Abstract
C17H16Cl2N2O4, monoclinic, P21/c (no. 14), a = 8.0893(1) Å, b = 26.1791(3) Å, c = 8.2311(1) Å, β = 98.002(1)°, V = 1726.13(4) Å3, Z = 4, Rgt(F) = 0.0343, wRref(F2) = 0.0904, T = 160 K.
Table 1 contains the crystallographic data. The list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Plate, colourless |
| Size: | 0.17 × 0.06 × 0.01 mm |
| Wavelength: μ: |
CuKα radiation (1.54184 Å) 3,61 mm−1 |
| Diffractometer: θmax, completeness: |
XtaLAB Synergy 77,3°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 20945, 3662, 0.029 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3282 |
| N(param)refined: | 233 |
| Programs: | CrysAlisPRO, 1 SHELX, 2 , 3 WinGX and ORTEP 4 |
1 Source of material
A mixture of 3,4,5-trimethoxybenzohydrazide (1.13 g, 5.0 mmol) and 3,4-dichlorobenzaldehyde (0.875 g, 5.0 mmol), in ethanol (8 mL), was heated under reflux for 2 h. On cooling, the precipitated crude product was filtered, washed with cold ethanol, dried and recrystallised from ethanol to yield 1.78 g (93 %) of the title compound as colourless plates. M. pt.: 461–463 K (uncorrected). 1H NMR (DMSO‑d6, 500.13 MHz): δ 11.44 (br. s, 1H, NH), 8.98 (s, 1H, CH=N), 8.06 (s, 1H, Ar–H), 7.70–7.762 (m, 2H, Ar–H), 7.22 (s, 2H, Ar–H), 3.84 (s, 6H, OCH3) and 3.72 (s, 3H, OCH3). 13C NMR (DMSO‑d6, 125.76 MHz): δ 162.84 (C=O), 151.20, 145.20, 141.86, 136.04, 133.24, 132.98, 131.08, 130.98, 128.88, 127.04, 109.66 (Ar–C & CH=N) and 61.80, 59.86 (OCH3). Analysis for C17H16Cl2N2O4 (M. Wt. 383.23): C, 53.22 (Calc. 53.28); H, 4.23 (Calc. 4.21); N, 7.28 (Calc. 7.31).
2 Experimental details
The C-bound H atoms were geometrically placed (C–H = 0.95–0.98 Å) and refined as riding with Uiso(H) = 1.2–1.5Ueq(C). The N-bound H atom was refined freely (N–H = 0.86(2) Å). Owing to poor agreement, one reflection, i.e. (10 5 0), was omitted from the final cycles of refinement.
3 Discussion
Aromatic and hetero-aromatic hydrazides and their hydrazone derivatives are of considerable interest for their remarkable chemotherapeutic properties. 5 , 6 Furthermore, carbohydrazide-hydrazone derivatives are frequently utilised as efficient intermediates for the synthesis of numerous bioactive heterocyclic compounds. 7 , 8 In this context, herein, the synthesis and single crystal X-ray structure of the title molecule, (I), is described; systematic name: N′-[(1E)-(3,4-dichlorophenyl)methylidene]-3,4,5-trimethoxybenzohydrazide.
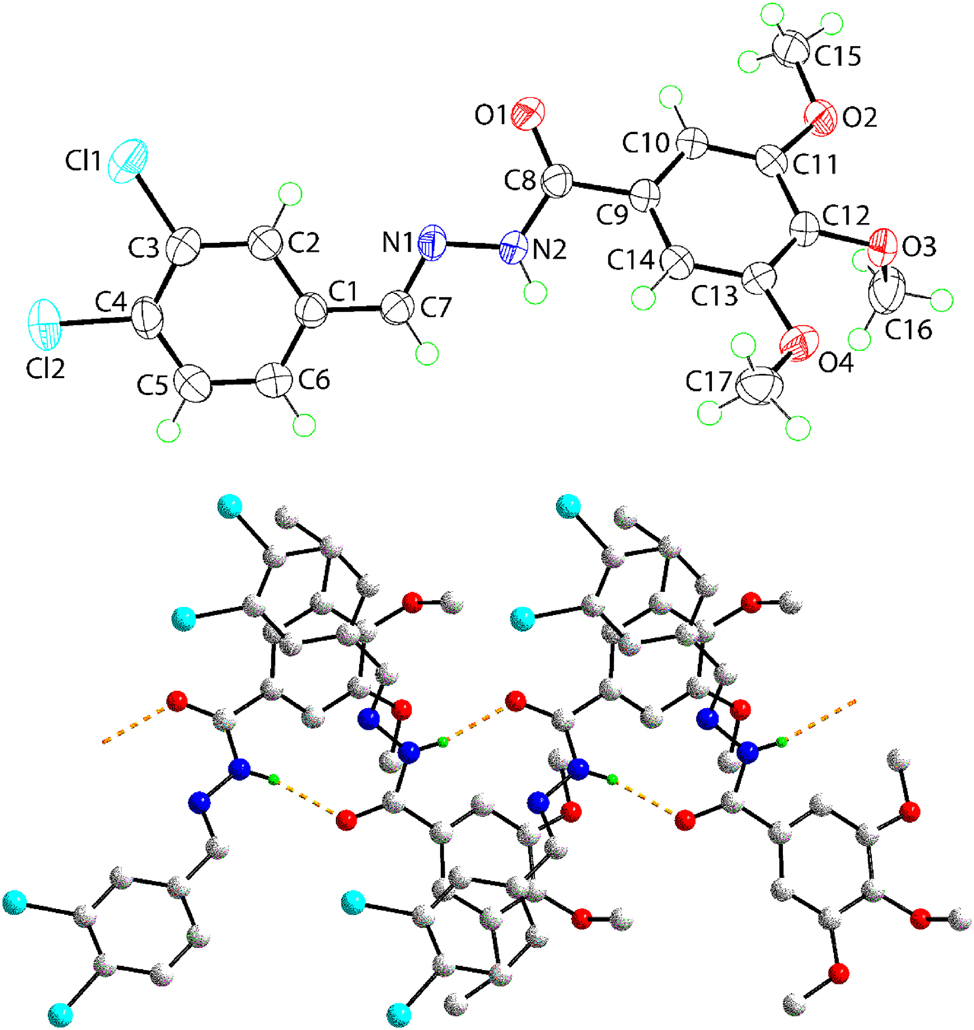
The molecular structure of (I) is shown in the upper view of figure (70 % probability ellipsoids). The molecule comprises a central, planar C2N2O residue [r.m.s. deviation for the fitted atoms = 0.0261 Å with the maximum deviation of 0.0377(10) Å being for atom C7] flanked at the C7 atom by a 3,4-dichlorophenyl group, and at the C8 atom by a 3,4,5-trimethoxyphenyl group. The dihedral angles between the central residue and the C6 rings of the 3,4-dichlorophenyl and 3,4,5-trimethoxyphenyl groups are 6.93(7) and 41.42(7) Å, respectively, indicating to close to co-planar and splayed dispositions, respectively. The C7–N1 [1.2798(19) Å] and C8–N2 [1.3551(18) Å] bond lengths, being close their respective double- and single-bond values, suggest limited delocalisation of π-electron density in the central residue. To limit steric hindrance in the 3,4,5-trimethoxyphenyl group, the central methoxy group occupies a position approximately normal to the C6 ring [the C16–O3–C12–C13 torsion angle = 94.38(17)°] compared to close to co-planar dispositions for the outer two methoxy groups [C15–O2–C11–C10 and C17–O4–C13–C12 = 4.0(2) and 164.46(13)°, respectively]. The amide group adopts an anti-conformation.
The most closely related structure in the crystallographic literature is of the isomeric compound, N′-(2,4-dichlorobenzylidene)-3,4,5-trimethoxybenzohydrazide, hereafter (II). 9 Not surprisingly, this molecule has a very similar conformation to that described for (I) with dihedral angles between the central residue and the C6 rings of the 2,4-dichlorophenyl and 3,4,5-trimethoxyphenyl groups being 2.72(10) and 41.06(9) Å, respectively. A similar electronic structure is also evident with the C7–N1 and C8–N2 bond lengths being 1.272(4) and 1.355(3) Å, respectively.
Conventional amide–N–H⋯O(amide) hydrogen-bonding [N2–H2n⋯O1 i : H2n⋯O1 i = 2.01(2) Å, N2⋯O1 i = 2.8525(16) Å with the angle subtended the H2n atom = 168(2)° for symmetry operation (i): x, 1/2−y, 1/2+z] is noted in the crystal. These interactions occur within a zigzag chain (glide symmetry) along the c-axis; a view of the chain is shown in the lower image of the figure (non-participating hydrogen atoms have been omitted for clarity). Prominent connections between chains to form a supramolecular layer in the bc-plane include methyl–C–H⋯O(methoxy) [C15–H15c⋯O3 ii : H15c⋯O3 ii = 2.48 Å, C15⋯O3 ii = 3.4426(19) Å with angle at H15c = 166° for (ii): 1−x, −y, 2−z] interactions. The closest contacts between layers appear to occur between two inclined 3,4-dichlorophenyl and 3,4,5-trimethoxyphenyl rings [Cg(C1–C6)⋯Cg(C9–C14) iii = 3.9909(9) Å with angle of inclination between rings = 26.88(7)° for (iii): −1+x, 1/2−y, −1/2+z]. However, with the angle of inclination being what it is, the shortest contact between ring-carbon atoms of 3.432(2)° occurs between the C6 and C12 iii atoms. Crystal (II) also crystallises in the P21/c space group with Z = 4 but is not isomorphous to (I). However, amide–N–H⋯O(amide) hydrogen-bonding occurs within a zigzag chain along the c-axis, as for (I).
It was thought of interest to compare the calculated Hirshfeld surfaces and the associated two-dimensional fingerprint plots for (I) and (II) by employing CrystalExplorer 10 and standard procedures. 11 The majority of Hirshfeld surface contacts for each of (I) and (II) involve hydrogen, amounting to 86.9 and 87.8 %, respectively. This similarity is reflected by identical contributions in each of (I) and (II) by H⋯H [29.5 %] and Cl⋯H/Hl [23.2 %] contacts; all contacts occur at distances beyond their respective sum of the van der Waals radii. The C⋯H/H⋯C [14.1 % for (I) and 12.9 % for (II)] and N⋯H/H⋯N [3.5 cf. 3.0 %] surface contacts are greater in the crystal of (I) but the converse is true for O⋯H/H⋯O [16.6 cf. 19.2 %] contacts. Of the surface contacts not involving hydrogen, the percentage contribution by C⋯C contacts are identical in both crystals, being 5.3 %. The next most important surface contacts are of the type N⋯C/C⋯N at 2.4 and 2.2 % for (I) and (II), respectively, followed by O⋯C/C⋯O [1.9 cf. 1.2 %]. The only other contacts in either crystals beyond 1 % contributions are Cl⋯C/C⋯Cl contacts which make a zero contribution in the crystal of (I) but 1.9 % in the crystal of (II).
-
Author contributions: All authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest: The authors declare no conflicts of interest.
-
Research funding: This study was financially supported by the the Princess Nourah bint Abdulrahman University Researchers Supporting Project No. PNURSP2025R3, from the Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
References
1. Rigaku Oxford Diffraction. CRYSALISPRO; Rigaku Corporation: Oxford, UK, 2024.Search in Google Scholar
2. Sheldrick, G. M. Shelxt – Integrated Space-group and Crystal-structure Determination. Acta Crystallogr. 2015, A71, 3–8. https://doi.org/10.1107/S2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/S2053229614024218.Search in Google Scholar PubMed PubMed Central
4. Farrugia, L. J. WinGX and Ortep for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. https://doi.org/10.1107/S0021889812029111.Search in Google Scholar
5. Popiołek, Ł. Updated Information on Antimicrobial Activity of hydrazide-hydrazones. Int. J. Mol. Sci. 2021 (22), 9389. https://doi.org/10.3390/ijms22179389.Search in Google Scholar PubMed PubMed Central
6. Onyeyilim, E. L.; Ezeokonkwo, M. A.; Ugwu, D. I.; Uzoewulu, C. P.; Eze, F. U.; Okonkwo, V. I.; Eze, C. C.; Ezugwu, J. A. Carbohydrazide Analogues: A Review of Synthesis and Biological Activities. Mini Rev. Med. Chem. 2022, 22, 661–682. https://doi.org/10.2174/1389557521666210831154935.Search in Google Scholar PubMed
7. El-Emam, A. A.; Alrashood, K. A.; Al-Omar, M. A.; Al-Tamimi, A. M. S. Synthesis and Antimicrobial Activity of N′-heteroarylidene-1-adamantylcarbohydrazides and (+-)-2-(1-adamantyl)-4-acetyl-5-[5-(4-substituted phenyl-3-isoxazolyl)]-1,3,4-oxadiazolines. Molecules 2012, 17, 3475–3483. https://doi.org/10.3390/molecules17033475.Search in Google Scholar PubMed PubMed Central
8. Ishii, M.; Jorge, S. D.; de Oliveira, A. A.; Palace-Berl, F.; Sonehara, I. Y.; Pasqualoto, K. F.; Tavares, L. C. Synthesis, Molecular Modeling and Preliminary Biological Evaluation of a Set of 3-acetyl-2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazole as Potential Antibacterial, anti-Trypanosoma Cruzi and Antifungal Agents. Bioorg. Med. Chem. 2011, 19, 6292–6301. https://doi.org/10.1016/j.bmc.2011.09.009.Search in Google Scholar PubMed
9. Han, Y.-Y. Crystal Structures and Antimicrobial Activities of N′-[2,5-dihydroxybenzylidene]-3,4,5-trimethoxybenzohydrazide, N′-[2,4-dichlorobenzylidene]-3,4,5-trimethoxybenzohydrazide, and 2,4-dichloro-N′-(2-hydroxy-3-methoxy-5-nitrobenzylidene) Benzohydrazide Ethanol Solvate. J. Chil. Chem. Soc. 2013, 58, 1858–1861. https://doi.org/10.4067/S0717-97072013000300014.Search in Google Scholar
10. Spackman, P. R.; Turner, M. J.; McKinnon, J. J.; Wolff, S. K.; Grimwood, D. J.; Jayatilaka, D.; Spackman, M. A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. https://doi.org/10.1107/S1600576721002910.Search in Google Scholar PubMed PubMed Central
11. Tan, S. L.; Jotani, M. M.; Tiekink, E. R. T. Utilizing Hirshfeld Surface Calculations, Non-covalent Interaction (NCI) Plots and the Calculation of Interaction Energies in the Analysis of Molecular Packing. Acta Crystallogr. 2019, E75, 308–318. https://doi.org/10.1107/S2056989019001129.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (1Z, 2Z)-3-phenyl-2-propenal 2-(4-bromo-2-fluorophenyl)hydrazone, C15H12BrFN2
- Refinement of crystal structure of 2-(2,3-dihydro-3-oxo-1 H -inden-1-ylidene)-1 H -indene-1,3(2 H )-dione C18H10O3
- The crystal structure of 3-(1-fluoro-2-(naphthalen-2-yl)-2-oxoethyl)-2-methoxy-3,4-dihydroisoquinolin-1(2H)-one, C22H18FNO3
- Crystal structure of the dinuclear copper(II) complex bis(μ2-2,2′ -{[1,3-phenylenebis-(methylene)]bis(oxy)}dibenzoaot-κ4O,O′:O′′,O′′′)-bis(dimethylformamide-κ1O)dicopper(II), C50H44Cu2N2O14
- Crystal structure of poly[triaqua-(μ9-biphenyl-3,3′,5,5′-tetracarboxylic-κ8 O,O:O,O′: O,O″:O,O‴)samarium(III)sodium(I)], C16H12NaSmO11
- The crystal structure of 5-benzyl-1-(4-fluorobenzyl)-4-((4-fluorobenzyl)oxy)-1,5-dihydro-2H-pyrrol-2-one, C25H21F2NO2
- The crystal structure of diammonium 2,5-dihydroxyterephthalate, C8H12N2O6
- Crystal structure of (E)-4-(4-(1H-1,2,4-triazol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[b]oxepin-5(2H)-one, C21H19N3O4
- Crystal structure of poly[oktakis(μ2-oxido-κ2O:O)-tetrakis(oxido-κ1O)-bis(μ2-1,1′-[1,4-phenylenebis(methylene)]di(1H-imidazole-κ2N:N′))-tetravanadium(V)-dizinc(II)] monohydrate, C28H30Zn2N8O13V4
- Crystal structure of acotiamide hydrochloride dimethylacetamide solvate (1/1), C25H40ClN5O6S
- Crystal structure of catena-poly[monoaqua (u2-(3-(3,5-dicarboxyphenyl)pyridine-2,6-dicarboxylic-k4O:O′:O″:N)zinc(II))] monohydrate, C15H11NO10Zn
- Crystal structure of dichlorido{2,6-bis(3,5-diisopropyl-N-pyrazolyl)pyridine}zinc(II), C23H33Cl2N5Zn
- Crystal structure of nitrato-κ2O,O′-[hydridotris(3,5-diethylpyrazol-1-yl)borato-κ3N,N′,N″]copper(II), C21H34BCuN7O3
- Crystal structure of 2,7-bis(3,5-diethyl-1H-pyrazol-4-yl)-benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetrone, C28H26N6O4
- Crystal structure of 2-(4-chlorophenyl)benzothiazole, C13H8ClNS
- Synthesis and crystal structure (3R,4′S)-4′-(3,5-dibromophenyl)-1′-methyl-2H-dispiro [benzofuran-3,3′-pyrrolidine-2′,2″-indene]-1″,2,3″-trione, C26H17Br2NO4
- Crystal structure of bis(((3a,7a-dihydro-1H-benzo[d][1,2,3]triazol-1-yl)methyl) triphenylphosphonium) tetrachloridomanganate(II), C50H42Cl4MnN6P2
- The crystal structure of 4,9-bis(4-chlorophenyl)-1,6-bis(2-cyanobenzyl)-2,4a,5,6,7,7a-hexahydro-1H-2,7,5-(epiprop[2]ene[1,1,3]triyl)pyrrolo[3,4-b]pyridine-3,10-dicarbonitrile, C40H26Cl2N6
- The crystal structure of poly((μ2-3-(3-nitro-4-carboxylphenyl)benzoate-κ3O, O′:O″)-μ2-1,4-bis(1-imidazolyl)benzene-κ2N:N′-cadmium(II)), C26H17N5O6Cd
- The crystal structure of 6-hydroxy-5H-pyrrolo[3,4-b]pyridine-5,7(6H)-dione monohydrate, C7H6N2O4
- Crystal structure of 4-((cyclohexylsulfonyl)methyl)-1,2,3,4-tetrahydrobenzo [4,5]imidazo[1,2-a]pyridine, C18H24N2O2S
- Crystal structure of 4,7-diphenyl-1,10-phenanthroline-κ2N,N′)-bis(2,4-di(fluorine)-1-phenylpyridine-κ2C,N)-iridium(III) hexafluorophosphate–dichloromethane (1/1), C47H30Cl2F10IrN4P
- Crystal structure of (4-(1-phenyl-1H-benzo[d]imidazol-2-yl)phenyl)boronic acid, C19H15BN2O2
- The crystal structure of (E)-(2-((pyridin-2-ylmethylene)amino)phenyl)arsonic acid, C12H11AsN2O3
- The crystal structure of N(benzyl(phenyl)carbomothioyl)benzamide, C21H18N2OS
- The crystal structure of bis(2-picolinium) hexachlorostannate dichloromethane monosolvate, C13H18Cl8N2Sn
- Crystal structure of poly[tetraaqua-bis(μ4-3–1-(carboxylatomethyl)-1H-1,2,4-triazole-3-carboxylato)-κ4O:O′,O″,N)zinc(II)], C5H7N3O6Zn
- The crystal structure of the co-crystal isonicotinamide – 2-(nitrophenyl)methanol (1/1), C6H6N2O·C7H7NO3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-fluorobenzoate hydrate, C23H25F2N3O6
- Crystal structure of [diaqua-{1H-benzo[d]imidazol-3-ium-5,6-dicarboxylato-κ2O,O′}magnesium(II)] C18H14MgN4O10
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl) (2-iodo-5-methoxyphenyl)methanone, C20H20INO5
- The crystal structure of 3,7,11-trimethylbenzo[5,6][1,4]thiazino[2,3,4-kl]phenothiazine 5,5,9,9-tetraoxide, C21H17NO4S2
- Crystal structure of tris(piperazine-1,4-diium)bis(2-hydroxy-1,2,3-propane-tricarboxylate) pentahydrate, C24H56N6O19
- Crystal structure of 2-chloro-5-((5-isopropyl-2-methylphenoxy)methyl)pyridine, C16H18ClNO
- Crystal structure of (E)-4-(4-(1H-pyrrol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[(b)]oxepin-5(2H)-one, C23H21NO4
- Crystal structure of (E)-N′-(3,4-dichlorobenzylidene)-3,4,5-trimethoxybenzohydrazide, C17H16Cl2N2O4
- The crystal structure of 2-(2-hydroxyphenyl)-3-(pyridin-2-yl)-2,3- dihydroquinazolin-4(1H)-one, C19H15N3O2
- Crystal structure of 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-8-methylchroman-4-one, C17H16O5
- Crystal structure of bis[(3,4-dimethoxybenzyl)triphenylphosphonium]di-μ2-bromido-dibromidodicopper(I)
- Crystal structure of bis [(1,3-dioxolan-2-ylmethyl)triphenylphosphonium] tetrabromidodicopper(I), C22H22Br2CuO2P
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid], C12H8N2O5
- The crystal structure of one-dimensional cooridnation polymer bis(thiocyanato)-bis((1E,2E)-1,2-bis(1-(pyridin-3-yl)ethylidene)-hydrazine κ2N:N)iron(II), (C30H28N10S2Fe)n
- Crystal structure of ((4-acetamidophenyl)sulfonyl)-l-alanine, C11H14N2O5S
- Crystal structure of [(1-naphthalen-1-yl-methyl)triphenylphosphonium] dichloridocopper(I), [C29H24P]+[CuCl2]−
- RbTm3S5: the first rubidium lanthanoid(III) sulfide with CsEr3Se5-type crystal structure
- Crystal structure of 2,2′-((ethane-1,2-diylbis(methylammoniumdiyl))bis(methylene))bis(pyridin-1-ium) diiodido-tris(μ2-iodido-κ2I:I)dicopper(II) chloride dihydrate, C16H30Cu2I6N4O2
- The crystal structure of 4-(trifluoromethyl)pyridine-2-carboxylic acid, C7H4F3NO2
- The crystal structure of (E)-2-ethoxy-1-methoxy-4-(2-(methylsulfonyl)vinyl)benzene, C12H16O4S
- Crystal structure of potassium 1H,1H,2H,2H-perfluorooctanesulfonate, C8H4O3F13SK
- Crystal structure of 4-(4-(quinolin-8-yloxy)-1,2,5-thiadiazol-3-yl)morpholine, C15H14O2N4S
- The crystal structure of 1,4-bis(bromomethyl)-2,5-dimethylbenzene, C10H12Br2
- The crystal structure of imidazo[4,5-e][1,3]diazepine-4,6,8-triamine methanol solvate, C7H11N7O
- The crystal structure of chlorido-bis(1,10-phenantroline-κ2N,N′)-(2-formylphenoxyacetato-κ2O,O) lead(II), C33H23N4O4ClPb
- Crystal structure of pyridinium tetrakis(1,1,1-trifluoro-2,4-pentadionato-κ2O,O′)yttrium(III) C20F12H16YO8C5H6N
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (1Z, 2Z)-3-phenyl-2-propenal 2-(4-bromo-2-fluorophenyl)hydrazone, C15H12BrFN2
- Refinement of crystal structure of 2-(2,3-dihydro-3-oxo-1 H -inden-1-ylidene)-1 H -indene-1,3(2 H )-dione C18H10O3
- The crystal structure of 3-(1-fluoro-2-(naphthalen-2-yl)-2-oxoethyl)-2-methoxy-3,4-dihydroisoquinolin-1(2H)-one, C22H18FNO3
- Crystal structure of the dinuclear copper(II) complex bis(μ2-2,2′ -{[1,3-phenylenebis-(methylene)]bis(oxy)}dibenzoaot-κ4O,O′:O′′,O′′′)-bis(dimethylformamide-κ1O)dicopper(II), C50H44Cu2N2O14
- Crystal structure of poly[triaqua-(μ9-biphenyl-3,3′,5,5′-tetracarboxylic-κ8 O,O:O,O′: O,O″:O,O‴)samarium(III)sodium(I)], C16H12NaSmO11
- The crystal structure of 5-benzyl-1-(4-fluorobenzyl)-4-((4-fluorobenzyl)oxy)-1,5-dihydro-2H-pyrrol-2-one, C25H21F2NO2
- The crystal structure of diammonium 2,5-dihydroxyterephthalate, C8H12N2O6
- Crystal structure of (E)-4-(4-(1H-1,2,4-triazol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[b]oxepin-5(2H)-one, C21H19N3O4
- Crystal structure of poly[oktakis(μ2-oxido-κ2O:O)-tetrakis(oxido-κ1O)-bis(μ2-1,1′-[1,4-phenylenebis(methylene)]di(1H-imidazole-κ2N:N′))-tetravanadium(V)-dizinc(II)] monohydrate, C28H30Zn2N8O13V4
- Crystal structure of acotiamide hydrochloride dimethylacetamide solvate (1/1), C25H40ClN5O6S
- Crystal structure of catena-poly[monoaqua (u2-(3-(3,5-dicarboxyphenyl)pyridine-2,6-dicarboxylic-k4O:O′:O″:N)zinc(II))] monohydrate, C15H11NO10Zn
- Crystal structure of dichlorido{2,6-bis(3,5-diisopropyl-N-pyrazolyl)pyridine}zinc(II), C23H33Cl2N5Zn
- Crystal structure of nitrato-κ2O,O′-[hydridotris(3,5-diethylpyrazol-1-yl)borato-κ3N,N′,N″]copper(II), C21H34BCuN7O3
- Crystal structure of 2,7-bis(3,5-diethyl-1H-pyrazol-4-yl)-benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetrone, C28H26N6O4
- Crystal structure of 2-(4-chlorophenyl)benzothiazole, C13H8ClNS
- Synthesis and crystal structure (3R,4′S)-4′-(3,5-dibromophenyl)-1′-methyl-2H-dispiro [benzofuran-3,3′-pyrrolidine-2′,2″-indene]-1″,2,3″-trione, C26H17Br2NO4
- Crystal structure of bis(((3a,7a-dihydro-1H-benzo[d][1,2,3]triazol-1-yl)methyl) triphenylphosphonium) tetrachloridomanganate(II), C50H42Cl4MnN6P2
- The crystal structure of 4,9-bis(4-chlorophenyl)-1,6-bis(2-cyanobenzyl)-2,4a,5,6,7,7a-hexahydro-1H-2,7,5-(epiprop[2]ene[1,1,3]triyl)pyrrolo[3,4-b]pyridine-3,10-dicarbonitrile, C40H26Cl2N6
- The crystal structure of poly((μ2-3-(3-nitro-4-carboxylphenyl)benzoate-κ3O, O′:O″)-μ2-1,4-bis(1-imidazolyl)benzene-κ2N:N′-cadmium(II)), C26H17N5O6Cd
- The crystal structure of 6-hydroxy-5H-pyrrolo[3,4-b]pyridine-5,7(6H)-dione monohydrate, C7H6N2O4
- Crystal structure of 4-((cyclohexylsulfonyl)methyl)-1,2,3,4-tetrahydrobenzo [4,5]imidazo[1,2-a]pyridine, C18H24N2O2S
- Crystal structure of 4,7-diphenyl-1,10-phenanthroline-κ2N,N′)-bis(2,4-di(fluorine)-1-phenylpyridine-κ2C,N)-iridium(III) hexafluorophosphate–dichloromethane (1/1), C47H30Cl2F10IrN4P
- Crystal structure of (4-(1-phenyl-1H-benzo[d]imidazol-2-yl)phenyl)boronic acid, C19H15BN2O2
- The crystal structure of (E)-(2-((pyridin-2-ylmethylene)amino)phenyl)arsonic acid, C12H11AsN2O3
- The crystal structure of N(benzyl(phenyl)carbomothioyl)benzamide, C21H18N2OS
- The crystal structure of bis(2-picolinium) hexachlorostannate dichloromethane monosolvate, C13H18Cl8N2Sn
- Crystal structure of poly[tetraaqua-bis(μ4-3–1-(carboxylatomethyl)-1H-1,2,4-triazole-3-carboxylato)-κ4O:O′,O″,N)zinc(II)], C5H7N3O6Zn
- The crystal structure of the co-crystal isonicotinamide – 2-(nitrophenyl)methanol (1/1), C6H6N2O·C7H7NO3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-fluorobenzoate hydrate, C23H25F2N3O6
- Crystal structure of [diaqua-{1H-benzo[d]imidazol-3-ium-5,6-dicarboxylato-κ2O,O′}magnesium(II)] C18H14MgN4O10
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl) (2-iodo-5-methoxyphenyl)methanone, C20H20INO5
- The crystal structure of 3,7,11-trimethylbenzo[5,6][1,4]thiazino[2,3,4-kl]phenothiazine 5,5,9,9-tetraoxide, C21H17NO4S2
- Crystal structure of tris(piperazine-1,4-diium)bis(2-hydroxy-1,2,3-propane-tricarboxylate) pentahydrate, C24H56N6O19
- Crystal structure of 2-chloro-5-((5-isopropyl-2-methylphenoxy)methyl)pyridine, C16H18ClNO
- Crystal structure of (E)-4-(4-(1H-pyrrol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[(b)]oxepin-5(2H)-one, C23H21NO4
- Crystal structure of (E)-N′-(3,4-dichlorobenzylidene)-3,4,5-trimethoxybenzohydrazide, C17H16Cl2N2O4
- The crystal structure of 2-(2-hydroxyphenyl)-3-(pyridin-2-yl)-2,3- dihydroquinazolin-4(1H)-one, C19H15N3O2
- Crystal structure of 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-8-methylchroman-4-one, C17H16O5
- Crystal structure of bis[(3,4-dimethoxybenzyl)triphenylphosphonium]di-μ2-bromido-dibromidodicopper(I)
- Crystal structure of bis [(1,3-dioxolan-2-ylmethyl)triphenylphosphonium] tetrabromidodicopper(I), C22H22Br2CuO2P
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid], C12H8N2O5
- The crystal structure of one-dimensional cooridnation polymer bis(thiocyanato)-bis((1E,2E)-1,2-bis(1-(pyridin-3-yl)ethylidene)-hydrazine κ2N:N)iron(II), (C30H28N10S2Fe)n
- Crystal structure of ((4-acetamidophenyl)sulfonyl)-l-alanine, C11H14N2O5S
- Crystal structure of [(1-naphthalen-1-yl-methyl)triphenylphosphonium] dichloridocopper(I), [C29H24P]+[CuCl2]−
- RbTm3S5: the first rubidium lanthanoid(III) sulfide with CsEr3Se5-type crystal structure
- Crystal structure of 2,2′-((ethane-1,2-diylbis(methylammoniumdiyl))bis(methylene))bis(pyridin-1-ium) diiodido-tris(μ2-iodido-κ2I:I)dicopper(II) chloride dihydrate, C16H30Cu2I6N4O2
- The crystal structure of 4-(trifluoromethyl)pyridine-2-carboxylic acid, C7H4F3NO2
- The crystal structure of (E)-2-ethoxy-1-methoxy-4-(2-(methylsulfonyl)vinyl)benzene, C12H16O4S
- Crystal structure of potassium 1H,1H,2H,2H-perfluorooctanesulfonate, C8H4O3F13SK
- Crystal structure of 4-(4-(quinolin-8-yloxy)-1,2,5-thiadiazol-3-yl)morpholine, C15H14O2N4S
- The crystal structure of 1,4-bis(bromomethyl)-2,5-dimethylbenzene, C10H12Br2
- The crystal structure of imidazo[4,5-e][1,3]diazepine-4,6,8-triamine methanol solvate, C7H11N7O
- The crystal structure of chlorido-bis(1,10-phenantroline-κ2N,N′)-(2-formylphenoxyacetato-κ2O,O) lead(II), C33H23N4O4ClPb
- Crystal structure of pyridinium tetrakis(1,1,1-trifluoro-2,4-pentadionato-κ2O,O′)yttrium(III) C20F12H16YO8C5H6N


