Abstract
Table 1 contains the crystallographic data. The list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Needle, clear light colourless |
|---|---|
| Size: | 0.19 × 0.18 × 0.15 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 0.86 mm−1 |
| Diffractometer, scan mode: | Bruker Smart APEX-II, φ and ω-scans |
| θmax, completeness: | 74.1°, >99 % |
| N (hkl)measured, N(hkl)unique, Rint: | 3732, 2,133, 0.023 |
| Criterion for Iobs, N(hkl)gt: | Iobs>2σ(Iobs), 1993 |
| N (param)refined: | 203 |
| Programs: | Bruker programs 1 , Olex2 2 , Shelx 3 , 4 |
1 Source of materials
The target compound, 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-8-methylchroman-4-one (abbreviated as the chroman-4-one derivative), was purchased from Jilin Chinese Academy of Sciences – Yanshen Technology Co., Ltd. Its isolation and purification were performed based on general procedures for flavonoid compounds. 5 , 6 The dried and powdered plant material was first macerated with n-hexane at room temperature to thoroughly remove non-polar components such as lipids and pigments. The resulting defatted plant residue was air-dried and then exhaustively extracted with ethyl acetate. The combined ethyl acetate extracts were concentrated in vacuo to afford a dark, paste-like crude extract. This crude extract was dry-loaded onto a silica gel column and subjected to elution with an N-hexane/ethyl acetate phase (95:5, v/v). Fractions were monitored, and similar fractions containing the target product were combined. After concentration, the enriched fraction was subjected to final purification by preparative thin-layer chromatography using a phase of dichloromethane/methanol (95:5, v/v). The target band was scraped from the plate and eluted with methanol or ethyl acetate. Concentration of the eluate yielded the high-purity target compound. The purified powder was dissolved in a minimal amount of hot ethyl acetate to form a saturated solution. The hot solution was filtered into a clean tube, the mouth of which was then sealed with Parafilm. Several small holes were pierced to allow for slow evaporation of the solvent. The apparatus was left undisturbed at room temperature, yielding colorless, block crystals in approximately one week.
2 Experimental details
A quality crystal of the title compound was collected using single-crystal X-ray diffraction analysis. The crystal structure was initially solved by employing Direct Methods within the Shelxt program. Subsequently, the structural model was refined using the Shelxl program through a full-matrix least-squares minimization on F2. All non-hydrogen atoms (C and O) were refined with anisotropic displacement parameters, whereas hydrogen atoms were placed in geometrically calculated positions and refined isotropically.
3 Comment
The chroman-4-one scaffold, a bicyclic heterocycle composed of a benzene ring with a ketone-bearing dihydropyran ring, is a privileged scaffold of fundamental importance in organic and medicinal chemistry. It not only forms the core structure of numerous natural products, such as flavonoids, but also serves as a highly versatile synthetic building block.
7
,
8
,
9
,
10
,
11
,
12
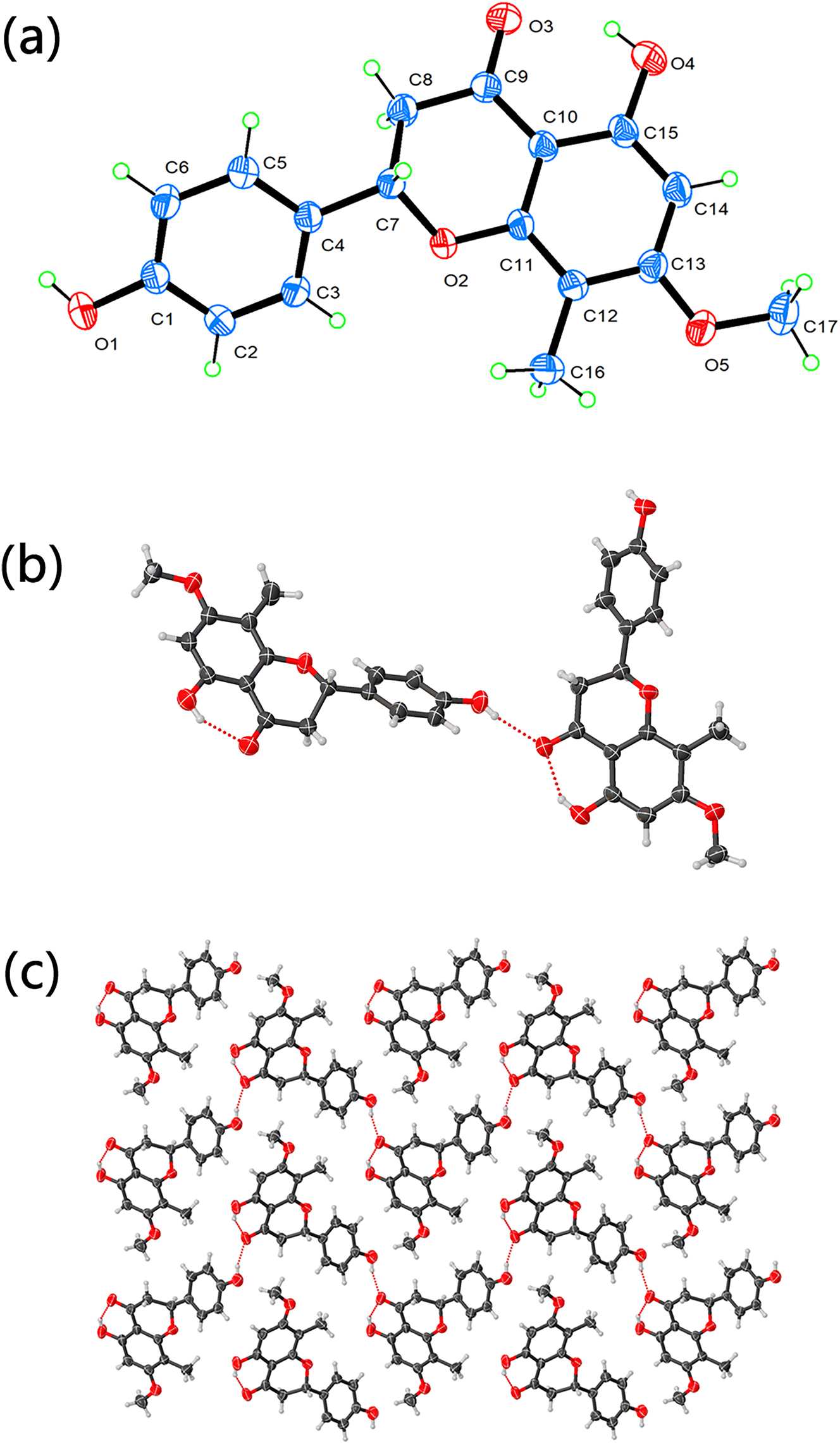
Consequently, its derivatives exhibit a broad spectrum of biological activities including anticancer, antioxidant, and anti-inflammatory properties, making it a pivotal intermediate for the design and discovery of novel lead compounds in drug development. Although the crystal structures of numerous chroman-4-one derivatives have been reported, that of 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-8-methylchroman-4-one has remained unreported. Single-crystal X-ray diffraction test proved that the title chroman-4-one derivative crystallizes in the monoclinic crystal system belonging to the P21/c space group. The asymmetric unit contains one complete chroman-4-one derivative molecule (see the figure, part a). The C–C bond lengths in the crystal are observed to be in the range of 1.374(4) Å to 1.518(3) Å, with the shortest and longest bonds being C14–C15 and C7–C8, respectively. Meanwhile, the C–O bond lengths vary from 1.258(3) Å to 1.437(3) Å, corresponding to the C9–O3 and C7–O2 bonds, respectively. These bond lengths are similar to the reported chroman-4-one derivatives.
13
,
14
,
15
,
16
,
17
,
18
The molecular structure of the chroman-4-one derivative can be regarded as comprising two principal moieties (hydrogen atoms are omitted for clarity in the following description). The first is the chroman-4-one scaffold (encompassing atoms O2–C7–C8–C9–C10–C11–C12–C13–C14–C15) along with its associated substituents: a carbonyl oxygen (O3), a hydroxyl group (O4), a methoxy group (C17–O5), and a methyl group (C16). The second moiety is a hydroxyl-substituted phenyl ring (C1–C2–C3–C4–C5–C6 with hydroxyl O1). These two moieties are connected by a C4–C7 single bond, resulting in a specific spatial orientation. This is defined by the C3–C4–C7–O2 dihedral angle of 2.6° and the C3–C4–C7–C8 dihedral angle of 119.2°. Notably, the C7–C8 linkage within the chroman-4-one core is a single bond, not a double or triple bond, which in turn establishes specific bond angles such as C8–C7–O2 (109.9°), C4–C7–O2 (107.0°), and C4–C7–C8 (114.1°). Consequently, these structural features dictate a non-planar conformation for the molecule, which disrupts the extended π-conjugated system across the entire structure. An analysis of the crystal packing, performed using the Platon and Mercury software packages,
19
,
20
reveals that the supramolecular structure is primarily stabilized by an extensive network of hydrogen bonds. The key interactions include O–H⋯O hydrogen bonds (O1–H1⋯
Acknowledgments
This work was supported by the Shandong Province Natural Science Foundation (No. ZR2021QB053 and ZR2023QB209), and research startup funds of Weifang University (No. 196100040020).
References
1. Bruker. Saint and Sadabs; Bruker AXS Inc.: Madison, Wisconsin, USA, 2000.Suche in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341, https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. Shelxl – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
5. Brito, I.; Borquez, J.; Simirgiotis, M.; Cardenas, A.; Lopez-Rodriguez, M. 4′,5–Dihy-Droxy-7-Meth-Oxy-Flavanone Dihydrate. Acta Crystallogr., Sect. E: Struct. Rep. Online 2012, E68, o32–o33.10.1107/S1600536811051221Suche in Google Scholar PubMed PubMed Central
6. Dos, S. G. S.; Dorigueto, A. C.; Landre, I. M.; Soares, M. G.; Martho, K.; Lima, R.; Pascon, R. C.; Vallim, M. A.; Capello, T. M.; Romoff, P.; Sartorelli, P.; Lago, J. H. Structural Crystalline Characterization of Sakuranetin–An Antimicrobial Flavanone from Twigs of Baccharis Retusa (Asteraceae). Molecules. 2014, 19, 7528–7542, https://doi.org/10.3390/molecules19067528.Suche in Google Scholar PubMed PubMed Central
7. Diana, E. J.; Kanchana, U. S.; Mathew, T. V. Current Developments in the Synthesis of 4-Chromanone-Derived Compounds. Org. Biomol. Chem. 2021, 19, 7995–8008; https://doi.org/10.1039/d1ob01352a.Suche in Google Scholar PubMed
8. Emami, S.; Ghanbarimasir, Z. Recent Advances of Chroman-4-One Derivatives: Synthetic Approaches and Bioactivities. Eur. J. Med. Chem. 2015, 93, 539–563, https://doi.org/10.1016/j.ejmech.2015.02.048.Suche in Google Scholar PubMed
9. Awantu, A. F.; Lenta, B. N.; Donfack, E. V.; Wansi, J. D.; Neumann, B.; Stammler, H. G.; Noungoue, D. T.; Tsamo, E.; Sewald, N. Flavonoids and Other Constituents of Hymenostegia Afzelii (Caesalpiniaceae). Phytochem. Let. 2011, 4, 315–319, https://doi.org/10.1016/j.phytol.2011.06.002.Suche in Google Scholar
10. Duong, T. H.; Beniddir, M. A.; Nguyen, V. K.; Aree, T.; Gallard, J. F.; Mac, D. H.; Nguyen, H. H.; Bui, X. H.; Boustie, J.; Nguyen, K. P.; Chavasiri, W.; Le Pogam, P. Sulfonic Acid-Containing Flavonoids from the Roots of Phyllanthus Acidus, J. Nat. Prod. 2018, 81, 2026–2031, https://doi.org/10.1021/acs.jnatprod.8b00322.Suche in Google Scholar PubMed
11. Zeng, F.; Wang, W.; Wu, Y.; Dey, M.; Ye, M.; Avery, M. A.; Khan, I. A.; Guo, D. A. Two Prenylated and C-Methylated Flavonoids from Tripterygium Wilfordii. Planta Med. 2010, 76, 1596–1599; https://doi.org/10.1055/s-0029-1241017.Suche in Google Scholar PubMed
12. Wang, C.; Xiong, W.; Reddy Perumalla, S.; Fang, J.; Calvin Sun, C. Solid-state Characterization of Optically Pure (+)Dihydromyricetin Extracted from Ampelopsis Grossedentata Leaves. Int. J. Pharm. 2016, 511, 245–252, https://doi.org/10.1016/j.ijpharm.2016.07.018.Suche in Google Scholar PubMed
13. Pagola, S.; Tracanna, M. I.; Amani, S. M.; González, A. M.; Raschi, A. B.; Romano, E.; Benavente, A. M.; Stephens, P. W. Sideroxylin from Miconia Ioneura: Monohydrate Crystal Structure from High Resolution X-Ray Powder Diffraction. Nat. Prod. Commun. 2008, 3, 759–764; https://doi.org/10.1177/1934578x0800300516.Suche in Google Scholar
14. Cisterna, J.; Bórquez, J.; Muñoz, R.; Simirgiotis, M.; Cárdenas, A.; Brito, I. Crystal Structure of 5,4′-Dihydroxy-7,3′-Dimethoxyflavanone, C17H16O6. Z. Kristallogr. – N. Cryst. Struct. 2019, 234, 405–407.10.1515/ncrs-2018-0295Suche in Google Scholar
15. Shoja, M. 4′,5-Dihydroxy-7-methoxyflavanone. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1990, 46, 1969–1971, https://doi.org/10.1107/s0108270190002979.Suche in Google Scholar
16. Nesterov, V. V.; Zakharov, L. N.; Nesterov, V. N.; Calderon, J. G.; Longo, A.; Zaman, K.; Choudhury, F. K.; Farrell, W.; Shulaev, V.; Richmond, M. G. 5,7-Dihydroxy-2-(4-hydroxyphenyl)chroman-4-one (Naringenin): X-Ray Diffraction Structures of the Naringenin Enantiomers and DFT Evaluation of the Preferred Ground-State Structures and Thermodynamics for Racemization. J. Mol. Struct. 2017, 1130, 994–1000, https://doi.org/10.1016/j.molstruc.2016.10.068.Suche in Google Scholar
17. Timmons, D. J.; Pacheco, M. R.; Fricke, K. A.; Slebodnick, C. Assembling Extended Structures with Flavonoids. Cryst. Growth Des. 2008, 8, 2765–2769; https://doi.org/10.1021/cg7009572.Suche in Google Scholar
18. Khandavilli, U. B. R.; Skořepová, E.; Sinha, A. S.; Bhogala, B. R.; Maguire, N. M.; Maguire, A. R.; Lawrence, S. E. Cocrystals and a Salt of the Bioactive Flavonoid: Naringenin. Cryst. Growth Des. 2018, 18, 4571–4577.10.1021/acs.cgd.8b00557Suche in Google Scholar
19. Macrae, C. F.; Edgington, P. R.; Mccabe, P.; Pidcock, E.; Shields, G. P.; Taylor, R.; Towler, M.; Van De Streek, J. Mercury: Visualization and Analysis of Crystal Structures. J. Appl. Crystallogr. 2006, 39, 453–457, https://doi.org/10.1107/s002188980600731x.Suche in Google Scholar
20. Spek, A. L. Structure Validation in Chemical Crystallography. Acta Crystallogr. D Biol. Crystallogr. 2009, 65, 148–155; https://doi.org/10.1107/s090744490804362x.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of (1Z, 2Z)-3-phenyl-2-propenal 2-(4-bromo-2-fluorophenyl)hydrazone, C15H12BrFN2
- Refinement of crystal structure of 2-(2,3-dihydro-3-oxo-1 H -inden-1-ylidene)-1 H -indene-1,3(2 H )-dione C18H10O3
- The crystal structure of 3-(1-fluoro-2-(naphthalen-2-yl)-2-oxoethyl)-2-methoxy-3,4-dihydroisoquinolin-1(2H)-one, C22H18FNO3
- Crystal structure of the dinuclear copper(II) complex bis(μ2-2,2′ -{[1,3-phenylenebis-(methylene)]bis(oxy)}dibenzoaot-κ4O,O′:O′′,O′′′)-bis(dimethylformamide-κ1O)dicopper(II), C50H44Cu2N2O14
- Crystal structure of poly[triaqua-(μ9-biphenyl-3,3′,5,5′-tetracarboxylic-κ8 O,O:O,O′: O,O″:O,O‴)samarium(III)sodium(I)], C16H12NaSmO11
- The crystal structure of 5-benzyl-1-(4-fluorobenzyl)-4-((4-fluorobenzyl)oxy)-1,5-dihydro-2H-pyrrol-2-one, C25H21F2NO2
- The crystal structure of diammonium 2,5-dihydroxyterephthalate, C8H12N2O6
- Crystal structure of (E)-4-(4-(1H-1,2,4-triazol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[b]oxepin-5(2H)-one, C21H19N3O4
- Crystal structure of poly[oktakis(μ2-oxido-κ2O:O)-tetrakis(oxido-κ1O)-bis(μ2-1,1′-[1,4-phenylenebis(methylene)]di(1H-imidazole-κ2N:N′))-tetravanadium(V)-dizinc(II)] monohydrate, C28H30Zn2N8O13V4
- Crystal structure of acotiamide hydrochloride dimethylacetamide solvate (1/1), C25H40ClN5O6S
- Crystal structure of catena-poly[monoaqua (u2-(3-(3,5-dicarboxyphenyl)pyridine-2,6-dicarboxylic-k4O:O′:O″:N)zinc(II))] monohydrate, C15H11NO10Zn
- Crystal structure of dichlorido{2,6-bis(3,5-diisopropyl-N-pyrazolyl)pyridine}zinc(II), C23H33Cl2N5Zn
- Crystal structure of nitrato-κ2O,O′-[hydridotris(3,5-diethylpyrazol-1-yl)borato-κ3N,N′,N″]copper(II), C21H34BCuN7O3
- Crystal structure of 2,7-bis(3,5-diethyl-1H-pyrazol-4-yl)-benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetrone, C28H26N6O4
- Crystal structure of 2-(4-chlorophenyl)benzothiazole, C13H8ClNS
- Synthesis and crystal structure (3R,4′S)-4′-(3,5-dibromophenyl)-1′-methyl-2H-dispiro [benzofuran-3,3′-pyrrolidine-2′,2″-indene]-1″,2,3″-trione, C26H17Br2NO4
- Crystal structure of bis(((3a,7a-dihydro-1H-benzo[d][1,2,3]triazol-1-yl)methyl) triphenylphosphonium) tetrachloridomanganate(II), C50H42Cl4MnN6P2
- The crystal structure of 4,9-bis(4-chlorophenyl)-1,6-bis(2-cyanobenzyl)-2,4a,5,6,7,7a-hexahydro-1H-2,7,5-(epiprop[2]ene[1,1,3]triyl)pyrrolo[3,4-b]pyridine-3,10-dicarbonitrile, C40H26Cl2N6
- The crystal structure of poly((μ2-3-(3-nitro-4-carboxylphenyl)benzoate-κ3O, O′:O″)-μ2-1,4-bis(1-imidazolyl)benzene-κ2N:N′-cadmium(II)), C26H17N5O6Cd
- The crystal structure of 6-hydroxy-5H-pyrrolo[3,4-b]pyridine-5,7(6H)-dione monohydrate, C7H6N2O4
- Crystal structure of 4-((cyclohexylsulfonyl)methyl)-1,2,3,4-tetrahydrobenzo [4,5]imidazo[1,2-a]pyridine, C18H24N2O2S
- Crystal structure of 4,7-diphenyl-1,10-phenanthroline-κ2N,N′)-bis(2,4-di(fluorine)-1-phenylpyridine-κ2C,N)-iridium(III) hexafluorophosphate–dichloromethane (1/1), C47H30Cl2F10IrN4P
- Crystal structure of (4-(1-phenyl-1H-benzo[d]imidazol-2-yl)phenyl)boronic acid, C19H15BN2O2
- The crystal structure of (E)-(2-((pyridin-2-ylmethylene)amino)phenyl)arsonic acid, C12H11AsN2O3
- The crystal structure of N(benzyl(phenyl)carbomothioyl)benzamide, C21H18N2OS
- The crystal structure of bis(2-picolinium) hexachlorostannate dichloromethane monosolvate, C13H18Cl8N2Sn
- Crystal structure of poly[tetraaqua-bis(μ4-3–1-(carboxylatomethyl)-1H-1,2,4-triazole-3-carboxylato)-κ4O:O′,O″,N)zinc(II)], C5H7N3O6Zn
- The crystal structure of the co-crystal isonicotinamide – 2-(nitrophenyl)methanol (1/1), C6H6N2O·C7H7NO3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-fluorobenzoate hydrate, C23H25F2N3O6
- Crystal structure of [diaqua-{1H-benzo[d]imidazol-3-ium-5,6-dicarboxylato-κ2O,O′}magnesium(II)] C18H14MgN4O10
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl) (2-iodo-5-methoxyphenyl)methanone, C20H20INO5
- The crystal structure of 3,7,11-trimethylbenzo[5,6][1,4]thiazino[2,3,4-kl]phenothiazine 5,5,9,9-tetraoxide, C21H17NO4S2
- Crystal structure of tris(piperazine-1,4-diium)bis(2-hydroxy-1,2,3-propane-tricarboxylate) pentahydrate, C24H56N6O19
- Crystal structure of 2-chloro-5-((5-isopropyl-2-methylphenoxy)methyl)pyridine, C16H18ClNO
- Crystal structure of (E)-4-(4-(1H-pyrrol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[(b)]oxepin-5(2H)-one, C23H21NO4
- Crystal structure of (E)-N′-(3,4-dichlorobenzylidene)-3,4,5-trimethoxybenzohydrazide, C17H16Cl2N2O4
- The crystal structure of 2-(2-hydroxyphenyl)-3-(pyridin-2-yl)-2,3- dihydroquinazolin-4(1H)-one, C19H15N3O2
- Crystal structure of 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-8-methylchroman-4-one, C17H16O5
- Crystal structure of bis[(3,4-dimethoxybenzyl)triphenylphosphonium]di-μ2-bromido-dibromidodicopper(I)
- Crystal structure of bis [(1,3-dioxolan-2-ylmethyl)triphenylphosphonium] tetrabromidodicopper(I), C22H22Br2CuO2P
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid], C12H8N2O5
- The crystal structure of one-dimensional cooridnation polymer bis(thiocyanato)-bis((1E,2E)-1,2-bis(1-(pyridin-3-yl)ethylidene)-hydrazine κ2N:N)iron(II), (C30H28N10S2Fe)n
- Crystal structure of ((4-acetamidophenyl)sulfonyl)-l-alanine, C11H14N2O5S
- Crystal structure of [(1-naphthalen-1-yl-methyl)triphenylphosphonium] dichloridocopper(I), [C29H24P]+[CuCl2]−
- RbTm3S5: the first rubidium lanthanoid(III) sulfide with CsEr3Se5-type crystal structure
- Crystal structure of 2,2′-((ethane-1,2-diylbis(methylammoniumdiyl))bis(methylene))bis(pyridin-1-ium) diiodido-tris(μ2-iodido-κ2I:I)dicopper(II) chloride dihydrate, C16H30Cu2I6N4O2
- The crystal structure of 4-(trifluoromethyl)pyridine-2-carboxylic acid, C7H4F3NO2
- The crystal structure of (E)-2-ethoxy-1-methoxy-4-(2-(methylsulfonyl)vinyl)benzene, C12H16O4S
- Crystal structure of potassium 1H,1H,2H,2H-perfluorooctanesulfonate, C8H4O3F13SK
- Crystal structure of 4-(4-(quinolin-8-yloxy)-1,2,5-thiadiazol-3-yl)morpholine, C15H14O2N4S
- The crystal structure of 1,4-bis(bromomethyl)-2,5-dimethylbenzene, C10H12Br2
- The crystal structure of imidazo[4,5-e][1,3]diazepine-4,6,8-triamine methanol solvate, C7H11N7O
- The crystal structure of chlorido-bis(1,10-phenantroline-κ2N,N′)-(2-formylphenoxyacetato-κ2O,O) lead(II), C33H23N4O4ClPb
- Crystal structure of pyridinium tetrakis(1,1,1-trifluoro-2,4-pentadionato-κ2O,O′)yttrium(III) C20F12H16YO8C5H6N
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of (1Z, 2Z)-3-phenyl-2-propenal 2-(4-bromo-2-fluorophenyl)hydrazone, C15H12BrFN2
- Refinement of crystal structure of 2-(2,3-dihydro-3-oxo-1 H -inden-1-ylidene)-1 H -indene-1,3(2 H )-dione C18H10O3
- The crystal structure of 3-(1-fluoro-2-(naphthalen-2-yl)-2-oxoethyl)-2-methoxy-3,4-dihydroisoquinolin-1(2H)-one, C22H18FNO3
- Crystal structure of the dinuclear copper(II) complex bis(μ2-2,2′ -{[1,3-phenylenebis-(methylene)]bis(oxy)}dibenzoaot-κ4O,O′:O′′,O′′′)-bis(dimethylformamide-κ1O)dicopper(II), C50H44Cu2N2O14
- Crystal structure of poly[triaqua-(μ9-biphenyl-3,3′,5,5′-tetracarboxylic-κ8 O,O:O,O′: O,O″:O,O‴)samarium(III)sodium(I)], C16H12NaSmO11
- The crystal structure of 5-benzyl-1-(4-fluorobenzyl)-4-((4-fluorobenzyl)oxy)-1,5-dihydro-2H-pyrrol-2-one, C25H21F2NO2
- The crystal structure of diammonium 2,5-dihydroxyterephthalate, C8H12N2O6
- Crystal structure of (E)-4-(4-(1H-1,2,4-triazol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[b]oxepin-5(2H)-one, C21H19N3O4
- Crystal structure of poly[oktakis(μ2-oxido-κ2O:O)-tetrakis(oxido-κ1O)-bis(μ2-1,1′-[1,4-phenylenebis(methylene)]di(1H-imidazole-κ2N:N′))-tetravanadium(V)-dizinc(II)] monohydrate, C28H30Zn2N8O13V4
- Crystal structure of acotiamide hydrochloride dimethylacetamide solvate (1/1), C25H40ClN5O6S
- Crystal structure of catena-poly[monoaqua (u2-(3-(3,5-dicarboxyphenyl)pyridine-2,6-dicarboxylic-k4O:O′:O″:N)zinc(II))] monohydrate, C15H11NO10Zn
- Crystal structure of dichlorido{2,6-bis(3,5-diisopropyl-N-pyrazolyl)pyridine}zinc(II), C23H33Cl2N5Zn
- Crystal structure of nitrato-κ2O,O′-[hydridotris(3,5-diethylpyrazol-1-yl)borato-κ3N,N′,N″]copper(II), C21H34BCuN7O3
- Crystal structure of 2,7-bis(3,5-diethyl-1H-pyrazol-4-yl)-benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetrone, C28H26N6O4
- Crystal structure of 2-(4-chlorophenyl)benzothiazole, C13H8ClNS
- Synthesis and crystal structure (3R,4′S)-4′-(3,5-dibromophenyl)-1′-methyl-2H-dispiro [benzofuran-3,3′-pyrrolidine-2′,2″-indene]-1″,2,3″-trione, C26H17Br2NO4
- Crystal structure of bis(((3a,7a-dihydro-1H-benzo[d][1,2,3]triazol-1-yl)methyl) triphenylphosphonium) tetrachloridomanganate(II), C50H42Cl4MnN6P2
- The crystal structure of 4,9-bis(4-chlorophenyl)-1,6-bis(2-cyanobenzyl)-2,4a,5,6,7,7a-hexahydro-1H-2,7,5-(epiprop[2]ene[1,1,3]triyl)pyrrolo[3,4-b]pyridine-3,10-dicarbonitrile, C40H26Cl2N6
- The crystal structure of poly((μ2-3-(3-nitro-4-carboxylphenyl)benzoate-κ3O, O′:O″)-μ2-1,4-bis(1-imidazolyl)benzene-κ2N:N′-cadmium(II)), C26H17N5O6Cd
- The crystal structure of 6-hydroxy-5H-pyrrolo[3,4-b]pyridine-5,7(6H)-dione monohydrate, C7H6N2O4
- Crystal structure of 4-((cyclohexylsulfonyl)methyl)-1,2,3,4-tetrahydrobenzo [4,5]imidazo[1,2-a]pyridine, C18H24N2O2S
- Crystal structure of 4,7-diphenyl-1,10-phenanthroline-κ2N,N′)-bis(2,4-di(fluorine)-1-phenylpyridine-κ2C,N)-iridium(III) hexafluorophosphate–dichloromethane (1/1), C47H30Cl2F10IrN4P
- Crystal structure of (4-(1-phenyl-1H-benzo[d]imidazol-2-yl)phenyl)boronic acid, C19H15BN2O2
- The crystal structure of (E)-(2-((pyridin-2-ylmethylene)amino)phenyl)arsonic acid, C12H11AsN2O3
- The crystal structure of N(benzyl(phenyl)carbomothioyl)benzamide, C21H18N2OS
- The crystal structure of bis(2-picolinium) hexachlorostannate dichloromethane monosolvate, C13H18Cl8N2Sn
- Crystal structure of poly[tetraaqua-bis(μ4-3–1-(carboxylatomethyl)-1H-1,2,4-triazole-3-carboxylato)-κ4O:O′,O″,N)zinc(II)], C5H7N3O6Zn
- The crystal structure of the co-crystal isonicotinamide – 2-(nitrophenyl)methanol (1/1), C6H6N2O·C7H7NO3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-fluorobenzoate hydrate, C23H25F2N3O6
- Crystal structure of [diaqua-{1H-benzo[d]imidazol-3-ium-5,6-dicarboxylato-κ2O,O′}magnesium(II)] C18H14MgN4O10
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl) (2-iodo-5-methoxyphenyl)methanone, C20H20INO5
- The crystal structure of 3,7,11-trimethylbenzo[5,6][1,4]thiazino[2,3,4-kl]phenothiazine 5,5,9,9-tetraoxide, C21H17NO4S2
- Crystal structure of tris(piperazine-1,4-diium)bis(2-hydroxy-1,2,3-propane-tricarboxylate) pentahydrate, C24H56N6O19
- Crystal structure of 2-chloro-5-((5-isopropyl-2-methylphenoxy)methyl)pyridine, C16H18ClNO
- Crystal structure of (E)-4-(4-(1H-pyrrol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[(b)]oxepin-5(2H)-one, C23H21NO4
- Crystal structure of (E)-N′-(3,4-dichlorobenzylidene)-3,4,5-trimethoxybenzohydrazide, C17H16Cl2N2O4
- The crystal structure of 2-(2-hydroxyphenyl)-3-(pyridin-2-yl)-2,3- dihydroquinazolin-4(1H)-one, C19H15N3O2
- Crystal structure of 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-8-methylchroman-4-one, C17H16O5
- Crystal structure of bis[(3,4-dimethoxybenzyl)triphenylphosphonium]di-μ2-bromido-dibromidodicopper(I)
- Crystal structure of bis [(1,3-dioxolan-2-ylmethyl)triphenylphosphonium] tetrabromidodicopper(I), C22H22Br2CuO2P
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid], C12H8N2O5
- The crystal structure of one-dimensional cooridnation polymer bis(thiocyanato)-bis((1E,2E)-1,2-bis(1-(pyridin-3-yl)ethylidene)-hydrazine κ2N:N)iron(II), (C30H28N10S2Fe)n
- Crystal structure of ((4-acetamidophenyl)sulfonyl)-l-alanine, C11H14N2O5S
- Crystal structure of [(1-naphthalen-1-yl-methyl)triphenylphosphonium] dichloridocopper(I), [C29H24P]+[CuCl2]−
- RbTm3S5: the first rubidium lanthanoid(III) sulfide with CsEr3Se5-type crystal structure
- Crystal structure of 2,2′-((ethane-1,2-diylbis(methylammoniumdiyl))bis(methylene))bis(pyridin-1-ium) diiodido-tris(μ2-iodido-κ2I:I)dicopper(II) chloride dihydrate, C16H30Cu2I6N4O2
- The crystal structure of 4-(trifluoromethyl)pyridine-2-carboxylic acid, C7H4F3NO2
- The crystal structure of (E)-2-ethoxy-1-methoxy-4-(2-(methylsulfonyl)vinyl)benzene, C12H16O4S
- Crystal structure of potassium 1H,1H,2H,2H-perfluorooctanesulfonate, C8H4O3F13SK
- Crystal structure of 4-(4-(quinolin-8-yloxy)-1,2,5-thiadiazol-3-yl)morpholine, C15H14O2N4S
- The crystal structure of 1,4-bis(bromomethyl)-2,5-dimethylbenzene, C10H12Br2
- The crystal structure of imidazo[4,5-e][1,3]diazepine-4,6,8-triamine methanol solvate, C7H11N7O
- The crystal structure of chlorido-bis(1,10-phenantroline-κ2N,N′)-(2-formylphenoxyacetato-κ2O,O) lead(II), C33H23N4O4ClPb
- Crystal structure of pyridinium tetrakis(1,1,1-trifluoro-2,4-pentadionato-κ2O,O′)yttrium(III) C20F12H16YO8C5H6N


