Abstract
A quantitative investigation of poly(vinyl) chloride (PVC) de-chlorination using Fe2O3, together with the impact of SiO2 addition on the co-pyrolysis of PVC and Fe2O3, was conducted below 673 K in an Ar atmosphere aiming to cut the emission of gaseous Cl⁻ products. It was found that chlorine in PVC can be fixed in FeCl2 by the reaction between PVC and Fe2O3. The co-pyrolysis of PVC and Fe2O3 proceeds in two stages with a temperature boundary of around 543 K. Below 543 K, a direct reaction occurs between PVC and Fe2O3, resulting in a small mass loss ratio and some extent of chlorine fixing ratio in FeCl2. However, above 543 K, PVC starts to decompose to release gaseous H2, HCl, etc., which react with Fe2O3 through two possible pathways to form FeCl2. In Pathway 1, first Fe2O3 is reduced to Fe3O4 by H2, followed by the chlorination of Fe3O4 to FeCl2 by HCl. In Pathway 2, first Fe2O3 is chlorinated to FeCl3 by HCl, followed by the reduction of FeCl3 to FeCl2 by H2. The chlorine fixing ratio in FeCl2 and the volatile generation ratio increase with decreasing PVC content in the initial mixtures. The addition of SiO2 promotes the chlorine fixing ratio in FeCl2 and volatile generation, and the impact gets stronger with decreasing PVC content in the mixtures. The chlorine fixing ratio is increased from 70.8 to 82.6% by SiO2 addition for the mixtures containing 25% PVC, whereas the difference in the chlorine fixing ratio in FeCl2 caused by SiO2 addition is negligible for the mixtures containing 90% PVC. Fayalite (Fe2SiO4) was not detected in the solid residues after the experiments. After separating FeCl2 using water leaching, the filter residue, a composite of iron oxide and conjugated polyene, can be used as a raw material for iron-making.
1 Introduction
Recycling waste plastics, especially chlorine-bearing waste plastics, has attracted tremendous attention globally due to environmental and technical challenges. Typical chlorine-bearing plastics, poly(vinyl) chloride (PVC), release corrosive hydrogen chloride upon sunlight irradiation in the environment or upon heat during recycling processes, such as incineration, causing secondary pollution and damage to recycling equipment. Therefore, waste PVC must be de-chlorinated before further recycling for chemicals or energy.
De-chlorination by utilizing sorbents, enhanced with superheat steam or acidic catalysts in some cases, to fix chlorine in water-soluble chlorides is considered an effective and eco-friendly way to mitigate gaseous chlorine-bearing substances. The resulting metal chlorides can be separated easily from the other solid products of PVC de-chlorination, conjugated polyene, by using water leaching. Conjugated polyene, containing only carbon and hydrogen, is suitable for oxide reduction or combustion for energy with less carbon dioxide emission compared to carbon.
Two types of sorbents, alkalic substances and metal oxides (transition metals or RE metals), have been investigated for PVC de-chlorination.
Alkalic substances utilized to capture chlorine from PVC include NaOH [1,2], NaHCO3 [3], CaO [3,4,5,6,7], Ca(OH)2 [5,6,7], CaCO3 [5,8,9,10], and Mg(OH)2 [6]. It has been confirmed that alkalic substances can absorb chlorine from PVC to prohibit the release of gaseous hydrogen chloride. The removal efficiency of chlorine is largely dependent on the sorbent properties and the mixing ratio of sorbent to PVC [5]. Calcium-bearing chemicals exhibit a better removal efficiency of chlorine than magnesium-containing substances [4,6]. Carbonates shift the peak temperature of de-hydrochlorination higher [10], and CaCO3 suppresses the emission of dioxin [8]. A higher sorbent to PVC ratio results in larger chlorine removal efficiency. Superheat steam is usually employed as the media to accelerate the breakage of PVC and trap HCl. Without the assistance of superheated steam, the de-chlorination efficiency of alkalic substances such as NaOH is inferior to metal oxides under the same experimental conditions [1]. Therefore, the operation conditions are usually very crucial largely because the hot corrosive chloride solution damages the equipment severely. Moreover, the operation cost can be hardly recovered because the economic value of obtained chlorides of alkali or alkali earth metals is lower than their respective alkalic substances.
Transition metal oxides and RE metal oxides are another group of additives used to absorb chlorine and catalyse the degradation of PVC. The results show that transition metal oxides (V2O5, MoO3, MnO2, CuO, TiO2, ZnO, Co3O4, and Cr2O3) generally decrease the onset temperature of PVC decomposition [11,12] and suppress the generation of aromatic compounds, whereas they increase the amounts of residual carbon and aliphatic hydrocarbons in volatiles [11]. Copper oxide (CuO), which can be chlorinated to CuCl2, promotes the formation of short-chain hydrocarbon and dioxin but decreases the aromatic hydrocarbon formation during PVC decomposition [13,14]. Zinc oxide promotes dehydrochlorination at low temperatures, i.e., 97.6% chlorine can be fixed in zinc chloride (ZnCl2) at around 473 K [15]. However, ZnCl2 evaporates when the temperature is increased further [4,16,17]. RE metal oxides, La2O3, Nd2O3, and CeO2, can fix the chlorine from PVC into insoluble oxychlorides, and the order of chlorine fixing ability is La2O3 > Nd2O3 ≫ CeO2 [16]. The oxides CeO2 and PbO2 show limited influence on the decomposition of PVC [12], while PbO can be chlorinated by PVC to form PbCl2, which is transferable into flue gas when the temperature is higher than 774 K [18,19].
For clean and eco-friendly recycling of waste PVC, sorbents that can fix chlorine in solid chlorides soluble in water with a large chlorine fixing ratio are highly desired. Our previous study shows that Fe3O4 can fix chlorine from PVC in FeCl2, an important chemical feedstock. Only 0.6 mass% HCl was released when co-pyrolyzing the mixture of 25 mass% PVC and 75 mass% Fe3O4 at 673 K. After separating FeCl2 from the other solid compounds by water leaching, the filter residue, a mixture of Fe3O4/Fe2O3 and conjugated polyene, was found to be suitable for iron-making [20]. In another study, Fe3O4 displayed the best de-chlorination efficiency for ex situ chlorine removal from liquid products generated by decomposing a model plastic mixture containing 10% PVC [21]. The promising outcome encourages further exploration of potential sorbent candidates to fix chlorine from PVC in metal chlorides.
Hematite (Fe2O3) is another abundant resource of iron. It is used as a filler or flame retardant for PVC plastics [22,23]. DFT calculations have demonstrated the fixing ability of Fe2O3 for chlorine from PVC [24]. Some experimental work qualitatively showed that the chlorination of Fe2O3 by PVC occurs below 673 K, and with further increase in the temperature, Fe2O3 is reduced in a step-wise manner to Fe3O4, FeO, and finally Fe [14,15,25]. The onset temperature of HCl release shifts lower [17,26], and the formation of conjugated polyene is hindered [16] but the generation of chlorobenzene is promoted by Fe2O3 addition [12]. An investigation of the thermal degradation behaviour of PVC and its stoichiometric mixtures with Fe3O4 and Fe2O3 suggests that both Fe3O4 and Fe2O3 have an inhibiting effect on the de-hydrochlorination of PVC [27]. The release of HCl could be inhibited by using K/Na/Ca-modified iron ore as oxygen carriers in the chemical looping conversion of waste PVC [28]. Furthermore, some secondary industrial wastes containing iron oxides and acidic compounds such as red mud [29] and electric arc furnace dust [17,30] have been tested for the ability of PVC de-chlorination as well, and the formation of FeCl2 and FeCl3 has been confirmed. All of these results indicate the possibility of utilizing Fe2O3 as a sorbent to fix chlorine from PVC in iron chlorides. However, the results of all these studies show a relatively low chlorine fixing ratio of PVC in iron chloride using Fe2O3. Therefore, further improvement in de-chlorination efficiency using Fe2O3 is required.
Acidic oxide SiO2, a co-existing component with Fe2O3 in nature and a filler in PVC-SiO2 composite to improve the mechanical properties of plastics, has been proven to exhibit a catalytic effect on PVC degradation [31]. However, so far, there are no reports on the effect of SiO2 on PVC degradation in the presence of Fe2O3.
In this study, the de-chlorination efficiency of PVC using Fe2O3 as the sorbent in the presence of SiO2 is investigated quantitatively to improve the de-chlorination efficiency of PVC using Fe2O3 and provide essential knowledge applicable to industrial practices. Finally, the fabrication of FeCl2 and metallic iron is confirmed after water leaching the solid residues of PVC de-chlorination to demonstrate the recycling of waste PVC using Fe2O3, in an eco-friendly and profitable way.
2 Experimental
Homogeneous mixtures of PVC and Fe2O3 with and without SiO2 were formulated from as-received raw material powders of high purity. The PVC content ranged from 25 to 90% and the ratio of Fe2O3 to SiO2 was fixed at 4:1 for the mixtures containing SiO2. All amounts are in mass unless specifically mentioned. PVC powders (Cl% ≈ 56.8%, 80 μm) were purchased from Yangli Mechanical and Electrical Materials Co. Ltd. Fe2O3 powders (purity 99.9%, ~400 nm) were supplied by SinoPharmo, and the pre-mixture of Fe2O3 and SiO2 (mixing ratio of Fe2O3/SiO2 was 4:1 by mass, ~400 nm) was obtained from Hengxing Chemicals Co. Ltd. After mixing, oxide powders attach onto the surface of PVC particles, and the covered surface area of PVC particles increases with decreasing content of PVC, and almost no open surface of PVC is left in the mixtures containing 25% PVC. The mixtures were pressed into cylinders of ɸ6 mm for better contacting conditions between PVC and oxides.
Two experimental settings were used to investigate the de-chlorination process. Thermogravimetry coupled with differential scanning calorimetry (TG-DSC) (Labsys Evo, Setaram), connected to a mass spectrometer (MS) (GSD350, Pfeiffer) with a stainless capillary at 523 K, was employed to monitor the mass change, heat flow, and off-gas composition from room temperature to 673 K. Around 8 mg of the mixtures was precisely weighed and placed into a corundum crucible (100 μL) located on the top of the measuring thermocouple inside the TG-DSC chamber. High-purity Ar gas was purged for 30 min to completely expel the air from the chamber before the sample was heated at a constant rate of 20 K·min−1.
An electric furnace was used to heat samples of a relatively large amount (~2 g) in an Ar atmosphere at a heating rate of 10 K·min−1 to a pre-set temperature ranging from 523 to 673 K to obtain solid residues for compositional and morphological analyses. For both settings, the off-gas was passed through an acetone bath to capture organic compounds and NaOH aqueous solution to neutralize HCl before being released into the atmosphere. X-ray diffraction (XRD) (Rigaku) was utilized for solid product identification, and the chemical bonds in the solid residues were confirmed using X-ray photoemission spectroscopy (XPS) (ESCALAB 250 XI, Thermo Fisher Scientific). The morphological structure of solid residues was observed using a scanning electron microscope and energy dispersive spectrometer (SEM-EDS) (SU-5000, Hitachi). The contents of iron chloride and total iron in the solid residues were determined using K2Cr2O7 titration after dissolving the solid residues in deionized water and HCl, respectively. The HCl in the off-gas, captured in NaOH solution, was quantitatively titrated using AgNO3 solution after the excess NaOH was neutralized with HNO3 acid.
Finally, the water-soluble FeCl2 in the solid residues after de-chlorination of PVC using Fe2O3 at 673 K was separated from the other solid products, a mixture of conjugated polyene and iron oxide with or without SiO2, using water leaching. The feasibility of metallic iron and FeCl2 fabrication was verified by heating the filtered residue to 1,673 K and evaporating the FeCl2 aqueous solution to 383 K, respectively.
The schematic diagram of the experimental settings is shown in Figure 1.
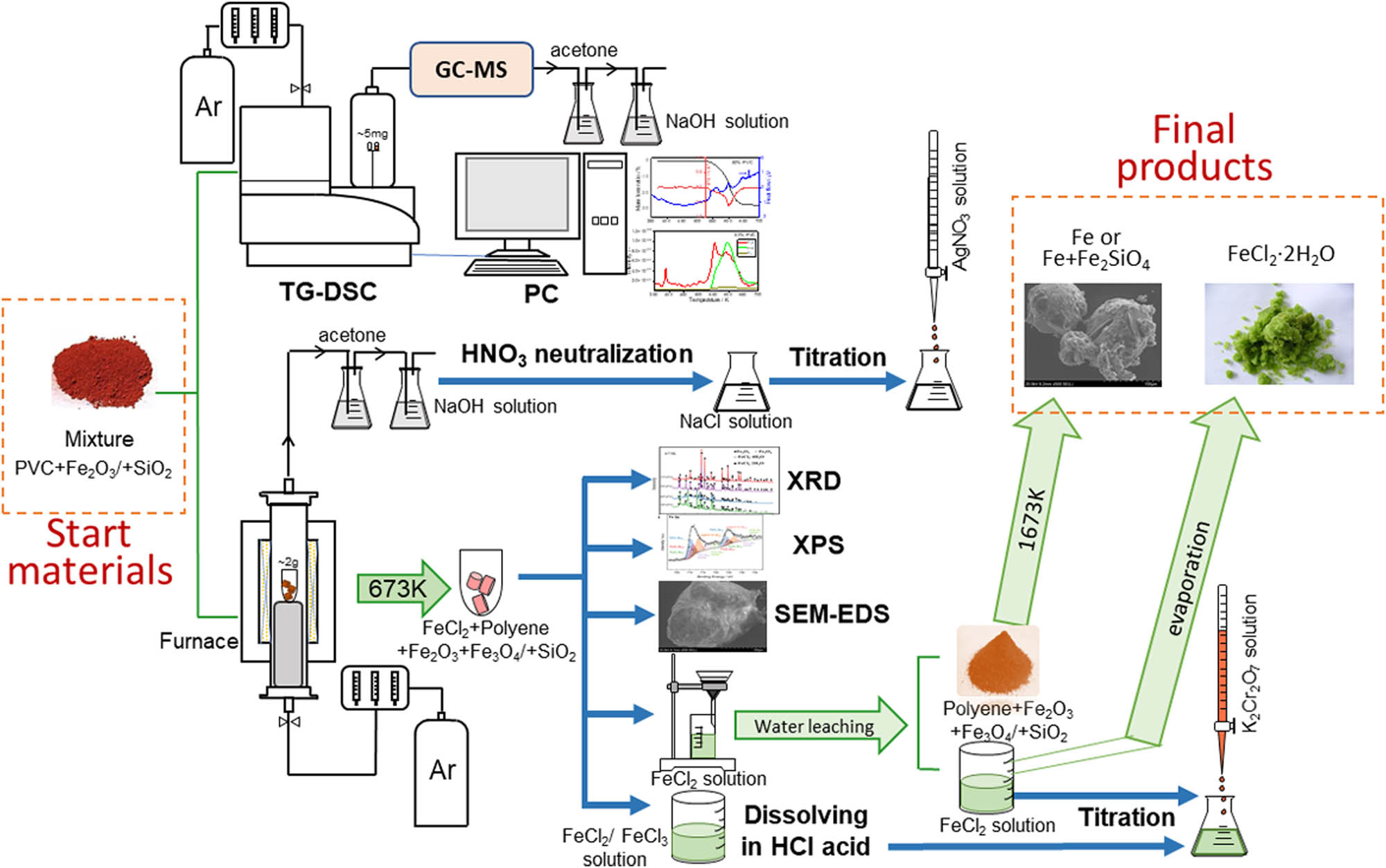
Schematic diagram of the experimental settings.
Two parameters, the mass loss ratio and chlorine fixing ratio in FeCl2, were used to evaluate the de-chlorination efficiency of PVC using Fe2O3. The mass loss ratio was determined according to equation (1):
There are two iron chlorides, FeCl2 (m.p. = 950 K, b.p. = 1,296 K) and FeCl3 (m.p. = 579 K, decomposition at 588 K), with different melting/boiling points. In the current experimental temperature range, FeCl2 is a solid, whereas FeCl3 evaporates or decomposes. The addition of Fe2O3 is to fix chlorine from PVC into solid chloride and to cut the emission of hazardous chlorine-bearing gases. Therefore, only the chlorine fixed in FeCl2 was considered the valid de-chlorination product. The ratio of chlorine fixed in FeCl2 was calculated using equation (2):
During the experiments, FeCl3 escaped from the reaction sites before being condensed onto the furnace tube and off-gas pipe where the temperature was <579 K, without passing through the NaOH solution. Hence, the amount of FeCl3 was determined by balancing the chlorine contained in FeCl2, HCl, and the original samples.
Thermodynamic calculation on the interactions among PVC decomposition products (mainly HCl and H2), Fe2O3, and SiO2 was conducted using FACTSageTM to assist in the analysis of experimental results.
3 Results and discussion
3.1 PVC de-chlorination using Fe2O3
PVC de-chlorination using Fe2O3 was conducted in the temperature range from 523 to 673 K with PVC contents set at 25, 50, and 90% mass. The composition of the solid residues after reactions was analysed using XRD and XPS. Figure 2 shows typical XRD (from 523 to 673 K) and XPS (673 K) results of the solid products for the mixture containing 50% PVC. It is verified that the chlorination of Fe2O3 occurred during the heating process, resulting in the generation of FeCl2. With increasing temperature, the peaks of Fe2O3 decay but the peaks of FeCl2·2H2O and FeCl2·4H2O become stronger. Weak peaks of Fe3O4 start to appear from 543 K as a result of Fe2O3 reduction by hydrogen (Figure 2a). In addition to Fe2O3, Fe3O4, and FeCl2, organic iron exists in the solid residue (Figure 2b), and this is in accordance with the XPS results of carbon bonds C≡O and C–O (Figure 2d). It is also evidenced from the XPS results that chlorine was cracked away from the PVC chain at 673 K, and only FeCl2 was detected as the solid chlorine-bearing product (Figure 2c).

XRD results of solid products at various temperatures (298–673 K) (a), and XPS results of Fe 2p (b), Cl 2p (c), and C 1s (d) of the solid products at 673 K for the mixture containing 50% Fe2O3 and 50% PVC.
Figure 3 shows changes in the mass loss ratio (Figure 3a) and the chlorine fixing ratio in FeCl2 (Figure 3b) with the temperature after heating PVC and Fe2O3 mixtures with different PVC contents. Typical TG, DTG, and heat flow results (Figure 3c), and MS results of H2, H2O, and HCl in the off-gas (Figure 3d) are shown as well for the mixture containing 50% PVC. There are three peaks of H2O at around 370, 550, and 590 K, corresponding to three exo-thermal peaks. The peaks of HCl and H2 were recorded in the same temperature range with the third peak of H2O (590 K), where the largest DTG peak was recorded as well. No chlorinated hydrocarbon was detected in the off-gas according to the MS results. The main components of the volatile are hydrocarbons such as methane and ethylene, similar to the results of our previous report [20]. This is probably because much more Fe2O3 was mixed with PVC in the present work than in the literature [12], in which the mixing ratio of Fe2O3 to PVC is 1:5. Hence, in the current work, the probability of chlorine reacting with Fe2O3 is high but the probability of forming chlorinated hydrocarbons is low.

Impact of temperature on the mass loss ratio (a). Chlorine fixing ratio in FeCl2 (b) for various PVC contents tested using electric furnace setting; and changes in the mass loss ratio, DTG, and heat flow (c). GC-MS results of H2O, H2, and HCl (d) with temperature tested using TG-DSC-GC-MS during co-pyrolysis of 50% PVC and 50% Fe2O3.
It seems the chlorination of Fe2O3 by PVC proceeds in two stages, distinguished at around 543 K. Below 543 K, no apparent mass loss and some extent of chlorine fixing ratio in FeCl2 was observed. In this stage, no volatiles, a sign of PVC decomposition, but only H2O was detected. The formation of FeCl2 and H2O is the result of direct reaction between PVC and Fe2O3 (equation (3a)) because PVC was in good contact with Fe2O3 in this stage:
When the temperature is above 543 K, the mass loss ratio and the chlorine fixing ratio in FeCl2 almost synchronically increase with increasing temperature, strongly indicating that the formation of FeCl2 in this stage is in accordance with PVC decomposition. The mass loss ratio increases but the ratio of chlorine fixed in FeCl2 decreases with increasing initial content of PVC. The peaks of H2O, H2, and HCl were recorded, suggesting that PVC starts to decompose significantly to release volatiles and cause huge mass loss. The two peaks of H2O at 550 and 590 K are attributed to the reduction of Fe2O3 to Fe3O4 by H2 and the chlorination of iron oxide to iron chloride by HCl, respectively, which is evidenced by the appearance of Fe3O4 peaks and strong peaks of FeCl2·2H2O and FeCl2·4H2O (Figure 2a). In this temperature range, the mass loss is predominantly determined by the decomposition of PVC, and more PVC content leads to more mass loss. However, with increasing initial PVC content, the surface coverage of PVC particles by Fe2O3 powders decreases, and hence the encountering chance for Fe2O3 with released HCl from PVC decomposition decreases, resulting in a low chlorine fixing ratio in FeCl2.
The highest chlorine fixing ratio in FeCl2 is 74.6%, which is achieved with the mixture containing 25% PVC at 673 K. This ratio is much lower than 96.5%, which is the chlorine fixing ratio in FeCl2 when co-pyrolyzing PVC with Fe3O4 under the same experimental conditions of temperature and PVC content [20]. It is obvious that Fe3O4 can fix more chlorine in FeCl2 than Fe2O3.
3.2 Impact of SiO2 on PVC de-chlorination using Fe2O3
The mass loss ratio and chlorine fixing ratio in FeCl2 after co-pyrolyzing PVC and Fe2O3 at 673 K with and without SiO2 addition were quantitatively investigated, and the results are shown in Figure 4. The composition of the initial mixtures is expressed using the PVC content and Fe/Cl molar ratio in the mixture. A high PVC content corresponds to a low Fe/Cl molar ratio.

Impact of SiO2 addition on the mass loss ratio and Cl fixing ratio in FeCl2 during PVC de-chlorination using Fe2O3 (green lines and symbols: with SiO2 addition, red lines and symbols: without SiO2 addition) at various PVC contents and Fe/Cl molar ratios. (a) Mass loss ratio vs PVC content; (b) Cl fixing ratio in FeCl2 vs PVC content; (c) mass loss ratio vs Fe/Cl molar ratio; and (d) Cl fixing ratio in FeCl2 vs Fe/Cl molar ratio.
In both cases, with and without SiO2 addition, the mass loss ratio increases with increasing PVC content (Figure 4a) or decreasing Fe/Cl ratio of the initial mixtures (Figure 4c). However, the chlorine fixing ratio in FeCl2 exhibits the opposite trend; it increases with decreasing PVC content (Figure 4b) or increasing Fe/Cl molar ratio (Figure 4d) in the initial mixtures.
For all the mixtures containing different PVC contents, the addition of SiO2 promotes the chlorine fixing ratio in FeCl2, and the difference in the chlorine fixing ratio in FeCl2 caused by SiO2 addition gets higher with decreasing PVC content (Figure 4b) or increasing Fe/Cl molar ratio in the mixtures (Figure 4d). In contrast, the impact of SiO2 on the mass loss ratio shows a turning point at a PVC content of around 50% or Fe/Cl molar ratio of around 0.5, which is the stoichiometric Fe/Cl molar ratio of FeCl2. SiO2 addition increases the mass loss ratio in the mixtures containing more PVC than 50%. However, SiO2 addition decreases the mass loss ratio for the mixtures with Fe/Cl molar ratio <0.5. This result indicates there are at least two-folds of SiO2 impact on the mass loss during chlorination of Fe2O3 by PVC and they are balanced in the mixture containing 50% PVC.
3.3 Identification of the FeCl2 formation pathway
In order to clarify the mechanism of PVC de-chlorination using Fe2O3 and the impact of SiO2 on the de-chlorination, thermodynamic calculations were done using FACTSageTM in the temperature range 500–673 K. The dominant zones of inorganic solid products are shown in Figure 5 (a: PVC + Fe2O3, b: PVC + Fe2O3 + SiO2). The input for the calculation was oxides, Fe2O3 or Fe2O3 + SiO2 (mass ratio of Fe2O3/SiO2 was 4:1), together with the decomposition products of PVC, mainly H2 and HCl, the amounts of which were determined according to the initial mixing ratio.

Thermodynamic calculation results of PVC + Fe2O3 (a) and PVC + Fe2O3 + SiO2 (b), and the identification of solid products obtained at 673 K for PVC + Fe2O3 (c) and PVC + Fe2O3 + SiO2 (d).
The gaseous products included H2O and FeCl3, and no SiCl4 or any other gaseous silicon compounds were present according to the FACTSageTM calculation results. The interaction between SiO2 and HCl can hardly occur under the current experimental conditions, as typically indicated by the formation of SiCl4 (reaction 3b) [32]:
The FACTSageTM calculation results of solid products are shown in Figure 5. There exists an inverted triangle area in both PVC + Fe2O3 and PVC + Fe2O3 + SiO2 reaction systems, which is found at PVC contents of around 61% (or 39% Fe2O3) or 56% (or 44% Fe2O3 and SiO2), respectively. In the reaction system of PVC + Fe2O3 (Figure 5a), FeCl2 and Fe3O4 co-exist in the inverted triangle area; the left side represents the FeCl2 dominant area, and the right side is the co-existing zone of FeCl2, Fe2O3, and Fe3O4. Similar results were observed in the reaction system of PVC + Fe2O3 + SiO2 (Figure 5b). SiO2 remains unreacted almost in the whole temperature and composition range, and fayalite (Fe2SiO4) is formed only within a tiny zone above 620 K. This is in agreement with the reported results that fayalite generation starts to occur at 673 K even from nano-sized iron oxide and silica [33]. Therefore, it is safe to conclude that SiO2 is not involved in the chemical reactions directly during the de-chlorination of PVC using Fe2O3 under the current experimental conditions.
The thermodynamic calculation results are consistent with the XRD analysis results of solid residues obtained at 673 K (Figure 5c and d). Four iron-bearing compounds, Fe2O3, Fe3O4, FeCl2·2H2O, and FeCl2·4H2O, are present in the solid residues after the experiments. The ferrous chloride hydrates, FeCl2·2H2O and FeCl2·4H2O, are the result of moisture absorbance from the air after the experiments. The relative peak intensity of FeCl2·2H2O and FeCl2·4H2O increases, whereas the peak intensity of iron oxides (Fe2O3 and Fe3O4) decreases with increasing PVC content in the initial mixtures. This is because, with increasing PVC content, less iron oxide is left so the percentage of iron chloride in the final solid residue increases. No fayalite (Fe2SiO4) but only SiO2 was detected (Figure 5d) because the mixtures prepared in the current study are not located within the narrow co-existing zone of Fe2SiO4, FeCl2, and SiO2 (Figure 5b). The broad peak at around 26° corresponds to conjugated polyene, which is the solid product of PVC decomposition.
The possible chemical reactions of PVC de-chlorination using Fe2O3 are analysed based on the thermodynamic data [32] and the experimental results. Below 543 K, the decomposition of PVC is very limited and PVC can directly contact Fe2O3; therefore, the generation of H2O and FeCl2 is the result of a direct reaction between the atoms on PVC chains, H and Cl, and Fe2O3 (equation 3a).
When the temperature is further increased above 543 K, PVC starts to decompose heavily and the direct contact between PVC and Fe2O3 is blocked by the generated FeCl2, and the interaction between the PVC gaseous decomposition products, H2 and HCl, with Fe2O3 dominating the process. Hence, the chlorination of Fe2O3 follows reactions (3c) and (3d), i.e. FeCl3 is generated first and then reduced by H2 resulting in the formation of FeCl2:
Another pathway of FeCl2 formation may be as follows: first, Fe2O3 is reduced by H2 to Fe3O4 (reaction 3e), and then Fe3O4 reacts with HCl following reactions (3f) and (3g). The product FeCl3 is reduced to FeCl2 according to reaction (3d):
The gases, HCl and H2, are the decomposition products of PVC (reaction 3h):
It should be mentioned that under the current experimental conditions, Fe2Cl6 can hardly be generated as shown in our previous paper [20].
Since Fe3O4 shows better fixing ability of chlorine in FeCl2 than Fe2O3 [20], and Fe2O3 can be easily reduced to Fe3O4 in the presence of H2, Pathway 2 is likely predominant for the formation of FeCl2 during co-pyrolysis of PVC and Fe2O3.
3.4 Mechanism analysis of PVC de-chlorination using Fe2O3 in the presence of SiO2
PVC de-chlorination using Fe2O3 was mechanistically analysed by quantifying the pyrolysis products. The distribution of the three constituent elements of PVC, Cl, H, and C, in the products was calculated using equation (4), and the results are shown in Figure 6 as a function of the initial PVC content in the mixtures without (Figure 6a) and with (Figure 6b) SiO2 addition. The pyrolysis products of pure PVC at 673 K are shown for comparison:

Mass balance of PVC components as a function of initial PVC content in the mixtures after reaction at 673 K (a: PVC + Fe2O3; b: PVC + Fe2O3 + SiO2); and impact of SiO2 on the chlorine distribution in Cl⁻ products (c).
The products completely originated from PVC, including conjugated polyene, volatiles, H2, and HCl. These substances are listed separately. Volatiles refer to all organic gaseous decomposition products of PVC, such as CH4. Since it is hard to separate H2 from volatiles in the off-gas, they are listed together. Hydrogen in H2O, as a result of reactions (3a, b, d, e, and f), was calculated using the iron titration results of iron chloride and iron oxides. Chlorine in FeCl2, FeCl3, and HCl were obtained from the titration results of iron and chlorine. Since both FeCl3 and HCl are vapour under the current experimental conditions, chlorine in FeCl3 is listed together with HCl.
Compared to pure PVC, for both mixtures (with and without SiO2) containing 90% PVC, the percentage of volatiles and H2 decreases but the percentage of conjugated polyene increases slightly. Then, with decreasing PVC content in the mixtures, more volatiles and H2 were released but less conjugated polyene was generated. However, the percentage of hydrogen in H2O increases to an extremely limited extent, partly due to the small atomic mass of hydrogen; the percentage of chlorine in FeCl2 increases, whereas the sum of HCl and FeCl3 decreases.
The addition of SiO2 favours the formation of FeCl2 and volatiles but suppresses the generation of conjugated polyene, HCl, and FeCl3. In the mixtures with high PVC content, sufficient H2 is supplied for the reduction of Fe2O3 and FeCl3, resulting in a high fixing ratio of chlorine in FeCl2 and the limited influence of SiO2 on chlorine transformation to FeCl2. Therefore, in the mixtures containing 90% PVC, the difference in FeCl2 formation caused by SiO2 addition is almost negligible (Figure 4b). However, the enhancement of volatile formation caused by SiO2 is high, leading to an increase in the mass loss ratio (Figure 4a). In contrast, in the mixtures with low PVC content, insufficient H2 is provided for the reduction of Fe2O3 and FeCl3, so that the enhancement of FeCl2 formation by SiO2 addition appears significant. As a result, the chlorine fixing ratio in FeCl2 is increased from 70.8 to 82.6% by SiO2 addition for the mixtures containing 25% PVC (Figure 4b). The favourable impact of SiO2 addition on FeCl2 generation surpasses the increase in volatile generation caused by SiO2 addition, leading to the overall decrease in the mass loss ratio (Figure 4a).
In order to provide more details on the changes in chlorine-containing products caused by SiO2 addition, the distribution of chlorine in the products, FeCl2, FeCl3, and HCl, is shown in Figure 6c for the mixtures containing 25% PVC after heating at 673 K because this pair of mixtures show the largest difference in the chlorine fixing ratio in FeCl2 (Figure 4b). The percentage of chlorine in the Cl⁻ products was calculated using equation (5):
The addition of SiO2 increases the chlorine fixing ratio in FeCl2 by decreasing the formation of gaseous FeCl3 and HCl, especially the generation of FeCl3 was suppressed by nearly 50% while a slight decrease in HCl was observed.
Summarizing the results, it is clear that the interaction between PVC and Fe2O3 proceeds in two stages. Below 543 K, the decomposition of PVC is very limited and PVC directly contacts with Fe2O3, so the formation of FeCl2/FeCl3 and H2O occurs through the atoms of H and Cl on the PVC chains with Fe2O3 (Figure 7a), resulting in a very limited mass loss ratio and a certain amount of FeCl2 formation. The generated FeCl2 distributes between PVC and Fe2O3, and therefore, the direct contact between PVC and Fe2O3 is blocked. When the temperature is further increased above 543 K, the decomposition of PVC releases gaseous products H2 and HCl (Figure 7b1), which can react with Fe2O3 to form Fe3O4, FeCl2/FeCl3, and H2O (Figure 7b2).

Proposed reaction mechanism between PVC and Fe2O3 in the presence of SiO2. (a) Interaction between PVC and Fe2O3 below 543 K. (b) Interaction between PVC and Fe2O3 above 543 K. -: chemical bond; ---: electrical attraction.
A mechanism of increased generation of FeCl2 and volatiles in the presence of oxides, iron oxide, and SiO2 is also proposed (Figure 7b3). The molecules of the oxides and conjugated polyene are electrically polar. The attraction between opposite-charged poles and repellence among same-charged poles occur and thus impact the conjugated polyene chain, which is very unstable immediately after the formation from PVC decomposition to crack down the bonds of C–H and C═C to release volatiles. Therefore, volatile generation was promoted in the presence of SiO2 and unreacted Fe2O3 (Figure 6a and b). In this case, SiO2 and Fe2O3 act as Lewis acid and Lewis base, respectively, to catalyse the breakage of conjugated polyene. Similarly, FeCl3 molecules are electrically polar and attracted by the opposite poles in SiO2 molecules, while the electrically positive pole of H in conjugated polyene is attracted as well; therefore, reaction (3c) occurs to form FeCl2 and HCl, increasing the chlorine fixing ratio in FeCl2.
In the sample without SiO2 addition, Fe and Cl distribute almost evenly on the surface of the conjugated polyene (top graph of Figure 8). When SiO2 is present, Fe and Cl distribute more in affinity with SiO2, which is evidenced by the element distribution of Si, O, Fe, and Cl, especially at the right bottom of the figures, indicating that the formation of FeCl2 near SiO2 particles is enhanced (bottom graph of Figure 8).

SEM-EDS images of FeCl2 distribution in the affinity of SiO2 particles after heating the mixture of PVC + Fe2O3 + SiO2 containing 75% PVC to 673 K.
The current results that the mass loss ratio is increased in the presence of oxides are in agreement with the observations reported by some other researchers. It has been pointed out that the acidic compounds, β-zeolite [1], SiO2 [31], and Al2O3 [16], impact catalytically PVC degradation. These substances can hardly react directly with PVC and/or its decomposition products; however, they change the decomposition products, especially increasing the release of hydrocarbon volatiles by acting on the chemical bonds in PVC. SiO2 in combination with Al2O3 can increase the yield of gaseous and liquid products, both branched aliphatic and aromatic hydrocarbons, from waste PE at 649 K, and the yield can be increased further by doping nano-TiO2 to the SiO2–Al2O3 structure [34]. Nano-hematite (α-Fe2O3) in the form of silica-supported granules has been proven a very effective catalyst in cracking petroleum vacuum residues in supercritical water media, with C–C bond cleavage promoted by the lattice oxygen of α-Fe2O3 [35]. The polyene synthesis from polycondensation aromatic intermediates is hindered by Fe2O3, resulting in volatiles and carbon formation [16].
Based on the above results, the mixtures containing 25% PVC with and without SiO2 addition were heated to 673 K in an Ar atmosphere to conduct PVC de-chlorination using Fe2O3. Then, water leaching was utilized to separate the solid residues, resulting in FeCl2 aqueous solution and the filtered residue. The chemical feedstock FeCl2·2H2O was obtained after the aqueous solution was evaporated at 383 K. The filtered residue was identified as a mixture of conjugated polyene and iron oxides, mainly Fe2O3 and a small part of Fe3O4, for the mixture without SiO2; whereas for the mixture with SiO2 addition, SiO2 was present in the filtered residue. Metallic iron was fabricated by heating the filtered residues to 1,873 K in an Ar atmosphere. The metallic iron yield is lower for the mixture containing SiO2 than that without SiO2 due to the formation of fayalite.
4 Conclusions
The de-chlorination of PVC using Fe2O3 and the impact of SiO2 addition on the process were quantitatively investigated below 673 K in an Ar atmosphere. The following conclusions can be drawn.
The chlorine in PVC can be fixed in FeCl2 through the interactions between PVC and Fe2O3, and the chlorine fixing ratio in FeCl2 increases with a decrease in the PVC content in the mixtures. There are two reaction stages at around 543 K. The direct reaction between PVC and Fe2O3 occurs below 543 K, resulting in the formation of FeCl2 and H2O, leading to a small mass loss ratio and some extent of chlorine fixing ratio in FeCl2. When the temperature is above 543 K, PVC decomposition occurs to release gaseous products H2 and HCl, and there are two possible reaction pathways between Fe2O3 and PVC decomposition products. In Pathway 1, first, Fe2O3 is chlorinated to FeCl3 by HCl, followed by the reduction of FeCl3 to FeCl2 by H2. In Pathway 2, first, Fe2O3 is reduced to Fe3O4 by H2, followed by chlorination of Fe3O4 to FeCl2 by HCl. Pathway 2 seems to be predominant, as demonstrated by the thermodynamic calculations and experimental results.
The addition of SiO2 promotes the release of volatiles and the chlorine fixing ratio in FeCl2 by reducing the generation of gaseous Cl⁻ products, especially FeCl3. The impact of SiO2 addition gets stronger with decreasing the PVC content in the mixtures. The difference in the chlorine fixing ratio caused by SiO2 addition is negligible for the mixture containing 90% PVC, in which sufficient H2 is available for the reduction reactions. However, the chlorine fixing ratio is increased from 70.8 to 82.6% by SiO2 addition for the mixtures containing 25% PVC, in which H2 supply is insufficient for the reduction of Fe2O3 and FeCl3. SiO2 remains unchanged and no fayalite is present in the solid residues in experiments below 673 K.
After PVC de-chlorination using Fe2O3 at 673 K, the solid residues can be separated using water leaching into FeCl2 aqueous solution and filtered residue composed of conjugated polyene and oxides. FeCl2·2H2O can be obtained by evaporating FeCl2 aqueous solution, and metallic iron can be fabricated by heating the filtered residue to 1,873 K in an Ar atmosphere.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (No. 51774206).
-
Author contribution: Lan Hong designed and organized the research work and edited the manuscript; Tai-lin Li conducted the experiments and Lin-hai Ye carried out the thermodynamic calculation and data analysis.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: Data will be made available on request.
References
[1] Hapipi, A. M., H. Suda, M. A. Uddin, and Y. Kato. Dechlorination of polyvinyl chloride under superheated steam with catalysts and adsorbents. Energy & Fuels, Vol. 32, No. 7, 2018, pp. 7792–7799.10.1021/acs.energyfuels.8b00838Search in Google Scholar
[2] Hungwe, D., S. Hosokawa, H. Xu, and Y. Yamasaki. The role of NaOH in the hydrothermal dehydrochlorination of polyvinyl chloride. Polymer Degradation and Stability, Vol. 208, 2023, id. 110266.10.1016/j.polymdegradstab.2023.110266Search in Google Scholar
[3] Torres, D., Y. Jiang, D. A. Sanchez-Monsalve, and G. A. Leeke. Hydrochloric acid removal from the thermogravimetric pyrolysis of PVC. Journal of Analytical and Applied Pyrolysis, Vol. 149, 2020, id. 104831.10.1016/j.jaap.2020.104831Search in Google Scholar
[4] Meng, T. T., H. Zhang, F. Lü, L. M. Shao, and P. J. He. Comparing the effects of different metal oxides on low temperature decomposition of PVC. Journal of Analytical and Applied Pyrolysis, Vol. 159, 2021, id. 105312.10.1016/j.jaap.2021.105312Search in Google Scholar
[5] Zhu, H. M., X. G. Jiang, J. H. Yan, Y. Chi, and K. F. Cen. TG-FTIR analysis of PVC thermal degradation and HCl removal. Journal of Analytical and Applied Pyrolysis, Vol. 82, No. 1, 2008, pp. 1–9.10.1016/j.jaap.2007.11.011Search in Google Scholar
[6] Wootthikanokkhan, J., A. Jaturapiree, and V. Meeyoo. Effect of metal compounds and experimental conditions on distribution of products from PVC pyrolysis. Journal of Polymers and the Environment, Vol. 11, 2003, pp. 1–6.Search in Google Scholar
[7] Nishibata, H., M. A. Uddin, and Y. Kato. Simultaneous degradation and dechlorination of poly (vinyl chloride) by a combination of superheated steam and CaO catalyst/adsorbent. Polymer Degradation and Stability, Vol. 179, 2020, id. 109225.10.1016/j.polymdegradstab.2020.109225Search in Google Scholar
[8] Sun, R. D., H. Irie, T. Nishikawa, A. Nakajima, T. Watanabe, and K. Hashimoto. Suppressing effect of CaCO3 on the dioxins emission from poly (vinyl chloride) (PVC) incineration. Polymer degradation and stability, Vol. 79, No. 2, 2003, pp. 253–256.10.1016/S0141-3910(02)00288-4Search in Google Scholar
[9] Zou, Y., D. Pau, Y. Li, Y. Zhang, and K. Li. Oxygen effects on the evolution of hydrogen chloride and chlorine gas during combustion of PVC-CaCO3 based cable. Fuel, Vol. 329, 2022, id. 125469.10.1016/j.fuel.2022.125469Search in Google Scholar
[10] Karayildirim, T., J. Yanik, M. Yuksel, M. Saglam, C. Vasile, and H. Bockhorn. The effect of some fillers on PVC degradation. Journal of Analytical and Applied Pyrolysis, Vol. 75, No. 2, 2006, pp. 112–119.10.1016/j.jaap.2005.04.012Search in Google Scholar
[11] Ji, M., L. Chen, J. Que, L. Zheng, Z. Chen, and Z. Wu. Effects of transition metal oxides on pyrolysis properties of PVC. Process Safety and Environmental Protection, Vol. 140, 2020, pp. 211–220.10.1016/j.psep.2020.04.010Search in Google Scholar
[12] Sivalingam, G., R. Karthik, and G. Madras. Effect of metal oxides on thermal degradation of poly (vinyl acetate) and poly (vinyl chloride) and their blends. Ind. Eng. Chem. Res., Vol. 42, 2003, pp. 3647–3653.10.1021/ie030009kSearch in Google Scholar
[13] Jafari, A. J. and J. D. Donaldson. Determination of HCl and VOC emission from thermal degradation of PVC in the absence and presence of copper, copper (II) oxide and copper (II) chloride. E-Journal of Chemistry, Vol. 6, No. 3, 2009, pp. 685–692.10.1155/2009/753835Search in Google Scholar
[14] Shibata, E., S. Yamamoto, E. Kasai, and T. Nakamura. Formation behavior of PCDD/Fs in PVC pyrolysis with copper oxide. Chemosphere, Vol. 50, No. 9, 2003, pp. 1235–1242.10.1016/S0045-6535(02)00580-5Search in Google Scholar
[15] Kosuda, T., T. Okada, S. Nozaka, Y. Matsuzawa, T. Shimizu, S. Hamanaka, et al. Characteristics and mechanism of low temperature dehydrochlorination of poly (vinyl chloride) in the presence of zinc (II) oxide. Polymer degradation and stability, Vol. 97, No. 4, 2012, pp. 584–591.10.1016/j.polymdegradstab.2012.01.009Search in Google Scholar
[16] Masuda, Y., T. Uda, O. Terakado, and M. Hirasawa. Pyrolysis study of poly (vinyl chloride)–metal oxide mixtures: quantitative product analysis and the chlorine fixing ability of metal oxides. Journal of Analytical and Applied Pyrolysis, Vol. 77, No. 2, 2006, pp. 159–168.10.1016/j.jaap.2006.03.001Search in Google Scholar
[17] Zhang, B., X. Y. Yan, K. Shibata, T. Uda, M. Tada, and M. Hirasawa. Thermogravimetric-mass spectrometric analysis of the reactions between oxide (ZnO, Fe2O3 or ZnFe2O4) and polyvinyl chloride under inert atmosphere. Materials Transactions, JIM, Vol. 41, No. 10, 2000, pp. 1342–1350.10.2320/matertrans1989.41.1342Search in Google Scholar
[18] Wang, S. J., H. Zhang, L. M. Shao, S. M. Liu, and P. J. He. Thermochemical reaction mechanism of lead oxide with poly (vinyl chloride) in waste thermal treatment. Chemosphere, Vol. 117, 2014, pp. 353–359.10.1016/j.chemosphere.2014.07.076Search in Google Scholar PubMed
[19] Wang, S. J., P. J. He, W. T. Lu, L. M. Shao, and H. Zhang. Comparison of Pb, Cd, Zn, and Cu chlorination during pyrolysis and incineration. Fuel, Vol. 194, 2017, pp. 257–265.10.1016/j.fuel.2017.01.035Search in Google Scholar
[20] Ye, L.-H., T.-L. Li, and L. Hong. Co-pyrolysis of Fe3O4-poly (vinyl chloride) (PVC) mixtures: mitigation of chlorine emission during PVC recycling. Waste Management, Vol. 126, 2021, pp. 832–842.10.1016/j.wasman.2021.04.021Search in Google Scholar PubMed
[21] Hubáček, J., J. Lederer, P. Kuráň, P. Koutník, Z. Gholami, M. Zbuzek, et al. Dechlorination during pyrolysis of plastics: the potential of stepwise pyrolysis in combination with metal sorbents. Fuel Processing Technology, Vol. 231, 2022, id. 107226.10.1016/j.fuproc.2022.107226Search in Google Scholar
[22] Mahmoud, K. A., E. Lacomme, M. I. Sayyed, Ö.F. Özpolat, and O. L. Tashlykov. Investigation of the gamma ray shielding properties for polyvinyl chloride reinforced with chalcocite and hematite minerals. Heliyon, Vol. 6, No. 3, 2020, id. e03560.10.1016/j.heliyon.2020.e03560Search in Google Scholar PubMed PubMed Central
[23] Sun, Y., M. Gao, Z. Chai, and H. Wang. Thermal behaviour of the flexible polyvinyl chloride including montmorillonite modified with iron oxide as flame retardant. Journal of Thermal Analysis and Calorimetry, Vol. 131, 2018, pp. 65–70.10.1007/s10973-017-6117-7Search in Google Scholar
[24] Ahmed, O. H., M. Altarawneh, Z. T. Jiang, M. Al-Harahsheh, and B. Z. Dlugogorski. Reactions of products from thermal degradation of PVC with nanoclusters of α-Fe2O3 (hematite). Chemical Engineering Journal, Vol. 323, 2017, pp. 396–405.10.1016/j.cej.2017.04.047Search in Google Scholar
[25] Lingaiah, N., M. A. Uddin, A. Muto, Y. Sakata, T. Imai, and K. Murata. Catalytic dechlorination of chloroorganic compounds from PVC-containing mixed plastic-derived oil. Applied Catalysis A: General, Vol. 207, No. 1–2, 2001, pp. 79–84.10.1016/S0926-860X(00)00656-6Search in Google Scholar
[26] Blazso, M. and E. Jakab. Effect of metals, metal oxides, and carboxylates on the thermal decomposition processes of poly (vinyl chloride). Journal of Analytical and Applied Pyrolysis, Vol. 49, No. 1–2, 1999, pp. 125–143.10.1016/S0165-2370(98)00123-5Search in Google Scholar
[27] Altarawneh, S., M. Al-Harahsheh, A. Buttress, C. Dodds, and S. Kingman. A thermo-kinetic investigation on the thermal degradation of polyvinyl chloride in the presence of magnetite and hematite. Thermochimica Acta, Vol. 718, 2022, id. 179390.10.1016/j.tca.2022.179390Search in Google Scholar
[28] Jiang, H., R. Huo, Z. Zhang, Y. Lin, Z. Zhao, J. Hu, et al. Dechlorination performance in chemical looping conversion of polyvinyl chloride plastic waste using K/Na/Ca-modified iron ore oxygen carriers. Journal of Environmental Chemical Engineering, Vol. 10, No. 2, 2022, id. 107314.10.1016/j.jece.2022.107314Search in Google Scholar
[29] Lingaiah, N., M. A. Uddin, A. Muto, T. Imai, and Y. Sakata. Removal of organic chlorine compounds by catalytic dehydrochlorination for the refinement of municipal waste plastic derived oil. Fuel, Vol. 80, No. 13, 2001, pp. 1901–1905.10.1016/S0016-2361(01)00046-1Search in Google Scholar
[30] Zhang, B., X. Yan, K. Shibata, M. Tada, and M. Hirasawa. Novel process for recycling metallic elements from mixtures of metal oxide wastes and waste polyvinyl chloride. High Temperature Materials and Processes, Vol. 18, No. 4, 1999, pp. 197–212.10.1515/HTMP.1999.18.4.197Search in Google Scholar
[31] Lingaiah, N., M. A. Uddin, K. Morikawa, A. Muto, K. Murata, and Y. Sakata. Catalytic dehydrochlorination of chloro-organic compounds from PVC containing waste plastics derived fuel oil over FeCl2/SiO2 catalyst. Green Chemistry, Vol. 3, No. 2, 2001, pp. 74–75.10.1039/b009471oSearch in Google Scholar
[32] Dean, J. A. Lange’s handbook of chemistry: thirteenth edition [M], McGraw-Hill, New York, 1985, pp. 1467–1494.Search in Google Scholar
[33] Coombes, M. J., E. J. Olivier, E. Prestat, S. J. Haigh, E. du Plessis, and J. H. Neethling. Iron-silica interaction during reduction of precipitated silica-promoted iron oxides using in situ XRD and TEM. Applied Catalyst A, General, Vol. 613, 2021, id. 118031.10.1016/j.apcata.2021.118031Search in Google Scholar
[34] Mondal, B. K., F. Guha, and M. N. Abser. Sol-gel derived Ti-doped mesoporous silica-alumina: an efficient catalyst to recover energy sources from environmental hazard waste plastics. Journal of Thermal Analysis and Calorimetry, Vol. 148, 2023, pp. 5257–5270.10.1007/s10973-023-12059-7Search in Google Scholar
[35] Hosseinpour, M., S. Fatemi, and S. J. Ahmadi. Catalytic Cracking of petroleum vacuum residue in supercritical water media: Impact of α-Fe2O3 in the form of free nanoparticles and silica-supported granules. Fuel, Vol. 159, 2015, pp. 538–549.10.1016/j.fuel.2015.06.086Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- De-chlorination of poly(vinyl) chloride using Fe2O3 and the improvement of chlorine fixing ratio in FeCl2 by SiO2 addition
- Reductive behavior of nickel and iron metallization in magnesian siliceous nickel laterite ores under the action of sulfur-bearing natural gas
- Study on properties of CaF2–CaO–Al2O3–MgO–B2O3 electroslag remelting slag for rack plate steel
- The origin of {113}<361> grains and their impact on secondary recrystallization in producing ultra-thin grain-oriented electrical steel
- Channel parameter optimization of one-strand slab induction heating tundish with double channels
- Effect of rare-earth Ce on the texture of non-oriented silicon steels
- Performance optimization of PERC solar cells based on laser ablation forming local contact on the rear
- Effect of ladle-lining materials on inclusion evolution in Al-killed steel during LF refining
- Analysis of metallurgical defects in enamel steel castings
- Effect of cooling rate and Nb synergistic strengthening on microstructure and mechanical properties of high-strength rebar
- Effect of grain size on fatigue strength of 304 stainless steel
- Analysis and control of surface cracks in a B-bearing continuous casting blooms
- Application of laser surface detection technology in blast furnace gas flow control and optimization
- Preparation of MoO3 powder by hydrothermal method
- The comparative study of Ti-bearing oxides introduced by different methods
- Application of MgO/ZrO2 coating on 309 stainless steel to increase resistance to corrosion at high temperatures and oxidation by an electrochemical method
- Effect of applying a full oxygen blast furnace on carbon emissions based on a carbon metabolism calculation model
- Characterization of low-damage cutting of alfalfa stalks by self-sharpening cutters made of gradient materials
- Thermo-mechanical effects and microstructural evolution-coupled numerical simulation on the hot forming processes of superalloy turbine disk
- Endpoint prediction of BOF steelmaking based on state-of-the-art machine learning and deep learning algorithms
- Effect of calcium treatment on inclusions in 38CrMoAl high aluminum steel
- Effect of isothermal transformation temperature on the microstructure, precipitation behavior, and mechanical properties of anti-seismic rebar
- Evolution of residual stress and microstructure of 2205 duplex stainless steel welded joints during different post-weld heat treatment
- Effect of heating process on the corrosion resistance of zinc iron alloy coatings
- BOF steelmaking endpoint carbon content and temperature soft sensor model based on supervised weighted local structure preserving projection
- Innovative approaches to enhancing crack repair: Performance optimization of biopolymer-infused CXT
- Structural and electrochromic property control of WO3 films through fine-tuning of film-forming parameters
- Influence of non-linear thermal radiation on the dynamics of homogeneous and heterogeneous chemical reactions between the cone and the disk
- Thermodynamic modeling of stacking fault energy in Fe–Mn–C austenitic steels
- Research on the influence of cemented carbide micro-textured structure on tribological properties
- Performance evaluation of fly ash-lime-gypsum-quarry dust (FALGQ) bricks for sustainable construction
- First-principles study on the interfacial interactions between h-BN and Si3N4
- Analysis of carbon emission reduction capacity of hydrogen-rich oxygen blast furnace based on renewable energy hydrogen production
- Just-in-time updated DBN BOF steel-making soft sensor model based on dense connectivity of key features
- Effect of tempering temperature on the microstructure and mechanical properties of Q125 shale gas casing steel
- Review Articles
- A review of emerging trends in Laves phase research: Bibliometric analysis and visualization
- Effect of bottom stirring on bath mixing and transfer behavior during scrap melting in BOF steelmaking: A review
- High-temperature antioxidant silicate coating of low-density Nb–Ti–Al alloy: A review
- Communications
- Experimental investigation on the deterioration of the physical and mechanical properties of autoclaved aerated concrete at elevated temperatures
- Damage evaluation of the austenitic heat-resistance steel subjected to creep by using Kikuchi pattern parameters
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part II
- Synthesis of aluminium (Al) and alumina (Al2O3)-based graded material by gravity casting
- Experimental investigation into machining performance of magnesium alloy AZ91D under dry, minimum quantity lubrication, and nano minimum quantity lubrication environments
- Numerical simulation of temperature distribution and residual stress in TIG welding of stainless-steel single-pass flange butt joint using finite element analysis
- Special Issue on A Deep Dive into Machining and Welding Advancements - Part I
- Electro-thermal performance evaluation of a prismatic battery pack for an electric vehicle
- Experimental analysis and optimization of machining parameters for Nitinol alloy: A Taguchi and multi-attribute decision-making approach
- Experimental and numerical analysis of temperature distributions in SA 387 pressure vessel steel during submerged arc welding
- Optimization of process parameters in plasma arc cutting of commercial-grade aluminium plate
- Multi-response optimization of friction stir welding using fuzzy-grey system
- Mechanical and micro-structural studies of pulsed and constant current TIG weldments of super duplex stainless steels and Austenitic stainless steels
- Stretch-forming characteristics of austenitic material stainless steel 304 at hot working temperatures
- Work hardening and X-ray diffraction studies on ASS 304 at high temperatures
- Study of phase equilibrium of refractory high-entropy alloys using the atomic size difference concept for turbine blade applications
- A novel intelligent tool wear monitoring system in ball end milling of Ti6Al4V alloy using artificial neural network
- A hybrid approach for the machinability analysis of Incoloy 825 using the entropy-MOORA method
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part II
- Innovations for sustainable chemical manufacturing and waste minimization through green production practices
- Topical Issue on Conference on Materials, Manufacturing Processes and Devices - Part I
- Characterization of Co–Ni–TiO2 coatings prepared by combined sol-enhanced and pulse current electrodeposition methods
- Hot deformation behaviors and microstructure characteristics of Cr–Mo–Ni–V steel with a banded structure
- Effects of normalizing and tempering temperature on the bainite microstructure and properties of low alloy fire-resistant steel bars
- Dynamic evolution of residual stress upon manufacturing Al-based diesel engine diaphragm
- Study on impact resistance of steel fiber reinforced concrete after exposure to fire
- Bonding behaviour between steel fibre and concrete matrix after experiencing elevated temperature at various loading rates
- Diffusion law of sulfate ions in coral aggregate seawater concrete in the marine environment
- Microstructure evolution and grain refinement mechanism of 316LN steel
- Investigation of the interface and physical properties of a Kovar alloy/Cu composite wire processed by multi-pass drawing
- The investigation of peritectic solidification of high nitrogen stainless steels by in-situ observation
- Microstructure and mechanical properties of submerged arc welded medium-thickness Q690qE high-strength steel plate joints
- Experimental study on the effect of the riveting process on the bending resistance of beams composed of galvanized Q235 steel
- Density functional theory study of Mg–Ho intermetallic phases
- Investigation of electrical properties and PTCR effect in double-donor doping BaTiO3 lead-free ceramics
- Special Issue on Thermal Management and Heat Transfer
- On the thermal performance of a three-dimensional cross-ternary hybrid nanofluid over a wedge using a Bayesian regularization neural network approach
- Time dependent model to analyze the magnetic refrigeration performance of gadolinium near the room temperature
- Heat transfer characteristics in a non-Newtonian (Williamson) hybrid nanofluid with Hall and convective boundary effects
- Computational role of homogeneous–heterogeneous chemical reactions and a mixed convective ternary hybrid nanofluid in a vertical porous microchannel
- Thermal conductivity evaluation of magnetized non-Newtonian nanofluid and dusty particles with thermal radiation
Articles in the same Issue
- Research Articles
- De-chlorination of poly(vinyl) chloride using Fe2O3 and the improvement of chlorine fixing ratio in FeCl2 by SiO2 addition
- Reductive behavior of nickel and iron metallization in magnesian siliceous nickel laterite ores under the action of sulfur-bearing natural gas
- Study on properties of CaF2–CaO–Al2O3–MgO–B2O3 electroslag remelting slag for rack plate steel
- The origin of {113}<361> grains and their impact on secondary recrystallization in producing ultra-thin grain-oriented electrical steel
- Channel parameter optimization of one-strand slab induction heating tundish with double channels
- Effect of rare-earth Ce on the texture of non-oriented silicon steels
- Performance optimization of PERC solar cells based on laser ablation forming local contact on the rear
- Effect of ladle-lining materials on inclusion evolution in Al-killed steel during LF refining
- Analysis of metallurgical defects in enamel steel castings
- Effect of cooling rate and Nb synergistic strengthening on microstructure and mechanical properties of high-strength rebar
- Effect of grain size on fatigue strength of 304 stainless steel
- Analysis and control of surface cracks in a B-bearing continuous casting blooms
- Application of laser surface detection technology in blast furnace gas flow control and optimization
- Preparation of MoO3 powder by hydrothermal method
- The comparative study of Ti-bearing oxides introduced by different methods
- Application of MgO/ZrO2 coating on 309 stainless steel to increase resistance to corrosion at high temperatures and oxidation by an electrochemical method
- Effect of applying a full oxygen blast furnace on carbon emissions based on a carbon metabolism calculation model
- Characterization of low-damage cutting of alfalfa stalks by self-sharpening cutters made of gradient materials
- Thermo-mechanical effects and microstructural evolution-coupled numerical simulation on the hot forming processes of superalloy turbine disk
- Endpoint prediction of BOF steelmaking based on state-of-the-art machine learning and deep learning algorithms
- Effect of calcium treatment on inclusions in 38CrMoAl high aluminum steel
- Effect of isothermal transformation temperature on the microstructure, precipitation behavior, and mechanical properties of anti-seismic rebar
- Evolution of residual stress and microstructure of 2205 duplex stainless steel welded joints during different post-weld heat treatment
- Effect of heating process on the corrosion resistance of zinc iron alloy coatings
- BOF steelmaking endpoint carbon content and temperature soft sensor model based on supervised weighted local structure preserving projection
- Innovative approaches to enhancing crack repair: Performance optimization of biopolymer-infused CXT
- Structural and electrochromic property control of WO3 films through fine-tuning of film-forming parameters
- Influence of non-linear thermal radiation on the dynamics of homogeneous and heterogeneous chemical reactions between the cone and the disk
- Thermodynamic modeling of stacking fault energy in Fe–Mn–C austenitic steels
- Research on the influence of cemented carbide micro-textured structure on tribological properties
- Performance evaluation of fly ash-lime-gypsum-quarry dust (FALGQ) bricks for sustainable construction
- First-principles study on the interfacial interactions between h-BN and Si3N4
- Analysis of carbon emission reduction capacity of hydrogen-rich oxygen blast furnace based on renewable energy hydrogen production
- Just-in-time updated DBN BOF steel-making soft sensor model based on dense connectivity of key features
- Effect of tempering temperature on the microstructure and mechanical properties of Q125 shale gas casing steel
- Review Articles
- A review of emerging trends in Laves phase research: Bibliometric analysis and visualization
- Effect of bottom stirring on bath mixing and transfer behavior during scrap melting in BOF steelmaking: A review
- High-temperature antioxidant silicate coating of low-density Nb–Ti–Al alloy: A review
- Communications
- Experimental investigation on the deterioration of the physical and mechanical properties of autoclaved aerated concrete at elevated temperatures
- Damage evaluation of the austenitic heat-resistance steel subjected to creep by using Kikuchi pattern parameters
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part II
- Synthesis of aluminium (Al) and alumina (Al2O3)-based graded material by gravity casting
- Experimental investigation into machining performance of magnesium alloy AZ91D under dry, minimum quantity lubrication, and nano minimum quantity lubrication environments
- Numerical simulation of temperature distribution and residual stress in TIG welding of stainless-steel single-pass flange butt joint using finite element analysis
- Special Issue on A Deep Dive into Machining and Welding Advancements - Part I
- Electro-thermal performance evaluation of a prismatic battery pack for an electric vehicle
- Experimental analysis and optimization of machining parameters for Nitinol alloy: A Taguchi and multi-attribute decision-making approach
- Experimental and numerical analysis of temperature distributions in SA 387 pressure vessel steel during submerged arc welding
- Optimization of process parameters in plasma arc cutting of commercial-grade aluminium plate
- Multi-response optimization of friction stir welding using fuzzy-grey system
- Mechanical and micro-structural studies of pulsed and constant current TIG weldments of super duplex stainless steels and Austenitic stainless steels
- Stretch-forming characteristics of austenitic material stainless steel 304 at hot working temperatures
- Work hardening and X-ray diffraction studies on ASS 304 at high temperatures
- Study of phase equilibrium of refractory high-entropy alloys using the atomic size difference concept for turbine blade applications
- A novel intelligent tool wear monitoring system in ball end milling of Ti6Al4V alloy using artificial neural network
- A hybrid approach for the machinability analysis of Incoloy 825 using the entropy-MOORA method
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part II
- Innovations for sustainable chemical manufacturing and waste minimization through green production practices
- Topical Issue on Conference on Materials, Manufacturing Processes and Devices - Part I
- Characterization of Co–Ni–TiO2 coatings prepared by combined sol-enhanced and pulse current electrodeposition methods
- Hot deformation behaviors and microstructure characteristics of Cr–Mo–Ni–V steel with a banded structure
- Effects of normalizing and tempering temperature on the bainite microstructure and properties of low alloy fire-resistant steel bars
- Dynamic evolution of residual stress upon manufacturing Al-based diesel engine diaphragm
- Study on impact resistance of steel fiber reinforced concrete after exposure to fire
- Bonding behaviour between steel fibre and concrete matrix after experiencing elevated temperature at various loading rates
- Diffusion law of sulfate ions in coral aggregate seawater concrete in the marine environment
- Microstructure evolution and grain refinement mechanism of 316LN steel
- Investigation of the interface and physical properties of a Kovar alloy/Cu composite wire processed by multi-pass drawing
- The investigation of peritectic solidification of high nitrogen stainless steels by in-situ observation
- Microstructure and mechanical properties of submerged arc welded medium-thickness Q690qE high-strength steel plate joints
- Experimental study on the effect of the riveting process on the bending resistance of beams composed of galvanized Q235 steel
- Density functional theory study of Mg–Ho intermetallic phases
- Investigation of electrical properties and PTCR effect in double-donor doping BaTiO3 lead-free ceramics
- Special Issue on Thermal Management and Heat Transfer
- On the thermal performance of a three-dimensional cross-ternary hybrid nanofluid over a wedge using a Bayesian regularization neural network approach
- Time dependent model to analyze the magnetic refrigeration performance of gadolinium near the room temperature
- Heat transfer characteristics in a non-Newtonian (Williamson) hybrid nanofluid with Hall and convective boundary effects
- Computational role of homogeneous–heterogeneous chemical reactions and a mixed convective ternary hybrid nanofluid in a vertical porous microchannel
- Thermal conductivity evaluation of magnetized non-Newtonian nanofluid and dusty particles with thermal radiation

