Abstract
C9H5ClN4, monoclinic, P21/c (no. 14), a = 8.1178(4) Å, b = 7.2367(4) Å, c = 15.2585(8) Å, β = 101.506(2)°, V = 878.36(8) Å3, Z = 4, Rgt(F) = 0.0466, wRref(F2) = 0.1170, T = 100 K.
Tables 1–3 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless, block, size 0.158×0.368×0.415 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 3.93 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω scans |
| 2θmax: | 55° |
| N(hkl)measured, N(hkl)unique: | 7339, 2002 |
| N(param)refined: | 135 |
| Programs: | SHELX [11], Bruker programs [12] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | Site | x | y | z | Uiso |
|---|---|---|---|---|---|
| H(4A) | 4e | 0.6846 | 0.8470 | 0.5411 | 0.018 |
| H(5A) | 4e | 0.8671 | 0.8971 | 0.4445 | 0.016 |
| H(6A) | 4e | 0.2139 | 0.5444 | 0.4288 | 0.013 |
| H(3A) | 4e | 0.415(3) | 0.718(3) | 0.487(2) | 0.015(6) |
| H(1N2) | 4e | 0.277(4) | 0.577(4) | 0.264(2) | 0.025(7) |
Fractional coordinates and atomic displacement parameters (Å2).
| Atom | Site | x | y | z | U11 | U22 | U33 | U12 | U13 | U23 |
|---|---|---|---|---|---|---|---|---|---|---|
| Cl(1) | 4e | 0.51976(6) | 0.67648(7) | 0.19164(3) | 0.0131(3) | 0.0206(3) | 0.0080(2) | −0.0055(2) | 0.0043(2) | −0.0017(2) |
| N(1) | 4e | 0.7187(2) | 0.8003(2) | 0.3346(1) | 0.0069(8) | 0.0141(8) | 0.0120(8) | 0.0005(6) | 0.0020(7) | 0.0009(7) |
| N(2) | 4e | 0.2987(2) | 0.6011(2) | 0.3197(1) | 0.0078(8) | 0.0140(8) | 0.0092(8) | −0.0015(7) | 0.0034(7) | −0.0021(7) |
| N(3) | 4e | −0.1660(2) | 0.3525(3) | 0.4286(1) | 0.0164(9) | 0.031(1) | 0.0175(9) | −0.0093(8) | 0.0069(8) | −0.0028(8) |
| N(4) | 4e | −0.0895(2) | 0.4870(3) | 0.1578(1) | 0.0103(9) | 0.025(1) | 0.020(1) | 0.0014(8) | 0.0014(7) | 0.0023(8) |
| C(1) | 4e | 0.5709(2) | 0.7225(3) | 0.3058(1) | 0.0066(9) | 0.0098(9) | 0.0090(9) | 0.0015(7) | 0.0028(7) | 0.0012(7) |
| C(2) | 4e | 0.4524(2) | 0.6831(3) | 0.3583(1) | 0.0063(9) | 0.0101(9) | 0.0107(9) | −0.0004(7) | 0.0034(7) | −0.0003(7) |
| C(3) | 4e | 0.4957(3) | 0.7335(3) | 0.4483(1) | 0.013(1) | 0.018(1) | 0.0100(9) | −0.0024(8) | 0.0056(8) | −0.0010(8) |
| C(4) | 4e | 0.6522(3) | 0.8134(3) | 0.4799(1) | 0.014(1) | 0.021(1) | 0.0088(9) | −0.0024(9) | 0.0013(8) | −0.0015(8) |
| C(5) | 4e | 0.7601(3) | 0.8438(3) | 0.4220(1) | 0.0093(9) | 0.018(1) | 0.012(1) | −0.0025(8) | −0.0003(8) | 0.0006(8) |
| C(6) | 4e | 0.1844(2) | 0.5410(3) | 0.3654(1) | 0.0093(9) | 0.0115(9) | 0.0120(9) | 0.0009(8) | 0.0041(7) | 0.0009(7) |
| C(7) | 4e | 0.0287(3) | 0.4752(3) | 0.3278(1) | 0.0092(9) | 0.0123(9) | 0.015(1) | 0.0002(8) | 0.0046(8) | 0.0002(8) |
| C(8) | 4e | −0.0788(3) | 0.4075(3) | 0.3837(1) | 0.0099(9) | 0.018(1) | 0.014(1) | −0.0019(8) | 0.0023(8) | −0.0030(8) |
| C(9) | 4e | −0.0362(2) | 0.4800(3) | 0.2333(1) | 0.0047(9) | 0.013(1) | 0.019(1) | −0.0008(7) | 0.0034(8) | 0.0007(8) |
Source of material
Method A: a mixture of 3-amino-2-chloropyridine (1.28 g, 0.01 mol), malononitrile (0.66 g, 0.01 mol), triethylorthoformate (1.48 g, 0.01 mol), and methanol (30 mL) containing acetic acid (1 mL) was refluxed for 8 h, the reaction mixture was filtered, the filtered solid was crystallized from ethanol. Method B: a mixture of 3-amino-2-chloropyridine (1.28 g, 0.01 mol) and 2-(ethoxymethylene)malononitrile (1.22 g, 0.01 mole) in methanol (20 mL) containing acetic acid (1 mL) was refluxed for 12 h, the reaction mixture was filtered off, the filtered solid was crystallized from ethanol to give: Yield: 89%, m.p. = 193.4°C; IR: cm−1: 3215 (NH), 3079 (CH arom.), 2975, 2864 (CH aliph.), 2218 (CN), 1614 (C = N), 759 (C—Cl) 1H—NMR (DMSO-d6, ppm): 7.2, 8.0 [m, 3H, Ar—H], 8.1 [s, 1H, CH, D2O-exchangeable], 10.9 [s, 1H, NH, D2O-exchangeable]. 13C—NMR (DMSO-d6, ppm): 55.6, 113.7(2), 122.1, 124.8, 136.7, 140.3, 146.9, 160.4. Anal. Calcd. for C9H5ClN4 (204.62, %): C, 52.83; H, 2.46; N, 27.38. Found (%): C, 52.48; H, 2.13; N, 27.71.
Experimental details
The hydrogen atoms H4A, H5A and H6A were idealized and refined using a riding model (AFIX 43 option of the SHELX program [11]).
Discussion
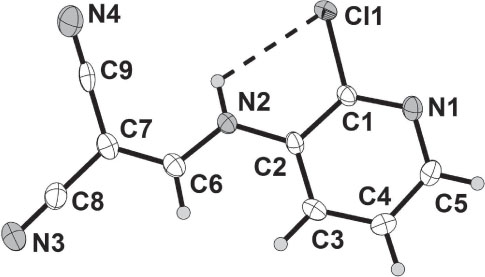
Pyridine derivatives were shown antibacterial [1], antimalarial [2], anticonvulsant [3], and cytotoxic agents [4–6]. These findings led us to continue our work on the synthesis of biologically active compounds [7–10]. The aim of this work was to, synthesize the corresponding compound having a biologically active methylenemalononitrile moiety. The crystal structure of target compound contains one molecule in the asymmetric unit. There is one intramolecular N—H⋯Cl hydrogen bond (figure). The crystal structure is stabilized by three intermolecular hydrogen bonds, of which N1, N3 and N4 work as hydrogen bond acceptors and N2, C6 and C4 work as hydrogen bond donors. The distance of the interactions between N2—H1N2⋯N1i is 2.51(3) Å, C4—H4A⋯N4ii is 2.58 Å and C6—H6A⋯N3iii is 2.4 Å and the angles are 138(3)°, 145° and 151°, respectively. Symmetry codes: (i) −x+1, y−1/2, −z+1/2; (ii) x+1, −y+3/2, z+1/2; (iii) −x, −y+1, −z+1.
Funding source: Deanship of Scientific Research
Award Identifier / Grant number: RGP-VPP-302
Funding statement: The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding of this research through the Research Group Project no. RGP-VPP-302.
Acknowledgements:
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding of this research through the Research Group Project no. RGP-VPP-302. The authors thank the responsible editor for supplying the figure.
References
1. Navin, B. P.; Suresh, N. A.; Faiyazalam, M. S.: Synthesis and antimicrobial activity of new pyridine derivatives-I. Med. Chem. Res. 20 (2011) 1033–1041.10.1007/s00044-010-9440-0Search in Google Scholar
2. Xue, J.; Diao, J.; Cai, G.; Deng, L.; Zheng, B.; Yao, Y.; Song, Y.: Antimalarial and structural studies of pyridine-containing inhibitors of 1-deoxyxylulose-5-phosphate reductoisomerase. ACS Med. Chem. Lett. 4 (2013) 278–282.10.1021/ml300419rSearch in Google Scholar PubMed PubMed Central
3. Kaminski, K.; Obniska, J.; Zagorska, A.; Maciag, D.: Synthesis, physicochemical and anticonvulsant properties of new N-(pyridine-2-yl) derivatives of 2-azaspiro[4.4]nonane and [4.5]decane-1,3-dione. Part II. Arch. Pharm. 339 (2006) 255–261.10.1002/chin.200635141Search in Google Scholar
4. Liu, M. C.; Lin, T. S.; Sartorelli, A. C.: Synthesis and antitumor activity of amino derivatives of pyridine-2-carboxaldehyde thiosemicarbazone. J. Med. Chem. 35 (1992) 3672–3677.10.1021/jm00098a012Search in Google Scholar PubMed
5. Audrey, E., Evans, T., Nathan, O. K.: Pyridine nucleotide transhydrogenase in normal human and leukemic leukocytes. J. Clin. Inves. 45 (1966) 1268–1272.10.1172/JCI105433Search in Google Scholar PubMed PubMed Central
6. Byeong-Seon, J.; Hoyoung, C.; Young-Shin, K.; Eung-Seok, L.: Synthesis of 2,4,6-tripyridyl pyridines, and evaluation of their antitumor cytotoxicity, topoisomerase I and II inhibitory activity, and structure-activity relationship. Bull. Korean Chem. Soc. 32 (2011) 3566–3570.10.5012/bkcs.2011.32.10.3566Search in Google Scholar
7. Ghorab, M. M.; Ragab, F. A.; Heiba, H. I.; Nissan, Y. M.; Ghorab, W. M: Novel brominated quinoline and pyrimidoquinoline derivatives as potential cytotoxic agents with synergistic effects of γ-radiation. Arch. Pharm. Res. 35 (2012) 1335–1346.10.1007/s12272-012-0803-6Search in Google Scholar PubMed
8. Ghorab, M. M.; Alsaid, M. S.; Nissan, Y. M: Dapson in heterocyclic chemistry, part V: synthesis, molecular docking and anticancer activity of some novel sulfonylbiscompounds carrying biologically active dihydropyridine, dihydroisoquinoline, 1,3-dithiolan, 1,3-dithian, acrylamide, pyrazole, pyrazolopyrimidine and benzochromenemoieties. Chem. Pharm. Bull. 60 (2012) 1019–1028.10.1248/cpb.c12-00292Search in Google Scholar PubMed
9. Ghorab, M. M.; Ceruso, M.; Alsaid, M. S.; Nissan, Y. M.; Arafa, R. K.; Supuran, C. T.: Novel sulfonamides bearing pyrrole and pyrrolopyrimidine moieties as carbonic anhydrase inhibitors: synthesis, cytotoxic activity and molecular modeling. Eur. J. Med. Chem. 87 (2014) 186–196.10.1016/j.ejmech.2014.09.059Search in Google Scholar PubMed
10. Ghorab, M. M., Ceruso, M., Alsaid, M. S., Nissan, Y. M., Supuran, C. T.: Carbonic anhydrase inhibitors: synthesis, molecular docking, cytotoxic and inhibition of the human carbonic anhydrase isoforms I, II, IX, XII with novel benzenesulfonamides incorporating pyrrole, pyrrolopyrimidine and fused pyrrolopyrimidine moieties. Biooorg. Med. Chem. 22 (2014) 3684–3695.10.1016/j.bmc.2014.05.009Search in Google Scholar PubMed
11. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
12. Bruker: APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA, 2009.Search in Google Scholar
©2016 Mostafa M. Ghorab et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Frontmatter
- Crystal structure of poly[aqua(μ2-2,5-bis(4-pyridyl)-1,3,4-oxadiazole-κ2N,N)(μ2-1,3-phenylenediacetato-κ3O,O′:O′′)cobalt(II)], C22H18CoN4O6
- Crystal structure of 2-amino-7,7-dimethyl-5-oxo-4-(3-phenoxy-phenyl)-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile
- Crystal structure of (E)-4,4′-(diazene-1,2-diyl)bis(1-nitro-1H-1,2,4-triazol-5(4H)-one)—acetonitrile (1:1), C6H5N11O6
- Crystal structure of potassium (E)-5-oxo-4-((5-oxo-1H-1,2,4-triazol-4(5H)-yl)diazenyl)-4,5-dihydro-1,2,4-triazol-1-ide – (E)-4,4-diazene-1,2-diylbis(2,4-dihydro-3H-1,2,4-triazol-3-one) – methanol (1/1/1), C9H11N16KO5
- Crystal structure of (N,N′-bis(2-(((2,6-diisopropylphenyl)imino)methyl)phenyl)benzene-1,2-diamido-κ4O,O′,O′′,O′′′)oxidovanadium(IV), C44H48N4OV
- Crystal structure of 5,5-bis(4-iodophenyl)-5H-cyclopenta[2,1-b:3,4-b′]dipyridine, C23H14I2N2
- Crystal structure of aquadichloridobis(1-((2-methyl-1H-imidazol-1-yl)methyl)-1H-benotriazole-κN)mercury(II), C22H24Cl2HgN10O
- Crystal structure of 2-[4-(1H-imidazol-1-yl)phenyl]-1H-benzimidazol-3-ium [2-(carboxymethyl)phenyl]acetate monohydrate, C26H24N4O5
- Crystal structure of 4,4′-bipiperidinium dichloride 0.12 hydrate, C10H22N2Cl2 · 0.12 H2O
- Crystal structure of catena-poly[tetraaqua(μ2-4,4′(E)-ethene-1,2-diyldipyridine-κ2N:N′)nickel(II)] bis(6-methyl-2-oxo-1,2-dihydro-pyridine-4-carboxylate) pentahydrate, C26H42N4O15Ni
- Crystal structure of ethyl-5-amino-1-(2,4-dinitrophenyl)-1H-pyrazole-4-carboxylate, C12H11N5O6
- Crystal structure of dicarbonyl(pyridin-2-olate-1-oxido-κ2O,O′)rhodium(I), C7H4NO4Rh
- Crystal structure of 1-(adamantan-1-yl)-3-(4-chlorophenyl)thiourea, C17H21ClN2S
- Crystal structure of 2-((2-chloropyridin-3-ylamino)methylene)malononitrile, C9H5ClN4
- Crystal structure of tetraaquabis(μ2-4-chlorobenzoato-κ2O:O,O′)bis(4-chlorobenzoato-κO)bis(1,10-phenanthroline-κ2N,N′)distrontium(II), C52H40Cl4N4O12Sr2
- Crystal structure of 3-hydroxy-3-phenyl-1,3-dihydro-2H-indol-2-one, C14H11NO2
- Crystal structures of bis(1,10-phenanthrolin-1-ium) aquapentakis(nitrato-κ2O,O′)neodym(III) monohydrate, C24H22N9NdO17
- Crystal structure of 2-ethyl-1-tert-butyl 3-oxo-2-[phenyl(tert-butoxycarbonylamino)methyl]-1,2-pyrrolidinedicarboxylate, C24H34N2O7
- Crystal structure of poly[diaquabis(μ4-benzene-1,3,5-tricarboxylato-κO1,κO2:κO3,κO4:κO5:κO6)-bis(μ2-4,4′-benzene-1,3-diylbis(4H-1,2,4-triazole-κ2N:N′)tricadmium(II)] tetrahydrate, C38H34Cd3N12O18
- Crystal structure of trans-dichlorido[1,3-bis(9-methyl-9H-fluoren-9-yl) benzimidazol-2-ylidene](pyridine)palladium(II) – a compound with anagostic CH–Pd interactions, C40H31Cl2N3Pd
- Crystal structure of catena-poly[(μ3-5-(4-(tetrazol-1-id-5-yl)phenoxy)benzene-1,3-dicarboxyato-κ3O:O′:N)(4-(3-(pyridin-4-yl)propyl)pyridinium-κN)zinc(II)], C28H22ZnN6O5
- Crystal structure of 3,6-di-2-pyridinyl-4-pyridazine carbonitrile, C15H9N5
- Crystal structure of 5,5,9,13-tetramethyltetracyclo[10·2·1·01,10·04,9]pentadecane-3,7,14-triol, C20H34O4
- Crystal structure of 2-amino-7-methyl-5-oxo-4-phenyl-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H12N2O3
- Crystal structure of the poly[(1,10-phenanthroline-κ2N,N′)(μ3-carboxylatophenoxyacetato-κ4O,O′:O′′;O′′′)lead(II)] monohydrate, C21H16N2O6Pb
- Crystal structure of the poly[(μ4-biphenyl-4,4′-dicarboxylato-κ4O:O′:O′′:O′′′) bis(μ3-8-(11-(oxysulfonyl)-4-silbenyl)-2-(oxysulfonyl)stilbene-κ4O:O′:O′′,O′′′) bis(1,10-phenanthroline-k2N,N′) dipraseodymium(III)], C94H64N4O16Pr2S4
- Crystal structure of 3-iodo-5-methoxy-7-(methoxymethoxy)-4-(3-methoxyphenoxy)-2H-chromen-2-one, C19H17IO7
- Crystal structure of poly[(5-carboxy-2,6-dimethylpyridinium-3-carboxylato-κO)tris(μ2-2,6-dimethylpyridinium-3,5-dicarboxylato-κ3O,O′:O′′)erbium(III)], C36H33ErN4O16
- This molecule targets at type 2 diabetes - a single crystal study on (2R,3S,5R)-2-(2,5-difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl] tetrahydro-2H-pyran-3-amine (Omarigliptin), C17H20F2N4O3S
- Crystal structure of poly[(μ2-2,2′-benzene-1,2-diyldiacetato-κ2O:O′), (μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′)zinc(II)], C22H19N5O4Zn
- Crystal structure of tetraaqua(μ2-3-(3,5-dicarboxyphenoxy)benzene-1,2-dicarboxylato-κ2O:O′)manganese(II) dihydrate, C16H20O15Mn
- Crystal structure of 4,4′-sulfonyldipyridine, C10H8N2O2S
- Crystal structure of 4-[(E)-(2-chloro-6-fluorobenzylidene)amino]-1,2-dihydro-2,3-dimethyl-1-phenylpyrazol-5-one, C18H15ClFN3O
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O, O′)(triphenylphosphine-κP)rhodium(I), C24H19NO3PRh
- Crystal structure of diaquabis(2-(3-bromophenyl)-5-carboxy-1H-imidazol-4-carboxylato-κ2O,N) cobalt(II) trihydrate, C22H22Br2CoN4O13
- Crystal structure of 4-(pyridin-4-ylmethylsulfonyl)pyridine, C11H10N2O2S
- Crystal structure of poly[(μ4-1-methyl-1H-tetrazole-5-thiolato-κ3S:S:N:N′)copper(I)], C2H3CuN4S
- Crystal structure of poly[diaquabis(μ2-4,4′-sulfinyldipyridine-κ2N,N′)zinc(II)] diperchlorate dihydrate, C20H24N4O14S2Cl2Zn
- Crystal structure of (1-((1-benzylpyrrolidin-2-yl-κN)methyl)-3-isopropyl-1H-imidazol-2(3H)-ylidene–κC)dibromidopalladium(II), C18H25Br2N3Pd
- Crystal structure of pentacalcium tetranitridovanadate(V) mononitride based on a powder diffraction study, Ca5[VN4]N
- Crystal structure of ((1-((1-benzylpyrrolidin-2-yl)methyl)-3-ethyl-1H-imidazol-2(3H)-ylidene)-κ2C,N)dichloridopalladium(II), C17H23Cl2N3Pd
- Crystal structure of 9-allyl-4,5-dichloro-12-cyano-9,10-dihydro-9,10-ethanoanthracen-12-yl acetate, C22H17Cl2NO2
- Crystal structure of 4-bromo-2-(8-(3-ethoxy-2-hydroxyphenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenol, C18H19BrN2O5
- Crystal structure of 3-tert-butyl-3-hydroxy-1,3-dihydro-2H-pyrrolo[3,2-c]pyridin-2-one, C11H14N2O2
- Crystal structure of diaquabis(3-(3,5-dibromophenyl)-5-(pyridin-2-yl)-1,2,4-triazol-4-ido-κ2N,N′)nickel(II) mono hydrate, C26H20Br4N8NiO3
- Crystal structure of 5-(adamantan-1-yl)-3-[(2-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2-ethyl-1-tert-butyl-2-((4-fluorophenyl)(tert-butoxycarbonylamino)methyl)-3-oxo-pyrrolidine-1,2-dicarboxylate, C24H33FN2O7
- Crystal structure of bis(μ2-2-fluorobenzoato-κ2O:O:O′) bis(μ2-2-fluorobenzoato-κ2O:O′)dinitrato-κ2O,O′ bis(1,10-phenathroline-κ2N,N′)diterbium(III), C52H32F4N6O14Tb2
- Crystal structure of tetrabutylammonium 4-aminobenzenesulfonate 2/3 hydrate, C22H42N2O3S · 2/3 H2O
- Crystal structure of tetraethylammonium 4-aminobenzenesulfonate, C14H26N2O3S
- Crystal structure of bis(guanidinium) 3,3′-oxybis(6-carboxybenzoate), C18H20N6O9
- Crystal structure of N′-(4-methoxybenzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide, C18H16N4O2
- Crystal structure of N′-(4-nitrobenzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide, C17H13N5O3
- Crystal structure of 5-((4-bromophenyl)(2-hydroxy-6-oxocyclohex-1-en-1-yl)methyl)-6-hydroxy-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, C19H19BrN2O5
- Crystal structure of 3-(((cyclohexyl(phenyl)methylidene)amino)oxy)-2-hydroxy-N-(propan-2-yl)propan-1-aminium chloride, C19H31ClN2O2
- Crystal structure of 6-hydroxy-5-((2-hydroxy-6-oxocyclohex-1-en-1-yl)(phenyl)methyl)-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, C19H20N2O5
- Crystal structure of 2-(4-(4-bromophenyl)thiazol-2-yl)isoindoline-1,3-dione, C17H9BrN2O2S
- Crystal structure of 2-(4-methylbenzoyl)pyrene, C24H16O
- Crystal structure of N-(5-bromo-4-(p-tolyl)thiazol-2-yl)-4-chlorobutanamide, C14H14BrClN2OS
- Crystal structure of 4,5-diphenylthiazol-2-amine, C15H12N2S
- Crystal structure of poly[bis(μ2-4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoato-κ3N:O,O′)-lead(II)], C28H18O4N8Pb
- Crystal structure of (Z)-4-(4-oxopent-2-en-2-ylamino)benzenesulfonamide, C11H14N2O3S
- Crystal structure of poly[(μ2-2-methyl-1-(4-(2-methyl-2H-benzo[d] imidazol-1(7aH)-yl)butyl)-1H-benzo[d]imidazole-κ2N:N′)bis(μ3-5-tert-butylbenzene-1,3-dicarboxylato-κ4O:O,O′:O′′,O′′′)dicadmium(II)] tetrahydrate, C44H54Cd2N4O12
- Crystal structure of catena-poly-[aqua(μ2-4,4′-bipyridine-κ2N:N′)bis(3′,5,5′-tricarboxybiphenyl-2-carboxylato-κ2O,O′)cadmium(II)], C42H28N2O17Cd
- Crystal structure of (2RS,3RS)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl)pentan-3-ol, C15H20ClN3O
- Crystal structure of N′-(4-(dimethylamino)benzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide, C19H19N5O
- Crystal structure of 3-(benzofuran-2-yl)-5-(4-fluorophenyl)-4,5-dihydro-1Hpyrazole-1-carbothioamide, C18H14FN3OS
- Crystal structure of ent-1β-acetoxy-7α,14α-di-hydroxy-7β,20-epoxykaur-16-en-15-one, C22H30O6
- Crystal structure of 1α,7β-dihydroxy-11β-acetoxy-ent-7β,20-epoxykaur-16-en-15-one, C22H30O6
- Crystal structure of 7β,14β,15β-trihydroxy-1α-acetoxy-7α,20-epoxy-ent-kaurane, C22H32O6
- Crystal structure of ent-1β,7α,11α-trihydroxy-7β,20-epoxykaur-16-en-15-one, C20H28O5
- Crystal structure of poly-[tetraaquabis(μ8-benzene-1,2,4,5-tetracarboxylato-1κ3O4:O6:O8:2κ4O2:O2:O5:O5:3κ4O1:O3:O5:O7)(di-μ3-hydroxido)-pentazinc(II)] decahydrate, C20H34O32Zn5
- Crystal structure of 1-(4-methylthiazol-2-yl)-3-propylthiourea, C8H13N3S2
- Crystal structure of 2-((dimethylamino)methylene)-5,5-Dimethylcyclohexane-1,3-dione, C11H17NO2
- Crystal structure of poly[1,4-bis(2-methylbenzimidazol)butane-κ2N:N′)bis(4,4′-oxybis(benzoato-κ4O,O′:O′′,O′′′)dicadmium] monohydrate, C48H38Cd2N4O10
- Crystal structure of 2-(3-(benzofuran-2-yl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-4-(4-chlorophenyl)thiazole, C26H17ClFN3OS
- Crystal structure of bis(μ3-isophthalato-κ3O:O′:O′′)(μ2-1,4-\ bis((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)dizinc(II), C22H19N2O4Zn
- Crystal structure of poly[bis(adipate-κ4O,O′:O′′, O′′′)(1,4-bis(2-methyl-1H-benzo[d]imidazol-1-yl)benzene-κ2N:N′)dizinc(II), C36H38N4O8Zn2
- Crystal structure of N-(2-(2-oxoindolin-4-yl)ethyl)-N-propylpropan-1-aminium tetraphenylborate, C40H45BN2O
- Crystal structure of 1-(2,3-dihydro-4-methyl-3-phenyl-2-thioxothiazol-5-yl)-1-ethanone, C12H11NOS2
- Crystal structure of 3-(adamantan-1-yl)-1-[(4-benzylpiperazin-1-yl)methyl]-4-[(E)-(2,6-difluorobenzylidene)amino]-1H-1,2,4-triazole-5(4H)-thione, C31H36F2N6S
- Crystal structure of 6-(4-chlorophenyl)-3-(thiophen-2-yl)-[1,2,4]triazolo[3,4-b][1,3,4]-thiadiazole, C13H7ClN4S2
- Crystal structure of bis(ethanaminium) poly[bis(hexaselenido-κ2Se1,Se6)palladate(II)], C4H16N2PdSe12
- Crystal structure of 2-(3-(benzofuran-2-yl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl)-4-phenylthiazole, C26H19N3OS
- Crystal structure of poly-[bis(μ3-5-hydroxyisophtalato-κ3O:O′:O′′)(μ2-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)benzene-κ2N:N′)dizinc(II)], C40H30N4O5Zn2
- Crystal structure of poly-[(μ-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)benzene-κ2N:N′)-bis(μ4-2,2′-(1,3-phenylene)diacetate-κ4O:O′:O′′:O′′′)dizinc(II)], C44H38N4O8Zn2
- Crystal structure of 5,17-bis-cyano-25,26,27,28-tetrapropyloxy-calix[4]arene, C42H46N2O4
- Crystal structure of poly-[μ-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)benzene-κ2N:N′)-bis(μ3-5-hydroxyisophthalate(2–)-κ3O,O′:O′′)dicadmium(II)] monohydrate, C64H54N8O11Cd2
- Crystal structure of poly-[μ-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)benzene-κ2N:N′)-bis(μ4-4,4′-sulfonyldibenzoato-κ4O:O′:O′′:O′′′)dicadmium(II)] monohydrate, C52H42N4O14Cd2
- Crystal structure of poly-[bis(μ4-adipato-κ4O:O′:O′′:O′′′)(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)dizinc(II)], C38H42N4O8Zn2
- Crystal structure of (2-(2-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- Crystal structure of 2-(4-Bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylic acid, C13H15BrO4
- Crystal structure of (2-(4-bromophenyl)-5-ethyl-1,3-dioxan-5-yl)methanol, C13H17BrO3
- Crystal structure of 3-((1,3,5,7-tetraoxo-6-(pyridin-3-ylmethyl)-3,3a,4,4a,5,6,7,7a,8,8a-decahydro-4,8-ethenopyrrolo[3,4-f]isoindol-2(1H)-yl)methyl)pyridin-1-ium-κN-trichloridocobalt(II) hemihydrate, C24H22Cl3CoN4O4.5
- Crystal structure of 4-chloro-4′,6′-dichloro-2,2′-[propane-1,3-diyldioxybis(nitrilomethylidyne)]-diphenol, C17H14Cl3N2O4
- Crystal structure of 2-amino-4-(3-trifluoromethylphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C17H13F3N2O2
- Crystal structure of bis(benzoato-κO)bis(4,4′-((1H-1,2,4-triazol-1-yl)methylene)dibenzonitrile-κN)zinc(II), C48H32N10O4Zn
- Crystal structure of 3-methyl-2H-chromen-2-one, C10H8O2
- Crystal structure of catena-poly-[aqua-(2-carboxy-5-(3-carboxy-5-carboxylatophenoxy)benzoato-κO)(μ2-4,4′-di(1H-imidazol-1-yl)-1,1′-biphenyl-κ2N:N′)cobalt(II)], C34H24N4O10Co
- Crystal structure of 4,4′-di(1H-imidazol-1-yl)-1,1′-biphenyl, C36H28N8
- Crystal structure of 1-(3-chloropropyl)piperidin-1-ium tetraphenylborate, C32H37BClN
- Crystal structure of dimethyl 5-(benzylamino)isophthalate, C17H17NO4
- Crystal structure of dimethyl 5-(dibenzylamino)isophthalate, C24H23NO4
- Crystal structure of N-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioamide, C21H29N3S
- Crystal structure of (2,2′(cyclohexane-1,2-diylbis(nitrilo(E)methylylidene))diphenolato-κ4O,O′,N,N′)dimethanolmanganese(III) bromide, C22H28BrMnN2O4
- Crystal structure of 3,5,7-tris(morpholinomethyl)tropolone·0.67 hydrate, C22H33N3O5·0.67H2O
- Crystal structure of biphenyl-2,3′,5,5′-tetracarboxylic acid – 4,4′-biphenyl-4,4′-diyldipyridine (3/2), C49H34N3O8
Articles in the same Issue
- Frontmatter
- Crystal structure of poly[aqua(μ2-2,5-bis(4-pyridyl)-1,3,4-oxadiazole-κ2N,N)(μ2-1,3-phenylenediacetato-κ3O,O′:O′′)cobalt(II)], C22H18CoN4O6
- Crystal structure of 2-amino-7,7-dimethyl-5-oxo-4-(3-phenoxy-phenyl)-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile
- Crystal structure of (E)-4,4′-(diazene-1,2-diyl)bis(1-nitro-1H-1,2,4-triazol-5(4H)-one)—acetonitrile (1:1), C6H5N11O6
- Crystal structure of potassium (E)-5-oxo-4-((5-oxo-1H-1,2,4-triazol-4(5H)-yl)diazenyl)-4,5-dihydro-1,2,4-triazol-1-ide – (E)-4,4-diazene-1,2-diylbis(2,4-dihydro-3H-1,2,4-triazol-3-one) – methanol (1/1/1), C9H11N16KO5
- Crystal structure of (N,N′-bis(2-(((2,6-diisopropylphenyl)imino)methyl)phenyl)benzene-1,2-diamido-κ4O,O′,O′′,O′′′)oxidovanadium(IV), C44H48N4OV
- Crystal structure of 5,5-bis(4-iodophenyl)-5H-cyclopenta[2,1-b:3,4-b′]dipyridine, C23H14I2N2
- Crystal structure of aquadichloridobis(1-((2-methyl-1H-imidazol-1-yl)methyl)-1H-benotriazole-κN)mercury(II), C22H24Cl2HgN10O
- Crystal structure of 2-[4-(1H-imidazol-1-yl)phenyl]-1H-benzimidazol-3-ium [2-(carboxymethyl)phenyl]acetate monohydrate, C26H24N4O5
- Crystal structure of 4,4′-bipiperidinium dichloride 0.12 hydrate, C10H22N2Cl2 · 0.12 H2O
- Crystal structure of catena-poly[tetraaqua(μ2-4,4′(E)-ethene-1,2-diyldipyridine-κ2N:N′)nickel(II)] bis(6-methyl-2-oxo-1,2-dihydro-pyridine-4-carboxylate) pentahydrate, C26H42N4O15Ni
- Crystal structure of ethyl-5-amino-1-(2,4-dinitrophenyl)-1H-pyrazole-4-carboxylate, C12H11N5O6
- Crystal structure of dicarbonyl(pyridin-2-olate-1-oxido-κ2O,O′)rhodium(I), C7H4NO4Rh
- Crystal structure of 1-(adamantan-1-yl)-3-(4-chlorophenyl)thiourea, C17H21ClN2S
- Crystal structure of 2-((2-chloropyridin-3-ylamino)methylene)malononitrile, C9H5ClN4
- Crystal structure of tetraaquabis(μ2-4-chlorobenzoato-κ2O:O,O′)bis(4-chlorobenzoato-κO)bis(1,10-phenanthroline-κ2N,N′)distrontium(II), C52H40Cl4N4O12Sr2
- Crystal structure of 3-hydroxy-3-phenyl-1,3-dihydro-2H-indol-2-one, C14H11NO2
- Crystal structures of bis(1,10-phenanthrolin-1-ium) aquapentakis(nitrato-κ2O,O′)neodym(III) monohydrate, C24H22N9NdO17
- Crystal structure of 2-ethyl-1-tert-butyl 3-oxo-2-[phenyl(tert-butoxycarbonylamino)methyl]-1,2-pyrrolidinedicarboxylate, C24H34N2O7
- Crystal structure of poly[diaquabis(μ4-benzene-1,3,5-tricarboxylato-κO1,κO2:κO3,κO4:κO5:κO6)-bis(μ2-4,4′-benzene-1,3-diylbis(4H-1,2,4-triazole-κ2N:N′)tricadmium(II)] tetrahydrate, C38H34Cd3N12O18
- Crystal structure of trans-dichlorido[1,3-bis(9-methyl-9H-fluoren-9-yl) benzimidazol-2-ylidene](pyridine)palladium(II) – a compound with anagostic CH–Pd interactions, C40H31Cl2N3Pd
- Crystal structure of catena-poly[(μ3-5-(4-(tetrazol-1-id-5-yl)phenoxy)benzene-1,3-dicarboxyato-κ3O:O′:N)(4-(3-(pyridin-4-yl)propyl)pyridinium-κN)zinc(II)], C28H22ZnN6O5
- Crystal structure of 3,6-di-2-pyridinyl-4-pyridazine carbonitrile, C15H9N5
- Crystal structure of 5,5,9,13-tetramethyltetracyclo[10·2·1·01,10·04,9]pentadecane-3,7,14-triol, C20H34O4
- Crystal structure of 2-amino-7-methyl-5-oxo-4-phenyl-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H12N2O3
- Crystal structure of the poly[(1,10-phenanthroline-κ2N,N′)(μ3-carboxylatophenoxyacetato-κ4O,O′:O′′;O′′′)lead(II)] monohydrate, C21H16N2O6Pb
- Crystal structure of the poly[(μ4-biphenyl-4,4′-dicarboxylato-κ4O:O′:O′′:O′′′) bis(μ3-8-(11-(oxysulfonyl)-4-silbenyl)-2-(oxysulfonyl)stilbene-κ4O:O′:O′′,O′′′) bis(1,10-phenanthroline-k2N,N′) dipraseodymium(III)], C94H64N4O16Pr2S4
- Crystal structure of 3-iodo-5-methoxy-7-(methoxymethoxy)-4-(3-methoxyphenoxy)-2H-chromen-2-one, C19H17IO7
- Crystal structure of poly[(5-carboxy-2,6-dimethylpyridinium-3-carboxylato-κO)tris(μ2-2,6-dimethylpyridinium-3,5-dicarboxylato-κ3O,O′:O′′)erbium(III)], C36H33ErN4O16
- This molecule targets at type 2 diabetes - a single crystal study on (2R,3S,5R)-2-(2,5-difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl] tetrahydro-2H-pyran-3-amine (Omarigliptin), C17H20F2N4O3S
- Crystal structure of poly[(μ2-2,2′-benzene-1,2-diyldiacetato-κ2O:O′), (μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′)zinc(II)], C22H19N5O4Zn
- Crystal structure of tetraaqua(μ2-3-(3,5-dicarboxyphenoxy)benzene-1,2-dicarboxylato-κ2O:O′)manganese(II) dihydrate, C16H20O15Mn
- Crystal structure of 4,4′-sulfonyldipyridine, C10H8N2O2S
- Crystal structure of 4-[(E)-(2-chloro-6-fluorobenzylidene)amino]-1,2-dihydro-2,3-dimethyl-1-phenylpyrazol-5-one, C18H15ClFN3O
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O, O′)(triphenylphosphine-κP)rhodium(I), C24H19NO3PRh
- Crystal structure of diaquabis(2-(3-bromophenyl)-5-carboxy-1H-imidazol-4-carboxylato-κ2O,N) cobalt(II) trihydrate, C22H22Br2CoN4O13
- Crystal structure of 4-(pyridin-4-ylmethylsulfonyl)pyridine, C11H10N2O2S
- Crystal structure of poly[(μ4-1-methyl-1H-tetrazole-5-thiolato-κ3S:S:N:N′)copper(I)], C2H3CuN4S
- Crystal structure of poly[diaquabis(μ2-4,4′-sulfinyldipyridine-κ2N,N′)zinc(II)] diperchlorate dihydrate, C20H24N4O14S2Cl2Zn
- Crystal structure of (1-((1-benzylpyrrolidin-2-yl-κN)methyl)-3-isopropyl-1H-imidazol-2(3H)-ylidene–κC)dibromidopalladium(II), C18H25Br2N3Pd
- Crystal structure of pentacalcium tetranitridovanadate(V) mononitride based on a powder diffraction study, Ca5[VN4]N
- Crystal structure of ((1-((1-benzylpyrrolidin-2-yl)methyl)-3-ethyl-1H-imidazol-2(3H)-ylidene)-κ2C,N)dichloridopalladium(II), C17H23Cl2N3Pd
- Crystal structure of 9-allyl-4,5-dichloro-12-cyano-9,10-dihydro-9,10-ethanoanthracen-12-yl acetate, C22H17Cl2NO2
- Crystal structure of 4-bromo-2-(8-(3-ethoxy-2-hydroxyphenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)phenol, C18H19BrN2O5
- Crystal structure of 3-tert-butyl-3-hydroxy-1,3-dihydro-2H-pyrrolo[3,2-c]pyridin-2-one, C11H14N2O2
- Crystal structure of diaquabis(3-(3,5-dibromophenyl)-5-(pyridin-2-yl)-1,2,4-triazol-4-ido-κ2N,N′)nickel(II) mono hydrate, C26H20Br4N8NiO3
- Crystal structure of 5-(adamantan-1-yl)-3-[(2-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2-ethyl-1-tert-butyl-2-((4-fluorophenyl)(tert-butoxycarbonylamino)methyl)-3-oxo-pyrrolidine-1,2-dicarboxylate, C24H33FN2O7
- Crystal structure of bis(μ2-2-fluorobenzoato-κ2O:O:O′) bis(μ2-2-fluorobenzoato-κ2O:O′)dinitrato-κ2O,O′ bis(1,10-phenathroline-κ2N,N′)diterbium(III), C52H32F4N6O14Tb2
- Crystal structure of tetrabutylammonium 4-aminobenzenesulfonate 2/3 hydrate, C22H42N2O3S · 2/3 H2O
- Crystal structure of tetraethylammonium 4-aminobenzenesulfonate, C14H26N2O3S
- Crystal structure of bis(guanidinium) 3,3′-oxybis(6-carboxybenzoate), C18H20N6O9
- Crystal structure of N′-(4-methoxybenzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide, C18H16N4O2
- Crystal structure of N′-(4-nitrobenzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide, C17H13N5O3
- Crystal structure of 5-((4-bromophenyl)(2-hydroxy-6-oxocyclohex-1-en-1-yl)methyl)-6-hydroxy-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, C19H19BrN2O5
- Crystal structure of 3-(((cyclohexyl(phenyl)methylidene)amino)oxy)-2-hydroxy-N-(propan-2-yl)propan-1-aminium chloride, C19H31ClN2O2
- Crystal structure of 6-hydroxy-5-((2-hydroxy-6-oxocyclohex-1-en-1-yl)(phenyl)methyl)-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, C19H20N2O5
- Crystal structure of 2-(4-(4-bromophenyl)thiazol-2-yl)isoindoline-1,3-dione, C17H9BrN2O2S
- Crystal structure of 2-(4-methylbenzoyl)pyrene, C24H16O
- Crystal structure of N-(5-bromo-4-(p-tolyl)thiazol-2-yl)-4-chlorobutanamide, C14H14BrClN2OS
- Crystal structure of 4,5-diphenylthiazol-2-amine, C15H12N2S
- Crystal structure of poly[bis(μ2-4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoato-κ3N:O,O′)-lead(II)], C28H18O4N8Pb
- Crystal structure of (Z)-4-(4-oxopent-2-en-2-ylamino)benzenesulfonamide, C11H14N2O3S
- Crystal structure of poly[(μ2-2-methyl-1-(4-(2-methyl-2H-benzo[d] imidazol-1(7aH)-yl)butyl)-1H-benzo[d]imidazole-κ2N:N′)bis(μ3-5-tert-butylbenzene-1,3-dicarboxylato-κ4O:O,O′:O′′,O′′′)dicadmium(II)] tetrahydrate, C44H54Cd2N4O12
- Crystal structure of catena-poly-[aqua(μ2-4,4′-bipyridine-κ2N:N′)bis(3′,5,5′-tricarboxybiphenyl-2-carboxylato-κ2O,O′)cadmium(II)], C42H28N2O17Cd
- Crystal structure of (2RS,3RS)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl)pentan-3-ol, C15H20ClN3O
- Crystal structure of N′-(4-(dimethylamino)benzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide, C19H19N5O
- Crystal structure of 3-(benzofuran-2-yl)-5-(4-fluorophenyl)-4,5-dihydro-1Hpyrazole-1-carbothioamide, C18H14FN3OS
- Crystal structure of ent-1β-acetoxy-7α,14α-di-hydroxy-7β,20-epoxykaur-16-en-15-one, C22H30O6
- Crystal structure of 1α,7β-dihydroxy-11β-acetoxy-ent-7β,20-epoxykaur-16-en-15-one, C22H30O6
- Crystal structure of 7β,14β,15β-trihydroxy-1α-acetoxy-7α,20-epoxy-ent-kaurane, C22H32O6
- Crystal structure of ent-1β,7α,11α-trihydroxy-7β,20-epoxykaur-16-en-15-one, C20H28O5
- Crystal structure of poly-[tetraaquabis(μ8-benzene-1,2,4,5-tetracarboxylato-1κ3O4:O6:O8:2κ4O2:O2:O5:O5:3κ4O1:O3:O5:O7)(di-μ3-hydroxido)-pentazinc(II)] decahydrate, C20H34O32Zn5
- Crystal structure of 1-(4-methylthiazol-2-yl)-3-propylthiourea, C8H13N3S2
- Crystal structure of 2-((dimethylamino)methylene)-5,5-Dimethylcyclohexane-1,3-dione, C11H17NO2
- Crystal structure of poly[1,4-bis(2-methylbenzimidazol)butane-κ2N:N′)bis(4,4′-oxybis(benzoato-κ4O,O′:O′′,O′′′)dicadmium] monohydrate, C48H38Cd2N4O10
- Crystal structure of 2-(3-(benzofuran-2-yl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-4-(4-chlorophenyl)thiazole, C26H17ClFN3OS
- Crystal structure of bis(μ3-isophthalato-κ3O:O′:O′′)(μ2-1,4-\ bis((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)dizinc(II), C22H19N2O4Zn
- Crystal structure of poly[bis(adipate-κ4O,O′:O′′, O′′′)(1,4-bis(2-methyl-1H-benzo[d]imidazol-1-yl)benzene-κ2N:N′)dizinc(II), C36H38N4O8Zn2
- Crystal structure of N-(2-(2-oxoindolin-4-yl)ethyl)-N-propylpropan-1-aminium tetraphenylborate, C40H45BN2O
- Crystal structure of 1-(2,3-dihydro-4-methyl-3-phenyl-2-thioxothiazol-5-yl)-1-ethanone, C12H11NOS2
- Crystal structure of 3-(adamantan-1-yl)-1-[(4-benzylpiperazin-1-yl)methyl]-4-[(E)-(2,6-difluorobenzylidene)amino]-1H-1,2,4-triazole-5(4H)-thione, C31H36F2N6S
- Crystal structure of 6-(4-chlorophenyl)-3-(thiophen-2-yl)-[1,2,4]triazolo[3,4-b][1,3,4]-thiadiazole, C13H7ClN4S2
- Crystal structure of bis(ethanaminium) poly[bis(hexaselenido-κ2Se1,Se6)palladate(II)], C4H16N2PdSe12
- Crystal structure of 2-(3-(benzofuran-2-yl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl)-4-phenylthiazole, C26H19N3OS
- Crystal structure of poly-[bis(μ3-5-hydroxyisophtalato-κ3O:O′:O′′)(μ2-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)benzene-κ2N:N′)dizinc(II)], C40H30N4O5Zn2
- Crystal structure of poly-[(μ-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)benzene-κ2N:N′)-bis(μ4-2,2′-(1,3-phenylene)diacetate-κ4O:O′:O′′:O′′′)dizinc(II)], C44H38N4O8Zn2
- Crystal structure of 5,17-bis-cyano-25,26,27,28-tetrapropyloxy-calix[4]arene, C42H46N2O4
- Crystal structure of poly-[μ-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)benzene-κ2N:N′)-bis(μ3-5-hydroxyisophthalate(2–)-κ3O,O′:O′′)dicadmium(II)] monohydrate, C64H54N8O11Cd2
- Crystal structure of poly-[μ-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)benzene-κ2N:N′)-bis(μ4-4,4′-sulfonyldibenzoato-κ4O:O′:O′′:O′′′)dicadmium(II)] monohydrate, C52H42N4O14Cd2
- Crystal structure of poly-[bis(μ4-adipato-κ4O:O′:O′′:O′′′)(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)dizinc(II)], C38H42N4O8Zn2
- Crystal structure of (2-(2-chlorophenyl)-5-methyl-1,3-dioxan-5-yl)methanol, C12H15ClO3
- Crystal structure of 2-(4-Bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylic acid, C13H15BrO4
- Crystal structure of (2-(4-bromophenyl)-5-ethyl-1,3-dioxan-5-yl)methanol, C13H17BrO3
- Crystal structure of 3-((1,3,5,7-tetraoxo-6-(pyridin-3-ylmethyl)-3,3a,4,4a,5,6,7,7a,8,8a-decahydro-4,8-ethenopyrrolo[3,4-f]isoindol-2(1H)-yl)methyl)pyridin-1-ium-κN-trichloridocobalt(II) hemihydrate, C24H22Cl3CoN4O4.5
- Crystal structure of 4-chloro-4′,6′-dichloro-2,2′-[propane-1,3-diyldioxybis(nitrilomethylidyne)]-diphenol, C17H14Cl3N2O4
- Crystal structure of 2-amino-4-(3-trifluoromethylphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C17H13F3N2O2
- Crystal structure of bis(benzoato-κO)bis(4,4′-((1H-1,2,4-triazol-1-yl)methylene)dibenzonitrile-κN)zinc(II), C48H32N10O4Zn
- Crystal structure of 3-methyl-2H-chromen-2-one, C10H8O2
- Crystal structure of catena-poly-[aqua-(2-carboxy-5-(3-carboxy-5-carboxylatophenoxy)benzoato-κO)(μ2-4,4′-di(1H-imidazol-1-yl)-1,1′-biphenyl-κ2N:N′)cobalt(II)], C34H24N4O10Co
- Crystal structure of 4,4′-di(1H-imidazol-1-yl)-1,1′-biphenyl, C36H28N8
- Crystal structure of 1-(3-chloropropyl)piperidin-1-ium tetraphenylborate, C32H37BClN
- Crystal structure of dimethyl 5-(benzylamino)isophthalate, C17H17NO4
- Crystal structure of dimethyl 5-(dibenzylamino)isophthalate, C24H23NO4
- Crystal structure of N-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioamide, C21H29N3S
- Crystal structure of (2,2′(cyclohexane-1,2-diylbis(nitrilo(E)methylylidene))diphenolato-κ4O,O′,N,N′)dimethanolmanganese(III) bromide, C22H28BrMnN2O4
- Crystal structure of 3,5,7-tris(morpholinomethyl)tropolone·0.67 hydrate, C22H33N3O5·0.67H2O
- Crystal structure of biphenyl-2,3′,5,5′-tetracarboxylic acid – 4,4′-biphenyl-4,4′-diyldipyridine (3/2), C49H34N3O8

