Abstract
The periodic four-layered model of the pure Cu(111) surface has been considered, and the effect of doping with palladium on CH4 dissociation has been investigated. The most stable adsorption geometries of CH x species (x = 1–4) and H atom on the PdCu(111) and pure Cu(111) surfaces have been obtained. Their computed adsorption energy results on the pure Cu(111) surface have been compared with the previously reported studies. Then, transition state geometries of CH4 dehydrogenation steps on both surfaces were calculated by the climbing image nudged elastic band method. Finally, the relative energy diagram for CH4 complete dehydrogenation has been represented. The results show that the PdCu(111) surface is more favorable than the Cu(111) surface in terms of the activation energies. The addition of Pd atoms to the Cu(111) surface significantly improves the catalytic activity. This knowledge can enable an efficient catalyst design at a lower cost using different strategies.
1 Introduction
One of the most basic examples of the transition from hydrocarbon-based compounds to renewable energy sources is natural gas. As it is known, natural gas is a fuel mainly containing methane, and the chemical formula of methane is CH4. Its heat of combustion is −890 kJ/mol. Moreover, it has a high energy content compared to other hydrocarbon fuels. Due to the advantageous properties of the CH4 molecule, it has been extensively studied for new applications and hydrogen production techniques [1,2]. The CH4 dissociation reaction is usually based on sequential hydrogen decomposition (or dissociation). The CH x species occurring at each stage of the reaction deactivate the catalysts [3,4,5]. Therefore, catalysts modified with transition metals are often used to improve catalysts’ activity and stability in the reaction. These catalysts are usually bimetallic or alloy groups. As the nickel and nickel-based catalysts are of low cost and have high catalytic effect, they have been often used for the dissociation reaction of CH4, which include Ni [6], Ni/γ-Al2O3 [7], Sn/Ni [8], Ni–M (M = Cu, Ru, Rh, Pd, Ag, Pt, and Au) [9], Ni/Fe-catalyzed carbon [10], pure and gold-alloyed Ni(111) [11], Ni(100) [12], NiPd [13], NiCu [14], NiM(111) (M = Co, Rh, Ir) [15], NiCo [16], MgO(001)-supported Ni4 [17], Ni(111) and Ru(0001) [18], Pt–Ni [19], Ni, Pd, Pt, and Cu [20]. The aforementioned results have shown that while bimetallic catalyst groups such as NiPt and NiPd have high catalytic activity on CH4 dissociation reaction, NiAu, NiSn, and Ni can be classified as catalysts or catalyst groups with low activity.
Zhao et al. [21] have revealed that the Pd-doped Ni catalyst has high stability and that the Ni(211) and Pd/Ni(211) surfaces are more favorable for CH4 dissociation than the Ni(100) and Pd/Ni(100) surfaces in terms of reaction energetics. Khettal et al. [22] have reported that the W–Cu(100) surface is more active thermodynamically and kinetically than other Cu-based catalysts for CH4 dissociation. Recently, Pd- and Cu-based catalysts have been extensively used for CH4 dissociation reaction. Rahmani et al. [23] have found that the activation barrier of the oxygen-covered surface of Cu(111) on CH4 dissociation reaction is lower than that of oxygen-covered surfaces of Ni(111). Meng et al. [24] have examined partial methane oxidation on two single atom alloy catalysts (A-model Pd/Cu(111) and D-model Pd/Cu(111)). They found that CH4 dissociation (CH4 → CH3) is the rate-determining step (RDS) of reaction on both catalysts and the activation energy values of RDS on these surfaces are very close to each other.
Solymosi et al. [25] have investigated the decomposition of methane on supported Pd catalysts and found that a significant part of hydrogen is dissolved through Pd crystals. Hou et al. [26] have studied the effects of Pd/Pt bimetal supported by the γ-Al2O3 surface on methane activation. They discovered that CH4 adsorption depends on the ratio of Pd/Pt. Moreover, the Pd-3Pt/γ-Al2O3 surface has been found to have the best catalytic activity among the modeling surfaces with 3Pd-Pt/γ-Al2O3, 2Pd-Pt/γ-Al2O3, and Pd-3Pt/γ-Al2O3. Li et al. [27] have proposed two possible reaction mechanisms on Ni(111) and Cu(111) surfaces. These reaction mechanisms are H-abstraction reaction (CH x + H → CH x−1 + H2) and direct dehydrogenation reaction (CH x + H → CH x−1 + 2H). They have shown that the direct dehydrogenation reaction on the Cu(111) surface is more favored than that on the Ni(111) surface. In the present research, the effect of Pd atoms doped onto different layers of the Cu(111) surface on CH4 dehydrogenation reaction has been studied. First, the adsorption mechanisms of CH x species and H atom on the PdCu(111) and pure Cu(111) surfaces were considered. Then, the activation and reaction energies for sequential dehydrogenation steps on the PdCu(111) and pure Cu(111) surfaces were calculated by the nudged elastic band method.
2 Methods
2.1 Computational method
All calculations were carried out via Quantum espresso code based on the density functional theory (DFT). For ab initio electronic structure calculations, the projector augmented wave potentials were used. The Perdew–Burke-Ernzerhof exchange–correlation functional [31] was used for the computation. The spin polarization term is used for accurate identification of total magnetization values. The Brillouin zone integrations were performed on a grid of sampling of 4 × 4 × 1 k-points of the Monkhorst–Pack. The charge density and the wave functions were expanded with kinetic cutoffs of 60 and 600 Ry, respectively, for the Cu(111) and PdCu(111) surfaces. Relaxations in material optimizations were introduced using the BFGS quasi-Newton algorithm. In all calculations, van der Waals (vdW) interaction was defined by the DFT-D2 force-field approach. The force on each atom and energy convergence during geometric optimization were less than 1 × 10−5 eV/Å and 1 × 10−6 eV. The transition state geometries and activation energies between reactants and products on the reaction pathway were obtained using the climbing image nudged elastic band (CINEB) method.
2.2 Surface model
Examining the catalyst’s active role in the CH4 dissociation reaction is necessary to understand the individual binding nature of atomic or molecular species adsorbed by surfaces. The catalyst effect on a chemical reaction has often been investigated on M(111) surfaces, where M denotes transition metals. In this context, the catalytic effect of the Cu(111) surface on decomposition reactions has been studied in the literature [24,27,28]. Besides, a periodically repeating four-layered (2 × 2) supercell can be constructed to analyze the adsorption mechanism of atomic and molecular structures (adsorbed species) on the PdCu(111) surface. Herein, the top two layers were relaxed, and the bottom layers were fixed at their ideal bulk positions. A 14 Å vacuum in the slab model was applied to prevent undesirable interactions between periodically repeating supercells. The Cu atoms in the second layer of the periodic slab model were substituted by Pd atoms.
Adsorption energy, E ads, between the surface and adsorbed species was calculated as follows:
where E adsorbates/slab is the total energy of the surface and the adsorbed species. E adsorbates is the total energy of adsorbates, which was isolated in the 12 × 12 × 12 cubic box. E slab is the total energy of the metal slab.
3 Results and discussion
3.1 Adsorption mechanism of CH x (x = 0–4) species and H atom
In this section, the most stable geometries of CH x (x = 0–4) species and H atom on PdCu(111) are represented. The possible adsorption sites on the PdCu(111) surface are shown in Figure 1. The Cu atoms have been labeled as Cu(n) (n = 1 → 5).

The possible adsorption sites of the PdCu(111) surface. The Cu and Pd atoms are shown in orange and dark blue colors, respectively.
Figure 1 indicates that the PdCu(111) surface has four high symmetric adsorption sites. The fcc and hcp sites are the threefold hollow sites of three Cu atoms. The bridge site is the twofold hollow site of two Cu atoms. The top site is above the Cu atom.
The preferred sites of CH x (x = 0–4) species and H atom were determined on the pure Cu(111) surface. The results have been compared to the previously reported studies [27,28]. The adsorption geometries of CH x (x = 0–4) and H on the PdCu(111) surface are given in Figure 2.

The stable adsorption geometries of CH x (x = 0–4) and H on the PdCu(111) surface. (a) CH4, (b) CH3, (c) CH2, (d) CH, (e) C, (f) H.
3.2 Adsorption mechanism of CH4 on the PdCu(111) and pure Cu(111) surfaces
The adsorption of the CH4 molecule on transition metal surfaces is usually a physisorption process.
CH4 is adsorbed onto the PdCu(111) surface by vdW interaction. The bond lengths and adsorption energies of CH x (x = 1–4), C, and H on the PdCu(111) and Cu(111) surfaces are given in Table 1. The most stable configuration of CH4 is shown in Figure 2a. In this configuration, three H atoms are parallel to the surface, and the other H atom is perpendicular to the C atom.
Adsorption energies (E ads, eV) and bond lengths for the most stable adsorption structures of CH x (x = 1–4), C, and H on the PdCu(111) and Cu(111) surfaces
| Species | E ads (eV) | Bond lengths (Å) | |
|---|---|---|---|
| Cu(111) | PdCu(111) | Adsorbed | |
| CH4 | −0.01 | 0.02 | C–H1 = 1.10 |
| C–H2 = 1.10 | |||
| C–H3 = 1.10 | |||
| C–H4 = 1.10 | |||
| CH3 | −1.83 | −1.84 | C–H1 = 1.10 |
| C–H2 = 1.10 | |||
| C–H3 = 1.10 | |||
| CH2 | −3.86 | −3.62 | C–H1 = 1.10 |
| C–H2 = 1.11 | |||
| CH | −5.38 | −5.45 | C–H = 1.09 |
| C | −5.34 | −5.10 | C–Cu(1) = 1.84 |
| C–Cu(2) = 1.84 | |||
| C–Cu(3) = 1.84 | |||
| H | −2.77 | −2.66 | H–Cu(1) = 1.72 |
| H–Cu(2) = 1.72 | |||
| H–Cu(3) = 1.71 | |||
The adsorption energy of the CH4 molecule was found to be −0.01 eV. CH4 on the pure Cu(111) surface has low adsorption energy. The adsorption energy of CH4 was calculated as −0.02 eV on the PdCu(111) surface, which is very close to the data in previous studies [27,28].
3.3 Adsorption mechanism of CH3 on the PdCu(111) and pure Cu(111) surfaces
The adsorption of CH3 onto the PdCu(111) surface has been considered on the fcc and hcp sites. Because CH3 does not exhibit a stable behavior on the top and bridge sites, CH3 interacts with the nearest neighbor Cu atoms on the fcc site. The adsorption energies of CH3 on the fcc and hcp sites are −1.84 and −1.81 eV, respectively. For CH3, the most stable geometry is at the fcc site (shown in Figure 2b). The bond lengths of C–H(1), C–H(2), and C–H(3) are 1.107, 1.106, and 1.107 Å, respectively.
The adsorption energies of CH3 on the pure Cu(111) surface are −1.83 eV on the fcc site and −1.82 eV on the hcp site. These outputs are in good agreement with the literature results [27,28]. The bond lengths of C–H(1), C–H(2), and C–H(3) are 1.107, 1.106, and 1.107 Å, respectively. Compared to the PdCu(111) surface, there is no significant difference in the bond lengths of CH3 on the pure Cu(111) surface.
3.4 Adsorption mechanism of CH2 on the PdCu(111) and pure Cu(111) surfaces
The adsorption of CH2 on the PdCu(111) surface has been investigated on all possible sites. Two stable geometries have been obtained. The calculated adsorption energies of the CH2 are −3.62 eV on the fcc site and −3.58 eV on the hcp site. The most stable geometry of CH2 is shown in Figure 2c. In this stable geometry, the H(1) atom is directly bonded to the Cu(3) surface atom, and the H(2) atom is relatively vertical on the bridge site. The bond lengths of C–H(1) and C–H(2) are 1.102 and 1.118 Å, respectively.
The adsorption energies of CH2 on the pure Cu(111) surface are −3.86 eV on the fcc site and −3.78 eV on the hcp site. This result obtained on the fcc site is consistent with the study reported by Li et al. [27]. The bond lengths of C–H(1) and C–H(2) are 1.102 and 1.121 Å, respectively. The bond length of C–H(2) on the pure Cu(111) surface is more elongated by 0.003 Å than the PdCu(111) surface, and the bond length of C–H(1) is the same on both surfaces. Moreover, the CH2 binds stronger on the pure Cu(111) surface compared to the PdCu(111) surface.
3.5 Adsorption mechanism of CH on the PdCu(111) and pure Cu(111) surfaces
When CH was being optimized on top and bridge sites of the PdCu(111) surface, it tended to move to the fcc or hcp sites, on which the adsorption energies of CH were calculated to be −5.45 and −5.40 eV, respectively. Therefore, the most stable geometry of CH is at the fcc site. The preferred geometry of CH is presented in Figure 2d. The bond length of C–H is 1.096 Å. CH prefers the fcc site on pure Cu(111), and the adsorption energy of CH is −5.38 eV, in agreement with previous studies [27,28]. The bond length of C–H on pure Cu(111) is 1.097 Å.
3.6 Adsorption mechanism of C and H on the PdCu(111) and pure Cu(111) surfaces
C and H atoms were optimized in all active sites on the PdCu(111) surface. The adsorption energy of H atom is close to each other on the fcc, hcp, bridge, and top sites. The most stable geometry of H is the fcc site (shown in Figure 2f). The adsorption energy of H on the fcc site is −2.66 eV. The C atom prefers to occupy the threefold hollow site of three Cu atoms denoted as the fcc site, and the adsorption energy of C at the fcc site is −5.10 eV. The preferred geometry of the C atom is plotted in Figure 2e.
The adsorption geometries of C and H atoms on the pure Cu(111) surface are similar to those on the PdCu(111) surface. Their most stable adsorption sites are at the fcc sites. The adsorption energies of C and H atoms are −5.34 and −2.70 eV, respectively. These adsorption energies are in good agreement with the literature results [27,28].
4 Transition states for the dissociation reaction of CH4
In this section, sequential dehydrogenation of the CH4 reaction is studied on the PdCu(111) and pure Cu(111) surfaces. According to the reaction mechanism considered, the reaction path has occurred through the same route on both surfaces. The most stable geometries of the initial and final states were selected to determine transition states (TS). Then, CH x dissociated to CH x−1 and H. The total energy of the system with CH x−1 (x = 1–4) species and H atom at an infinite distance was considered as the initial state of the next dehydrogenation step [11,30].
For the reaction of CH x → CH x−1 + H on surfaces, the activation energies (E a) and reaction energy (E r) were calculated with the following formulas:
where E IS and E FS are the total energies of surface together with the adsorbed CH x and the dissociated CH x−1 + H species, respectively; E TS is the total energy of transition states.
According to the calculations, the activation energies of all possible reaction steps for CH4 dehydrogenation are summarized in Table 2. Moreover, the pure Cu(111) surface results are in good agreement with the previously reported data [27,28].
Activation energies (eV) of sequential dehydrogenation of CH4 on the pure Cu(111) and PdCu(111) surfaces
| Surface | CH4 → CH3 + H | CH3 → CH2 + H | CH2 → CH + H | CH → C + H |
|---|---|---|---|---|
| Cu(111) (vdW) in this study | 1.32 | 1.24 | 0.87 | 2.03 |
| Cu(111) (vdW) | 1.31a | 1.26a | 0.93a | 1.97a |
| Cu(111) (no vdW) | 1.54b | 1.34b | 0.96b | 1.98b |
| 1.57c | 1.36c | 0.94c | 1.84c | |
| PdCu(111) (vdW) in this study | 0.84 | 1.11 | 0.80 | 1.98 |
4.1 Transition states for CH4 → CH3 + H reaction
CH4 sequential dehydrogenation reaction begins with a breakaway of a single H from the CH4 molecule. This transition state is called TS1, and the related initial state, transition state, and final state geometries are shown in Figure 3, respectively. Moreover, H atoms attached to the CH4 molecule have been labeled as H(n) (n = 1 → 4).
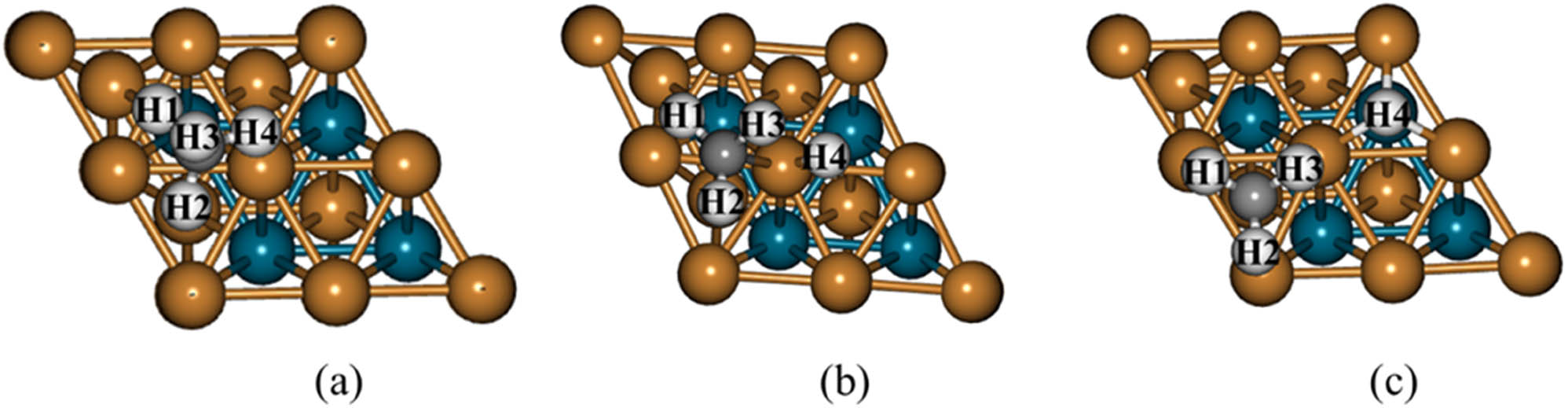
The optimized initial and final state geometries and the transition state geometry of CH4 → CH3 + H dehydrogenation step on PdCu(111). (a) CH4, (b) TS1, (c) CH3 + H.
CH4 on the NiPd(111) surface has been adsorbed to the surface by vdW interaction. This geometry was selected as the initial state of the first dehydrogenation step (Figure 3a). CH3 has been placed at the fcc site for the final state, and H has been located at the hcp site based on the information that it has nearly the same stability at every site on the surface. The co-adsorbed geometry of CH3 and H is represented in Figure 3c. In the transition state (TS1), the dissociated H(4) atom moved to the top site. As shown in Figure 3b, the distance between H(4) and C atom is 1.930 Å. In the final state, this value increases to 3.10 Å. The activation and reaction energies of the first dehydrogenation step (CH4 → CH3 + H) were calculated to be 0.84 and 0.34 eV, respectively. This reaction is endothermic by 0.34 eV.
Similar to the above first dehydrogenation step of CH4 dissociation, the reaction path was created for the pure Cu(111) surface. The calculated activation and reaction energies of the first dehydrogenation step are 1.32 and 0.72 eV, respectively, which are nearly the same as that on the pure Cu(111) surface [27].
4.2 Transition states for CH3 → CH2 + H reaction
In the second dehydrogenation step, removal of a single H atom from the CH3 molecule has been investigated. The initial, transition, and final states on the PdCu(111) surface are plotted in Figure 4. The fcc site was selected as the initial state for CH3. For the final state, the co-adsorbed species CH2 and H have been placed at the fcc and hcp sites, respectively. In the transition state (TS2), H(1) and H(2) atoms moved toward the bridge site and top site, respectively. The dissociated H(3) atom migrates toward the top site. Moreover, the distance between H(3) and C atoms is 2.12 Å. In the final state, this value increases to 3.05 Å (Figure 4c).

The optimized initial and final state geometries and the transition state geometry of CH3 → CH2 + H dehydrogenation step on PdCu(111). (a) CH3, (b) TS2, (c) CH2 + H.
The activation and reaction energies were calculated to be 1.11 and 0.52 eV, respectively. This reaction is endothermic. On pure Cu(111), the activation and reaction energy values are 1.24 and 0.54 eV, respectively. Moreover, the obtained activation energy value on the pure Cu(111) surface is in agreement with the result (1.26 eV) of Li et al. [28].
4.3 Transition states for CH2 → CH + H reaction
In the third step of the dehydrogenation reaction, CH2 adsorbed on the PdCu(111) surface starts breaking down into CH and H species. For CH2, the fcc site on the surface was selected as the initial state (Figure 5a). The co-adsorbed geometry of CH and H is shown in Figure 5c, and they have been placed at the fcc and hcp sites, respectively, as the final state.

The optimized initial and final state geometries and the transition state geometry of CH2 → CH + H dehydrogenation step on PdCu(111). (a) CH2, (b) TS3, (c) CH + H.
In the transition state (TS3), the dissociated H(2) atom migrated toward the top site (Figure 5b). The calculated distance C–H(2) is 1.96 Å. In the final state, the H(2) atom exceeding over the activation energy is at a distance of 2.97 Å from the C atom (shown in Figure 7c). The reaction energy was calculated to be 0.38 eV. This reaction is endothermic. The activation energies of the third dehydrogenation step on the PdCu(111) and pure Cu(111) surfaces were calculated to be 0.80 and 0.87 eV, respectively, which is approximate to that (0.93 eV) on pure Cu(111) [28]. Moreover, the obtained reaction energy value on the pure Cu(111) surface is 0.56 eV and endothermic.
4.4 Transition states for CH → C + H reaction
In the final step of the dehydrogenation reaction, the CH molecule’s most stable adsorption site is presented in Figure 6a. In this geometry, the C atom is bound to the nearest surface atoms, and the H atom is in the perpendicular position to the C atom. CH adsorbed to the fcc site on PdCu(111) was selected as the initial state. In the final state geometry, C and H atoms have been placed at the fcc and hcp sites (Figure 6c).

The optimized initial and final state geometries and the transition state geometry of CH → C + H dehydrogenation step on PdCu(111). (a) CH, (b) TS4, (c) C + H.
In the transition state (TS4), the H atom migrated toward the top site of the PdCu(111) surface (Figure 6b). The calculated distance C–H is 1.97 Å. The reaction energy is 0.54 eV, and this reaction is endothermic. The activation energies of the final dehydrogenation step on the PdCu(111) and pure Cu(111) surfaces were calculated to be 1.97 and 2.03 eV, respectively, which is approximate to that (1.98 eV) on pure Cu(111) [28]. Moreover, the calculated reaction energy value on the pure Cu(111) surface is 0.61 eV and endothermic. According to the activation energies of both surfaces, the relative energy diagram is given in Figure 7 by Table 3.

Relative energy diagram of CH4 complete dehydrogenation on the pure Cu(111) and PdCu(111) surfaces.
Relative energy diagram results for CH4 dissociation on the Cu(111) and PdCu(111) surfaces
| Reaction steps | Activation energies (E a, eV) | |
|---|---|---|
| Cu(111) | PdCu(111) | |
| CH4 → CH3 + H | 1.32 | 0.84 |
| CH3 + H → CH2 + 2H | 2.72 | 1.48 |
| CH2 + 2H → CH + 3H | 2.86 | 1.71 |
| CH + 3H → C + 4H | 4.10 | 3.18 |
The activation energies of the first dehydrogenation step (CH4 → CH3 + H) are 1.32 and 0.84 eV, respectively, on pure Cu(111) and PdCu(111). The activation energies of the second dehydrogenation step (CH3 + H → CH2 + 2H) are 2.72 and 1.48 eV, respectively, on pure Cu(111) and PdCu(111). The activation energies of the third step (CH2 + 2H → CH + 3H) are 2.86 and 1.71 eV on pure Cu(111) and PdCu(111), respectively. The activation energies of the last step (CH + 3H → C + 4H) are 4.10 and 3.18 eV, respectively, on pure Cu(111) and PdCu(111). As mentioned above, the activation energies of all dissociation steps are much lower on the PdCu(111) surface. We can see that the highest activation energy is CH dissociation. In other words, the RDS of the reaction is the final step for both surfaces.
Based on the CH4 complete dissociation, we can see that the bonding mechanism between Pd and Cu atoms improves the catalytic activity of reaction, so the reaction route should be on the surface where Pd atoms take an active role. Moreover, the adsorption energies of the CH x (x = 1–4) on both surfaces point out that these molecules are more weakly adsorbed onto the PdCu surface than the Cu(111) surface. This trend in adsorption energies can be a reason for the decreases in activation energies.
5 Conclusion
In this study, the sequential dehydrogenation reaction of CH4 has been studied through DFT calculations on the PdCu(111) and pure Cu(111) surfaces. The adsorption energies and the most stable adsorption geometries were calculated on both surfaces. The obtained results show that CH x (x = 0–4) species and H atom on PdCu(111) are more weakly adsorbed than that on the pure Cu(111) surface. Then, all possible states of the reaction mechanism are given in Table 1. The transition states of each dehydrogenation reaction step have been investigated by the CINEB method. The results have revealed that the PdCu(111) surface is more active than the pure Cu(111) surface. Significantly, the first step of the dehydrogenation on PdCu(111) is lower by 0.48 eV than the pure Cu(111) surface. The second and third steps are lower, approximately 0.1 eV than the pure Cu(111) surface. The final step occurs in the same way on both surfaces.
Acknowledgments
All calculations reported in this study were performed at TUBITAK ULAKBIM, High Performance and Grid Computing Center (TRUBA resources).
References
[1] He Y, Zhou W, Chen B. Methane storage in metal–organic frameworks. Chem Soc Rev. 2014;43:5657–78.10.1039/C4CS00032CSearch in Google Scholar
[2] Pucker J, Zwart R, Jungmeier G. Greenhouse gas and energy analysis of substitute natural gas from biomass for space heat. Biomass Bioenergy. 2012;38:95–101.10.1016/j.biombioe.2011.02.040Search in Google Scholar
[3] Bartholomew CH, Agrawal PK, Katzer JR. Sulfur poisoning of metals. Adv Catal. 1982;31:135–242.10.1016/S0360-0564(08)60454-XSearch in Google Scholar
[4] Bartholomew CH. Mechanisms of catalyst deactivation. Appl Catal A. 2001;212:17–60.10.1016/S0926-860X(00)00843-7Search in Google Scholar
[5] Tian B, Liu T, Yang Y, Li K, Wu Z, Wang Y. CH4 dissociation in the early stage of graphene growth on Fe–Cu(100) surface: theoretical insights. Appl Surf Sci. 2018;427:953–60.10.1016/j.apsusc.2017.09.088Search in Google Scholar
[6] Wang SG, Cao DB, Li YW, Wang J, Jiao H. CH4 dissociation on Ni surfaces: density functional theory study. Surf Sci. 2006;600:3226–34.10.1016/j.susc.2006.06.008Search in Google Scholar
[7] Li J, Croiset E, Ricardez-Sandoval L. Effect of metal–support interface during CH4 and H2 dissociation on Ni/γ-Al2O3: a density functional theory study. J Phys Chem C. 2013;117:16907–20.10.1021/jp402421qSearch in Google Scholar
[8] Nikolla E, Schwank J, Linic S. Comparative study of the kinetics of methane steam reforming on supported Ni and Sn/Ni alloy catalysts: the impact of the formation of Ni alloy on chemistry. J Catal. 2009;263:220–7.10.1016/j.jcat.2009.02.006Search in Google Scholar
[9] Fan C, Zhu YA, Xu Y, Zhou Y, Zhou XG, Chen D. Origin of synergistic effect over Ni-based bimetallic surfaces: a density functional theory study. J Chem Phys. 2012;137:014703.10.1063/1.4731811Search in Google Scholar PubMed
[10] Fan C, Zhou XG, Chen D, Cheng HY, Zhu YA. Toward CH4 dissociation and C diffusion during Ni/Fe-catalyzed carbon nanofiber growth: a density functional theory study. Chem Phys. 2011;134:134704.10.1063/1.3575193Search in Google Scholar PubMed
[11] Kratzer P, Hammer B, Norskov JK. A theoretical study of CH4 dissociation on pure and gold‐alloyed Ni(111) surfaces. J Chem Phys. 1996;105:5595–604.10.1063/1.472399Search in Google Scholar
[12] Zhu YA, Dai YC, Chen D, Yuan WK. First-principles calculations of CH4 dissociation on Ni(100) surface along different reaction pathways. J Mol Catal A. 2007;264:299–308.10.1016/j.molcata.2006.09.043Search in Google Scholar
[13] Li K, Zhou Z, Wang Y, Wu Z. A theoretical study of CH4 dissociation on NiPd(111) surface. Surf Sci. 2013;612:63–8.10.1016/j.susc.2013.02.012Search in Google Scholar
[14] Liu H, Zhang R, Yan R, Li J, Wang B, Xie K. Insight into CH4 dissociation on NiCu catalyst: a first-principles study. Appl Surf Sci. 2012;258:8177–84.10.1016/j.apsusc.2012.05.017Search in Google Scholar
[15] Li K, Jiao M, Wang Y, Wu Z. CH4 dissociation on NiM(111) (M = Co, Rh, Ir) surface: a first-principles study. Surf Sci. 2013;617:149–55.10.1016/j.susc.2013.08.004Search in Google Scholar
[16] Liu H, Zhang R, Yan R, Wang B, Xie K. CH4 dissociation on NiCo(111) surface: a first-principles study. Appl Surf Sci. 2011;257:8955–64.10.1016/j.apsusc.2011.05.073Search in Google Scholar
[17] Liu H, Teng B, Fan M, Wang B, Zhang Y, Harris HG. CH4 dissociation on the perfect and defective MgO(001) supported Ni4. Fuel. 2014;123:285–92.10.1016/j.fuel.2014.01.087Search in Google Scholar
[18] Egeberg RC, Ullmann S, Alstrup I, Mullins CB, Chorkendorff I. Dissociation of CH4 on Ni(111) and Ru(0001). Surf Sci. 2002;497:183–93.10.1016/S0039-6028(01)01428-5Search in Google Scholar
[19] Roy S, Hariharan S, Tiwari AK. Pt–Ni subsurface alloy catalysts: an improved performance toward CH4 dissociation. J Phys Chem C. 2018;122:10857–70.10.1021/acs.jpcc.8b01705Search in Google Scholar
[20] Liao MS, Au CT, Ng CF. Methane dissociation on Ni, Pd, Pt and Cu metal(111) surfaces – a theoretical comparative study. Chem Phys Lett. 1997;272:445–52.10.1016/S0009-2614(97)00555-1Search in Google Scholar
[21] Zhao Y, Shenggang LI, Yuhan S. Theoretical study on the dissociative adsorption of CH4 on Pd-doped Ni surfaces. Chin J Catal. 2013;34:911–22.10.1016/S1872-2067(12)60565-8Search in Google Scholar
[22] Khettal H, Haroun MF, Boukelkoul M. Theoretical study of CH4 adsorption and dissociation on W–Cu(100) surface. Comput Theor Chem. 2020;1186:112890.10.1016/j.comptc.2020.112890Search in Google Scholar
[23] Rahmani-Didar B, Balbuena PB. Methane dehydrogenation on Cu and Ni surfaces with low and moderate oxygen coverage. Int J Quantum Chem. 2020;120:26065.10.1002/qua.26065Search in Google Scholar
[24] Meng Y, Ding C, Gao X, Zhang K, Wang J, Li Z. Adsorption of Pd on the Cu(111) surface and its catalysis of methane partial oxidation: a density functional theory study. Appl Surf Sci. 2020;513:145724.10.1016/j.apsusc.2020.145724Search in Google Scholar
[25] Solymosi F, Erdohelyi A, Cserenyi J, Felvegi AJ. Decomposition of CH4 over supported Pd catalysts. Catalysis Today. 1994;147:272–8.10.1006/jcat.1994.1138Search in Google Scholar
[26] Hou M, Zhang X, Fu C, Cen W, Chen J. Effects of a Pd/Pt bimetal supported by a γ-Al2O3 surface on methane activation. Phys Chem Chem Phys. 2020;22:4692–8.10.1039/C9CP05920BSearch in Google Scholar
[27] Li K, He C, Jiao M, Wang Y, Wu Z. A first-principles study on the role of hydrogen in early stage of graphene growth during the CH4 dissociation on Cu(111) and Ni(111) surfaces. Carbon. 2014;74:255–65.10.1016/j.carbon.2014.03.030Search in Google Scholar
[28] Gajewski G, Pao CW. Ab initio calculations of the reaction pathways for methane decomposition over the Cu(111) surface. J Chem Phys. 2011;135:064707.10.1063/1.3624524Search in Google Scholar PubMed
[29] Gajewski G, Pao CW. Ab initio calculations of the reaction pathways for methane decomposition over the Cu(111) surface. J Chem Phys. 2011;135(6):064707.10.1063/1.3624524Search in Google Scholar
[30] Akça A, Genç AE, Kutlu B. BH4 dissociation on various metal(111) surfaces: a DFT study. Appl Surf Sci. 2019;473:681–92.10.1016/j.apsusc.2018.12.134Search in Google Scholar
[31] Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett. 1996;77:3865–8.10.1103/PhysRevLett.77.3865Search in Google Scholar PubMed
© 2020 Aykan Akça, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Model of electric charge distribution in the trap of a close-contact TENG system
- Dynamics of Online Collective Attention as Hawkes Self-exciting Process
- Enhanced Entanglement in Hybrid Cavity Mediated by a Two-way Coupled Quantum Dot
- The nonlinear integro-differential Ito dynamical equation via three modified mathematical methods and its analytical solutions
- Diagnostic model of low visibility events based on C4.5 algorithm
- Electronic temperature characteristics of laser-induced Fe plasma in fruits
- Comparative study of heat transfer enhancement on liquid-vapor separation plate condenser
- Characterization of the effects of a plasma injector driven by AC dielectric barrier discharge on ethylene-air diffusion flame structure
- Impact of double-diffusive convection and motile gyrotactic microorganisms on magnetohydrodynamics bioconvection tangent hyperbolic nanofluid
- Dependence of the crossover zone on the regularization method in the two-flavor Nambu–Jona-Lasinio model
- Novel numerical analysis for nonlinear advection–reaction–diffusion systems
- Heuristic decision of planned shop visit products based on similar reasoning method: From the perspective of organizational quality-specific immune
- Two-dimensional flow field distribution characteristics of flocking drainage pipes in tunnel
- Dynamic triaxial constitutive model for rock subjected to initial stress
- Automatic target recognition method for multitemporal remote sensing image
- Gaussons: optical solitons with log-law nonlinearity by Laplace–Adomian decomposition method
- Adaptive magnetic suspension anti-rolling device based on frequency modulation
- Dynamic response characteristics of 93W alloy with a spherical structure
- The heuristic model of energy propagation in free space, based on the detection of a current induced in a conductor inside a continuously covered conducting enclosure by an external radio frequency source
- Microchannel filter for air purification
- An explicit representation for the axisymmetric solutions of the free Maxwell equations
- Floquet analysis of linear dynamic RLC circuits
- Subpixel matching method for remote sensing image of ground features based on geographic information
- K-band luminosity–density relation at fixed parameters or for different galaxy families
- Effect of forward expansion angle on film cooling characteristics of shaped holes
- Analysis of the overvoltage cooperative control strategy for the small hydropower distribution network
- Stable walking of biped robot based on center of mass trajectory control
- Modeling and simulation of dynamic recrystallization behavior for Q890 steel plate based on plane strain compression tests
- Edge effect of multi-degree-of-freedom oscillatory actuator driven by vector control
- The effect of guide vane type on performance of multistage energy recovery hydraulic turbine (MERHT)
- Development of a generic framework for lumped parameter modeling
- Optimal control for generating excited state expansion in ring potential
- The phase inversion mechanism of the pH-sensitive reversible invert emulsion from w/o to o/w
- 3D bending simulation and mechanical properties of the OLED bending area
- Resonance overvoltage control algorithms in long cable frequency conversion drive based on discrete mathematics
- The measure of irregularities of nanosheets
- The predicted load balancing algorithm based on the dynamic exponential smoothing
- Influence of different seismic motion input modes on the performance of isolated structures with different seismic measures
- A comparative study of cohesive zone models for predicting delamination fracture behaviors of arterial wall
- Analysis on dynamic feature of cross arm light weighting for photovoltaic panel cleaning device in power station based on power correlation
- Some probability effects in the classical context
- Thermosoluted Marangoni convective flow towards a permeable Riga surface
- Simultaneous measurement of ionizing radiation and heart rate using a smartphone camera
- On the relations between some well-known methods and the projective Riccati equations
- Application of energy dissipation and damping structure in the reinforcement of shear wall in concrete engineering
- On-line detection algorithm of ore grade change in grinding grading system
- Testing algorithm for heat transfer performance of nanofluid-filled heat pipe based on neural network
- New optical solitons of conformable resonant nonlinear Schrödinger’s equation
- Numerical investigations of a new singular second-order nonlinear coupled functional Lane–Emden model
- Circularly symmetric algorithm for UWB RF signal receiving channel based on noise cancellation
- CH4 dissociation on the Pd/Cu(111) surface alloy: A DFT study
- On some novel exact solutions to the time fractional (2 + 1) dimensional Konopelchenko–Dubrovsky system arising in physical science
- An optimal system of group-invariant solutions and conserved quantities of a nonlinear fifth-order integrable equation
- Mining reasonable distance of horizontal concave slope based on variable scale chaotic algorithms
- Mathematical models for information classification and recognition of multi-target optical remote sensing images
- Hopkinson rod test results and constitutive description of TRIP780 steel resistance spot welding material
- Computational exploration for radiative flow of Sutterby nanofluid with variable temperature-dependent thermal conductivity and diffusion coefficient
- Analytical solution of one-dimensional Pennes’ bioheat equation
- MHD squeezed Darcy–Forchheimer nanofluid flow between two h–distance apart horizontal plates
- Analysis of irregularity measures of zigzag, rhombic, and honeycomb benzenoid systems
- A clustering algorithm based on nonuniform partition for WSNs
- An extension of Gronwall inequality in the theory of bodies with voids
- Rheological properties of oil–water Pickering emulsion stabilized by Fe3O4 solid nanoparticles
- Review Article
- Sine Topp-Leone-G family of distributions: Theory and applications
- Review of research, development and application of photovoltaic/thermal water systems
- Special Issue on Fundamental Physics of Thermal Transports and Energy Conversions
- Numerical analysis of sulfur dioxide absorption in water droplets
- Special Issue on Transport phenomena and thermal analysis in micro/nano-scale structure surfaces - Part I
- Random pore structure and REV scale flow analysis of engine particulate filter based on LBM
- Prediction of capillary suction in porous media based on micro-CT technology and B–C model
- Energy equilibrium analysis in the effervescent atomization
- Experimental investigation on steam/nitrogen condensation characteristics inside horizontal enhanced condensation channels
- Experimental analysis and ANN prediction on performances of finned oval-tube heat exchanger under different air inlet angles with limited experimental data
- Investigation on thermal-hydraulic performance prediction of a new parallel-flow shell and tube heat exchanger with different surrogate models
- Comparative study of the thermal performance of four different parallel flow shell and tube heat exchangers with different performance indicators
- Optimization of SCR inflow uniformity based on CFD simulation
- Kinetics and thermodynamics of SO2 adsorption on metal-loaded multiwalled carbon nanotubes
- Effect of the inner-surface baffles on the tangential acoustic mode in the cylindrical combustor
- Special Issue on Future challenges of advanced computational modeling on nonlinear physical phenomena - Part I
- Conserved vectors with conformable derivative for certain systems of partial differential equations with physical applications
- Some new extensions for fractional integral operator having exponential in the kernel and their applications in physical systems
- Exact optical solitons of the perturbed nonlinear Schrödinger–Hirota equation with Kerr law nonlinearity in nonlinear fiber optics
- Analytical mathematical schemes: Circular rod grounded via transverse Poisson’s effect and extensive wave propagation on the surface of water
- Closed-form wave structures of the space-time fractional Hirota–Satsuma coupled KdV equation with nonlinear physical phenomena
- Some misinterpretations and lack of understanding in differential operators with no singular kernels
- Stable solutions to the nonlinear RLC transmission line equation and the Sinh–Poisson equation arising in mathematical physics
- Calculation of focal values for first-order non-autonomous equation with algebraic and trigonometric coefficients
- Influence of interfacial electrokinetic on MHD radiative nanofluid flow in a permeable microchannel with Brownian motion and thermophoresis effects
- Standard routine techniques of modeling of tick-borne encephalitis
- Fractional residual power series method for the analytical and approximate studies of fractional physical phenomena
- Exact solutions of space–time fractional KdV–MKdV equation and Konopelchenko–Dubrovsky equation
- Approximate analytical fractional view of convection–diffusion equations
- Heat and mass transport investigation in radiative and chemically reacting fluid over a differentially heated surface and internal heating
- On solitary wave solutions of a peptide group system with higher order saturable nonlinearity
- Extension of optimal homotopy asymptotic method with use of Daftardar–Jeffery polynomials to Hirota–Satsuma coupled system of Korteweg–de Vries equations
- Unsteady nano-bioconvective channel flow with effect of nth order chemical reaction
- On the flow of MHD generalized maxwell fluid via porous rectangular duct
- Study on the applications of two analytical methods for the construction of traveling wave solutions of the modified equal width equation
- Numerical solution of two-term time-fractional PDE models arising in mathematical physics using local meshless method
- A powerful numerical technique for treating twelfth-order boundary value problems
- Fundamental solutions for the long–short-wave interaction system
- Role of fractal-fractional operators in modeling of rubella epidemic with optimized orders
- Exact solutions of the Laplace fractional boundary value problems via natural decomposition method
- Special Issue on 19th International Symposium on Electromagnetic Fields in Mechatronics, Electrical and Electronic Engineering
- Joint use of eddy current imaging and fuzzy similarities to assess the integrity of steel plates
- Uncertainty quantification in the design of wireless power transfer systems
- Influence of unequal stator tooth width on the performance of outer-rotor permanent magnet machines
- New elements within finite element modeling of magnetostriction phenomenon in BLDC motor
- Evaluation of localized heat transfer coefficient for induction heating apparatus by thermal fluid analysis based on the HSMAC method
- Experimental set up for magnetomechanical measurements with a closed flux path sample
- Influence of the earth connections of the PWM drive on the voltage constraints endured by the motor insulation
- High temperature machine: Characterization of materials for the electrical insulation
- Architecture choices for high-temperature synchronous machines
- Analytical study of air-gap surface force – application to electrical machines
- High-power density induction machines with increased windings temperature
- Influence of modern magnetic and insulation materials on dimensions and losses of large induction machines
- New emotional model environment for navigation in a virtual reality
- Performance comparison of axial-flux switched reluctance machines with non-oriented and grain-oriented electrical steel rotors
- Erratum
- Erratum to “Conserved vectors with conformable derivative for certain systems of partial differential equations with physical applications”
Articles in the same Issue
- Regular Articles
- Model of electric charge distribution in the trap of a close-contact TENG system
- Dynamics of Online Collective Attention as Hawkes Self-exciting Process
- Enhanced Entanglement in Hybrid Cavity Mediated by a Two-way Coupled Quantum Dot
- The nonlinear integro-differential Ito dynamical equation via three modified mathematical methods and its analytical solutions
- Diagnostic model of low visibility events based on C4.5 algorithm
- Electronic temperature characteristics of laser-induced Fe plasma in fruits
- Comparative study of heat transfer enhancement on liquid-vapor separation plate condenser
- Characterization of the effects of a plasma injector driven by AC dielectric barrier discharge on ethylene-air diffusion flame structure
- Impact of double-diffusive convection and motile gyrotactic microorganisms on magnetohydrodynamics bioconvection tangent hyperbolic nanofluid
- Dependence of the crossover zone on the regularization method in the two-flavor Nambu–Jona-Lasinio model
- Novel numerical analysis for nonlinear advection–reaction–diffusion systems
- Heuristic decision of planned shop visit products based on similar reasoning method: From the perspective of organizational quality-specific immune
- Two-dimensional flow field distribution characteristics of flocking drainage pipes in tunnel
- Dynamic triaxial constitutive model for rock subjected to initial stress
- Automatic target recognition method for multitemporal remote sensing image
- Gaussons: optical solitons with log-law nonlinearity by Laplace–Adomian decomposition method
- Adaptive magnetic suspension anti-rolling device based on frequency modulation
- Dynamic response characteristics of 93W alloy with a spherical structure
- The heuristic model of energy propagation in free space, based on the detection of a current induced in a conductor inside a continuously covered conducting enclosure by an external radio frequency source
- Microchannel filter for air purification
- An explicit representation for the axisymmetric solutions of the free Maxwell equations
- Floquet analysis of linear dynamic RLC circuits
- Subpixel matching method for remote sensing image of ground features based on geographic information
- K-band luminosity–density relation at fixed parameters or for different galaxy families
- Effect of forward expansion angle on film cooling characteristics of shaped holes
- Analysis of the overvoltage cooperative control strategy for the small hydropower distribution network
- Stable walking of biped robot based on center of mass trajectory control
- Modeling and simulation of dynamic recrystallization behavior for Q890 steel plate based on plane strain compression tests
- Edge effect of multi-degree-of-freedom oscillatory actuator driven by vector control
- The effect of guide vane type on performance of multistage energy recovery hydraulic turbine (MERHT)
- Development of a generic framework for lumped parameter modeling
- Optimal control for generating excited state expansion in ring potential
- The phase inversion mechanism of the pH-sensitive reversible invert emulsion from w/o to o/w
- 3D bending simulation and mechanical properties of the OLED bending area
- Resonance overvoltage control algorithms in long cable frequency conversion drive based on discrete mathematics
- The measure of irregularities of nanosheets
- The predicted load balancing algorithm based on the dynamic exponential smoothing
- Influence of different seismic motion input modes on the performance of isolated structures with different seismic measures
- A comparative study of cohesive zone models for predicting delamination fracture behaviors of arterial wall
- Analysis on dynamic feature of cross arm light weighting for photovoltaic panel cleaning device in power station based on power correlation
- Some probability effects in the classical context
- Thermosoluted Marangoni convective flow towards a permeable Riga surface
- Simultaneous measurement of ionizing radiation and heart rate using a smartphone camera
- On the relations between some well-known methods and the projective Riccati equations
- Application of energy dissipation and damping structure in the reinforcement of shear wall in concrete engineering
- On-line detection algorithm of ore grade change in grinding grading system
- Testing algorithm for heat transfer performance of nanofluid-filled heat pipe based on neural network
- New optical solitons of conformable resonant nonlinear Schrödinger’s equation
- Numerical investigations of a new singular second-order nonlinear coupled functional Lane–Emden model
- Circularly symmetric algorithm for UWB RF signal receiving channel based on noise cancellation
- CH4 dissociation on the Pd/Cu(111) surface alloy: A DFT study
- On some novel exact solutions to the time fractional (2 + 1) dimensional Konopelchenko–Dubrovsky system arising in physical science
- An optimal system of group-invariant solutions and conserved quantities of a nonlinear fifth-order integrable equation
- Mining reasonable distance of horizontal concave slope based on variable scale chaotic algorithms
- Mathematical models for information classification and recognition of multi-target optical remote sensing images
- Hopkinson rod test results and constitutive description of TRIP780 steel resistance spot welding material
- Computational exploration for radiative flow of Sutterby nanofluid with variable temperature-dependent thermal conductivity and diffusion coefficient
- Analytical solution of one-dimensional Pennes’ bioheat equation
- MHD squeezed Darcy–Forchheimer nanofluid flow between two h–distance apart horizontal plates
- Analysis of irregularity measures of zigzag, rhombic, and honeycomb benzenoid systems
- A clustering algorithm based on nonuniform partition for WSNs
- An extension of Gronwall inequality in the theory of bodies with voids
- Rheological properties of oil–water Pickering emulsion stabilized by Fe3O4 solid nanoparticles
- Review Article
- Sine Topp-Leone-G family of distributions: Theory and applications
- Review of research, development and application of photovoltaic/thermal water systems
- Special Issue on Fundamental Physics of Thermal Transports and Energy Conversions
- Numerical analysis of sulfur dioxide absorption in water droplets
- Special Issue on Transport phenomena and thermal analysis in micro/nano-scale structure surfaces - Part I
- Random pore structure and REV scale flow analysis of engine particulate filter based on LBM
- Prediction of capillary suction in porous media based on micro-CT technology and B–C model
- Energy equilibrium analysis in the effervescent atomization
- Experimental investigation on steam/nitrogen condensation characteristics inside horizontal enhanced condensation channels
- Experimental analysis and ANN prediction on performances of finned oval-tube heat exchanger under different air inlet angles with limited experimental data
- Investigation on thermal-hydraulic performance prediction of a new parallel-flow shell and tube heat exchanger with different surrogate models
- Comparative study of the thermal performance of four different parallel flow shell and tube heat exchangers with different performance indicators
- Optimization of SCR inflow uniformity based on CFD simulation
- Kinetics and thermodynamics of SO2 adsorption on metal-loaded multiwalled carbon nanotubes
- Effect of the inner-surface baffles on the tangential acoustic mode in the cylindrical combustor
- Special Issue on Future challenges of advanced computational modeling on nonlinear physical phenomena - Part I
- Conserved vectors with conformable derivative for certain systems of partial differential equations with physical applications
- Some new extensions for fractional integral operator having exponential in the kernel and their applications in physical systems
- Exact optical solitons of the perturbed nonlinear Schrödinger–Hirota equation with Kerr law nonlinearity in nonlinear fiber optics
- Analytical mathematical schemes: Circular rod grounded via transverse Poisson’s effect and extensive wave propagation on the surface of water
- Closed-form wave structures of the space-time fractional Hirota–Satsuma coupled KdV equation with nonlinear physical phenomena
- Some misinterpretations and lack of understanding in differential operators with no singular kernels
- Stable solutions to the nonlinear RLC transmission line equation and the Sinh–Poisson equation arising in mathematical physics
- Calculation of focal values for first-order non-autonomous equation with algebraic and trigonometric coefficients
- Influence of interfacial electrokinetic on MHD radiative nanofluid flow in a permeable microchannel with Brownian motion and thermophoresis effects
- Standard routine techniques of modeling of tick-borne encephalitis
- Fractional residual power series method for the analytical and approximate studies of fractional physical phenomena
- Exact solutions of space–time fractional KdV–MKdV equation and Konopelchenko–Dubrovsky equation
- Approximate analytical fractional view of convection–diffusion equations
- Heat and mass transport investigation in radiative and chemically reacting fluid over a differentially heated surface and internal heating
- On solitary wave solutions of a peptide group system with higher order saturable nonlinearity
- Extension of optimal homotopy asymptotic method with use of Daftardar–Jeffery polynomials to Hirota–Satsuma coupled system of Korteweg–de Vries equations
- Unsteady nano-bioconvective channel flow with effect of nth order chemical reaction
- On the flow of MHD generalized maxwell fluid via porous rectangular duct
- Study on the applications of two analytical methods for the construction of traveling wave solutions of the modified equal width equation
- Numerical solution of two-term time-fractional PDE models arising in mathematical physics using local meshless method
- A powerful numerical technique for treating twelfth-order boundary value problems
- Fundamental solutions for the long–short-wave interaction system
- Role of fractal-fractional operators in modeling of rubella epidemic with optimized orders
- Exact solutions of the Laplace fractional boundary value problems via natural decomposition method
- Special Issue on 19th International Symposium on Electromagnetic Fields in Mechatronics, Electrical and Electronic Engineering
- Joint use of eddy current imaging and fuzzy similarities to assess the integrity of steel plates
- Uncertainty quantification in the design of wireless power transfer systems
- Influence of unequal stator tooth width on the performance of outer-rotor permanent magnet machines
- New elements within finite element modeling of magnetostriction phenomenon in BLDC motor
- Evaluation of localized heat transfer coefficient for induction heating apparatus by thermal fluid analysis based on the HSMAC method
- Experimental set up for magnetomechanical measurements with a closed flux path sample
- Influence of the earth connections of the PWM drive on the voltage constraints endured by the motor insulation
- High temperature machine: Characterization of materials for the electrical insulation
- Architecture choices for high-temperature synchronous machines
- Analytical study of air-gap surface force – application to electrical machines
- High-power density induction machines with increased windings temperature
- Influence of modern magnetic and insulation materials on dimensions and losses of large induction machines
- New emotional model environment for navigation in a virtual reality
- Performance comparison of axial-flux switched reluctance machines with non-oriented and grain-oriented electrical steel rotors
- Erratum
- Erratum to “Conserved vectors with conformable derivative for certain systems of partial differential equations with physical applications”

