[33] Hess B, Bekker H, Berendsen HJ, Fraaije JG. LINCS: a linear constraint solver for molecular simulations. J Comput Chem. 1997;18(12):1463–9.10.1002/(SICI)1096-987X(199709)18:12<1463::AID-JCC4>3.0.CO;2-HSuche in Google Scholar
[34] Darden T, Perera L, Li L, Pedersen L. New tricks for modelers from the crystallography toolkit: the particle mesh Ewald algorithm and its use in nucleic acid simulations. Structure. 1999;7(3):R55–60.10.1016/S0969-2126(99)80033-1Suche in Google Scholar
[35] Muldoon MJ, Aki SNVK, Anderson JL, Dixon JK, Brennecke JF. Improving carbon dioxide solubility in ionic liquids. J Phys Chem B. 2007;111(30):9001–8.10.1021/jp071897qSuche in Google Scholar PubMed
[36] Cadena C, Anthony JL, Shah JK, Morrow TI, Brennecke JF, Maginn EJ. Why is CO2 so soluble in imidazolium-based ionic liquids? J Am Chem Soc. 2004;126(16):5300–8.10.1021/ja039615xSuche in Google Scholar PubMed
[37] Sistla YS, Jain L, Khanna A. Validation and prediction of solubility parameters of ionic liquids for CO2 capture. Sep Purif Technol. 2012;97:51–64.10.1016/j.seppur.2012.01.050Suche in Google Scholar
[38] Mahurin SM, Lee JS, Baker GA, Luo H, Dai S. Performance of nitrile-containing anions in task-specific ionic liquids for improved CO2/N2 separation. J Memberane Sci. 2010;353(1–2):177–6.10.1016/j.memsci.2010.02.045Suche in Google Scholar
[39] Mahurin SM, Hillesheim PC, Yeary JS, Jiang DE, Dai S. High CO2 solubility, permeability and selectivity in ionic liquids with the tetracyanoborate anion. RSC Adv. 2012;2(31):11813–6.10.1039/c2ra22342bSuche in Google Scholar
[40] Martínez L, Andrade R, Birgin EG, Martínez JM. PACKMOL: a package for building initial configurations for molecular dynamics simulations. J Comput Chem. 2009;30(13):2157–7.10.1002/jcc.21224Suche in Google Scholar PubMed
[41] Kanakubo M, Umecky T, Hiejima Y, Aizawa T, Nanjo H, Kameda Y. Solution structures of 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid saturated with CO2: experimental evidence of specific anion−CO2 interaction. J Phys Chem B. 2005;109:13847–3.10.1021/jp052354oSuche in Google Scholar PubMed
[42] Babarao R, Sheng D, Jiang D. Understanding the high solubility of CO2 in an ionic liquid with the tetracyanoborate anion. J Phys Chem B. 2011;115(32):9789–5.10.1021/jp205399rSuche in Google Scholar PubMed
[43] Zhang X, Huo F, Liu Z, Wang W, Shi W, Maginn EJ. Absorption of CO2 in the ionic liquid 1-n-hexyl-3-methylimidazolium tris (pentafluoroethyl) trifluorophosphate ([hmim][FEP]): a molecular view by computer simulations. J Phys Chem B. 2009;113(21):7591–7.10.1021/jp900403qSuche in Google Scholar PubMed
© 2022 Xingang Jia et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop
Artikel in diesem Heft
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop

