Abstract
The present study explores the structural, phase stability, mechanical, and electrical properties of Mg–Ho intermetallic phases, namely Mg24Ho5, Mg2Ho, and MgHo. The investigation is conducted using the first-principles plane-wave pseudopotential method within the framework of density functional theory, as implemented in the Vienna Ab initio Simulation Package. The primary objective of this research is to illuminate the phase stability and mechanical behavior of these compounds, which are of paramount importance for their potential applications in magnesium alloys. The study determines the formation enthalpy (ΔH) and elastic constants (C ij ) for each intermetallic phase and calculates the elastic moduli of the corresponding polycrystalline materials. The findings of this study reveal that the MgHo phase exhibits the highest absolute value of formation enthalpy (ΔH = −8.01 kJ·mol−1), indicating its superior stability among the three investigated intermetallic phases. As the concentration of Ho in Mg increases, the G/B ratio for the phases decreases from 1.02 to 0.60 (>0.57), suggesting that the intermetallic phases are stable, albeit brittle. The elastic anisotropy index (A U), derived from the elastic constants (C ij ), follows an ascending order of Mg24Ho5, Mg2Ho, and MgHo, signifying that MgHo possesses the most favorable elastic anisotropy among the studied phases.
1 Introduction
Magnesium and its alloys are the lightest structural materials with advantages including low density, high strength, good machinability, and stable dimensional accuracy. They are widely used in aerospace, automotive, and biomedical applications [1,2]. Nonetheless, at elevated temperatures, the mechanical properties of magnesium alloys degrade, resulting in a substantial reduction in strength and creep resistance as temperature rises. These limitations significantly impede their further advancement and application. Rare earth elements have been used in magnesium alloys to improve their mechanical properties, casting characteristics, and welding attributes [3]. Holmium (Ho) has improved the mechanical properties and corrosion resistance of magnesium alloys [4], making them suitable for a wide range of marine engineering applications. These alloys can be effectively utilized in offshore platform structures, hull structural components, and the casings of detectors. As an added element in magnesium alloy, Ho will combine with magnesium to form alloy phases such as MgHo, Mg2Ho, and Mg24Ho5. The distributions, particle sizes, and properties of the intermetallic phases would exhibit significant influence on the corrosion and mechanical properties of Mg alloys. Thus, it is essential to comprehensively investigate the properties of the Mg–Ho phases for the development of new Mg alloys.
Density functional theory (DFT), as a precise computational theory, has gained widespread acceptance for secondary phase calculations and has become a cornerstone technology in materials science computation. Currently, the development of Mg alloys is insufficient on the first-principles methods to predict the properties of Mg alloys. Existing studies have primarily focused on investigating the enthalpy of formation, density of states, and elastic properties of the MgHo phase [5]. This computational approach significantly reduces the time required for scientific research.
In this article, the enthalpy of formation and electronic properties of Mg–Ho intermetallic phases was investigated by DFT. In addition, the DFT was used to calculate the elastic constants and further obtain the bulk modulus, shear modulus, Young’s modulus, and Poisson’s ratio. These calculation results allowed for the successful prediction of the mechanical properties of the Mg–Ho alloy phase. The predictive capabilities of DFT offer a significant advantage, furnishing more robust theoretical insights crucial for the prospective design and application of magnesium alloys.
2 Basis for calculation
In this work, based on the DFT, the Vienna Ab-initio Simulation Package based on the plane wave pseudopotential method was used for the first-principles calculation [6]. The Perdew–Burke–Enzerho (PBE) functional form of the generalized gradient approximation (GGA) was employed to specify the exchange-correlation energy [7]. The cut-off of 910.0 eV was implemented, and distinct k-points grids were utilized in the Brillouin zone, specifically 8 × 8 × 8, 5 × 5 × 4, and 4 × 4 × 4. The self-consistent iterative method was employed for total energy calculations with the following convergence criteria: the total energy convergence value for the system was set at 1.0 × 10−5 eV/atom, the tolerance offset was maintained below 0.001 Å, and the stress deviation was limited to less than 0.05 GPa.
3 Results and discussion
3.1 Crystal structure and formation enthalpy
The crystal structures of the Mg–Ho intermetallic phases are depicted in Figure 1. A summary of the crystal structure parameters and lattice constants can be found in Table 1. The discrepancy between the calculated and experimental values is within 2%, which can be attributed to the fact that the calculations are performed at 0 K and 0 Pa, while the experimental measurements are conducted under different temperature and pressure conditions. Furthermore, the calculated values are slightly higher than their experimental counterpart [8]. This overestimation can be explained by the use of the GGA-PBE functional in the calculations, which is a semi-local functional. The approximation method employed to handle the electron exchange-correlation energy may not adequately capture the many-body effect, potentially leading to an underestimation of the material’s internal pressure. Consequently, this underestimation results in an overestimation of the calculated lattice constant [9].

Crystal structures of Mg–Ho intermetallic phases: (a) MgHo, (b) Mg2Ho, and (c) Mg24Ho5.
Structural parameters, lattice constant, volume (V) and density (ρ), and formation enthalpy (ΔH) of Mg–Ho intermetallic phases
| Phase | Crystal structure | Space group | Lattice constants (Å) | Volume (Å3) | Density (g·cm−3) | ΔH (kJ·mol−1) | |
|---|---|---|---|---|---|---|---|
| This work | Experiment | ||||||
| MgHo | Cubic | PM−3m | a = 3.81 | a = 3.77 [10] | 55.31 | 5.68 | −8.01 |
| Mg2Ho | Hexagonal | P63/MMC | a = 6.06 | a = 6.02 [11] | 311.93 | 4.55 | −7.64 |
| c = 9.82 | c = 9.75 | ||||||
| Mg24Ho5 | Cubic | I−43m | a = 11.28 | a = 11.23 [12] | 717.77 | 3.25 | −3.90 |
In general, the alloying ability can be assessed by considering the value of formation enthalpy. Typically, a negative formation enthalpy indicates a stronger alloying capability [13]. The formation enthalpy for each phase can be determined using the following equation:
where E
tot represents the total energy of each Mg–Ho intermetallic phase, N
A and N
B denote the number of atoms of A and B in the unit cell, and
3.2 Electronic properties
To gain insight into the electronic properties of Mg–Ho intermetallic phases, an analysis was performed by optimizing the total and partial density of states (DOS) for each phase. As illustrated in Figure 2, the formed intermetallic phases tend to become more stable. The density of states for the three phases exhibited similarities and mainly ranged from −10 to 20 eV; the electron hybridization occurred among Mg-s, p, and Ho-d, f orbitals. The degree of orbital electron hybridization is indicative of the intermetallic phases’ stability, with stronger hybridization indicating greater stability. The partial DOS analysis revealed various degrees of hybridization in the −10–20 eV region for the three phases, affirming their stability. The total DOS increased in the order of Mg24Ho5 < Mg2Ho < MgHo, indicating that MgHo exhibited the highest degree of hybridization and superior stability. The pseudogaps were utilized to discern the strength of covalent bonds in the Mg alloys, identified by the width between the peaks on either side of the Fermi level. Generally, a wider pseudogap signifies a stronger covalent bond [14]. Figure 2 shows that the MgHo phase had a slightly larger pseudogap width compared to the Mg2Ho and Mg24Ho5 phases, indicating a higher covalent bond strength in the MgHo phase.
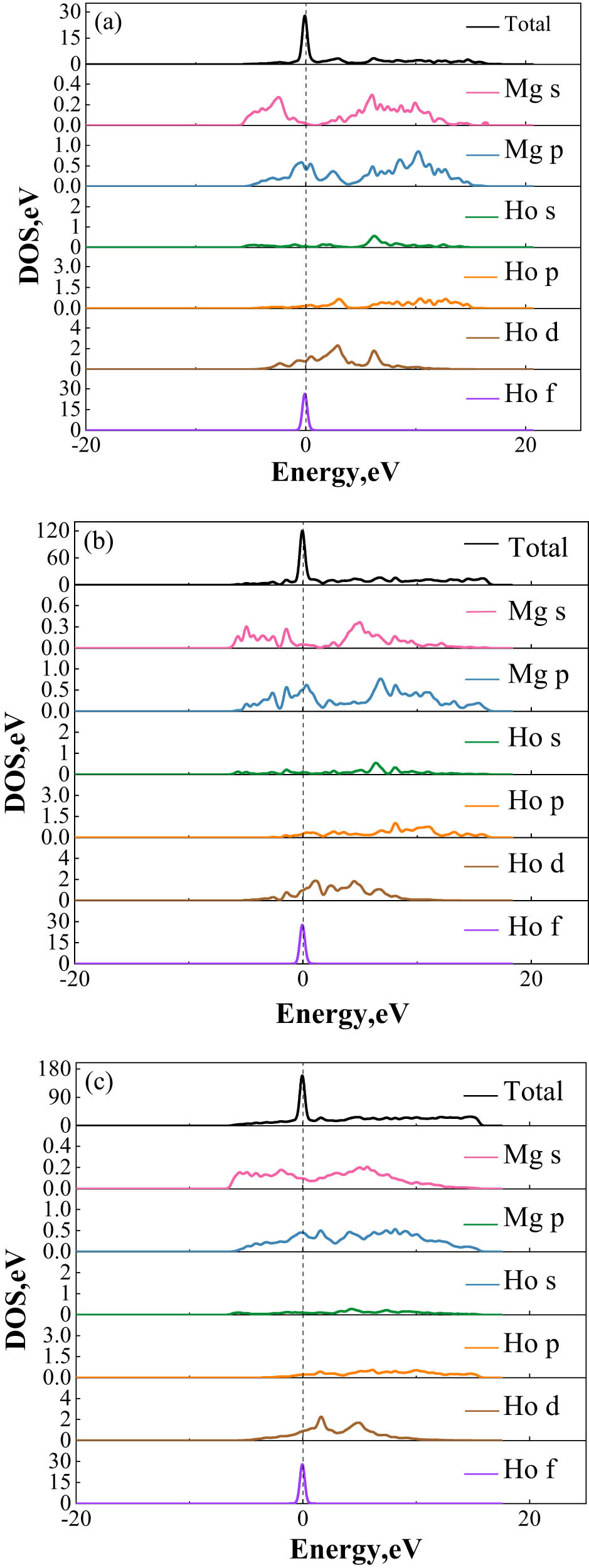
Density of states of MgHo (a), Mg2Ho (b), and Mg24Ho5 (c) phases.
3.3 Elastic properties
Elastic constants are commonly employed for characterizing the elastic properties of materials, and they represent the fundamental physical constants of materials. The cubic crystal system has three independent elastic constants: C11, C12, and C44 [14]. To remain stable, their elastic constants must satisfy the following stability conditions: C11 − C12 > 0, C11 > 0, C44 > 0, and C11 + 2C12 > 0. Conversely, the hexagonal system entails five independent elastic constants, namely C11, C12, C13, C33, and C44 [15]. For this system, the mechanical stability criteria involve C11 > 0, C44 > 0, C11 > C12, and (C11 + 2C12) C33 > 2C13 2. Table 2 enumerates the elastic constants (C ij ) for Mg24Ho5, Mg2Ho, and MgHo phases. Notably, these intermetallic phases exhibited structural stability and satisfactorily met the mechanical stability criteria.
Elastic constants C ij of Mg–Ho intermetallic phases
| Phase | C 11 (GPa) | C 12 (GPa) | C 13 (GPa) | C 33 (GPa) | C 44 (GPa) |
|---|---|---|---|---|---|
| MgHo | 34.03 | 21.42 | — | — | 39.36 |
| Mg2Ho | 57.65 | 15.45 | 9.83 | 80.03 | 25.42 |
| Mg24Ho5 | 64.48 | 16.80 | — | — | 17.32 |
Bulk modulus (B), shear modulus (G), Young’s modulus (E), Poisson’s ratio (ν), theoretical hardness (Hv), and universal anisotropic index (A U) for each phase, utilizing the following formulas [16,17]:
The calculated results are listed in Table 3. The bulk modulus serves as an indicator of a material’s resistance to volume change caused by external pressure. From Table 3, the bulk modulus of these three phases increased as the Ho concentration decreased. The shear modulus and Young’s modulus offer insights into a material’s hardness to some extent. Generally, higher values of shear modulus and Young’s modulus correspond to greater material hardness. The material’s hardness can be assessed through its theoretical hardness. As shown in Table 3, the shear modulus, Young’s modulus, and theoretical hardness for the three phases follow the sequence of Mg24Ho5 < Mg2Ho < MgHo. Based on the comparative analysis, it can be inferred that although the Mg–Ho intermetallic phases exhibit satisfactory mechanical properties, their slightly lower elastic moduli indicate that the Mg17Al12 phase in the AZ91 magnesium alloy possesses superior stiffness and resistance to deformation [18]. This finding holds significant implications for material selection and engineering applications where specific mechanical performance criteria must be satisfied. The insight gained from this study can guide future research and development efforts, which could be directed towards enhancing the elastic properties of Mg–Ho intermetallic phases or optimizing their integration within magnesium alloy systems to achieve the desired balance of properties. Such endeavors would contribute to the development of advanced magnesium alloys with tailored mechanical characteristics, thereby expanding their potential applications in various industries.
Bulk modulus B (GPa), shear modulus G (GPa), Young’s modulus E (GPa), Poisson’s ratio (v), theoretical hardness H v (GPa), and the universal anisotropic index (A U) of Mg–Ho intermetallic phases
| Phase | B V | B R | B | G V | G R | G | E | v | G/B | H V | A U |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MgHo | 25.62 | 25.62 | 25.62 | 26.14 | 12.71 | 26.14 | 58.52 | 0.12 | 1.02 | 6.62 | 5.289 |
| Mg2Ho | 29.51 | 29.18 | 29.35 | 25.07 | 24.42 | 24.74 | 57.95 | 0.17 | 0.85 | 5.45 | 0.144 |
| Mg24Ho5 | 32.69 | 32.69 | 32.69 | 19.93 | 19.45 | 19.69 | 49.19 | 0.25 | 0.60 | 3.28 | 0.124 |
Material brittleness and ductility can be evaluated through the ratio of B/G or Poisson’s ratio. Typically, materials with G/B > 0.5 [19] are classified as brittle. Additionally, Poisson’s ratio is less than 1/3 [19], so the material is considered brittle.
In the Mg–Ho binary alloy system, three distinct phases can form, including MgHo, Mg2Ho, and Mg24Ho5. As the concentration of Ho increases, the proportion of the MgHo phase correspondingly rises based on the Mg–Ho phase diagram. According to the data presented in Table 3, the shear modulus (G) and Young’s modulus (E) of the MgHo phase are superior to those of the Mg24Ho5 and Mg2Ho phases. This result indicates that in the fabrication of Mg–Ho binary phase alloys, an increment of Ho can effectively improve the mechanical properties of the alloy.
Fang et al. [20] suggest that the incorporation of rare earth elements can significantly influence the elastic anisotropy of magnesium with a hexagonal close-packed crystal structure. Moreover, the nature and concentration of these rare earth elements play a pivotal role in determining the resulting elastic properties. For example, Ho, a rare earth element, has been demonstrated to have a notable impact on the elastic anisotropy of magnesium. To further investigate this phenomenon, the present study calculates the universal anisotropy index (A U) for three alloy phases to characterize the elastic anisotropy of each phase. Deviations of the universal anisotropy index (A U) from zero are indicative of the degree of elastic anisotropy, with greater deviations corresponding to higher levels of anisotropy. As shown in Table 3, MgHo exhibited the highest A U value among the studied phases, signifying its superior anisotropic behavior. This finding underscores the importance of considering the specific rare earth element and its concentration when designing magnesium alloys with tailored elastic properties for various engineering applications (Table 4).
Elastic compliance constants Mg–Ho intermetallic phases
| Phase | Elastic compliance constants (GPa−1) | |||||
|---|---|---|---|---|---|---|
| S 11 | S 12 | S 13 | S 33 | S 44 | S 66 | |
| Mg1Ho1 | 0.0572 | −0.0220 | — | — | 0.0254 | — |
| Mg2Ho1 | 0.0189 | −0.0048 | −0.0017 | 0.0129 | 0.0393 | 0.0474 |
| Mg24Ho5 | 0.0175 | −0.0037 | — | — | 0.0595 | — |
To gain deeper insights into the mechanical anisotropy of the intermetallic phases, three-dimensional (3D) surface profiles of Young’s modulus were generated, as depicted in Figure 3. Therefore, the corresponding reciprocal calculations of Young’s modulus for Mg–Ho intermetallic phases are outlined as follows:

3D surface profiles of Young’s modulus for Mg–Ho intermetallic phases: MgHo (a), Mg2Ho (b), and Mg24Ho5 (c).
For cubic crystals:
For hexagonal crystals:
here, S ij represents the elastic flexibility constant, and l 1, l 2, and l 3 are the direction cosines in spherical coordinates.
The 3D diagram for MgHo resembles an eight-petal shape, signifying higher anisotropy, consistent with A U. In contrast, Mg2Ho and Mg24Ho5 exhibit lower anisotropy.
4 Conclusions
In this study, we conducted comprehensive investigations into the stability, elastic properties, and electronic characteristics of Mg–Ho intermetallic phases through DFT calculations. The results can be summarized as follows:
The formation enthalpy (ΔH) of each phase was meticulously computed. The MgHo phase exhibited the highest absolute values, indicating its remarkable stability among the three intermetallic phases.
The result through calculated DOS is in good agreement with the formation enthalpy. Further examination of the DOS affirmed that MgHo displayed the strongest hybridization, underlining its superior stability.
The elastic properties of each intermetallic phase show that these three phases are mechanically stable, brittle, hard, and anisotropic.
Acknowledgement
This work was acknowledged by Jiangsu Ocean University, which provided high-performance computers to perform all first-principles calculations.
-
Funding information: The work was financially supported by the National Natural Science Foundation of China (Grant No. 51971100), the Lianyungang 521 Scientific Research Project (Grant No. LYG06521202312), and the National Key Research and Development Program of Lianyungang (Grant No. 23CY036).
-
Author contributions: Yuting Yang: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. As the first author, Yuting Yang led the study, performed the DFT calculations, analyzed the results, and wrote the initial draft of the manuscript. Mengqin He: Conceptualization, Methodology, Data curation, Validation. Mengqin He collaborated with Yuting Yang in constructing the paper's framework, processing data, and ensuring the accuracy and quality of the information. Yi Luo: Supervision, Validation, Formal analysis. Yi Luo provided theoretical guidance and expertise in data analysis, contributing to the interpretation of results. Yuhang Gu: Methodology, Software, Validation. Yuhang Gu assisted in the construction of the model and literature review. Yunfei Ding: Supervision, Project administration, Funding acquisition, Writing – review & editing. As the corresponding author, Yunfei Ding provided overall guidance, secured funding for the project, and participated in the review and editing of the manuscript to ensure its scientific rigor.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: Not applicable.
References
[1] Xu, T., Y. Yang, X. Peng, J. Song, and F. Pan. Overview of advancement and development trend on magnesium alloy. Journal of Magnesium and Alloys, Vol. 7, No. 3, 2019, pp. 536–544.10.1016/j.jma.2019.08.001Search in Google Scholar
[2] Gao, Y., C. Wu, W. Feng, H. He, H. He, J. Yang, et al. Effects of the rare earth y on the structural and tensile properties of Mg-based Alloy: A first-principles study. Crystals, Vol. 11, No. 8, 2021, id. 1003.10.3390/cryst11081003Search in Google Scholar
[3] Hu, Z., Q. Hua, H. Yan, X. Jie, and Y. Rao. Effect of trace Sm addition on microstructure and mechanical properties of AZ61 magnesium alloys. Rare Metal Materials and Engineering, Vol. 45, No. 9, 2016, pp. 2275–2281.10.1016/S1875-5372(17)30016-4Search in Google Scholar
[4] Tong, X., D. Zhang, J. Lin, Y. Dai, Y. Luan, Q. Sun, et al. Development of biodegradable Zn–1Mg–0.1 RE (RE = Er, Dy, and Ho) alloys for biomedical applications. Acta Biomaterialia, Vol. 117, 2020, pp. 384–399.10.1016/j.actbio.2020.09.036Search in Google Scholar PubMed
[5] Tao, X., Y. Ouyang, H. Liu, Y. Feng, Y. Du, Y. He, et al. Phase stability of magnesium-rare earth binary systems from first-principles calculations. Journal of Alloys and Compounds, Vol. 509, No. 24, 2011, pp. 6899–6907.10.1016/j.jallcom.2011.03.177Search in Google Scholar
[6] Kresse, G. and J. Furthmüller. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Computational Materials Science, Vol. 6, No. 1, 1996, pp. 15–50.10.1016/0927-0256(96)00008-0Search in Google Scholar
[7] Perdew, J. P. and Y. Wang. Accurate and simple analytic representation of the electron-gas correlation energy. Physical Review B, Vol. 45, No. 23, 1992, id. 13244.10.1103/PhysRevB.45.13244Search in Google Scholar
[8] Anua, N. N., R. Ahmed, M. A. Saeed, A. Shaari, and U. H. Bakhtiar. DFT investigations of structural and electronic properties of gallium arsenide (GaAs)[C]. AIP Conference Proceedings, Vol. 1482, No. 1, 2012, pp. 64–68. American Institute of Physics.10.1063/1.4757439Search in Google Scholar
[9] Chen, H. L., L. Lin, P. L. Mao, and Z. Liu. Phase stability, electronic, elastic and thermodynamic properties of Al-RE intermetallics in Mg-Al-RE alloy: A first principles study. Journal of Magnesium and Alloys, Vol. 3, No. 3, 2015, pp. 197–202.10.1016/j.jma.2015.08.003Search in Google Scholar
[10] Iandelli, A. and A. Palenzona. Atomic size of rare earths in intermetallic compounds. MX compounds of CsCl type. Journal of the Less Common Metals, Vol. 9, No. 4, 1965, pp. 1–6.10.1016/0022-5088(65)90028-7Search in Google Scholar
[11] Ebner, M. A. and K. J. Range. Studies on AB2-type intermetallic compounds, III. High-pressure synthesis and single-crystal structure refinement of the Laves-Friauf phases HoMg2, TmMg2 and YbMg2. Journal of Alloys and Compounds, Vol. 236, No. 1–2, 1996, pp. 50–57.10.1016/0925-8388(95)02167-1Search in Google Scholar
[12] Kripyakevich, P. I., V. I. Evdokimenko, and E. I. Gladyshevskii. Compounds with the α-Mn superstructure in the rare earth-magnesium system. Kristallografiya, Vol. 9, No. 3, 1964, pp. 410–411.Search in Google Scholar
[13] Fu, C. L., X. Wang, Y. Y. Ye, and K. M. Ho. Phase stability, bonding mechanism, and elastic constants of Mo5Si3 by first-principles calculation. Intermetallics, Vol. 7, No. 2, 1999, pp. 179–184.10.1016/S0966-9795(98)00018-1Search in Google Scholar
[14] Takagiwa, Y. and K. Kimura. Metallic–covalent bonding conversion and thermoelectric properties of Al-based icosahedral quasicrystals and approximants. Science and Technology of Advanced Materials, Vol. 15, No. 4, 2014, id. 044802.10.1088/1468-6996/15/4/044802Search in Google Scholar PubMed PubMed Central
[15] Panda, K. B. and K. S. R. Chandran. Determination of elastic constants of titanium diboride (TiB2) from first principles using FLAPW implementation of the density functional theory. Computational Materials Science, Vol. 35, No. 2, 2006, pp. 134–150.10.1016/j.commatsci.2005.03.012Search in Google Scholar
[16] Mian, S. A., M. Muzammil, G. Rahman, and E. Ahmed. The study of structural, elastic, electronic and optical properties of CsY I (Y = F, Cl, Br) using density functional theory. Materials Science-Poland, Vol. 35, No. 1, 2017, pp. 197–210.10.1515/msp-2017-0017Search in Google Scholar
[17] Marmier, A., Z. A. D. Lethbridge, R. I. Walton, C. W. Smith, S. C. Parker, and K. E. Evans. ElAM: A computer program for the analysis and representation of anisotropic elastic properties. Computer Physics Communications, Vol. 181, No. 12, 2010, pp. 2102–2115.10.1016/j.cpc.2010.08.033Search in Google Scholar
[18] Huang, Z., Y. Zhao, and H. Hou. Structural, thermodynamics and elastic properties of Mg17Al12, Al2Y and Al4Ba phases by first-principles calculations. Journal of Central South University, Vol. 19, No. 6, 2012, pp. 1475–1481.10.1007/s11771-012-1164-5Search in Google Scholar
[19] Vaitheeswaran, G., V. Kanchana, A. Svane, and A. Delin. Elastic properties of MgCNi3 – a superconducting perovskite. Journal of Physics: Condensed Matter, Vol. 19, No. 32, 2007, id. 326214.10.1088/0953-8984/19/32/326214Search in Google Scholar
[20] Fang, C., J. Zhang, Y. Huang, J. L. Xue, and X. Y. Dong. Orientation dependence of elastic properties of Mg binary alloys: A first-principles study. Computational Condensed Matter, Vol. 22, 2020, id. e00447.10.1016/j.cocom.2019.e00447Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- De-chlorination of poly(vinyl) chloride using Fe2O3 and the improvement of chlorine fixing ratio in FeCl2 by SiO2 addition
- Reductive behavior of nickel and iron metallization in magnesian siliceous nickel laterite ores under the action of sulfur-bearing natural gas
- Study on properties of CaF2–CaO–Al2O3–MgO–B2O3 electroslag remelting slag for rack plate steel
- The origin of {113}<361> grains and their impact on secondary recrystallization in producing ultra-thin grain-oriented electrical steel
- Channel parameter optimization of one-strand slab induction heating tundish with double channels
- Effect of rare-earth Ce on the texture of non-oriented silicon steels
- Performance optimization of PERC solar cells based on laser ablation forming local contact on the rear
- Effect of ladle-lining materials on inclusion evolution in Al-killed steel during LF refining
- Analysis of metallurgical defects in enamel steel castings
- Effect of cooling rate and Nb synergistic strengthening on microstructure and mechanical properties of high-strength rebar
- Effect of grain size on fatigue strength of 304 stainless steel
- Analysis and control of surface cracks in a B-bearing continuous casting blooms
- Application of laser surface detection technology in blast furnace gas flow control and optimization
- Preparation of MoO3 powder by hydrothermal method
- The comparative study of Ti-bearing oxides introduced by different methods
- Application of MgO/ZrO2 coating on 309 stainless steel to increase resistance to corrosion at high temperatures and oxidation by an electrochemical method
- Effect of applying a full oxygen blast furnace on carbon emissions based on a carbon metabolism calculation model
- Characterization of low-damage cutting of alfalfa stalks by self-sharpening cutters made of gradient materials
- Thermo-mechanical effects and microstructural evolution-coupled numerical simulation on the hot forming processes of superalloy turbine disk
- Endpoint prediction of BOF steelmaking based on state-of-the-art machine learning and deep learning algorithms
- Effect of calcium treatment on inclusions in 38CrMoAl high aluminum steel
- Effect of isothermal transformation temperature on the microstructure, precipitation behavior, and mechanical properties of anti-seismic rebar
- Evolution of residual stress and microstructure of 2205 duplex stainless steel welded joints during different post-weld heat treatment
- Effect of heating process on the corrosion resistance of zinc iron alloy coatings
- BOF steelmaking endpoint carbon content and temperature soft sensor model based on supervised weighted local structure preserving projection
- Innovative approaches to enhancing crack repair: Performance optimization of biopolymer-infused CXT
- Structural and electrochromic property control of WO3 films through fine-tuning of film-forming parameters
- Influence of non-linear thermal radiation on the dynamics of homogeneous and heterogeneous chemical reactions between the cone and the disk
- Thermodynamic modeling of stacking fault energy in Fe–Mn–C austenitic steels
- Research on the influence of cemented carbide micro-textured structure on tribological properties
- Performance evaluation of fly ash-lime-gypsum-quarry dust (FALGQ) bricks for sustainable construction
- First-principles study on the interfacial interactions between h-BN and Si3N4
- Analysis of carbon emission reduction capacity of hydrogen-rich oxygen blast furnace based on renewable energy hydrogen production
- Just-in-time updated DBN BOF steel-making soft sensor model based on dense connectivity of key features
- Effect of tempering temperature on the microstructure and mechanical properties of Q125 shale gas casing steel
- Review Articles
- A review of emerging trends in Laves phase research: Bibliometric analysis and visualization
- Effect of bottom stirring on bath mixing and transfer behavior during scrap melting in BOF steelmaking: A review
- High-temperature antioxidant silicate coating of low-density Nb–Ti–Al alloy: A review
- Communications
- Experimental investigation on the deterioration of the physical and mechanical properties of autoclaved aerated concrete at elevated temperatures
- Damage evaluation of the austenitic heat-resistance steel subjected to creep by using Kikuchi pattern parameters
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part II
- Synthesis of aluminium (Al) and alumina (Al2O3)-based graded material by gravity casting
- Experimental investigation into machining performance of magnesium alloy AZ91D under dry, minimum quantity lubrication, and nano minimum quantity lubrication environments
- Numerical simulation of temperature distribution and residual stress in TIG welding of stainless-steel single-pass flange butt joint using finite element analysis
- Special Issue on A Deep Dive into Machining and Welding Advancements - Part I
- Electro-thermal performance evaluation of a prismatic battery pack for an electric vehicle
- Experimental analysis and optimization of machining parameters for Nitinol alloy: A Taguchi and multi-attribute decision-making approach
- Experimental and numerical analysis of temperature distributions in SA 387 pressure vessel steel during submerged arc welding
- Optimization of process parameters in plasma arc cutting of commercial-grade aluminium plate
- Multi-response optimization of friction stir welding using fuzzy-grey system
- Mechanical and micro-structural studies of pulsed and constant current TIG weldments of super duplex stainless steels and Austenitic stainless steels
- Stretch-forming characteristics of austenitic material stainless steel 304 at hot working temperatures
- Work hardening and X-ray diffraction studies on ASS 304 at high temperatures
- Study of phase equilibrium of refractory high-entropy alloys using the atomic size difference concept for turbine blade applications
- A novel intelligent tool wear monitoring system in ball end milling of Ti6Al4V alloy using artificial neural network
- A hybrid approach for the machinability analysis of Incoloy 825 using the entropy-MOORA method
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part II
- Innovations for sustainable chemical manufacturing and waste minimization through green production practices
- Topical Issue on Conference on Materials, Manufacturing Processes and Devices - Part I
- Characterization of Co–Ni–TiO2 coatings prepared by combined sol-enhanced and pulse current electrodeposition methods
- Hot deformation behaviors and microstructure characteristics of Cr–Mo–Ni–V steel with a banded structure
- Effects of normalizing and tempering temperature on the bainite microstructure and properties of low alloy fire-resistant steel bars
- Dynamic evolution of residual stress upon manufacturing Al-based diesel engine diaphragm
- Study on impact resistance of steel fiber reinforced concrete after exposure to fire
- Bonding behaviour between steel fibre and concrete matrix after experiencing elevated temperature at various loading rates
- Diffusion law of sulfate ions in coral aggregate seawater concrete in the marine environment
- Microstructure evolution and grain refinement mechanism of 316LN steel
- Investigation of the interface and physical properties of a Kovar alloy/Cu composite wire processed by multi-pass drawing
- The investigation of peritectic solidification of high nitrogen stainless steels by in-situ observation
- Microstructure and mechanical properties of submerged arc welded medium-thickness Q690qE high-strength steel plate joints
- Experimental study on the effect of the riveting process on the bending resistance of beams composed of galvanized Q235 steel
- Density functional theory study of Mg–Ho intermetallic phases
- Investigation of electrical properties and PTCR effect in double-donor doping BaTiO3 lead-free ceramics
- Special Issue on Thermal Management and Heat Transfer
- On the thermal performance of a three-dimensional cross-ternary hybrid nanofluid over a wedge using a Bayesian regularization neural network approach
- Time dependent model to analyze the magnetic refrigeration performance of gadolinium near the room temperature
- Heat transfer characteristics in a non-Newtonian (Williamson) hybrid nanofluid with Hall and convective boundary effects
- Computational role of homogeneous–heterogeneous chemical reactions and a mixed convective ternary hybrid nanofluid in a vertical porous microchannel
- Thermal conductivity evaluation of magnetized non-Newtonian nanofluid and dusty particles with thermal radiation
Articles in the same Issue
- Research Articles
- De-chlorination of poly(vinyl) chloride using Fe2O3 and the improvement of chlorine fixing ratio in FeCl2 by SiO2 addition
- Reductive behavior of nickel and iron metallization in magnesian siliceous nickel laterite ores under the action of sulfur-bearing natural gas
- Study on properties of CaF2–CaO–Al2O3–MgO–B2O3 electroslag remelting slag for rack plate steel
- The origin of {113}<361> grains and their impact on secondary recrystallization in producing ultra-thin grain-oriented electrical steel
- Channel parameter optimization of one-strand slab induction heating tundish with double channels
- Effect of rare-earth Ce on the texture of non-oriented silicon steels
- Performance optimization of PERC solar cells based on laser ablation forming local contact on the rear
- Effect of ladle-lining materials on inclusion evolution in Al-killed steel during LF refining
- Analysis of metallurgical defects in enamel steel castings
- Effect of cooling rate and Nb synergistic strengthening on microstructure and mechanical properties of high-strength rebar
- Effect of grain size on fatigue strength of 304 stainless steel
- Analysis and control of surface cracks in a B-bearing continuous casting blooms
- Application of laser surface detection technology in blast furnace gas flow control and optimization
- Preparation of MoO3 powder by hydrothermal method
- The comparative study of Ti-bearing oxides introduced by different methods
- Application of MgO/ZrO2 coating on 309 stainless steel to increase resistance to corrosion at high temperatures and oxidation by an electrochemical method
- Effect of applying a full oxygen blast furnace on carbon emissions based on a carbon metabolism calculation model
- Characterization of low-damage cutting of alfalfa stalks by self-sharpening cutters made of gradient materials
- Thermo-mechanical effects and microstructural evolution-coupled numerical simulation on the hot forming processes of superalloy turbine disk
- Endpoint prediction of BOF steelmaking based on state-of-the-art machine learning and deep learning algorithms
- Effect of calcium treatment on inclusions in 38CrMoAl high aluminum steel
- Effect of isothermal transformation temperature on the microstructure, precipitation behavior, and mechanical properties of anti-seismic rebar
- Evolution of residual stress and microstructure of 2205 duplex stainless steel welded joints during different post-weld heat treatment
- Effect of heating process on the corrosion resistance of zinc iron alloy coatings
- BOF steelmaking endpoint carbon content and temperature soft sensor model based on supervised weighted local structure preserving projection
- Innovative approaches to enhancing crack repair: Performance optimization of biopolymer-infused CXT
- Structural and electrochromic property control of WO3 films through fine-tuning of film-forming parameters
- Influence of non-linear thermal radiation on the dynamics of homogeneous and heterogeneous chemical reactions between the cone and the disk
- Thermodynamic modeling of stacking fault energy in Fe–Mn–C austenitic steels
- Research on the influence of cemented carbide micro-textured structure on tribological properties
- Performance evaluation of fly ash-lime-gypsum-quarry dust (FALGQ) bricks for sustainable construction
- First-principles study on the interfacial interactions between h-BN and Si3N4
- Analysis of carbon emission reduction capacity of hydrogen-rich oxygen blast furnace based on renewable energy hydrogen production
- Just-in-time updated DBN BOF steel-making soft sensor model based on dense connectivity of key features
- Effect of tempering temperature on the microstructure and mechanical properties of Q125 shale gas casing steel
- Review Articles
- A review of emerging trends in Laves phase research: Bibliometric analysis and visualization
- Effect of bottom stirring on bath mixing and transfer behavior during scrap melting in BOF steelmaking: A review
- High-temperature antioxidant silicate coating of low-density Nb–Ti–Al alloy: A review
- Communications
- Experimental investigation on the deterioration of the physical and mechanical properties of autoclaved aerated concrete at elevated temperatures
- Damage evaluation of the austenitic heat-resistance steel subjected to creep by using Kikuchi pattern parameters
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part II
- Synthesis of aluminium (Al) and alumina (Al2O3)-based graded material by gravity casting
- Experimental investigation into machining performance of magnesium alloy AZ91D under dry, minimum quantity lubrication, and nano minimum quantity lubrication environments
- Numerical simulation of temperature distribution and residual stress in TIG welding of stainless-steel single-pass flange butt joint using finite element analysis
- Special Issue on A Deep Dive into Machining and Welding Advancements - Part I
- Electro-thermal performance evaluation of a prismatic battery pack for an electric vehicle
- Experimental analysis and optimization of machining parameters for Nitinol alloy: A Taguchi and multi-attribute decision-making approach
- Experimental and numerical analysis of temperature distributions in SA 387 pressure vessel steel during submerged arc welding
- Optimization of process parameters in plasma arc cutting of commercial-grade aluminium plate
- Multi-response optimization of friction stir welding using fuzzy-grey system
- Mechanical and micro-structural studies of pulsed and constant current TIG weldments of super duplex stainless steels and Austenitic stainless steels
- Stretch-forming characteristics of austenitic material stainless steel 304 at hot working temperatures
- Work hardening and X-ray diffraction studies on ASS 304 at high temperatures
- Study of phase equilibrium of refractory high-entropy alloys using the atomic size difference concept for turbine blade applications
- A novel intelligent tool wear monitoring system in ball end milling of Ti6Al4V alloy using artificial neural network
- A hybrid approach for the machinability analysis of Incoloy 825 using the entropy-MOORA method
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part II
- Innovations for sustainable chemical manufacturing and waste minimization through green production practices
- Topical Issue on Conference on Materials, Manufacturing Processes and Devices - Part I
- Characterization of Co–Ni–TiO2 coatings prepared by combined sol-enhanced and pulse current electrodeposition methods
- Hot deformation behaviors and microstructure characteristics of Cr–Mo–Ni–V steel with a banded structure
- Effects of normalizing and tempering temperature on the bainite microstructure and properties of low alloy fire-resistant steel bars
- Dynamic evolution of residual stress upon manufacturing Al-based diesel engine diaphragm
- Study on impact resistance of steel fiber reinforced concrete after exposure to fire
- Bonding behaviour between steel fibre and concrete matrix after experiencing elevated temperature at various loading rates
- Diffusion law of sulfate ions in coral aggregate seawater concrete in the marine environment
- Microstructure evolution and grain refinement mechanism of 316LN steel
- Investigation of the interface and physical properties of a Kovar alloy/Cu composite wire processed by multi-pass drawing
- The investigation of peritectic solidification of high nitrogen stainless steels by in-situ observation
- Microstructure and mechanical properties of submerged arc welded medium-thickness Q690qE high-strength steel plate joints
- Experimental study on the effect of the riveting process on the bending resistance of beams composed of galvanized Q235 steel
- Density functional theory study of Mg–Ho intermetallic phases
- Investigation of electrical properties and PTCR effect in double-donor doping BaTiO3 lead-free ceramics
- Special Issue on Thermal Management and Heat Transfer
- On the thermal performance of a three-dimensional cross-ternary hybrid nanofluid over a wedge using a Bayesian regularization neural network approach
- Time dependent model to analyze the magnetic refrigeration performance of gadolinium near the room temperature
- Heat transfer characteristics in a non-Newtonian (Williamson) hybrid nanofluid with Hall and convective boundary effects
- Computational role of homogeneous–heterogeneous chemical reactions and a mixed convective ternary hybrid nanofluid in a vertical porous microchannel
- Thermal conductivity evaluation of magnetized non-Newtonian nanofluid and dusty particles with thermal radiation

