Abstract
The loss of boron (B) in the rack plate steel during the electroslag remelting process has a significant impact on its mechanical properties. Therefore, it is necessary to design appropriate slags to suppress the loss of B. CaF2–CaO–Al2O3 phase diagram was calculated to determine the basic slag. The influence of MgO and B2O3 on the performance of the basic slag was studied to determine the optimal amount of MgO addition. The equilibrium reactions between rack plate steel and the 50.48 mass% CaF2–24.81 mass% CaO–24.71 mass% Al2O3–2 mass% MgO-y mass% B2O3 (y was 1–10) slag were studied to examine the variation in B content in the steel. Results indicate the presence of a temperature-qualified eutectic point in the CaF2–CaO–Al2O3 phase diagram. With an increase in MgO content in the slag, the melting temperature of the basic slag initially decreases and then increases, while the electrical conductivity decreases. On the other hand, B2O3 reduces the melting point of the slag and increases the equilibrium B content in the steel. The 50.48 mass% CaF2–24.81 mass% CaO–24.71 mass% Al2O3–2 mass% MgO-y mass% B2O3 (y was 5–7) slag can achieve the target range of B content in the steel, and its viscosity meets the requirements of electric slag remelting.
1 Introduction
The growing energy crisis has promoted nations worldwide to focus on the development and utilization of marine resources. Among these resources, deep-sea oil and gas exploration has garnered significant attention as a pivotal sector for energy security. Self-elevating drilling platforms, serving as iconic facilities for deep-sea oil and gas operations, require thick-grade rack plate steel plates in their spud legs to withstand complex loads and multiple corrosive conditions. These steel plates necessitate high strength, high toughness, fatigue resistance, corrosion resistance, low-temperature toughness, resistance to lamellar tearing, and excellent weldability [1,2,3,4,5,6,7]. Therefore, the development of rack plate steel appears to be particularly significant.
As the terminal smelting process of high-end special alloy steel, electroslag remelting (ESR) is favoured by metallurgical workers due to its advantages of dense microstructure, uniform composition and structure, and smooth surface of the ingots [8]. The rack plate steel is therefore usually produced with ESR. However, in the process of remelts B-containing rack plate steel, the loss of boron (B) in the alloy exhibits a serious adverse effect on the properties of the alloy [9]. As we all know, the composition of slag has a considerable influence on the oxidation loss of alloy elements in steel. Shi [10] believed that the oxidation during ESR is affected by many factors such as alloy composition, remelting atmosphere, and slag composition. Chang et al. [11] found that reducing the content of Al2O3 in slag can effectively reduce oxygen content. Duan et al. [12] found that adding CaO into the slag can inhibit the loss of Al in the alloy. The loss of alloy element in steel can also be inhibited by addition of oxide of the element into slag [13,14]. However, so far, there has been little literature on the suppression of B element oxidation in B-containing rack plate steel during ESR. It is therefore necessary to design an appropriate slag for smelting B-containing rack plate steel.
In the past, metallurgists used a trial and error approach to design slag systems [15,16], which involved adjusting the composition of the slag based on traditional slag or existing slag to test the feasibility of the modified slag. However, the trial and error method often resulted in a wide range of potential slag systems, leading to instability in the actual production process. Moreover, this method is costly and time-consuming. In contrast, the use of Factsage thermodynamic software can effectively reduce the research cost of metallurgical problems and narrow down the range of designed slags. In this study, Factsage software was employed to investigate the effects of different MgO and B2O3 contents in the slag on the performance of the basic slag, as well as the influence of B content in the alloy. The suitable slag systems for remelting boron-containing rack plate steel were designed, which provides a reference for the design of slag used in electric slag remelting processes.
2 Calculation method
2.1 Determination of basic slag
The melting temperature of the slag is crucial for the entire process of electric slag remelting. Therefore, the melting temperature is the primary consideration when designing slag. Generally, the melting temperature of the slag used in ESR should be lower than that of the alloy steel by 100–200°C [17]. Table 1 shows the composition of the boron-containing rack plate steel, and melting temperature of rack plate steel was calculated using an empirical formula summarized by Chen [18], resulting in a calculated melting point of 1,509°C. Therefore, the melting point range of suitable slag for remelting rack plate steel is within the range of 1,309–1,409°C.
Chemical compositions of rack plate steel (mass%)
| C | Si | Mn | P | S | Ni | Cr | Mo | Ti | Cu | V | Al | N | B | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max. | 0.12 | 0.25 | 1.40 | ≤0.01 | ≤0.003 | 2.90 | 0.55 | 0.65 | 0.013 | 0.35 | 0.07 | 0.08 | ≤0.004 | 0.0015 |
| Min. | 0.09 | 0.19 | 1.10 | 2.50 | 0.45 | 0.50 | 0.007 | 0.15 | 0.05 | 0.04 | 0.001 | |||
| Goal | 0.1 | 0.22 | 1.2 | 0.01 | 0.001 | 2.7 | 0.5 | 0.55 | 0.01 | 0.25 | 0.06 | 0.055 | 0.003 | 0.0012 |
Designing a suitable melting slag with the aid of phase diagrams is a widely accepted approach [19,20,21,22]. In this study, CaF2, CaO, and Al2O3 were chosen as the fundamental constituents of the ESR slag. The CaF2–CaO–Al2O3 ternary phase diagram was calculated using FactSage 8.2, and is shown in Figure 1. The black dot is a eutectic point with a eutectic temperature of 1344.57°C. When selecting the slag composition, it is desirable to choose components close to the eutectic point to suppress the liquid chromatography during solidification. On the other hand, the slag composition should be away from the eutectic point to promote selective crystallization [9]. Additionally, the slag composition should be designed within a relatively stable temperature range to prevent significant variation in the slag melting temperature caused by changes in composition. Based on the above considerations, a composition of 50.48 mass% CaF2–24.81 mass% CaO–24.71 mass% Al2O3 (denoted as the red dot) was selected as the basic slag.

Phase diagram of CaF2–CaO–Al2O3.
2.2 Physical properties of the slag
2.2.1 Melting temperature and viscosity
The addition of MgO to the slag can reduce gas permeability of slag [9]. Meanwhile, according to equation (1), the addition of B2O3 to the slag can suppress the oxidation of B in the alloy. The impact of MgO and B2O3 on the performance of slag was investigated to determine the optimal amounts. The liquid phase region of the slag system was explored using the Phase Diagram mode in Factsage 8.2. The melting temperature of the slag system was calculated using the Equilib mode. Considering that solid particles will be precipitated in the slag at low temperatures, and these solid particles have a great influence on the viscosity of the slags, therefore, the viscosity of the slag system was calculated by the Viscosity mode of Factsage 8.2 and was corrected by equation (2). Equation (2) has been applied in literature [23,24].
where η and η 0 are the viscosity of the solid-containing and the solid-free melt, respectively; f is the volume fraction of solid particles in the melt, which can be replaced with the mass fraction; and for spherical particles of a uniform size, a and n are 1.35 and 2.5, respectively [25].
2.2.2 Electrical conductivity
Ogino et al. [26] summarized an electrical conductivity calculation formula for multicomponent slag systems with an error within 10%, but the influence of MgO was not considered. Dong et al. [27] made a modification to the formula and obtained equations (3) and (4). The modified equation has been applied in in literature [28,29,30,31]. By comparing the conductivity measurement value of the ternary slags, it is found that the average error of equation (3) was 6.71%. Therefore, it is reliable to use equations (3) and (4) to calculate the conductivity of the slag.
where κ is the electrical conductivity of the slag, S·m−1; T is the temperature, K, and the applicable range is 1,823–2,053 K; x(i) is the mole fraction of i, and the applicable range is x(Al2O3) = 0–0.5, x(CaO) = 0–0.65, x(MgO) = 0–0.1, x(SiO2) = 0–0.17, x(TiO2) = 0–0.18, x(ZrO2) = 0–0.15.
Ogino [32] obtained the electrical conductivity values of 60 mass% CaF2–20 mass% CaO–20 mass% Al2O3 slag and 40 mass% CaF2–30 mass% CaO–30 mass% Al2O3 slag at 1,600°C, which were 352 and 217 S·m−1, respectively. According to production experience, the electrical conductivity of 60 mass% CaF2–20 mass% CaO–20 mass% Al2O3 slag resulted in energy waste, while the low electrical conductivity of 40 mass% CaF2–30 mass% CaO–30 mass% Al2O3 slag resulted in insufficient reaction. Therefore, the appropriate conductivity should be within the range of 217–352 S·m−1, and the median value (284.5 S·m−1) may be the best choice.
3 Slag-steel equilibration experiments
The equilibrium reactions between 50.48 mass% CaF2–24.81 mass% CaO–24.71 mass% Al2O3-x mass% MgO-y mass% B2O3 and rack steel (containing 0.0012 mass% B) were calculated using the equilib mode of Factsage 8.2. The influence of B2O3 and MgO on the B content in the rack steel was investigated. The reaction temperature was set at 1,600°C, and the oxygen partial pressure was set to 1 × 10−10 atm. Additionally, a slag-steel mass ratio of 6:1 was used to simulate the actual refining process. Based on the physical properties of the slag and the calculated results of the slag-steel equilibrium reactions, suitable slags were selected. Subsequently, slag-steel equilibrium experiments were conducted to test the smelting performance of the designed slags.
Before the slag was pre-melted, the analytical-grade reagents were calcined in a muffle furnace at 1,273 K for 8 h to remove hydroxide and moisture. The slag made by Table 2 was put into a graphite crucible lined with a 0.2 mm thick molybdenum film and held at 1,600°C for 1 h in a MoSi2 furnace. The pre-melt slag was then removed for air cooling and crushed.
Composition of the designed slags (mass%)
| CaF2 | CaO | Al2O3 | MgO | B2O3 | |
|---|---|---|---|---|---|
| 1# | 48.97 | 24.07 | 23.96 | 2 | 1.00 |
| 2# | 48.46 | 23.82 | 23.72 | 2 | 2.00 |
| 3# | 47.96 | 23.57 | 23.47 | 2 | 3.00 |
| 4# | 46.95 | 23.07 | 22.98 | 2 | 5.00 |
| 5# | 45.94 | 22.58 | 22.48 | 2 | 7.00 |
150 g rack steel (32 mm diameter and 27 mm height) and 20 g pre-melt slag were placed into a MgO crucible (35 mm inner diameter and 50 mm height) with a 0.2 mm thick molybdenum film. The molybdenum film was fixed above the steel by a corundum rod, so it would not fall into the steel liquid. A graphite crucible (60 mm inner diameter and 130 mm height) containing the MgO crucible was then placed in MoSi2 furnace at 1,600°C, as shown in Figure 2. After 1 h of reaction, it was removed from the furnace. Argon gas was blown in the furnace at a flow rate of 2,000 mL·min−1 during the experiments. The Optical Emission Spectrometer was used to determine the B content in the steel sample after the reaction.

Schematic diagram of the equilibration experiment device.
4 Results and discussions
4.1 Effect of MgO on the properties of CaF2–CaO–Al2O3 slag
4.1.1 Effect of MgO on the liquid phase zone
The polythermal liquidus projections of CaF2–CaO–Al2O3-x mass% MgO (x ≤ 7) slag at 1,200–1,600°C are shown in Figure 3. Liquid phase zone at 1,250°C expands first and then contracts, and other liquid phase zones with different temperatures exhibit a similar trend as MgO content increases in the slag. It is worth noting that liquid phase zone at 1,350°C expands towards the Al2O3 direction, whereas liquid phase zone at 1,400°C tends to expand towards CaF2 when MgO content is less than 2 mass%. The liquid phase zone at 1,200, 1,250, and 1,300°C disappear upon the addition of 2 mass%, 4 mass%, and 7 mass% MgO, respectively. Correspondingly, the liquid phase regions at other temperatures progressively diminish as MgO content increases, which means that variations in MgO content lead to significant changes in the melting temperatures of slag.

Polythermal liquidus projection of CaF2–CaO–Al2O3-x mass% MgO (x ≤ 6) slag at 1,200–1,600°C: (a) 1 mass% MgO; (b) 2 mass% MgO; (c) 3 mass% MgO; (d) 4 mass% MgO; (e) 5 mass% MgO; (f) 6 mass% MgO; and (g) 7 mass% MgO.
Based on the calculation results presented in Section 2.1, the melting point of the slag should be in the range from 1,309 to 1,409°C, with a possible optimal value around 1,350°C. The liquid phase zone of CaF2–CaO–Al2O3 slag with different w(MgO) at 1,350°C were calculated, and the results are shown in Figure 4. With the increase in MgO, the liquid phase area initially expands and then starts to decrease at 3 mass% MgO. When MgO reaches 7 mass%, the liquid phase area accounts for only 1.3%. A small liquid phase area indicates that ESR process is difficult to conduct.

Liquid phase area of CaF2–Al2O3–CaO slag with different w(MgO) at 1,350°C.
The phase diagrams of the CaF2-Al2O3-CaO-x mass% MgO slag (x ≤ 7) with liquid phase zone at 1,350°C are shown in Figure 5. The main primary phases in the slags are CaF2-HT (high temperature CaF2), Ca12Al14F2O32, and Ca4Al6F2O12. When MgO content rises to 2 mass%, monoxide#2 (the main components are MgO) forms in the slag. Spinel appears first in CaF2-Al2O3-CaO-3 mass% MgO slag. When the MgO reaches 4 mass%, Ca4Al6F2O12 disappears, but spinel and monoxide#2 continued to increase. At the same time, the liquid phase area at 1,350°C decreases. In summary, the addition of MgO may elevate the content of MgO·Al2O3 inclusion [33], thus affecting the cleanliness of the ingot.

Phase diagrams of CaF2–CaO–Al2O3-x mass% MgO slag: (a) 1 mass% MgO; (b) 2 mass% MgO; (c) 3 mass% MgO; (d) 4 mass% MgO; (e) 5 mass% MgO; (f) 6 mass% MgO; and (g) 7 mass% MgO. (The area circled by red lines is liquid area at 1,350°C).
4.1.2 Effect of MgO on the melting temperature
The effect of MgO on the melting temperature of the basic slag is shown in Figure 6. The melting point first decreases from 1,351 to 1281.5°C when MgO content increases from 0 mass% to 5 mass%. However, the melting point of the slag containing 7 mass% MgO is 1411.4°C. The significant change in melting temperature emphasizes the importance of strict control over the MgO content.

Melting temperature of the basic slag with different w(MgO).
4.1.3 Effect of MgO on the electrical conductivity and viscosity
The electrical conductivity (at 1,600°C) and viscosity (at 1,800°C) of the basic slag are illustrated in Figure 7. When MgO content changes from 0 mass% to 7 mass%, the viscosity of slag increases from 0.0113 to 0.0125 Pa·s. In contrast, the conductivity of the slag decreases with the increase in the MgO content, which is consistent with the findings of Presoly et al. [34]. The conductivity and viscosity of the slag exhibit opposite trends, as described in previous literature [35,36]. Yan et al. [37] attributed this phenomenon to an increase in melt polymerization degree and the cation consumption. The increase in viscosity of the slag hampers the movement of charged ions within the slag, resulting in a decrease in electrical conductivity of the slag. The electrical conductivity of the slag containing 1 mass% MgO is 284.3 S·m−1 which was very close to the median value (284.5 S·m−1). In addition, Zhang et al. [38] found that B2O3 can enhance the electrical conductivity of aluminosilicate slag. Therefore, the MgO content should be less than 6%.

Electrical conductivity and viscosity of the basic slag with different w(MgO).
The addition of 3 mass% MgO to CaF2–CaO–Al2O3 slag results in a maximum liquid phase region at 1,350°C. The addition of 5 mass% MgO makes the lowest melting temperature of the slag, which is favourable for rapid melting of the ESR slag. These findings suggest that the melting performance of the slag containing 4 mass% MgO is also satisfactory. Furthermore, the electrical conductivity and viscosity of the slag with 4 mass% MgO content are also appropriate. This implies that the slag containing 4 mass% MgO seems to be optimal.
4.2 Effect of B2O3 on the performance of CaF2–CaO–Al2O3-4 mass% MgO slag
4.2.1 Effect of B2O3 on the liquid phase zone
The polythermal liquidus projections of CaF2–CaO–Al2O3-4 mass% MgO-y mass% B2O3 slag was calculated and presented in Figure 8. The addition of B2O3 evidently increases the liquid phase region of the slag, with liquid phase region appearing at 1,200°C when 3 mass% B2O3 is added to the slag. The most pronounced changes occur at 1,350°C, where another liquid phase region is generated on the CaF2-Al2O3 boundary when 1 mass%B2O3 is added in the slag. Similarly, when the B2O3 content increases to 2 mass%, both liquid phase regions expand and merge to form a larger liquid phase region. In summary, B2O3 can significantly expand the liquid phase region of CaF2–CaO–Al2O3 slag and promote the rapid melting of the slag.

Polythermal liquidus projections of CaF2–CaO–Al2O3-4 mass% MgO-y mass% B2O3 (y ≤ 5) slag at 1,200–1,600°C: (a) 1 mass% B2O3; (b) 2 mass% B2O3; (c) 3 mass% B2O3; (d) 4 mass% B2O3; and (e) 5 mass% B2O3.
In order to analyse the influence of B2O3 on the liquid phase region more intuitively, the calculation of the lowest eutectic temperature and liquid phase area at 1,350°C of CaF2–CaO–Al2O3-4 mass% MgO-y mass% B2O3 (y ≤ 5) slag was performed, and the results are shown in Figure 9. With an increase in the B2O3 content in the slag, the eutectic temperature decreases from 1247.34 to 1172.48°C. Correspondingly, the proportion of the liquid phase area at 1,350°C of the slag increases from 11 to 37%.

The low eutectic temperature and liquid phase area at 1,350°C of CaF2–CaO–Al2O3-4 mass% MgO-y mass% B2O3 slag.
The phase diagrams of CaF2–CaO–Al2O3-4 mass% MgO-y mass% B2O3 (y ≤ 5) slag are shown in Figure 10. The main phases in slags are CaF2-HT, monoxide (the main components are CaO and MgO), Ca12Al14F2O32, and spinel (the main component is MgAl2O4). With the increase in B2O3 content in the slag, the area of CaF2-HT gradually increases, but monoxide and spinel decreases. This leads to a decrease in the melting temperature of the slag and an increase in the liquid phase area. In summary, B2O3 can facilitate the melting of the slag, thereby contributing to the attainment of a smooth surface of the steel ingot.

Phase diagrams of CaF2–CaO–Al2O3-4 mass% MgO-y mass% B2O3 slag: (a) 1 mass% B2O3; (b) 2 mass% B2O3; (c) 3 mass% B2O3; (d) 4 mass% B2O3; and (e) 5 mass% B2O3 (The area circled by red lines is liquid area at 1,350°C).
4.2.2 Effect of B2O3 on the melting temperature
The melting temperature of the slag with different w(B2O3) are shown in Figure 11. When MgO is less than 5 mass%, the melting temperature of the slag slightly decreases within the range of 1,330–1,270°C with the increase in the B2O3 content. When MgO is more than 5 mass%, the melting temperature of the slag initially decreased sharply and then gradually decreased with the increase in the B2O3 content. This indicates that B2O3 could decrease the melting temperature of the slag. When the B2O3 content is constant, the melting temperature of the slag decreases initially and then increases with the increase in MgO, which is consistent with the results described in Section 3.1.2. The effect of B2O3 in reducing the melting temperature is stronger than that of MgO. The red dotted line represents the upper limit of the melting temperature required (1,409°C), and the blue dotted line represents the lower limit of the melting temperature required (1,309°C). The slag with 4 mass% MgO has a melting point lower than 1,309°C, whereas the slag containing 1 mass% and 2 mass% MgO has a more suitable melting point. Considering that MgO has the effect of inhibiting the transfer of oxygen to the slag, it was decided to add 2 mass% MgO instead of 1 mass% MgO to the studied slag.
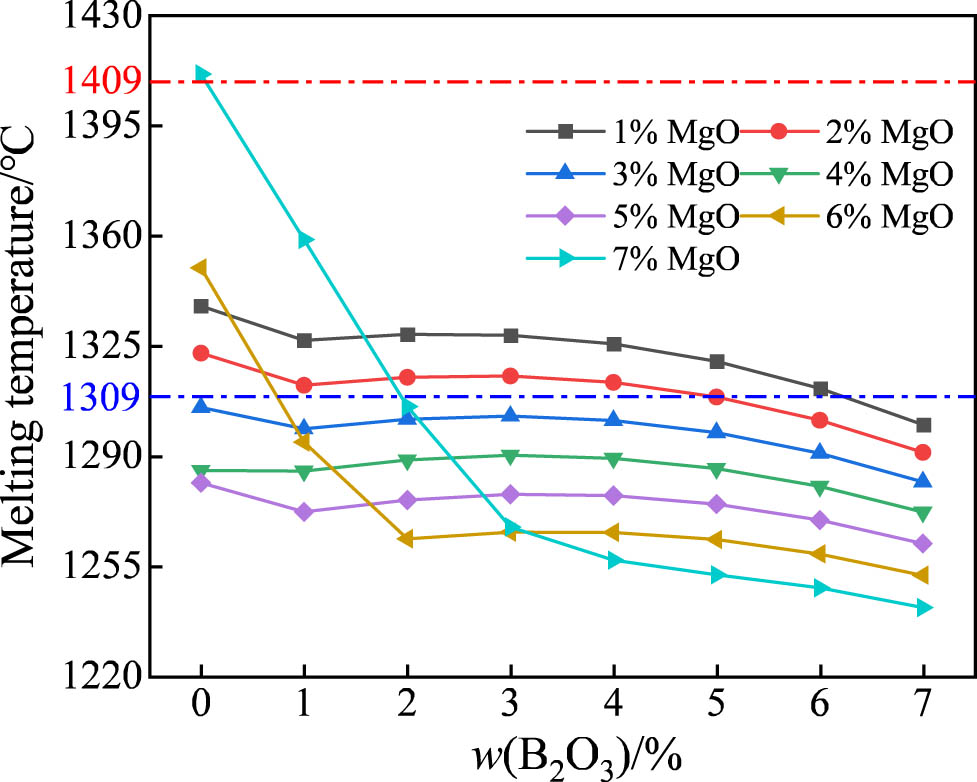
Melting temperature of the basic slag with different w(MgO) and w(B2O3).
4.3 Effect of B2O3 on the equilibrium B content in the steel
During the ESR process of B-containing rack plate steel, the molten metal film located at the tip of the electrode reacts with the slag as equation (1), and the equilibrium constant of the reaction is expressed as equation (5). Equation (5) is calculated by reactions of 2B(s) + 1.5O2 = B2O3(l), B(s) = [B], and 1/2O2 = [O]. The ∆G θ of these equations are standard molar Gibbs free energies.
where K B represents the equilibrium constant for equation (1), a (B2O3) denotes the activity of B2O3, f [B] denotes the interaction coefficient of B, and T represents the temperature in Kelvin, K.
The effect of B2O3 addition in the 50.48 mass% CaF2–24.81 mass% CaO–24.71 mass% Al2O3–2 mass% MgO slag on the equilibrium B content in the rack steel (containing 0.0012 mass% B) at 1,600°C was calculated. And the oxygen partial pressure was set to 1 × 10−10 atm. In Figure 12, the blue and red lines represent the upper and lower limits of target B content, respectively. The increase in B2O3 significantly increases the B content in the steel ingot which is consistent with the conclusions reported in literature [13,14]. The greater the amount of B2O3 in the slag, the greater the increase in B content in the alloy. From the thermodynamic calculation results, it can be observed that adding 4.7–6.0 mass% B2O3 to CaF2–CaO–Al2O3–2 mass% MgO slag enables the equilibrium B content in the rack steel to fall within the target range when

The effect of varied w(MgO) and w(B2O3) in the basic slag on equilibrium content of B.
Based on the calculation results of the melting temperature and electrical conductivity of the CaF2–CaO–Al2O3-x mass% MgO-y mass% B2O3 slag, it can be concluded that the addition of 2 mass% MgO to the slag is appropriate. The calculation results of the equilibrium reaction show that adding 4.7–6.0 mass% B2O3 to the slag can achieve the desired range of B content in the alloy. Due to the instability of the operational and reaction processes, the oxygen partial pressure may increase or decrease. Therefore, in this study, it is proposed to add 1 mass%, 2 mass%, 3 mass%, 5 mass%, and 7 mass% B2O3 in the slag to investigate the influence of B2O3 on the equilibrium B content in rack steel. The composition of the designed slag is shown in Table 2.
The experimental results of the B content in the steel after the equilibrium reaction between the designed slag and the rack steel are shown in Figure 12. The B content in the alloy increases with an increase in the w(B2O3), which is consistent with the thermodynamic calculation results. It is worth noting that the experimental values of B content in the alloy are consistently lower than the calculated values. This may be attributed to the entry of atmospheric oxygen into the furnace tube during crucible placement, thereby disrupting the argon atmosphere in the experimental furnace. Therefore, these experimental results can be understood. Furthermore, incomplete progress of the slag-steel reaction can also lead to deviations in the experimental results. Although the experimental values are slightly lower than the calculated values, the overall trend of both are very similar, indicating that the experimental results are still reliable. When w(B2O3) is 5 mass%, the B content in the steel just reaches the lower limit of the target range. However, when w(B2O3) is 7 mass%, the B content in the steel exceeds the upper limit of the target range. This calculation result closely matches the experimental result. It is initially believed that adding 5 mass% to 7 mass% B2O3 in the slag can meet the B content requirement in the rack steel. This provides important theoretical references for practical smelting, but the feasibility of the designed slag still requires further validation through ESR experiments.
The composition of final slags after equilibrium experiments are as seen in Table 3. After normalization, it can be clearly seen that the CaF2 in the slag volatilizes about 4 mass% after the test. There is no significant change in the content of CaO and Al2O3. However, the MgO content increases, possibly because the MgO crucible is eroded by the slag at high temperatures. The content of B2O3 in the slag 1#–4# increases indicating that the B in the steel is burned. However, the B2O3 content in slag 5# decreases, which indicates that the B in the steel is not oxidized. Therefore, 7 mass% B2O3 addition seems to completely suppress the loss of B in the rack steel. The occurrence of SiO2 in the slag is related to Si in rack steel. During the smelting process, a small amount of oxygen may enter the steel liquid, so FeO is detected.
Composition of final slags after experiments (mass%)
| CaF2 | CaO | Al2O3 | MgO | B2O3 | SiO2 | FeO | |
|---|---|---|---|---|---|---|---|
| 1# | 44.85 | 24.48 | 23.18 | 4.56 | 1.62 | 1.23 | 0.08 |
| 2# | 44.03 | 23.92 | 22.78 | 4.91 | 2.93 | 1.31 | 0.12 |
| 3# | 43.88 | 23.37 | 22.39 | 5.17 | 3.54 | 1.37 | 0.28 |
| 4# | 42.94 | 22.37 | 22.74 | 5.13 | 5.11 | 1.42 | 0.29 |
| 5# | 42.19 | 22.25 | 23.01 | 4.67 | 6.13 | 1.43 | 0.32 |
4.4 Effect of B2O3 on the viscosity
The viscosity of the designed slags is shown in Figure 13. The viscosity of the slags decreased with the increase in temperature, while increased with the increase in B2O3 at high temperature (more than 1,320°C). Geng et al. [39] and Huang et al. [40] found B2O3 as a network generating oxide, enhancing the degree of polymerization of the slag and strengthening and complicating its network structure at high temperatures. The viscosity of slag at 1,440°C varied in the range from 0.042 to 0.048 Pa·s and the viscosity of slag changed slightly with temperature, which would improve the stability of the ESR and obtain the ingots with great surface quality.

The viscosity–temperature curve of slags.
5 Conclusion
The eutectic temperature of the CaF2–CaO–Al2O3 slag is determined to be 1344.57°C, which is suitable for the remelting of rack steel. A slag composition containing 50.48 mass% CaF2, 24.81 mass% CaO, and 24.71 mass% Al2O3 closely approximates the eutectic composition, making it more suitable for electro-slag remelting of rack steel.
With an increase in MgO content, the liquid phase area at 1,350°C of CaF2–CaO–Al2O3-x mass% MgO (x ≤ 7) increases first and then decreases, and melting temperature first decreases and then increases. With the increase in B2O3 content, the liquid phase area at 1,350°C of CaF2–CaO–Al2O3-4 mass% MgO-y mass% B2O3 (y ≤ 5) slag increases, and the melting temperature decreases. The CaF2–CaO–Al2O3–2 mass% MgO slag exhibits suitable characteristics such as melting point, electrical conductivity, and viscosity for the remelting of rack steel.
The results of the slag–steel equilibrium reaction show that 50.48 mass% CaF2–24.81 mass% CaO–24.71 mass% Al2O3–2 mass% MgO-y mass% B2O3 (y was 5–7) slag can achieve the desired level of B content in rack steel. Furthermore, the viscosity of the designed slags meets the requirements for ESR.
Acknowledgements
This paper is supported by National Natural Science Foundation of China (NSFC) under grant Nos 52174317 and 52274337.
-
Funding information: This project is supported by the National Natural Science Foundation of China (Grant Nos 52174317 and 52274337).
-
Author contributions: Yong-jiao Zhang: Conceptualization, Software, Investigation, Formal Analysis, Writing – Original Draft; Ling-zhong Kong: Conceptualization, Funding Acquisition, Resources, Supervision, Writing – Review & Editing. Xi-min Zang: Conceptualization, Funding Acquisition, Resources, Supervision, Writing – Review & Editing. Shi-sen Li: Conceptualization; Investigation; Supervision; Writing – Review & Editing.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The authors confirm that the data supporting the findings of this study are available within the article [and/or its supplementary materials].
References
[1] Zhang, P. and H. Ding. Bearing capacity of the bucket spudcan foundation for offshore jack-up drilling platforms. Petroleum Exploration and Development, Vol. 38, 2011, pp. 237–242.Search in Google Scholar
[2] Ahmed, K. S., A. K. Keng, and K. C. Ghee. Stress and stiffness analysis of a 7-teeth pinion/rack jacking system of an offshore jack-up rig. Engineering Failure Analysis, Vol. 115, 2020, id. 104623.Search in Google Scholar
[3] Myers, P. T., F. P. Brennan, and W. D. Dover. The effect of rack/rib plate on the stress concentration factors in jack-up chords. Marine Structures, Vol. 14, 2001, pp. 485–505.Search in Google Scholar
[4] Zhang, P., X. Yu, and H. Ding. Spudcan bearing capacity calculation of the offshore jack-up drilling platform during the preloading process. Petroleum Exploration and Development, Vol. 38, 2011, pp. 613–619.Search in Google Scholar
[5] Pargeter, R. J. The weldability of steels used in jack-up drilling platforms. Marine structures, Vol. 2, 1989, pp. 255–264.Search in Google Scholar
[6] Liu, D., B. Cheng, and Y. Chen. Strengthening and toughening of a heavy plate steel for shipbuilding with yield strength of approximately 690 MPa. Metallurgical and Materials Transactions A, Vol. 44, 2013, pp. 440–455.Search in Google Scholar
[7] Wei, Y., G. S. Li, and Q. W. Cai. Effect of a novel gradient temperature rolling process on deformation, microstructure and mechanical properties of ultra-heavy plate. Journal of Materials Processing Technology, Vol. 217, 2015, pp. 317–326.Search in Google Scholar
[8] Stetsenko, N. V., A. N. Korotkov, and E. I. Moshkevich. Improving metal quality and increasing efficiency in ESR. Metallurgist, Vol. 19, 1975, pp. 361–364.Search in Google Scholar
[9] Paju, M. Effects of boron protection methods on properties of steel. Ironmaking & Steelmaking, Vol. 19, 1992, pp. 495–500.Search in Google Scholar
[10] Shi, C.B. Deoxidation of electroslag remelting (ESR) – A review. ISIJ International, Vol. 60, 2020, pp. 1083–1096.Search in Google Scholar
[11] Chang, L. Z., X. F. Shi, and J. Q. Cong. Study on mechanism of oxygen increase and countermeasure to control oxygen content during electroslag remelting process. Ironmaking & Steelmaking, Vol. 41, 2014, pp. 182–186.Search in Google Scholar
[12] Duan, S. C., M. J. Lee, J. H. Park, and H. J. Guo. Effect of temperature on the oxidation behavior of Al and Ti in Inconel® 718 alloy by ESR slag with different amounts of CaO. JOM, Vol. 74, 2022, pp. 1228–1236.Search in Google Scholar
[13] Peng, L. Z., Z. H. Jiang, X. Geng, F. B. Liu, and H. B. Li. Effect of B2O3 on the crystallization behavior of CaF2-based slag for electroslag remelting 9CrMoCoB steel. Metals, Vol. 9, 2019, id. 1331.Search in Google Scholar
[14] Duan, S. C., X. Shi, F. Wang, M. C. Zhang, Y. Sun, H. J. Guo, et al. A review of methodology development for controlling loss of alloying elements during the electroslag remelting process. Metallurgical and Materials Transactions B, Vol. 50, 2019, pp. 3055–3071.Search in Google Scholar
[15] Mahto, D. and A. Kumar. Novel method of productivity improvement and waste reduction through recycling of submerged arc welding slag. Jordan Journal of Mechanical and Industrial Engineering, Vol. 4, 2010, pp. 451–466.Search in Google Scholar
[16] Wen, Q. Y., Z. H. Wu, P. X. Zhang, and X. D. Jiang. Improved artificial neural network for data analysis and property prediction in slag glass–ceramic. Journal of the American Ceramic Society, Vol. 88, 2005, pp. 1765–1769.Search in Google Scholar
[17] Mitchell, A. The chemistry of ESR slags. Canadian Metallurgical Quarterly, Vol. 20, 1981, pp. 101–112.Search in Google Scholar
[18] Chen, E. P. Iron-based, nickel-based and cobalt-based alloy melting point calculation methods and empirical formulas. Special Steel, Vol. 2, 1992, pp. 28–30 (in Chinese).Search in Google Scholar
[19] Ai, X. B., H. Bai, L. H. Zhao, D. Q. Cang, and Q. Tang. Thermodynamic analysis and formula optimization of steel slag-based ceramic materials by FACTsage software. International Journal of Minerals, Metallurgy, and Materials, Vol. 30, 2013, pp. 379–385.Search in Google Scholar
[20] Dai, B. and J. Zhang. The research of blast furnace slag fluidity by viscosity experiment and phase diagram analysis. Metalurgia International, Vol. 17, 2012, pp. 25–29.Search in Google Scholar
[21] Bennett, J. and K. S. Kwong. Thermodynamic studies of MgO saturated EAF slag. Ironmaking & steelmaking, Vol. 37, 2010, pp. 529–535.Search in Google Scholar
[22] Rusen, A., A. Geveci, Y. A. Topkaya, and B. Derin. Effects of some additives on copper losses to matte smelting slag. JOM, Vol. 68, 2016, pp. 2323–2331.Search in Google Scholar
[23] Seok, S. H., S. M. Jung, Y. S. Lee, and D. J. Min. Viscosity of highly basic slags. ISIJ International, Vol. 48, 2007, pp. 1090–1096.Search in Google Scholar
[24] Massoudi, M. and P. Wang. Slag behavior in gasifiers. Part II: Constitutive modeling of slag. Energies, Vol. 6, 2013, pp. 807–838.Search in Google Scholar
[25] Wright, S., L. Zhang, S. Sun, and S. Jahanshahi. Viscosity of a CaO-MgO-Al2O3-SiO2 melt containing spinel particles at 1,646K. Metallurgical and Materials Transactions B, Vol. 31, 2000, pp. 97–104.Search in Google Scholar
[26] Ogino, K., H. Hashimoto, and S. Hara. Measurement of the electrical conductivity of ESR fluxes containing fluoride by four electrodes method with alternating current. Tetsu-to-Hagane, Vol. 64, 1978, pp. 225–231 (in Japanese).Search in Google Scholar
[27] Dong, Y. W., Z. H. Jiang, H. B. Li, G. Q. Shao, A. Yu, R. Chen, and Z. W. Song. Conductivity calculation of slag containing fluoride used for electroslag metallurgy. Journal of Materials and Metallurgy, Vol. 11, 2012, pp. 274–277 (in Chinese).Search in Google Scholar
[28] Wang, H. T., Y. H. Han, L. J. Cao, and L. G. Zhu. Optimization of electroslag remelting slag system of H13 hot work die steel. Iron and Steel, Vol. 57, 2022, pp. 111–122 (in Chinese).Search in Google Scholar
[29] Miao, Z. Q., G. G. Cheng, S. J. Li, L. Chen, Z. Q. Liu, and C. W. Li. Mathematical model of electrode immersing depth in industrial electroslag remelting process and application. Iron and Steel, Vol. 53, 2018, pp. 25–29 (in Chinese).Search in Google Scholar
[30] Xiao, A. P., Z. Zhang, D. S. Li, Z. G. Wang, and Y. W. Dong. Influence of ESR Slag on Cleanness of GCr15 Bearing Steel. Special Steel, Vol. 43, 2022, pp. 34–37 (in Chinese).Search in Google Scholar
[31] Qu, M. L., G. G. Cheng, S. J. Li, L. Chen, Q. Z. Yan, and M. Liang. Mathematical Model of Electrode Immersing Depth in 2.3 t Electro-Slag Ingot Remelting Process and Application. Special Steel, Vol. 38, 2017, pp. 5–8 (in Chinese).Search in Google Scholar
[32] Ogino, K. Electro-slag remelting slag. Bulletin of the Japan Institute of Metals, Vol. 18, 1979, pp. 684–693 (in Japanese).Search in Google Scholar
[33] Dong, Y. W., Z. H. Jiang, Y. L. Cao, A. Yu, and D. Hou. Effect of slag on inclusions during electroslag remelting process of die steel. Metallurgical and Materials Transactions B, Vol. 45, 2014, pp. 1315–1324.Search in Google Scholar
[34] Presoly, P., J. Korp, and R. Schneider. Electrical conductivity and corresponding specific energy consumption of new MgO-containing ESR-slags. Archives of metallurgy and materials, Vol. 53, 2008, pp. 567–574.Search in Google Scholar
[35] Zhang, G. H. and K. C. Chou. Correlation between viscosity and electrical conductivity of aluminosilicate melts. Metallurgical and Materials Transactions B, Vol. 43, 2012, pp. 849–855.Search in Google Scholar
[36] Li, W. L., X. Z. Cao, T. Jiang, H. Yang, and X. X. Xue. Relation between electrical conductivity and viscosity of CaO-SiO2-Al2O3-MgO system. ISIJ International, Vol. 56, 2016, pp. 205–209.Search in Google Scholar
[37] Yan, X. B., W. J. Pan, X. S. Wang, X. B. Zhang, S. P. He, and Q. Wang. Electrical conductivity, viscosity and structure of CaO-Al2O3-based mold slags for continuous casting of high-Al steels. Metallurgical and Materials Transactions B, Vol. 52, 2021, pp. 2526–2535.Search in Google Scholar
[38] Zhang, C., Y. Kong, T. Wu, G. D. Bao, J. Lei, and H. C. Wang. Effect of B2O3 on the structure and properties of aluminate slag. Metallurgical Research & Technology, Vol. 119, 2022, id. 507.Search in Google Scholar
[39] Geng, X., X. R. Tao, Z. H. Jiang, L. Z. Peng, F. B. Liu, and H. B. Li. Effect of B2O3 on ESR slag viscosity for remelting 9CrMoCoB Steel. ISIJ International, Vol. 62, 2022, pp. 1070–1077.Search in Google Scholar
[40] Huang, Y., C. B. Shi, X. X. Wan, Y. J. Liang, J. Li, and S. J. Liu. Viscosity and surface tension of CaF2–CaO–Al2O3-based slag with varying SiO2 and B2O3 contents for ESR of rotor steel. Journal of Iron and Steel Research International, Vol. 30, 2023, pp. 74–81.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- De-chlorination of poly(vinyl) chloride using Fe2O3 and the improvement of chlorine fixing ratio in FeCl2 by SiO2 addition
- Reductive behavior of nickel and iron metallization in magnesian siliceous nickel laterite ores under the action of sulfur-bearing natural gas
- Study on properties of CaF2–CaO–Al2O3–MgO–B2O3 electroslag remelting slag for rack plate steel
- The origin of {113}<361> grains and their impact on secondary recrystallization in producing ultra-thin grain-oriented electrical steel
- Channel parameter optimization of one-strand slab induction heating tundish with double channels
- Effect of rare-earth Ce on the texture of non-oriented silicon steels
- Performance optimization of PERC solar cells based on laser ablation forming local contact on the rear
- Effect of ladle-lining materials on inclusion evolution in Al-killed steel during LF refining
- Analysis of metallurgical defects in enamel steel castings
- Effect of cooling rate and Nb synergistic strengthening on microstructure and mechanical properties of high-strength rebar
- Effect of grain size on fatigue strength of 304 stainless steel
- Analysis and control of surface cracks in a B-bearing continuous casting blooms
- Application of laser surface detection technology in blast furnace gas flow control and optimization
- Preparation of MoO3 powder by hydrothermal method
- The comparative study of Ti-bearing oxides introduced by different methods
- Application of MgO/ZrO2 coating on 309 stainless steel to increase resistance to corrosion at high temperatures and oxidation by an electrochemical method
- Effect of applying a full oxygen blast furnace on carbon emissions based on a carbon metabolism calculation model
- Characterization of low-damage cutting of alfalfa stalks by self-sharpening cutters made of gradient materials
- Thermo-mechanical effects and microstructural evolution-coupled numerical simulation on the hot forming processes of superalloy turbine disk
- Endpoint prediction of BOF steelmaking based on state-of-the-art machine learning and deep learning algorithms
- Effect of calcium treatment on inclusions in 38CrMoAl high aluminum steel
- Effect of isothermal transformation temperature on the microstructure, precipitation behavior, and mechanical properties of anti-seismic rebar
- Evolution of residual stress and microstructure of 2205 duplex stainless steel welded joints during different post-weld heat treatment
- Effect of heating process on the corrosion resistance of zinc iron alloy coatings
- BOF steelmaking endpoint carbon content and temperature soft sensor model based on supervised weighted local structure preserving projection
- Innovative approaches to enhancing crack repair: Performance optimization of biopolymer-infused CXT
- Structural and electrochromic property control of WO3 films through fine-tuning of film-forming parameters
- Influence of non-linear thermal radiation on the dynamics of homogeneous and heterogeneous chemical reactions between the cone and the disk
- Thermodynamic modeling of stacking fault energy in Fe–Mn–C austenitic steels
- Research on the influence of cemented carbide micro-textured structure on tribological properties
- Performance evaluation of fly ash-lime-gypsum-quarry dust (FALGQ) bricks for sustainable construction
- First-principles study on the interfacial interactions between h-BN and Si3N4
- Analysis of carbon emission reduction capacity of hydrogen-rich oxygen blast furnace based on renewable energy hydrogen production
- Just-in-time updated DBN BOF steel-making soft sensor model based on dense connectivity of key features
- Effect of tempering temperature on the microstructure and mechanical properties of Q125 shale gas casing steel
- Review Articles
- A review of emerging trends in Laves phase research: Bibliometric analysis and visualization
- Effect of bottom stirring on bath mixing and transfer behavior during scrap melting in BOF steelmaking: A review
- High-temperature antioxidant silicate coating of low-density Nb–Ti–Al alloy: A review
- Communications
- Experimental investigation on the deterioration of the physical and mechanical properties of autoclaved aerated concrete at elevated temperatures
- Damage evaluation of the austenitic heat-resistance steel subjected to creep by using Kikuchi pattern parameters
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part II
- Synthesis of aluminium (Al) and alumina (Al2O3)-based graded material by gravity casting
- Experimental investigation into machining performance of magnesium alloy AZ91D under dry, minimum quantity lubrication, and nano minimum quantity lubrication environments
- Numerical simulation of temperature distribution and residual stress in TIG welding of stainless-steel single-pass flange butt joint using finite element analysis
- Special Issue on A Deep Dive into Machining and Welding Advancements - Part I
- Electro-thermal performance evaluation of a prismatic battery pack for an electric vehicle
- Experimental analysis and optimization of machining parameters for Nitinol alloy: A Taguchi and multi-attribute decision-making approach
- Experimental and numerical analysis of temperature distributions in SA 387 pressure vessel steel during submerged arc welding
- Optimization of process parameters in plasma arc cutting of commercial-grade aluminium plate
- Multi-response optimization of friction stir welding using fuzzy-grey system
- Mechanical and micro-structural studies of pulsed and constant current TIG weldments of super duplex stainless steels and Austenitic stainless steels
- Stretch-forming characteristics of austenitic material stainless steel 304 at hot working temperatures
- Work hardening and X-ray diffraction studies on ASS 304 at high temperatures
- Study of phase equilibrium of refractory high-entropy alloys using the atomic size difference concept for turbine blade applications
- A novel intelligent tool wear monitoring system in ball end milling of Ti6Al4V alloy using artificial neural network
- A hybrid approach for the machinability analysis of Incoloy 825 using the entropy-MOORA method
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part II
- Innovations for sustainable chemical manufacturing and waste minimization through green production practices
- Topical Issue on Conference on Materials, Manufacturing Processes and Devices - Part I
- Characterization of Co–Ni–TiO2 coatings prepared by combined sol-enhanced and pulse current electrodeposition methods
- Hot deformation behaviors and microstructure characteristics of Cr–Mo–Ni–V steel with a banded structure
- Effects of normalizing and tempering temperature on the bainite microstructure and properties of low alloy fire-resistant steel bars
- Dynamic evolution of residual stress upon manufacturing Al-based diesel engine diaphragm
- Study on impact resistance of steel fiber reinforced concrete after exposure to fire
- Bonding behaviour between steel fibre and concrete matrix after experiencing elevated temperature at various loading rates
- Diffusion law of sulfate ions in coral aggregate seawater concrete in the marine environment
- Microstructure evolution and grain refinement mechanism of 316LN steel
- Investigation of the interface and physical properties of a Kovar alloy/Cu composite wire processed by multi-pass drawing
- The investigation of peritectic solidification of high nitrogen stainless steels by in-situ observation
- Microstructure and mechanical properties of submerged arc welded medium-thickness Q690qE high-strength steel plate joints
- Experimental study on the effect of the riveting process on the bending resistance of beams composed of galvanized Q235 steel
- Density functional theory study of Mg–Ho intermetallic phases
- Investigation of electrical properties and PTCR effect in double-donor doping BaTiO3 lead-free ceramics
- Special Issue on Thermal Management and Heat Transfer
- On the thermal performance of a three-dimensional cross-ternary hybrid nanofluid over a wedge using a Bayesian regularization neural network approach
- Time dependent model to analyze the magnetic refrigeration performance of gadolinium near the room temperature
- Heat transfer characteristics in a non-Newtonian (Williamson) hybrid nanofluid with Hall and convective boundary effects
- Computational role of homogeneous–heterogeneous chemical reactions and a mixed convective ternary hybrid nanofluid in a vertical porous microchannel
- Thermal conductivity evaluation of magnetized non-Newtonian nanofluid and dusty particles with thermal radiation
Articles in the same Issue
- Research Articles
- De-chlorination of poly(vinyl) chloride using Fe2O3 and the improvement of chlorine fixing ratio in FeCl2 by SiO2 addition
- Reductive behavior of nickel and iron metallization in magnesian siliceous nickel laterite ores under the action of sulfur-bearing natural gas
- Study on properties of CaF2–CaO–Al2O3–MgO–B2O3 electroslag remelting slag for rack plate steel
- The origin of {113}<361> grains and their impact on secondary recrystallization in producing ultra-thin grain-oriented electrical steel
- Channel parameter optimization of one-strand slab induction heating tundish with double channels
- Effect of rare-earth Ce on the texture of non-oriented silicon steels
- Performance optimization of PERC solar cells based on laser ablation forming local contact on the rear
- Effect of ladle-lining materials on inclusion evolution in Al-killed steel during LF refining
- Analysis of metallurgical defects in enamel steel castings
- Effect of cooling rate and Nb synergistic strengthening on microstructure and mechanical properties of high-strength rebar
- Effect of grain size on fatigue strength of 304 stainless steel
- Analysis and control of surface cracks in a B-bearing continuous casting blooms
- Application of laser surface detection technology in blast furnace gas flow control and optimization
- Preparation of MoO3 powder by hydrothermal method
- The comparative study of Ti-bearing oxides introduced by different methods
- Application of MgO/ZrO2 coating on 309 stainless steel to increase resistance to corrosion at high temperatures and oxidation by an electrochemical method
- Effect of applying a full oxygen blast furnace on carbon emissions based on a carbon metabolism calculation model
- Characterization of low-damage cutting of alfalfa stalks by self-sharpening cutters made of gradient materials
- Thermo-mechanical effects and microstructural evolution-coupled numerical simulation on the hot forming processes of superalloy turbine disk
- Endpoint prediction of BOF steelmaking based on state-of-the-art machine learning and deep learning algorithms
- Effect of calcium treatment on inclusions in 38CrMoAl high aluminum steel
- Effect of isothermal transformation temperature on the microstructure, precipitation behavior, and mechanical properties of anti-seismic rebar
- Evolution of residual stress and microstructure of 2205 duplex stainless steel welded joints during different post-weld heat treatment
- Effect of heating process on the corrosion resistance of zinc iron alloy coatings
- BOF steelmaking endpoint carbon content and temperature soft sensor model based on supervised weighted local structure preserving projection
- Innovative approaches to enhancing crack repair: Performance optimization of biopolymer-infused CXT
- Structural and electrochromic property control of WO3 films through fine-tuning of film-forming parameters
- Influence of non-linear thermal radiation on the dynamics of homogeneous and heterogeneous chemical reactions between the cone and the disk
- Thermodynamic modeling of stacking fault energy in Fe–Mn–C austenitic steels
- Research on the influence of cemented carbide micro-textured structure on tribological properties
- Performance evaluation of fly ash-lime-gypsum-quarry dust (FALGQ) bricks for sustainable construction
- First-principles study on the interfacial interactions between h-BN and Si3N4
- Analysis of carbon emission reduction capacity of hydrogen-rich oxygen blast furnace based on renewable energy hydrogen production
- Just-in-time updated DBN BOF steel-making soft sensor model based on dense connectivity of key features
- Effect of tempering temperature on the microstructure and mechanical properties of Q125 shale gas casing steel
- Review Articles
- A review of emerging trends in Laves phase research: Bibliometric analysis and visualization
- Effect of bottom stirring on bath mixing and transfer behavior during scrap melting in BOF steelmaking: A review
- High-temperature antioxidant silicate coating of low-density Nb–Ti–Al alloy: A review
- Communications
- Experimental investigation on the deterioration of the physical and mechanical properties of autoclaved aerated concrete at elevated temperatures
- Damage evaluation of the austenitic heat-resistance steel subjected to creep by using Kikuchi pattern parameters
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part II
- Synthesis of aluminium (Al) and alumina (Al2O3)-based graded material by gravity casting
- Experimental investigation into machining performance of magnesium alloy AZ91D under dry, minimum quantity lubrication, and nano minimum quantity lubrication environments
- Numerical simulation of temperature distribution and residual stress in TIG welding of stainless-steel single-pass flange butt joint using finite element analysis
- Special Issue on A Deep Dive into Machining and Welding Advancements - Part I
- Electro-thermal performance evaluation of a prismatic battery pack for an electric vehicle
- Experimental analysis and optimization of machining parameters for Nitinol alloy: A Taguchi and multi-attribute decision-making approach
- Experimental and numerical analysis of temperature distributions in SA 387 pressure vessel steel during submerged arc welding
- Optimization of process parameters in plasma arc cutting of commercial-grade aluminium plate
- Multi-response optimization of friction stir welding using fuzzy-grey system
- Mechanical and micro-structural studies of pulsed and constant current TIG weldments of super duplex stainless steels and Austenitic stainless steels
- Stretch-forming characteristics of austenitic material stainless steel 304 at hot working temperatures
- Work hardening and X-ray diffraction studies on ASS 304 at high temperatures
- Study of phase equilibrium of refractory high-entropy alloys using the atomic size difference concept for turbine blade applications
- A novel intelligent tool wear monitoring system in ball end milling of Ti6Al4V alloy using artificial neural network
- A hybrid approach for the machinability analysis of Incoloy 825 using the entropy-MOORA method
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part II
- Innovations for sustainable chemical manufacturing and waste minimization through green production practices
- Topical Issue on Conference on Materials, Manufacturing Processes and Devices - Part I
- Characterization of Co–Ni–TiO2 coatings prepared by combined sol-enhanced and pulse current electrodeposition methods
- Hot deformation behaviors and microstructure characteristics of Cr–Mo–Ni–V steel with a banded structure
- Effects of normalizing and tempering temperature on the bainite microstructure and properties of low alloy fire-resistant steel bars
- Dynamic evolution of residual stress upon manufacturing Al-based diesel engine diaphragm
- Study on impact resistance of steel fiber reinforced concrete after exposure to fire
- Bonding behaviour between steel fibre and concrete matrix after experiencing elevated temperature at various loading rates
- Diffusion law of sulfate ions in coral aggregate seawater concrete in the marine environment
- Microstructure evolution and grain refinement mechanism of 316LN steel
- Investigation of the interface and physical properties of a Kovar alloy/Cu composite wire processed by multi-pass drawing
- The investigation of peritectic solidification of high nitrogen stainless steels by in-situ observation
- Microstructure and mechanical properties of submerged arc welded medium-thickness Q690qE high-strength steel plate joints
- Experimental study on the effect of the riveting process on the bending resistance of beams composed of galvanized Q235 steel
- Density functional theory study of Mg–Ho intermetallic phases
- Investigation of electrical properties and PTCR effect in double-donor doping BaTiO3 lead-free ceramics
- Special Issue on Thermal Management and Heat Transfer
- On the thermal performance of a three-dimensional cross-ternary hybrid nanofluid over a wedge using a Bayesian regularization neural network approach
- Time dependent model to analyze the magnetic refrigeration performance of gadolinium near the room temperature
- Heat transfer characteristics in a non-Newtonian (Williamson) hybrid nanofluid with Hall and convective boundary effects
- Computational role of homogeneous–heterogeneous chemical reactions and a mixed convective ternary hybrid nanofluid in a vertical porous microchannel
- Thermal conductivity evaluation of magnetized non-Newtonian nanofluid and dusty particles with thermal radiation

