Abstract
C16H12NaSmO11, monoclinic, P21/c (no. 14), a = 6.9833(14) Å, b = 17.482(3) Å, c = 14.875(4) Å, β = 112.23(3)∘, V = 1681.0(7) Å3, Z = 4, Rgt(F) = 0.0343, wRref(F2) = 0.0759, T = 293(2) K.
Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
![Figure 1:
Coordination environment of the Sm(III) and Na(I) ions in title compound. [symmetry codes: A 1 + x, 1.5 - y, 1/2 + z; B -x, 1/2 + y, 1.5 - z; C x, 1.5 - y, -1/2 + z; D 1 - x, 1 - y, 2 - z; E 1 - x, 1/2 + y, 2.5 - z].](/document/doi/10.1515/ncrs-2025-0259/asset/graphic/j_ncrs-2025-0259_fig_001.jpg)
Coordination environment of the Sm(III) and Na(I) ions in title compound. [symmetry codes: A 1 + x, 1.5 - y, 1/2 + z; B -x, 1/2 + y, 1.5 - z; C x, 1.5 - y, -1/2 + z; D 1 - x, 1 - y, 2 - z; E 1 - x, 1/2 + y, 2.5 - z].
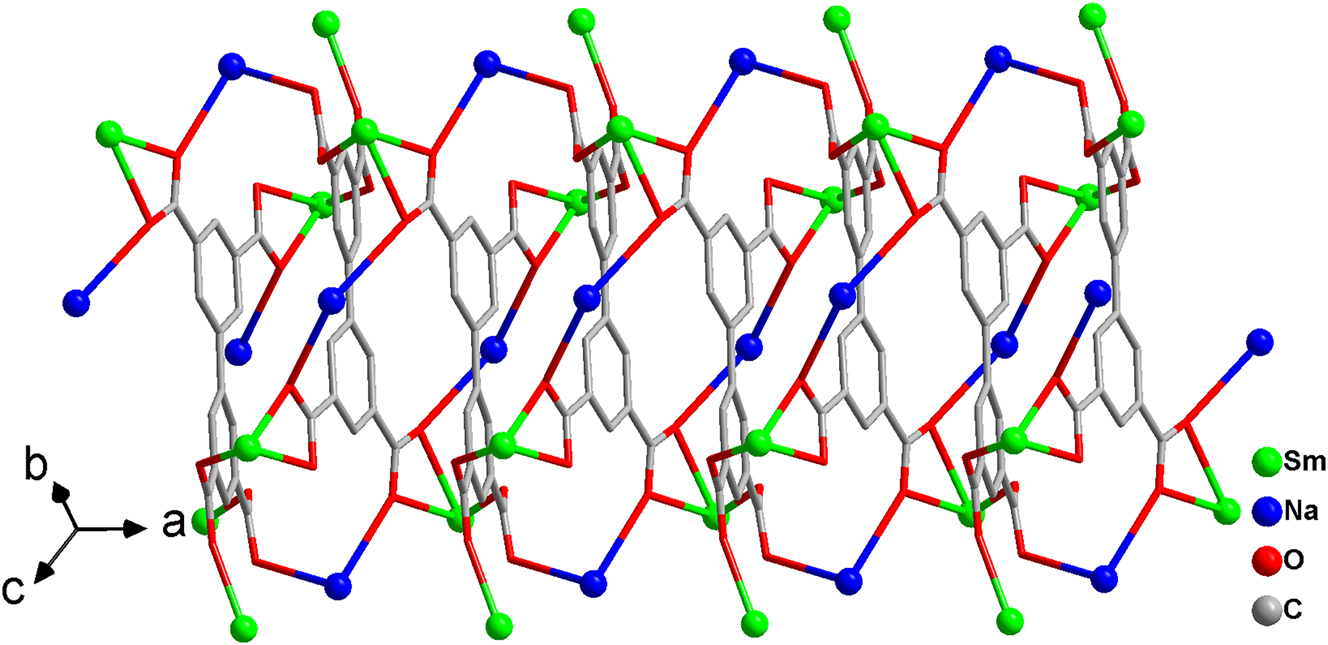
The one-dimensional chain running along the a-axis.
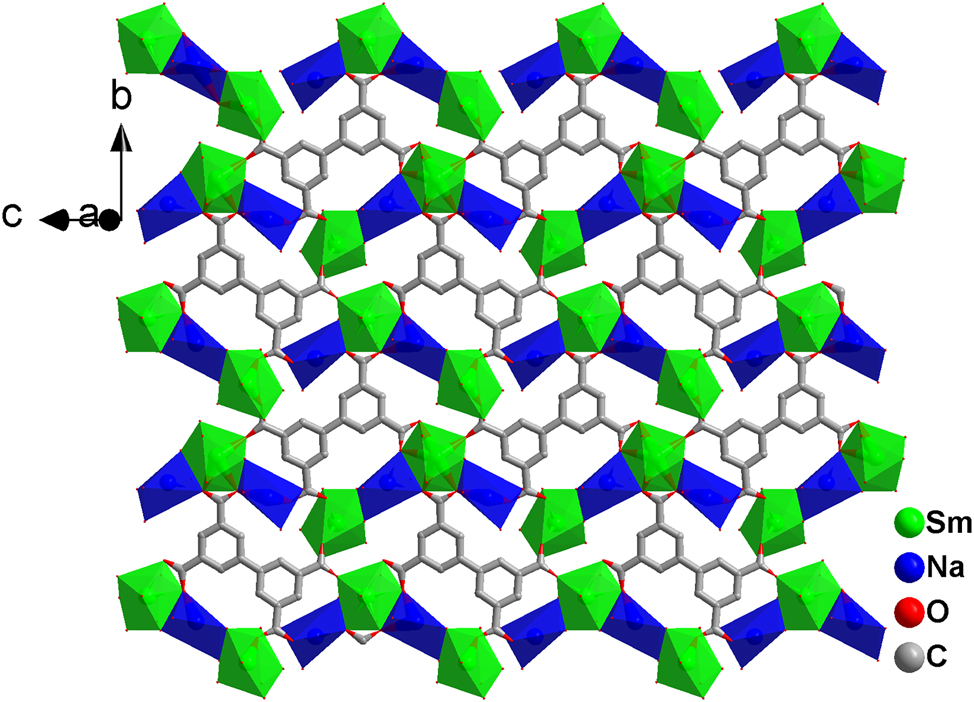
Two-dimensional layered structure of the title compound.
Data collection and handling.
| Crystal: | Colorless prism |
| Size: | 0.33 × 0.30 × 0.30 mm |
| Wavelength: μ: |
Mo Kα radiation (0.71073 Å) 3.59 mm−1 |
| Diffractometer, scan mode: θmax, completeness: |
Bruker APEX-II, φ and ω scans 25.5°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 7348, 3081, 0.051 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2944 |
| N(param)refined: | 262 |
| Programs: | Bruker, 1 SHELX, 2 , 3 Diamond 4 |
1 Source of materials
All chemical reagents were purchased from commercial sources without further purification. The mixture of samarium(III) nitrate hexahydrate (0.088 g, 0.2 mmol) and biphenyl-3,3′,5,5′-tetracarboxylicacid (H4bpta) (0.033 g, 0.1 mmol) were dissolved in 8 mL CH3CN/H2O (1:2 vol ratio). Then the solution was adjusted to approximately pH 7.5 with dilute NaOH solution. The resulting solution was sealed in a 15 ml Teflon-lined autoclave for 72 h at 433 K. Colourless block crystals of the title compound were obtained after cooling to room temperature.
2 Experimental details
The structure was solved with the ShelXT 2 structure solution program and refined with the ShelXL. 3 The hydrogen atoms on C atoms were positioned geometrically and refined using the riding model, with C–H = 0.93 Å and Uiso(H) = 1.2Ueq(C), and hydrogen atoms of water were located from a difference Fourier map with O–H = 0.82 Å and Uiso(H) = 1.5Ueq(O).
3 Comment
Metal-organic frameworks (MOFs) have attracted increasing attention due to their diverse structures with strong designability, high porosity and large specific surface area, and excellent optical properties, showing broad application prospects in gas storage, 5 separation, 6 catalysis, 7 sensing, 8 and optoelectronic materials. 9 Among them, lanthanide based MOFs (Ln–MOFs) have become an important platform for the design of multifunctional materials, thanks to the unique 4f electronic configuration (such as excellent luminescent properties, high coordination numbers, and magnetism). 10 However, the coordination behavior of rare earth ions is easily restricted by the ligand configuration. How to achieve stable high-dimensional Ln–MOF structures through ligand design and further regulate their physicochemical properties remains one of the key challenges in this field. 11
Rigid carboxylate ligands, which combine the structural directivity of rigid ligands and the conformational adaptability of ligands, 12 provide an ideal choice for constructing novel Ln–MOFs. For example, rigid dicarboxylate or polycarboxylate ligands 13 , 14 , 15 feature several hard O,O-chelating sites, can bind several metal ions. In addition, the carboxylate groups can adopt monodentate and chelating or bridging modes, thus forming unique topological networks or functionalized channel structures with rare earth ions. Moreover, the variable conformations of such ligands may induce dynamic responses of the framework to external stimuli (such as temperature, pressure, or guest molecules). 16
In this paper, a rigid planar biphenyl-3,3′,5,5-tetracarboxylicacid (H4bpta) ligand was strategically employed to react with samarium nitrate under alkaline conditions, yielding a heteronuclear Na(I)–Sm(III)-based MOF featuring a three-dimensional network architecture. Single crystal X-ray diffraction analysis reveals that the title complex crystallizes in the space group P21/c. The asymmetric unit comprises one crystallographically unique Sm(III) ion, one Na(I) ion, one fully deprotonated bpta4− ligand, and three coordinating water molecules. As shown in Figure 1, the Sm(III) center presents a nine-coordinate tricapped triangular prismatic geometry, coordinating with seven carboxylate oxygen atoms from five bpta4− ligands and two water molecules (O1, O3A, O4B, O5C, O6C, O7D, O8D, O9, and O10; symmetry codes: A 1 + x, 1.5 − y, 1/2 + z; B −x, 1/2 + y, 1.5 − z; C x, 1.5 − y, −1/2 + z; D 1 − x, 1 − y, 2 − z). The Sm–O bond lengths range from 2.308(3) to 2.672(4) Å, consistent with previously reported samarium complexes. 17 , 18 , 19 , 20 Notably, the Na(I) ion exhibits a distorted octahedral coordination environment, where the equatorial plane is formed by three carboxylate oxygen atoms (O5, O7E, and O8D; symmetry codes: E 1 − x, 1/2 + y, 2.5 − z) from bpta4− ligands and a bridging water molecule (O10), and two axial positions are occupied by an oxygen atom (O2A) from bpta4− and a terminal water molecule (O11). The Na–O bond lengths are 2.720(4) to 2.877(5) Å, while the O–Sm–O angles range from 50.83(11)° to 152.37(14)°.
In the crystal structure, the bpta4− ligand exhibits a μ9- κ11−coordination mode, simultaneously linking five Sm(III) and four Na(I) ions. The 3,3′-carboxylate groups adopt syn-anti and μ–O bridging configurations to connect the heteronuclear metal centers, forming a one-dimensional zigzag [SmNa(COO)2] n chain with Na⋯Sm separations of 4.283–4.348 Å. Along the a axis direction, sequential Sm(III) and Na(I) ions are further connected through the carboxylate groups of the coupled bpta4− ligands, constructing a one-dimensional chain structure with a 22-membered ring (Figure 2).
On the bc-plane, Sm(III)- and Na(I)-centered polyhedra are connected via shared edges to form an asymmetric tetraatomic metal cluster unit. These clusters are further linked by carboxylate groups into infinite one-dimensional chains extending along the c-axis. Adjacent chains are bridged by bpta4− ligands, constructing two-dimensional layer (Figure 3). Such two-dimensional layered structures stack to form a three-dimensional network structure.
Acknowledgments
This work was financially supported by the Natural Science Foundation of Shanxi Province (No. 202203021222318), Program for the Innovative Talents of Higher Education Institutions of Shanxi (No. 2021 L568) and Key Research and Development Project of Lyuliang (No. 2023GXYF10).
References
1. Bruker. Apex2, Saint and Sadabs; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Suche in Google Scholar
2. Sheldrick, G. M. Shelxtl-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Brandenburg, K. Diamond. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Suche in Google Scholar
5. Xie, W. P.; Fu, Q. .J.; Yang, L. Z.; Yan, L. T.; Zhang, J.; Zhao, X. B. Methane Storage and Purification of Natural Gas in Metal-organic Frameworks. ChemSusChem 2025, 18, 202401382; https://doi.org/10.1002/cssc.202401382.Suche in Google Scholar PubMed
6. Zhang, Y. Q.; Zhang, Y. J.; Hu, T. P. A Porous three-dimensional Cu–MOF: Preparation and Application in Supercapacitors, Low Temperature Hydrogen Storage and Gas Separation. Inorg. Chim. Acta 2025, 575, 122414; https://doi.org/10.1016/j.ica.2024.122414.Suche in Google Scholar
7. Zheng, D. Y.; Gao, J.; Diao, Y.; Li, M.; Huang, X. C. MOF Confinement Enables Selective Synthesis of Novel Oxazoles from Indole and Formaldehyde. Chem. Commun. 2025, 61, 2520–2523; https://doi.org/10.1039/d4cc06486k.Suche in Google Scholar PubMed
8. Su, F.; Cheng, P. Z.; Liu, Y. H.; Liu, X. Y.; Sun, Y.; Li, S. X.; Sun, L.; Wang, Z. J. Heterometal-Organic Frameworks as Highly Sensitive and Highly Selective Luminescent Probes to Detect Cu2+, H2PO4-ions and CTC in Aqueous Solutions. J. Mol. Struct. 2025, 1322, 140591; https://doi.org/10.1016/j.molstruc.2024.140591.Suche in Google Scholar
9. Mao, Z.; Zhang, S. Y.; Zhao, D.; Weng, X.; Kang, C.; Fang, H.; Zeng, Y. J. Magnetic and Optoelectronic Modulation of Cu–MOF-74 Films by Quantum Dots. J. Mater. Chem. C 2023, 11, 11204–11212; https://doi.org/10.1039/d3tc01879b.Suche in Google Scholar
10. Younis, S. A.; Bhardwaj, N.; Bhardwaj, S. K.; Sanjeev, K.; Kim, K. H.; Deep, A. Rare Earth Metal-organic Frameworks (RE–MOFs): Synthesis, Properties, and Biomedical Applications. Coord. Chem. Rev. 2021, 429, 213620; https://doi.org/10.1016/j.ccr.2020.213620.Suche in Google Scholar
11. Shi, Q.; Xu, Y. H.; Chen, M. H.; Zhao, P.; Deng, W. B.; Xiong, J.; Guo, K.; Feng, Y. Q.; Zhang, B. A Versatile Synthetic Strategy Towards Rare Earth Based Metal-organic Frameworks. Sci. China Chem. 2025, 68, 1362–1371; https://doi.org/10.1007/s11426-024-2322-9.Suche in Google Scholar
12. Su, F.; Zhou, C. Y.; Han, C.; Wu, L. T.; Wu, X.; Sun, L.; Su, J.; Feng, S. S.; Lu, L. P.; Zhu, M. L. Binuclear Mn2+ Complexes of a Biphenyltetracarboxylic Acid with Variable N-donor Ligands: Syntheses, Structures, and Magnetic Properties. CrystEngComm 2018, 20, 1818–1831; https://doi.org/10.1039/c8ce00058a.Suche in Google Scholar
13. Tang, Y.; Ji, G. F.; Li, H. N.; Gao, H.; He, C.; Zhao, L.; Duan, C. Y. Ligand-Regulated Photoinduced Electron Transfer Within metal-organic Frameworks for Efficient Photocatalysis. Inorg. Chem. Front. 2023, 10, 5439–5451; https://doi.org/10.1039/d3qi01120h.Suche in Google Scholar
14. Dong, L. Z.; Zhang, L.; Liu, J.; Huang, Q.; Lu, M.; Ji, W. X.; Lan, Y. Q. Stable Heterometallic Cluster–based Organic Framework Catalysts for Artificial Photosynthesis. Angew. Chem., Int. Ed. 2020, 59, 2659–2663; https://doi.org/10.1002/anie.201913284.Suche in Google Scholar PubMed
15. Yang, X. F.; Zhu, H. B.; Liu, M. Transition-Metal-Based (Zn2+ and Cd2+) Metal-organic Frameworks as Fluorescence “turn-off” Sensors for Highly Sensitive and Selective Detection of Hydrogen Sulfide. Inorg. Chim. Acta 2017, 466, 410–416; https://doi.org/10.1016/j.ica.2017.06.067.Suche in Google Scholar
16. Wang, Y. N.; Li, H. J.; He, X. L.; Xu, Z. Q. Application in Anticounterfeiting for Multistimuli Smart Luminescent Materials Based on MOF-on-MOF. Inorg. Chem. 2021, 60, 15001–15009; https://doi.org/10.1021/acs.inorgchem.1c02455.Suche in Google Scholar PubMed
17. Yin, L.; Huang, J. B.; Yue, T. C.; Wang, L. L.; Wang, D. Z. Two 2D metal-organic Frameworks Based on Purine Carboxylic Acid Ligands for Photocatalytic Oxidation of Sulfides and CO2 Chemical Fixation. Inorg. Chem. 2024, 63, 9109–9118; https://doi.org/10.1021/acs.inorgchem.4c00333.Suche in Google Scholar PubMed
18. Song, J. Y.; Zhang, X. Y.; Zhang, C. L.; Zhao, B. B.; Huang, S. J.; Liu, X. Crystal Structure of (diaqua-bis (phenanthroline-κ2 N,N′))-tetrakis (m2-3,4,5,6-tetrafluorophthalato-κ4 O,O:O′: O″; κ2 O:O′)dierbium(III) phenanthroline(1/2), C80H38Er2F16N8O18. Z. Kristallogr. – N. Cryst. Struct. 2023, 238, 441–443; https://doi.org/10.1515/ncrs-2023-0023.Suche in Google Scholar
19. Cheng, Z.; Zhang, X. Y.; Liu, X.; Yang, X. R. Crystal structure of poly[hexaqua-pentakis(μ4-2,2′-bipyridine-4,4′-dicarboxylato-κ4 O:O′:O″: O‴)(μ2-2,2′-bipyridine-4,4′-dicarboxylato-κ2 O:O) tetraytterbium(III)]hydrate, C36H26N6O16Yb2. Z. Kristallogr. – N. Cryst. Struct. 2025, 240, 7–9; https://doi.org/10.1515/ncrs-2024-0278.Suche in Google Scholar
20. Zhang, L.; Song, T.; Xu, J.; Sun, J.; Zeng, S.; Wu, Y.; Fan, Y.; Wang, L. Polymorphic Ln(III) and BPTC-based Porous metal–organic Frameworks with Visible, NIR Photoluminescent and Magnetic Properties. CrystEngComm 2014, 16, 2440; https://doi.org/10.1039/C3CE42181C.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of (1Z, 2Z)-3-phenyl-2-propenal 2-(4-bromo-2-fluorophenyl)hydrazone, C15H12BrFN2
- Refinement of crystal structure of 2-(2,3-dihydro-3-oxo-1 H -inden-1-ylidene)-1 H -indene-1,3(2 H )-dione C18H10O3
- The crystal structure of 3-(1-fluoro-2-(naphthalen-2-yl)-2-oxoethyl)-2-methoxy-3,4-dihydroisoquinolin-1(2H)-one, C22H18FNO3
- Crystal structure of the dinuclear copper(II) complex bis(μ2-2,2′ -{[1,3-phenylenebis-(methylene)]bis(oxy)}dibenzoaot-κ4O,O′:O′′,O′′′)-bis(dimethylformamide-κ1O)dicopper(II), C50H44Cu2N2O14
- Crystal structure of poly[triaqua-(μ9-biphenyl-3,3′,5,5′-tetracarboxylic-κ8 O,O:O,O′: O,O″:O,O‴)samarium(III)sodium(I)], C16H12NaSmO11
- The crystal structure of 5-benzyl-1-(4-fluorobenzyl)-4-((4-fluorobenzyl)oxy)-1,5-dihydro-2H-pyrrol-2-one, C25H21F2NO2
- The crystal structure of diammonium 2,5-dihydroxyterephthalate, C8H12N2O6
- Crystal structure of (E)-4-(4-(1H-1,2,4-triazol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[b]oxepin-5(2H)-one, C21H19N3O4
- Crystal structure of poly[oktakis(μ2-oxido-κ2O:O)-tetrakis(oxido-κ1O)-bis(μ2-1,1′-[1,4-phenylenebis(methylene)]di(1H-imidazole-κ2N:N′))-tetravanadium(V)-dizinc(II)] monohydrate, C28H30Zn2N8O13V4
- Crystal structure of acotiamide hydrochloride dimethylacetamide solvate (1/1), C25H40ClN5O6S
- Crystal structure of catena-poly[monoaqua (u2-(3-(3,5-dicarboxyphenyl)pyridine-2,6-dicarboxylic-k4O:O′:O″:N)zinc(II))] monohydrate, C15H11NO10Zn
- Crystal structure of dichlorido{2,6-bis(3,5-diisopropyl-N-pyrazolyl)pyridine}zinc(II), C23H33Cl2N5Zn
- Crystal structure of nitrato-κ2O,O′-[hydridotris(3,5-diethylpyrazol-1-yl)borato-κ3N,N′,N″]copper(II), C21H34BCuN7O3
- Crystal structure of 2,7-bis(3,5-diethyl-1H-pyrazol-4-yl)-benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetrone, C28H26N6O4
- Crystal structure of 2-(4-chlorophenyl)benzothiazole, C13H8ClNS
- Synthesis and crystal structure (3R,4′S)-4′-(3,5-dibromophenyl)-1′-methyl-2H-dispiro [benzofuran-3,3′-pyrrolidine-2′,2″-indene]-1″,2,3″-trione, C26H17Br2NO4
- Crystal structure of bis(((3a,7a-dihydro-1H-benzo[d][1,2,3]triazol-1-yl)methyl) triphenylphosphonium) tetrachloridomanganate(II), C50H42Cl4MnN6P2
- The crystal structure of 4,9-bis(4-chlorophenyl)-1,6-bis(2-cyanobenzyl)-2,4a,5,6,7,7a-hexahydro-1H-2,7,5-(epiprop[2]ene[1,1,3]triyl)pyrrolo[3,4-b]pyridine-3,10-dicarbonitrile, C40H26Cl2N6
- The crystal structure of poly((μ2-3-(3-nitro-4-carboxylphenyl)benzoate-κ3O, O′:O″)-μ2-1,4-bis(1-imidazolyl)benzene-κ2N:N′-cadmium(II)), C26H17N5O6Cd
- The crystal structure of 6-hydroxy-5H-pyrrolo[3,4-b]pyridine-5,7(6H)-dione monohydrate, C7H6N2O4
- Crystal structure of 4-((cyclohexylsulfonyl)methyl)-1,2,3,4-tetrahydrobenzo [4,5]imidazo[1,2-a]pyridine, C18H24N2O2S
- Crystal structure of 4,7-diphenyl-1,10-phenanthroline-κ2N,N′)-bis(2,4-di(fluorine)-1-phenylpyridine-κ2C,N)-iridium(III) hexafluorophosphate–dichloromethane (1/1), C47H30Cl2F10IrN4P
- Crystal structure of (4-(1-phenyl-1H-benzo[d]imidazol-2-yl)phenyl)boronic acid, C19H15BN2O2
- The crystal structure of (E)-(2-((pyridin-2-ylmethylene)amino)phenyl)arsonic acid, C12H11AsN2O3
- The crystal structure of N(benzyl(phenyl)carbomothioyl)benzamide, C21H18N2OS
- The crystal structure of bis(2-picolinium) hexachlorostannate dichloromethane monosolvate, C13H18Cl8N2Sn
- Crystal structure of poly[tetraaqua-bis(μ4-3–1-(carboxylatomethyl)-1H-1,2,4-triazole-3-carboxylato)-κ4O:O′,O″,N)zinc(II)], C5H7N3O6Zn
- The crystal structure of the co-crystal isonicotinamide – 2-(nitrophenyl)methanol (1/1), C6H6N2O·C7H7NO3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-fluorobenzoate hydrate, C23H25F2N3O6
- Crystal structure of [diaqua-{1H-benzo[d]imidazol-3-ium-5,6-dicarboxylato-κ2O,O′}magnesium(II)] C18H14MgN4O10
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl) (2-iodo-5-methoxyphenyl)methanone, C20H20INO5
- The crystal structure of 3,7,11-trimethylbenzo[5,6][1,4]thiazino[2,3,4-kl]phenothiazine 5,5,9,9-tetraoxide, C21H17NO4S2
- Crystal structure of tris(piperazine-1,4-diium)bis(2-hydroxy-1,2,3-propane-tricarboxylate) pentahydrate, C24H56N6O19
- Crystal structure of 2-chloro-5-((5-isopropyl-2-methylphenoxy)methyl)pyridine, C16H18ClNO
- Crystal structure of (E)-4-(4-(1H-pyrrol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[(b)]oxepin-5(2H)-one, C23H21NO4
- Crystal structure of (E)-N′-(3,4-dichlorobenzylidene)-3,4,5-trimethoxybenzohydrazide, C17H16Cl2N2O4
- The crystal structure of 2-(2-hydroxyphenyl)-3-(pyridin-2-yl)-2,3- dihydroquinazolin-4(1H)-one, C19H15N3O2
- Crystal structure of 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-8-methylchroman-4-one, C17H16O5
- Crystal structure of bis[(3,4-dimethoxybenzyl)triphenylphosphonium]di-μ2-bromido-dibromidodicopper(I)
- Crystal structure of bis [(1,3-dioxolan-2-ylmethyl)triphenylphosphonium] tetrabromidodicopper(I), C22H22Br2CuO2P
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid], C12H8N2O5
- The crystal structure of one-dimensional cooridnation polymer bis(thiocyanato)-bis((1E,2E)-1,2-bis(1-(pyridin-3-yl)ethylidene)-hydrazine κ2N:N)iron(II), (C30H28N10S2Fe)n
- Crystal structure of ((4-acetamidophenyl)sulfonyl)-l-alanine, C11H14N2O5S
- Crystal structure of [(1-naphthalen-1-yl-methyl)triphenylphosphonium] dichloridocopper(I), [C29H24P]+[CuCl2]−
- RbTm3S5: the first rubidium lanthanoid(III) sulfide with CsEr3Se5-type crystal structure
- Crystal structure of 2,2′-((ethane-1,2-diylbis(methylammoniumdiyl))bis(methylene))bis(pyridin-1-ium) diiodido-tris(μ2-iodido-κ2I:I)dicopper(II) chloride dihydrate, C16H30Cu2I6N4O2
- The crystal structure of 4-(trifluoromethyl)pyridine-2-carboxylic acid, C7H4F3NO2
- The crystal structure of (E)-2-ethoxy-1-methoxy-4-(2-(methylsulfonyl)vinyl)benzene, C12H16O4S
- Crystal structure of potassium 1H,1H,2H,2H-perfluorooctanesulfonate, C8H4O3F13SK
- Crystal structure of 4-(4-(quinolin-8-yloxy)-1,2,5-thiadiazol-3-yl)morpholine, C15H14O2N4S
- The crystal structure of 1,4-bis(bromomethyl)-2,5-dimethylbenzene, C10H12Br2
- The crystal structure of imidazo[4,5-e][1,3]diazepine-4,6,8-triamine methanol solvate, C7H11N7O
- The crystal structure of chlorido-bis(1,10-phenantroline-κ2N,N′)-(2-formylphenoxyacetato-κ2O,O) lead(II), C33H23N4O4ClPb
- Crystal structure of pyridinium tetrakis(1,1,1-trifluoro-2,4-pentadionato-κ2O,O′)yttrium(III) C20F12H16YO8C5H6N
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of (1Z, 2Z)-3-phenyl-2-propenal 2-(4-bromo-2-fluorophenyl)hydrazone, C15H12BrFN2
- Refinement of crystal structure of 2-(2,3-dihydro-3-oxo-1 H -inden-1-ylidene)-1 H -indene-1,3(2 H )-dione C18H10O3
- The crystal structure of 3-(1-fluoro-2-(naphthalen-2-yl)-2-oxoethyl)-2-methoxy-3,4-dihydroisoquinolin-1(2H)-one, C22H18FNO3
- Crystal structure of the dinuclear copper(II) complex bis(μ2-2,2′ -{[1,3-phenylenebis-(methylene)]bis(oxy)}dibenzoaot-κ4O,O′:O′′,O′′′)-bis(dimethylformamide-κ1O)dicopper(II), C50H44Cu2N2O14
- Crystal structure of poly[triaqua-(μ9-biphenyl-3,3′,5,5′-tetracarboxylic-κ8 O,O:O,O′: O,O″:O,O‴)samarium(III)sodium(I)], C16H12NaSmO11
- The crystal structure of 5-benzyl-1-(4-fluorobenzyl)-4-((4-fluorobenzyl)oxy)-1,5-dihydro-2H-pyrrol-2-one, C25H21F2NO2
- The crystal structure of diammonium 2,5-dihydroxyterephthalate, C8H12N2O6
- Crystal structure of (E)-4-(4-(1H-1,2,4-triazol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[b]oxepin-5(2H)-one, C21H19N3O4
- Crystal structure of poly[oktakis(μ2-oxido-κ2O:O)-tetrakis(oxido-κ1O)-bis(μ2-1,1′-[1,4-phenylenebis(methylene)]di(1H-imidazole-κ2N:N′))-tetravanadium(V)-dizinc(II)] monohydrate, C28H30Zn2N8O13V4
- Crystal structure of acotiamide hydrochloride dimethylacetamide solvate (1/1), C25H40ClN5O6S
- Crystal structure of catena-poly[monoaqua (u2-(3-(3,5-dicarboxyphenyl)pyridine-2,6-dicarboxylic-k4O:O′:O″:N)zinc(II))] monohydrate, C15H11NO10Zn
- Crystal structure of dichlorido{2,6-bis(3,5-diisopropyl-N-pyrazolyl)pyridine}zinc(II), C23H33Cl2N5Zn
- Crystal structure of nitrato-κ2O,O′-[hydridotris(3,5-diethylpyrazol-1-yl)borato-κ3N,N′,N″]copper(II), C21H34BCuN7O3
- Crystal structure of 2,7-bis(3,5-diethyl-1H-pyrazol-4-yl)-benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetrone, C28H26N6O4
- Crystal structure of 2-(4-chlorophenyl)benzothiazole, C13H8ClNS
- Synthesis and crystal structure (3R,4′S)-4′-(3,5-dibromophenyl)-1′-methyl-2H-dispiro [benzofuran-3,3′-pyrrolidine-2′,2″-indene]-1″,2,3″-trione, C26H17Br2NO4
- Crystal structure of bis(((3a,7a-dihydro-1H-benzo[d][1,2,3]triazol-1-yl)methyl) triphenylphosphonium) tetrachloridomanganate(II), C50H42Cl4MnN6P2
- The crystal structure of 4,9-bis(4-chlorophenyl)-1,6-bis(2-cyanobenzyl)-2,4a,5,6,7,7a-hexahydro-1H-2,7,5-(epiprop[2]ene[1,1,3]triyl)pyrrolo[3,4-b]pyridine-3,10-dicarbonitrile, C40H26Cl2N6
- The crystal structure of poly((μ2-3-(3-nitro-4-carboxylphenyl)benzoate-κ3O, O′:O″)-μ2-1,4-bis(1-imidazolyl)benzene-κ2N:N′-cadmium(II)), C26H17N5O6Cd
- The crystal structure of 6-hydroxy-5H-pyrrolo[3,4-b]pyridine-5,7(6H)-dione monohydrate, C7H6N2O4
- Crystal structure of 4-((cyclohexylsulfonyl)methyl)-1,2,3,4-tetrahydrobenzo [4,5]imidazo[1,2-a]pyridine, C18H24N2O2S
- Crystal structure of 4,7-diphenyl-1,10-phenanthroline-κ2N,N′)-bis(2,4-di(fluorine)-1-phenylpyridine-κ2C,N)-iridium(III) hexafluorophosphate–dichloromethane (1/1), C47H30Cl2F10IrN4P
- Crystal structure of (4-(1-phenyl-1H-benzo[d]imidazol-2-yl)phenyl)boronic acid, C19H15BN2O2
- The crystal structure of (E)-(2-((pyridin-2-ylmethylene)amino)phenyl)arsonic acid, C12H11AsN2O3
- The crystal structure of N(benzyl(phenyl)carbomothioyl)benzamide, C21H18N2OS
- The crystal structure of bis(2-picolinium) hexachlorostannate dichloromethane monosolvate, C13H18Cl8N2Sn
- Crystal structure of poly[tetraaqua-bis(μ4-3–1-(carboxylatomethyl)-1H-1,2,4-triazole-3-carboxylato)-κ4O:O′,O″,N)zinc(II)], C5H7N3O6Zn
- The crystal structure of the co-crystal isonicotinamide – 2-(nitrophenyl)methanol (1/1), C6H6N2O·C7H7NO3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-fluorobenzoate hydrate, C23H25F2N3O6
- Crystal structure of [diaqua-{1H-benzo[d]imidazol-3-ium-5,6-dicarboxylato-κ2O,O′}magnesium(II)] C18H14MgN4O10
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl) (2-iodo-5-methoxyphenyl)methanone, C20H20INO5
- The crystal structure of 3,7,11-trimethylbenzo[5,6][1,4]thiazino[2,3,4-kl]phenothiazine 5,5,9,9-tetraoxide, C21H17NO4S2
- Crystal structure of tris(piperazine-1,4-diium)bis(2-hydroxy-1,2,3-propane-tricarboxylate) pentahydrate, C24H56N6O19
- Crystal structure of 2-chloro-5-((5-isopropyl-2-methylphenoxy)methyl)pyridine, C16H18ClNO
- Crystal structure of (E)-4-(4-(1H-pyrrol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[(b)]oxepin-5(2H)-one, C23H21NO4
- Crystal structure of (E)-N′-(3,4-dichlorobenzylidene)-3,4,5-trimethoxybenzohydrazide, C17H16Cl2N2O4
- The crystal structure of 2-(2-hydroxyphenyl)-3-(pyridin-2-yl)-2,3- dihydroquinazolin-4(1H)-one, C19H15N3O2
- Crystal structure of 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-8-methylchroman-4-one, C17H16O5
- Crystal structure of bis[(3,4-dimethoxybenzyl)triphenylphosphonium]di-μ2-bromido-dibromidodicopper(I)
- Crystal structure of bis [(1,3-dioxolan-2-ylmethyl)triphenylphosphonium] tetrabromidodicopper(I), C22H22Br2CuO2P
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid], C12H8N2O5
- The crystal structure of one-dimensional cooridnation polymer bis(thiocyanato)-bis((1E,2E)-1,2-bis(1-(pyridin-3-yl)ethylidene)-hydrazine κ2N:N)iron(II), (C30H28N10S2Fe)n
- Crystal structure of ((4-acetamidophenyl)sulfonyl)-l-alanine, C11H14N2O5S
- Crystal structure of [(1-naphthalen-1-yl-methyl)triphenylphosphonium] dichloridocopper(I), [C29H24P]+[CuCl2]−
- RbTm3S5: the first rubidium lanthanoid(III) sulfide with CsEr3Se5-type crystal structure
- Crystal structure of 2,2′-((ethane-1,2-diylbis(methylammoniumdiyl))bis(methylene))bis(pyridin-1-ium) diiodido-tris(μ2-iodido-κ2I:I)dicopper(II) chloride dihydrate, C16H30Cu2I6N4O2
- The crystal structure of 4-(trifluoromethyl)pyridine-2-carboxylic acid, C7H4F3NO2
- The crystal structure of (E)-2-ethoxy-1-methoxy-4-(2-(methylsulfonyl)vinyl)benzene, C12H16O4S
- Crystal structure of potassium 1H,1H,2H,2H-perfluorooctanesulfonate, C8H4O3F13SK
- Crystal structure of 4-(4-(quinolin-8-yloxy)-1,2,5-thiadiazol-3-yl)morpholine, C15H14O2N4S
- The crystal structure of 1,4-bis(bromomethyl)-2,5-dimethylbenzene, C10H12Br2
- The crystal structure of imidazo[4,5-e][1,3]diazepine-4,6,8-triamine methanol solvate, C7H11N7O
- The crystal structure of chlorido-bis(1,10-phenantroline-κ2N,N′)-(2-formylphenoxyacetato-κ2O,O) lead(II), C33H23N4O4ClPb
- Crystal structure of pyridinium tetrakis(1,1,1-trifluoro-2,4-pentadionato-κ2O,O′)yttrium(III) C20F12H16YO8C5H6N


