Abstract
C40H26Cl2N6, triclinic,
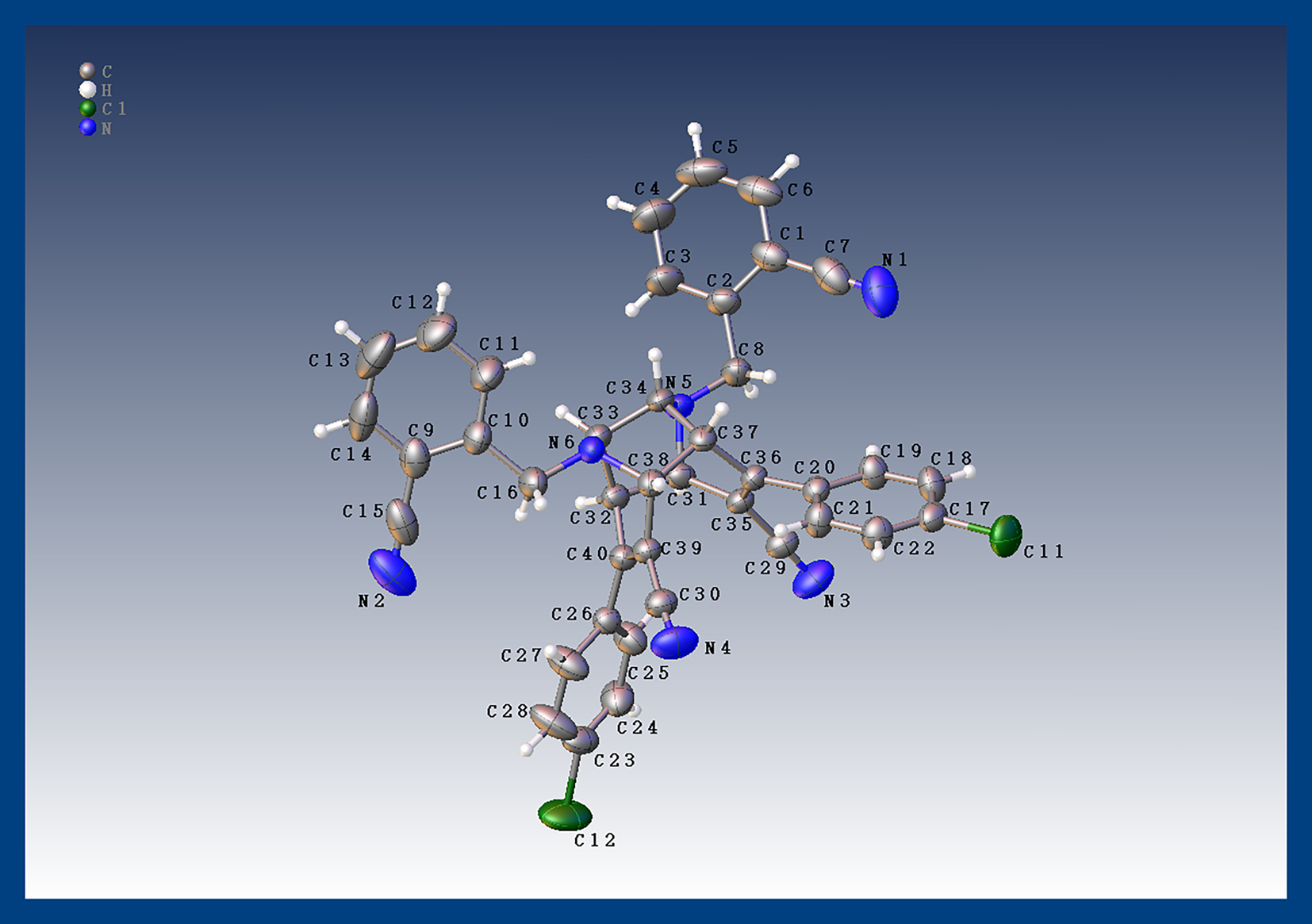
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.23 × 0.20 × 0.18 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.24 mm−1 |
| Diffractometer, scan mode: | Bruker Apex-II, φ and ω scans |
| θmax, completeness: | 25.0°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 28,687, 5,838, 0.080 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 4,358 |
| N(param)refined: | 433 |
| Programs: | Bruker, 1 Olex2, 2 Shelx 3 , 4 |
1 Source of materials
The 2-cyanobenzyl bromide (7.84 g; 40 mmol) was added to the acetonitrile (40 mL) solution of 3-cyanopyridine (4.16 g; 40 mmol) to allow the mixture to react for 6–7 h. After the reaction is finished and cooled, dichloromethane is added to precipitate the product, the product is filtered and rinsed three times with dichloromethane (10 mL*3) to obtain 1-(2-cyanobenzyl)-3-cyano-1,4-dihydropyridine bromide, which is a white powder. The resulting 1-(2-cyanobenzyl)-3-cyano-1,4-dihydropyridine bromide (3.00 g; 10 mmol) is suspended in anhydrous THF (70 mL). After the addition of CuI (0.10 g; 0.5 mmol), a solution of 4-chlorophenylmagnesium bromide (1 M) in THF (10 mL, 10 mmol) was added dropwise to the suspension. After 1–1.5 h, the reaction solution was treated with a saturated aqueous solution of ammonium chloride (60 mL), then extracted with dichloromethane (50 mL*3) and water (150 mL), and distilled under reduced pressure to obtain the crude product. The 1-(2-cyanobenzyl)-3-cyano-4-(4-chlorophenyl)-1,4-dihydropyridine was obtained by methanol recrystallization. The 1-(2-cyanobenzyl)-3-cyano-4-(4-chlorophenyl)-1,4-dihydropyridine (1.23 g; 3.7 mmol) was dissolved in ethanol (30 mL) under stirring. Under room temperature and nitrogen protection, irradiation was performed with a blue LED light (10 W, 410 nm), and the reaction ended after 10 h. The solvent was removed by reduced pressure concentration and separated and purified by rapid chromatography (petroleum ether/ethyl acetate rations of 10:1 to 8:1) to obtain the title product (total yield of 41 %). Single crystals of the title compound were obtained by crystallization from a mixture of dichloromethane (1 mL) and n-hexane (2 mL).
2 Experimental details
Using Olex2, 2 the structure was solved with the SHELXT 3 structure solution program and refined with the SHELXL 4 refinement package. All hydrogen atoms were placed in the calculated positions.
3 Comment
Cage compounds are a unique molecular structure with significant applications in the medical field, serving various functions such as anti–HIV, 5 anticancer, 6 and acting as drug linkers. 7 The synthesized title compound is a 3,12-diazatetracyclododecane-diene derivative, which is a novel cage compound. 8 Based on the original compound, 3-cyanopyridine, we synthesized the structure of the new compound described in this paper with moderate yields. The reaction route adopted in this paper has good substrate applicability and functional group compatibility.
The title compound has a unique open-ended cage structure, with two dihydropyridine planes located in different planes, separated by a dihedral angle of 160.8°. In the crystal structure, the bond lengths of C–N are in the range of 1.137(4) Å–1.470(4) Å, and the bond lengths of C–Cl are 1.727(3) Å and 1.736(3) Å, respectively. The molecule is assembled by intermolecular interactions between benzene rings. 9
-
Author contributions: Author has accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. SAINT. Program for Data Extraction and Reduction; Bruker AXS, Inc: Madison, WI, 2001.Suche in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. Shelxtl – Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3–8.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Cryst. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
5. Hilgeroth, A.; Lilie, H. Structure-activity Relationships of First Bishydroxymethyl-Substituted Cage Dimeric 4-Aryl-1,4-Dihydropyridines as HIV-1 Protease Inhibitors. Eur. J. Med. Chem. 2003, 38, 495–499; https://doi.org/10.1016/s0223-5234(03)00060-6.Suche in Google Scholar PubMed
6. Richter, M.; Molnár, J.; Hilgeroth, A. Biological Evaluation of Bishydroxymethyl-Substituted Cage Dimeric 1,4-Dihydropyridines as a Novel Class of P-Glycoprotein Modulating Agents in Cancer Cells. J. Med. Chem. 2006, 49, 2838–2840; https://doi.org/10.1021/jm058046w.Suche in Google Scholar PubMed
7. Vujcic, B.; Wyllie, J.; Tania, B. J.; White, K. F.; Cromwell, S.; Lupton, D. W.; Dutton, J. L.; Soares da Costa, T. P.; Houston, S. D. Cage Hydrocarbons as Linkers in Dimeric Drug Design: Case Studies with Trimethoprim and Tedizolid. Bioorg. Med. Chem. Lett. 2023, 80, 129086; https://doi.org/10.1016/j.bmcl.2022.129086.Suche in Google Scholar PubMed
8. Zhong, Q. D.; Wang, Y. X.; Lan, H. Y.; Zhu, H.; Fan, Q. W. Construction of 3,12-Diazatetracyclododecane-Dienes through Unexpected Visible-Light-Induced Radical Cascade Cyclization. J. Org. Chem. 2022, 87, 10937–10946; https://doi.org/10.1021/acs.joc.2c01225.Suche in Google Scholar PubMed
9. Zheng, J. Y.; Tang, W. H.; Pan, H. Y. The Crystal Structure ofN′-(tert-Butyl)-N′-(3,5-Dimethylbenzoyl)-3-Methoxy-N,2-Dimethylbenzohydrazide, C23H30N2O3. Z. Kristallogr. N. Cryst. Struct. 2024, 239, 843–845; https://doi.org/10.1515/ncrs-2024-0173.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of (1Z, 2Z)-3-phenyl-2-propenal 2-(4-bromo-2-fluorophenyl)hydrazone, C15H12BrFN2
- Refinement of crystal structure of 2-(2,3-dihydro-3-oxo-1 H -inden-1-ylidene)-1 H -indene-1,3(2 H )-dione C18H10O3
- The crystal structure of 3-(1-fluoro-2-(naphthalen-2-yl)-2-oxoethyl)-2-methoxy-3,4-dihydroisoquinolin-1(2H)-one, C22H18FNO3
- Crystal structure of the dinuclear copper(II) complex bis(μ2-2,2′ -{[1,3-phenylenebis-(methylene)]bis(oxy)}dibenzoaot-κ4O,O′:O′′,O′′′)-bis(dimethylformamide-κ1O)dicopper(II), C50H44Cu2N2O14
- Crystal structure of poly[triaqua-(μ9-biphenyl-3,3′,5,5′-tetracarboxylic-κ8 O,O:O,O′: O,O″:O,O‴)samarium(III)sodium(I)], C16H12NaSmO11
- The crystal structure of 5-benzyl-1-(4-fluorobenzyl)-4-((4-fluorobenzyl)oxy)-1,5-dihydro-2H-pyrrol-2-one, C25H21F2NO2
- The crystal structure of diammonium 2,5-dihydroxyterephthalate, C8H12N2O6
- Crystal structure of (E)-4-(4-(1H-1,2,4-triazol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[b]oxepin-5(2H)-one, C21H19N3O4
- Crystal structure of poly[oktakis(μ2-oxido-κ2O:O)-tetrakis(oxido-κ1O)-bis(μ2-1,1′-[1,4-phenylenebis(methylene)]di(1H-imidazole-κ2N:N′))-tetravanadium(V)-dizinc(II)] monohydrate, C28H30Zn2N8O13V4
- Crystal structure of acotiamide hydrochloride dimethylacetamide solvate (1/1), C25H40ClN5O6S
- Crystal structure of catena-poly[monoaqua (u2-(3-(3,5-dicarboxyphenyl)pyridine-2,6-dicarboxylic-k4O:O′:O″:N)zinc(II))] monohydrate, C15H11NO10Zn
- Crystal structure of dichlorido{2,6-bis(3,5-diisopropyl-N-pyrazolyl)pyridine}zinc(II), C23H33Cl2N5Zn
- Crystal structure of nitrato-κ2O,O′-[hydridotris(3,5-diethylpyrazol-1-yl)borato-κ3N,N′,N″]copper(II), C21H34BCuN7O3
- Crystal structure of 2,7-bis(3,5-diethyl-1H-pyrazol-4-yl)-benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetrone, C28H26N6O4
- Crystal structure of 2-(4-chlorophenyl)benzothiazole, C13H8ClNS
- Synthesis and crystal structure (3R,4′S)-4′-(3,5-dibromophenyl)-1′-methyl-2H-dispiro [benzofuran-3,3′-pyrrolidine-2′,2″-indene]-1″,2,3″-trione, C26H17Br2NO4
- Crystal structure of bis(((3a,7a-dihydro-1H-benzo[d][1,2,3]triazol-1-yl)methyl) triphenylphosphonium) tetrachloridomanganate(II), C50H42Cl4MnN6P2
- The crystal structure of 4,9-bis(4-chlorophenyl)-1,6-bis(2-cyanobenzyl)-2,4a,5,6,7,7a-hexahydro-1H-2,7,5-(epiprop[2]ene[1,1,3]triyl)pyrrolo[3,4-b]pyridine-3,10-dicarbonitrile, C40H26Cl2N6
- The crystal structure of poly((μ2-3-(3-nitro-4-carboxylphenyl)benzoate-κ3O, O′:O″)-μ2-1,4-bis(1-imidazolyl)benzene-κ2N:N′-cadmium(II)), C26H17N5O6Cd
- The crystal structure of 6-hydroxy-5H-pyrrolo[3,4-b]pyridine-5,7(6H)-dione monohydrate, C7H6N2O4
- Crystal structure of 4-((cyclohexylsulfonyl)methyl)-1,2,3,4-tetrahydrobenzo [4,5]imidazo[1,2-a]pyridine, C18H24N2O2S
- Crystal structure of 4,7-diphenyl-1,10-phenanthroline-κ2N,N′)-bis(2,4-di(fluorine)-1-phenylpyridine-κ2C,N)-iridium(III) hexafluorophosphate–dichloromethane (1/1), C47H30Cl2F10IrN4P
- Crystal structure of (4-(1-phenyl-1H-benzo[d]imidazol-2-yl)phenyl)boronic acid, C19H15BN2O2
- The crystal structure of (E)-(2-((pyridin-2-ylmethylene)amino)phenyl)arsonic acid, C12H11AsN2O3
- The crystal structure of N(benzyl(phenyl)carbomothioyl)benzamide, C21H18N2OS
- The crystal structure of bis(2-picolinium) hexachlorostannate dichloromethane monosolvate, C13H18Cl8N2Sn
- Crystal structure of poly[tetraaqua-bis(μ4-3–1-(carboxylatomethyl)-1H-1,2,4-triazole-3-carboxylato)-κ4O:O′,O″,N)zinc(II)], C5H7N3O6Zn
- The crystal structure of the co-crystal isonicotinamide – 2-(nitrophenyl)methanol (1/1), C6H6N2O·C7H7NO3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-fluorobenzoate hydrate, C23H25F2N3O6
- Crystal structure of [diaqua-{1H-benzo[d]imidazol-3-ium-5,6-dicarboxylato-κ2O,O′}magnesium(II)] C18H14MgN4O10
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl) (2-iodo-5-methoxyphenyl)methanone, C20H20INO5
- The crystal structure of 3,7,11-trimethylbenzo[5,6][1,4]thiazino[2,3,4-kl]phenothiazine 5,5,9,9-tetraoxide, C21H17NO4S2
- Crystal structure of tris(piperazine-1,4-diium)bis(2-hydroxy-1,2,3-propane-tricarboxylate) pentahydrate, C24H56N6O19
- Crystal structure of 2-chloro-5-((5-isopropyl-2-methylphenoxy)methyl)pyridine, C16H18ClNO
- Crystal structure of (E)-4-(4-(1H-pyrrol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[(b)]oxepin-5(2H)-one, C23H21NO4
- Crystal structure of (E)-N′-(3,4-dichlorobenzylidene)-3,4,5-trimethoxybenzohydrazide, C17H16Cl2N2O4
- The crystal structure of 2-(2-hydroxyphenyl)-3-(pyridin-2-yl)-2,3- dihydroquinazolin-4(1H)-one, C19H15N3O2
- Crystal structure of 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-8-methylchroman-4-one, C17H16O5
- Crystal structure of bis[(3,4-dimethoxybenzyl)triphenylphosphonium]di-μ2-bromido-dibromidodicopper(I)
- Crystal structure of bis [(1,3-dioxolan-2-ylmethyl)triphenylphosphonium] tetrabromidodicopper(I), C22H22Br2CuO2P
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid], C12H8N2O5
- The crystal structure of one-dimensional cooridnation polymer bis(thiocyanato)-bis((1E,2E)-1,2-bis(1-(pyridin-3-yl)ethylidene)-hydrazine κ2N:N)iron(II), (C30H28N10S2Fe)n
- Crystal structure of ((4-acetamidophenyl)sulfonyl)-l-alanine, C11H14N2O5S
- Crystal structure of [(1-naphthalen-1-yl-methyl)triphenylphosphonium] dichloridocopper(I), [C29H24P]+[CuCl2]−
- RbTm3S5: the first rubidium lanthanoid(III) sulfide with CsEr3Se5-type crystal structure
- Crystal structure of 2,2′-((ethane-1,2-diylbis(methylammoniumdiyl))bis(methylene))bis(pyridin-1-ium) diiodido-tris(μ2-iodido-κ2I:I)dicopper(II) chloride dihydrate, C16H30Cu2I6N4O2
- The crystal structure of 4-(trifluoromethyl)pyridine-2-carboxylic acid, C7H4F3NO2
- The crystal structure of (E)-2-ethoxy-1-methoxy-4-(2-(methylsulfonyl)vinyl)benzene, C12H16O4S
- Crystal structure of potassium 1H,1H,2H,2H-perfluorooctanesulfonate, C8H4O3F13SK
- Crystal structure of 4-(4-(quinolin-8-yloxy)-1,2,5-thiadiazol-3-yl)morpholine, C15H14O2N4S
- The crystal structure of 1,4-bis(bromomethyl)-2,5-dimethylbenzene, C10H12Br2
- The crystal structure of imidazo[4,5-e][1,3]diazepine-4,6,8-triamine methanol solvate, C7H11N7O
- The crystal structure of chlorido-bis(1,10-phenantroline-κ2N,N′)-(2-formylphenoxyacetato-κ2O,O) lead(II), C33H23N4O4ClPb
- Crystal structure of pyridinium tetrakis(1,1,1-trifluoro-2,4-pentadionato-κ2O,O′)yttrium(III) C20F12H16YO8C5H6N
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of (1Z, 2Z)-3-phenyl-2-propenal 2-(4-bromo-2-fluorophenyl)hydrazone, C15H12BrFN2
- Refinement of crystal structure of 2-(2,3-dihydro-3-oxo-1 H -inden-1-ylidene)-1 H -indene-1,3(2 H )-dione C18H10O3
- The crystal structure of 3-(1-fluoro-2-(naphthalen-2-yl)-2-oxoethyl)-2-methoxy-3,4-dihydroisoquinolin-1(2H)-one, C22H18FNO3
- Crystal structure of the dinuclear copper(II) complex bis(μ2-2,2′ -{[1,3-phenylenebis-(methylene)]bis(oxy)}dibenzoaot-κ4O,O′:O′′,O′′′)-bis(dimethylformamide-κ1O)dicopper(II), C50H44Cu2N2O14
- Crystal structure of poly[triaqua-(μ9-biphenyl-3,3′,5,5′-tetracarboxylic-κ8 O,O:O,O′: O,O″:O,O‴)samarium(III)sodium(I)], C16H12NaSmO11
- The crystal structure of 5-benzyl-1-(4-fluorobenzyl)-4-((4-fluorobenzyl)oxy)-1,5-dihydro-2H-pyrrol-2-one, C25H21F2NO2
- The crystal structure of diammonium 2,5-dihydroxyterephthalate, C8H12N2O6
- Crystal structure of (E)-4-(4-(1H-1,2,4-triazol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[b]oxepin-5(2H)-one, C21H19N3O4
- Crystal structure of poly[oktakis(μ2-oxido-κ2O:O)-tetrakis(oxido-κ1O)-bis(μ2-1,1′-[1,4-phenylenebis(methylene)]di(1H-imidazole-κ2N:N′))-tetravanadium(V)-dizinc(II)] monohydrate, C28H30Zn2N8O13V4
- Crystal structure of acotiamide hydrochloride dimethylacetamide solvate (1/1), C25H40ClN5O6S
- Crystal structure of catena-poly[monoaqua (u2-(3-(3,5-dicarboxyphenyl)pyridine-2,6-dicarboxylic-k4O:O′:O″:N)zinc(II))] monohydrate, C15H11NO10Zn
- Crystal structure of dichlorido{2,6-bis(3,5-diisopropyl-N-pyrazolyl)pyridine}zinc(II), C23H33Cl2N5Zn
- Crystal structure of nitrato-κ2O,O′-[hydridotris(3,5-diethylpyrazol-1-yl)borato-κ3N,N′,N″]copper(II), C21H34BCuN7O3
- Crystal structure of 2,7-bis(3,5-diethyl-1H-pyrazol-4-yl)-benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetrone, C28H26N6O4
- Crystal structure of 2-(4-chlorophenyl)benzothiazole, C13H8ClNS
- Synthesis and crystal structure (3R,4′S)-4′-(3,5-dibromophenyl)-1′-methyl-2H-dispiro [benzofuran-3,3′-pyrrolidine-2′,2″-indene]-1″,2,3″-trione, C26H17Br2NO4
- Crystal structure of bis(((3a,7a-dihydro-1H-benzo[d][1,2,3]triazol-1-yl)methyl) triphenylphosphonium) tetrachloridomanganate(II), C50H42Cl4MnN6P2
- The crystal structure of 4,9-bis(4-chlorophenyl)-1,6-bis(2-cyanobenzyl)-2,4a,5,6,7,7a-hexahydro-1H-2,7,5-(epiprop[2]ene[1,1,3]triyl)pyrrolo[3,4-b]pyridine-3,10-dicarbonitrile, C40H26Cl2N6
- The crystal structure of poly((μ2-3-(3-nitro-4-carboxylphenyl)benzoate-κ3O, O′:O″)-μ2-1,4-bis(1-imidazolyl)benzene-κ2N:N′-cadmium(II)), C26H17N5O6Cd
- The crystal structure of 6-hydroxy-5H-pyrrolo[3,4-b]pyridine-5,7(6H)-dione monohydrate, C7H6N2O4
- Crystal structure of 4-((cyclohexylsulfonyl)methyl)-1,2,3,4-tetrahydrobenzo [4,5]imidazo[1,2-a]pyridine, C18H24N2O2S
- Crystal structure of 4,7-diphenyl-1,10-phenanthroline-κ2N,N′)-bis(2,4-di(fluorine)-1-phenylpyridine-κ2C,N)-iridium(III) hexafluorophosphate–dichloromethane (1/1), C47H30Cl2F10IrN4P
- Crystal structure of (4-(1-phenyl-1H-benzo[d]imidazol-2-yl)phenyl)boronic acid, C19H15BN2O2
- The crystal structure of (E)-(2-((pyridin-2-ylmethylene)amino)phenyl)arsonic acid, C12H11AsN2O3
- The crystal structure of N(benzyl(phenyl)carbomothioyl)benzamide, C21H18N2OS
- The crystal structure of bis(2-picolinium) hexachlorostannate dichloromethane monosolvate, C13H18Cl8N2Sn
- Crystal structure of poly[tetraaqua-bis(μ4-3–1-(carboxylatomethyl)-1H-1,2,4-triazole-3-carboxylato)-κ4O:O′,O″,N)zinc(II)], C5H7N3O6Zn
- The crystal structure of the co-crystal isonicotinamide – 2-(nitrophenyl)methanol (1/1), C6H6N2O·C7H7NO3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-fluorobenzoate hydrate, C23H25F2N3O6
- Crystal structure of [diaqua-{1H-benzo[d]imidazol-3-ium-5,6-dicarboxylato-κ2O,O′}magnesium(II)] C18H14MgN4O10
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl) (2-iodo-5-methoxyphenyl)methanone, C20H20INO5
- The crystal structure of 3,7,11-trimethylbenzo[5,6][1,4]thiazino[2,3,4-kl]phenothiazine 5,5,9,9-tetraoxide, C21H17NO4S2
- Crystal structure of tris(piperazine-1,4-diium)bis(2-hydroxy-1,2,3-propane-tricarboxylate) pentahydrate, C24H56N6O19
- Crystal structure of 2-chloro-5-((5-isopropyl-2-methylphenoxy)methyl)pyridine, C16H18ClNO
- Crystal structure of (E)-4-(4-(1H-pyrrol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[(b)]oxepin-5(2H)-one, C23H21NO4
- Crystal structure of (E)-N′-(3,4-dichlorobenzylidene)-3,4,5-trimethoxybenzohydrazide, C17H16Cl2N2O4
- The crystal structure of 2-(2-hydroxyphenyl)-3-(pyridin-2-yl)-2,3- dihydroquinazolin-4(1H)-one, C19H15N3O2
- Crystal structure of 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-8-methylchroman-4-one, C17H16O5
- Crystal structure of bis[(3,4-dimethoxybenzyl)triphenylphosphonium]di-μ2-bromido-dibromidodicopper(I)
- Crystal structure of bis [(1,3-dioxolan-2-ylmethyl)triphenylphosphonium] tetrabromidodicopper(I), C22H22Br2CuO2P
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid], C12H8N2O5
- The crystal structure of one-dimensional cooridnation polymer bis(thiocyanato)-bis((1E,2E)-1,2-bis(1-(pyridin-3-yl)ethylidene)-hydrazine κ2N:N)iron(II), (C30H28N10S2Fe)n
- Crystal structure of ((4-acetamidophenyl)sulfonyl)-l-alanine, C11H14N2O5S
- Crystal structure of [(1-naphthalen-1-yl-methyl)triphenylphosphonium] dichloridocopper(I), [C29H24P]+[CuCl2]−
- RbTm3S5: the first rubidium lanthanoid(III) sulfide with CsEr3Se5-type crystal structure
- Crystal structure of 2,2′-((ethane-1,2-diylbis(methylammoniumdiyl))bis(methylene))bis(pyridin-1-ium) diiodido-tris(μ2-iodido-κ2I:I)dicopper(II) chloride dihydrate, C16H30Cu2I6N4O2
- The crystal structure of 4-(trifluoromethyl)pyridine-2-carboxylic acid, C7H4F3NO2
- The crystal structure of (E)-2-ethoxy-1-methoxy-4-(2-(methylsulfonyl)vinyl)benzene, C12H16O4S
- Crystal structure of potassium 1H,1H,2H,2H-perfluorooctanesulfonate, C8H4O3F13SK
- Crystal structure of 4-(4-(quinolin-8-yloxy)-1,2,5-thiadiazol-3-yl)morpholine, C15H14O2N4S
- The crystal structure of 1,4-bis(bromomethyl)-2,5-dimethylbenzene, C10H12Br2
- The crystal structure of imidazo[4,5-e][1,3]diazepine-4,6,8-triamine methanol solvate, C7H11N7O
- The crystal structure of chlorido-bis(1,10-phenantroline-κ2N,N′)-(2-formylphenoxyacetato-κ2O,O) lead(II), C33H23N4O4ClPb
- Crystal structure of pyridinium tetrakis(1,1,1-trifluoro-2,4-pentadionato-κ2O,O′)yttrium(III) C20F12H16YO8C5H6N


