Abstract
C18H10O3, monoclinic, P21/n (no. 14), a = 9.9981(2) Å, b = 9.3873(2) Å, c = 13.7621(3) Å, β = 102.841(2)°, V = 1,259.34(5) Å3, Z = 4, R gt (F) = 0.0463, wR ref (F2) = 0.1235, T = 293 K.
The molecular structure is shown in the Figure 1. Table 1 contains the crystallographic data.
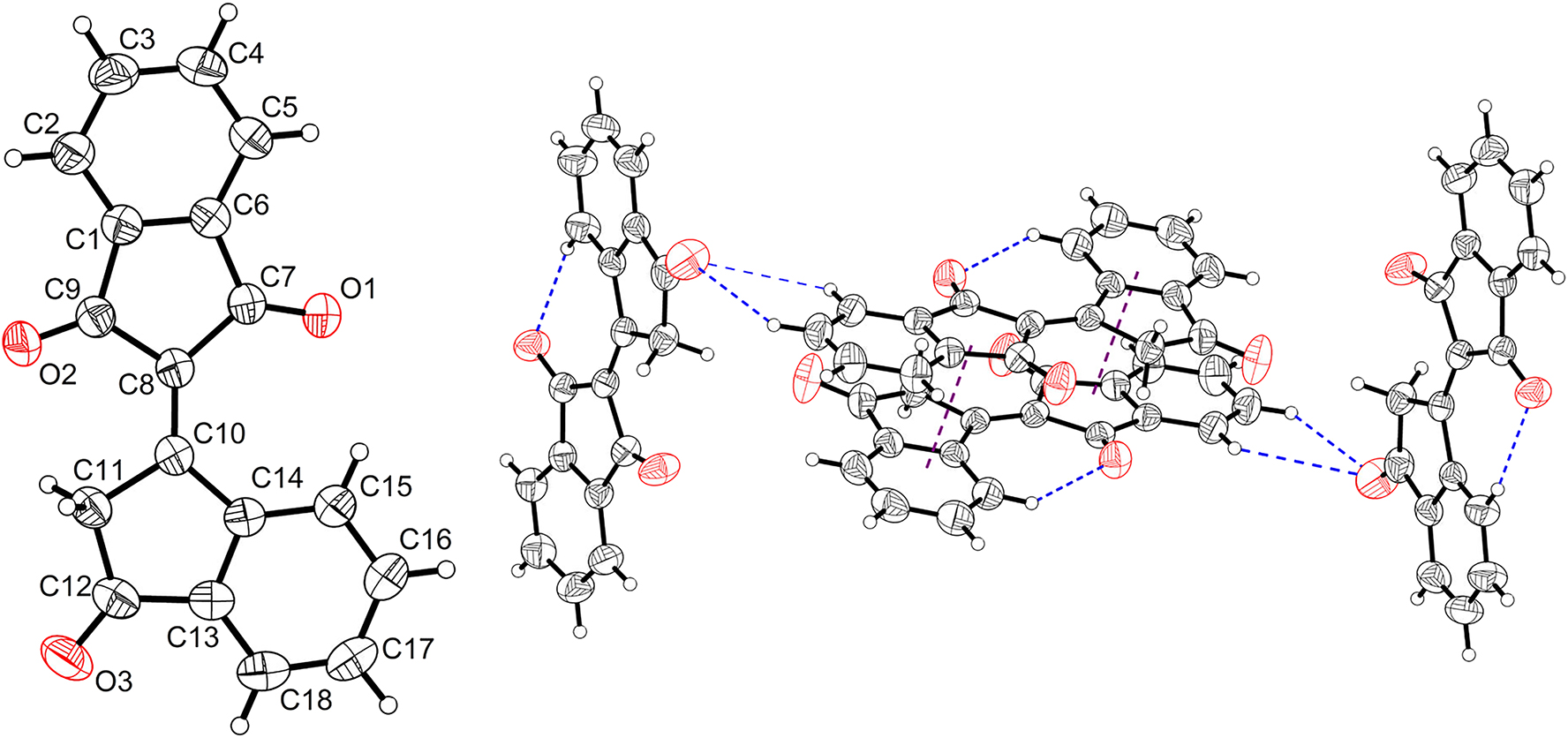
A view of the molecule. Displacement ellipsoids are drawn at the 50 % probability level and H atoms are shown as small spheres of arbitrary radii.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.28 × 0.16 × 0.14 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 0.81 mm−1 |
| Diffractometer, scan mode: | Rigaku, ω scans |
| θmax, completeness: | 71.5°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 5382, 2407, 0.023 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2,044 |
| N(param)refined: | 191 |
| Programs: | Rigaku 1 , SHELX 2 , 3 , 4 , Olex2 5 , Diamond 6 |
1 Source of material
The title compound, 2-(2,3-dihydro-3-oxo-1H-inden-1-ylidene)-1H-indene-1,3(2H)-dione, also known as bindone. It was synthesized according to the literature method with a slight modification. 7 1,3-Indanedione (1.2 g, 7 mmol) was charged in 100 mL two-neck round bottle and mixed with 35 mL anhydrous ethanol. After 10 min stirring, anhydrous sodium acetate (760 mg, 9.3 mmol) was added to the solution. Immediately, the solution color was turned to be violet. Then the solution was heated to reflux (about 80 °C) for about 5 h. And the solution color would be turned to deep violet. The reaction progress was monitored by thin–layer chromatography (TLC). When the starting 1,3-indanedione disappeared completely on the TLC, the reaction was diluted by adding water (20 mL). Subsequently, the reaction mixture was acidified by concentrated hydrochloric acid until large amount solid precipitate from the reaction solution appeared, which was collected by filtration and washed with distilled water. The crude product was purified by flash column chromatography on silica gel (200–300 mesh), using dichloromethane and hexane (50:1) as the eluent to give the title compound as a browness solid with the yield of 65 %. 1H NMR (400 MHz, CDCl3): δ 9.67 (d, J = 8.0 Hz, 1 H), 8.04–7.93 (m, 3H), 7.88–7.85 (m, 3H), 7.74 (td, J = 7.2, 0.8 Hz, 1 H), 4.16 (s, 2H). The condensation is kinetically or thermodynamically controlled. Therefore, the 1,3-indanedione involved condensation always confused chemists whenever there exists other active methylene compounds (such as 3,5-difluorophenylacetonitrile). Actually, the product is bindone instead of the condensed product between bindone and 3,5-difluorophenylacetonitrile. 8 The growth of a single crystal was carried out by dissolving bindone in ethanol and sealed in 10 mL vial with parafilm at room temperature. The holes in the parafilm were used to control a slow evaporation of solvent. Regular shaped crystals can be obtained.
2 Experimental details
Single-crystal X-ray diffraction measurements of the title compound were carried out on Rigaku SuperNova with mirror monochromatic Cu Kα X-ray source (λ = 1.54184 Å) at room temperature. Data reduction was carried out using CrysAlisPRO and empirical absorption corrections were made using SADABS. 1 The structure was solved by using the programs of SHELXT and refined by SHELXL through the Olex2 interface (Table 1). 2 , 3 , 4 , 5 Hydrogen atoms were placed geometrically and refined using a riding model approximation, assigned isotropic thermal parameters with d(C–H) = 0.93 or 0.97 Å (–CH, –CH2). Uiso(H) = 1.2Ueq(C) for CH and Uiso(H) = 1.5Ueq(C) for CH2. 2 One outlier reflection (7, 1, 8) was omitted from the final cycles of refinement owing to the large deviation. The molecular graphics were drawn using DIAMOND software with 50 % probability ellipsoids (Figure 1). 6 The structure was examined using the ADDSYM subroutine of PLATON 51222 to ensure that no additional symmetry could be applied to the models. 9
3 Comment
The title compound, bindone, is a popular intermediate in cycloaddition to configure spiroconjugated cyclic ketones. When a fluorescent molecular framework was incorporated in bindone, the reasonable designed cyclo rigidity of the molecule could be used to modulate the emission efficiency effectively. Therefore, many highly efficient fluorescent dyes were configured aiming for various applications in chemosensor, biological imaging, and dye-sensitized solar cells, etc. 10 , 11 , 12 , 13 , 14 In the structure of bindone, there exist two sets of multiple aromatic systems and three carbonyl groups. The strong electron withdrawing character of carbonyl activates the hydrogen atoms of methylene, which is significant when bonding to various fluorophores. Owing to the reactive methylene, highly fluorescent dyes can be developed toward the application in optical device fabrication.
In the title crystal structure, the asymmetric unit contains only one molecule, which is different from that of literature published. 7 Obviously, the bindone crystallized in monoclinic system with the space group P21/n (no. 14). On the contrary, the literatured bindone crystallized in orthorhombic system with the space group Fdd2 (no. 43). In the two 1,3-indanedione parts (C1/C2/C3/C4/C5/C6/C7/C8/C9/O1/O2 and C11/C12/C13 /C14/C15/C16/C17/C18/O3), the double ring structure of indanedione is coplanar with the root mean square error (RMSD) in distance estimated to be 0.026 and 0.039 Å, respectively. The two indanedione cyclic parts are bonded by a typical double bond, which is estimated to be 1.354 Å. In addition, the bond of C10–C11 and C12–C11 has the typical single C–C bond character with the bond length of 1.520 and 1.503 Å, which is slightly shorter than the typical single bond length 1.54 Å due to the adjacent electron-withdrawing carbonly (C12–O3). Conversely, the bond length of C7–C8/C8–C9 is 1.497/1.493 Å and also shorter than that of C10–C11/C12–C11, indicating the conjugation between C8–C10 (1.354 Å) and C7–O1 (1.220 Å)/C9–O2 (1.218 Å). Therefore, the two 1,3-indanedione parts (C1/C2/C3/C4/C5/C6/C7/C8/C9/O1/O2, and C11/C12/C13/C14/C15/C16/C17/C18/O3) should be coplanar theoretically. However, the two planes are twisted with the angle of 8.27°. At the same time, the twist between the two planes was also inhibited and fixed by an intramolecular hydrogen bond C15–H15⋯O1i1 (i1: x, y, z). The distance between electron donor (C15) and acceptor (O1) was estimated to be 2.953 Å and the C–H⋯O angle was 137.9°, which is well inside the interval of 3.0–4.0 Å and quoted by Desiraju. 15 And C–H⋯O angles are also in agreement with the above mentioned survey. Totally, the comprehensive repelling and locking derived from C15–H15⋯O1 generates a slightly twist between the two indanedione. In contrast to that of bindone in literature, the twisted angle is similar to values from the literature (8.5 and 10.0°). And the only left reactive methylene can be applied in construction the controllable molecule rigidity, specifically optical photophysical properties can be effectively developed. 16 , 17 , 18 , 19 The adjacent parallel molecules are packed by strong π⋯π interactions. The distance between the two indanedione planes (C1/C2/C3/C4/C5/C6/C7/C8/C9/O1 /O2i1, and C11/C12/C13/C14/C15/C16/C17/C18/O3i2 i2: 1−x, −y, 1−z) is determined to be 3.602 Å and the parallel shift is 1.024 Å, corresponding to the parallel-displaced geometry (Figure 1). It is interesting that another indanedione plane does not involve the π⋯π interaction. The cohesion of adjacent non-parallel molecules is totally contributed by intramolecular hydrogen bonds. Four hydrogen bonds are found (C2–H2⋯O1i3, C3–H3⋯O2i4, C11–H11A⋯O3i4, C15–H15⋯O2i4, i3: 1/2 + x, 3/2−y, 1/2 + z; i4: x, 1 + y, z) with the C⋯O contacts ranging from 3.289 to 3.828 Å, C–H⋯O angles ranging from 122.3 to 161.5° (Figure 1, right). Furthermore, the adjacent non-parallel molecules are almost perpendicular to each other due to the hydrogen bond interaction, yielding to its unique packing model. It also indicates that there exist significant intramolecular interactions between the monoclinic (this work) and orthorhombic (literature: CCDC 1456538) crystal system. Among various intermolecular interactions, the hydrogen bond established in the crystal is also significant to the optical properties of fluorescent dyes. 20 , 21 , 22 , 23 Therefore, reasonable introduction of inter- and/or intra-molecular hydrogen bonds into the crystal lattice is an effective methodology to inhibit the ration of nonradiative channels. 24 , 25 Weaker intramolecular interactions benefit to maintain the highly emissive character of crystals. The contrary had been proved true: stronger interactions could inactivate the excitated state of dye molecule and quench the fluorescence. 26 , 27 , 28
In the crystal, the molecules are regularly packed by various intermolecular forces, including hydrogen bonds and π⋯π interactions. The driving forces that pack the dye molecules parallel to each other along the axis a is based on the stronger π⋯π interaction. While the hydrogen bonds join the parallel molecules to form the network. The weaker hydrogen bonds are the determining factor to optical properties of the compound in crystal state. 29 , 30 , 31 The perpendicular conformation of adjacent non-parallel molecules inhibit a tight regular packing model in some degree, and thus avoiding significant fluorescence quenching. 32 , 33 , 34 , 35 , 36 The ratio of radiative and nonradiative channels is significantly influenced by the external environment (various interactions). 37 , 38 In case of bindone, it is an useful intermediate to design the dye with unique fluorescent properties in solid and crystal state. 39 , 40 It indicates that reasonable configuration of inter- and intra-molecular interactions by introducing heteroatoms and/or aromatic system is the effective strategy in dye designing. 41 , 42 , 43 In conclusion, weak intra- and inter-molecular non-covalent interactions were identified in the crystal structure, including hydrogen bonds and T-type intermolecular π⋯π stacking.
Acknowledgments
The authors appreciates the financial supporting by the Department of Science and Technology of Henan province (252102230148), Research Project of Zhengzhou Science and Technology Burear (zkz202204), and Open Laboratory Project of Zhengzhou Uniuversity of Technology (2024), the Basic Reasearch and Applied Basic R. The authors also gratefully thanks Prof. Xiaochuan Li (Henan Normal University) for his assistance with the lab facilities supportation/chemical purification, single crystal X-ray diffraction data acquisition and providing his expertise on crystal structure solving.
References
1. Agilent. CrysAlisPRO. Agilent Technologies Ltd: Yarnton, Oxfordshire, England., 2014.Search in Google Scholar
2. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Sheldrick, G. M. A Short History of SHELX. Acta Crystallogr. 2008, A64, 112–122. https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
4. Sheldrick, G. M. SHELXTL – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
5. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
6. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver 4.0; Crystal Impact GbR: Bonn, Germany, 2015.Search in Google Scholar
7. Wilbuer, J.; Schnakenburg, G.; Esser, B. Syntheses, Structures and Optoelectronic Properties of Spiroconjugated Cyclic Ketones. Eur. J. Org Chem. 2016, 14, 2404–2412. https://doi.org/10.1002/ejoc.201600235.Search in Google Scholar
8. Lv, R.; Chen, D.; Liao, X.; Chen, L.; Chen, Y. A Terminally Tetrafluorinated Nonfullerene Acceptor for Well-Performing Alloy Ternary Solar Cells. Adv. Funct. Mater. 2019, 29, 1805872. https://doi.org/10.1002/adfm.201805872.Search in Google Scholar
9. Spek, A. L. Structure Validation in Chemical Crystalloxgraphy. Acta Cryst. 2014, D65, 148–155.10.1107/S090744490804362XSearch in Google Scholar PubMed PubMed Central
10. Li, X.; Liu, X.; Li, F. Configuration of Super-Fast Cu2+-Responsive Chemosensor by Attaching Diaminomaleonitrile to BODIPY Scaffold for High-Contrast Fluorescence Imaging of Living Cells. Spectrochim. Acta, Part A 2024, 304, 123377. https://doi.org/10.1016/j.saa.2023.123377.Search in Google Scholar PubMed
11. Zheng, X.; Liu, X.; Liu, L.; Li, X.; Jiang, S.; Niu, C.; Xie, P.; Liu, G.; Cao, Z.; Ren, Y.; Qin, Y.; Wang, J. Multi-Stimuli-Induced Mechanical Bending and Reversible Fluorescence Switching in a Single Organic Crystal. Angew. Chem., Int. Ed. 2022, 61, e202113073. https://doi.org/10.1002/anie.202113073.Search in Google Scholar PubMed
12. Li, X.; Han, Y.; Sun, S.; Shan, D.; Ma, X.; He, G.; Mergu, N.; Park, J. S.; Kim, C. H.; Son, Y. A. A Diaminomaleonitrile-Appended BODIPY Chemosensor for the Selective Detection of Cu2+ via Oxidative Cyclization and Imaging in SiHa Cells and Zebrafish. Spectrochim. Acta, Part A 2020, 233, 118179. https://doi.org/10.1016/j.saa.2020.118179.Search in Google Scholar PubMed
13. Xie, P.; Zhou, Y.; Li, X.; Liu, X.; Liu, L.; Cao, Z.; Wang, J.; Zheng, X. Strong Dual-State Emission of Unsymmetrical and Symmetrical Thiazolothiazole-Bridged Imidazolium Salts. Chin. Chem. Lett. 2023, 34, 107582. https://doi.org/10.1016/j.cclet.2022.06.005.Search in Google Scholar
14. Li, X.; Tian, G.; Shao, D.; Xu, Y.; Wang, Y.; Ji, G.; Ryu, J.; Son, Y. A. A BODIPY Based Emission Signal Turn-On Probe Toward Multiple Heavy Metals. Mol. Cryst. Liq. Cryst. 2020, 706, 38–46. https://doi.org/10.1080/15421406.2020.1743436.Search in Google Scholar
15. Desiraju, G. R. The C–H⋯O Hydrogen Bond in Crystals: What is it? Acc. Chem. Res. 1991, 24, 290–296. https://doi.org/10.1021/ar00010a002.Search in Google Scholar
16. Li, X.; Li, F.; Ji, G. A Fluorescent Turn-On Sensor Toward Multiple Heavy Metal Ions Based on Meso-Anisole Modified BODIPY Scaffold. J. Fluoresc. 2023, 33, 631–637; https://doi.org/10.1007/s10895-022-03110-1.Search in Google Scholar PubMed
17. Li, Y.; Liu, K.; Zhang, W.; Wang, Y.; Wang, B.; Wang, Y.; Li, X. Two 3D Ln(III)-mOFs Based on Phosphineoxide Ligand: Synthesis, Structure Luminescent and Photocatalytic Properties. J. Fluoresc. 2023, 33, 2119–2129. https://doi.org/10.1007/s10895-023-03218-y.Search in Google Scholar PubMed
18. Cao, Z.; Yang, F.; Wu, D.; Wu, L.; Liu, L.; Liu, G.; Li, X.; Zheng, X.; Zheng, X.; Qu, D. Supramolecular Aggregates Constructed by Pillar[5]arene-Based Host-Guest Interaction with Aggregation-Induced Emission. Polym. Chem. 2023, 14, 1318–1322; https://doi.org/10.1039/d3py00026e.Search in Google Scholar
19. Li, X.; Yao, C.; Jiang, W. Emission and Energy Transfer Investigation of Non-Conjugated Total Carbon Configuration Between BODIPY and Naphthalimide. J. Chem. Sci. 2023, 135, 65. https://doi.org/10.1007/s12039-023-02181-2.Search in Google Scholar
20. Li, X.; Qian, Q.; Jiang, W. Photo-Induced Fluorochromism of a Star-Shaped Photochromic Dye with 2,4-Dimethylthiazole Attaching to Triangle Terthiophene. J. Fluoresc. 2023, 33, 1907–1915. https://doi.org/10.1007/s10895-023-03196-1.Search in Google Scholar PubMed
21. Ji, G.; Hou, Q.; Jiang, W.; Li, X. Investigating the Properties of Double Triangle Terthiophene Configured Dumbbell-Like Photochromic Dye with Ethyne and 1,3-Butadiene Bridge. J. Fluoresc. 2023, 33, 1495–1503. https://doi.org/10.1007/s10895-023-03171-w.Search in Google Scholar PubMed
22. Li, X.; Zou, Y.; Heo, G.; Son, Y. A. Emission Shift of an Imidazole Bridged Diethylaminocoumarin and Diphenyl. Mol. Cryst. Liq. Cryst. 2020, 704, 48–56. https://doi.org/10.1080/15421406.2020.1741801.Search in Google Scholar
23. Zheng, X.; Wang, G.; Liu, L.; Li, X.; Xie, P.; Fan, Y.; Cao, Z.; Niu, C.; Tian, D.; Xie, L. Hydrogen-Bonding Induced Multicolor and Thermochromic Emissions of Triphenylamines. Chem. Eur. J. 2025, 31, e202500643. https://doi.org/10.1002/chem.202500643.Search in Google Scholar PubMed
24. Liu, Y.; Li, X.; Min, K. S.; Son, Y. A. Emission Behavior of Naphthalimide-Coumarin Cassette. Mol. Cryst. Liq. Cryst. 2018, 662, 139–146. https://doi.org/10.1080/15421406.2018.1466533.Search in Google Scholar
25. Kong, Y.; Liu, X.; Jiang, W.; Li, X. Photochromic Properties of Triangle Terthiophene- and Triphenylamine-Configured Diarylethene Type Dye with Propeller-Like Conformation. Chem. Pap. 2025, 79, 2401–2409; https://doi.org/10.1007/s11696-025-03934-8.Search in Google Scholar
26. Li, X.; Liao, M.; Sun, J.; Heo, G.; Son, Y. A. Thiophene Modulated BODIPY Dye as a Light Harvester. Mol. Cryst. Liq. Cryst. 2019, 679, 127–136. https://doi.org/10.1080/15421406.2019.1597557.Search in Google Scholar
27. Li, X.; Cai, Q.; Zhang, J.; Kim, H.; Son, Y. A. An “Electron Lock” Toward the Photochromic Activity of Phenylacetylene Appended Bisthienylethene. Mol. Cryst. Liq. Cryst. 2020, 706, 141–149. https://doi.org/10.1080/15421406.2020.1743450.Search in Google Scholar
28. Liu, Y.; Li, X.; Kim, H.; Son, Y. A. Investigation of Fluorescent Optical Properties of Fluorine-Boron Cored Dye. Mol. Cryst. Liq. Cryst. 2018, 677, 27–33. https://doi.org/10.1080/15421406.2019.1597508.Search in Google Scholar
29. Ji, G.; Hou, Q.; Zhang, J.; Li, X. Investigation of Triangle Terthiophene and Hydroxyphenylbenzothiazole Configured Fluorescent Dye with a Triple Bond Bridge. J. Fluoresc. 2023, 33, 153–159. https://doi.org/10.1007/s10895-022-03049-3.Search in Google Scholar PubMed
30. Li, X.; Han, Y.; Kim, M.; Son, Y. A. A BODIPY-based Highly Emissive Dye with Thiophene-Based Branch Harvesting the Light. Mol. Cryst. Liq. Cryst. 2018, 662, 157–164; https://doi.org/10.1080/15421406.2018.1467613.Search in Google Scholar
31. Liu, Y.; Li, X.; Min, K. S.; Son, Y. A. Highly Fluorescent Response of 4-(2,5-Dimethylthiophen-3-yl)-2-hydroxyphenylbenzothiazole Toward BF3⋅Et2O and Zn2+. Mol. Cryst. Liq. Cryst. 2018, 662, 132–138; https://doi.org/10.1080/15421406.2018.1466531.Search in Google Scholar
32. Li, X.; Guo, X.; Chen, Y.; Cui, T.; Xing, L. Double 3-Ethyl-2,4-dimethylpyrrole Configured Fluorescent Dye with Fluorine-Boron as the Bridge. J. Fluoresc. 2021, 31, 1797–1803; https://doi.org/10.1007/s10895-021-02819-9.Search in Google Scholar PubMed
33. Li, X.; Zhou, Q.; Heo, G.; Son, Y. A. 2,4-Dimethylpyrrole Configured Fluorine-Boron Complexes. Mol. Cryst. Liq. Cryst. 2018, 677, 34–41. https://doi.org/10.1080/15421406.2019.1597509.Search in Google Scholar
34. He, W.; Li, X.; Kim, H.; Son, Y. A. Shifting the Emission of Proton Transfer Fluorescence with Fluorine-Boron as the Rotation Lock. Mol. Cryst. Liq. Cryst. 2020, 704, 41–47. https://doi.org/10.1080/15421406.2020.1741800.Search in Google Scholar
35. Li, X.; Han, Y.; Kim, M. J.; Son, Y. A. Reversed Photochromism Reactivity of Malononitrile Attached Bisthienylthene. Mol. Cryst. Liq. Cryst. 2018, 662, 147–156. https://doi.org/10.1080/15421406.2018.1466534.Search in Google Scholar
36. Li, X.; Wang, Y.; Jia, C.; Kim, H.; Son, Y. A. Photochromic Reactivity Induced by Electron Distribution: Active or Inactive. Mol. Cryst. Liq. Cryst. 2019, 689, 83–91. https://doi.org/10.1080/15421406.2019.1597556.Search in Google Scholar
37. Ji, G.; Hou, Q.; Jiang, W.; Li, X. Investigating the Properties of Triangle Terthiophene and Triphenylamine Configured Propeller-Like Photochromic Dye with Ethyne Bridge. J. Fluoresc. 2025, 35, 933–941; https://doi.org/10.1007/s10895-023-03557-w.Search in Google Scholar PubMed
38. Li, X.; Liu, X. A Sensitive Probe of Meso-Cyanophenyl Substituted BODIPY Derivative as Fluorescent Chemosensor for the Detection of Multiple Heavy Metal Ions. J. Fluoresc. 2025, 35, 1089–1098; https://doi.org/10.1007/s10895-024-03581-4.Search in Google Scholar PubMed
39. Li, X.; Han, Y.; Min, K.; Son, Y. A. Configuration of White Light Emission by Courmarin and Naphthalimide. Mol. Cryst. Liq. Cryst. 2018, 660, 10–16; https://doi.org/10.1080/15421406.2018.1452861.Search in Google Scholar
40. Qin, X.; Li, H.; Wang, Y.; Li, Y.; Li, X. Conjugated Iminodibenzyl Dyes Incorporating Phenolic Hydroxyl Group and Strong Electron Donating or Accepting Groups for Facilitating ESIPT and Proton Transfer in Six‑ or Seven‑Membered Cycles. J. Fluoresc. 2025, https://doi.org/10.1007/s10895-025-04285-z, In press.Search in Google Scholar PubMed
41. Liu, Y.; Li, X.; Sun, S.; Ji, G.; Son, Y. A. Crystal Structure of 2,7-Diiodo-1,3,6,8-tetramethyl-bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine, C14H14B2F4I2N4. Z. Kristallogr. New Cryst. Struct. 2020, 235, 371–372; https://doi.org/10.1515/ncrs-2019-0678.Search in Google Scholar
42. He, W.; Liu, Y.; Sun, S.; Ji, G.; Li, X. Crystal Structure of 2-Bromo-1,3,6,8-tetramethylBOPHY (BOPHY = Bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4. Z. Kristallogr. New Cryst. Struct. 2021, 236, 949–952; https://doi.org/10.1515/ncrs-2021-0163.Search in Google Scholar
43. Liu, Y.; Sun, S.; Ji, G.; Li, X.; Son, Y. A. Crystal Structure of 2-Phenylethynyl-1,3,6,8-tetramethylBOPHY (BOPHY = Bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C22H20B2F4N4. Z. Kristallogr. -New Cryst. Struct. 2021, 236, 749–752; https://doi.org/10.1515/ncrs-2021-0074.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (1Z, 2Z)-3-phenyl-2-propenal 2-(4-bromo-2-fluorophenyl)hydrazone, C15H12BrFN2
- Refinement of crystal structure of 2-(2,3-dihydro-3-oxo-1 H -inden-1-ylidene)-1 H -indene-1,3(2 H )-dione C18H10O3
- The crystal structure of 3-(1-fluoro-2-(naphthalen-2-yl)-2-oxoethyl)-2-methoxy-3,4-dihydroisoquinolin-1(2H)-one, C22H18FNO3
- Crystal structure of the dinuclear copper(II) complex bis(μ2-2,2′ -{[1,3-phenylenebis-(methylene)]bis(oxy)}dibenzoaot-κ4O,O′:O′′,O′′′)-bis(dimethylformamide-κ1O)dicopper(II), C50H44Cu2N2O14
- Crystal structure of poly[triaqua-(μ9-biphenyl-3,3′,5,5′-tetracarboxylic-κ8 O,O:O,O′: O,O″:O,O‴)samarium(III)sodium(I)], C16H12NaSmO11
- The crystal structure of 5-benzyl-1-(4-fluorobenzyl)-4-((4-fluorobenzyl)oxy)-1,5-dihydro-2H-pyrrol-2-one, C25H21F2NO2
- The crystal structure of diammonium 2,5-dihydroxyterephthalate, C8H12N2O6
- Crystal structure of (E)-4-(4-(1H-1,2,4-triazol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[b]oxepin-5(2H)-one, C21H19N3O4
- Crystal structure of poly[oktakis(μ2-oxido-κ2O:O)-tetrakis(oxido-κ1O)-bis(μ2-1,1′-[1,4-phenylenebis(methylene)]di(1H-imidazole-κ2N:N′))-tetravanadium(V)-dizinc(II)] monohydrate, C28H30Zn2N8O13V4
- Crystal structure of acotiamide hydrochloride dimethylacetamide solvate (1/1), C25H40ClN5O6S
- Crystal structure of catena-poly[monoaqua (u2-(3-(3,5-dicarboxyphenyl)pyridine-2,6-dicarboxylic-k4O:O′:O″:N)zinc(II))] monohydrate, C15H11NO10Zn
- Crystal structure of dichlorido{2,6-bis(3,5-diisopropyl-N-pyrazolyl)pyridine}zinc(II), C23H33Cl2N5Zn
- Crystal structure of nitrato-κ2O,O′-[hydridotris(3,5-diethylpyrazol-1-yl)borato-κ3N,N′,N″]copper(II), C21H34BCuN7O3
- Crystal structure of 2,7-bis(3,5-diethyl-1H-pyrazol-4-yl)-benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetrone, C28H26N6O4
- Crystal structure of 2-(4-chlorophenyl)benzothiazole, C13H8ClNS
- Synthesis and crystal structure (3R,4′S)-4′-(3,5-dibromophenyl)-1′-methyl-2H-dispiro [benzofuran-3,3′-pyrrolidine-2′,2″-indene]-1″,2,3″-trione, C26H17Br2NO4
- Crystal structure of bis(((3a,7a-dihydro-1H-benzo[d][1,2,3]triazol-1-yl)methyl) triphenylphosphonium) tetrachloridomanganate(II), C50H42Cl4MnN6P2
- The crystal structure of 4,9-bis(4-chlorophenyl)-1,6-bis(2-cyanobenzyl)-2,4a,5,6,7,7a-hexahydro-1H-2,7,5-(epiprop[2]ene[1,1,3]triyl)pyrrolo[3,4-b]pyridine-3,10-dicarbonitrile, C40H26Cl2N6
- The crystal structure of poly((μ2-3-(3-nitro-4-carboxylphenyl)benzoate-κ3O, O′:O″)-μ2-1,4-bis(1-imidazolyl)benzene-κ2N:N′-cadmium(II)), C26H17N5O6Cd
- The crystal structure of 6-hydroxy-5H-pyrrolo[3,4-b]pyridine-5,7(6H)-dione monohydrate, C7H6N2O4
- Crystal structure of 4-((cyclohexylsulfonyl)methyl)-1,2,3,4-tetrahydrobenzo [4,5]imidazo[1,2-a]pyridine, C18H24N2O2S
- Crystal structure of 4,7-diphenyl-1,10-phenanthroline-κ2N,N′)-bis(2,4-di(fluorine)-1-phenylpyridine-κ2C,N)-iridium(III) hexafluorophosphate–dichloromethane (1/1), C47H30Cl2F10IrN4P
- Crystal structure of (4-(1-phenyl-1H-benzo[d]imidazol-2-yl)phenyl)boronic acid, C19H15BN2O2
- The crystal structure of (E)-(2-((pyridin-2-ylmethylene)amino)phenyl)arsonic acid, C12H11AsN2O3
- The crystal structure of N(benzyl(phenyl)carbomothioyl)benzamide, C21H18N2OS
- The crystal structure of bis(2-picolinium) hexachlorostannate dichloromethane monosolvate, C13H18Cl8N2Sn
- Crystal structure of poly[tetraaqua-bis(μ4-3–1-(carboxylatomethyl)-1H-1,2,4-triazole-3-carboxylato)-κ4O:O′,O″,N)zinc(II)], C5H7N3O6Zn
- The crystal structure of the co-crystal isonicotinamide – 2-(nitrophenyl)methanol (1/1), C6H6N2O·C7H7NO3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-fluorobenzoate hydrate, C23H25F2N3O6
- Crystal structure of [diaqua-{1H-benzo[d]imidazol-3-ium-5,6-dicarboxylato-κ2O,O′}magnesium(II)] C18H14MgN4O10
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl) (2-iodo-5-methoxyphenyl)methanone, C20H20INO5
- The crystal structure of 3,7,11-trimethylbenzo[5,6][1,4]thiazino[2,3,4-kl]phenothiazine 5,5,9,9-tetraoxide, C21H17NO4S2
- Crystal structure of tris(piperazine-1,4-diium)bis(2-hydroxy-1,2,3-propane-tricarboxylate) pentahydrate, C24H56N6O19
- Crystal structure of 2-chloro-5-((5-isopropyl-2-methylphenoxy)methyl)pyridine, C16H18ClNO
- Crystal structure of (E)-4-(4-(1H-pyrrol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[(b)]oxepin-5(2H)-one, C23H21NO4
- Crystal structure of (E)-N′-(3,4-dichlorobenzylidene)-3,4,5-trimethoxybenzohydrazide, C17H16Cl2N2O4
- The crystal structure of 2-(2-hydroxyphenyl)-3-(pyridin-2-yl)-2,3- dihydroquinazolin-4(1H)-one, C19H15N3O2
- Crystal structure of 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-8-methylchroman-4-one, C17H16O5
- Crystal structure of bis[(3,4-dimethoxybenzyl)triphenylphosphonium]di-μ2-bromido-dibromidodicopper(I)
- Crystal structure of bis [(1,3-dioxolan-2-ylmethyl)triphenylphosphonium] tetrabromidodicopper(I), C22H22Br2CuO2P
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid], C12H8N2O5
- The crystal structure of one-dimensional cooridnation polymer bis(thiocyanato)-bis((1E,2E)-1,2-bis(1-(pyridin-3-yl)ethylidene)-hydrazine κ2N:N)iron(II), (C30H28N10S2Fe)n
- Crystal structure of ((4-acetamidophenyl)sulfonyl)-l-alanine, C11H14N2O5S
- Crystal structure of [(1-naphthalen-1-yl-methyl)triphenylphosphonium] dichloridocopper(I), [C29H24P]+[CuCl2]−
- RbTm3S5: the first rubidium lanthanoid(III) sulfide with CsEr3Se5-type crystal structure
- Crystal structure of 2,2′-((ethane-1,2-diylbis(methylammoniumdiyl))bis(methylene))bis(pyridin-1-ium) diiodido-tris(μ2-iodido-κ2I:I)dicopper(II) chloride dihydrate, C16H30Cu2I6N4O2
- The crystal structure of 4-(trifluoromethyl)pyridine-2-carboxylic acid, C7H4F3NO2
- The crystal structure of (E)-2-ethoxy-1-methoxy-4-(2-(methylsulfonyl)vinyl)benzene, C12H16O4S
- Crystal structure of potassium 1H,1H,2H,2H-perfluorooctanesulfonate, C8H4O3F13SK
- Crystal structure of 4-(4-(quinolin-8-yloxy)-1,2,5-thiadiazol-3-yl)morpholine, C15H14O2N4S
- The crystal structure of 1,4-bis(bromomethyl)-2,5-dimethylbenzene, C10H12Br2
- The crystal structure of imidazo[4,5-e][1,3]diazepine-4,6,8-triamine methanol solvate, C7H11N7O
- The crystal structure of chlorido-bis(1,10-phenantroline-κ2N,N′)-(2-formylphenoxyacetato-κ2O,O) lead(II), C33H23N4O4ClPb
- Crystal structure of pyridinium tetrakis(1,1,1-trifluoro-2,4-pentadionato-κ2O,O′)yttrium(III) C20F12H16YO8C5H6N
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (1Z, 2Z)-3-phenyl-2-propenal 2-(4-bromo-2-fluorophenyl)hydrazone, C15H12BrFN2
- Refinement of crystal structure of 2-(2,3-dihydro-3-oxo-1 H -inden-1-ylidene)-1 H -indene-1,3(2 H )-dione C18H10O3
- The crystal structure of 3-(1-fluoro-2-(naphthalen-2-yl)-2-oxoethyl)-2-methoxy-3,4-dihydroisoquinolin-1(2H)-one, C22H18FNO3
- Crystal structure of the dinuclear copper(II) complex bis(μ2-2,2′ -{[1,3-phenylenebis-(methylene)]bis(oxy)}dibenzoaot-κ4O,O′:O′′,O′′′)-bis(dimethylformamide-κ1O)dicopper(II), C50H44Cu2N2O14
- Crystal structure of poly[triaqua-(μ9-biphenyl-3,3′,5,5′-tetracarboxylic-κ8 O,O:O,O′: O,O″:O,O‴)samarium(III)sodium(I)], C16H12NaSmO11
- The crystal structure of 5-benzyl-1-(4-fluorobenzyl)-4-((4-fluorobenzyl)oxy)-1,5-dihydro-2H-pyrrol-2-one, C25H21F2NO2
- The crystal structure of diammonium 2,5-dihydroxyterephthalate, C8H12N2O6
- Crystal structure of (E)-4-(4-(1H-1,2,4-triazol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[b]oxepin-5(2H)-one, C21H19N3O4
- Crystal structure of poly[oktakis(μ2-oxido-κ2O:O)-tetrakis(oxido-κ1O)-bis(μ2-1,1′-[1,4-phenylenebis(methylene)]di(1H-imidazole-κ2N:N′))-tetravanadium(V)-dizinc(II)] monohydrate, C28H30Zn2N8O13V4
- Crystal structure of acotiamide hydrochloride dimethylacetamide solvate (1/1), C25H40ClN5O6S
- Crystal structure of catena-poly[monoaqua (u2-(3-(3,5-dicarboxyphenyl)pyridine-2,6-dicarboxylic-k4O:O′:O″:N)zinc(II))] monohydrate, C15H11NO10Zn
- Crystal structure of dichlorido{2,6-bis(3,5-diisopropyl-N-pyrazolyl)pyridine}zinc(II), C23H33Cl2N5Zn
- Crystal structure of nitrato-κ2O,O′-[hydridotris(3,5-diethylpyrazol-1-yl)borato-κ3N,N′,N″]copper(II), C21H34BCuN7O3
- Crystal structure of 2,7-bis(3,5-diethyl-1H-pyrazol-4-yl)-benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetrone, C28H26N6O4
- Crystal structure of 2-(4-chlorophenyl)benzothiazole, C13H8ClNS
- Synthesis and crystal structure (3R,4′S)-4′-(3,5-dibromophenyl)-1′-methyl-2H-dispiro [benzofuran-3,3′-pyrrolidine-2′,2″-indene]-1″,2,3″-trione, C26H17Br2NO4
- Crystal structure of bis(((3a,7a-dihydro-1H-benzo[d][1,2,3]triazol-1-yl)methyl) triphenylphosphonium) tetrachloridomanganate(II), C50H42Cl4MnN6P2
- The crystal structure of 4,9-bis(4-chlorophenyl)-1,6-bis(2-cyanobenzyl)-2,4a,5,6,7,7a-hexahydro-1H-2,7,5-(epiprop[2]ene[1,1,3]triyl)pyrrolo[3,4-b]pyridine-3,10-dicarbonitrile, C40H26Cl2N6
- The crystal structure of poly((μ2-3-(3-nitro-4-carboxylphenyl)benzoate-κ3O, O′:O″)-μ2-1,4-bis(1-imidazolyl)benzene-κ2N:N′-cadmium(II)), C26H17N5O6Cd
- The crystal structure of 6-hydroxy-5H-pyrrolo[3,4-b]pyridine-5,7(6H)-dione monohydrate, C7H6N2O4
- Crystal structure of 4-((cyclohexylsulfonyl)methyl)-1,2,3,4-tetrahydrobenzo [4,5]imidazo[1,2-a]pyridine, C18H24N2O2S
- Crystal structure of 4,7-diphenyl-1,10-phenanthroline-κ2N,N′)-bis(2,4-di(fluorine)-1-phenylpyridine-κ2C,N)-iridium(III) hexafluorophosphate–dichloromethane (1/1), C47H30Cl2F10IrN4P
- Crystal structure of (4-(1-phenyl-1H-benzo[d]imidazol-2-yl)phenyl)boronic acid, C19H15BN2O2
- The crystal structure of (E)-(2-((pyridin-2-ylmethylene)amino)phenyl)arsonic acid, C12H11AsN2O3
- The crystal structure of N(benzyl(phenyl)carbomothioyl)benzamide, C21H18N2OS
- The crystal structure of bis(2-picolinium) hexachlorostannate dichloromethane monosolvate, C13H18Cl8N2Sn
- Crystal structure of poly[tetraaqua-bis(μ4-3–1-(carboxylatomethyl)-1H-1,2,4-triazole-3-carboxylato)-κ4O:O′,O″,N)zinc(II)], C5H7N3O6Zn
- The crystal structure of the co-crystal isonicotinamide – 2-(nitrophenyl)methanol (1/1), C6H6N2O·C7H7NO3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-fluorobenzoate hydrate, C23H25F2N3O6
- Crystal structure of [diaqua-{1H-benzo[d]imidazol-3-ium-5,6-dicarboxylato-κ2O,O′}magnesium(II)] C18H14MgN4O10
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl) (2-iodo-5-methoxyphenyl)methanone, C20H20INO5
- The crystal structure of 3,7,11-trimethylbenzo[5,6][1,4]thiazino[2,3,4-kl]phenothiazine 5,5,9,9-tetraoxide, C21H17NO4S2
- Crystal structure of tris(piperazine-1,4-diium)bis(2-hydroxy-1,2,3-propane-tricarboxylate) pentahydrate, C24H56N6O19
- Crystal structure of 2-chloro-5-((5-isopropyl-2-methylphenoxy)methyl)pyridine, C16H18ClNO
- Crystal structure of (E)-4-(4-(1H-pyrrol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[(b)]oxepin-5(2H)-one, C23H21NO4
- Crystal structure of (E)-N′-(3,4-dichlorobenzylidene)-3,4,5-trimethoxybenzohydrazide, C17H16Cl2N2O4
- The crystal structure of 2-(2-hydroxyphenyl)-3-(pyridin-2-yl)-2,3- dihydroquinazolin-4(1H)-one, C19H15N3O2
- Crystal structure of 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-8-methylchroman-4-one, C17H16O5
- Crystal structure of bis[(3,4-dimethoxybenzyl)triphenylphosphonium]di-μ2-bromido-dibromidodicopper(I)
- Crystal structure of bis [(1,3-dioxolan-2-ylmethyl)triphenylphosphonium] tetrabromidodicopper(I), C22H22Br2CuO2P
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid], C12H8N2O5
- The crystal structure of one-dimensional cooridnation polymer bis(thiocyanato)-bis((1E,2E)-1,2-bis(1-(pyridin-3-yl)ethylidene)-hydrazine κ2N:N)iron(II), (C30H28N10S2Fe)n
- Crystal structure of ((4-acetamidophenyl)sulfonyl)-l-alanine, C11H14N2O5S
- Crystal structure of [(1-naphthalen-1-yl-methyl)triphenylphosphonium] dichloridocopper(I), [C29H24P]+[CuCl2]−
- RbTm3S5: the first rubidium lanthanoid(III) sulfide with CsEr3Se5-type crystal structure
- Crystal structure of 2,2′-((ethane-1,2-diylbis(methylammoniumdiyl))bis(methylene))bis(pyridin-1-ium) diiodido-tris(μ2-iodido-κ2I:I)dicopper(II) chloride dihydrate, C16H30Cu2I6N4O2
- The crystal structure of 4-(trifluoromethyl)pyridine-2-carboxylic acid, C7H4F3NO2
- The crystal structure of (E)-2-ethoxy-1-methoxy-4-(2-(methylsulfonyl)vinyl)benzene, C12H16O4S
- Crystal structure of potassium 1H,1H,2H,2H-perfluorooctanesulfonate, C8H4O3F13SK
- Crystal structure of 4-(4-(quinolin-8-yloxy)-1,2,5-thiadiazol-3-yl)morpholine, C15H14O2N4S
- The crystal structure of 1,4-bis(bromomethyl)-2,5-dimethylbenzene, C10H12Br2
- The crystal structure of imidazo[4,5-e][1,3]diazepine-4,6,8-triamine methanol solvate, C7H11N7O
- The crystal structure of chlorido-bis(1,10-phenantroline-κ2N,N′)-(2-formylphenoxyacetato-κ2O,O) lead(II), C33H23N4O4ClPb
- Crystal structure of pyridinium tetrakis(1,1,1-trifluoro-2,4-pentadionato-κ2O,O′)yttrium(III) C20F12H16YO8C5H6N

