Abstract
C26H17N5O6Cd, triclinic,
Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.20 × 0.15 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.01 mm−1 |
| Diffractometer, scan mode: | Oxford Xcalibur, ω scan |
| θmax, completeness: | 25.1°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 6,314, 4,068, 0.031 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 3491 |
| N(param)refined: | 343 |
| Programs: | Oxford, 1 Olex2, 2 SHELX 3 , 4 |
1 Source of materials
All chemicals were purchased from commercial sources and used as received. A mixture of Cd(NO3)2·4H2O (0.0492 g, 0.2 mmol), 3-(3-nitro-4-carboxylphenyl)benzoate (H2nba) (0.0281 g, 0.1 mmol), 1,4-bis(1-imidazolyl)benzene (bib) (0.0213 g, 0.1 mmol), H2O (6 mL), and C2H5OH (3 mL) were placed in a Teflon-lined stainless-steel vessel (25 mL), which was sealed and heated at 140° for 4 days. After cooling to room temperature, light yellow crystals of I were collected by filtration, washed with distilled water, and then dried in air (yield: 68 % based on Cd). IR data (cm−1): 3062 (w), 1584 (m), 1518 (s), 1405 (w), 1341 (m), 1146 (w), 1106 (w), 844 (m), 784 (m), 724 (s), 646 (w). Elemental analysis calc. for C26H17N5O6Cd, Mr = 607.84: C, 51.41; H, 2.80; N, 11.51. Found: C, 51.34; H, 2.67; N, 11.47 (CCDC number 2045767).
2 Experimental details
CrysAlisPro 1.171.39.46 (Rigaku Oxford Diffraction, 2018) empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm was used. 1 Using Olex2, 2 the structure was solved with the ShelXT 3 structure solution program and refined with the ShelXL 4 refinement package. Carbon-bound hydrogen atoms were placed in calculated positions (d = 0.93 Å for CH and were included in the refinement in the riding model approximation, with Uiso(H) set to 1.2 Ueq(C) for –CH). The structure was examined using the ADDSYM subroutine of PLATON 5 to ensure that no additional symmetry could be applied to the models.
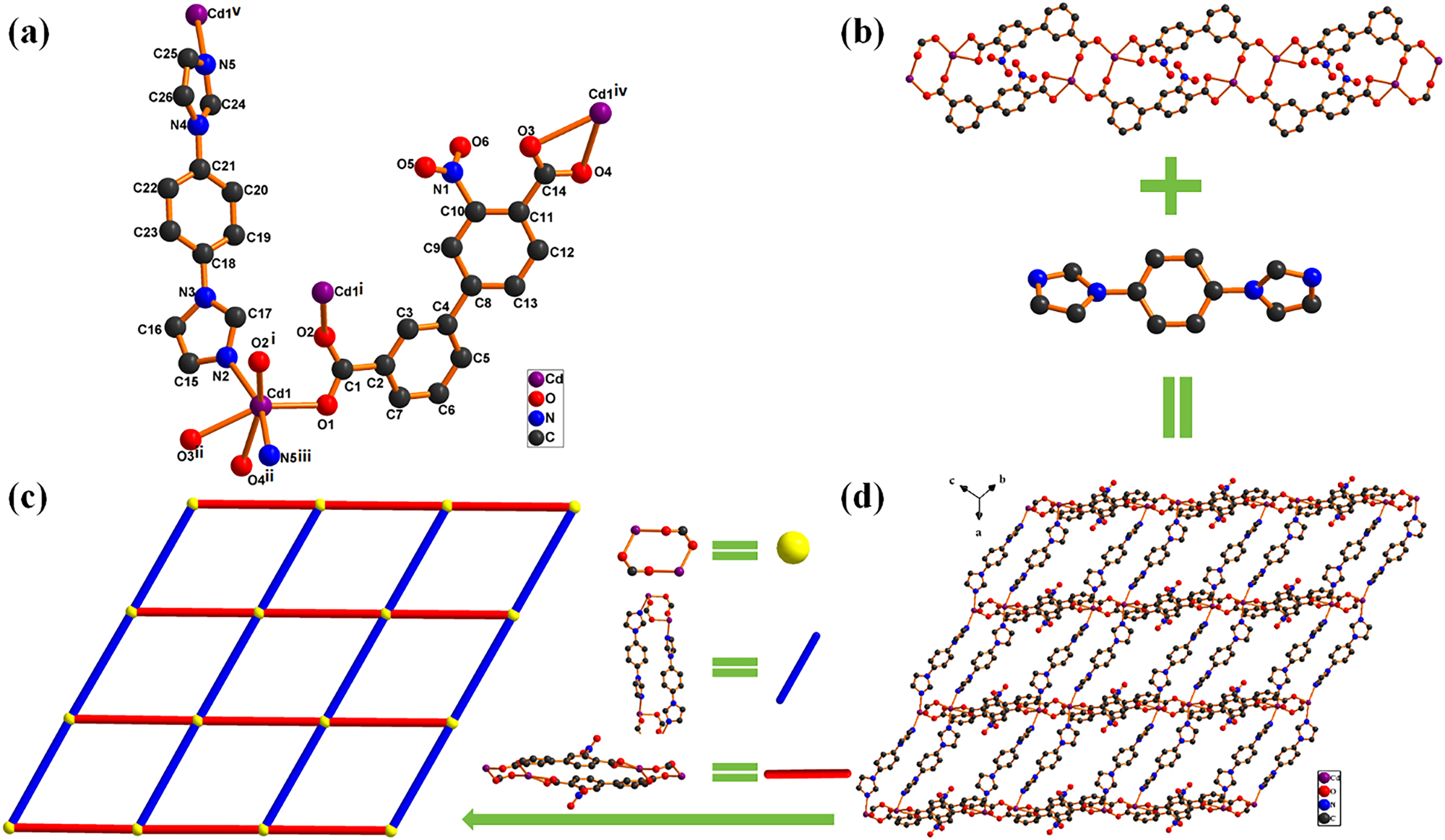
3 Comment
The crystal engineering based on metal-organic frameworks (MOFs) or coordination polymers (CPs) obtains great interest for appealing structures and potential applications in gas storage, microelectronics, ion exchange, chemical separations, nonlinear optics, heterogeneous catalysis, and so on. 6 Generally, structures of such materials are dependent on many factors, such as metal ion, template, metal-ligand ratio, pH, counteranion, and number of coordination sites provided by organic ligands. 7 In assembly of MOFs or CPs, the use of ligands is very important in obtaining the desired MOFs. 8 Compared to other biphenyl carboxylic acids, such as 4,4′-biphenyl-dicarboxyli acid, 2,4′-biphenyl-tetracarboxylic acid, 3,3′,5,5′-biphenyl-dicarboxylic acid, and 3,3′,4,4′-biphenyl- tetracarboxylic acid, biphenyl-3,4′-dicarboxylic acid (H2bpda) is less common, and the angle between carboxyl groups makes large porous coordination polymers possible. 9 , 10 , 11 , 12 Among this kind of ligands, biphenyl-based tetra-, tri-, and bicarboxylates are especially interesting because the free rotation of two phenyl rings can afford different coordination conformations depending on the dihedral angle between them. 13 As indicated by a CSD (Cambridge Structure Database) survey with the help of CSD version 5.46 (November 2024) only 38 H2bpba-based coordination complexes have been sporadically characterized and reported until now. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22
H2nba features two biphenyl rings and a nitro group in the 3-position. H2nba was selected as a ligand, which has the following characteristics: (i) the two carboxyl groups have four O-donor atoms with the dihedral angle 180°, are able to establish bridges between several metal nodes through variety of coordination modes and conjugated aromatic ring allows them to become rigid linkers. (ii) The nitro group in H2nba provides more hydrogen bonding donor or acceptor sites for supramolecular interactions. In particular, the two nitro O donors play a key role in the formation of potential hydrogen bonded or N–O⋯π interactions. (iii) The ditopic linear rod-like topology introduces low steric hindrance, thus, allowing formation of a porous and stabilized framework. (iv) Biphenyl ring functions as a big space mediator for transmitting exchange interaction between metal centers. (v) Moreover, the two aromatic rings of H2nba may take part in π–π and C–H⋯π stacking interactions. Despite that numerous MOFs or CPs have been synthesized based on 2,4′-, 4,4′-, and 3,4′-biphenyldicarboxylate acid, little attention has been focused on its derivatives, such as H2nba. To the best of our knowledge and in a search of the Cambridge Crystal Database (CSD), however, there is only one crystal structure report of H2nba as ligand for the construction of MOFs or CPs by our research group to date. 23 , 24 In addition, it is known that a strategy to increase the chemical stability of CPs/MOFs is to strengthen the metal-ligand bond using linkers such as inflexible neutral nitrogen-containing pyridine, pyrazolates or imidazolates instead of carboxylates. In this work, we used H2nba and bib as organic ligands for Cd(II) centers.
Single-crystal X-ray diffraction analysis shows that I crystallizes in the triclinic crystal system with space group
-
Research funding: This research was funded by the Guangxi Natural Science Foundation (Grant Nos. 2025GXNSFHA069144 and 2025GXNSFAA069072), the Guangxi Science and Technology Major Project (Grant No. AB25069382), the Beibu Gulf University High-level Talent Research Start-up Project in 2024 (Grant No. 24KYQD04), the Research Fund of Guangxi Education Department (2025KY0481), the Innovative Training Program for Guangxi Province College Students (Grant No. 202411607371), and the Guangxi Key Laboratory of Green Chemical Materials and Safety Technology, Beibu Gulf University (Grant No. 2023ZZKT01).
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Oxford Diffraction Ltd. CrysAlisPRO; Rigaku Oxford Diffraction. Version 1.171.39.6a: England, 2018.Search in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Shelxtl – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Spek, A. L. Structure Validation in Chemical Crystallography. Acta Crystallogr. 2009, D65, 148–155; https://doi.org/10.1107/s090744490804362x.Search in Google Scholar
6. Sun, Z.; Ding, Y.; Tian, L.; Zhang, X. Two Threefold Interpenetrated Metal-Organic Frameworks (CoII, NiII) with (65.8) Topology Constructed from biphenyl-3,4′-dicarboxylic Acid. J. Coord. Chem. 2013, 66 (5), 763–771; https://doi.org/10.1080/00958972.2013.767895.Search in Google Scholar
7. Yu, Z. A Novel Mn(II) Coordination Polymer Based on Carboxylate and Tris[4-(1Himidazol-1-yl)-Phenyl]Amine Coligands with a Rare Hms Topology: Solvothermal Synthesis, Crystal Structure, and Luminescent Property. Synth. Reacti. Inorg. M. 2016, 46 (10), 1495–1498; https://doi.org/10.1080/15533174.2015.1136960.Search in Google Scholar
8. Guo, F.; Zhu, B.; Zhang, X.; Song, Y.; Wu, P. Synthesis and Crystal Structures of Two New Zigzag Versus Helical Coordination Polymers Based on Diphenic Acid. J. Coord. Chem. 2010, 63 (7), 1130–1138; https://doi.org/10.1080/00958971003735465.Search in Google Scholar
9. Feng, L.; Chen, Z.; Liao, T.; Li, P.; Jia, Y.; Liu, X.; Yang, Y.; Zhou, Y. Supramolecular Isomerism of Metal-Organic Frameworks Derived from a Bicarboxylate Linker with Two Distinct Binding Motifs. Cryst. Growth Des. 2009, 9 (3), 1505–1510; https://doi.org/10.1021/cg801026y.Search in Google Scholar
10. Zou, G.; Wang, X.; Xie, B. Construction of Two New Cd(II) Coordination Polymers via Isomeric Biphenyldicarboxylates and Imidazole-Containing Ligands. Synth. Reacti. Inorg. M. 2014, 44 (4), 498–502; https://doi.org/10.1080/15533174.2013.778879.Search in Google Scholar
11. Guo, F.; Wang, F.; Yang, H.; Zhang, X.; Zhang, J. Tuning Structural Topologies of Three Photoluminescent Metal-Organic Frameworks via Isomeric Biphenyldicarboxylates. Inorg. Chem. 2012, 51, 9677–9682; https://doi.org/10.1021/ic3008969.Search in Google Scholar PubMed
12. Li, J.-R.; Zhou, H.-C. Metal-Organic Hendecahedra Assembled from Dinuclear Paddlewheel Nodes and Mixtures of Ditopic Linkers with 120° and 90° Bend Angles. Angew. Chem. Int. Ed. 2009, 48, 8465–8468; https://doi.org/10.1002/anie.200904722.Search in Google Scholar PubMed
13. Sun, D.; Han, L.-L.; Yuan, S.; Deng, Y.-K.; Xu, M.-Z.; Sun, D.-F. Four New Cd(II) Coordination Polymers with Mixed Multidentate N-Donors and Biphenyl-Based Polycarboxylate Ligands: Syntheses, Structures, and Photoluminescent Properties. Cryst. Growth Des. 2013, 13, 377–385; https://doi.org/10.1021/cg301573c.Search in Google Scholar
14. Du, P.; Yuan, X.; Sun, Z.; Chen, P. Two Coordination Polymers: Luminescent, Photocatalytic Property and Enhancement Activity on Prostate Cancer Combined with 125I Radioactive Particle Placement. Polym. Bull. 2023, 80, 883–894; https://doi.org/10.1007/s00289-021-04055-4.Search in Google Scholar
15. Guo, F.; Zhu, B.; Zhang, X. Synthesis and Crystal Structures of Two New Coordination Polymers Based on Diphenic Acid. J. Inorg. Organomet. Polym. 2010, 20, 118–123; https://doi.org/10.1007/s10904-009-9321-5.Search in Google Scholar
16. Yang, L. Synthesis and Crystal Structure of Three New Zn(II), Ni(II) and Mn(II) Coordination Polymers Based on Asymmetric Dicarboxylate Ligand. J. Inorg. Organomet. Polym. 2012, 22, 709–716; https://doi.org/10.1007/s10904-011-9626-z.Search in Google Scholar
17. Guo, F. Two New Zn(II) Coordination Polymers Constructed from Asymmetric Biphenyl-Dicarboxylate and Imidazole-Containing Ligands: Structures and Photoluminescent Properties. J. Inorg. Organomet. Polym. 2013, 23, 472–476; https://doi.org/10.1007/s10904-012-9802-9.Search in Google Scholar
18. Peng, J.-H.; Wang, X.-L.; Ran, L.; Song, J.-L.; Zhang, X.; Li, H.-Y. Crystal Structures and Anti-breast Cancer Activities of Two New Coordination Polymers [Co2(bpdc)2(bibt)1.5](H2O)2 and [Gd(bpdc)2(Hbpp)](H2O)3. Struct. Chem. 2018, 29, 1671–1675; https://doi.org/10.1007/s11224-018-1145-x.Search in Google Scholar
19. Wang, S.; Ma, J.; Li, L.; Chen, T.; Sun, Z.; Luo, J. Guest Exchange Dynamics in a Flexible Bilayer Porous Coordination Polymer. Inor. Chem. Commun. 2012, 16, 65–69; https://doi.org/10.1016/j.inoche.2011.11.032.Search in Google Scholar
20. Jia, W.; Luo, J.; Zhu, M. A Molecular Basketwork: Self-Assembly of Coordination Polymers from Zn(II) and Biphenyl-3,4′-dicarboxylate Regulated by Different Flexible Bridging and Chelating N-Donor Ancillary Ligands. Cryst. Growth Des. 2011, 11, 2386–2397; https://doi.org/10.1021/cg200142f.Search in Google Scholar
21. Zhou, X.; Zhang, M.-N.; Zhang, D.-C.; Li, X.; Yao, Y.-F. Syntheses, Crystal Structures and Fluorescence Sensing of Lanthanide Complexes with 3,4′-Biphenyldicarboxylic Acid and N-donor Ligands. Chinese J. Inorg. Chem. 2018, 34 (7), 1285–1292.Search in Google Scholar
22. Wang, F.-M. Poly[(μ3-Biphenyl-3,4′-Dicarboxyl-ato-κ4O3:O3′: O4,O4″)(1H-Imidazo[4,5-F] [1,10]Phenanthroline-κ2N7,N8)manganese(II)]. Acta Crystallogr., Sect.E: Struct. Rep. Online 2010, 66, m1677; https://doi.org/10.1107/s1600536810045587.Search in Google Scholar PubMed PubMed Central
23. Xu, T. Y.; Li, J. M.; Han, Y. H.; Wang, A. R.; He, K. H.; Shi, Z. F. A New 3D Four-fold Interpenetrated Dia-like Luminescent Zn(II)-Based Metal-Organic Framwork: The Sensitive Detection of Fe3+, Cr2O72−, and CrO42− in Water, and Nitrobenzene in Ethanol. New J. Chem. 2020, 44, 4011–4022; https://doi.org/10.1039/c9nj06056a.Search in Google Scholar
24. Li, J.; Wang, A.; Qiu, S.; Wang, X.; Li, J. A 12-Connected [Y4((μ3-OH)4]8+ Cluster-based Luminescent Metal-Organic Framework for Selective Turn-on Detection of F− in H2O. Molecules 2023, 28, 1893; https://doi.org/10.3390/molecules28041893.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (1Z, 2Z)-3-phenyl-2-propenal 2-(4-bromo-2-fluorophenyl)hydrazone, C15H12BrFN2
- Refinement of crystal structure of 2-(2,3-dihydro-3-oxo-1 H -inden-1-ylidene)-1 H -indene-1,3(2 H )-dione C18H10O3
- The crystal structure of 3-(1-fluoro-2-(naphthalen-2-yl)-2-oxoethyl)-2-methoxy-3,4-dihydroisoquinolin-1(2H)-one, C22H18FNO3
- Crystal structure of the dinuclear copper(II) complex bis(μ2-2,2′ -{[1,3-phenylenebis-(methylene)]bis(oxy)}dibenzoaot-κ4O,O′:O′′,O′′′)-bis(dimethylformamide-κ1O)dicopper(II), C50H44Cu2N2O14
- Crystal structure of poly[triaqua-(μ9-biphenyl-3,3′,5,5′-tetracarboxylic-κ8 O,O:O,O′: O,O″:O,O‴)samarium(III)sodium(I)], C16H12NaSmO11
- The crystal structure of 5-benzyl-1-(4-fluorobenzyl)-4-((4-fluorobenzyl)oxy)-1,5-dihydro-2H-pyrrol-2-one, C25H21F2NO2
- The crystal structure of diammonium 2,5-dihydroxyterephthalate, C8H12N2O6
- Crystal structure of (E)-4-(4-(1H-1,2,4-triazol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[b]oxepin-5(2H)-one, C21H19N3O4
- Crystal structure of poly[oktakis(μ2-oxido-κ2O:O)-tetrakis(oxido-κ1O)-bis(μ2-1,1′-[1,4-phenylenebis(methylene)]di(1H-imidazole-κ2N:N′))-tetravanadium(V)-dizinc(II)] monohydrate, C28H30Zn2N8O13V4
- Crystal structure of acotiamide hydrochloride dimethylacetamide solvate (1/1), C25H40ClN5O6S
- Crystal structure of catena-poly[monoaqua (u2-(3-(3,5-dicarboxyphenyl)pyridine-2,6-dicarboxylic-k4O:O′:O″:N)zinc(II))] monohydrate, C15H11NO10Zn
- Crystal structure of dichlorido{2,6-bis(3,5-diisopropyl-N-pyrazolyl)pyridine}zinc(II), C23H33Cl2N5Zn
- Crystal structure of nitrato-κ2O,O′-[hydridotris(3,5-diethylpyrazol-1-yl)borato-κ3N,N′,N″]copper(II), C21H34BCuN7O3
- Crystal structure of 2,7-bis(3,5-diethyl-1H-pyrazol-4-yl)-benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetrone, C28H26N6O4
- Crystal structure of 2-(4-chlorophenyl)benzothiazole, C13H8ClNS
- Synthesis and crystal structure (3R,4′S)-4′-(3,5-dibromophenyl)-1′-methyl-2H-dispiro [benzofuran-3,3′-pyrrolidine-2′,2″-indene]-1″,2,3″-trione, C26H17Br2NO4
- Crystal structure of bis(((3a,7a-dihydro-1H-benzo[d][1,2,3]triazol-1-yl)methyl) triphenylphosphonium) tetrachloridomanganate(II), C50H42Cl4MnN6P2
- The crystal structure of 4,9-bis(4-chlorophenyl)-1,6-bis(2-cyanobenzyl)-2,4a,5,6,7,7a-hexahydro-1H-2,7,5-(epiprop[2]ene[1,1,3]triyl)pyrrolo[3,4-b]pyridine-3,10-dicarbonitrile, C40H26Cl2N6
- The crystal structure of poly((μ2-3-(3-nitro-4-carboxylphenyl)benzoate-κ3O, O′:O″)-μ2-1,4-bis(1-imidazolyl)benzene-κ2N:N′-cadmium(II)), C26H17N5O6Cd
- The crystal structure of 6-hydroxy-5H-pyrrolo[3,4-b]pyridine-5,7(6H)-dione monohydrate, C7H6N2O4
- Crystal structure of 4-((cyclohexylsulfonyl)methyl)-1,2,3,4-tetrahydrobenzo [4,5]imidazo[1,2-a]pyridine, C18H24N2O2S
- Crystal structure of 4,7-diphenyl-1,10-phenanthroline-κ2N,N′)-bis(2,4-di(fluorine)-1-phenylpyridine-κ2C,N)-iridium(III) hexafluorophosphate–dichloromethane (1/1), C47H30Cl2F10IrN4P
- Crystal structure of (4-(1-phenyl-1H-benzo[d]imidazol-2-yl)phenyl)boronic acid, C19H15BN2O2
- The crystal structure of (E)-(2-((pyridin-2-ylmethylene)amino)phenyl)arsonic acid, C12H11AsN2O3
- The crystal structure of N(benzyl(phenyl)carbomothioyl)benzamide, C21H18N2OS
- The crystal structure of bis(2-picolinium) hexachlorostannate dichloromethane monosolvate, C13H18Cl8N2Sn
- Crystal structure of poly[tetraaqua-bis(μ4-3–1-(carboxylatomethyl)-1H-1,2,4-triazole-3-carboxylato)-κ4O:O′,O″,N)zinc(II)], C5H7N3O6Zn
- The crystal structure of the co-crystal isonicotinamide – 2-(nitrophenyl)methanol (1/1), C6H6N2O·C7H7NO3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-fluorobenzoate hydrate, C23H25F2N3O6
- Crystal structure of [diaqua-{1H-benzo[d]imidazol-3-ium-5,6-dicarboxylato-κ2O,O′}magnesium(II)] C18H14MgN4O10
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl) (2-iodo-5-methoxyphenyl)methanone, C20H20INO5
- The crystal structure of 3,7,11-trimethylbenzo[5,6][1,4]thiazino[2,3,4-kl]phenothiazine 5,5,9,9-tetraoxide, C21H17NO4S2
- Crystal structure of tris(piperazine-1,4-diium)bis(2-hydroxy-1,2,3-propane-tricarboxylate) pentahydrate, C24H56N6O19
- Crystal structure of 2-chloro-5-((5-isopropyl-2-methylphenoxy)methyl)pyridine, C16H18ClNO
- Crystal structure of (E)-4-(4-(1H-pyrrol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[(b)]oxepin-5(2H)-one, C23H21NO4
- Crystal structure of (E)-N′-(3,4-dichlorobenzylidene)-3,4,5-trimethoxybenzohydrazide, C17H16Cl2N2O4
- The crystal structure of 2-(2-hydroxyphenyl)-3-(pyridin-2-yl)-2,3- dihydroquinazolin-4(1H)-one, C19H15N3O2
- Crystal structure of 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-8-methylchroman-4-one, C17H16O5
- Crystal structure of bis[(3,4-dimethoxybenzyl)triphenylphosphonium]di-μ2-bromido-dibromidodicopper(I)
- Crystal structure of bis [(1,3-dioxolan-2-ylmethyl)triphenylphosphonium] tetrabromidodicopper(I), C22H22Br2CuO2P
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid], C12H8N2O5
- The crystal structure of one-dimensional cooridnation polymer bis(thiocyanato)-bis((1E,2E)-1,2-bis(1-(pyridin-3-yl)ethylidene)-hydrazine κ2N:N)iron(II), (C30H28N10S2Fe)n
- Crystal structure of ((4-acetamidophenyl)sulfonyl)-l-alanine, C11H14N2O5S
- Crystal structure of [(1-naphthalen-1-yl-methyl)triphenylphosphonium] dichloridocopper(I), [C29H24P]+[CuCl2]−
- RbTm3S5: the first rubidium lanthanoid(III) sulfide with CsEr3Se5-type crystal structure
- Crystal structure of 2,2′-((ethane-1,2-diylbis(methylammoniumdiyl))bis(methylene))bis(pyridin-1-ium) diiodido-tris(μ2-iodido-κ2I:I)dicopper(II) chloride dihydrate, C16H30Cu2I6N4O2
- The crystal structure of 4-(trifluoromethyl)pyridine-2-carboxylic acid, C7H4F3NO2
- The crystal structure of (E)-2-ethoxy-1-methoxy-4-(2-(methylsulfonyl)vinyl)benzene, C12H16O4S
- Crystal structure of potassium 1H,1H,2H,2H-perfluorooctanesulfonate, C8H4O3F13SK
- Crystal structure of 4-(4-(quinolin-8-yloxy)-1,2,5-thiadiazol-3-yl)morpholine, C15H14O2N4S
- The crystal structure of 1,4-bis(bromomethyl)-2,5-dimethylbenzene, C10H12Br2
- The crystal structure of imidazo[4,5-e][1,3]diazepine-4,6,8-triamine methanol solvate, C7H11N7O
- The crystal structure of chlorido-bis(1,10-phenantroline-κ2N,N′)-(2-formylphenoxyacetato-κ2O,O) lead(II), C33H23N4O4ClPb
- Crystal structure of pyridinium tetrakis(1,1,1-trifluoro-2,4-pentadionato-κ2O,O′)yttrium(III) C20F12H16YO8C5H6N
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (1Z, 2Z)-3-phenyl-2-propenal 2-(4-bromo-2-fluorophenyl)hydrazone, C15H12BrFN2
- Refinement of crystal structure of 2-(2,3-dihydro-3-oxo-1 H -inden-1-ylidene)-1 H -indene-1,3(2 H )-dione C18H10O3
- The crystal structure of 3-(1-fluoro-2-(naphthalen-2-yl)-2-oxoethyl)-2-methoxy-3,4-dihydroisoquinolin-1(2H)-one, C22H18FNO3
- Crystal structure of the dinuclear copper(II) complex bis(μ2-2,2′ -{[1,3-phenylenebis-(methylene)]bis(oxy)}dibenzoaot-κ4O,O′:O′′,O′′′)-bis(dimethylformamide-κ1O)dicopper(II), C50H44Cu2N2O14
- Crystal structure of poly[triaqua-(μ9-biphenyl-3,3′,5,5′-tetracarboxylic-κ8 O,O:O,O′: O,O″:O,O‴)samarium(III)sodium(I)], C16H12NaSmO11
- The crystal structure of 5-benzyl-1-(4-fluorobenzyl)-4-((4-fluorobenzyl)oxy)-1,5-dihydro-2H-pyrrol-2-one, C25H21F2NO2
- The crystal structure of diammonium 2,5-dihydroxyterephthalate, C8H12N2O6
- Crystal structure of (E)-4-(4-(1H-1,2,4-triazol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[b]oxepin-5(2H)-one, C21H19N3O4
- Crystal structure of poly[oktakis(μ2-oxido-κ2O:O)-tetrakis(oxido-κ1O)-bis(μ2-1,1′-[1,4-phenylenebis(methylene)]di(1H-imidazole-κ2N:N′))-tetravanadium(V)-dizinc(II)] monohydrate, C28H30Zn2N8O13V4
- Crystal structure of acotiamide hydrochloride dimethylacetamide solvate (1/1), C25H40ClN5O6S
- Crystal structure of catena-poly[monoaqua (u2-(3-(3,5-dicarboxyphenyl)pyridine-2,6-dicarboxylic-k4O:O′:O″:N)zinc(II))] monohydrate, C15H11NO10Zn
- Crystal structure of dichlorido{2,6-bis(3,5-diisopropyl-N-pyrazolyl)pyridine}zinc(II), C23H33Cl2N5Zn
- Crystal structure of nitrato-κ2O,O′-[hydridotris(3,5-diethylpyrazol-1-yl)borato-κ3N,N′,N″]copper(II), C21H34BCuN7O3
- Crystal structure of 2,7-bis(3,5-diethyl-1H-pyrazol-4-yl)-benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetrone, C28H26N6O4
- Crystal structure of 2-(4-chlorophenyl)benzothiazole, C13H8ClNS
- Synthesis and crystal structure (3R,4′S)-4′-(3,5-dibromophenyl)-1′-methyl-2H-dispiro [benzofuran-3,3′-pyrrolidine-2′,2″-indene]-1″,2,3″-trione, C26H17Br2NO4
- Crystal structure of bis(((3a,7a-dihydro-1H-benzo[d][1,2,3]triazol-1-yl)methyl) triphenylphosphonium) tetrachloridomanganate(II), C50H42Cl4MnN6P2
- The crystal structure of 4,9-bis(4-chlorophenyl)-1,6-bis(2-cyanobenzyl)-2,4a,5,6,7,7a-hexahydro-1H-2,7,5-(epiprop[2]ene[1,1,3]triyl)pyrrolo[3,4-b]pyridine-3,10-dicarbonitrile, C40H26Cl2N6
- The crystal structure of poly((μ2-3-(3-nitro-4-carboxylphenyl)benzoate-κ3O, O′:O″)-μ2-1,4-bis(1-imidazolyl)benzene-κ2N:N′-cadmium(II)), C26H17N5O6Cd
- The crystal structure of 6-hydroxy-5H-pyrrolo[3,4-b]pyridine-5,7(6H)-dione monohydrate, C7H6N2O4
- Crystal structure of 4-((cyclohexylsulfonyl)methyl)-1,2,3,4-tetrahydrobenzo [4,5]imidazo[1,2-a]pyridine, C18H24N2O2S
- Crystal structure of 4,7-diphenyl-1,10-phenanthroline-κ2N,N′)-bis(2,4-di(fluorine)-1-phenylpyridine-κ2C,N)-iridium(III) hexafluorophosphate–dichloromethane (1/1), C47H30Cl2F10IrN4P
- Crystal structure of (4-(1-phenyl-1H-benzo[d]imidazol-2-yl)phenyl)boronic acid, C19H15BN2O2
- The crystal structure of (E)-(2-((pyridin-2-ylmethylene)amino)phenyl)arsonic acid, C12H11AsN2O3
- The crystal structure of N(benzyl(phenyl)carbomothioyl)benzamide, C21H18N2OS
- The crystal structure of bis(2-picolinium) hexachlorostannate dichloromethane monosolvate, C13H18Cl8N2Sn
- Crystal structure of poly[tetraaqua-bis(μ4-3–1-(carboxylatomethyl)-1H-1,2,4-triazole-3-carboxylato)-κ4O:O′,O″,N)zinc(II)], C5H7N3O6Zn
- The crystal structure of the co-crystal isonicotinamide – 2-(nitrophenyl)methanol (1/1), C6H6N2O·C7H7NO3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-fluorobenzoate hydrate, C23H25F2N3O6
- Crystal structure of [diaqua-{1H-benzo[d]imidazol-3-ium-5,6-dicarboxylato-κ2O,O′}magnesium(II)] C18H14MgN4O10
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl) (2-iodo-5-methoxyphenyl)methanone, C20H20INO5
- The crystal structure of 3,7,11-trimethylbenzo[5,6][1,4]thiazino[2,3,4-kl]phenothiazine 5,5,9,9-tetraoxide, C21H17NO4S2
- Crystal structure of tris(piperazine-1,4-diium)bis(2-hydroxy-1,2,3-propane-tricarboxylate) pentahydrate, C24H56N6O19
- Crystal structure of 2-chloro-5-((5-isopropyl-2-methylphenoxy)methyl)pyridine, C16H18ClNO
- Crystal structure of (E)-4-(4-(1H-pyrrol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[(b)]oxepin-5(2H)-one, C23H21NO4
- Crystal structure of (E)-N′-(3,4-dichlorobenzylidene)-3,4,5-trimethoxybenzohydrazide, C17H16Cl2N2O4
- The crystal structure of 2-(2-hydroxyphenyl)-3-(pyridin-2-yl)-2,3- dihydroquinazolin-4(1H)-one, C19H15N3O2
- Crystal structure of 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-8-methylchroman-4-one, C17H16O5
- Crystal structure of bis[(3,4-dimethoxybenzyl)triphenylphosphonium]di-μ2-bromido-dibromidodicopper(I)
- Crystal structure of bis [(1,3-dioxolan-2-ylmethyl)triphenylphosphonium] tetrabromidodicopper(I), C22H22Br2CuO2P
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid], C12H8N2O5
- The crystal structure of one-dimensional cooridnation polymer bis(thiocyanato)-bis((1E,2E)-1,2-bis(1-(pyridin-3-yl)ethylidene)-hydrazine κ2N:N)iron(II), (C30H28N10S2Fe)n
- Crystal structure of ((4-acetamidophenyl)sulfonyl)-l-alanine, C11H14N2O5S
- Crystal structure of [(1-naphthalen-1-yl-methyl)triphenylphosphonium] dichloridocopper(I), [C29H24P]+[CuCl2]−
- RbTm3S5: the first rubidium lanthanoid(III) sulfide with CsEr3Se5-type crystal structure
- Crystal structure of 2,2′-((ethane-1,2-diylbis(methylammoniumdiyl))bis(methylene))bis(pyridin-1-ium) diiodido-tris(μ2-iodido-κ2I:I)dicopper(II) chloride dihydrate, C16H30Cu2I6N4O2
- The crystal structure of 4-(trifluoromethyl)pyridine-2-carboxylic acid, C7H4F3NO2
- The crystal structure of (E)-2-ethoxy-1-methoxy-4-(2-(methylsulfonyl)vinyl)benzene, C12H16O4S
- Crystal structure of potassium 1H,1H,2H,2H-perfluorooctanesulfonate, C8H4O3F13SK
- Crystal structure of 4-(4-(quinolin-8-yloxy)-1,2,5-thiadiazol-3-yl)morpholine, C15H14O2N4S
- The crystal structure of 1,4-bis(bromomethyl)-2,5-dimethylbenzene, C10H12Br2
- The crystal structure of imidazo[4,5-e][1,3]diazepine-4,6,8-triamine methanol solvate, C7H11N7O
- The crystal structure of chlorido-bis(1,10-phenantroline-κ2N,N′)-(2-formylphenoxyacetato-κ2O,O) lead(II), C33H23N4O4ClPb
- Crystal structure of pyridinium tetrakis(1,1,1-trifluoro-2,4-pentadionato-κ2O,O′)yttrium(III) C20F12H16YO8C5H6N


