Abstract
The effect of lining materials (Al2O3 and Al2O3–MgO·Al2O3) of ladle on evolution of non-metallic inclusions in aluminum-killed (Al-killed) steel during ladle furnace refining without Ca treatment was investigated through industrial experiments. The results showed that non-metallic inclusions experienced the changes from Al2O3 → MgO–Al2O3 → CaO–Al2O3. During the refining process using either of the two ladle lining materials, for all non-metallic inclusions, the vast majority are distributed in the high Al2O3 area of the CaO–Al2O3–MgO phase diagram, with very little or none in the low melting point zone. Non-metallic inclusions are mainly smaller than 3 μm, while those larger than 3 μm consisted primarily of MgO·Al2O3 and CaO–Al2O3 inclusions. The use of an Al2O3–MgO·Al2O3-lining ladle is more effective in reducing the number density of inclusions in the steel. However, during the refining process, the Al2O3-lining ladle does not have a significant impact on the presence of MgO–Al2O3 and CaO–Al2O3 inclusions in the molten steel. The Al2O3–MgO·Al2O3-lining ladle does not have a significant effect on MgO–Al2O3 inclusions, but it does promote the formation of CaO–Al2O3 and CaS inclusions in the molten steel.
1 Introduction
In the process of refining aluminum-killed (Al-killed) steel, various types of inclusions are commonly encountered, including Al2O3, MgO–Al2O3, and CaO–Al2O3 [1–4]. During the refining process of Al-killed steel, Al2O3 inclusions are inevitably formed in the molten steel due to the use of aluminum for deoxidation [5,6]. As the refining progresses, MgO–Al2O3 and CaO–Al2O3 inclusions gradually appear in the steel. Some studies have confirmed [7–10] that even without the addition of Mg and Ca, MgO–Al2O3 and CaO–Al2O3 inclusions can still be formed in the molten steel. MgO–Al2O3 inclusions, owing to their high melting point and sharp geometric structure, can seriously damage the fatigue performance of steel products [2,3]. CaO–Al2O3 inclusions, typically larger in size, are also considered to have the potential to cause defects in the products [11,12]. The control of non-metallic inclusions has become imperative for achieving higher steel product quality. However, the nature of inclusions is primarily determined by the chemical composition of the steel, which, in turn, is directly influenced by the ladle lining and the refining slag. Hence, the choice of ladle-lining refractory and refining slag indirectly impacts the inclusions [13]. During refining process of Al-killed steel, the refractory used in the ladle lining come into extensive real-time contact with the molten steel. Therefore, the influence of ladle material on inclusions in the steel has gained increasing attention. Harada [14,15] investigated the reaction mechanism between MgO–C refractory and Al-killed steel, and observed the presence of MgO and MgO–Al2O3 inclusions in the steel and the formation of a spinel layer at the steel–refractory interface. Liu [7,13] discovered that [Al] in the molten steel could reduce MgO in the MgO–C refractory, leading to an increase in dissolved [Mg] in the steel and the formation of MgO·Al2O3 inclusions. Similar results were obtained by Chi et al. [16], Brabie [17], Jansson et al. [18], and others who studied the interaction between MgO-based refractories and molten steel. Huang et al. [19], through research on the dissolution kinetics of MgO-based refractories in steel, found that the reaction of MgO with [Al] in the steel occurs concurrently with the decomposition of MgO refractories, contributing to an increase in Mg in the steel. Deng et al. [20] studied the effect of spinel refractories on inclusions in Al-killed steel, and found that there is no transformation of Al2O3 inclusions in the molten steel. This is due to relatively low activity of MgO in spinel refractories, which is not easily reduced by [Al]. The studies in these literature have mainly focused on MgO–C refractory, while the study of the effect of other refractory on inclusions in steel is still incomplete. Therefore, it is necessary to systematically study the effect of ladles with different refractories on inclusions in steel.
In this article, industrial sampling was carried out during the refining and continuous casting process of Al-killed steel with Al2O3-lined ladle and Al2O3–MgO–Al2O3-lined ladle. The effect of two different lined refractories on various inclusions is also analyzed.
2 Methods
2.1 Industrial production and sampling
The production process of Al-killed steel in this study was basic oxygen furnace (BOF) → ladle furnace refining (LF) → continuous casting (CC). The operations of the refining process included: (1) After tapping from BOF, aluminum and lime were added to the ladle for deoxidation and slag making. (2) The steel was heated using electrodes and alloyed with aluminum, lime, and ferromanganese alloy. The molten steel is heated for 20 min. The slag basicity (w(CaO)/w(SiO2)) was greater than 11. (3) Argon was blown to stir the steel for 6 min before LF departure. (4) Continuous casting was started after LF departure, and the basicity of the flux in tundish was 2.3–2.6.
Samples of steel and slag were collected at various points in the production process. The sampling nodes were LF arrival (LF1), end of electrode heating (LF2), LF departure (LF3), and casting tundish (CC1) (about 100 tons of molten steel was poured), as shown in Figure 1.
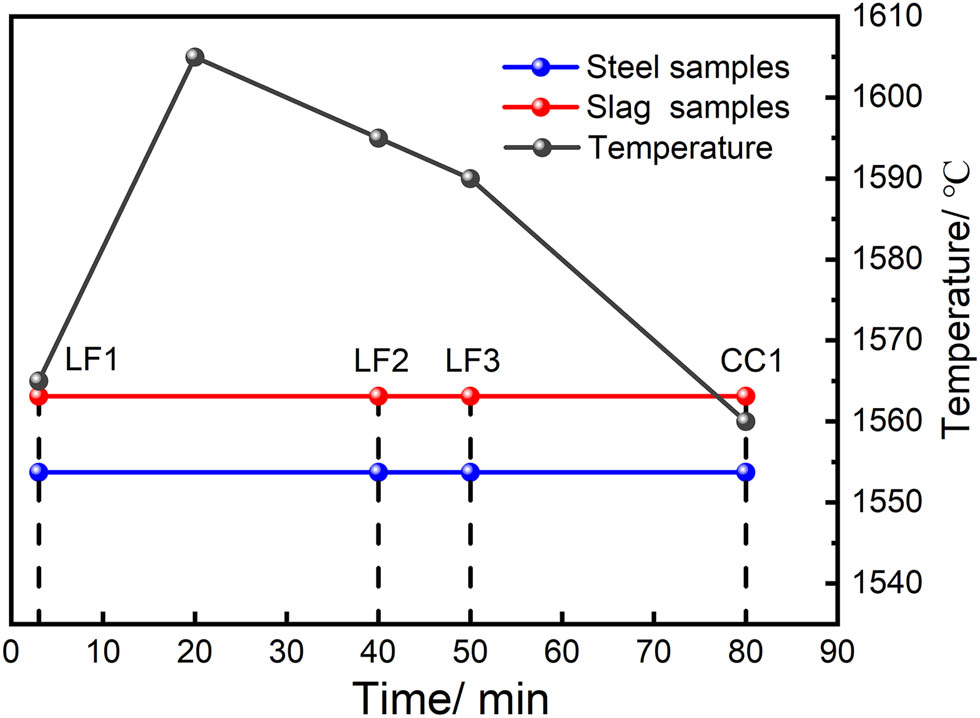
Sampling nodes for the refining and casting process. (In the figure, the horizontal axis represents the refining time. The red and blue marks represent the collection of slag and steel samples at a certain time point, respectively).
2.2 Analysis methods
Steel samples were analyzed and detected for inclusions with an equivalent diameter greater than 1 μm, including their size, morphology, and composition, using a field-emission electron probe microanalyzer (Shimadzu/EPMA 8050G). The scanning area for each sample was set to 15 mm2.
The content of carbon [C] and sulfur [S] in the steel samples was determined using a high-frequency infrared ray carbon sulfur analyzer (LECO CS744). The content of [Si], [Mn], [P], [Ca], and [Al] were measured using an inductively coupled plasma mass spectrometer (ICP-MS/NexION 1000G). The oxide composition of the refining slag and tundish flux was determined through inductively coupled plasma-atomic emission spectrometry (ICP-MS/NexION 1000G).
3 Results
3.1 Composition of samples from refining and casting processes
The composition of the molten steel refined using different lined ladles is shown in Table 1. It is clear that when an Al2O3-lining ladle is used, the [Al] content in the molten steel initially increases due to the addition of aluminum for deoxygenation. As the refining process proceeds, the [Al] content gradually decreases. At the same time, the contents of [Si] and [Mn] tend to increase. The reason for this phenomenon is that [Mn] in the molten steel participates in steel–slag reactions, resulting in the formation of MnO (as shown in equation (1)). In addition, the [Al] in the molten steel reacts with MnO in the slag (equation (2)). These two reactions lead to an increase in [Si] and [Mn] content in the molten steel. Since calcium treatment is not carried out during the refining process, the [Ca] content in the molten steel remains very low throughout the process [21,22].
Molten steel composition at various sampling points (wt%)
| Material | Process | C | Si | Mn | P | S | Ca | Al | Als |
|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | LF1 | 0.040 | 0.004 | 0.128 | 0.011 | 0.033 | 0.0001 | 0.061 | 0.051 |
| LF2 | 0.032 | 0.005 | 0.137 | 0.011 | 0.022 | 0.0001 | 0.076 | 0.074 | |
| LF3 | 0.060 | 0.013 | 0.290 | 0.011 | 0.011 | 0.0001 | 0.050 | 0.047 | |
| CC1 | 0.068 | 0.013 | 0.281 | 0.012 | 0.011 | 0.0004 | 0.039 | 0.034 | |
| Al2O3–MgO·Al2O3 | LF1 | 0.041 | 0.004 | 0.095 | 0.009 | 0.029 | 0.0001 | 0.055 | 0.037 |
| LF2 | 0.042 | 0.012 | 0.216 | 0.010 | 0.015 | 0.0001 | 0.098 | 0.096 | |
| LF3 | 0.065 | 0.021 | 0.280 | 0.009 | 0.006 | 0.0001 | 0.055 | 0.054 | |
| CC1 | 0.073 | 0.020 | 0.279 | 0.010 | 0.006 | 0.0002 | 0.052 | 0.050 |
Als: acid soluble aluminum in molten steel.
As shown in Table 1, it can be observed that the composition changes in the molten steel during LF refining are consistent when using both ladles. The decrease in [S] content in the molten steel during refining, indicates that the refining slag has some desulfurization capacity. However, the key difference lies in the removal rate of [S] in the molten steel. The removal rate of [S] was 66% when using an Al2O3-lined ladle, while it was 79% when using an Al2O3–MgO–Al2O3-lined ladle. (desulfurization rate: ratio of sulfur removal to initial sulfur content). This suggests that the use of an Al2O3–MgO·Al2O3-lining ladle is more effective in desulfurizing the molten steel.
The chemical compositions of the refining slag and tundish flux are shown in Table 2. The basicity (w(CaO)/w(SiO2)) of the refining slag during the refining process ranges from 11.38 to 37.42, while the basicity of the tundish flux ranges from 2.35 to 2.60. The sulfur content in the slag gradually increases during LF refining. Conversely, the content of MnO and SiO2 in the slag decreases as the refining process progresses.
Composition of refining slag and tundish flux at various sampling points (wt%)
| Material | Process | SiO2 | CaO | MgO | Al2O3 | MnO | P2O5 | T.Fe | S | R |
|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | LF1 | 3.71 | 42.21 | 4.55 | 41.86 | 1.33 | 0.22 | 4.47 | 0.14 | 11.38 |
| LF2 | 1.95 | 57.22 | 8.30 | 32.72 | 0.13 | 0.01 | 0.15 | 0.51 | 29.34 | |
| LF3 | 1.50 | 56.13 | 8.74 | 34.23 | 0.07 | / | 0.25 | 0.48 | 37.42 | |
| CC1 | 15.81 | 37.12 | 11.20 | 30.78 | 0.95 | 0.06 | 1.41 | 0.08 | 2.35 | |
| Al2O3–MgO·Al2O3 | LF1 | 3.09 | 42.45 | 4.23 | 43.65 | 1.56 | 0.07 | 2.61 | 0.19 | 13.74 |
| LF2 | 2.57 | 51.91 | 4.65 | 31.56 | 0.92 | / | 2.90 | 0.44 | 20.20 | |
| LF3 | 2.17 | 52.86 | 5.01 | 34.90 | 0.15 | / | 0.40 | 0.62 | 24.36 | |
| CC1 | 14.97 | 38.98 | 5.28 | 29.60 | 0.82 | 0.033 | 0.75 | 0.14 | 2.60 |
R = w(CaO)/w(SiO2).
3.2 Transformation of non-metallic inclusions
3.2.1 Types and number ratio of non-metallic inclusions
In the refining of Al-killed steel, the addition of aluminum blocks during steel tapping promotes strong deoxidation, as shown in equation (3). However, when a large quantity of aluminum blocks is added at once, it leads to the rapid formation of clustered Al2O3 inclusions in the molten steel, as depicted in Figure 2(a). These Al2O3 clusters have poor wettability with the molten steel, causing them to float upward into the slag during the refining process [23]. Consequently, small-sized block-shaped Al2O3 inclusions are left behind in the molten steel. As the steel interacts with the slag and ladle lining, the content of [Mg] and [Ca] in the molten steel increases, resulting in the transformation of Al2O3 inclusions, as shown in equations (4) and (5). This leads to the formation of MgO–Al2O3 and CaO–Al2O3 series inclusions, which exhibit irregular or spherical shapes, as observed in Figures 2 and 3 [24,25].
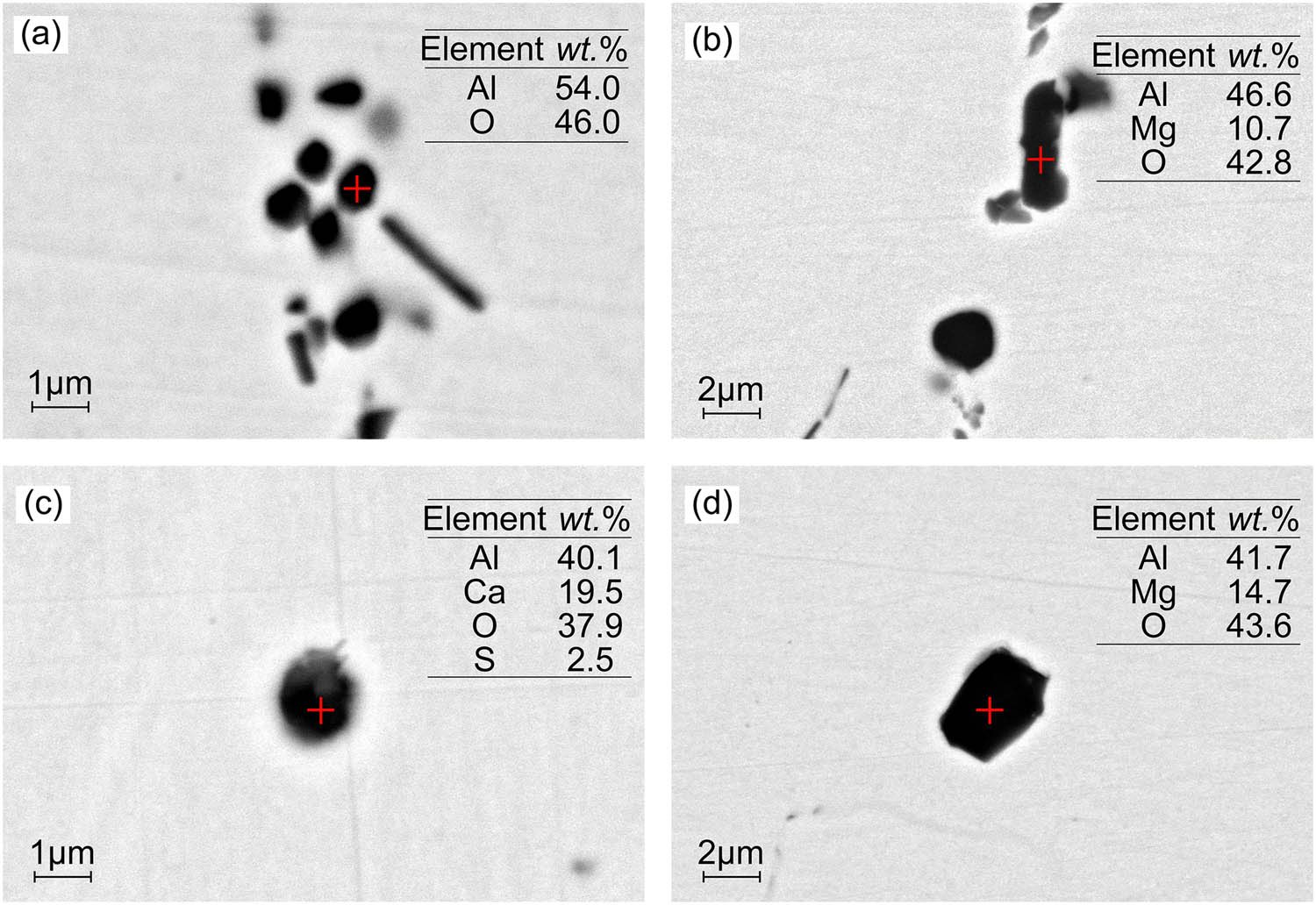
Morphology and composition of inclusions (Al2O3-lining ladle: (a) LF1, 10,000×, (b) LF2, 5,000×, (c) LF3, 10,000×, and (d) CC1, 5,000×). (The morphology and composition of typical inclusions in steel at different process stages when using Al2O3-lining ladle during LF refining of Al-killed steel. The inclusion composition is the mass fraction).
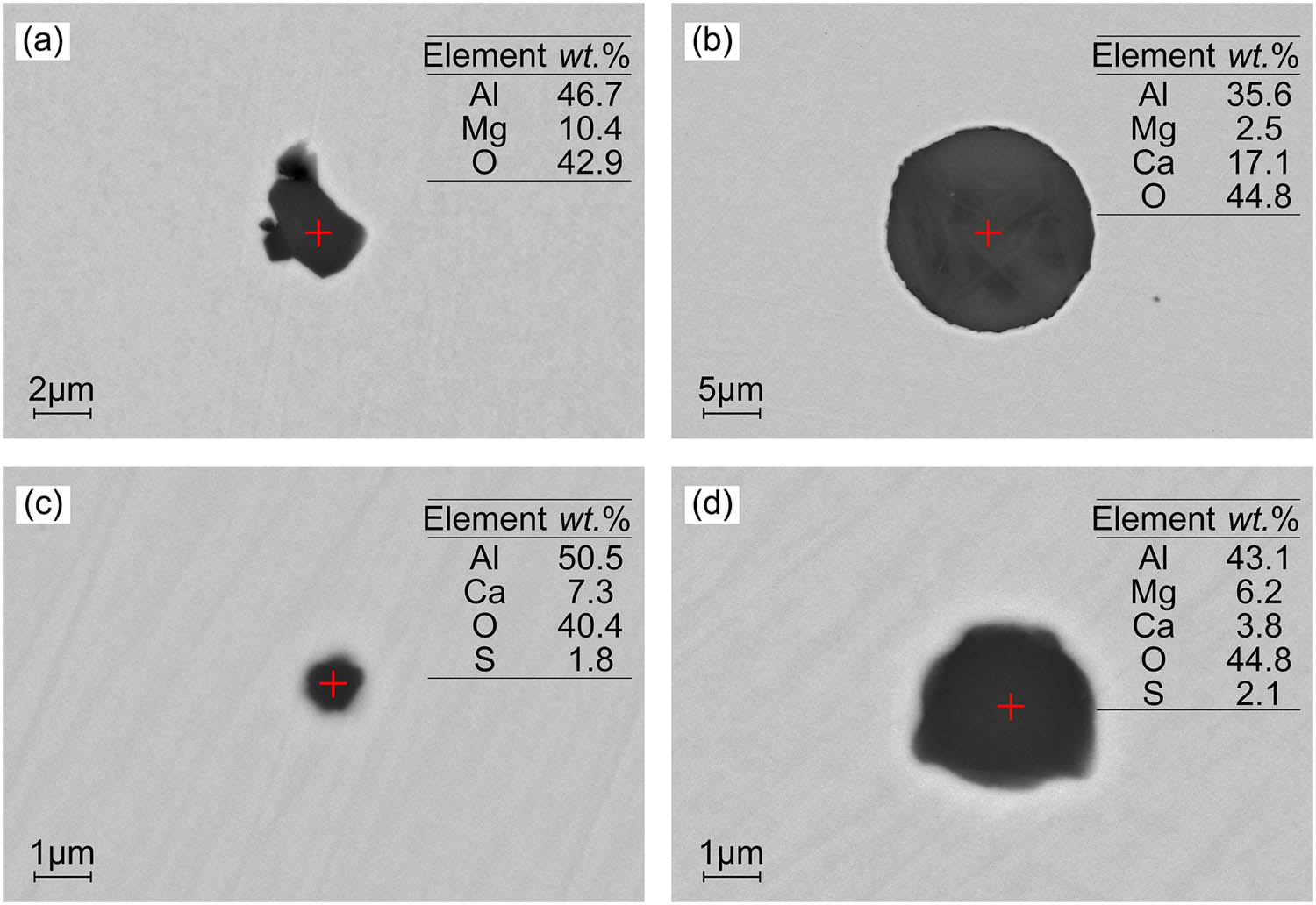
Morphology and composition of inclusions (Al2O3–MgO·Al2O3-lining ladle: (a) LF1, 5,000×, (b) LF2, 2,000×, (c) LF3, 10,000×, and (d) CC1, 10,000×). (The morphology and composition of typical inclusions in steel at different process stages when using Al2O3–MgO·Al2O3-lining ladle during LF refining of Al-killed steel. The inclusion composition is the mass fraction).
Figure 4 shows information on the number ratio of different types of non-metallic inclusions at various sampling points during two industrial experiments. It was evident that non-metallic oxide inclusions in the molten steel can be classified into three types: Al2O3, MgO–Al2O3, and CaO–Al2O3.
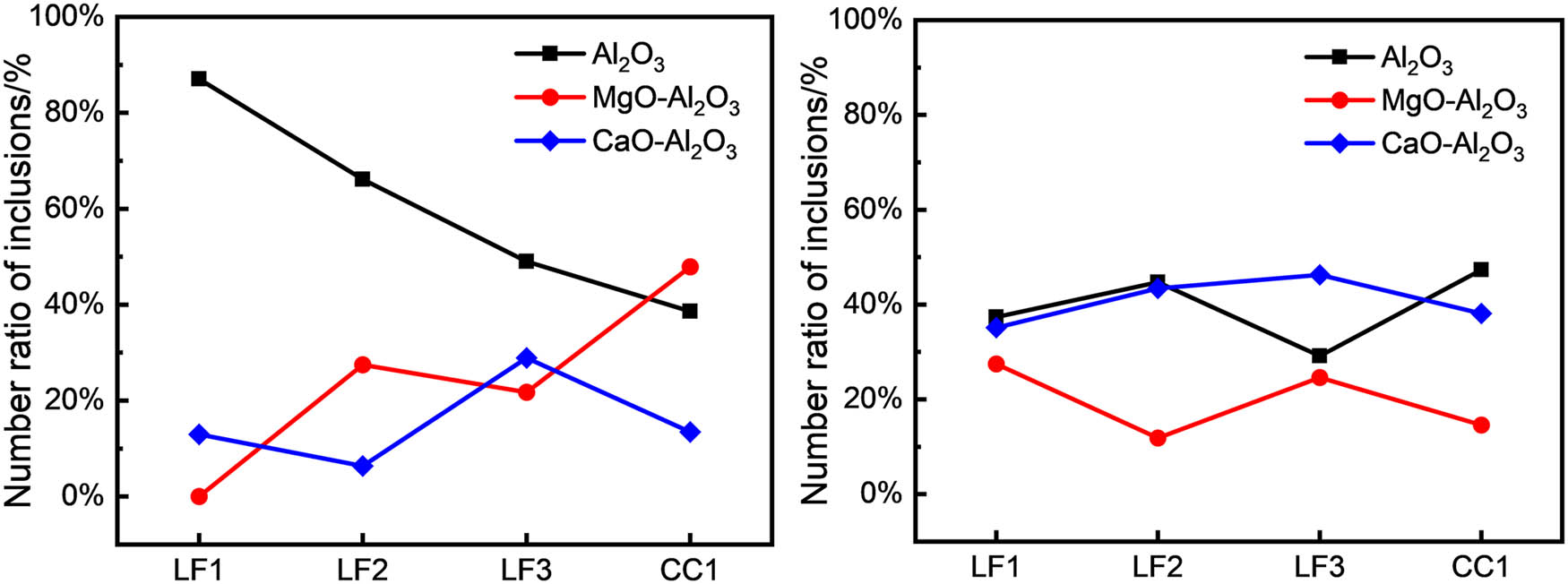
Number ratio of non-metallic inclusions. (a) Al2O3-lining ladle, (b) Al2O3–MgO·Al2O3-lining ladle.
In the case of refining with an Al2O3-lining ladle, the number ratio of non-metallic inclusions changed as shown in Figure 4(a). When LF arrived, 87% of the inclusions were Al2O3, while 13% were CaO–Al2O3 inclusions. As the LF refining progressed, the ratio of Al2O3 inclusions decreased to 49%, MgO–Al2O3 inclusions were 22%, and CaO–Al2O3 inclusions increased to 29%. During casting, the ratio of Al2O3 inclusions decreased to 38%, MgO–Al2O3 inclusions increased to 48%, and CaO–Al2O3 inclusions decreased to 13%.
For the refining of ladle-lining with Al2O3–MgO·Al2O3, the variation of the number ratio of non-metallic inclusions is shown in Figure 4(b). The ratio of CaO–Al2O3 inclusions remains relatively stable during LF refining, ranging between 35 and 46%. In contrast, Al2O3 and MgO–Al2O3 inclusions exhibit almost opposite trends. The ratio of Al2O3 inclusions increased toward the end of electrode heating, decreased at LF departure, and then rose again. Meanwhile, the ratio of MgO–Al2O3 inclusions decreased initially and then increased. During the casting, the ratio of Al2O3 inclusions increased significantly, which could be attributed to the secondary oxidation of molten steel. Consequently, the ratio of MgO–Al2O3 and CaO–Al2O3 decreased during the casting.
3.2.2 Composition and size distribution of inclusions
The compositions of inclusions have been plotted on the CaO–Al2O3–MgO phase diagram in the molten steel at different sampling points. The evolution paths of inclusions in steel for both experiments are depicted in Figures 5 and 6, respectively. For the Al2O3-lining ladle, it is observed that upon LF arrival, the inclusions in the molten steel are primarily composed of Al2O3. After LF heating, the inclusions mainly consist of Al2O3 and MgO–Al2O3 inclusions, indicating a transformation from Al2O3 inclusions to MgO–Al2O3 inclusions during refining, as shown in Figure 5(a) and (b). Upon LF departure, the dominant inclusions in the molten steel are CaO–Al2O3 and MgO–Al2O3. Notably, MgO–Al2O3 inclusions tend to have larger sizes, while CaO–Al2O3 inclusions generally have smaller sizes, as illustrated in Figure 5(c). During casting, MgO–Al2O3 inclusions continue to be present in the molten steel, while the quantity of CaO–Al2O3 inclusions significantly decreases, indicating that CaO–Al2O3 series inclusions are more easily removed from the steel during tundish casting, as shown in Figure 5(d).
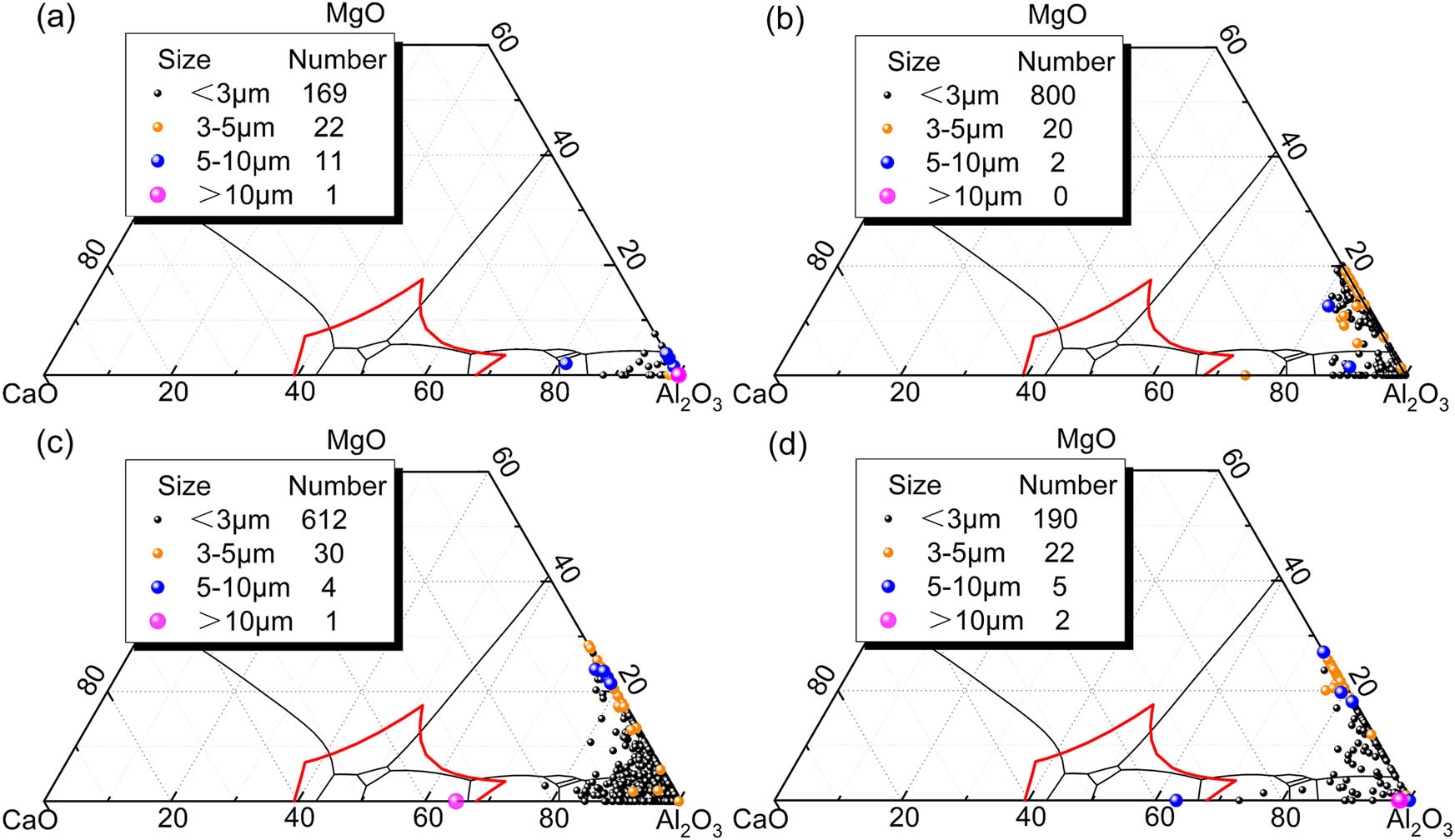
Composition and size distribution of non-metallic inclusions (Al2O3-lining ladle, (a) LF1, (b) LF2, (c) LF3, and (d) CC1).
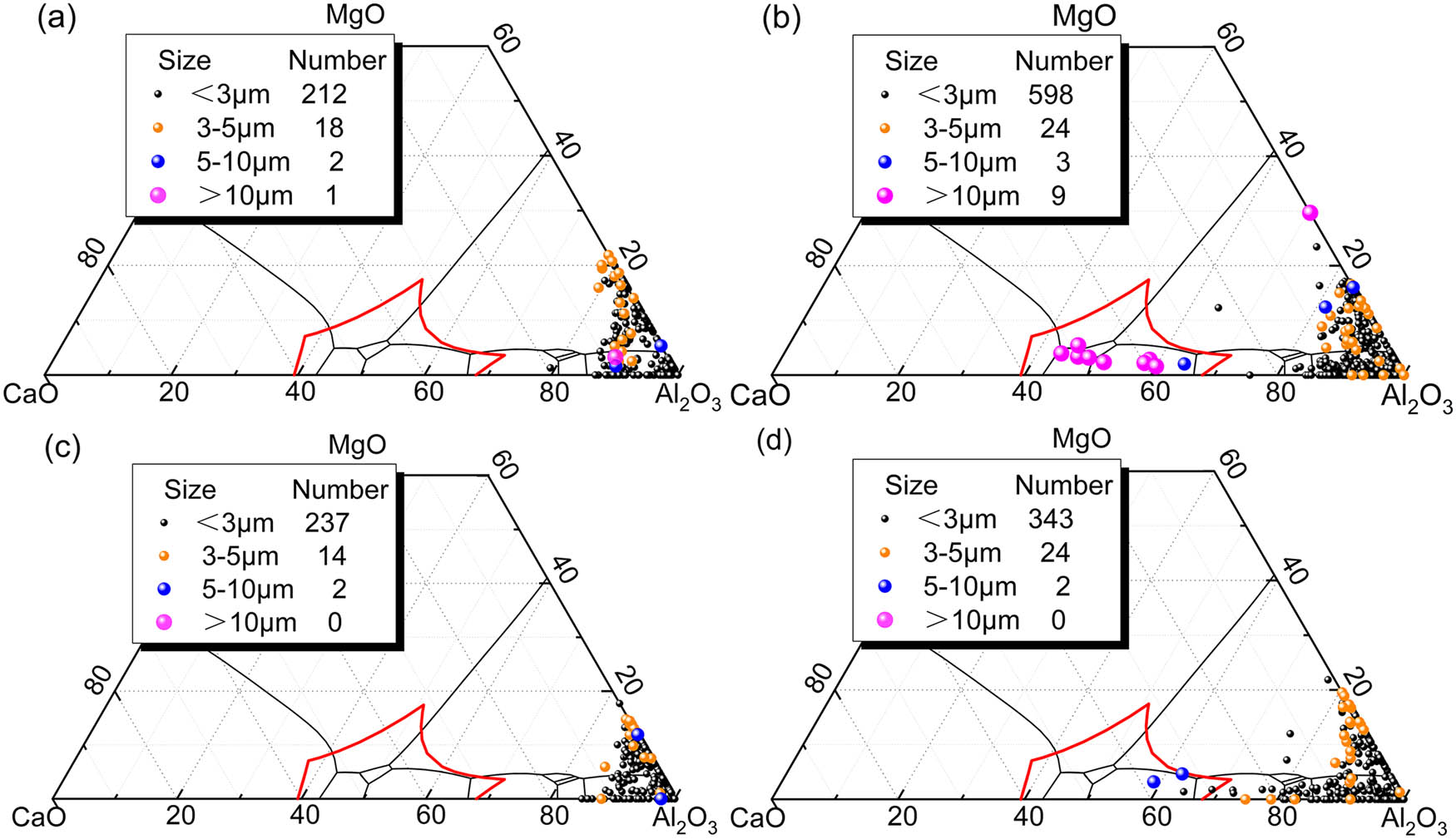
Composition and size distribution of non-metallic inclusions (Al2O3–MgO·Al2O3-lining ladle, (a) LF1, (b) LF2, (c) LF3, and (d) CC1).
In the case of using an Al2O3–MgO·Al2O3-lining ladle, the molten steel contains three types of inclusions: Al2O3, MgO–Al2O3, and CaO–Al2O3, upon LF arrival. Large-sized inclusions are predominantly MgO–Al2O3, as seen in Figure 6(a). After electrode heating, the MgO content in MgO–Al2O3 inclusions decreases, and CaO–Al2O3 series inclusions increase. Some larger-sized CaO–Al2O3 series inclusions appear in the low-melting zone, suggesting a trend of transformation from MgO–Al2O3 inclusions to CaO–Al2O3 during refining with Al2O3–MgO·Al2O3-lining ladle. After LF refining, the low-melting CaO–Al2O3 inclusions are no longer present, and only MgO–Al2O3 inclusions and the high-melting CaO–Al2O3 inclusions remain in the molten steel, as shown in Figure 6(b) and (c). During casting, the inclusions tend to concentrate in the CaO–Al2O3 and MgO–Al2O3 regions with higher Al2O3 content. Figure 6(d) illustrates that there is only a small quantity of CaO–Al2O3 series inclusions present in the low-melting region. In comparison to using an Al2O3-lining ladle, the removal of CaO–Al2O3 inclusions is not as prominent during this step.
3.2.3 Number density and average size of non-metallic inclusions
Two steel samples were analyzed for non-metallic inclusions number density and average size. As shown in Figure 7(a), the number densities of inclusions in both experiments were quite similar at LF arrival, 13.5 and 15.5 mm−2, respectively. During the refining process, the number density of inclusions in steel with Al2O3–MgO·Al2O3-lining ladle is much lower than in the case of Al2O3-lining ladle. Especially, the former has an inclusion number density of 14.8 mm−2 during LF departure, while the latter is 43.1 mm−2. This suggests that the use of Al2O3–MgO–Al2O3 lining-ladle is beneficial to the cleanliness of the molten steel. During casting, the steel refined with an Al2O3-lining ladle experiences a significant decline in inclusion number density from 43.1 to 14.6 mm−2, as shown in Figure 5(c) and (d). This reduction is attributed to the removal of CaO–Al2O3 inclusions. Conversely, the steel refined with Al2O3–MgO·Al2O3-lining ladle experienced an increase in inclusion density during the casting, rising from 16.8 to 24.6 mm−2.
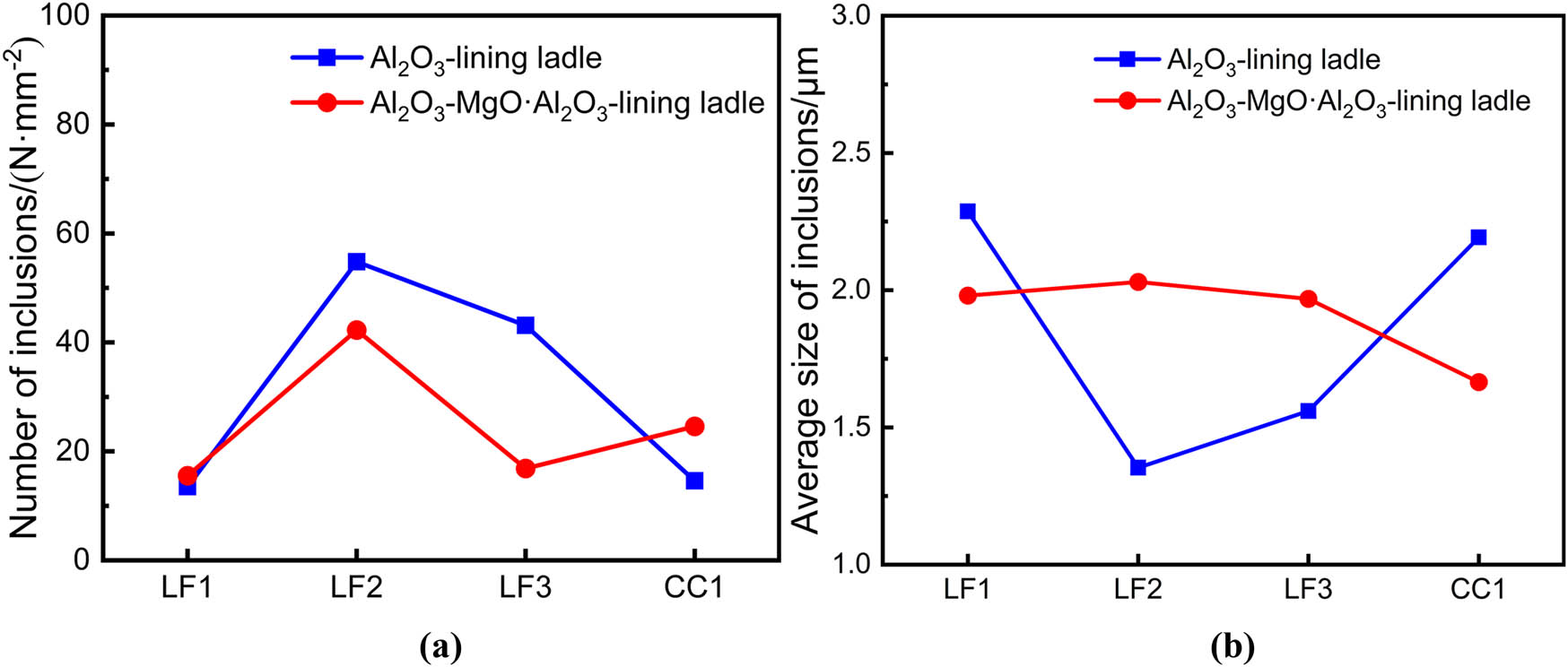
Number density (a) and average size of non-metallic inclusions (b).
Based on analyzing the average inclusion size from both experiments as shown in Figure 7(b), it is observed that at LF arrival, the inclusions in steel have a similar average size from the two experiments, ranging from 2.0 to 2.3 μm. However, as the refining process progressed and reached LF departure, the steel refined with an Al2O3-lining ladle exhibited notably smaller average inclusion sizes compared to the Al2O3–MgO·Al2O3-lining ladle. At the end of electrode heating and LF departure, the average inclusion sizes were 1.35 and 1.56 μm for the Al2O3-lining ladle. This suggests that the use of an Al2O3-lining ladle effectively controlled the growth of inclusions. During the LF departure and casting process, CaO–Al2O3 inclusions are removed from the steel in the Al2O3-lining ladle. However, there are many large-sized MgO–Al2O3 inclusions in the steel, resulting in an increase in the average inclusion size to 2.2 μm. In contrast, the average size of inclusions in the steel refined with an Al2O3–MgO·Al2O3-lining ladle decreased from 1.9 to 1.6 μm during the LF departure and casting stages.
3.3 Number and number ratio of CaS inclusions in steel
The number of CaS inclusions in steel samples from two experiments was counted, as well as its ratio among all inclusions. Only composite inclusions with a CaS greater than 5 wt% were considered. The results are presented in Figure 8. The number of CaS inclusions in the steel was relatively low when the Al2O3-lining ladle was used. The number of CaS inclusions peaked at LF departure at 3.9% of all inclusions.
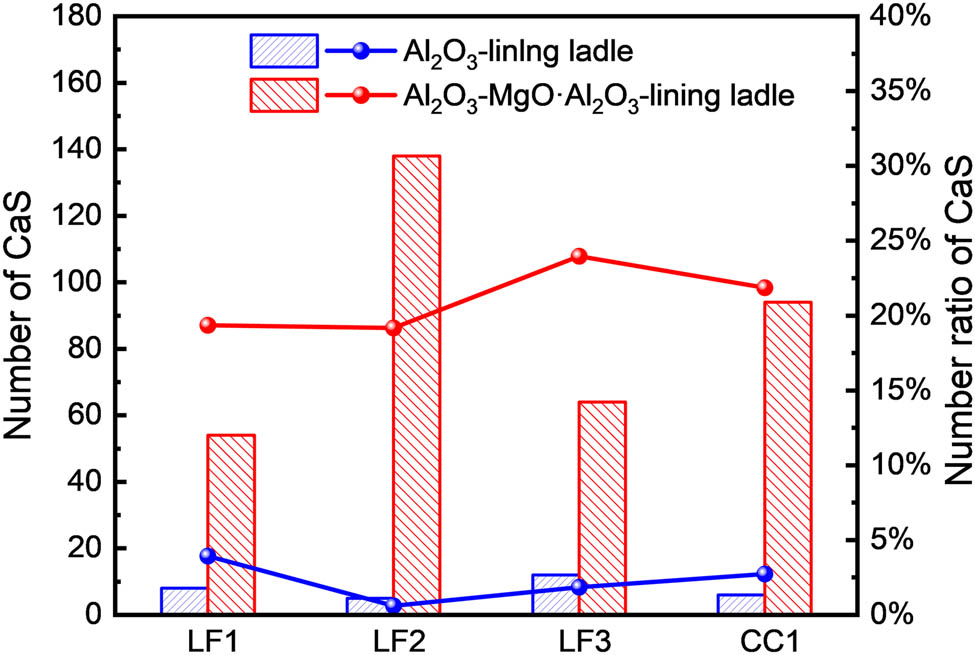
Number and number ratio of CaS inclusions. (The column shape in the figure represents the total number of CaS inclusions detected on the steel sample when using two types of ladle liners. The red and black spherical markings indicate the proportion of CaS inclusions to all inclusions).
Conversely, when using an Al2O3–MgO·Al2O3-lining ladle, the quantity of CaS inclusions at all sampling points is significantly higher. Upon LF arrival, the number of CaS inclusions in the steel sample is 54, accounting for 19.3% of the total. After the end of electrode heating, lime is added to the ladle for slag making, which leads to an increase in the number of CaS inclusions. At this point, the number of CaS is 138, accounting for 19.1% of all inclusions. During LF departure and Tundish casting, the number of CaS in the steel decreases but remains significantly higher than in steel refined with an Al2O3-lining ladle. This indicates that refining Al-killed steel using an Al2O3-lining ladle effectively prevents the formation of CaS inclusions in the steel.
4 Discussion
4.1 Effect of ladle-lining refractories on the formation of MgO–Al2O3 inclusions
In the refining process of Al-killed steel, the formation of MgO–Al2O3 inclusions is common. These inclusions are formed due to the interaction between [Al] in the steel and MgO in the refining slag or ladle-lining materials, as described in equation (6). When [Mg] is reduced and enters the steel, it further reacts with [Al] or Al2O3 in the steel to form MgO–Al2O3, as shown in equations (7) and (8) [25]. Therefore, it is widely accepted that the refining slag and MgO-containing refractory materials are the primary sources of MgO–Al2O3 inclusions in steel.
The transformation and number ratios of MgO·Al2O3 inclusions in steel are shown in Figures 4 and 5. When an Al2O3-lining ladle was used, the refractory was not able to provide Mg into the steel. At LF arrival, the melting of the refining slag was incomplete and the steel–slag reaction had not yet occurred. However, as the slag–steel reaction progressed, the formation of MgO·Al2O3 inclusions became inevitable. The number ratio of MgO·Al2O3 inclusions in the steel did not show a significant advantage over the case where an Al2O3–MgO·Al2O3-lined ladle was used. Therefore, the Al2O3-lining ladle can prevent the formation of MgO·Al2O3 inclusions in the initial stages of refining, but it does not effectively control their formation during the refining process. In the case of using an Al2O3–MgO·Al2O3-lining ladle, the molten steel already contains a large number of MgO·Al2O3 inclusions at LF arrival due to the exfoliation of the ladle-lining. During the LF refining process, the MgO·Al2O3 inclusions were reduced by transformation to CaO–Al2O3 system inclusions or absorption by the refining slag. This is due to the fact that the heating before steel tapping intensifies the slag–steel reaction, and the blowing and stirring of molten steel intensifies the scouring of molten steel on the refractory material. Therefore, the effect of Al2O3–MgO·Al2O3-lining ladle on MgO·Al2O3 inclusions is mainly the spalling of the lining material. The use of Al2O3–MgO·Al2O3-lining ladle is detrimental to the reduction of MgO·Al2O3 inclusions in molten steel.
4.2 Effect of ladle-lining refractories on the formation of CaO–Al2O3 inclusions
In the process of LF refining of Al-killed steel, it is common to introduce calcium wire into the ladle for inclusions modification. This is aimed at transforming spinel inclusions into calcium-aluminate inclusions or intermediate products such as CaO–Al2O3–MgO inclusions [26]. In reality, CaO–Al2O3 series inclusions can form in the steel even without calcium treatment during the refining process [2,3,6–10]. As the reaction between the slag and steel progresses to a certain extent, [Ca] will be introduced into the steel due to the reduction of CaO in the slag by [Al], as described in equation (9). The stability of MgO–Al2O3 inclusions is affected by the presence of a small amount of [Ca] in the steel. When [Ca] enters the steel, it replaces Mg in the MgO–Al2O3 inclusions, resulting in inclusions with a core of MgO–Al2O3 surrounded by CaO–Al2O3, as shown in equation (10). Furthermore, [Ca] in the steel can react directly with Al2O3 inclusions, leading to the formation of CaO–Al2O3 inclusions, as shown in equation (11) [3].
The LF refining process for Al-killed steel in this study did not involve calcium treatment. The steel contained both CaO–Al2O3 inclusions and modified intermediate products, CaO–Al2O3–MgO inclusions, as mentioned before. When using an Al2O3-lining ladle for refining, the proportion of CaO–Al2O3 inclusions in the steel was initially low. This was due to the absence of MgO–Al2O3 inclusions in the steel upon LF arrival, which prevented equation (10). The CaO–Al2O3 inclusions in the steel are mainly formed through equations (9)–(11). As the refining process progresses, the reactions of between the slag and steel result in the emergence of MgO–Al2O3 inclusions. This leads to an increase in the quantity of CaO–Al2O3 inclusions in the steel, as described in equation (10). The transformation of MgO–Al2O3 inclusions into CaO–Al2O3 inclusions occurs gradually from the surface toward the center of the inclusions as reported in refs [3,27]. The Ca content in the steel is very low and is not sufficient to completely transform the MgO–Al2O3 inclusions. Therefore, the steel contains a significant amount of CaO–Al2O3–MgO intermediate products coexisting in the steel, which is in line with the reference findings [25]. The Al2O3-lining ladle can only reduce the formation of CaO–Al2O3 inclusions at the beginning of the refining process. On the other hand, in an Al2O3–MgO·Al2O3-lining ladle, the steel undergoes equations (9)–(11) simultaneously throughout the refining process. Figure 4(b) shows that the steel contains a higher quantity of CaO–Al2O3 inclusions. In addition, it was observed that CaO–Al2O3 inclusions with higher Ca content appeared in the low-melting region of the ternary phase diagram during the refining process. This observation suggests that the using of an Al2O3–MgO·Al2O3-lining ladle is more favorable for the formation of CaO–Al2O3 inclusions compared to an Al2O3-lining ladle.
4.3 Effect of ladle-lining refractories on formation of CaS inclusions
The desulfurization process in molten steel typically occurs at the steel–slag interface, and CaO is commonly used as the desulfurizing agent. Therefore, the refining slag used in the process of Al-killed steel refining often has higher basicity [28]. The desulfurization reaction in the Al-killed steel refining process is represented by equation (12). Liu’s research [24] showed that the use of magnesium-containing ladle-linings in the refining process of Al-killed steel can add magnesium to molten steel. This suggests that in addition to Mg in the refining slag, Mg in the ladle can also participate in the desulfurization reaction, as shown in equations (12)–(16). The MgO·Al2O3 in the ladle lining is reduced by [Al], and [Mg] enters the molten steel. Subsequently, [Mg] directly reacts with [S] in the molten steel to form MgS. Due to the thermodynamic instability of MgS, it further reacts with CaO in the slag to generate CaS and MgO. While the desulfurization capability of Mg is weaker than that of Ca, the use of ladle materials containing Mg introduces more Mg into the molten steel due to the larger contact area between the steel and the refractory lining. Therefore, the desulfurization effect of Mg should not be underestimated [22,24,29,30].
When refining Al-killed steel using an Al2O3-lining ladle, the introduction of Mg into the molten steel relies solely on the reaction with the steel and slag. Therefore, the desulfurization process at the slag–steel interface occurs only through the first method as shown in equation (12). However, when using an Al2O3–MgO·Al2O3-lining ladle for refining, the desulfurization reactions proceed through both methods simultaneously. Therefore, using an Al2O3–MgO·Al2O3-lining ladle during LF refining can improve the desulfurization reaction rate, resulting in increased CaS content in the steel. This finding is consistent with the present results.
5 Conclusion
The effect of two different types of ladle-lining refractories on non-metallic inclusions in steel was investigated by sampling Al-killed steel during refining and casting. However, there was a wide range of refractories used for ladle lining and only two of them were investigated in this study. In the future, it is necessary to investigate the effect of more types of ladle-lining refractories on inclusions in steel.
The evolution of inclusions during the refining and casting of Al2O3-lining ladles follows the sequence Al2O3 → MgO·Al2O3 → CaO–Al2O3. For Al2O3–MgO·Al2O3-lining ladles, all three types of inclusions were observed in the steel throughout the experiments.
In both industrial samples, the composition of inclusions was concentrated in the high Al2O3 reign of the CaO–Al2O3·MgO phase diagram. Most of the CaO–Al2O3 inclusions did not transform to the low-melting point region. The size of inclusions were mainly concentrated in the range of less than 3 μm.
When using an Al2O3 ladle, the Al2O3 inclusions in the steel gradually transformed into MgO·Al2O3 and CaO–Al2O3 inclusions. At the end of refining, the steel still contained predominantly Al2O3 inclusions. When using an Al2O3–MgO·Al2O3-lining ladle, the quantity ratio of CaO–Al2O3 inclusions in steel was higher, and these inclusions dominated during the refining process.
The Al2O3–MgO·Al2O3-lining ladle was more conducive to improving steel cleanliness, while Al2O3 was more conducive to reducing the average size of inclusions.
The effectiveness of molten steel desulfurization was enhanced when using an Al2O3–MgO·Al2O3-lining ladle. However, there was a significant increase in CaS inclusions in the steel compared to when using an Al2O3-lining ladle.
Acknowledgments:
The authors gratefully acknowledge the fundamental support from the National Natural Science Foundation of China (52274341, 52074197) and State Key Laboratory of Refractories and Metallurgy, Wuhan University of Science and Technology.
-
Funding information: This work was financially supported by the National Natural Science Foundation of China (52274341, 52074197) and State Key Laboratory of Refractories and Metallurgy, Wuhan University of Science and Technology.
-
Author contributions: Fu-bin Gao: factory experiments and sampling; Xinbo Yan: data processing and analysis, manuscript writing; Fuming Wang, Jianli Li, Xinhua Wang: manuscript revision.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All authors can confirm that all data used in this article can be published by the Journal “High Temperature Materials and Processes.”
References
[1] Zhang, L. and B. G. Thomas. State of the art in evaluation and control of steel cleanliness. ISIJ International, Vol. 43, No. 3, 2003, pp. 271–291.10.2355/isijinternational.43.271Search in Google Scholar
[2] Jiang, M., X. Wang, B. Chen, and W. Wang. Formation of MgO·Al2O3 inclusions in high strength alloyed structural steel refined by CaO–SiO2–Al2O3–MgO slag. ISIJ International, Vol. 48, No. 7, 2008, pp. 885–890.10.2355/isijinternational.48.885Search in Google Scholar
[3] Deng, Z. and M. Zhu. Evolution mechanism of non-metallic inclusions in Al-killed alloyed steel during secondary refining process. ISIJ International, Vol. 53, No. 3, 2013, pp. 450–458.10.2355/isijinternational.53.450Search in Google Scholar
[4] Beskow, K. and D. Sichen. Ladle glaze: major source of oxide inclusions during ladle treatment of steel. Ironmaking & Steelmaking, Vol. 31, No. 5, 2004, pp. 393–400.10.1179/030192304225018244Search in Google Scholar
[5] Guo, J., S. Chen, and Z. Cheng. Mechanism of non-metallic inclusion formation and modification and their deformation during compact strip production (CSP) process for aluminum-killed steel. ISIJ International, Vol. 53, No. 12, 2013, pp. 2142–2151.10.2355/isijinternational.53.2142Search in Google Scholar
[6] Herrera, M., F. Castro, M. Castro, M. Méndez, H. Solís, A. Castellá, et al. Modification of Al2O3 inclusions in medium carbon aluminium killed steels by AlCaFe additions. Ironmaking & Steelmaking, Vol. 33, No. 1, 2006, pp. 45–51.10.1179/174328106X80000Search in Google Scholar
[7] Liu, C., F. Huang, J. Suo, and X. Wang. Effect of magnesia-carbon refractory on the kinetics of MgO·Al2O3 spinel inclusion generation in extra-low oxygen steels. Metallurgical and Materials Transactions B-Process Metallurgy and Materials Processing Science, Vol. 47, No. 2, 2016, pp. 989–998.10.1007/s11663-015-0540-6Search in Google Scholar
[8] Yang, S., Q. Wang, L. Zhang, J. Li, and K. Peaslee. Formation and modification of MgO·Al2O3-based inclusions in alloy steels. Metallurgical and Materials Transactions B-Process Metallurgy and Materials Processing Science, Vol. 43, No. 4, 2012, pp. 731–750.10.1007/s11663-012-9663-1Search in Google Scholar
[9] Liu, C., S. Kitamura, X. Gao, and S. Ueda. Change in composition of inclusions through the reaction between Al-killed steel and the slag of CaO and MgO saturation. ISIJ International, Vol. 59, No. 2, 2019, pp. 268–276.10.2355/isijinternational.ISIJINT-2018-584Search in Google Scholar
[10] Mu, H., T. Zhang, B. Webler, and R. Fruehan. Reduction of CaO and MgO slag components by Al in liquid Fe. Metallurgical and Materials Transactions B-Process Metallurgy and Materials Processing Science, Vol. 49, No. 4, 2018, pp. 1665–1674.10.1007/s11663-018-1294-8Search in Google Scholar
[11] Park, J. H. and H. Todoroki. Control of MgO·Al2O3 spinel inclusions in stainless steels. ISIJ International, Vol. 50, No. 10, 2010, pp. 1333–1346.10.2355/isijinternational.50.1333Search in Google Scholar
[12] Larsson, M., A. Melander, and A. Nordgren. Effect of inclusions on fatigue behaviour of hardened spring steel. Journal of Materials Science & Technology, Vol. 9, No. 3, 1993, pp. 235–245.10.1179/mst.1993.9.3.235Search in Google Scholar
[13] Liu, C., F. Huang, and X. Wang. The effect of refining slag and refractory on inclusion transformation in extra low oxygen steels. Metallurgical and Materials Transactions B-Process Metallurgy and Materials Processing Science, Vol. 47, No. 2, 2016, pp. 999–1009.10.1007/s11663-016-0592-2Search in Google Scholar
[14] Harada, A., G. Miyano, N. Maruoka, H. Shibata, and S. Kitamura. Dissolution behavior of Mg from MgO into molten steel deoxidized by Al. ISIJ International, Vol. 54, No. 10, 2014, pp. 2230–2238.10.2355/isijinternational.54.2230Search in Google Scholar
[15] Harada, A., G. Miyano, N. Maruoka, H. Shibata, and S. Kitamura. Kinetic analysis of compositional changes in inclusions during ladle refining. ISIJ International, Vol. 5911, 2014, pp. 2569–2577.10.2355/isijinternational.54.2569Search in Google Scholar
[16] Chi, Y., Z. Deng, and M. Zhu. Effects of refractory and ladle glaze on evolution of non-metallic inclusions in Al-killed steel. ISIJ International, Vol. 88, No. 9, 2019, id. 1600470.10.1002/srin.201600470Search in Google Scholar
[17] Brabie, V. Mechanism of reaction between refractory materials and aluminum deoxidised molten steel. ISIJ International, Vol. 36, No. S, 1996, pp. S109–S112.10.2355/isijinternational.36.Suppl_S109Search in Google Scholar
[18] Jansson, S., V. Brabie, and P. Jonsson. Magnesia–carbon refractory dissolution in Al killed low carbon steel. Ironmaking & Steelmaking, Vol. 33, No. 5, 2006, pp. 389–397.10.1179/174328106X113977Search in Google Scholar
[19] Huang, F., L. Zhang, Y. Ren, and Y. Zhang. Kinetic modeling for the dissolution of MgO lining refractory in Al-killed steels. Metallurgical and Materials Transactions B-Process Metallurgy and Materials Processing Science, Vol. 48, No. 4, 2017, pp. 2195–2206.10.1007/s11663-017-0996-7Search in Google Scholar
[20] Deng, Z., S. Du, and M. Zhu. Effect of refractory on nonmetallic inclusions in Al-killed steel. Metallurgical and Materials Transactions B-Process Metallurgy and Materials Processing Science, Vol. 47, No. 5, 2016, pp. 3158–3167.10.1007/s11663-016-0746-2Search in Google Scholar
[21] Kobayashi, S. Thermodynamic fundamentals for alumina-content control of oxide inclusions in Mn–Si deoxidation of molten steel. ISIJ International, Vol. 39, No. 7, 1999, pp. 664–670.10.2355/isijinternational.39.664Search in Google Scholar
[22] Z., Deng, B. Glaser, M. A. Bombeck, and S. Du. Effects of temperature and holding time on the sintering of ladle filler sand with liquid steel. Steel Research International, Vol. 87, No. 7, 2016, pp. 921–929.10.1002/srin.201500277Search in Google Scholar
[23] Ogino, K., K. Nogi, and O. Yamase. Effects of selenium and tellurium on the surface tension of molten iron and the wettability of alumina by molten iron. Transactions of the Iron and Steel Institute of Japan, Vol. 23, No. 3, 1983, pp. 234–239.10.2355/isijinternational1966.23.234Search in Google Scholar
[24] Liu, C., M. Guo, S. Kitamura, X. Gao, and S. Ueda. Composition changes of inclusions by reaction with slag and refractory: a review. ISIJ International, Vol. 60, No. 9, 2020, pp. 1835–1848.10.2355/isijinternational.ISIJINT-2019-695Search in Google Scholar
[25] Gao, F., M. Jiang, F. Wang, J. Li, and X. Zhang. Influence of slag and refractory materials on inclusions during the ladle refining of low carbon aluminum killed steel. Metals, Vol. 13, No. 5, 2023, pp. 866–875.10.3390/met13050866Search in Google Scholar
[26] Deng, Z., Z. Liu, M. Zhu, and L. Huo. Formation, evolution and removal of MgO·Al2O3 spinel inclusions in steel. ISIJ International, Vol. 61, No. 1, 2021, pp. 1–15.10.2355/isijinternational.ISIJINT-2020-352Search in Google Scholar
[27] Tabatabaei, Y., K. S. Coley, G. A. Irons, and S. Sun. A multilayer model for alumina inclusion transformation by calcium in the ladle furnace. Metallurgical and Materials Transactions B-Process Metallurgy and Materials Processing Science, Vol. 49, No. 1, 2018, pp. 375–387.10.1007/s11663-017-1120-8Search in Google Scholar
[28] Yu, H., G. Qiu, L. Hao, Y. Zhao, and X. Wang. Effect of Al2O3 content in refining slag on non-metallic inclusions in Al-killed steel. Steelmaking, Vol. 39, No. 2, 2023, pp. 18–23.Search in Google Scholar
[29] Liu, C., M. Yagi, X. Gao, S. Kim, F. Huang, S. Ueda, et al. Dissolution behavior of Mg from magnesia-chromite refractory into Al-killed molten steel. Metallurgical and Materials Transactions B-Process Metallurgy and Materials Processing Science, Vol. 49, No. 5, 2018, pp. 2298–2307.10.1007/s11663-018-1301-0Search in Google Scholar
[30] Su, J., Z. Dou, T. Zhang, and Y. Liu. Effect of magnesium injection process on hot metal desulfurization. Journal of Iron and Steel Research International, Vol. 27, No. 12, 2020, pp. 1391–1399.10.1007/s42243-020-00507-9Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- De-chlorination of poly(vinyl) chloride using Fe2O3 and the improvement of chlorine fixing ratio in FeCl2 by SiO2 addition
- Reductive behavior of nickel and iron metallization in magnesian siliceous nickel laterite ores under the action of sulfur-bearing natural gas
- Study on properties of CaF2–CaO–Al2O3–MgO–B2O3 electroslag remelting slag for rack plate steel
- The origin of {113}<361> grains and their impact on secondary recrystallization in producing ultra-thin grain-oriented electrical steel
- Channel parameter optimization of one-strand slab induction heating tundish with double channels
- Effect of rare-earth Ce on the texture of non-oriented silicon steels
- Performance optimization of PERC solar cells based on laser ablation forming local contact on the rear
- Effect of ladle-lining materials on inclusion evolution in Al-killed steel during LF refining
- Analysis of metallurgical defects in enamel steel castings
- Effect of cooling rate and Nb synergistic strengthening on microstructure and mechanical properties of high-strength rebar
- Effect of grain size on fatigue strength of 304 stainless steel
- Analysis and control of surface cracks in a B-bearing continuous casting blooms
- Application of laser surface detection technology in blast furnace gas flow control and optimization
- Preparation of MoO3 powder by hydrothermal method
- The comparative study of Ti-bearing oxides introduced by different methods
- Application of MgO/ZrO2 coating on 309 stainless steel to increase resistance to corrosion at high temperatures and oxidation by an electrochemical method
- Effect of applying a full oxygen blast furnace on carbon emissions based on a carbon metabolism calculation model
- Characterization of low-damage cutting of alfalfa stalks by self-sharpening cutters made of gradient materials
- Thermo-mechanical effects and microstructural evolution-coupled numerical simulation on the hot forming processes of superalloy turbine disk
- Endpoint prediction of BOF steelmaking based on state-of-the-art machine learning and deep learning algorithms
- Effect of calcium treatment on inclusions in 38CrMoAl high aluminum steel
- Effect of isothermal transformation temperature on the microstructure, precipitation behavior, and mechanical properties of anti-seismic rebar
- Evolution of residual stress and microstructure of 2205 duplex stainless steel welded joints during different post-weld heat treatment
- Effect of heating process on the corrosion resistance of zinc iron alloy coatings
- BOF steelmaking endpoint carbon content and temperature soft sensor model based on supervised weighted local structure preserving projection
- Innovative approaches to enhancing crack repair: Performance optimization of biopolymer-infused CXT
- Structural and electrochromic property control of WO3 films through fine-tuning of film-forming parameters
- Influence of non-linear thermal radiation on the dynamics of homogeneous and heterogeneous chemical reactions between the cone and the disk
- Thermodynamic modeling of stacking fault energy in Fe–Mn–C austenitic steels
- Research on the influence of cemented carbide micro-textured structure on tribological properties
- Performance evaluation of fly ash-lime-gypsum-quarry dust (FALGQ) bricks for sustainable construction
- First-principles study on the interfacial interactions between h-BN and Si3N4
- Analysis of carbon emission reduction capacity of hydrogen-rich oxygen blast furnace based on renewable energy hydrogen production
- Just-in-time updated DBN BOF steel-making soft sensor model based on dense connectivity of key features
- Effect of tempering temperature on the microstructure and mechanical properties of Q125 shale gas casing steel
- Review Articles
- A review of emerging trends in Laves phase research: Bibliometric analysis and visualization
- Effect of bottom stirring on bath mixing and transfer behavior during scrap melting in BOF steelmaking: A review
- High-temperature antioxidant silicate coating of low-density Nb–Ti–Al alloy: A review
- Communications
- Experimental investigation on the deterioration of the physical and mechanical properties of autoclaved aerated concrete at elevated temperatures
- Damage evaluation of the austenitic heat-resistance steel subjected to creep by using Kikuchi pattern parameters
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part II
- Synthesis of aluminium (Al) and alumina (Al2O3)-based graded material by gravity casting
- Experimental investigation into machining performance of magnesium alloy AZ91D under dry, minimum quantity lubrication, and nano minimum quantity lubrication environments
- Numerical simulation of temperature distribution and residual stress in TIG welding of stainless-steel single-pass flange butt joint using finite element analysis
- Special Issue on A Deep Dive into Machining and Welding Advancements - Part I
- Electro-thermal performance evaluation of a prismatic battery pack for an electric vehicle
- Experimental analysis and optimization of machining parameters for Nitinol alloy: A Taguchi and multi-attribute decision-making approach
- Experimental and numerical analysis of temperature distributions in SA 387 pressure vessel steel during submerged arc welding
- Optimization of process parameters in plasma arc cutting of commercial-grade aluminium plate
- Multi-response optimization of friction stir welding using fuzzy-grey system
- Mechanical and micro-structural studies of pulsed and constant current TIG weldments of super duplex stainless steels and Austenitic stainless steels
- Stretch-forming characteristics of austenitic material stainless steel 304 at hot working temperatures
- Work hardening and X-ray diffraction studies on ASS 304 at high temperatures
- Study of phase equilibrium of refractory high-entropy alloys using the atomic size difference concept for turbine blade applications
- A novel intelligent tool wear monitoring system in ball end milling of Ti6Al4V alloy using artificial neural network
- A hybrid approach for the machinability analysis of Incoloy 825 using the entropy-MOORA method
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part II
- Innovations for sustainable chemical manufacturing and waste minimization through green production practices
- Topical Issue on Conference on Materials, Manufacturing Processes and Devices - Part I
- Characterization of Co–Ni–TiO2 coatings prepared by combined sol-enhanced and pulse current electrodeposition methods
- Hot deformation behaviors and microstructure characteristics of Cr–Mo–Ni–V steel with a banded structure
- Effects of normalizing and tempering temperature on the bainite microstructure and properties of low alloy fire-resistant steel bars
- Dynamic evolution of residual stress upon manufacturing Al-based diesel engine diaphragm
- Study on impact resistance of steel fiber reinforced concrete after exposure to fire
- Bonding behaviour between steel fibre and concrete matrix after experiencing elevated temperature at various loading rates
- Diffusion law of sulfate ions in coral aggregate seawater concrete in the marine environment
- Microstructure evolution and grain refinement mechanism of 316LN steel
- Investigation of the interface and physical properties of a Kovar alloy/Cu composite wire processed by multi-pass drawing
- The investigation of peritectic solidification of high nitrogen stainless steels by in-situ observation
- Microstructure and mechanical properties of submerged arc welded medium-thickness Q690qE high-strength steel plate joints
- Experimental study on the effect of the riveting process on the bending resistance of beams composed of galvanized Q235 steel
- Density functional theory study of Mg–Ho intermetallic phases
- Investigation of electrical properties and PTCR effect in double-donor doping BaTiO3 lead-free ceramics
- Special Issue on Thermal Management and Heat Transfer
- On the thermal performance of a three-dimensional cross-ternary hybrid nanofluid over a wedge using a Bayesian regularization neural network approach
- Time dependent model to analyze the magnetic refrigeration performance of gadolinium near the room temperature
- Heat transfer characteristics in a non-Newtonian (Williamson) hybrid nanofluid with Hall and convective boundary effects
- Computational role of homogeneous–heterogeneous chemical reactions and a mixed convective ternary hybrid nanofluid in a vertical porous microchannel
- Thermal conductivity evaluation of magnetized non-Newtonian nanofluid and dusty particles with thermal radiation
Articles in the same Issue
- Research Articles
- De-chlorination of poly(vinyl) chloride using Fe2O3 and the improvement of chlorine fixing ratio in FeCl2 by SiO2 addition
- Reductive behavior of nickel and iron metallization in magnesian siliceous nickel laterite ores under the action of sulfur-bearing natural gas
- Study on properties of CaF2–CaO–Al2O3–MgO–B2O3 electroslag remelting slag for rack plate steel
- The origin of {113}<361> grains and their impact on secondary recrystallization in producing ultra-thin grain-oriented electrical steel
- Channel parameter optimization of one-strand slab induction heating tundish with double channels
- Effect of rare-earth Ce on the texture of non-oriented silicon steels
- Performance optimization of PERC solar cells based on laser ablation forming local contact on the rear
- Effect of ladle-lining materials on inclusion evolution in Al-killed steel during LF refining
- Analysis of metallurgical defects in enamel steel castings
- Effect of cooling rate and Nb synergistic strengthening on microstructure and mechanical properties of high-strength rebar
- Effect of grain size on fatigue strength of 304 stainless steel
- Analysis and control of surface cracks in a B-bearing continuous casting blooms
- Application of laser surface detection technology in blast furnace gas flow control and optimization
- Preparation of MoO3 powder by hydrothermal method
- The comparative study of Ti-bearing oxides introduced by different methods
- Application of MgO/ZrO2 coating on 309 stainless steel to increase resistance to corrosion at high temperatures and oxidation by an electrochemical method
- Effect of applying a full oxygen blast furnace on carbon emissions based on a carbon metabolism calculation model
- Characterization of low-damage cutting of alfalfa stalks by self-sharpening cutters made of gradient materials
- Thermo-mechanical effects and microstructural evolution-coupled numerical simulation on the hot forming processes of superalloy turbine disk
- Endpoint prediction of BOF steelmaking based on state-of-the-art machine learning and deep learning algorithms
- Effect of calcium treatment on inclusions in 38CrMoAl high aluminum steel
- Effect of isothermal transformation temperature on the microstructure, precipitation behavior, and mechanical properties of anti-seismic rebar
- Evolution of residual stress and microstructure of 2205 duplex stainless steel welded joints during different post-weld heat treatment
- Effect of heating process on the corrosion resistance of zinc iron alloy coatings
- BOF steelmaking endpoint carbon content and temperature soft sensor model based on supervised weighted local structure preserving projection
- Innovative approaches to enhancing crack repair: Performance optimization of biopolymer-infused CXT
- Structural and electrochromic property control of WO3 films through fine-tuning of film-forming parameters
- Influence of non-linear thermal radiation on the dynamics of homogeneous and heterogeneous chemical reactions between the cone and the disk
- Thermodynamic modeling of stacking fault energy in Fe–Mn–C austenitic steels
- Research on the influence of cemented carbide micro-textured structure on tribological properties
- Performance evaluation of fly ash-lime-gypsum-quarry dust (FALGQ) bricks for sustainable construction
- First-principles study on the interfacial interactions between h-BN and Si3N4
- Analysis of carbon emission reduction capacity of hydrogen-rich oxygen blast furnace based on renewable energy hydrogen production
- Just-in-time updated DBN BOF steel-making soft sensor model based on dense connectivity of key features
- Effect of tempering temperature on the microstructure and mechanical properties of Q125 shale gas casing steel
- Review Articles
- A review of emerging trends in Laves phase research: Bibliometric analysis and visualization
- Effect of bottom stirring on bath mixing and transfer behavior during scrap melting in BOF steelmaking: A review
- High-temperature antioxidant silicate coating of low-density Nb–Ti–Al alloy: A review
- Communications
- Experimental investigation on the deterioration of the physical and mechanical properties of autoclaved aerated concrete at elevated temperatures
- Damage evaluation of the austenitic heat-resistance steel subjected to creep by using Kikuchi pattern parameters
- Topical Issue on Focus of Hot Deformation of Metaland High Entropy Alloys - Part II
- Synthesis of aluminium (Al) and alumina (Al2O3)-based graded material by gravity casting
- Experimental investigation into machining performance of magnesium alloy AZ91D under dry, minimum quantity lubrication, and nano minimum quantity lubrication environments
- Numerical simulation of temperature distribution and residual stress in TIG welding of stainless-steel single-pass flange butt joint using finite element analysis
- Special Issue on A Deep Dive into Machining and Welding Advancements - Part I
- Electro-thermal performance evaluation of a prismatic battery pack for an electric vehicle
- Experimental analysis and optimization of machining parameters for Nitinol alloy: A Taguchi and multi-attribute decision-making approach
- Experimental and numerical analysis of temperature distributions in SA 387 pressure vessel steel during submerged arc welding
- Optimization of process parameters in plasma arc cutting of commercial-grade aluminium plate
- Multi-response optimization of friction stir welding using fuzzy-grey system
- Mechanical and micro-structural studies of pulsed and constant current TIG weldments of super duplex stainless steels and Austenitic stainless steels
- Stretch-forming characteristics of austenitic material stainless steel 304 at hot working temperatures
- Work hardening and X-ray diffraction studies on ASS 304 at high temperatures
- Study of phase equilibrium of refractory high-entropy alloys using the atomic size difference concept for turbine blade applications
- A novel intelligent tool wear monitoring system in ball end milling of Ti6Al4V alloy using artificial neural network
- A hybrid approach for the machinability analysis of Incoloy 825 using the entropy-MOORA method
- Special Issue on Recent Developments in 3D Printed Carbon Materials - Part II
- Innovations for sustainable chemical manufacturing and waste minimization through green production practices
- Topical Issue on Conference on Materials, Manufacturing Processes and Devices - Part I
- Characterization of Co–Ni–TiO2 coatings prepared by combined sol-enhanced and pulse current electrodeposition methods
- Hot deformation behaviors and microstructure characteristics of Cr–Mo–Ni–V steel with a banded structure
- Effects of normalizing and tempering temperature on the bainite microstructure and properties of low alloy fire-resistant steel bars
- Dynamic evolution of residual stress upon manufacturing Al-based diesel engine diaphragm
- Study on impact resistance of steel fiber reinforced concrete after exposure to fire
- Bonding behaviour between steel fibre and concrete matrix after experiencing elevated temperature at various loading rates
- Diffusion law of sulfate ions in coral aggregate seawater concrete in the marine environment
- Microstructure evolution and grain refinement mechanism of 316LN steel
- Investigation of the interface and physical properties of a Kovar alloy/Cu composite wire processed by multi-pass drawing
- The investigation of peritectic solidification of high nitrogen stainless steels by in-situ observation
- Microstructure and mechanical properties of submerged arc welded medium-thickness Q690qE high-strength steel plate joints
- Experimental study on the effect of the riveting process on the bending resistance of beams composed of galvanized Q235 steel
- Density functional theory study of Mg–Ho intermetallic phases
- Investigation of electrical properties and PTCR effect in double-donor doping BaTiO3 lead-free ceramics
- Special Issue on Thermal Management and Heat Transfer
- On the thermal performance of a three-dimensional cross-ternary hybrid nanofluid over a wedge using a Bayesian regularization neural network approach
- Time dependent model to analyze the magnetic refrigeration performance of gadolinium near the room temperature
- Heat transfer characteristics in a non-Newtonian (Williamson) hybrid nanofluid with Hall and convective boundary effects
- Computational role of homogeneous–heterogeneous chemical reactions and a mixed convective ternary hybrid nanofluid in a vertical porous microchannel
- Thermal conductivity evaluation of magnetized non-Newtonian nanofluid and dusty particles with thermal radiation

