Abstract
This research comprehensively explores the synthesis of Co–Ni–TiO2 coatings employing a hybrid methodology that integrates sol-enhanced and pulse current electrodeposition techniques. The investigation examined the surface morphology and intrinsic properties of Co–Ni–TiO2 coatings, revealing the significant influence of pulse duty cycle variations on the characteristics of coatings. A detailed analysis indicates that a pulse duty cycle of 0.4 optimized the coating’s performance, offering superior attributes compared to other duty cycle settings. The study elucidates that lower duty cycles foster hydrogen evolution reactions on the cathode surface, culminating in the formation of a porous, needle-like coating structure. Conversely, higher duty cycles are found to mitigate the effects of material replenishment, thereby affecting the coating’s quality and performance. The findings of this investigation not only shed light on the critical relationship between the pulse duty cycle and the properties of Co–Ni–TiO2 coatings but also lay a foundational framework for the further refinement and optimization of these coatings for advanced applications.
1 Introduction
Cobalt-nickel (Co–Ni) coatings [1,2,3] are extensively utilized across various industries owing to their remarkable physical and chemical advantages, alongside notable mechanical characteristics. These coatings are renowned for possessing low internal stress, elevated hardness, and superior wear resistance, making them promising protective decorative layers. The fabrication of Co–Ni coatings with desirable properties can be achieved through several established methods, including but not limited to chemical plating [4,5,6], electroplating [7,8,9], pulsed electrodeposition, chemical vapor deposition [10,11,12,13], plasma spraying [14,15,16,17], and electroless plating [18,19,20].
Recent advancements in the field of electrodeposited Co–Ni coating research have highlighted enhancing their performance through nanoparticle reinforcement [21,22,23,24] and the adoption of pulse electrodeposition techniques [25,26]. Pulse electrodeposition [27], in particular, has been shown to significantly improve electrochemical polarization, ensure the timely replenishment of metal ions and nanoparticles near the cathode, and mitigate issues related to concentration polarization and internal stress compared to traditional direct current electrodeposition methods. Furthermore, the introduction of nanoparticles within the coating matrix has been identified as a viable strategy to enhance overall coating performance by facilitating the even distribution of these particles. Despite these advantages, the propensity of nanoparticles to aggregate due to their high-energy surfaces poses a substantial challenge.
Addressing this concern, our research group has made significant strides by developing a novel chemical synthesis approach for producing titanium dioxide (TiO2) sol [28,29,30] characterized by its excellent dispersibility and stability. The introduction of this TiO2 sol into the sol-enhanced electrodeposition process enables the in situ generation and co-deposition of nanoparticles alongside metal ions, effectively circumventing the issue of aggregation and ensuring the uniform distribution of nanoparticles within the coatings.
In this study, we explore the synergistic effects of combining pulse current electrodeposition with sol-enhanced electrodeposition techniques to fabricate Co–Ni–TiO2 coatings of superior quality. Our investigation delves into how varying concentrations of TiO2 sol influence the coatings’ microstructure, phase composition, hardness, wear resistance, and corrosion resistance. The outcomes of this research underscore the potential of sol-enhanced and pulse electrodeposition methods in augmenting the mechanical properties and corrosion resistance of Co–Ni–TiO2 coatings, thereby presenting a promising pathway for the development of advanced coating technologies.
2 Experimental
2.1 Pulse current electrodeposition of Co–Ni–TiO2 coatings
The preparation of TiO2 sol was accurately conducted following specific methodologies outlined in our previously published work [28,29]: 8.68 mL of tetrabutylorthotitanate [Ti(OBu)4] was dissolved in a mixture solution of 35 mL ethanol and 2.82 mL diethanolamine. After magnetic stirring for 2 h, it was hydrolyzed by adding a mixture of 0.45 mL deionized water and 4.5 mL ethanol dropwise under magnetic stirring.
In the current study, Co–Ni–TiO2 coatings were synthesized utilizing a pulse current electrodeposition technique. This method was applied to a copper sheet substrate, which served as the cathode. Simultaneously, a high-purity cobalt plate was employed as the anode to facilitate the deposition process. To achieve precise control over the electrodeposition process, an adjustable pulse power supply was utilized. This setup allowed for the adjustment of current pulses, which is essential for the uniform deposition of the Co–Ni–TiO2 coatings. During the electrodeposition process, a meticulously measured volume of 5 mL·L−1 of TiO2 sol was incrementally introduced into the electrolytic solutions. This mixture was continuously stirred at a speed of 500 rpm to ensure a homogeneous distribution of the sol within the solution.
The electrodeposition bath consisted of an electrolyte solution that was formulated to include 70 g·L−1 nickel sulfate (NiSO4), 50 g·L−1 nickel chloride (NiCl), 60 g·L−1 cobalt sulfate (CoSO4), and 50 g·L−1 boric acid (H3BO3). Additionally, to enhance the quality of the coating, sodium dodecyl sulfate (C12H25SO4Na) at a concentration of 0.015 g·L−1 and saccharin (C7H5NO3S) at 0.15 g·L−1 were added as surfactants. The electrodeposition was conducted at a temperature of 50°C. For this experiment, a current density of 25 mA·cm−2 was maintained. Moreover, the solution was stirred at a speed of 300 rpm for 30 min. The process parameters, including different duty cycles, were varied to investigate their effects on the morphology and properties of the resulting coatings.
2.2 Characterization of the coatings
Co–Ni–TiO2 coatings were characterized to assess their microstructural, mechanical, and electrochemical properties. Surface and wear track morphologies were examined using a scanning electron microscope (SEM, Hitachi SU-8010) and an energy-dispersive X-ray spectroscope (Horiba, X-Max 50).
The phase composition of the coatings was meticulously determined using a D8 Advance-Bruker AXS X-ray diffractometer (XRD). This analysis was critical for identifying the crystalline structures present in the coatings, which directly impact their mechanical properties. The microhardness of the coatings was measured using a sclerometer. Wear properties were evaluated using a friction instrument (MSR-2T, Lanzhou, China). For these tests, zirconia balls of 4 mm diameter were employed under a load of 5 N at a wear rate of 10 mm·s−1. For each sample, the tested hardness was the average of five tests conducted randomly on the sample surface.
Electrochemical measurements were performed in a 3.5 wt% sodium chloride (NaCl) solution to evaluate the coatings’ corrosion resistance. These tests were conducted at a scan rate of 1 mV·s−1 using an electrochemical workstation (CS2350, Corrtest, Wuhan, China) in a standard three-electrode cell configuration.
3 Results and discussion
3.1 Structural and phase characterization
Various duty cycles of pulse electrodepositions were utilized to fabricate Co–Ni–TiO2 coatings. Figure 1 illustrates the SEM and XRD analyses of the Co–Ni-5 mL·L−1 TiO2 coatings synthesized at different duty cycles. At a low duty cycle of 0.2, the SEM images reveal that the Co–Ni–TiO2 coating exhibits a loosely structured needle-like morphology. This can be attributed to the short duty cycle, which induces a higher cathodic overpotential, thereby enhancing the hydrogen evolution reaction at the cathode. This reaction significantly influences the morphology of the coating’s surface, leading to the formation of a comparatively loose structure. When the duty cycle is increased to 0.4, this modification reduces the cathodic overpotential by lowering the current density applied during the pulse duration, which in turn, diminishes the hydrogen evolution reactions and lowers their effects on the surface structures. Nonetheless, the cathodic overpotential remains sufficiently high to favor the nucleation rate of the Co–Ni–TiO2 coating. In this scenario, the nucleation predominates over grain growth, resulting in a more refined surface morphology characterized by smaller grain sizes. As the duty cycle increases to 0.6 and 0.8, the cathodic overpotential decreases further, leading to a slowdown in the nucleation process. Under these conditions, the grain growth process takes precedence over nucleation. Additionally, the decrease in the pulse current turn-off time restricts the supply of metal ions near the cathode surface and reduces the number of nucleation sites, both of which favor grain growth and lead to grain coarsening.

Surface SEM morphologies of Co–Ni–TiO2 coatings at different duty cycles of (a) 0.2, (b) 0.4, (c) 0.6, and (d) 0.8; (e) X-ray diffraction patterns of Co–Ni–TiO2 coatings at different duty cycles.
Figure 1e shows the XRD patterns of the Co–Ni–TiO2 coatings prepared under various duty cycles. The diffraction peaks are observed at (111), (200), and (220) planes at a duty cycle of 0.2. The hydrogen evolution reactions at lower duty cycles could alter the textures of the XRD diffractograms, resulting in prominent peaks at (111) and (200). The incorporation of Ni atoms into the Co lattice structure shifts the characteristic peak of Co from the standard peak positions. As the duty cycle is increased, the diffraction peaks at (111) and (200) planes vanish, while the intensity of the peak at (220) plane grows. This pattern shows the necessity of regulating the duty cycle to achieve optimal coating deposition while mitigating the detrimental effects on hydrogen evolution reactions and grain coarsening.
Figure 2 demonstrates the SEM images of Co–Ni–TiO2 coatings obtained under varying duty cycles. It reveals that at a duty cycle of 0.2, cracking between the coating and substrate is observed. This phenomenon can be attributed to the fact that at lower duty cycles, the elevated cathodic overpotential causes an intense hydrogen evolution reaction. This gas-release process could result in a porous, needle-like structure. Consequently, this leads to a reduction in the adhesion strength of the coating and a decrease in coating thickness due to the consumption of applied current in the side reactions. As the duty cycle is increased to 0.4, the reduced extent of the hydrogen evolution reaction is conducive to the deposition of the coating and enhances the deposition efficiency, leading to a thicker coating. Further increase in the duty cycle results in a thinner coating thickness. The shortened off-time of the pulse current is detrimental to the replenishment of metal ions at the cathode surface. Therefore, it slows down the deposition of metal ions and reduces the efficiency of deposition on the cathode surface, which in turn leads to a thinner coating.

Cross-sectional morphology of Co–Ni–TiO2 coatings at different duty cycles of (a) 0.2, (b) 0.4, (c) 0.6, and (d) 0.8.
3.2 Mechanical and corrosion performance
Figure 3 presents the mechanical testing outcomes. Figure 3a elucidates the hardness of Co–Ni–TiO2 coatings synthesized at varying duty cycles, indicating an initial increase in hardness followed by a decrease as the duty cycle increases. The decrease in hardness at a duty cycle of 0.2 is linked to the loose surface structure formed by the vigorous hydrogen evolution reactions. Elevating the duty cycle to 0.4 mitigates the hydrogen evolution reaction, enhancing the quality of the coating and, consequently, its hardness. This improvement in hardness can be attributed to the effects of fine-grain and dispersion strengthening on the coating’s performance in hardness tests. Under these conditions, the replenishment of TiO2 nanoparticles resulting from the relaxation time during pulse current could increase the number of nanoparticles incorporated into the composite layer with significant dispersion. The increased nucleation sites and the appropriately increased applied current could help to properly refine the coating surface and give a fine-grain straightening effect. The increased dispersion strengthening by the well-distributed nanoparticles also contributes to better mechanical performance.

(a) Microhardness and (b) average friction coefficients of Co–Ni–TiO2 coatings with different duty cycles. The morphology of the wear track of the Co–Ni–TiO2 composite coating prepared at duty cycles of (c) 0.2, (d) 0.4, (e) 0.6, and (f) 0.8.
However, a reduction in coating hardness is observed at a duty cycle of 0.8, likely due to a decrease in the fine-grain strengthening effect. Figure 3b shows the average friction coefficient of the Co–Ni–TiO2 coating at various duty cycles, revealing an initial decrease followed by an increase in the friction coefficient with increasing duty cycles. This behavior is corroborated by the wear track width measurements displayed in Figure 3c–f, where the width decreases from 369 μm at a duty cycle of 0.2–255 μm at 0.4 but increases to 375 μm at a duty cycle of 0.8.
The corrosion behavior of the Co–Ni–TiO2 coating under different duty cycles was also examined. Figure 4 depicts the potentiodynamic polarization curves of the coatings fabricated at varied duty cycles, indicating that the corrosion resistance of the Co–Ni coating initially improves with increasing duty cycle but deteriorates at a duty cycle over 0.6. Table 1 presents the Tafel curve fitting results, indicating a corrosion rate of 0.071 mm·a−1 for the Co–Ni–TiO2 coating at a duty cycle of 0.2. The formation of a loose needle-like structure on the coating’s surface at this duty cycle facilitates the penetration of the corrosive medium, thereby diminishing the coating’s corrosion resistance. Elevating the duty cycle to 0.4 significantly reduces the corrosion rate to 0.034 mm·a−1, signifying improved corrosion resistance at this higher duty cycle. The optimal corrosion resistance observed at a duty cycle of 0.4 can be ascribed to its compact surface, which effectively blocks corrosion attacks. However, further increasing the duty cycle to 0.8 leads to an increase in the corrosion rate to 0.072 mm·a−1, as the coarsened surface lowers the coating’s density and corrosion resistance.
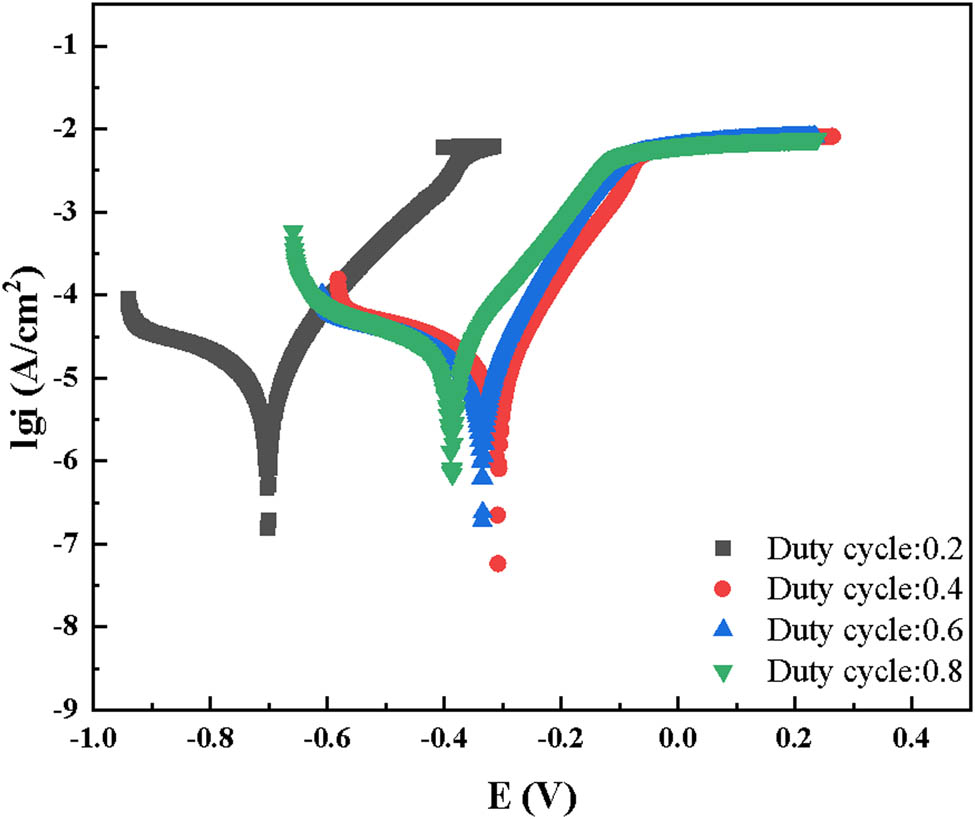
Potentiodynamic polarization curves of the Co–Ni–TiO2 coating under different duty cycles.
Fitting results of potential polarization curves of Co–Ni–TiO2
| Duty cycle | E corr (V) | I corr (μA·cm−2) | Corrosion rate (mm·a−1) |
|---|---|---|---|
| 0.2 | −0.70 | 3.30 | 0.071 |
| 0.4 | −0.31 | 1.59 | 0.034 |
| 0.6 | −0.33 | 2.04 | 0.044 |
| 0.8 | −0.34 | 3.33 | 0.072 |
4 Conclusions
In this study, Co–Ni–TiO2 coatings were synthesized via an innovative method that combined pulse current with sol-enhanced electrodeposition techniques, which exhibited superior characteristics. The synthesis of Co–Ni–TiO2 coatings was conducted across varying pulse duty cycles to ascertain the optimal conditions for achieving a compact surface morphology. It was demonstrated that employing a pulse duty cycle of 0.4 significantly enhanced surface compactness. Furthermore, the application of this specific pulse duty cycle in the fabrication process of Co–Ni–TiO2 coatings achieved the most diminutive grain size, alongside optimizing mechanical robustness and corrosion resistance. The outcomes of this comprehensive study shed light on the intricate relationship between the duty cycle parameters and the resultant properties of Co–Ni–TiO2 coatings. This investigation lays a foundational framework for the future optimization and enhancement of these coatings, aiming to maximize their performance and applicability in various industrial sectors.
Acknowledgments
This work was supported by the Jiangsu University of Science and Technology Undergraduate Innovation and Entrepreneurship Fund.
-
Author contributions: Zihan Liu, Songling Zheng, Zengcheng Miao, Jiaming Liu: Investigation and methodology; Zhen He and Jiajian Guan: Writing, reviewing and Investigation.
-
Conflict of interest: Authors state no conflict of interest.
References
[1] Wu, G., N. Li, D. Zhou, and K. Mitsuo. Electrodeposited Co–Ni–Al2O3 composite coatings. Surface and Coatings Technology, Vol. 176, 2004, pp. 157–164.Search in Google Scholar
[2] Ma, C., S. C. Wang, L. P. Wang, F. C. Walsh, and R. J. K. Wood. The electrodeposition and characterisation of low-friction and wear-resistant Co-Ni-P coatings. Surface and Coatings Technology, Vol. 235, 2013, pp. 495–505.Search in Google Scholar
[3] Adesina, O. S., B. A. Obadele, G. A. Farotade, D .A. Isadare, A. A. Adediran, and P. P. Ikubanni. Influence of phase composition and microstructure on corrosion behavior of laser based Ti–Co–Ni ternary coatings on Ti–6Al–4V alloy. Journal of Alloys and Compounds, Vol. 827, 2020, id. 154245.Search in Google Scholar
[4] Hao, W. S. Study of wear-resistance of chemical plating on the surface of 65Mn steel. Journal of Daqing Petroleum Institute, Vol. 28, 2004, id. 91.Search in Google Scholar
[5] Niederprüm, H. Chemical nickel plating. Angewandte Chemie International Edition in English, Vol. 14, No. 9, 1975, pp. 614–620.Search in Google Scholar
[6] Li, F., Y. Kang, D. Zhang, D. Sichen. Nickel coating on some organic and carbon fibres by chemical plating. International Journal of Materials Research (formerly Zeitschrift fuer Metallkunde), Vol. 99, 2008, pp. 84–91.Search in Google Scholar
[7] Andricacos, P. C., C. Uzoh, J. O. Dukovic, J. Horkans, and H. Deligianni. Damascene copper electroplating for chip interconnections. Ibm Journal of Research & Development, Vol. 42, 1998, pp. 567–574.Search in Google Scholar
[8] Chan, F. T. S. Application of a hybrid case-based reasoning approach in electroplating industry. Expert Systems with Applications, Vol. 29, 2005, pp. 121–130.Search in Google Scholar
[9] Zhang, Z., A. Kitada, K. Fukami, and K. Murase. Aluminum electroplating on AZ31 magnesium alloy with acetic anhydride pretreatment. Acta Metallurgica Sinica, Vol. 35, 2022, id. 11.Search in Google Scholar
[10] Seah, C. M., S. P. Chai, and A. R. Mohamed. Mechanisms of graphene growth by chemical vapour deposition on transition metals. Carbon: An International Journal Sponsored by the American Carbon Society, Vol. 70, 2014, pp. 1–21.Search in Google Scholar
[11] Chuang, A. T., B. O. Boskovic, and J. Robertson. Freestanding carbon nanowalls by microwave plasma-enhanced chemical vapour deposition. Diamond & Related Materials, Vol. 15, 2006, pp. 1103–1106.Search in Google Scholar
[12] Choy, K. L. Chemical vapour deposition of coatings. Progress in Materials Science, Vol. 48, No. 2, 2003, pp. 57–170.Search in Google Scholar
[13] Espinoza-Pérez, L. J., et al. Plasma enhanced chemical vapour deposition of ZrO_2 and YSZ coatings. Materials Science and Technology: MST: A publication of the Institute of Metals, Vol. 39, 2023, pp. 1977–1987.Search in Google Scholar
[14] Ducheyne, S. R. R. Plasma spraying induced changes of calcium phosphate ceramic characteristics and the effect on in vitro stability. Journal of Materials Science: Materials in Medicine, Vol. 3, 1992, pp. 33–42.Search in Google Scholar
[15] Vardelle, M., A. Vardelle, P. Fauchais, K. I. Li, B. Dussoubs, and N. J. Themelis. Controlling particle injection in plasma spraying. Journal of Thermal Spray Technology, Vol. 10, 2001, pp. 267–284.Search in Google Scholar
[16] Ma, W., Y. Ma, S. K. Gong, H. B. Xu, and X. Q. Cao. Thermal cycling behavior of lanthanum-cerium oxide thermal barrier coatings prepared by air plasma spraying. Key Engineering Materials, Vol. 336–338, 2007, pp. 1759–1761.Search in Google Scholar
[17] Guan, Y., Y. Xu, Z. Y. Zheng, and X. H. Tong. Microstructure and tribological properties of nano- and submicron-structured MoS2 coating deposited by plasma spraying. Transactions of the China Welding Institution, Vol. 12, 2005, pp. 51–54.Search in Google Scholar
[18] Wang, Y., J. Gou, H. Zhang, X. Yang, H. Zhang, and X. Li. Ni-P-SBR composite-electroless-plating enables Si anode with high conductivity and elasticity for high performance Li-ion batteries application. Journal of Energy Chemistry, Vol. 59, 2023, pp. 59–66.Search in Google Scholar
[19] Akram, S., A. Javid, and M. Ashraf. Silver electroless plating on aminated graphene oxide-based cotton fabric for electromagnetic interference shielding and bioactivity. Materials Science and Engineering: B, Vol. 288, 2023, id. 116159.Search in Google Scholar
[20] Bai-Yu, Z. Comparative Study on microstructure and properties of electroless Ni-P and Ni-Cu-P coatings deposited on PEEK. Materials Protection, Vol. 56, 2023, id. 127.Search in Google Scholar
[21] Li, Q., X. Yang, L. Zhang, J. Wang, and B. Chen. Corrosion resistance and mechanical properties of pulse electrodeposited Ni-TiO2 composite coating for sintered NdFeB magnet. Journal of Alloys & Compounds, Vol. 482, 2009, id. 339.Search in Google Scholar
[22] Setiawan, A. R., M. Noorprajuda, A. Ramelan, and R. Suratman. Preparation of Zn-ZrO2 nanocomposite coating by DC and pulsed current electrodeposition technique with low current density. Materials Science Forum, Vol. 827, 2015, pp. 332–337.Search in Google Scholar
[23] Zhang, D., P. Zhang, J. Chen, Z. He, and M. Wu. Fabrication and characterization of Ni–Fe–P–TiO2 nanocomposite coatings. International Journal of Modern Physics B, Vol. 36, 2022, id. 2240049.Search in Google Scholar
[24] Ghaderi, M., M. Rezagholizadeh, A. Heidary, and S. M. Monirvaghefi. The effect of Al2O3 nanoparticles on tribological and corrosion behavior of electroless Ni–B–Al2O3 composite coating. Protection of Metals and Physical Chemistry of Surfaces, Vol. 52, 2016, pp. 854–858.Search in Google Scholar
[25] Nielsch, K., F. Müller, A. P. Li, and U. Gösele. Uniform nickel deposition into ordered alumina pores by pulsed electrodeposition. Advanced Materials, Vol. 12, No. 8, 2000, pp. 582–586.Search in Google Scholar
[26] Beattie, S. D. and J. R. Dahn. Single bath, pulsed electrodeposition of copper-tin alloy negative electrodes for lithium-ion batteries. Journal of the Electrochemical Society, Vol. 150, 2003, id. A894.Search in Google Scholar
[27] Vazquez-Arenas, J., T. Treeratanaphitak, and M. Pritzker. Formation of Co–Ni alloy coatings under direct current, pulse current and pulse-reverse plating conditions. Electrochimica Acta, Vol. 62, 2012, pp. 63–72.Search in Google Scholar
[28] Wang, Y., Z. Miao, S. Zheng, J. Chen, and Z. He. An investigation into electrodeposited Co− Ni− TiO2 films with improved mechanical and corrosion properties. Coatings, Vol. 13, 2023, id. 783.Search in Google Scholar
[29] Wang, Y., W. Gao, Z. He, Y. Jin, Y. Qiao, G. Cheng, et al. Cu–Sn–Zn nanocomposite coatings prepared by TiO2 sol-enhanced electrodeposition. Journal of Applied Electrochemistry, Vol. 50, 2020, pp. 75–885.Search in Google Scholar
[30] Zhang, W., D. Cao, Y. Qiao, Z. He, Y. Wang, X. Li, et al. Microstructure and properties of duplex Ni-P-TiO 2/Ni-P nanocomposite coatings. Materials Research, Vol. 22, 2019, id. e20180748.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- De-chlorination of poly(vinyl) chloride using Fe2O3 and the improvement of chlorine fixing ratio in FeCl2 by SiO2 addition
- Reductive behavior of nickel and iron metallization in magnesian siliceous nickel laterite ores under the action of sulfur-bearing natural gas
- Study on properties of CaF2–CaO–Al2O3–MgO–B2O3 electroslag remelting slag for rack plate steel
- The origin of {113}<361> grains and their impact on secondary recrystallization in producing ultra-thin grain-oriented electrical steel
- Channel parameter optimization of one-strand slab induction heating tundish with double channels
- Effect of rare-earth Ce on the texture of non-oriented silicon steels
- Performance optimization of PERC solar cells based on laser ablation forming local contact on the rear
- Effect of ladle-lining materials on inclusion evolution in Al-killed steel during LF refining
- Analysis of metallurgical defects in enamel steel castings
- Effect of cooling rate and Nb synergistic strengthening on microstructure and mechanical properties of high-strength rebar
- Effect of grain size on fatigue strength of 304 stainless steel
- Analysis and control of surface cracks in a B-bearing continuous casting blooms
- Application of laser surface detection technology in blast furnace gas flow control and optimization
- Preparation of MoO3 powder by hydrothermal method
- The comparative study of Ti-bearing oxides introduced by different methods
- Application of MgO/ZrO2 coating on 309 stainless steel to increase resistance to corrosion at high temperatures and oxidation by an electrochemical method
- Effect of applying a full oxygen blast furnace on carbon emissions based on a carbon metabolism calculation model
- Characterization of low-damage cutting of alfalfa stalks by self-sharpening cutters made of gradient materials
- Thermo-mechanical effects and microstructural evolution-coupled numerical simulation on the hot forming processes of superalloy turbine disk
- Endpoint prediction of BOF steelmaking based on state-of-the-art machine learning and deep learning algorithms
- Effect of calcium treatment on inclusions in 38CrMoAl high aluminum steel
- Effect of isothermal transformation temperature on the microstructure, precipitation behavior, and mechanical properties of anti-seismic rebar
- Evolution of residual stress and microstructure of 2205 duplex stainless steel welded joints during different post-weld heat treatment
- Effect of heating process on the corrosion resistance of zinc iron alloy coatings
- BOF steelmaking endpoint carbon content and temperature soft sensor model based on supervised weighted local structure preserving projection
- Innovative approaches to enhancing crack repair: Performance optimization of biopolymer-infused CXT
- Structural and electrochromic property control of WO3 films through fine-tuning of film-forming parameters
- Influence of non-linear thermal radiation on the dynamics of homogeneous and heterogeneous chemical reactions between the cone and the disk
- Thermodynamic modeling of stacking fault energy in Fe–Mn–C austenitic steels
- Research on the influence of cemented carbide micro-textured structure on tribological properties
- Performance evaluation of fly ash-lime-gypsum-quarry dust (FALGQ) bricks for sustainable construction
- First-principles study on the interfacial interactions between h-BN and Si3N4
- Analysis of carbon emission reduction capacity of hydrogen-rich oxygen blast furnace based on renewable energy hydrogen production
- Just-in-time updated DBN BOF steel-making soft sensor model based on dense connectivity of key features
- Effect of tempering temperature on the microstructure and mechanical properties of Q125 shale gas casing steel
- Review Articles
- A review of emerging trends in Laves phase research: Bibliometric analysis and visualization
- Effect of bottom stirring on bath mixing and transfer behavior during scrap melting in BOF steelmaking: A review
- High-temperature antioxidant silicate coating of low-density Nb–Ti–Al alloy: A review
- Communications
- Experimental investigation on the deterioration of the physical and mechanical properties of autoclaved aerated concrete at elevated temperatures
- Damage evaluation of the austenitic heat-resistance steel subjected to creep by using Kikuchi pattern parameters
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part II
- Synthesis of aluminium (Al) and alumina (Al2O3)-based graded material by gravity casting
- Experimental investigation into machining performance of magnesium alloy AZ91D under dry, minimum quantity lubrication, and nano minimum quantity lubrication environments
- Numerical simulation of temperature distribution and residual stress in TIG welding of stainless-steel single-pass flange butt joint using finite element analysis
- Special Issue on A Deep Dive into Machining and Welding Advancements - Part I
- Electro-thermal performance evaluation of a prismatic battery pack for an electric vehicle
- Experimental analysis and optimization of machining parameters for Nitinol alloy: A Taguchi and multi-attribute decision-making approach
- Experimental and numerical analysis of temperature distributions in SA 387 pressure vessel steel during submerged arc welding
- Optimization of process parameters in plasma arc cutting of commercial-grade aluminium plate
- Multi-response optimization of friction stir welding using fuzzy-grey system
- Mechanical and micro-structural studies of pulsed and constant current TIG weldments of super duplex stainless steels and Austenitic stainless steels
- Stretch-forming characteristics of austenitic material stainless steel 304 at hot working temperatures
- Work hardening and X-ray diffraction studies on ASS 304 at high temperatures
- Study of phase equilibrium of refractory high-entropy alloys using the atomic size difference concept for turbine blade applications
- A novel intelligent tool wear monitoring system in ball end milling of Ti6Al4V alloy using artificial neural network
- A hybrid approach for the machinability analysis of Incoloy 825 using the entropy-MOORA method
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part II
- Innovations for sustainable chemical manufacturing and waste minimization through green production practices
- Topical Issue on Conference on Materials, Manufacturing Processes and Devices - Part I
- Characterization of Co–Ni–TiO2 coatings prepared by combined sol-enhanced and pulse current electrodeposition methods
- Hot deformation behaviors and microstructure characteristics of Cr–Mo–Ni–V steel with a banded structure
- Effects of normalizing and tempering temperature on the bainite microstructure and properties of low alloy fire-resistant steel bars
- Dynamic evolution of residual stress upon manufacturing Al-based diesel engine diaphragm
- Study on impact resistance of steel fiber reinforced concrete after exposure to fire
- Bonding behaviour between steel fibre and concrete matrix after experiencing elevated temperature at various loading rates
- Diffusion law of sulfate ions in coral aggregate seawater concrete in the marine environment
- Microstructure evolution and grain refinement mechanism of 316LN steel
- Investigation of the interface and physical properties of a Kovar alloy/Cu composite wire processed by multi-pass drawing
- The investigation of peritectic solidification of high nitrogen stainless steels by in-situ observation
- Microstructure and mechanical properties of submerged arc welded medium-thickness Q690qE high-strength steel plate joints
- Experimental study on the effect of the riveting process on the bending resistance of beams composed of galvanized Q235 steel
- Density functional theory study of Mg–Ho intermetallic phases
- Investigation of electrical properties and PTCR effect in double-donor doping BaTiO3 lead-free ceramics
- Special Issue on Thermal Management and Heat Transfer
- On the thermal performance of a three-dimensional cross-ternary hybrid nanofluid over a wedge using a Bayesian regularization neural network approach
- Time dependent model to analyze the magnetic refrigeration performance of gadolinium near the room temperature
- Heat transfer characteristics in a non-Newtonian (Williamson) hybrid nanofluid with Hall and convective boundary effects
- Computational role of homogeneous–heterogeneous chemical reactions and a mixed convective ternary hybrid nanofluid in a vertical porous microchannel
- Thermal conductivity evaluation of magnetized non-Newtonian nanofluid and dusty particles with thermal radiation
Articles in the same Issue
- Research Articles
- De-chlorination of poly(vinyl) chloride using Fe2O3 and the improvement of chlorine fixing ratio in FeCl2 by SiO2 addition
- Reductive behavior of nickel and iron metallization in magnesian siliceous nickel laterite ores under the action of sulfur-bearing natural gas
- Study on properties of CaF2–CaO–Al2O3–MgO–B2O3 electroslag remelting slag for rack plate steel
- The origin of {113}<361> grains and their impact on secondary recrystallization in producing ultra-thin grain-oriented electrical steel
- Channel parameter optimization of one-strand slab induction heating tundish with double channels
- Effect of rare-earth Ce on the texture of non-oriented silicon steels
- Performance optimization of PERC solar cells based on laser ablation forming local contact on the rear
- Effect of ladle-lining materials on inclusion evolution in Al-killed steel during LF refining
- Analysis of metallurgical defects in enamel steel castings
- Effect of cooling rate and Nb synergistic strengthening on microstructure and mechanical properties of high-strength rebar
- Effect of grain size on fatigue strength of 304 stainless steel
- Analysis and control of surface cracks in a B-bearing continuous casting blooms
- Application of laser surface detection technology in blast furnace gas flow control and optimization
- Preparation of MoO3 powder by hydrothermal method
- The comparative study of Ti-bearing oxides introduced by different methods
- Application of MgO/ZrO2 coating on 309 stainless steel to increase resistance to corrosion at high temperatures and oxidation by an electrochemical method
- Effect of applying a full oxygen blast furnace on carbon emissions based on a carbon metabolism calculation model
- Characterization of low-damage cutting of alfalfa stalks by self-sharpening cutters made of gradient materials
- Thermo-mechanical effects and microstructural evolution-coupled numerical simulation on the hot forming processes of superalloy turbine disk
- Endpoint prediction of BOF steelmaking based on state-of-the-art machine learning and deep learning algorithms
- Effect of calcium treatment on inclusions in 38CrMoAl high aluminum steel
- Effect of isothermal transformation temperature on the microstructure, precipitation behavior, and mechanical properties of anti-seismic rebar
- Evolution of residual stress and microstructure of 2205 duplex stainless steel welded joints during different post-weld heat treatment
- Effect of heating process on the corrosion resistance of zinc iron alloy coatings
- BOF steelmaking endpoint carbon content and temperature soft sensor model based on supervised weighted local structure preserving projection
- Innovative approaches to enhancing crack repair: Performance optimization of biopolymer-infused CXT
- Structural and electrochromic property control of WO3 films through fine-tuning of film-forming parameters
- Influence of non-linear thermal radiation on the dynamics of homogeneous and heterogeneous chemical reactions between the cone and the disk
- Thermodynamic modeling of stacking fault energy in Fe–Mn–C austenitic steels
- Research on the influence of cemented carbide micro-textured structure on tribological properties
- Performance evaluation of fly ash-lime-gypsum-quarry dust (FALGQ) bricks for sustainable construction
- First-principles study on the interfacial interactions between h-BN and Si3N4
- Analysis of carbon emission reduction capacity of hydrogen-rich oxygen blast furnace based on renewable energy hydrogen production
- Just-in-time updated DBN BOF steel-making soft sensor model based on dense connectivity of key features
- Effect of tempering temperature on the microstructure and mechanical properties of Q125 shale gas casing steel
- Review Articles
- A review of emerging trends in Laves phase research: Bibliometric analysis and visualization
- Effect of bottom stirring on bath mixing and transfer behavior during scrap melting in BOF steelmaking: A review
- High-temperature antioxidant silicate coating of low-density Nb–Ti–Al alloy: A review
- Communications
- Experimental investigation on the deterioration of the physical and mechanical properties of autoclaved aerated concrete at elevated temperatures
- Damage evaluation of the austenitic heat-resistance steel subjected to creep by using Kikuchi pattern parameters
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part II
- Synthesis of aluminium (Al) and alumina (Al2O3)-based graded material by gravity casting
- Experimental investigation into machining performance of magnesium alloy AZ91D under dry, minimum quantity lubrication, and nano minimum quantity lubrication environments
- Numerical simulation of temperature distribution and residual stress in TIG welding of stainless-steel single-pass flange butt joint using finite element analysis
- Special Issue on A Deep Dive into Machining and Welding Advancements - Part I
- Electro-thermal performance evaluation of a prismatic battery pack for an electric vehicle
- Experimental analysis and optimization of machining parameters for Nitinol alloy: A Taguchi and multi-attribute decision-making approach
- Experimental and numerical analysis of temperature distributions in SA 387 pressure vessel steel during submerged arc welding
- Optimization of process parameters in plasma arc cutting of commercial-grade aluminium plate
- Multi-response optimization of friction stir welding using fuzzy-grey system
- Mechanical and micro-structural studies of pulsed and constant current TIG weldments of super duplex stainless steels and Austenitic stainless steels
- Stretch-forming characteristics of austenitic material stainless steel 304 at hot working temperatures
- Work hardening and X-ray diffraction studies on ASS 304 at high temperatures
- Study of phase equilibrium of refractory high-entropy alloys using the atomic size difference concept for turbine blade applications
- A novel intelligent tool wear monitoring system in ball end milling of Ti6Al4V alloy using artificial neural network
- A hybrid approach for the machinability analysis of Incoloy 825 using the entropy-MOORA method
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part II
- Innovations for sustainable chemical manufacturing and waste minimization through green production practices
- Topical Issue on Conference on Materials, Manufacturing Processes and Devices - Part I
- Characterization of Co–Ni–TiO2 coatings prepared by combined sol-enhanced and pulse current electrodeposition methods
- Hot deformation behaviors and microstructure characteristics of Cr–Mo–Ni–V steel with a banded structure
- Effects of normalizing and tempering temperature on the bainite microstructure and properties of low alloy fire-resistant steel bars
- Dynamic evolution of residual stress upon manufacturing Al-based diesel engine diaphragm
- Study on impact resistance of steel fiber reinforced concrete after exposure to fire
- Bonding behaviour between steel fibre and concrete matrix after experiencing elevated temperature at various loading rates
- Diffusion law of sulfate ions in coral aggregate seawater concrete in the marine environment
- Microstructure evolution and grain refinement mechanism of 316LN steel
- Investigation of the interface and physical properties of a Kovar alloy/Cu composite wire processed by multi-pass drawing
- The investigation of peritectic solidification of high nitrogen stainless steels by in-situ observation
- Microstructure and mechanical properties of submerged arc welded medium-thickness Q690qE high-strength steel plate joints
- Experimental study on the effect of the riveting process on the bending resistance of beams composed of galvanized Q235 steel
- Density functional theory study of Mg–Ho intermetallic phases
- Investigation of electrical properties and PTCR effect in double-donor doping BaTiO3 lead-free ceramics
- Special Issue on Thermal Management and Heat Transfer
- On the thermal performance of a three-dimensional cross-ternary hybrid nanofluid over a wedge using a Bayesian regularization neural network approach
- Time dependent model to analyze the magnetic refrigeration performance of gadolinium near the room temperature
- Heat transfer characteristics in a non-Newtonian (Williamson) hybrid nanofluid with Hall and convective boundary effects
- Computational role of homogeneous–heterogeneous chemical reactions and a mixed convective ternary hybrid nanofluid in a vertical porous microchannel
- Thermal conductivity evaluation of magnetized non-Newtonian nanofluid and dusty particles with thermal radiation

