Abstract
Based on the raw material of ammonium tetramolybdate in the experiment, molybdenum trioxide was prepared by hydrothermal synthesis, and the effects of different pH, hydrothermal reaction time, filling degree, and calcination temperature on molybdenum trioxide powder were studied. Meanwhile, the molybdenum trioxide powder was characterized through scanning electron microscopy, transmission electron microscopy, X-ray diffraction, and thermal analysis differential scanning calorimetry analysis so as to study the morphology and phase changes during the experiment. As is evident from the research findings, MoO3 powders with uniform and suitable size, smooth and clear surface, good dispersibility, and no adhesion can be obtained at the conditions of pH = 1, 16 h hydrothermal reaction, 90% of filling degree, and 550°C of calcination temperature. In the calcination process, MoO3 crystal undergone crystal transformation and was completely transformed from h-MoO3 to α-MoO3 at the calcination temperature of 350°C.
1 Introduction
As a high-performance material with high melting point, excellent strength, strong hardness, good wear resistance, outstanding electrical conductivity, small expansion coefficient, and superior thermal shock resistance as well as thermal fatigue resistance, molybdenum metal has become one of the indispensable raw materials for modern high-tech development [1,2,3,4,5,6,7,8]. Now, it is widely used in fields such as metallurgy, machinery, petroleum, chemical industry, national defense, aviation, aerospace, electronics, nuclear industry, etc. [5,9,10,11,12]. By means of calcinating ammonium molybdate, molybdenum trioxide is prepared and then reduced to obtain the molybdenum powder [6,13,14]. According to the research studies on the refining mechanism of molybdenum powder, the morphology of molybdenum powder “inherits” from that of molybdenum trioxide to some extent. That means both the size and shape of molybdenum trioxide will greatly affect the preparations of molybdenum powder and molybdenum alloy [9,14,15,16,17,18]. MoO3 prepared based on the traditional process is so rough (coarse particles, poor size uniformity) that it can no longer meet the needs of nano-molybdenum powder, high-purity molybdenum powder, and high-performance molybdenum alloy, so it is of great significance to study the preparation process for MoO3 powder [10,19,20,21,22,23,24,25]. At present, there are many methods to prepare nano-MoO3 materials, including vapor deposition, precipitation, hydrothermal, solvothermal, and sol–gel method. For the sake of obtaining high-quality MoO3, MoO3 powder was prepared through hydrothermal synthesis in this experiment, and the effects of different hydrothermal times, pH values, calcination temperatures, and filling degrees on the morphology of MoO3 powder were studied.
2 Materials and methods
Ammonium tetramolybdate and dilute nitric acid solution, after being added into the autoclave, were stirred evenly for hydrothermal reaction, and then the MoO3 powder was obtained via washing, filtering, drying, and calcinating the hydrothermal synthesis product. After that, the effects of different pH values, hydrothermal reaction times, filling degrees, and calcination temperatures on molybdenum trioxide powder were studied.
In the experiment, the phase analysis of the powder was performed using a D8 ADVANCE X-ray diffractometer, the morphology of MoO3 powder was analyzed using a Regulus 8220 scanning electron microscope and a JEM-2100 URP/JEM-2100 transmission electron microscope, and thermal analysis of the samples was carried out by using DSC 204F differential scanning calorimetry.
3 Microstructure
3.1 Effects of different pH values on MoO3 powder
During the hydrothermal process, the conversion of (NH4)2Mo4O13·2H2O solution to MoO3 is the reaction of H+ electrophilic addition followed by dehydration:
During the hydrothermal process, H+ is involved in the reaction and its pH value has a great influence on the morphology of MoO3 powder. As shown in Figure 1, which is an observation of the morphology of MoO3 powders with pH values of 0, 0.5, 1, 1.5, and 2, the pH value seriously affects the morphology of MoO3 powder. Specifically, at the pH value of 0 (Figure 1a), the grains take on the shape of a complete hexagonal prism with basically equal side lengths and height at around 15 µm. When increasing the pH value to 0.5 (Figure 1b), fine crystals begin to appear, which are distributed in the vicinity of the hexagonal prisms. The hexagonal prism disappears, and MoO3 in flaky structure comes out once the pH value reaches 1. MoO3 in Figure 1c has a uniform size without adhesion, which is not only the best state among all products with a pH value of 0–2, but also the best pH value of MoO3 powder prepared by the hydrothermal method. With the increase of the pH value (Figure 1d), large bulk MoO3 and small unreacted (NH4)2Mo4O13·2H2O appear (Figure 1e). With the further increase of the pH value, a large amount of unreacted (NH4)2Mo4O13·2H2O begins to appear, as shown in Figure 1e. A study of the reaction process and the hydrothermal method’s preparation of MoO3 revealed that the pH of the solution has a significant impact on the hydrothermal reaction progress due to the involvement of H+ in the reaction. When the pH is lower than 1, rod-shaped MoO3 is formed, and the ideal MoO3 form appears as the pH reaches 1. With the further increase of the pH value, unreacted (NH4)2Mo4O13·2H2O begins to appear because H+ is insufficient to support the hydrothermal reaction, and the pH of the supernatant after the reaction at pH 1 is measured to be greater than 4.

SEM images of MoO3 powder synthesized by hydrothermal synthesis at different pH values. (a) pH = 0 (180°C × 16 h). (b) pH = 0.5 (180°C × 16 h). (c) pH = 1 (180°C × 16 h) (d) pH = 1.5 (180°C × 16 h). (e) pH = 2 (180°C × 16 h).
3.2 Effects of different hydrothermal reaction time on MoO3 powder
In view of needing to both ensure the complete reaction process and prevent the powder particles from being too large due to the long hydrothermal time, proper control of the hydrothermal time is critical to the hydrothermal process. Because the time is too short to observe the difference between the powders visually, 8 h is selected as the interval (as shown in Figure 2) to observe the morphology of MoO3 at the reaction time of 8, 16, 24, 32, 40, and 48 h.

SEM images of MoO3 powder synthesized by hydrothermal reaction at different reaction times. (a) 180°C × 8 h (pH = 1.0). (b) 180°C × 16 h (pH = 1.0). (c) 180°C × 24 h (pH = 1.0). (d) 180°C × 32 h (pH = 1.0). (e) 180°C × 40 h (pH = 1.0) (f) 180°C × 48 h (pH = 1.0).
In Figure 2a, at the beginning of the reaction, there are still a lot of flaky (NH4)2Mo4O13·2H2O, but the flaky (NH4)2Mo4O13·2H2O basically disappeared in Figure 2b. With the increase of hydrothermal time, the size of MoO3 grows further in Figure 2c–e, and finally, larger flaky MoO3 appears at 48 h in Figure 2f. The comparison discovers that 16 h is a more suitable reaction time.
3.3 Effects of different filling degrees on MoO3 powder
Filling degree refers to the percentage of the liquid volume in the lining of the hydrothermal kettle to the total volume. Different filling degrees have a great influence on the MoO3 powder prepared in the experiment, and pressure generated by the lining with different filling degrees during the experiment is varied, so that different growth conditions of the MoO3 powder are produced. The effects of 45, 60, 75, and 90% of filling degrees on the morphology of prepared MoO3 powders were studied, as shown in Figure 3. In Figure 3a, due to the small filling degree, the size of MoO3 powder is large with poor uniformityn of MoO3 powder decreases while the size uniformity improves (Figure 3b and c). The size and size uniformity of MoO3 powder (Figure 3d) are optimal when the final filling degree reaches 90%.
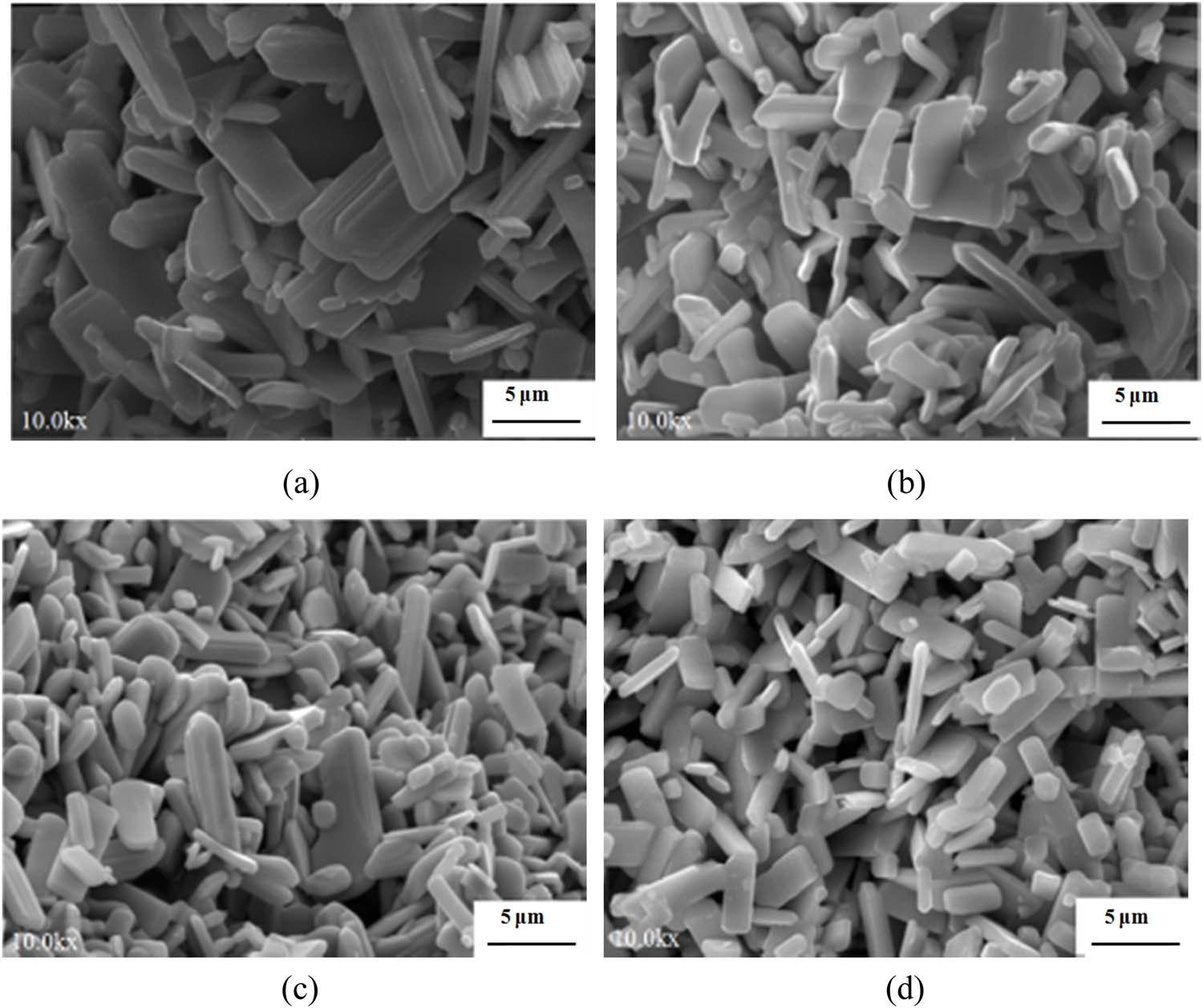
SEM images of MoO3 powder synthesized by hydrothermal method with different filling degrees. (a) 45% (180°C × 16 h pH = 1.0). (b) 60% (180°C × 16 h pH = 1.0); (c) 75% (180°C × 16 h pH = 1.0). (d) 90% (180°C × 16 h pH = 1.0).
3.4 Effects of different calcination temperatures on MoO3 powder
The hydrothermally generated MoO3 is composed of [MoO6] primitives, which are in the shape of an octahedron and connected in different ways. On this basis, MoO3 is in two different states. When the vertices are connected, the Mo–O–Mo bond is developed, so that the formed MoO3 is in a hexagonal metastable state. When the common edge is connected, two Mo–O–Mo bonds are developed, which, compared with the two octahedrons connected by the common point, is a stable orthorhombic structure with lower energy. Therefore, calcination is necessary to make MoO3 in a stable orthorhombic structure.
Calcination is an essential link in the preparation of MoO3 powder. After the drying and calcination of powder with a filling degree of 90% at 180°C × 16 h and pH = 1.0, there is still a large amount of crystal water in the dried powder, and MoO3 generated after hydrothermal is unstable that it needs to remove part of the crystal water by calcination. The MoO3 powders calcined at 250, 350, 450, 500, 550, and 600°C were observed and analyzed.
Figure 4 presents the SEM images of the hydrothermally synthesized MoO3 powders at different calcination temperatures. When the temperature is lower than 500°C, the MoO3 powder appears as nearly circular flakes (Figure 4a–d) and tends to agglomerate, and the grain size is as small as 2–5 µm. When the temperature increases to 550°C, the MoO3 powder particles become lath-like (Figure 4e). With the further temperature increase, the lath-like MoO3 continues to grow and shows a block-like structure (Figure 4f). When the calcination temperature reaches 550°C, MoO3 powder is completely transformed with a small and uniform size, which is a suitable calcination temperature that can ensure complete transformation without making the calcined MoO3 powder grow into a larger lath shape.

SEM images of MoO3 powder synthesized by hydrothermal reaction at different calcination temperatures.
4 Results and discussion
The XRD patterns of the hydrothermally synthesized MoO3 powders at different calcination temperatures are shown in Figure 5. At the calcination temperature of 250°C (Figure 5a), MoO3 powder basically presents the form of a metastable hexagonal phase, in which the lattice constants are a = b = 1.0531 nm and c = 1.4876 nm, and crystal planes with strong diffraction peaks are (110), (200), (210), (300), (220), (310), and (404) (JCPDS 21-0569), but there are also orthorhombic MoO3 powders. When the calcination temperature increases to 350°C (Figure 5b), the MoO3 powder is stably orthorhombic, and the positions of the main diffraction peaks are 12.8°, 23.30°, 26.65°, 27.4°, and 39.0°, corresponding to the orthorhombic MoO3 (020), (110), (040), (021), and (060) crystal planes (JCPDS: 05-0508), in which the lattice constants are a = 0.3963 nm, b = 1.3856 nm, c = 0.3697, and α = β = γ = 90°. When the calcination temperature is higher than 350°C (as shown in Figure 5c–f), the MoO3 powders all exist in the form of an orthorhombic phase, and the intensity of diffraction peaks is strengthened with the increase of calcination temperature, but their positions are not changed, and the intensity of individual diffraction peaks is strong, indicating that MoO3 crystals grow preferentially during the growth. According to the analysis of this experiment, only metastable h-MoO3 powder can be obtained in the stage of hydrothermal synthesis. Under subcritical or supercritical hydrothermal conditions, the reaction in hydrothermal synthesis is at the molecular level, so the activity of reactants is enhanced, the dissolution-recrystallization process is promoted, some new chemical reactions are realized, and metastable molybdenum materials are obtained. The growth of MoO3 crystal is divided into two stages: first, ammonium tetramolybdate reacts with nitric acid to generate a molybdenum trioxide crystal nucleus (as in formula (1)); second, once the crystal nucleus is developed, a solid–liquid interface is formed, and the crystal nucleus grows on it

XRD patterns of MoO3 powder synthesized by hydrothermal reaction at different calcination temperatures (A 250°C, B 350°C, C 450°C, D 500°C, E 550°C, F 600°C).
The growth process of h-MoO3 crystals is quite complicated because it is controlled by the combined effect of the single-nuclear layer mechanism, the multi-nuclear layer mechanism, and the diffusion-controlled growth mechanism rather than only one mechanism. In the hydrothermal synthesis stage of this experiment, only the thermodynamically metastable phase h-MoO3 was obtained instead of transforming into the thermodynamically stable phase α-MoO3. This is because under the hydrothermal conditions of this experiment, the external energy was lower than the activation energy needed for converting h-MoO3 into α-MoO3, leading to the existence of MoO3 at the metastable hexagonal phase. As the external energy is obtained during the calcination, h-MoO3 begins to transform to the α-MoO3 in a stable state (as shown in Figure 5a). When the temperature continued to rise to 350°C, h-MoO3 was all transformed into stable α-MoO3 after obtaining enough energy (Figure 5b) [21,22,26].
Figure 6 shows the DSC analysis curve of MoO3 powder. There is an obvious endothermic peak between 300 and 400°C, in which the crystal transformation of h-MoO3 into α-MoO3 occurred; for the exothermic peak between 400 and 500°C, it is caused by the decomposition of ammonium tetramolybdate with incomplete reaction; the DSC curve is relatively flat after 500°C, indicating that the decomposition of organic matter is complete. Therefore, only the temperature for the calcination of MoO3 powder at 550°C can meet the complete decomposition of ammonium tetramolybdate as well as a complete transformation of MoO3.

DSC analysis of MoO3 powder.
In order to further determine the crystal type of calcined molybdenum trioxide powder, TEM analysis was conducted on the powder calcined at different temperatures. Figure 7 shows the TEM image of MoO3 powder calcined at 250°C and its diffraction pattern, and Figure 8 shows the TEM image of MoO3 powder calcined at 250°C and its diffraction pattern. According to Figure 7a, MoO3 calcined at 250°C is in the form of flakes, and the diffraction pattern is marked as a hexagonal phase (Figure 7b), while as presented in Figure 8a, the MoO3 calcined at 550°C shows a lath-shaped diffraction pattern and is marked as an orthorhombic phase (Figure 8b).

TEM image, diffraction pattern, and crystal structure of roasted MoO3 powder at 250°C. (a) TEM. (b) The diffraction pattern.

TEM image, diffraction pattern, and crystal structure of roasted MoO3 powder at 550°C. (a) TEM. (b) The diffraction pattern.
5 Conclusion
MoO3 powder in smaller and uniform size and good dispersion can be obtained when pH = 1; after 16 h of hydrothermal reaction, the MoO3 crystal grows completely, and MoO3 crystal changes from cotton wool to flake, but cohesion will occur in the condition of long-time reaction (such as 48 h).
With the increase of filling degree, MoO3 crystal size becomes small and uniform, and MoO3 powder with better quality can be obtained at the filling degree of 90%.
Through the investigation of the hydrothermal process for MoO3 powder, it has been determined that the optimal method for preparing MoO3 powder involves hydrothermal treatment at 180°C under pH conditions of 1 and 16, a filling degree of 90%, and calcination at 550°C. This process yields uniformly fine particles with a smooth, unblemished surface, excellent dispersion, and no adhesiveness. The final product exhibits an optimal particle morphology and a stable crystal structure for MoO3 powder.
Acknowledgments
Due to the strong support from relevant personnel and related projects during the submission period, a number of additional projects have been added.
-
Funding information: This work was supported by the University Synergy Innovation Program of Anhui Province (GXXT-2022-086), Provincial “Six Excellent One Top” Project Material Physics Excellent Engineer Education and Training Program (2022zybj086), Anhui University Students Innovation and Entrepreneurship Training Program (S202310373088), Natural Science Foundation of Anhui Higher Education Institutions of China (2023AH050320), Huaibei Normal University postgraduate education teaching reform research project (2023jgxm011), Huaibei Longtu Aluminum Co., Ltd., cooperation project (2023340603000194), research project on Undergraduate Education and Teaching Reform at Huaibei Normal University (2023jxyj023), Laboratory Open Project at Huaibei Normal University (2023syskf021), and National Joint Engineering Research Center for abrasion control and molding of metal materials (Project No. HKDNM202105).
-
Author contributions: ZXW, XLJ, and CCP jointly designed the experiment, ZXW conducted the experiment and manuscript writing, WMJ, DHJ, YZ, and FMY assisted in the experiment, and CS, LQZ, TZF, LQ, and WHW conducted the evaluation and final review and revision of the experiment.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
[1] Schade, P. 100 years of doped tungsten wire. International Journal of Refractory Metals and Hard Materials, Vol. 28, 2010, pp. 648–660.10.1016/j.ijrmhm.2010.05.003Search in Google Scholar
[2] Banik, S., K. Pradhan, and I. Das. Systematic analysis of metamagnetic transitions in A-site disordered Sm0.5(Ca0.5−Sr)MnO3 compounds: A combined experimental and model Hamiltonian study. Journal of Alloys and Compounds, Vol. 862, 2021, id. 158515.10.1016/j.jallcom.2020.158515Search in Google Scholar
[3] Cockeram, B. V. The mechanical properties and fracture mechanisms of wrought low carbon arc cast (LCAC), molybdenum–0.5pct titanium–0.1pct zirconium (TZM), and oxide dispersion strengthened (ODS) molybdenum flat products. Materials Science and Engineering: A, Vol. 418, 2006, pp. 120–136.10.1016/j.msea.2005.11.030Search in Google Scholar
[4] Cockeram, B. V. The role of stress state on the fracture toughness and toughening mechanisms of wrought molybdenum and molybdenum alloys. Materials Science and Engineering: A, Vol. 528, 2010, pp. 288–308.10.1016/j.msea.2010.09.009Search in Google Scholar
[5] Dönni, A., V. Y. Pomjakushin, L. Zhang, K. Yamaura, and A. A. Belik. Magnetic properties and ferrimagnetic structures of Mn self-doped perovskite solid solutions (Ho1−xMnx)MnO3. Journal of Alloys and Compounds, Vol. 857, 2021, p. 158230.10.1016/j.jallcom.2020.158230Search in Google Scholar
[6] Sun, G.-D. and G.-H. Zhang. Novel pathway to prepare Mo nanopowder via hydrogen reduction of MoO2 containing mo nanoseeds produced by reducing MoO3 with carbon black. JOM, Vol. 72, 2019, pp. 347–353.10.1007/s11837-019-03445-4Search in Google Scholar
[7] Tian, L., I. Anderson, T. Riedemann, and A. Russell. Roles of miRNAs in regulating the differentiation and maturation of myeloid-derived suppressor cells. Acta Materialia, Vol. 77, 2014, pp. 151–153.10.1016/j.mehy.2014.05.006Search in Google Scholar PubMed
[8] Primig, S., H. Leitner, W. Knabl, A. Lorich, H. Clemens, R., and Stickler. Influence of the heating rate on the recrystallization behavior of molybdenum. Materials Science and Engineering: A, Vol. 535, 2012, pp. 316–324.10.1016/j.msea.2011.12.099Search in Google Scholar
[9] Fan, J., M. Lu, H. Cheng, J. Tian, and B. Huang. Effect of alloying elements Ti, Zr on the property and microstructure of molybdenum. International Journal of Refractory Metals and Hard Materials, Vol. 27, 2009, pp. 78–82.10.1016/j.ijrmhm.2008.03.006Search in Google Scholar
[10] Heyuan Zhou, J. T., L. Yang, J. Wang, B. Ding, Y. Pan, X. Yu, et al. Independent thickness and lateral size sorting of two-dimensional materials. Science China Materials, Vol. 64, 2021, pp. 2739–2746.10.1007/s40843-021-1690-2Search in Google Scholar
[11] Wang, L., J. Sun, G. Liu, Y. Sun, and G. Zhang. Influences of annealing temperature on microstructure and mechanical properties of Mo–La2O3. International Journal of Refractory Metals and Hard Materials, Vol. 29, 2011, pp. 522–527.10.1016/j.ijrmhm.2011.03.003Search in Google Scholar
[12] Sun, T.-L., L.-J. Xu, S.-Z. Wei, K.-M. Pan, W.-H. Li, Y.-C. Zhou, et al. Microstructure and compression properties of fine Al2O3 particles dispersion strengthened molybdenum alloy. Transactions of Nonferrous Metals Society of China, Vol. 30, 2020, pp. 3307–3321.10.1016/S1003-6326(20)65463-2Search in Google Scholar
[13] Cui, C., X. Zhu, S. Liu, Q. Li, M. Zhang, G. Zhu, et al. Effect of nano-sized ZrO2 on high temperature performance of Mo-ZrO2 alloy. Journal of Alloys and Compounds, Vol. 768, 2018, pp. 81–87.10.1016/j.jallcom.2018.07.214Search in Google Scholar
[14] Chaopeng, C., Z. xiangwei, L. Shulong, and L. Qiang. Effect of nano-sized ZrO2 on the recrystallization of Mo alloy. Journal of Alloys and Compounds, Vol. 752, 2018, pp. 308–316.10.1016/j.jallcom.2018.04.155Search in Google Scholar
[15] Chaopeng, C., Z. Xiangwei, L. Qiang, Z. Min, and Z. Guangping. Study on the erosion of Mo/ZrO2 alloys in glass melting process. High Temperature Materials and Processes, Vol. 39, 2020, pp. 595–598.10.1515/htmp-2020-0061Search in Google Scholar
[16] Cockeram, B. V., R. W. Smith, N. Hashimoto, and L. L. Snead. The swelling, microstructure, and hardening of wrought LCAC, TZM, and ODS molybdenum following neutron irradiation. Journal of Nuclear Materials, Vol. 418, 2011, pp. 121–136.10.1016/j.jnucmat.2011.05.055Search in Google Scholar
[17] Cui, C., Y. Gao, S. Wei, G. Zhang, Y. Zhou, X. Zhu, et al. Study on preparation and properties of molybdenum alloys reinforced by nano-sized ZrO2 particles. Applied Physics A, Vol. 122, 2016, id. 214.10.1007/s00339-016-9743-1Search in Google Scholar
[18] Cui, C. and X. Zhu. The refining mechanism of ZrO2-doped molybdenum powder during the reduction process. JOM, Vol. 73, 2021, pp. 1646–1651.10.1007/s11837-021-04595-0Search in Google Scholar
[19] Xu, L., Y.-H. Xiong, J.-W. Meng, J.-B. Wang, Z.-S. Hua, Y.-P. Tian, et al. Effects of projections on radiation dose for and image quality of chest digital radiography for children. Transactions of Nonferrous Metals Society of China, Vol. 31, 2021, pp. 1496–1502.10.1016/S1003-6326(21)65593-0Search in Google Scholar
[20] Tian, Q.-H., X.-D. Gan, D.-W. Yu, F.-H. Cui, and X.-Y. Guo. One-step and selective extraction of nickel from nickel-based superalloy by molten zinc. Transactions of Nonferrous Metals Society of China, Vol. 31, 2021, pp. 1828–1841.10.1016/S1003-6326(21)65620-0Search in Google Scholar
[21] Peng, J., J. Shen, X. Yu, H. Tang, Zulfiqar, and Q. Liu. Construction of LSPR-enhanced 0D/2D CdS/MoO3− S-scheme heterojunctions for visible-light-driven photocatalytic H2 evolution. Chinese Journal of Catalysis, Vol. 42, 2021, pp. 87–96.10.1016/S1872-2067(20)63595-1Search in Google Scholar
[22] Mazumdar, D., K. Das, and I. Das. Schottky-like anomaly in the heat capacity and magnetocaloric effect of charge-ordered single-crystalline (Sm, Ca, Sr)MnO3compound. Journal of Magnetism and Magnetic Materials, Vol. 540, 2021, id. 168447.10.1016/j.jmmm.2021.168447Search in Google Scholar
[23] Ma, B.-B., S.-J. Chen, Y.-W. Huang, Z.-Z. Nie, X.-B. Qiu, X.-Q. Xie, et al. Electrochemical lithium storage performance of three-dimensional foam-like biocarbon/MoS2 composites. Transactions of Nonferrous Metals Society of China, Vol. 31, 2021, pp. 255–264.10.1016/S1003-6326(21)65492-4Search in Google Scholar
[24] Li, X.-B., T. Wu, Q.-S. Zhou, T.-G. Qi, Z.-H. Peng, and G.-H. Liu. Kinetics of oxidation roasting of molybdenite with different particle sizes. Transactions of Nonferrous Metals Society of China, Vol. 31, 2021, pp. 842–852.10.1016/S1003-6326(21)65543-7Search in Google Scholar
[25] Jin, S., H. Li, K. Chu, X. Yu, X. Guan, X. Pu, et al. High room-temperature TCR of La0.7(K0.25Sr0.05)MnO3:xAg2O composites obtained at optimized Ag2O ratio. Journal of Alloys and Compounds, Vol. 873, 2021, id. 159762.10.1016/j.jallcom.2021.159762Search in Google Scholar
[26] Nagyne-Kovacs, T., L. Studnicka, I. E. Lukacs, K. Laszlo, P. Pasierb, I. M. Szilagyi, et al. Hydrothermal synthesis and gas sensing of monoclinic MoO(3) nanosheets. Nanomaterials (Basel), Vol. 10, 2020, id. 891.10.3390/nano10050891Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- De-chlorination of poly(vinyl) chloride using Fe2O3 and the improvement of chlorine fixing ratio in FeCl2 by SiO2 addition
- Reductive behavior of nickel and iron metallization in magnesian siliceous nickel laterite ores under the action of sulfur-bearing natural gas
- Study on properties of CaF2–CaO–Al2O3–MgO–B2O3 electroslag remelting slag for rack plate steel
- The origin of {113}<361> grains and their impact on secondary recrystallization in producing ultra-thin grain-oriented electrical steel
- Channel parameter optimization of one-strand slab induction heating tundish with double channels
- Effect of rare-earth Ce on the texture of non-oriented silicon steels
- Performance optimization of PERC solar cells based on laser ablation forming local contact on the rear
- Effect of ladle-lining materials on inclusion evolution in Al-killed steel during LF refining
- Analysis of metallurgical defects in enamel steel castings
- Effect of cooling rate and Nb synergistic strengthening on microstructure and mechanical properties of high-strength rebar
- Effect of grain size on fatigue strength of 304 stainless steel
- Analysis and control of surface cracks in a B-bearing continuous casting blooms
- Application of laser surface detection technology in blast furnace gas flow control and optimization
- Preparation of MoO3 powder by hydrothermal method
- The comparative study of Ti-bearing oxides introduced by different methods
- Application of MgO/ZrO2 coating on 309 stainless steel to increase resistance to corrosion at high temperatures and oxidation by an electrochemical method
- Effect of applying a full oxygen blast furnace on carbon emissions based on a carbon metabolism calculation model
- Characterization of low-damage cutting of alfalfa stalks by self-sharpening cutters made of gradient materials
- Thermo-mechanical effects and microstructural evolution-coupled numerical simulation on the hot forming processes of superalloy turbine disk
- Endpoint prediction of BOF steelmaking based on state-of-the-art machine learning and deep learning algorithms
- Effect of calcium treatment on inclusions in 38CrMoAl high aluminum steel
- Effect of isothermal transformation temperature on the microstructure, precipitation behavior, and mechanical properties of anti-seismic rebar
- Evolution of residual stress and microstructure of 2205 duplex stainless steel welded joints during different post-weld heat treatment
- Effect of heating process on the corrosion resistance of zinc iron alloy coatings
- BOF steelmaking endpoint carbon content and temperature soft sensor model based on supervised weighted local structure preserving projection
- Innovative approaches to enhancing crack repair: Performance optimization of biopolymer-infused CXT
- Structural and electrochromic property control of WO3 films through fine-tuning of film-forming parameters
- Influence of non-linear thermal radiation on the dynamics of homogeneous and heterogeneous chemical reactions between the cone and the disk
- Thermodynamic modeling of stacking fault energy in Fe–Mn–C austenitic steels
- Research on the influence of cemented carbide micro-textured structure on tribological properties
- Performance evaluation of fly ash-lime-gypsum-quarry dust (FALGQ) bricks for sustainable construction
- First-principles study on the interfacial interactions between h-BN and Si3N4
- Analysis of carbon emission reduction capacity of hydrogen-rich oxygen blast furnace based on renewable energy hydrogen production
- Just-in-time updated DBN BOF steel-making soft sensor model based on dense connectivity of key features
- Effect of tempering temperature on the microstructure and mechanical properties of Q125 shale gas casing steel
- Review Articles
- A review of emerging trends in Laves phase research: Bibliometric analysis and visualization
- Effect of bottom stirring on bath mixing and transfer behavior during scrap melting in BOF steelmaking: A review
- High-temperature antioxidant silicate coating of low-density Nb–Ti–Al alloy: A review
- Communications
- Experimental investigation on the deterioration of the physical and mechanical properties of autoclaved aerated concrete at elevated temperatures
- Damage evaluation of the austenitic heat-resistance steel subjected to creep by using Kikuchi pattern parameters
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part II
- Synthesis of aluminium (Al) and alumina (Al2O3)-based graded material by gravity casting
- Experimental investigation into machining performance of magnesium alloy AZ91D under dry, minimum quantity lubrication, and nano minimum quantity lubrication environments
- Numerical simulation of temperature distribution and residual stress in TIG welding of stainless-steel single-pass flange butt joint using finite element analysis
- Special Issue on A Deep Dive into Machining and Welding Advancements - Part I
- Electro-thermal performance evaluation of a prismatic battery pack for an electric vehicle
- Experimental analysis and optimization of machining parameters for Nitinol alloy: A Taguchi and multi-attribute decision-making approach
- Experimental and numerical analysis of temperature distributions in SA 387 pressure vessel steel during submerged arc welding
- Optimization of process parameters in plasma arc cutting of commercial-grade aluminium plate
- Multi-response optimization of friction stir welding using fuzzy-grey system
- Mechanical and micro-structural studies of pulsed and constant current TIG weldments of super duplex stainless steels and Austenitic stainless steels
- Stretch-forming characteristics of austenitic material stainless steel 304 at hot working temperatures
- Work hardening and X-ray diffraction studies on ASS 304 at high temperatures
- Study of phase equilibrium of refractory high-entropy alloys using the atomic size difference concept for turbine blade applications
- A novel intelligent tool wear monitoring system in ball end milling of Ti6Al4V alloy using artificial neural network
- A hybrid approach for the machinability analysis of Incoloy 825 using the entropy-MOORA method
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part II
- Innovations for sustainable chemical manufacturing and waste minimization through green production practices
- Topical Issue on Conference on Materials, Manufacturing Processes and Devices - Part I
- Characterization of Co–Ni–TiO2 coatings prepared by combined sol-enhanced and pulse current electrodeposition methods
- Hot deformation behaviors and microstructure characteristics of Cr–Mo–Ni–V steel with a banded structure
- Effects of normalizing and tempering temperature on the bainite microstructure and properties of low alloy fire-resistant steel bars
- Dynamic evolution of residual stress upon manufacturing Al-based diesel engine diaphragm
- Study on impact resistance of steel fiber reinforced concrete after exposure to fire
- Bonding behaviour between steel fibre and concrete matrix after experiencing elevated temperature at various loading rates
- Diffusion law of sulfate ions in coral aggregate seawater concrete in the marine environment
- Microstructure evolution and grain refinement mechanism of 316LN steel
- Investigation of the interface and physical properties of a Kovar alloy/Cu composite wire processed by multi-pass drawing
- The investigation of peritectic solidification of high nitrogen stainless steels by in-situ observation
- Microstructure and mechanical properties of submerged arc welded medium-thickness Q690qE high-strength steel plate joints
- Experimental study on the effect of the riveting process on the bending resistance of beams composed of galvanized Q235 steel
- Density functional theory study of Mg–Ho intermetallic phases
- Investigation of electrical properties and PTCR effect in double-donor doping BaTiO3 lead-free ceramics
- Special Issue on Thermal Management and Heat Transfer
- On the thermal performance of a three-dimensional cross-ternary hybrid nanofluid over a wedge using a Bayesian regularization neural network approach
- Time dependent model to analyze the magnetic refrigeration performance of gadolinium near the room temperature
- Heat transfer characteristics in a non-Newtonian (Williamson) hybrid nanofluid with Hall and convective boundary effects
- Computational role of homogeneous–heterogeneous chemical reactions and a mixed convective ternary hybrid nanofluid in a vertical porous microchannel
- Thermal conductivity evaluation of magnetized non-Newtonian nanofluid and dusty particles with thermal radiation
Articles in the same Issue
- Research Articles
- De-chlorination of poly(vinyl) chloride using Fe2O3 and the improvement of chlorine fixing ratio in FeCl2 by SiO2 addition
- Reductive behavior of nickel and iron metallization in magnesian siliceous nickel laterite ores under the action of sulfur-bearing natural gas
- Study on properties of CaF2–CaO–Al2O3–MgO–B2O3 electroslag remelting slag for rack plate steel
- The origin of {113}<361> grains and their impact on secondary recrystallization in producing ultra-thin grain-oriented electrical steel
- Channel parameter optimization of one-strand slab induction heating tundish with double channels
- Effect of rare-earth Ce on the texture of non-oriented silicon steels
- Performance optimization of PERC solar cells based on laser ablation forming local contact on the rear
- Effect of ladle-lining materials on inclusion evolution in Al-killed steel during LF refining
- Analysis of metallurgical defects in enamel steel castings
- Effect of cooling rate and Nb synergistic strengthening on microstructure and mechanical properties of high-strength rebar
- Effect of grain size on fatigue strength of 304 stainless steel
- Analysis and control of surface cracks in a B-bearing continuous casting blooms
- Application of laser surface detection technology in blast furnace gas flow control and optimization
- Preparation of MoO3 powder by hydrothermal method
- The comparative study of Ti-bearing oxides introduced by different methods
- Application of MgO/ZrO2 coating on 309 stainless steel to increase resistance to corrosion at high temperatures and oxidation by an electrochemical method
- Effect of applying a full oxygen blast furnace on carbon emissions based on a carbon metabolism calculation model
- Characterization of low-damage cutting of alfalfa stalks by self-sharpening cutters made of gradient materials
- Thermo-mechanical effects and microstructural evolution-coupled numerical simulation on the hot forming processes of superalloy turbine disk
- Endpoint prediction of BOF steelmaking based on state-of-the-art machine learning and deep learning algorithms
- Effect of calcium treatment on inclusions in 38CrMoAl high aluminum steel
- Effect of isothermal transformation temperature on the microstructure, precipitation behavior, and mechanical properties of anti-seismic rebar
- Evolution of residual stress and microstructure of 2205 duplex stainless steel welded joints during different post-weld heat treatment
- Effect of heating process on the corrosion resistance of zinc iron alloy coatings
- BOF steelmaking endpoint carbon content and temperature soft sensor model based on supervised weighted local structure preserving projection
- Innovative approaches to enhancing crack repair: Performance optimization of biopolymer-infused CXT
- Structural and electrochromic property control of WO3 films through fine-tuning of film-forming parameters
- Influence of non-linear thermal radiation on the dynamics of homogeneous and heterogeneous chemical reactions between the cone and the disk
- Thermodynamic modeling of stacking fault energy in Fe–Mn–C austenitic steels
- Research on the influence of cemented carbide micro-textured structure on tribological properties
- Performance evaluation of fly ash-lime-gypsum-quarry dust (FALGQ) bricks for sustainable construction
- First-principles study on the interfacial interactions between h-BN and Si3N4
- Analysis of carbon emission reduction capacity of hydrogen-rich oxygen blast furnace based on renewable energy hydrogen production
- Just-in-time updated DBN BOF steel-making soft sensor model based on dense connectivity of key features
- Effect of tempering temperature on the microstructure and mechanical properties of Q125 shale gas casing steel
- Review Articles
- A review of emerging trends in Laves phase research: Bibliometric analysis and visualization
- Effect of bottom stirring on bath mixing and transfer behavior during scrap melting in BOF steelmaking: A review
- High-temperature antioxidant silicate coating of low-density Nb–Ti–Al alloy: A review
- Communications
- Experimental investigation on the deterioration of the physical and mechanical properties of autoclaved aerated concrete at elevated temperatures
- Damage evaluation of the austenitic heat-resistance steel subjected to creep by using Kikuchi pattern parameters
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part II
- Synthesis of aluminium (Al) and alumina (Al2O3)-based graded material by gravity casting
- Experimental investigation into machining performance of magnesium alloy AZ91D under dry, minimum quantity lubrication, and nano minimum quantity lubrication environments
- Numerical simulation of temperature distribution and residual stress in TIG welding of stainless-steel single-pass flange butt joint using finite element analysis
- Special Issue on A Deep Dive into Machining and Welding Advancements - Part I
- Electro-thermal performance evaluation of a prismatic battery pack for an electric vehicle
- Experimental analysis and optimization of machining parameters for Nitinol alloy: A Taguchi and multi-attribute decision-making approach
- Experimental and numerical analysis of temperature distributions in SA 387 pressure vessel steel during submerged arc welding
- Optimization of process parameters in plasma arc cutting of commercial-grade aluminium plate
- Multi-response optimization of friction stir welding using fuzzy-grey system
- Mechanical and micro-structural studies of pulsed and constant current TIG weldments of super duplex stainless steels and Austenitic stainless steels
- Stretch-forming characteristics of austenitic material stainless steel 304 at hot working temperatures
- Work hardening and X-ray diffraction studies on ASS 304 at high temperatures
- Study of phase equilibrium of refractory high-entropy alloys using the atomic size difference concept for turbine blade applications
- A novel intelligent tool wear monitoring system in ball end milling of Ti6Al4V alloy using artificial neural network
- A hybrid approach for the machinability analysis of Incoloy 825 using the entropy-MOORA method
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part II
- Innovations for sustainable chemical manufacturing and waste minimization through green production practices
- Topical Issue on Conference on Materials, Manufacturing Processes and Devices - Part I
- Characterization of Co–Ni–TiO2 coatings prepared by combined sol-enhanced and pulse current electrodeposition methods
- Hot deformation behaviors and microstructure characteristics of Cr–Mo–Ni–V steel with a banded structure
- Effects of normalizing and tempering temperature on the bainite microstructure and properties of low alloy fire-resistant steel bars
- Dynamic evolution of residual stress upon manufacturing Al-based diesel engine diaphragm
- Study on impact resistance of steel fiber reinforced concrete after exposure to fire
- Bonding behaviour between steel fibre and concrete matrix after experiencing elevated temperature at various loading rates
- Diffusion law of sulfate ions in coral aggregate seawater concrete in the marine environment
- Microstructure evolution and grain refinement mechanism of 316LN steel
- Investigation of the interface and physical properties of a Kovar alloy/Cu composite wire processed by multi-pass drawing
- The investigation of peritectic solidification of high nitrogen stainless steels by in-situ observation
- Microstructure and mechanical properties of submerged arc welded medium-thickness Q690qE high-strength steel plate joints
- Experimental study on the effect of the riveting process on the bending resistance of beams composed of galvanized Q235 steel
- Density functional theory study of Mg–Ho intermetallic phases
- Investigation of electrical properties and PTCR effect in double-donor doping BaTiO3 lead-free ceramics
- Special Issue on Thermal Management and Heat Transfer
- On the thermal performance of a three-dimensional cross-ternary hybrid nanofluid over a wedge using a Bayesian regularization neural network approach
- Time dependent model to analyze the magnetic refrigeration performance of gadolinium near the room temperature
- Heat transfer characteristics in a non-Newtonian (Williamson) hybrid nanofluid with Hall and convective boundary effects
- Computational role of homogeneous–heterogeneous chemical reactions and a mixed convective ternary hybrid nanofluid in a vertical porous microchannel
- Thermal conductivity evaluation of magnetized non-Newtonian nanofluid and dusty particles with thermal radiation

