Abstract
C22H33N3O5·0.67H2O, monoclinic, P21/c, a = 11.9915(6) Å, b = 18.9934(10) Å, c = 10.5332(5) Å, β = 112.155(2)°, V = 2221.91(19) Å3, Z = 4, Rgt(F) = 0.0417, wRref(F2) = 0.1139, T = 100(2) K.
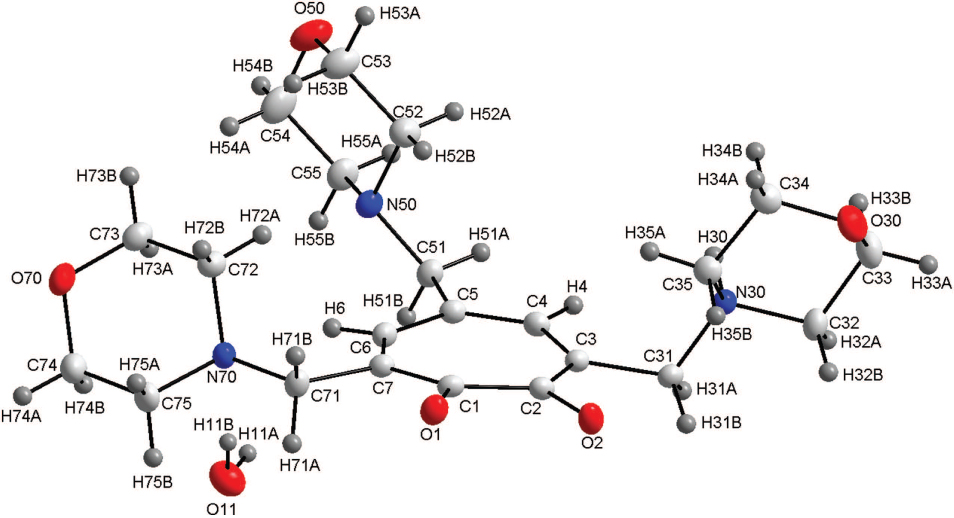
The asymmetric unit of the title structure is shown in the figure. Tables 1 and 2 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow, plate Size 0.44 × 0.31 × 0.08 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.9 cm−1 |
| Diffractometer, scan mode: | Bruker APEX II, φ and ω |
| 2θmax, completeness: | 55.4°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 47649, 5344, 0.045 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4142 |
| N(param)refined: | 292 |
| Programs: | Bruker programs [35], SHELX [36], WinGX [37], publCIF [38], PLATON [39] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.39736(8) | 0.11061(5) | 0.21802(9) | 0.0216(2) |
| O2 | 0.28806(8) | 0.22558(5) | 0.11278(9) | 0.0226(2) |
| O30 | −0.02112(8) | 0.31672(6) | −0.35103(10) | 0.0286(2) |
| O70 | 0.86587(9) | −0.08961(5) | 0.22705(10) | 0.0280(2) |
| O50 | 0.73553(10) | 0.06074(6) | −0.36038(11) | 0.0348(3) |
| N50 | 0.71576(10) | 0.14669(6) | −0.14634(11) | 0.0192(2) |
| N30 | 0.23099(9) | 0.31518(6) | −0.18441(11) | 0.0164(2) |
| N70 | 0.71684(9) | 0.02655(6) | 0.23533(10) | 0.0179(2) |
| C4 | 0.50472(11) | 0.24913(7) | −0.05380(12) | 0.0177(3) |
| H4 | 0.5025 | 0.2825 | −0.1194 | 0.021* |
| C51 | 0.69056(11) | 0.21264(7) | −0.08938(13) | 0.0192(3) |
| H51A | 0.6597 | 0.2471 | −0.1623 | 0.023* |
| H51B | 0.765 | 0.2309 | −0.0222 | 0.023* |
| C3 | 0.41070(11) | 0.25544(7) | −0.00536(12) | 0.0165(3) |
| C1 | 0.44476(11) | 0.14465(7) | 0.14896(12) | 0.0169(3) |
| C5 | 0.59955(11) | 0.20249(7) | −0.02175(12) | 0.0170(3) |
| C32 | 0.15966(12) | 0.38163(7) | −0.21589(14) | 0.0227(3) |
| H32A | 0.2105 | 0.4206 | −0.2201 | 0.027* |
| H32B | 0.1299 | 0.3914 | −0.1439 | 0.027* |
| C35 | 0.15039(11) | 0.25409(7) | −0.19074(13) | 0.0201(3) |
| H35B | 0.1202 | 0.2579 | −0.1176 | 0.024* |
| H35A | 0.1958 | 0.2106 | −0.1779 | 0.024* |
| C75 | 0.77975(13) | −0.01676(8) | 0.35678(14) | 0.0247(3) |
| H75A | 0.7275 | −0.0545 | 0.3628 | 0.03* |
| H75B | 0.8008 | 0.0118 | 0.4388 | 0.03* |
| C7 | 0.55900(11) | 0.12085(7) | 0.14749(12) | 0.0171(3) |
| C2 | 0.38015(11) | 0.21045(7) | 0.08333(12) | 0.0172(3) |
| C53 | 0.63868(14) | 0.05226(8) | −0.31342(17) | 0.0316(3) |
| H53B | 0.6606 | 0.017 | −0.2416 | 0.038* |
| H53A | 0.5678 | 0.0357 | −0.3885 | 0.038* |
| C6 | 0.62381(11) | 0.14724(7) | 0.07443(13) | 0.0181(3) |
| H6 | 0.6968 | 0.1245 | 0.0917 | 0.022* |
| C31 | 0.33490(11) | 0.32147(7) | −0.04899(12) | 0.0176(3) |
| H31B | 0.3038 | 0.3334 | 0.021 | 0.021* |
| H31A | 0.3861 | 0.36 | −0.0544 | 0.021* |
| C34 | 0.04610(12) | 0.25248(8) | −0.32732(14) | 0.0239(3) |
| H34B | 0.0762 | 0.2454 | −0.3999 | 0.029* |
| H34A | −0.0063 | 0.2133 | −0.3292 | 0.029* |
| C52 | 0.60946(12) | 0.12047(8) | −0.25896(14) | 0.0250(3) |
| H52A | 0.5829 | 0.1552 | −0.3319 | 0.03* |
| H52B | 0.5446 | 0.1128 | −0.2267 | 0.03* |
| C74 | 0.89261(13) | −0.04732(8) | 0.34678(15) | 0.0282(3) |
| H74B | 0.9459 | −0.0093 | 0.3448 | 0.034* |
| H74A | 0.9341 | −0.0757 | 0.4274 | 0.034* |
| C73 | 0.80074(12) | −0.04976(7) | 0.10683(14) | 0.0243(3) |
| H73B | 0.78 | −0.0799 | 0.0269 | 0.029* |
| H73A | 0.8514 | −0.0121 | 0.0969 | 0.029* |
| C55 | 0.81192(12) | 0.15699(8) | −0.19832(15) | 0.0250(3) |
| H55B | 0.8839 | 0.174 | −0.1252 | 0.03* |
| H55A | 0.7874 | 0.1919 | −0.2708 | 0.03* |
| C33 | 0.05461(13) | 0.37450(8) | −0.35204(15) | 0.0285(3) |
| H33A | 0.0078 | 0.4176 | −0.3713 | 0.034* |
| H33B | 0.085 | 0.3676 | −0.4244 | 0.034* |
| C71 | 0.60834(12) | 0.05853(7) | 0.24306(13) | 0.0197(3) |
| H71A | 0.6263 | 0.0739 | 0.3365 | 0.024* |
| H71B | 0.5462 | 0.0228 | 0.2219 | 0.024* |
| C72 | 0.68697(12) | −0.01874(7) | 0.11388(14) | 0.0218(3) |
| H72A | 0.6452 | 0.0086 | 0.0318 | 0.026* |
| H72B | 0.6339 | −0.0564 | 0.1185 | 0.026* |
| C54 | 0.83852(14) | 0.08795(9) | −0.25301(17) | 0.0340(4) |
| H54B | 0.9026 | 0.095 | −0.2868 | 0.041* |
| H54A | 0.8663 | 0.0539 | −0.179 | 0.041* |
| O11a | 0.90093(14) | 0.13948(9) | 0.36490(16) | 0.0327(4) |
| H11Ba | 0.848(2) | 0.1085(13) | 0.324(3) | 0.056(9)* |
| H11Aa | 0.914(3) | 0.1613(14) | 0.301(3) | 0.057(9)* |
| H30 | 0.2614(15) | 0.3083(10) | −0.2560(19) | 0.041(5)* |
aOccupancy: 0.667
Source of material
A mixture of tropolone (0.46 g, 3.73 mmol) and morpholine (1.18 mL, 13.54 mmol) was treated with 40% aqueous formaldehyde (0.80 mL, 9.61 mmol). After stirring at 60°C for 7 min, a reddish liquid was formed. A yellow precipitate was obtained overnight at ambient temperature. The crude product was crystallized from a ethyl acetate and hexane mixture (1:1), to form 3,5,7-tri(morpholinomethyl) tropolone as yellow crystals, (1.45 g, 3.47 mmol, 92.85% yield). MS: m/z 420.3; 1H NMR (300 MHz, CD2Cl2) δ 7.87,(s, 2H), 3.76–3.72 (m, 8H), 3. 71 (s, 4H), 3.70–3.66 (m, 4H), 3.51 (s, 2H), 2.54 (dd, J = 5.4, 3.9 Hz, 8H), 2.50–2.43 (m, 4H) ppm; 13C NMR (300 Mhz, CD2Cl2) δ 168.35, 138.91, 135.70, 132.86, 67.10, 66.94, 59.46, 53,73, 53.35, 52.76.
Experimental details
Aromatic and methylene hydrogen atoms were positioned geometrically and allowed to ride on their parent atoms, with Uiso(H) = 1.2Ueq(C) and Uiso(H) = 1.5Ueq(C) with C—H distances of 0.93 Å and 0.97 Å respectively. The hydrogen atoms of the water molecule were located from the electron density map.
Discussion
Tropolone, a hydroxide derivative of tropone, is a functional moiety found in a large number of natural products such as colchicine used for the treatment of gout [1, 2]. Tropolone and its derivatives have been reported as antimicrobial agents. It also possess antiviral, antitumor, antioxidant and enzyme inhibiting properties [3–7]. The title compound exhibit antiviral activities [7], while the isopropyl derivatives of tropolone (β-thujaplicin and γ-thujaplicin) were reported to have antifungal and antibacterial properties [6]. Thus these derivatives are used in the preservation of wood [8, 9].
The title compound consists of a tropolone core with three methyl morpholine moieties bound to it at C3, C5 and C7 of the backbone, with additional water (0.67 occupancy) completing the asymmetric unit, as shown in the figure. Earlier in the 1970′s, Shimanouchi et al. reported the molecular structure of tropolone [10, 11]. When comparing this structure to that of tropolone, some slight deviations were observed within the tropolone core. The C1—O1 and C2—O2 bond distances of the title compound of 1.257(2) Å and 1.285(2) Å compare well to that reported for the tropolone structure with C1—O1 and C2—O2 calculated as 1.2603(5) Å and 1.3333(7) Å respectively. The C1—C2 bond distance of the structure reported here and the structure of tropolone are determined as 1.496(2) Å and 1.4542(5) Å respectively and also compare well. All the bond distances and angles are in agreement to similar structures in literature [12, 13]. 3,5,7-tris(morpholinomethyl)tropolone was coordinated to the rhodium(I) showing bond angles and bond distances in the metal complex similar to the uncoordinated molecule [14]. Although, the C—O distances of the coordinated ligand is slightly longer at 1.31 Å (1.257 Å and 1.287 Å for the uncoordinated molecule) and the C1—C2 distance is slightly shorter at 1.44 Å (1.496 Å for the uncoordinated molecule), but still within the normal range. Schutte et al. synthesized the rhenium(I) tricarbonyl complexes with the tropolone and 3,5,7-tribromotropolone ligands coordinated to the metal centre [15–18]. Overall the C1—O1/C2—O2 and the C1—C2 bond distances in these structures vary from 1.276(3) Å to 1.307(8) Å and from 1.462(6) Å to 1.477(8) Å respectively. It is clear that even when these ligand systems are coordinated to a metal centre the bond distances don't vary significantly. In the title compound, the tropolone ring (defined by the atoms C1—C2—C3—C4—C5—C6—C7) is almost planar with the maximum deviation observed for C1 with a distance of −0.0710(9) Å from the plane. The torsion angle O1—C1—C2—O2 of 3.8(2)° indicate the slight twisting of the oxygen atoms with respect to the tropolone plane. The torsion angle in the rhodium(I) structure reported by Hill et al. has a smaller torsion angle of 1.8°, illustrating the effect of the coordination of the metal cenre. Moreover, there exist extensive inter- and intramolecular hydrogen interactions in this molecule. Seven C—H⋯O (two intramolecular, five intermolecular), two N—H⋯O (intermolecular), one C—H⋯N (intramolecular), one O—H⋯N (intramolecular) and one O—H⋯O (intermolecular) hydrogen interactions are observed. These hydrogen interactions seems to enhance the solid state ordering of the structure with the molecules packing in alternating layers when viewed along the ab-plane. These tropolone type ligand systems and other O,O′, N,O and N,N′ bidentate ligand systems form part of an ongoing study [19–34].
Acknowledgements:
Financial assistance from the University of the Free State is gratefully acknowledged. We also express our gratitude towards SASOL, PETLabs Pharmaceuticals, the South African National Research Foundation (SA-NRF/THRIP), Inkaba YeAfrica and the University of the Free State Strategic Academic Initiative (Advanced Biomolecular Systems) for financial support of this project. This work is based on the research supported in part by the National Research Foundation of South Africa (Grant specific unique reference number (UID) 84913). The Grantholder acknowledges that opinions, findings and conclusions or recommendations expressed in any publication generated by the NRF supported research are that of the authors, and that the NRF accepts no liability whatsoever in this regard.
References
1. Dewar, M. J. S.: Tropolone. Nature 166 (1950) 790–791.10.1038/166790a0Search in Google Scholar
2. Doering, W. E.; Knox, L. H. J.: Tropolone. J. Am. Chem. Soc. 73 (1951) 828–838.10.1021/ja01146a101Search in Google Scholar
3. Birkenshaw, J. H.: Chemistry of Fungi. Ann. Rev. Biochem. 22 (1953) 371–398.10.1146/annurev.bi.22.070153.002103Search in Google Scholar
4. Davison, J.; Al Fahad, A.; Cai, M.; Song, Z.; Yehia, S. Y.; Lazarus, C. M.; Bailey, A. M.; Simpson, T. J.; Cox, R. J.: Genetic, molecular, and biochemical basis of fungal tropolone biosynthesis. Proc. Natl. Acad. Sci. USA 109 (2012) 7642–7647.10.1073/pnas.1201469109Search in Google Scholar
5. Jacobsen, F. E.; Lewis, J. A.; Heroux, K. J.; Cohen, S. M.: Characterization and evaluation of pyrone and tropolone chelators for use in metalloprotein inhibitors. Inorg. Chim. Acta 360 (2007) 264–272.10.1016/j.ica.2006.07.044Search in Google Scholar
6. Inamori, Y.; Tsujibo, H.; Ohishi, H.; Ishii, F.; Mizugaki, M.; Aso, H.; Ishida, N.: Cytotoxic effect of hinokitiol and tropolone on the growth of mammalian cells and on blastogenesis of mouse splenic T cells. Biol. Pharm. Bull. 16 (1993) 521–523.10.1248/bpb.16.521Search in Google Scholar
7. Boguszewska-Chachulska, A. M.; Krawczyk, M.; Najda, A.; Kopanska, K.; Stankiewicz-Drogon, A.; Zagórski-Ostoja, W.; Bretner, M.: Searching for a new anti-HCV therapy: Synthesis and properties of tropolone derivatives. Biochem. and Biophys. Res. Comm. 341 (2006) 641–647.10.1016/j.bbrc.2006.01.015Search in Google Scholar
8. Anderson, T. R.; Slotkin, T. A.: Maturation of the adrenal medulla–IV. Effects of morphine. Biochem. Pharmacol. 24 (1975) 1469–1474.10.1016/0006-2952(75)90020-9Search in Google Scholar
9. Makar, A. B.; McMartin, K. E.; Palese, M.; Tephly, T. R.: Formate assay in body fluids: application in methanol poisoning. Biochem. Med. 13 (1975) 117–126.10.1016/0006-2944(75)90147-7Search in Google Scholar
10. Shimanouchi, H.; Sasada, Y.: An X-ray structure analysis of tropolone. Tetrahedron Lett. 11 (1970) 2421–2424.10.1016/S0040-4039(01)98245-0Search in Google Scholar
11. Shimanouchi, H.; Sasada, Y.: The crystal and molecular structure of tropolone. Acta Crystallogr. B29 (1973) 81–90.10.1107/S0567740873002013Search in Google Scholar
12. Steyl, G.; Roodt, A.: Molecular and crystallographic study of tropolone type derivatives by ab initio Hartree-Fock calculations. South Afri. J. Chem. 59 (2006) 21–27.Search in Google Scholar
13. Lyczko, K.; Lyczko, M.: 2-Hydroxy-7-nitrocyclohepta-2,4,6-trien-1-one. Acta Crystallogr. E69 (2013) o536–o536.10.1107/S1600536813006594Search in Google Scholar PubMed PubMed Central
14. Hill, T. N.; Steyl, G.: Dicarbonyl[2-hydroxy-3,5,7-tris(morpholinomethyl)cyclohepta-2,4,6-trienonato(1-)-κ2O1,O2]rhodium(I). Acta Crystallogr. E64 (2008) m1580–m1581.10.1107/S160053680803780XSearch in Google Scholar PubMed PubMed Central
15. Schutte, M.; Visser, H. G.; Roodt, A.: Coordinated aqua vs methanol substitution kinetics in fac-Re(I) tricarbonyl tropolonato complexes. Inorg. Chem. 51 (2012) 11996–12006.10.1021/ic301891uSearch in Google Scholar PubMed
16. Schutte, M.; Visser, H. G.; Steyl, G.: Tetraethylammonium-bromidotricarbonyl[3,5,7-tribromotropolonato(1-)-κ2O,O′]rhenate(I). Acta. Crystallogr. 63 (2007) m3195–m3196.10.1107/S1600536807053457Search in Google Scholar
17. Schutte, M.; Visser, H. G.; Roodt, A.: Aquatricarbonyl-(3,5,7-tribromotropolonato)rhenium(I) methanol solvate. Acta Crystallogr. E64 (2008) m1610–m1611.10.1107/S1600536808038737Search in Google Scholar PubMed PubMed Central
18. Schutte, M.; Visser, H. G.; Roodt, A.: Tetraethylammonium-bromidotricarbonyl(tropolonato)rhenate (I). Acta Crystallogr. 66 (2010) m859–m860.10.1107/S1600536810024505Search in Google Scholar PubMed PubMed Central
19. Manicum, A.-L.; Schutte-Smith, M.; Kemp, G.; Visser, H. G.: Illustration of the electronic influence of coordinated β-diketone type ligands: a kinetic and structural study. Polyhedron 85 (2015) 190–195.10.1016/j.poly.2014.08.005Search in Google Scholar
20. Manicum, A.-L.; Visser, H. G.; Engelbrecht, I.; Roodt, A.: Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(cyclohexyldiphenylphosphine-κP) rhenium(I), C26H28O5PRe. Z. Kristallogr. NCS 230 (2015) 150–152.10.1515/ncrs-2014-9013Search in Google Scholar
21. Schutte, M.; Kemp, G.; Visser, H. G.; Roodt, A.: Tuning the reactivity in classic low-spin d6 rhenium(I) tricarbonyl radiopharmaceutical synthon by selective bidentate ligand variation (L,L′-Bid; L,L′ = N,N′, N,O and O,O′ donor atom sets) in fac-[Re(CO)3(L,L′-Bid)(MeOH)]n complexes. Inorg. Chem. 50 (2011) 12486–12498.10.1021/ic2013792Search in Google Scholar PubMed
22. Brink, A.; Roodt, A.; Steyl, G.; Visser, H. G.: Steric vs electronic anomaly observed from iodomethane oxidative addition to tertiary phosphine modified Rhodium(I) acetylacetonato complexes following progressive phenyl replacement by cyclohexyl [PR(3) = PPh(3), PPh(2)Cy, PPhCy(2) and PCy(3)]. Dalton Trans. 39 (2010) 5572–5578.10.1039/b922083fSearch in Google Scholar
23. Roodt, A.; Basson S. S.; Leipoldt, J. G.: Studies on the substitution reactions of dioxotetracyanometalate systems with bidentate ligands: kinetics of the reaction between aquaoxotetracyanotungstate(IV) and 2-pyridinecarboxylate ions. Polyhedron 13 (1994) 599–607.10.1016/S0277-5387(00)84737-3Search in Google Scholar
24. Van Aswegen, K. G.; Leipoldt, J. G.; Potgieter, I. M.; Lamprecht, G. J.; Roodt, A.; van Zyl, G. J.: The crystal structure of the acetone adduct of trans-methyliodo-8 hydroxyquinolinatocarbonyltriphenylphosphinerhodium( III). Trans. Metal Chem. 16 (1991) 369–371.10.1007/BF01024085Search in Google Scholar
25. Kemp, G.; Roodt, A.; Purcell, W.; Koch, K. R.: Unprecedented N,S,O co-ordination of the doubly deprotonated anion of N-benzoyl-N′-phenylthiourea (H2L2) bridging two rhodium(I) centres: crystal structure of the acetone solvate of [(PPh3)2(CO)Rh(μ-L2-κN′:κO,S)Rh(PPh3)(CO)]. Dalton Trans. (1997) 4481–4484.10.1039/a705887jSearch in Google Scholar
26. Sacht, C.; Datt, M. S.; Otto, S.; Roodt, A.: Synthesis, characterisation and coordination chemistry of novel chiral N,N-dialkyl-N′-menthyloxycarbonylthioureas. Crystal and molecular structures of N,N-diethyl-N′-(-)-(3R)-menthyloxycarbonylthiourea and cis-(S,S)-[Pt(L)Cl(DMSO)] [where HL = N-(+)-(3R)-menthyloxycarbonyl-N′-morpholinothiourea or N-benzoyl-N′,N′-diethylthiourea]. Dalton Trans. (2000) 4579–4586.10.1039/b007589mSearch in Google Scholar
27. Erasmus, J. J. C.; Lamprecht, G. J.; Swarts, J. C.; Roodt, A.; Oskarsson, A.: (E)-1,3-Diferrocenyl-2-buten-1-one-Water (4/1). Acta Crystallogr. 52 (1996) 3000–3002.10.1107/S0108270196004258Search in Google Scholar
28. Viljoen, J. A.; Visser, H. G.; Roodt, A.; Steyn, M.: Di-μ-hydroxido-bis[tris(1,1,1,5,5,5- hexafluoroacetylacetonato-κ2O,O′)-hafnium(IV)] acetone solvate. Acta Crystallogr. E65 (2009) m1367–m1368.10.1107/S1600536809041658Search in Google Scholar PubMed PubMed Central
29. Molokoane, P. P.; Schutte, M.; Steyl, G.: 2-Ethyl-3-hydroxy-1-isopropyl-4-pyridone. Acta Crystallogr. E68 (2012) o3235–o3236.10.1107/S1600536812044091Search in Google Scholar PubMed PubMed Central
30. Schutte-Smith, M.; Visser, H. G.: The versatility of pyridine-2,5-dicarboxylic acid in the synthesis of fac-M(CO)3 complexes (M = Re, 99mTc): Reactivity towards substitution reactions and derivatization after coordination to a metal. Polyhedron 89 (2015) 122–128.10.1016/j.poly.2015.01.007Search in Google Scholar
31. Schutte-Smith, M.; Muller, T. J.; Visser, H. G.; Roodt, A.: Distorted octahedral environments in tricarbonylrhenium(I) complexes of 5-[2-(2,4,6-trimethylphenyl)diazen-1-yl]quinolin-8-olate and 5,7-bis[2-(2-methylphenyl)diazen-1-yl]quinolin-8-olate. Acta Crystallogr. C69 (2013) 1467–1471.10.1107/S0108270113027947Search in Google Scholar PubMed
32. Twala, T. N.; Schutte-Smith, M.; Roodt, A.; Visser, H. G.: Activation of the manganese(I) tricarbonyl core by selective variation of bidentate ligands (L,L′-Bid = N,N′ and N,O donor atom sets) in fac-[Mn(CO)3(L,L′-Bid)(CH3OH)]n complexes. Dalton Trans. 44 (2015) 3278–3288.10.1039/C4DT03524KSearch in Google Scholar
33. van der Westhuizen, H. J.; Meijboom, R.; Schutte, M.; Roodt, A.: Mechanism for the formation of substituted manganese(V) cyanidonitrido complexes: Crystallographic and kinetic study of the substitution reactions of trans-[MnN(H2O)(CN)4]2− with monodentate pyridine and bidentate pyridine-carbocylate ligands. Inorg. Chem. 49 (2010) 9599–9608.10.1021/ic101274qSearch in Google Scholar PubMed
34. Schutte, M.; Visser, H. G.; Roodt, A.; Braband, H.: N-Benzylisatin. Acta Crystallogr. E68 o777.10.1107/S1600536812006575Search in Google Scholar PubMed PubMed Central
35. Bruker. APEX2, SAINT and SADABS. Brucker AXS Inc., Madison, Wisconsin, USA, 2012.Search in Google Scholar
36. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
37. Farrugia, L. J.: WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 45 (2012) 849–854.10.1107/S0021889812029111Search in Google Scholar
38. Westrip, S. P.: publCIF: software for editing, validating and formatting crystallographic information files. J. Appl. Crystallogr. 43 (2010) 920–925.10.1107/S0021889810022120Search in Google Scholar
39. Spek, A. L.: Structure validation in chemical crystallography. Acta Crystallogr. D65 (2009) 148–155.10.1107/S090744490804362XSearch in Google Scholar PubMed PubMed Central
©2016 Marietjie Schutte-Smith et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Frontmatter
- Crystal structure of poly[aqua(μ2-2,5-bis(4-pyridyl)-1,3,4-oxadiazole-κ2N,N)(μ2-1,3-phenylenediacetato-κ3O,O′:O′′)cobalt(II)], C22H18CoN4O6
- Crystal structure of 2-amino-7,7-dimethyl-5-oxo-4-(3-phenoxy-phenyl)-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile
- Crystal structure of (E)-4,4′-(diazene-1,2-diyl)bis(1-nitro-1H-1,2,4-triazol-5(4H)-one)—acetonitrile (1:1), C6H5N11O6
- Crystal structure of potassium (E)-5-oxo-4-((5-oxo-1H-1,2,4-triazol-4(5H)-yl)diazenyl)-4,5-dihydro-1,2,4-triazol-1-ide – (E)-4,4-diazene-1,2-diylbis(2,4-dihydro-3H-1,2,4-triazol-3-one) – methanol (1/1/1), C9H11N16KO5
- Crystal structure of (N,N′-bis(2-(((2,6-diisopropylphenyl)imino)methyl)phenyl)benzene-1,2-diamido-κ4O,O′,O′′,O′′′)oxidovanadium(IV), C44H48N4OV
- Crystal structure of 5,5-bis(4-iodophenyl)-5H-cyclopenta[2,1-b:3,4-b′]dipyridine, C23H14I2N2
- Crystal structure of aquadichloridobis(1-((2-methyl-1H-imidazol-1-yl)methyl)-1H-benotriazole-κN)mercury(II), C22H24Cl2HgN10O
- Crystal structure of 2-[4-(1H-imidazol-1-yl)phenyl]-1H-benzimidazol-3-ium [2-(carboxymethyl)phenyl]acetate monohydrate, C26H24N4O5
- Crystal structure of 4,4′-bipiperidinium dichloride 0.12 hydrate, C10H22N2Cl2 · 0.12 H2O
- Crystal structure of catena-poly[tetraaqua(μ2-4,4′(E)-ethene-1,2-diyldipyridine-κ2N:N′)nickel(II)] bis(6-methyl-2-oxo-1,2-dihydro-pyridine-4-carboxylate) pentahydrate, C26H42N4O15Ni
- Crystal structure of ethyl-5-amino-1-(2,4-dinitrophenyl)-1H-pyrazole-4-carboxylate, C12H11N5O6
- Crystal structure of dicarbonyl(pyridin-2-olate-1-oxido-κ2O,O′)rhodium(I), C7H4NO4Rh
- Crystal structure of 1-(adamantan-1-yl)-3-(4-chlorophenyl)thiourea, C17H21ClN2S
- Crystal structure of 2-((2-chloropyridin-3-ylamino)methylene)malononitrile, C9H5ClN4
- Crystal structure of tetraaquabis(μ2-4-chlorobenzoato-κ2O:O,O′)bis(4-chlorobenzoato-κO)bis(1,10-phenanthroline-κ2N,N′)distrontium(II), C52H40Cl4N4O12Sr2
- Crystal structure of 3-hydroxy-3-phenyl-1,3-dihydro-2H-indol-2-one, C14H11NO2
- Crystal structures of bis(1,10-phenanthrolin-1-ium) aquapentakis(nitrato-κ2O,O′)neodym(III) monohydrate, C24H22N9NdO17
- Crystal structure of 2-ethyl-1-tert-butyl 3-oxo-2-[phenyl(tert-butoxycarbonylamino)methyl]-1,2-pyrrolidinedicarboxylate, C24H34N2O7
- Crystal structure of poly[diaquabis(μ4-benzene-1,3,5-tricarboxylato-κO1,κO2:κO3,κO4:κO5:κO6)-bis(μ2-4,4′-benzene-1,3-diylbis(4H-1,2,4-triazole-κ2N:N′)tricadmium(II)] tetrahydrate, C38H34Cd3N12O18
- Crystal structure of trans-dichlorido[1,3-bis(9-methyl-9H-fluoren-9-yl) benzimidazol-2-ylidene](pyridine)palladium(II) – a compound with anagostic CH–Pd interactions, C40H31Cl2N3Pd
- Crystal structure of catena-poly[(μ3-5-(4-(tetrazol-1-id-5-yl)phenoxy)benzene-1,3-dicarboxyato-κ3O:O′:N)(4-(3-(pyridin-4-yl)propyl)pyridinium-κN)zinc(II)], C28H22ZnN6O5
- Crystal structure of 3,6-di-2-pyridinyl-4-pyridazine carbonitrile, C15H9N5
- Crystal structure of 5,5,9,13-tetramethyltetracyclo[10·2·1·01,10·04,9]pentadecane-3,7,14-triol, C20H34O4
- Crystal structure of 2-amino-7-methyl-5-oxo-4-phenyl-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H12N2O3
- Crystal structure of the poly[(1,10-phenanthroline-κ2N,N′)(μ3-carboxylatophenoxyacetato-κ4O,O′:O′′;O′′′)lead(II)] monohydrate, C21H16N2O6Pb
- Crystal structure of the poly[(μ4-biphenyl-4,4′-dicarboxylato-κ4O:O′:O′′:O′′′) bis(μ3-8-(11-(oxysulfonyl)-4-silbenyl)-2-(oxysulfonyl)stilbene-κ4O:O′:O′′,O′′′) bis(1,10-phenanthroline-k2N,N′) dipraseodymium(III)], C94H64N4O16Pr2S4
- Crystal structure of 3-iodo-5-methoxy-7-(methoxymethoxy)-4-(3-methoxyphenoxy)-2H-chromen-2-one, C19H17IO7
- Crystal structure of poly[(5-carboxy-2,6-dimethylpyridinium-3-carboxylato-κO)tris(μ2-2,6-dimethylpyridinium-3,5-dicarboxylato-κ3O,O′:O′′)erbium(III)], C36H33ErN4O16
- This molecule targets at type 2 diabetes - a single crystal study on (2R,3S,5R)-2-(2,5-difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl] tetrahydro-2H-pyran-3-amine (Omarigliptin), C17H20F2N4O3S
- Crystal structure of poly[(μ2-2,2′-benzene-1,2-diyldiacetato-κ2O:O′), (μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′)zinc(II)], C22H19N5O4Zn
- Crystal structure of tetraaqua(μ2-3-(3,5-dicarboxyphenoxy)benzene-1,2-dicarboxylato-κ2O:O′)manganese(II) dihydrate, C16H20O15Mn
- Crystal structure of 4,4′-sulfonyldipyridine, C10H8N2O2S
- Crystal structure of 4-[(E)-(2-chloro-6-fluorobenzylidene)amino]-1,2-dihydro-2,3-dimethyl-1-phenylpyrazol-5-one, C18H15ClFN3O
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O, O′)(triphenylphosphine-κP)rhodium(I), C24H19NO3PRh
- Crystal structure of diaquabis(2-(3-bromophenyl)-5-carboxy-1H-imidazol-4-carboxylato-κ2O,N) cobalt(II) trihydrate, C22H22Br2CoN4O13
- Crystal structure of 4-(pyridin-4-ylmethylsulfonyl)pyridine, C11H10N2O2S
- Crystal structure of poly[(μ4-1-methyl-1H-tetrazole-5-thiolato-κ3S:S:N:N′)copper(I)], C2H3CuN4S
- Crystal structure of poly[diaquabis(μ2-4,4′-sulfinyldipyridine-κ2N,N′)zinc(II)] diperchlorate dihydrate, C20H24N4O14S2Cl2Zn
- Crystal structure of (1-((1-benzylpyrrolidin-2-yl-κN)methyl)-3-isopropyl-1H-imidazol-2(3H)-ylidene–κC)dibromidopalladium(II), C18H25Br2N3Pd
- Crystal structure of pentacalcium tetranitridovanadate(V) mononitride based on a powder diffraction study, Ca5[VN4]N
- Crystal structure of ((1-((1-benzylpyrrolidin-2-yl)methyl)-3-ethyl-1H-imidazol-2(3H)-ylidene)-κ2C,N)dichloridopalladium(II), C17H23Cl2N3Pd
- Crystal structure of 9-allyl-4,5-dichloro-12-cyano-9,10-dihydro-9,10-ethanoanthracen-12-yl acetate, C22H17Cl2NO2
- Crystal structure of 4-bromo-2-(8-(3-ethoxy-2-hydroxyphenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenol, C18H19BrN2O5
- Crystal structure of 3-tert-butyl-3-hydroxy-1,3-dihydro-2H-pyrrolo[3,2-c]pyridin-2-one, C11H14N2O2
- Crystal structure of diaquabis(3-(3,5-dibromophenyl)-5-(pyridin-2-yl)-1,2,4-triazol-4-ido-κ2N,N′)nickel(II) mono hydrate, C26H20Br4N8NiO3
- Crystal structure of 5-(adamantan-1-yl)-3-[(2-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2-ethyl-1-tert-butyl-2-((4-fluorophenyl)(tert-butoxycarbonylamino)methyl)-3-oxo-pyrrolidine-1,2-dicarboxylate, C24H33FN2O7
- Crystal structure of bis(μ2-2-fluorobenzoato-κ2O:O:O′) bis(μ2-2-fluorobenzoato-κ2O:O′)dinitrato-κ2O,O′ bis(1,10-phenathroline-κ2N,N′)diterbium(III), C52H32F4N6O14Tb2
- Crystal structure of tetrabutylammonium 4-aminobenzenesulfonate 2/3 hydrate, C22H42N2O3S · 2/3 H2O
- Crystal structure of tetraethylammonium 4-aminobenzenesulfonate, C14H26N2O3S
- Crystal structure of bis(guanidinium) 3,3′-oxybis(6-carboxybenzoate), C18H20N6O9
- Crystal structure of N′-(4-methoxybenzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide, C18H16N4O2
- Crystal structure of N′-(4-nitrobenzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide, C17H13N5O3
- Crystal structure of 5-((4-bromophenyl)(2-hydroxy-6-oxocyclohex-1-en-1-yl)methyl)-6-hydroxy-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, C19H19BrN2O5
- Crystal structure of 3-(((cyclohexyl(phenyl)methylidene)amino)oxy)-2-hydroxy-N-(propan-2-yl)propan-1-aminium chloride, C19H31ClN2O2
- Crystal structure of 6-hydroxy-5-((2-hydroxy-6-oxocyclohex-1-en-1-yl)(phenyl)methyl)-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, C19H20N2O5
- Crystal structure of 2-(4-(4-bromophenyl)thiazol-2-yl)isoindoline-1,3-dione, C17H9BrN2O2S
- Crystal structure of 2-(4-methylbenzoyl)pyrene, C24H16O
- Crystal structure of N-(5-bromo-4-(p-tolyl)thiazol-2-yl)-4-chlorobutanamide, C14H14BrClN2OS
- Crystal structure of 4,5-diphenylthiazol-2-amine, C15H12N2S
- Crystal structure of poly[bis(μ2-4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoato-κ3N:O,O′)-lead(II)], C28H18O4N8Pb
- Crystal structure of (Z)-4-(4-oxopent-2-en-2-ylamino)benzenesulfonamide, C11H14N2O3S
- Crystal structure of poly[(μ2-2-methyl-1-(4-(2-methyl-2H-benzo[d] imidazol-1(7aH)-yl)butyl)-1H-benzo[d]imidazole-κ2N:N′)bis(μ3-5-tert-butylbenzene-1,3-dicarboxylato-κ4O:O,O′:O′′,O′′′)dicadmium(II)] tetrahydrate, C44H54Cd2N4O12
- Crystal structure of catena-poly-[aqua(μ2-4,4′-bipyridine-κ2N:N′)bis(3′,5,5′-tricarboxybiphenyl-2-carboxylato-κ2O,O′)cadmium(II)], C42H28N2O17Cd
- Crystal structure of (2RS,3RS)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl)pentan-3-ol, C15H20ClN3O
- Crystal structure of N′-(4-(dimethylamino)benzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide, C19H19N5O
- Crystal structure of 3-(benzofuran-2-yl)-5-(4-fluorophenyl)-4,5-dihydro-1Hpyrazole-1-carbothioamide, C18H14FN3OS
- Crystal structure of ent-1β-acetoxy-7α,14α-di-hydroxy-7β,20-epoxykaur-16-en-15-one, C22H30O6
- Crystal structure of 1α,7β-dihydroxy-11β-acetoxy-ent-7β,20-epoxykaur-16-en-15-one, C22H30O6
- Crystal structure of 7β,14β,15β-trihydroxy-1α-acetoxy-7α,20-epoxy-ent-kaurane, C22H32O6
- Crystal structure of ent-1β,7α,11α-trihydroxy-7β,20-epoxykaur-16-en-15-one, C20H28O5
- Crystal structure of poly-[tetraaquabis(μ8-benzene-1,2,4,5-tetracarboxylato-1κ3O4:O6:O8:2κ4O2:O2:O5:O5:3κ4O1:O3:O5:O7)(di-μ3-hydroxido)-pentazinc(II)] decahydrate, C20H34O32Zn5
- Crystal structure of 1-(4-methylthiazol-2-yl)-3-propylthiourea, C8H13N3S2
- Crystal structure of 2-((dimethylamino)methylene)-5,5-Dimethylcyclohexane-1,3-dione, C11H17NO2
- Crystal structure of poly[1,4-bis(2-methylbenzimidazol)butane-κ2N:N′)bis(4,4′-oxybis(benzoato-κ4O,O′:O′′,O′′′)dicadmium] monohydrate, C48H38Cd2N4O10
- Crystal structure of 2-(3-(benzofuran-2-yl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-4-(4-chlorophenyl)thiazole, C26H17ClFN3OS
- Crystal structure of bis(μ3-isophthalato-κ3O:O′:O′′)(μ2-1,4-\ bis((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)dizinc(II), C22H19N2O4Zn
- Crystal structure of poly[bis(adipate-κ4O,O′:O′′, O′′′)(1,4-bis(2-methyl-1H-benzo[d]imidazol-1-yl)benzene-κ2N:N′)dizinc(II), C36H38N4O8Zn2
- Crystal structure of N-(2-(2-oxoindolin-4-yl)ethyl)-N-propylpropan-1-aminium tetraphenylborate, C40H45BN2O
- Crystal structure of 1-(2,3-dihydro-4-methyl-3-phenyl-2-thioxothiazol-5-yl)-1-ethanone, C12H11NOS2
- Crystal structure of 3-(adamantan-1-yl)-1-[(4-benzylpiperazin-1-yl)methyl]-4-[(E)-(2,6-difluorobenzylidene)amino]-1H-1,2,4-triazole-5(4H)-thione, C31H36F2N6S
- Crystal structure of 6-(4-chlorophenyl)-3-(thiophen-2-yl)-[1,2,4]triazolo[3,4-b][1,3,4]-thiadiazole, C13H7ClN4S2
- Crystal structure of bis(ethanaminium) poly[bis(hexaselenido-κ2Se1,Se6)palladate(II)], C4H16N2PdSe12
- Crystal structure of 2-(3-(benzofuran-2-yl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl)-4-phenylthiazole, C26H19N3OS
- Crystal structure of poly-[bis(μ3-5-hydroxyisophtalato-κ3O:O′:O′′)(μ2-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)benzene-κ2N:N′)dizinc(II)], C40H30N4O5Zn2
- Crystal structure of poly-[(μ-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)benzene-κ2N:N′)-bis(μ4-2,2′-(1,3-phenylene)diacetate-κ4O:O′:O′′:O′′′)dizinc(II)], C44H38N4O8Zn2
- Crystal structure of 5,17-bis-cyano-25,26,27,28-tetrapropyloxy-calix[4]arene, C42H46N2O4
- Crystal structure of poly-[μ-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)benzene-κ2N:N′)-bis(μ3-5-hydroxyisophthalate(2–)-κ3O,O′:O′′)dicadmium(II)] monohydrate, C64H54N8O11Cd2
- Crystal structure of poly-[μ-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)benzene-κ2N:N′)-bis(μ4-4,4′-sulfonyldibenzoato-κ4O:O′:O′′:O′′′)dicadmium(II)] monohydrate, C52H42N4O14Cd2
- Crystal structure of poly-[bis(μ4-adipato-κ4O:O′:O′′:O′′′)(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)dizinc(II)], C38H42N4O8Zn2
- Crystal structure of (2-(2-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- Crystal structure of 2-(4-Bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylic acid, C13H15BrO4
- Crystal structure of (2-(4-bromophenyl)-5-ethyl-1,3-dioxan-5-yl)methanol, C13H17BrO3
- Crystal structure of 3-((1,3,5,7-tetraoxo-6-(pyridin-3-ylmethyl)-3,3a,4,4a,5,6,7,7a,8,8a-decahydro-4,8-ethenopyrrolo[3,4-f]isoindol-2(1H)-yl)methyl)pyridin-1-ium-κN-trichloridocobalt(II) hemihydrate, C24H22Cl3CoN4O4.5
- Crystal structure of 4-chloro-4′,6′-dichloro-2,2′-[propane-1,3-diyldioxybis(nitrilomethylidyne)]-diphenol, C17H14Cl3N2O4
- Crystal structure of 2-amino-4-(3-trifluoromethylphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C17H13F3N2O2
- Crystal structure of bis(benzoato-κO)bis(4,4′-((1H-1,2,4-triazol-1-yl)methylene)dibenzonitrile-κN)zinc(II), C48H32N10O4Zn
- Crystal structure of 3-methyl-2H-chromen-2-one, C10H8O2
- Crystal structure of catena-poly-[aqua-(2-carboxy-5-(3-carboxy-5-carboxylatophenoxy)benzoato-κO)(μ2-4,4′-di(1H-imidazol-1-yl)-1,1′-biphenyl-κ2N:N′)cobalt(II)], C34H24N4O10Co
- Crystal structure of 4,4′-di(1H-imidazol-1-yl)-1,1′-biphenyl, C36H28N8
- Crystal structure of 1-(3-chloropropyl)piperidin-1-ium tetraphenylborate, C32H37BClN
- Crystal structure of dimethyl 5-(benzylamino)isophthalate, C17H17NO4
- Crystal structure of dimethyl 5-(dibenzylamino)isophthalate, C24H23NO4
- Crystal structure of N-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioamide, C21H29N3S
- Crystal structure of (2,2′(cyclohexane-1,2-diylbis(nitrilo(E)methylylidene))diphenolato-κ4O,O′,N,N′)dimethanolmanganese(III) bromide, C22H28BrMnN2O4
- Crystal structure of 3,5,7-tris(morpholinomethyl)tropolone·0.67 hydrate, C22H33N3O5·0.67H2O
- Crystal structure of biphenyl-2,3′,5,5′-tetracarboxylic acid – 4,4′-biphenyl-4,4′-diyldipyridine (3/2), C49H34N3O8
Articles in the same Issue
- Frontmatter
- Crystal structure of poly[aqua(μ2-2,5-bis(4-pyridyl)-1,3,4-oxadiazole-κ2N,N)(μ2-1,3-phenylenediacetato-κ3O,O′:O′′)cobalt(II)], C22H18CoN4O6
- Crystal structure of 2-amino-7,7-dimethyl-5-oxo-4-(3-phenoxy-phenyl)-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile
- Crystal structure of (E)-4,4′-(diazene-1,2-diyl)bis(1-nitro-1H-1,2,4-triazol-5(4H)-one)—acetonitrile (1:1), C6H5N11O6
- Crystal structure of potassium (E)-5-oxo-4-((5-oxo-1H-1,2,4-triazol-4(5H)-yl)diazenyl)-4,5-dihydro-1,2,4-triazol-1-ide – (E)-4,4-diazene-1,2-diylbis(2,4-dihydro-3H-1,2,4-triazol-3-one) – methanol (1/1/1), C9H11N16KO5
- Crystal structure of (N,N′-bis(2-(((2,6-diisopropylphenyl)imino)methyl)phenyl)benzene-1,2-diamido-κ4O,O′,O′′,O′′′)oxidovanadium(IV), C44H48N4OV
- Crystal structure of 5,5-bis(4-iodophenyl)-5H-cyclopenta[2,1-b:3,4-b′]dipyridine, C23H14I2N2
- Crystal structure of aquadichloridobis(1-((2-methyl-1H-imidazol-1-yl)methyl)-1H-benotriazole-κN)mercury(II), C22H24Cl2HgN10O
- Crystal structure of 2-[4-(1H-imidazol-1-yl)phenyl]-1H-benzimidazol-3-ium [2-(carboxymethyl)phenyl]acetate monohydrate, C26H24N4O5
- Crystal structure of 4,4′-bipiperidinium dichloride 0.12 hydrate, C10H22N2Cl2 · 0.12 H2O
- Crystal structure of catena-poly[tetraaqua(μ2-4,4′(E)-ethene-1,2-diyldipyridine-κ2N:N′)nickel(II)] bis(6-methyl-2-oxo-1,2-dihydro-pyridine-4-carboxylate) pentahydrate, C26H42N4O15Ni
- Crystal structure of ethyl-5-amino-1-(2,4-dinitrophenyl)-1H-pyrazole-4-carboxylate, C12H11N5O6
- Crystal structure of dicarbonyl(pyridin-2-olate-1-oxido-κ2O,O′)rhodium(I), C7H4NO4Rh
- Crystal structure of 1-(adamantan-1-yl)-3-(4-chlorophenyl)thiourea, C17H21ClN2S
- Crystal structure of 2-((2-chloropyridin-3-ylamino)methylene)malononitrile, C9H5ClN4
- Crystal structure of tetraaquabis(μ2-4-chlorobenzoato-κ2O:O,O′)bis(4-chlorobenzoato-κO)bis(1,10-phenanthroline-κ2N,N′)distrontium(II), C52H40Cl4N4O12Sr2
- Crystal structure of 3-hydroxy-3-phenyl-1,3-dihydro-2H-indol-2-one, C14H11NO2
- Crystal structures of bis(1,10-phenanthrolin-1-ium) aquapentakis(nitrato-κ2O,O′)neodym(III) monohydrate, C24H22N9NdO17
- Crystal structure of 2-ethyl-1-tert-butyl 3-oxo-2-[phenyl(tert-butoxycarbonylamino)methyl]-1,2-pyrrolidinedicarboxylate, C24H34N2O7
- Crystal structure of poly[diaquabis(μ4-benzene-1,3,5-tricarboxylato-κO1,κO2:κO3,κO4:κO5:κO6)-bis(μ2-4,4′-benzene-1,3-diylbis(4H-1,2,4-triazole-κ2N:N′)tricadmium(II)] tetrahydrate, C38H34Cd3N12O18
- Crystal structure of trans-dichlorido[1,3-bis(9-methyl-9H-fluoren-9-yl) benzimidazol-2-ylidene](pyridine)palladium(II) – a compound with anagostic CH–Pd interactions, C40H31Cl2N3Pd
- Crystal structure of catena-poly[(μ3-5-(4-(tetrazol-1-id-5-yl)phenoxy)benzene-1,3-dicarboxyato-κ3O:O′:N)(4-(3-(pyridin-4-yl)propyl)pyridinium-κN)zinc(II)], C28H22ZnN6O5
- Crystal structure of 3,6-di-2-pyridinyl-4-pyridazine carbonitrile, C15H9N5
- Crystal structure of 5,5,9,13-tetramethyltetracyclo[10·2·1·01,10·04,9]pentadecane-3,7,14-triol, C20H34O4
- Crystal structure of 2-amino-7-methyl-5-oxo-4-phenyl-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H12N2O3
- Crystal structure of the poly[(1,10-phenanthroline-κ2N,N′)(μ3-carboxylatophenoxyacetato-κ4O,O′:O′′;O′′′)lead(II)] monohydrate, C21H16N2O6Pb
- Crystal structure of the poly[(μ4-biphenyl-4,4′-dicarboxylato-κ4O:O′:O′′:O′′′) bis(μ3-8-(11-(oxysulfonyl)-4-silbenyl)-2-(oxysulfonyl)stilbene-κ4O:O′:O′′,O′′′) bis(1,10-phenanthroline-k2N,N′) dipraseodymium(III)], C94H64N4O16Pr2S4
- Crystal structure of 3-iodo-5-methoxy-7-(methoxymethoxy)-4-(3-methoxyphenoxy)-2H-chromen-2-one, C19H17IO7
- Crystal structure of poly[(5-carboxy-2,6-dimethylpyridinium-3-carboxylato-κO)tris(μ2-2,6-dimethylpyridinium-3,5-dicarboxylato-κ3O,O′:O′′)erbium(III)], C36H33ErN4O16
- This molecule targets at type 2 diabetes - a single crystal study on (2R,3S,5R)-2-(2,5-difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl] tetrahydro-2H-pyran-3-amine (Omarigliptin), C17H20F2N4O3S
- Crystal structure of poly[(μ2-2,2′-benzene-1,2-diyldiacetato-κ2O:O′), (μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′)zinc(II)], C22H19N5O4Zn
- Crystal structure of tetraaqua(μ2-3-(3,5-dicarboxyphenoxy)benzene-1,2-dicarboxylato-κ2O:O′)manganese(II) dihydrate, C16H20O15Mn
- Crystal structure of 4,4′-sulfonyldipyridine, C10H8N2O2S
- Crystal structure of 4-[(E)-(2-chloro-6-fluorobenzylidene)amino]-1,2-dihydro-2,3-dimethyl-1-phenylpyrazol-5-one, C18H15ClFN3O
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O, O′)(triphenylphosphine-κP)rhodium(I), C24H19NO3PRh
- Crystal structure of diaquabis(2-(3-bromophenyl)-5-carboxy-1H-imidazol-4-carboxylato-κ2O,N) cobalt(II) trihydrate, C22H22Br2CoN4O13
- Crystal structure of 4-(pyridin-4-ylmethylsulfonyl)pyridine, C11H10N2O2S
- Crystal structure of poly[(μ4-1-methyl-1H-tetrazole-5-thiolato-κ3S:S:N:N′)copper(I)], C2H3CuN4S
- Crystal structure of poly[diaquabis(μ2-4,4′-sulfinyldipyridine-κ2N,N′)zinc(II)] diperchlorate dihydrate, C20H24N4O14S2Cl2Zn
- Crystal structure of (1-((1-benzylpyrrolidin-2-yl-κN)methyl)-3-isopropyl-1H-imidazol-2(3H)-ylidene–κC)dibromidopalladium(II), C18H25Br2N3Pd
- Crystal structure of pentacalcium tetranitridovanadate(V) mononitride based on a powder diffraction study, Ca5[VN4]N
- Crystal structure of ((1-((1-benzylpyrrolidin-2-yl)methyl)-3-ethyl-1H-imidazol-2(3H)-ylidene)-κ2C,N)dichloridopalladium(II), C17H23Cl2N3Pd
- Crystal structure of 9-allyl-4,5-dichloro-12-cyano-9,10-dihydro-9,10-ethanoanthracen-12-yl acetate, C22H17Cl2NO2
- Crystal structure of 4-bromo-2-(8-(3-ethoxy-2-hydroxyphenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenol, C18H19BrN2O5
- Crystal structure of 3-tert-butyl-3-hydroxy-1,3-dihydro-2H-pyrrolo[3,2-c]pyridin-2-one, C11H14N2O2
- Crystal structure of diaquabis(3-(3,5-dibromophenyl)-5-(pyridin-2-yl)-1,2,4-triazol-4-ido-κ2N,N′)nickel(II) mono hydrate, C26H20Br4N8NiO3
- Crystal structure of 5-(adamantan-1-yl)-3-[(2-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2-ethyl-1-tert-butyl-2-((4-fluorophenyl)(tert-butoxycarbonylamino)methyl)-3-oxo-pyrrolidine-1,2-dicarboxylate, C24H33FN2O7
- Crystal structure of bis(μ2-2-fluorobenzoato-κ2O:O:O′) bis(μ2-2-fluorobenzoato-κ2O:O′)dinitrato-κ2O,O′ bis(1,10-phenathroline-κ2N,N′)diterbium(III), C52H32F4N6O14Tb2
- Crystal structure of tetrabutylammonium 4-aminobenzenesulfonate 2/3 hydrate, C22H42N2O3S · 2/3 H2O
- Crystal structure of tetraethylammonium 4-aminobenzenesulfonate, C14H26N2O3S
- Crystal structure of bis(guanidinium) 3,3′-oxybis(6-carboxybenzoate), C18H20N6O9
- Crystal structure of N′-(4-methoxybenzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide, C18H16N4O2
- Crystal structure of N′-(4-nitrobenzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide, C17H13N5O3
- Crystal structure of 5-((4-bromophenyl)(2-hydroxy-6-oxocyclohex-1-en-1-yl)methyl)-6-hydroxy-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, C19H19BrN2O5
- Crystal structure of 3-(((cyclohexyl(phenyl)methylidene)amino)oxy)-2-hydroxy-N-(propan-2-yl)propan-1-aminium chloride, C19H31ClN2O2
- Crystal structure of 6-hydroxy-5-((2-hydroxy-6-oxocyclohex-1-en-1-yl)(phenyl)methyl)-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, C19H20N2O5
- Crystal structure of 2-(4-(4-bromophenyl)thiazol-2-yl)isoindoline-1,3-dione, C17H9BrN2O2S
- Crystal structure of 2-(4-methylbenzoyl)pyrene, C24H16O
- Crystal structure of N-(5-bromo-4-(p-tolyl)thiazol-2-yl)-4-chlorobutanamide, C14H14BrClN2OS
- Crystal structure of 4,5-diphenylthiazol-2-amine, C15H12N2S
- Crystal structure of poly[bis(μ2-4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoato-κ3N:O,O′)-lead(II)], C28H18O4N8Pb
- Crystal structure of (Z)-4-(4-oxopent-2-en-2-ylamino)benzenesulfonamide, C11H14N2O3S
- Crystal structure of poly[(μ2-2-methyl-1-(4-(2-methyl-2H-benzo[d] imidazol-1(7aH)-yl)butyl)-1H-benzo[d]imidazole-κ2N:N′)bis(μ3-5-tert-butylbenzene-1,3-dicarboxylato-κ4O:O,O′:O′′,O′′′)dicadmium(II)] tetrahydrate, C44H54Cd2N4O12
- Crystal structure of catena-poly-[aqua(μ2-4,4′-bipyridine-κ2N:N′)bis(3′,5,5′-tricarboxybiphenyl-2-carboxylato-κ2O,O′)cadmium(II)], C42H28N2O17Cd
- Crystal structure of (2RS,3RS)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl)pentan-3-ol, C15H20ClN3O
- Crystal structure of N′-(4-(dimethylamino)benzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide, C19H19N5O
- Crystal structure of 3-(benzofuran-2-yl)-5-(4-fluorophenyl)-4,5-dihydro-1Hpyrazole-1-carbothioamide, C18H14FN3OS
- Crystal structure of ent-1β-acetoxy-7α,14α-di-hydroxy-7β,20-epoxykaur-16-en-15-one, C22H30O6
- Crystal structure of 1α,7β-dihydroxy-11β-acetoxy-ent-7β,20-epoxykaur-16-en-15-one, C22H30O6
- Crystal structure of 7β,14β,15β-trihydroxy-1α-acetoxy-7α,20-epoxy-ent-kaurane, C22H32O6
- Crystal structure of ent-1β,7α,11α-trihydroxy-7β,20-epoxykaur-16-en-15-one, C20H28O5
- Crystal structure of poly-[tetraaquabis(μ8-benzene-1,2,4,5-tetracarboxylato-1κ3O4:O6:O8:2κ4O2:O2:O5:O5:3κ4O1:O3:O5:O7)(di-μ3-hydroxido)-pentazinc(II)] decahydrate, C20H34O32Zn5
- Crystal structure of 1-(4-methylthiazol-2-yl)-3-propylthiourea, C8H13N3S2
- Crystal structure of 2-((dimethylamino)methylene)-5,5-Dimethylcyclohexane-1,3-dione, C11H17NO2
- Crystal structure of poly[1,4-bis(2-methylbenzimidazol)butane-κ2N:N′)bis(4,4′-oxybis(benzoato-κ4O,O′:O′′,O′′′)dicadmium] monohydrate, C48H38Cd2N4O10
- Crystal structure of 2-(3-(benzofuran-2-yl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-4-(4-chlorophenyl)thiazole, C26H17ClFN3OS
- Crystal structure of bis(μ3-isophthalato-κ3O:O′:O′′)(μ2-1,4-\ bis((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)dizinc(II), C22H19N2O4Zn
- Crystal structure of poly[bis(adipate-κ4O,O′:O′′, O′′′)(1,4-bis(2-methyl-1H-benzo[d]imidazol-1-yl)benzene-κ2N:N′)dizinc(II), C36H38N4O8Zn2
- Crystal structure of N-(2-(2-oxoindolin-4-yl)ethyl)-N-propylpropan-1-aminium tetraphenylborate, C40H45BN2O
- Crystal structure of 1-(2,3-dihydro-4-methyl-3-phenyl-2-thioxothiazol-5-yl)-1-ethanone, C12H11NOS2
- Crystal structure of 3-(adamantan-1-yl)-1-[(4-benzylpiperazin-1-yl)methyl]-4-[(E)-(2,6-difluorobenzylidene)amino]-1H-1,2,4-triazole-5(4H)-thione, C31H36F2N6S
- Crystal structure of 6-(4-chlorophenyl)-3-(thiophen-2-yl)-[1,2,4]triazolo[3,4-b][1,3,4]-thiadiazole, C13H7ClN4S2
- Crystal structure of bis(ethanaminium) poly[bis(hexaselenido-κ2Se1,Se6)palladate(II)], C4H16N2PdSe12
- Crystal structure of 2-(3-(benzofuran-2-yl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl)-4-phenylthiazole, C26H19N3OS
- Crystal structure of poly-[bis(μ3-5-hydroxyisophtalato-κ3O:O′:O′′)(μ2-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)benzene-κ2N:N′)dizinc(II)], C40H30N4O5Zn2
- Crystal structure of poly-[(μ-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)benzene-κ2N:N′)-bis(μ4-2,2′-(1,3-phenylene)diacetate-κ4O:O′:O′′:O′′′)dizinc(II)], C44H38N4O8Zn2
- Crystal structure of 5,17-bis-cyano-25,26,27,28-tetrapropyloxy-calix[4]arene, C42H46N2O4
- Crystal structure of poly-[μ-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)benzene-κ2N:N′)-bis(μ3-5-hydroxyisophthalate(2–)-κ3O,O′:O′′)dicadmium(II)] monohydrate, C64H54N8O11Cd2
- Crystal structure of poly-[μ-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)benzene-κ2N:N′)-bis(μ4-4,4′-sulfonyldibenzoato-κ4O:O′:O′′:O′′′)dicadmium(II)] monohydrate, C52H42N4O14Cd2
- Crystal structure of poly-[bis(μ4-adipato-κ4O:O′:O′′:O′′′)(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)dizinc(II)], C38H42N4O8Zn2
- Crystal structure of (2-(2-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- Crystal structure of 2-(4-Bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylic acid, C13H15BrO4
- Crystal structure of (2-(4-bromophenyl)-5-ethyl-1,3-dioxan-5-yl)methanol, C13H17BrO3
- Crystal structure of 3-((1,3,5,7-tetraoxo-6-(pyridin-3-ylmethyl)-3,3a,4,4a,5,6,7,7a,8,8a-decahydro-4,8-ethenopyrrolo[3,4-f]isoindol-2(1H)-yl)methyl)pyridin-1-ium-κN-trichloridocobalt(II) hemihydrate, C24H22Cl3CoN4O4.5
- Crystal structure of 4-chloro-4′,6′-dichloro-2,2′-[propane-1,3-diyldioxybis(nitrilomethylidyne)]-diphenol, C17H14Cl3N2O4
- Crystal structure of 2-amino-4-(3-trifluoromethylphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C17H13F3N2O2
- Crystal structure of bis(benzoato-κO)bis(4,4′-((1H-1,2,4-triazol-1-yl)methylene)dibenzonitrile-κN)zinc(II), C48H32N10O4Zn
- Crystal structure of 3-methyl-2H-chromen-2-one, C10H8O2
- Crystal structure of catena-poly-[aqua-(2-carboxy-5-(3-carboxy-5-carboxylatophenoxy)benzoato-κO)(μ2-4,4′-di(1H-imidazol-1-yl)-1,1′-biphenyl-κ2N:N′)cobalt(II)], C34H24N4O10Co
- Crystal structure of 4,4′-di(1H-imidazol-1-yl)-1,1′-biphenyl, C36H28N8
- Crystal structure of 1-(3-chloropropyl)piperidin-1-ium tetraphenylborate, C32H37BClN
- Crystal structure of dimethyl 5-(benzylamino)isophthalate, C17H17NO4
- Crystal structure of dimethyl 5-(dibenzylamino)isophthalate, C24H23NO4
- Crystal structure of N-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioamide, C21H29N3S
- Crystal structure of (2,2′(cyclohexane-1,2-diylbis(nitrilo(E)methylylidene))diphenolato-κ4O,O′,N,N′)dimethanolmanganese(III) bromide, C22H28BrMnN2O4
- Crystal structure of 3,5,7-tris(morpholinomethyl)tropolone·0.67 hydrate, C22H33N3O5·0.67H2O
- Crystal structure of biphenyl-2,3′,5,5′-tetracarboxylic acid – 4,4′-biphenyl-4,4′-diyldipyridine (3/2), C49H34N3O8

