Abstract
C21H18N2OS, monoclinic, P21/c, a = 8.4888(2) Å, b = 10.2133(3) Å, c = 19.9581(6) Å, β = 97.015(2)°, V = 1717.39(8) Å3, Z = 4, T = 150(2) K, R gt (F) = 0.0361, wR ref (F2) = 0.0913.
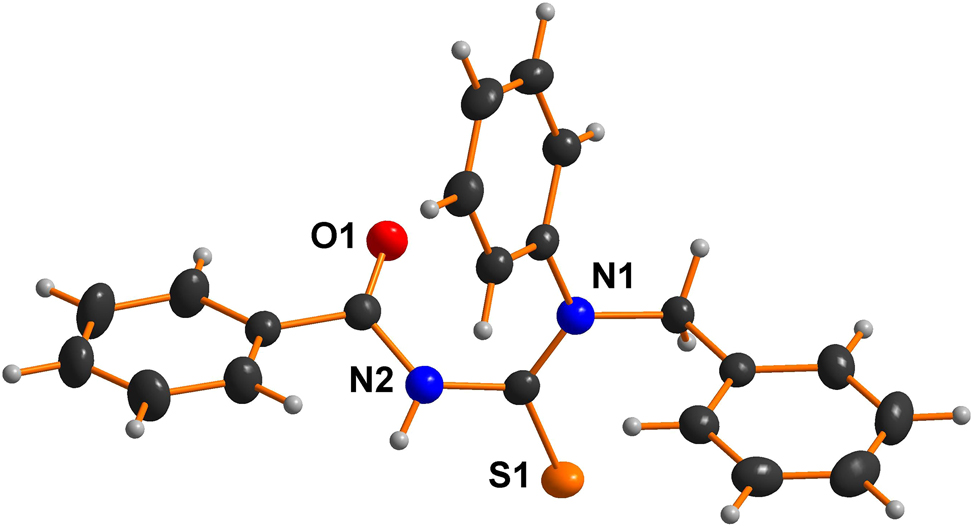
Thermal ellipsoids are plotted at a 50 % probability level in the figure. All hydrogen atoms are omitted for clarity. Table 1 contains the crystallographic data, and the list of the atoms, including atomic coordinates and displacement parameters, can be found in the CIF-file attached to this article.
Data collection and handling.
| Crystal: | Colourless blade |
| Size: | 0.35 × 0.19 × 0.09 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.20 mm−1 |
| Diffractometer, scan mode: | XtaLAB Synergy R, ω scans |
| θmax, completeness: | 31.1°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 28370, 4474, 0.036 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3,714 |
| N(param)refined: | 226 |
| Programs: | Rigaku, 1 Olex2, 2 SHELX 3 , 4 |
1 Source of materials
To ammonium thiocyanate (17 mmol) that was dissolved in hot acetone (10 mL), benzoyl chloride (17 mmol) was added dropwise, continuously stirring for 20 min and followed by the filtration of the resultant mixture. The carbonyl isothiocyanate filtrate was collected and heated at 65 °C for 30 min, followed by the slow addition of N-benzylaniline, with stirring for 30 min at ambient temperature. 5 On completion, as confirmed by TLC, the thiourea ligand was precipitated with distilled water, filtered, and rinsed with water successfully. The final product was recrystallized from n-hexane and ethyl acetate solution (9:1) with obtained yields of 80 %. 6 IR (KBr, cm −1 ): 3252 (C–H), 2955 (C–Hasy), 2876 (C–Hsym), 1692 (C=O), 1149 (C=S). UV–vis (solvent: CH3OH): ε (λmax 230 nm) 1065.7 M−1cm−1. HRMS (ES + ): 347.1224 [M+H]+ (found), 347.1218 (calcd), mp:153.4–154.9 °C.
2 Experimental details
All the hydrogen atoms were positioned geometrically and refined discernibly using a riding model with fixed C–HAromatic = 0.97 Å. The H atoms isotropic displacement parameters were fixed; Uiso(H) = 1.2Ueq (C) for aromatic, allowing them to ride on the parent atom. The graphics were obtained by using the DIAMOND program with 50 % probability ellipsoids.
3 Comment
Thiourea, often known as thiocarbamide, is an organosulfur molecule with the chemical formula SC(NH2)2. Thioureas are chelating agents and have been widely utilized in analytical chemistry and medicine. 7 , 8 , 9 Their compounds have demonstrated exceptional versatility in various academic, medical, industrial, biological, commercial, and agricultural applications. 10 , 11 The unique characteristics of these compounds facilitate their effective modification as binding agents, greatly improving their biological activity. These compounds demonstrate a diverse array of biological roles, including their application as anesthetic agents, 12 anticarcinogens, 13 , 14 anticonvulsants, 15 and antitubercular agents. 16 The thiourea moiety’s bond lengths and angles represent thiourea derivatives. 17 , 18 The bond lengths between C1 and O1, as well as C2 and S1, display the properties typical of a double bond, with measured distances of 1.219(1) Å and 1.670(1) Å, respectively. The lengths of C–N bonds show a notable reduction, with specific measurements recorded as follows: 1.345(1) Å for C2–N1, 1.446(1) Å for C3–N1, 1.382(2) Å for C1–N2, and 1.404(2) Å for C2–N2. This data indicates the likely existence of partial double-bond character in these bonds. The elongation of the C2–N2 bond relative to the C2–N1 bond is a phenomenon that has been extensively documented in various thiourea derivatives. 5 , 6 , 19 , 20 , 21 The observed elongation can be explained by the electron-withdrawing influence on the carbonyl group. 22 , 23 The compound in question exhibits three intermolecular hydrogen bonds, C11–H11⋯N1 exhibits a bond length of 2.8718(15) Å, C9–H9A…O1 has a bond length of 3.1714(14) Å and C9–H9B⋯S1 with the bond length of 3.0041(12) Å.
Funding source: National Research Foundation of South Africa
Award Identifier / Grant number: 129468
Funding source: Tshwane University of Technology
Funding source: The University of Pretoria
Acknowledgements
We would like to thank the National Research Foundation of South Africa (Grant No. 129468), Tshwane University of Technology, and the University of Pretoria for institutional and financial support. The authors would like to express their gratitude towards NCP Chlorchem for financial assistance.
References
1. System, C. S. Rigaku Oxford Diffraction; CrysAlisPRO Software System Yarnton: UK, 2022.Search in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Cryst. 2009, 42, 339–341.10.1107/S0021889808042726Search in Google Scholar
3. Sheldrick, G. M. SHELXT – Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
5. Douglass, I. B.; Dains, F. B. Some Derivatives of Benzoyl and Furoyl Isothiocyanates and their use in Synthesizing Heterocyclic Compounds. J. Am. Chem. Soc 1934, 56, 719–721; https://doi.org/10.1021/ja01318a057.Search in Google Scholar
6. Adam, F.; Fatihah, F.; Ameram, N. N.; Subramaniam, N.; Mubarrakh, S. A. The Synthesis and Characterization of 2-methyl-N-((4-methylpyridine-2yl) Carbamothioyl) Benzamide: Its Preparation with Antibacterial Study. J. Phys. Sci. 2016, 27 (2), 83–101; https://doi.org/10.21315/jps2016.27.2.7.Search in Google Scholar
7. Arslan, A.; Duran, N.; Borekci, G.; Ozer, C. K.; Akbay, C. Antimicrobial Activity of Some Thiourea Derivatives and Their Nickel and Copper Complexes. Molecule 2009, 14, 519–527; https://doi.org/10.3390/molecules14010519.Search in Google Scholar PubMed PubMed Central
8. Makhakhayi, L.; Malan, F. P.; Tembu, V. J.; Nkambule, C. M.; Manicum, A. The Crystal Structure of Fac-tricarbonyl(N-benzoyl-N,N-cyclohexylmethylcarbamimidothioato-κ2S,O)-(pyridine-κN)rhenium(I), C23H24N3O4ReS. Z. Kristallogr. New Cryst. Struct. 2023, 238 (4), 697–699; https://doi.org/10.1515/ncrs-2023-0157.Search in Google Scholar
9. Komane, W. K.; Mokolokolo, P.; Vatsha, B.; Manicum, A. The Crystal Structure of Fac-tricarbonyl (N′-benzoyl-N,N-diphenylcarbamimidothioato-κ2S,O)-(pyrazole-κN) rhenium(I)-methanol(1/1) C26H23O4N4SRe. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 767–769; https://doi.org/10.1515/ncrs-2021-0046.Search in Google Scholar
10. Khan, E.; Khan, S.; Gul, Z.; Muhammad, M. Medicinal Importance, Coordination Chemistry with Selected Metals (Cu, Ag, Au) and Chemosensing of Thiourea Derivatives. A Review. Crit. Rev. Anal. Chem. 2021, 51 (8), 812–834; https://doi.org/10.1080/10408347.2020.1777523.Search in Google Scholar PubMed
11. Mohapatra, R. K.; Das, P. K.; Pradhan, M. K.; El–Ajaily, M. M.; Das, D.; Salem, H. F.; E–Zahan, M. K.; Badhei, G.; Parhi, P. K.; Maihub, A. A.; -E-Zahan, M. K. Recent Advances in Urea-and Thiourea-based Metal Complexes: Biological, Sensor, Optical, and Corrosion Inhibition Studies. Comm. Inorg. Chem. 2019, 39 (3), 127–187; https://doi.org/10.1080/02603594.2019.1594204.Search in Google Scholar
12. Citi, V.; Martelli, A.; Bucci, M.; Piragine, E.; Testai, L.; Vellecco, V.; Calderone, V. Searching for Novel Hydrogen Sulfide Donors: the Vascular Effects of Two Thiourea Derivatives. Pharmacol. Res. 2020, 159, 105039–105047; https://doi.org/10.1016/j.phrs.2020.105039.Search in Google Scholar PubMed
13. Arafa, W. A. A.; Ghoneim, A. A.; Mourad, A. K. N-Naphthoyl Thiourea Derivatives: an Efficient Ultrasonic-Assisted Synthesis, Reaction, and In-vitro Anticancer Evaluations. ACS Omega 2022, 7 (7), 6210–6222; https://doi.org/10.1021/acsomega.1c06718.Search in Google Scholar PubMed PubMed Central
14. Makhakhayi, L.; Malan, F. P.; Senzani, S.; Tukulula, M.; Davison, C.; de la Mare, J. A.; Nkambule, C. M.; Tembu, V. J.; Manicum, A. Synthesis, Characterisation, X-ray Diffraction and Biological Evaluation of New Thiourea Derivatives Against Mycobacterium Tuberculosis and Cervical Cancer. J. Mol. Struct. 2024, 1314, 138818–138825; https://doi.org/10.1016/j.molstruc.2024.138818.Search in Google Scholar
15. Ozbek, O.; Gurdere, M. B. A Review on the Synthesis and Applications of Molecules as Anticonvulsant Drug Agent Candidates. Med. Chem. Res. 2020, 29 (9), 1553–1578; https://doi.org/10.1007/s00044-020-02595-4.Search in Google Scholar
16. Tapera, M.; Kekeçmuhammed, H.; Sahin, K.; Krishna, V. S.; Lherbet, C.; Homberset, H.; Sarıpınar, E. Synthesis, Characterization, Anti-tuberculosis Activity and Molecular Modeling Studies of Thiourea Derivatives Bearing Aminoguanidine Moiety. J. Mol. Struct. 2022, 1270, 133899–133943.10.1016/j.molstruc.2022.133899Search in Google Scholar
17. Allen, F. H.; Motherwell, W. D. S. Applications of the Cambridge Structural Database in Organic Chemistry and Crystal Chemistry. Acta Crystallogr. 2002, B58, 407–422; https://doi.org/10.1107/s0108768102004895.Search in Google Scholar PubMed
18. Yusof, M. S. M.; Hamid, M. A.; Ramli, R. M. H. R.; Yamin, B. M. Synthesis and Characterisation a Series of N-(3,4-dichlorophenyl)-N′-(2,3 and 4-methylbenzoyl)thiourea Derivatives. J. Mol. Struct. 2010, 975 (1), 280–284.10.1016/j.molstruc.2010.04.037Search in Google Scholar
19. Xian, L. N-(3-Methyl-phen-yl)-N′-(4-nitro-benzo-yl)thio-urea. Acta Crystallogr. 2008, E64, o1969; https://doi.org/10.1107/s1600536808029425.Search in Google Scholar
20. Yamin, B. M. 1-(2-Methylbenzoyl)-3-m-tolylthiourea. Acta Crystallogr. 2008, E64, o1227–o1227; https://doi.org/10.1107/s1600536808012300.Search in Google Scholar PubMed PubMed Central
21. Jusoh, R. H.; Khairul, W. M.; Yusof, M. S. M.; Kadir, M. A.; Yamin, B. M. Structural and Spectroscopic Studies of Novel Methylbenzoylthiourea Derivatives. MJAS 2011, 15 (1), 70–80.Search in Google Scholar
22. Yusof, M. S. M.; Soh, S. K. C.; Ngah, N.; Yamin, B. M. 1-Benzoyl-3-(6-methylpyridin-2-yl)thiourea. Acta Crystallogr. 2006, E62, o1446–o1448; https://doi.org/10.1107/s1600536806009366.Search in Google Scholar
23. Dillen, J.; Woldu, K. R. N,N-(Heptane-2,6-diyl)-N′-(3,4,5-methoxybenzoyl)thiourea. Acta. Crystallogr. 2006, E62, o5225–o5227; https://doi.org/10.1107/s1600536806043054..Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (1Z, 2Z)-3-phenyl-2-propenal 2-(4-bromo-2-fluorophenyl)hydrazone, C15H12BrFN2
- Refinement of crystal structure of 2-(2,3-dihydro-3-oxo-1 H -inden-1-ylidene)-1 H -indene-1,3(2 H )-dione C18H10O3
- The crystal structure of 3-(1-fluoro-2-(naphthalen-2-yl)-2-oxoethyl)-2-methoxy-3,4-dihydroisoquinolin-1(2H)-one, C22H18FNO3
- Crystal structure of the dinuclear copper(II) complex bis(μ2-2,2′ -{[1,3-phenylenebis-(methylene)]bis(oxy)}dibenzoaot-κ4O,O′:O′′,O′′′)-bis(dimethylformamide-κ1O)dicopper(II), C50H44Cu2N2O14
- Crystal structure of poly[triaqua-(μ9-biphenyl-3,3′,5,5′-tetracarboxylic-κ8 O,O:O,O′: O,O″:O,O‴)samarium(III)sodium(I)], C16H12NaSmO11
- The crystal structure of 5-benzyl-1-(4-fluorobenzyl)-4-((4-fluorobenzyl)oxy)-1,5-dihydro-2H-pyrrol-2-one, C25H21F2NO2
- The crystal structure of diammonium 2,5-dihydroxyterephthalate, C8H12N2O6
- Crystal structure of (E)-4-(4-(1H-1,2,4-triazol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[b]oxepin-5(2H)-one, C21H19N3O4
- Crystal structure of poly[oktakis(μ2-oxido-κ2O:O)-tetrakis(oxido-κ1O)-bis(μ2-1,1′-[1,4-phenylenebis(methylene)]di(1H-imidazole-κ2N:N′))-tetravanadium(V)-dizinc(II)] monohydrate, C28H30Zn2N8O13V4
- Crystal structure of acotiamide hydrochloride dimethylacetamide solvate (1/1), C25H40ClN5O6S
- Crystal structure of catena-poly[monoaqua (u2-(3-(3,5-dicarboxyphenyl)pyridine-2,6-dicarboxylic-k4O:O′:O″:N)zinc(II))] monohydrate, C15H11NO10Zn
- Crystal structure of dichlorido{2,6-bis(3,5-diisopropyl-N-pyrazolyl)pyridine}zinc(II), C23H33Cl2N5Zn
- Crystal structure of nitrato-κ2O,O′-[hydridotris(3,5-diethylpyrazol-1-yl)borato-κ3N,N′,N″]copper(II), C21H34BCuN7O3
- Crystal structure of 2,7-bis(3,5-diethyl-1H-pyrazol-4-yl)-benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetrone, C28H26N6O4
- Crystal structure of 2-(4-chlorophenyl)benzothiazole, C13H8ClNS
- Synthesis and crystal structure (3R,4′S)-4′-(3,5-dibromophenyl)-1′-methyl-2H-dispiro [benzofuran-3,3′-pyrrolidine-2′,2″-indene]-1″,2,3″-trione, C26H17Br2NO4
- Crystal structure of bis(((3a,7a-dihydro-1H-benzo[d][1,2,3]triazol-1-yl)methyl) triphenylphosphonium) tetrachloridomanganate(II), C50H42Cl4MnN6P2
- The crystal structure of 4,9-bis(4-chlorophenyl)-1,6-bis(2-cyanobenzyl)-2,4a,5,6,7,7a-hexahydro-1H-2,7,5-(epiprop[2]ene[1,1,3]triyl)pyrrolo[3,4-b]pyridine-3,10-dicarbonitrile, C40H26Cl2N6
- The crystal structure of poly((μ2-3-(3-nitro-4-carboxylphenyl)benzoate-κ3O, O′:O″)-μ2-1,4-bis(1-imidazolyl)benzene-κ2N:N′-cadmium(II)), C26H17N5O6Cd
- The crystal structure of 6-hydroxy-5H-pyrrolo[3,4-b]pyridine-5,7(6H)-dione monohydrate, C7H6N2O4
- Crystal structure of 4-((cyclohexylsulfonyl)methyl)-1,2,3,4-tetrahydrobenzo [4,5]imidazo[1,2-a]pyridine, C18H24N2O2S
- Crystal structure of 4,7-diphenyl-1,10-phenanthroline-κ2N,N′)-bis(2,4-di(fluorine)-1-phenylpyridine-κ2C,N)-iridium(III) hexafluorophosphate–dichloromethane (1/1), C47H30Cl2F10IrN4P
- Crystal structure of (4-(1-phenyl-1H-benzo[d]imidazol-2-yl)phenyl)boronic acid, C19H15BN2O2
- The crystal structure of (E)-(2-((pyridin-2-ylmethylene)amino)phenyl)arsonic acid, C12H11AsN2O3
- The crystal structure of N(benzyl(phenyl)carbomothioyl)benzamide, C21H18N2OS
- The crystal structure of bis(2-picolinium) hexachlorostannate dichloromethane monosolvate, C13H18Cl8N2Sn
- Crystal structure of poly[tetraaqua-bis(μ4-3–1-(carboxylatomethyl)-1H-1,2,4-triazole-3-carboxylato)-κ4O:O′,O″,N)zinc(II)], C5H7N3O6Zn
- The crystal structure of the co-crystal isonicotinamide – 2-(nitrophenyl)methanol (1/1), C6H6N2O·C7H7NO3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-fluorobenzoate hydrate, C23H25F2N3O6
- Crystal structure of [diaqua-{1H-benzo[d]imidazol-3-ium-5,6-dicarboxylato-κ2O,O′}magnesium(II)] C18H14MgN4O10
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl) (2-iodo-5-methoxyphenyl)methanone, C20H20INO5
- The crystal structure of 3,7,11-trimethylbenzo[5,6][1,4]thiazino[2,3,4-kl]phenothiazine 5,5,9,9-tetraoxide, C21H17NO4S2
- Crystal structure of tris(piperazine-1,4-diium)bis(2-hydroxy-1,2,3-propane-tricarboxylate) pentahydrate, C24H56N6O19
- Crystal structure of 2-chloro-5-((5-isopropyl-2-methylphenoxy)methyl)pyridine, C16H18ClNO
- Crystal structure of (E)-4-(4-(1H-pyrrol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[(b)]oxepin-5(2H)-one, C23H21NO4
- Crystal structure of (E)-N′-(3,4-dichlorobenzylidene)-3,4,5-trimethoxybenzohydrazide, C17H16Cl2N2O4
- The crystal structure of 2-(2-hydroxyphenyl)-3-(pyridin-2-yl)-2,3- dihydroquinazolin-4(1H)-one, C19H15N3O2
- Crystal structure of 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-8-methylchroman-4-one, C17H16O5
- Crystal structure of bis[(3,4-dimethoxybenzyl)triphenylphosphonium]di-μ2-bromido-dibromidodicopper(I)
- Crystal structure of bis [(1,3-dioxolan-2-ylmethyl)triphenylphosphonium] tetrabromidodicopper(I), C22H22Br2CuO2P
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid], C12H8N2O5
- The crystal structure of one-dimensional cooridnation polymer bis(thiocyanato)-bis((1E,2E)-1,2-bis(1-(pyridin-3-yl)ethylidene)-hydrazine κ2N:N)iron(II), (C30H28N10S2Fe)n
- Crystal structure of ((4-acetamidophenyl)sulfonyl)-l-alanine, C11H14N2O5S
- Crystal structure of [(1-naphthalen-1-yl-methyl)triphenylphosphonium] dichloridocopper(I), [C29H24P]+[CuCl2]−
- RbTm3S5: the first rubidium lanthanoid(III) sulfide with CsEr3Se5-type crystal structure
- Crystal structure of 2,2′-((ethane-1,2-diylbis(methylammoniumdiyl))bis(methylene))bis(pyridin-1-ium) diiodido-tris(μ2-iodido-κ2I:I)dicopper(II) chloride dihydrate, C16H30Cu2I6N4O2
- The crystal structure of 4-(trifluoromethyl)pyridine-2-carboxylic acid, C7H4F3NO2
- The crystal structure of (E)-2-ethoxy-1-methoxy-4-(2-(methylsulfonyl)vinyl)benzene, C12H16O4S
- Crystal structure of potassium 1H,1H,2H,2H-perfluorooctanesulfonate, C8H4O3F13SK
- Crystal structure of 4-(4-(quinolin-8-yloxy)-1,2,5-thiadiazol-3-yl)morpholine, C15H14O2N4S
- The crystal structure of 1,4-bis(bromomethyl)-2,5-dimethylbenzene, C10H12Br2
- The crystal structure of imidazo[4,5-e][1,3]diazepine-4,6,8-triamine methanol solvate, C7H11N7O
- The crystal structure of chlorido-bis(1,10-phenantroline-κ2N,N′)-(2-formylphenoxyacetato-κ2O,O) lead(II), C33H23N4O4ClPb
- Crystal structure of pyridinium tetrakis(1,1,1-trifluoro-2,4-pentadionato-κ2O,O′)yttrium(III) C20F12H16YO8C5H6N
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (1Z, 2Z)-3-phenyl-2-propenal 2-(4-bromo-2-fluorophenyl)hydrazone, C15H12BrFN2
- Refinement of crystal structure of 2-(2,3-dihydro-3-oxo-1 H -inden-1-ylidene)-1 H -indene-1,3(2 H )-dione C18H10O3
- The crystal structure of 3-(1-fluoro-2-(naphthalen-2-yl)-2-oxoethyl)-2-methoxy-3,4-dihydroisoquinolin-1(2H)-one, C22H18FNO3
- Crystal structure of the dinuclear copper(II) complex bis(μ2-2,2′ -{[1,3-phenylenebis-(methylene)]bis(oxy)}dibenzoaot-κ4O,O′:O′′,O′′′)-bis(dimethylformamide-κ1O)dicopper(II), C50H44Cu2N2O14
- Crystal structure of poly[triaqua-(μ9-biphenyl-3,3′,5,5′-tetracarboxylic-κ8 O,O:O,O′: O,O″:O,O‴)samarium(III)sodium(I)], C16H12NaSmO11
- The crystal structure of 5-benzyl-1-(4-fluorobenzyl)-4-((4-fluorobenzyl)oxy)-1,5-dihydro-2H-pyrrol-2-one, C25H21F2NO2
- The crystal structure of diammonium 2,5-dihydroxyterephthalate, C8H12N2O6
- Crystal structure of (E)-4-(4-(1H-1,2,4-triazol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[b]oxepin-5(2H)-one, C21H19N3O4
- Crystal structure of poly[oktakis(μ2-oxido-κ2O:O)-tetrakis(oxido-κ1O)-bis(μ2-1,1′-[1,4-phenylenebis(methylene)]di(1H-imidazole-κ2N:N′))-tetravanadium(V)-dizinc(II)] monohydrate, C28H30Zn2N8O13V4
- Crystal structure of acotiamide hydrochloride dimethylacetamide solvate (1/1), C25H40ClN5O6S
- Crystal structure of catena-poly[monoaqua (u2-(3-(3,5-dicarboxyphenyl)pyridine-2,6-dicarboxylic-k4O:O′:O″:N)zinc(II))] monohydrate, C15H11NO10Zn
- Crystal structure of dichlorido{2,6-bis(3,5-diisopropyl-N-pyrazolyl)pyridine}zinc(II), C23H33Cl2N5Zn
- Crystal structure of nitrato-κ2O,O′-[hydridotris(3,5-diethylpyrazol-1-yl)borato-κ3N,N′,N″]copper(II), C21H34BCuN7O3
- Crystal structure of 2,7-bis(3,5-diethyl-1H-pyrazol-4-yl)-benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetrone, C28H26N6O4
- Crystal structure of 2-(4-chlorophenyl)benzothiazole, C13H8ClNS
- Synthesis and crystal structure (3R,4′S)-4′-(3,5-dibromophenyl)-1′-methyl-2H-dispiro [benzofuran-3,3′-pyrrolidine-2′,2″-indene]-1″,2,3″-trione, C26H17Br2NO4
- Crystal structure of bis(((3a,7a-dihydro-1H-benzo[d][1,2,3]triazol-1-yl)methyl) triphenylphosphonium) tetrachloridomanganate(II), C50H42Cl4MnN6P2
- The crystal structure of 4,9-bis(4-chlorophenyl)-1,6-bis(2-cyanobenzyl)-2,4a,5,6,7,7a-hexahydro-1H-2,7,5-(epiprop[2]ene[1,1,3]triyl)pyrrolo[3,4-b]pyridine-3,10-dicarbonitrile, C40H26Cl2N6
- The crystal structure of poly((μ2-3-(3-nitro-4-carboxylphenyl)benzoate-κ3O, O′:O″)-μ2-1,4-bis(1-imidazolyl)benzene-κ2N:N′-cadmium(II)), C26H17N5O6Cd
- The crystal structure of 6-hydroxy-5H-pyrrolo[3,4-b]pyridine-5,7(6H)-dione monohydrate, C7H6N2O4
- Crystal structure of 4-((cyclohexylsulfonyl)methyl)-1,2,3,4-tetrahydrobenzo [4,5]imidazo[1,2-a]pyridine, C18H24N2O2S
- Crystal structure of 4,7-diphenyl-1,10-phenanthroline-κ2N,N′)-bis(2,4-di(fluorine)-1-phenylpyridine-κ2C,N)-iridium(III) hexafluorophosphate–dichloromethane (1/1), C47H30Cl2F10IrN4P
- Crystal structure of (4-(1-phenyl-1H-benzo[d]imidazol-2-yl)phenyl)boronic acid, C19H15BN2O2
- The crystal structure of (E)-(2-((pyridin-2-ylmethylene)amino)phenyl)arsonic acid, C12H11AsN2O3
- The crystal structure of N(benzyl(phenyl)carbomothioyl)benzamide, C21H18N2OS
- The crystal structure of bis(2-picolinium) hexachlorostannate dichloromethane monosolvate, C13H18Cl8N2Sn
- Crystal structure of poly[tetraaqua-bis(μ4-3–1-(carboxylatomethyl)-1H-1,2,4-triazole-3-carboxylato)-κ4O:O′,O″,N)zinc(II)], C5H7N3O6Zn
- The crystal structure of the co-crystal isonicotinamide – 2-(nitrophenyl)methanol (1/1), C6H6N2O·C7H7NO3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-fluorobenzoate hydrate, C23H25F2N3O6
- Crystal structure of [diaqua-{1H-benzo[d]imidazol-3-ium-5,6-dicarboxylato-κ2O,O′}magnesium(II)] C18H14MgN4O10
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl) (2-iodo-5-methoxyphenyl)methanone, C20H20INO5
- The crystal structure of 3,7,11-trimethylbenzo[5,6][1,4]thiazino[2,3,4-kl]phenothiazine 5,5,9,9-tetraoxide, C21H17NO4S2
- Crystal structure of tris(piperazine-1,4-diium)bis(2-hydroxy-1,2,3-propane-tricarboxylate) pentahydrate, C24H56N6O19
- Crystal structure of 2-chloro-5-((5-isopropyl-2-methylphenoxy)methyl)pyridine, C16H18ClNO
- Crystal structure of (E)-4-(4-(1H-pyrrol-1-yl)benzylidene)-6,8-dimethoxy-3,4-dihydrobenzo[(b)]oxepin-5(2H)-one, C23H21NO4
- Crystal structure of (E)-N′-(3,4-dichlorobenzylidene)-3,4,5-trimethoxybenzohydrazide, C17H16Cl2N2O4
- The crystal structure of 2-(2-hydroxyphenyl)-3-(pyridin-2-yl)-2,3- dihydroquinazolin-4(1H)-one, C19H15N3O2
- Crystal structure of 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-8-methylchroman-4-one, C17H16O5
- Crystal structure of bis[(3,4-dimethoxybenzyl)triphenylphosphonium]di-μ2-bromido-dibromidodicopper(I)
- Crystal structure of bis [(1,3-dioxolan-2-ylmethyl)triphenylphosphonium] tetrabromidodicopper(I), C22H22Br2CuO2P
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid], C12H8N2O5
- The crystal structure of one-dimensional cooridnation polymer bis(thiocyanato)-bis((1E,2E)-1,2-bis(1-(pyridin-3-yl)ethylidene)-hydrazine κ2N:N)iron(II), (C30H28N10S2Fe)n
- Crystal structure of ((4-acetamidophenyl)sulfonyl)-l-alanine, C11H14N2O5S
- Crystal structure of [(1-naphthalen-1-yl-methyl)triphenylphosphonium] dichloridocopper(I), [C29H24P]+[CuCl2]−
- RbTm3S5: the first rubidium lanthanoid(III) sulfide with CsEr3Se5-type crystal structure
- Crystal structure of 2,2′-((ethane-1,2-diylbis(methylammoniumdiyl))bis(methylene))bis(pyridin-1-ium) diiodido-tris(μ2-iodido-κ2I:I)dicopper(II) chloride dihydrate, C16H30Cu2I6N4O2
- The crystal structure of 4-(trifluoromethyl)pyridine-2-carboxylic acid, C7H4F3NO2
- The crystal structure of (E)-2-ethoxy-1-methoxy-4-(2-(methylsulfonyl)vinyl)benzene, C12H16O4S
- Crystal structure of potassium 1H,1H,2H,2H-perfluorooctanesulfonate, C8H4O3F13SK
- Crystal structure of 4-(4-(quinolin-8-yloxy)-1,2,5-thiadiazol-3-yl)morpholine, C15H14O2N4S
- The crystal structure of 1,4-bis(bromomethyl)-2,5-dimethylbenzene, C10H12Br2
- The crystal structure of imidazo[4,5-e][1,3]diazepine-4,6,8-triamine methanol solvate, C7H11N7O
- The crystal structure of chlorido-bis(1,10-phenantroline-κ2N,N′)-(2-formylphenoxyacetato-κ2O,O) lead(II), C33H23N4O4ClPb
- Crystal structure of pyridinium tetrakis(1,1,1-trifluoro-2,4-pentadionato-κ2O,O′)yttrium(III) C20F12H16YO8C5H6N


