Abstract
In recent times, research on the synthesis of noble metal nanoparticles (NPs) has developed rapidly and attracted considerable attention. The use of plant extracts is the preferred mode for the biological synthesis of NPs due to the presence of biologically active constituents. Aloe vera is a plant endowed with therapeutic benefits especially in skincare due to its unique curative properties. The present study focused on an environmental friendly and rapid method of phytosynthesis of silver nanoparticles (Ag-NPs) using A. vera gel extract as a reductant. The synthesized Ag-NPs were characterized by transmission electron microscopy (TEM), UV-Vis spectroscopy, Fourier transform infrared (FTIR), and dynamic light scattering (DLS). TEM micrographs showed spherical-shaped synthesized Ag-NPs with a diameter of 50–100 nm. The UV-Vis spectrum displayed a broad absorption peak of surface plasmon resonance (SPR) at 450 nm. The mean size and size distribution of the formed Ag-NPs were investigated using the DLS technique. Antibacterial studies revealed zones of inhibition by Ag-NPs of A. vera (9 and 7 mm) against Pseudomonas aeruginosa and Escherichia coli, respectively. Furthermore, the antifungal activity was screened, based on the diameter of the growth inhibition zone using the synthesized Ag-NPs for different fungal strains. Anticancer activity of the synthesized Ag-NPs against the mouse melanoma F10B16 cell line revealed 100% inhibition with Ag-NPs at a concentration of 100 µg mL−1. The phytosynthesized Ag-NPs demonstrated a marked antimicrobial activity and also exhibited a potent cytotoxic effect against mouse melanoma F10B16 cells. The key findings of this study indicate that synthesized Ag-NPs exhibit profound therapeutic activity and could be potentially ideal alternatives in medicinal applications.
1 Introduction
Nanotechnology includes the synthesis of nanoparticles (NPs) with varied morphology, size, and controlled dispersity to be used for their benefits in several ways. It has the potential to impact human society for being widely used in various fields of science and technology [1].
Antimicrobial, dental therapy, wound healing, surgery function, catalyst, and biomedical devices are just a few applications of metal NPs [2]. Because of their unique optical and electrical properties, NP-based drugs have been found to be more efficacious [3,4]. Surface plasmon resonance (SPR) is a well-known property of NPs that increases their effectiveness [5].
Silver nanoparticles (Ag-NPs) remarkably, have a narrow plasmon resonance, a high surface-to-volume ratio, special physicochemical properties, and a wide range of applications in medical research, microelectronics, and biological activities [6,7]. AgNPs have garnered considerable interest among other metal NPs due to their wide use in several commercial and pharmacologically significant products [8,9]. Considering the synthesis, the traditional methods such as the physical, thermal, hydrothermal, and chemical synthesis modes are expensive, extremely hazardous and make use of toxic chemicals. Therefore, the emphasis is on a green synthesis approach making use of biological resources for the efficient formulation of NPs [10,11,12]. The cornerstone of this sustainable method is the use of renewable materials and environmentally benign compounds as reducing/capping agents to synthesize green nanoparticles [13]. Methods of green synthesis have been effectively utilized to synthesize NPs using different biomolecules, such as vitamins, yeasts, enzymes, algae, biodegradable polymers, and microorganisms, and plant parts such as leaf, stem, gum, fruit, bark, shells, roots, buds, and flowers [12,14].
Aloe vera (A. vera) is a succulent plant with thorns in its branches with a waxy coating that grows easily in arid conditions [15,16]. A. vera leaves have three layers: the outer layer is a thick and protective layer which has a high proportion of cellulose; the middle layer contains major flavanone (aloin A and B), and the inner layer has a fresh gel that contains an acetylated glucomannan, sugars, vitamins (A, B, C, and E), amino acids, proteins, and anthraquinones [17,18,19]. A. vera gel is utilized in many cosmetic products especially skincare. It is also considered to be effective for treating burns and wounds, which is known for its soothing effect on the affected skin. It can also be used to treat a wide range of health-related disorders [20]. The antimicrobial property and biomedical applications of AgNPs synthesized with A. vera gel extract are well investigated. However, very few studies have reported and documented the use of A. vera gel to synthesize AgNPs for biomedical applications.
With this premise, the present study aimed at the synthesis of A. vera gel extract for phytosynthesis of AgNPs and the investigation of their promising biomedical applications based on the antimicrobial and anticancer activities.
2 Materials and methods
2.1 Identification of plant
Aloe vera was collected from a local park at King Saud University, girls campus, Riyadh, Saudi Arabia, and identified at the Taxonomy Laboratory No. 116, Botany Department, King Saud University, Riyadh.
2.2 Preparation of plant extract
The healthy large outer leaves close to the ground were selected. They were then cut carefully at a slight angle, and the leaves were placed upright in a slightly tilted container for roughly 10 min, allowing much of the sap and the gel to drain out. About 20 g of the collected gel was weighed and transferred into a 500 mL conical flask containing 100 mL of deionized water, mixed well, and heated for 45 min in a water bath. The resulting solution was centrifuged at 5,000 rpm for 15 min, filtered using Whatman number 1 filter paper, and then the filtrate was stored at 4°C for further use.
2.3 Synthesis of Ag-NPs
An aqueous solution of silver nitrate (AgNO3) was prepared using 2 mM AgNO3 powder dissolved in 100 mL of deionized water at a fixed ratio. The reaction mixture was prepared by taking 10 mL of the above-prepared filtrated extract obtained from the plant and 40 mL of the AgNO3 solution, with incubation at 80°C for up to 45 min. The reduction of Ag ions was observed by the change of color from colorless to light yellow and then to dark brown (Figure 1).
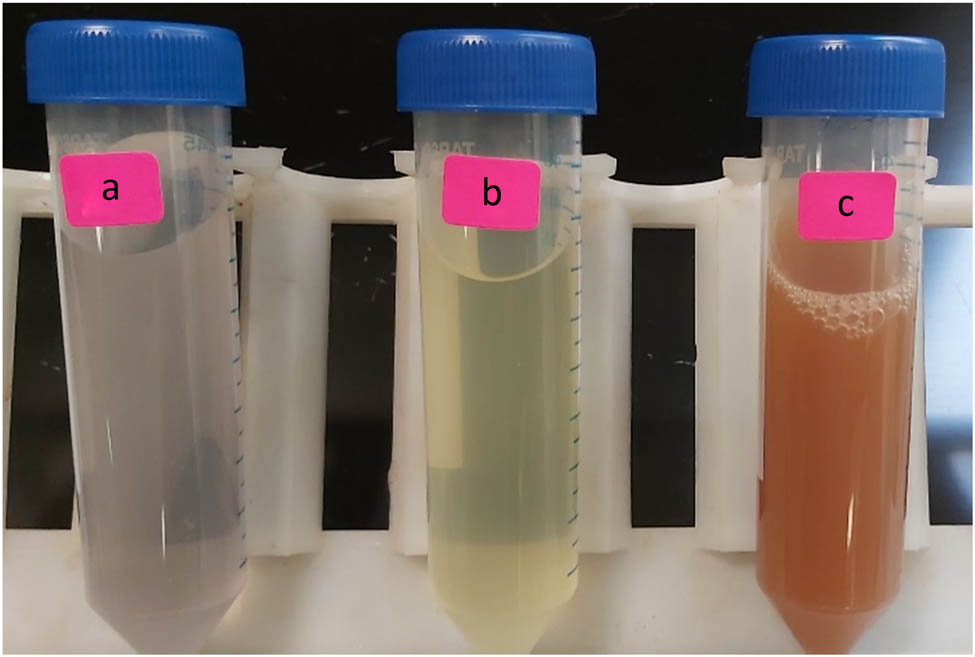
A. vera gel extract (a), a mixture of aqueous silver nitrate and gel extract after 10 min (b), and after 45 min (c), production of Ag-NPs using A. vera gel extract as green reducing.
2.4 Characterization of synthesized Ag-NPs
The absorption spectrum and SPR of the formed Ag-NPs were recorded using a UV-Vis (PerkinElmer, Waltham, MA, USA). The functional groups in the Ag-NP samples and A. vera gel extract were investigated via Fourier transform infrared (FTIR) spectroscopy (Nicolet 6700, Thermo Scientific, USA), using the potassium bromide (KBr) pellet technique. Transmission electron microscopy (TEM) (JEOL-JEM-1011, Japan) was used to examine the size and morphology of the phytosynthesized Ag-NPs with an acceleration voltage of 80 kV. The particle size distribution of the NPs, hydrodynamic diameter, and polydispersity index (PdI) were determined and measured by dynamic light scattering (DLS) particle size analyzer (ZEN3600 Malvern, Nano series, HT Laser, Malvern, UK).
2.5 AgNP biomedical activities
2.5.1 Cytotoxicity of synthesized Ag-NPs
The anti-proliferative activity of the Ag-NPs against mouse melanoma B16F10 cell lines was analyzed by the MTT assay using a 96-well plate supplemented with different concentrations of A. vera extract and Ag-NPs. The plates were incubated for 5 h at 37°C. Then, 0.1 mL of 1% trypan blue exclusion test was added. Cells were seeded in a 96-well plate at a density of 1 × 105 cells/well in a 90 µL Dulbecco’s modified Eagle’s medium. The cells were then allowed to settle before starting treated with different (3.125, 6.25, 12.5, 25, 50, and 100 µg mL−1) concentrations of the samples. The treated cells were allowed to grow further for 24 h. The experiment was performed on four replicates. Specifically, the non-viable cells adopted a blue color while the live cells did not take on the color of the dye. Then, the numbers of blue-stained and unstained cells were determined. The cell viability of the untreated cells was 100% and that of the treated cells was below 100%.
2.5.2 Antibacterial activity
Nutrient agar medium was prepared by dissolving 14 g of agar powder in 500 mL of distilled water and then autoclaved. A total of 20 mL of prepared agar was poured into each Petri dish, which was left to stand for 15 min for the agar to solidify, then the plates were inoculated overnight with human pathogens, such as the Gram-negative strain, Escherichia coli ATCC35218, and the gram-positive strains, Staphylococcus aureus ATCC 43300, Enterococcus faecalis ATCC 29212, and Bacillus cereus ATCC 11778 (clinical isolate) obtained from King Khalid University Hospital, Riyadh, Saudi Arabia. All organisms were tested simultaneously by the disc diffusion method [21]. Synthesized Ag-NPs and pure extract of A. vera were added steadily until the wells were full, followed by incubation at 37°C for 24 h. The diameter of the zone of inhibition was measured.
2.5.3 Antifungal activity
The samples were assayed for antifungal activity against bipolar heterothallic, Fusarium oxysporum and Macrophomina. These fungal strains were grown on a potato dextrose agar plate at 28°C. A total of 500 mL of the medium, which was prepared by dissolving 19 g of agar in 500 mL of distilled water, was autoclaved. A volume of 250 μL of synthesized Ag-NPs and pure extract of A. vera, separately, were added to a sterile petri dish, followed by pouring of the sterilized medium with gentle mixing. Fungal discs with a diameter of 6 mm, which were grown for 7 days from cultures of the above-mentioned fungi, were placed aseptically in the middle of the plate. The plates were incubated for 7−14 days at 28°C. The antifungal activity was estimated by measuring the diameter of the inhibition zone.
3 Results and discussion
3.1 UV-Vis spectral analysis
The phytosynthesis of Ag-NPs using A. vera gel extract as a reducing and stabilizing agent was primarily evidenced by the change in color of the reaction from light yellow to brown (Figure 1). The change in color confirmed the formation of Ag-NPs, due to the excitation of SPR vibration in these particles [22]. The position of the SPR band in UV-Vis spectra is sensitive to particle shape, size, interaction with the medium, local refractive index, and the extent of charge transfer between medium and the particles [23,24]. The UV-Vis spectral analysis identified a broad peak at approximately 450 nm, indicating the phytosynthesis of Ag-NPs (Figure 2). From the UV spectrum, it was observed that the absorption peak was broader, indicating the presence of particles with a wide size distribution. This result is in line with the findings of a previous study [25].
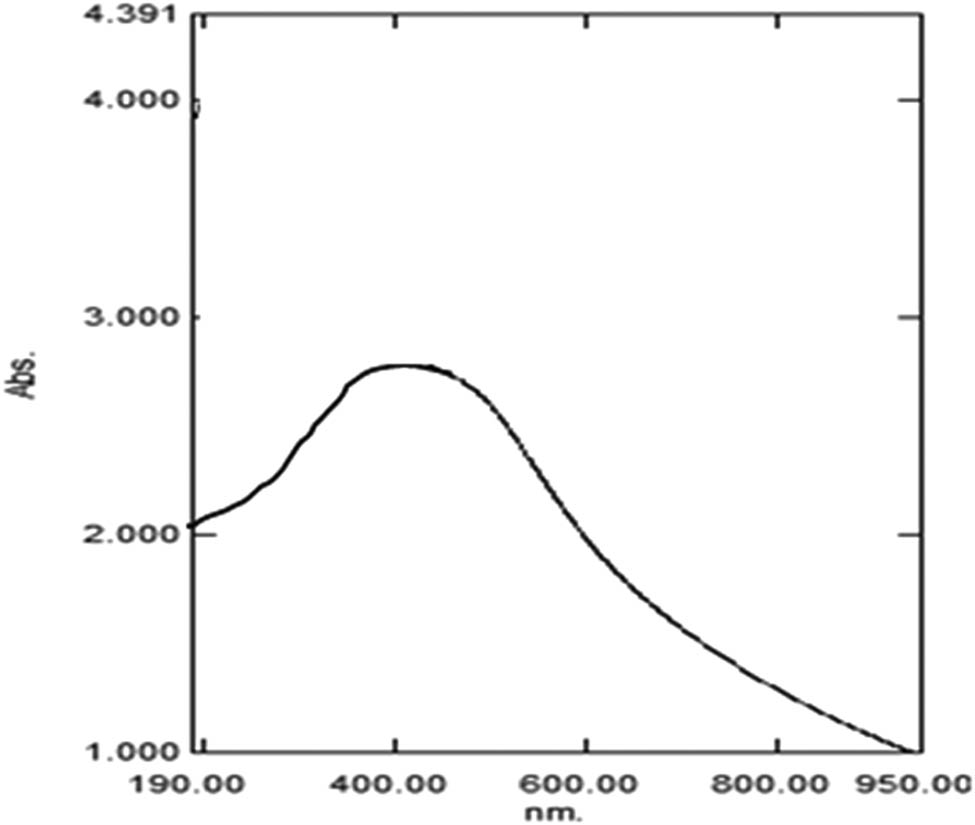
UV-Vis spectra of Ag-NPs produced by A. vera extract.
3.2 FTIR analysis
The FTIR absorption spectra of the A. vera gel aqueous extract and the formation of Ag-NPs are shown in Figure 3. The top spectrum of Figure 3 presents the FT-IR spectrum of pure A. vera gel extract, showing absorption peaks at 340.733, 1621.13, 1362.92, 1035.99, 777.12, 523.46, and 468.84 cm−1. These absorbance bands were identified to be associated with the stretching vibrations for O–H (hydrogen-bonded alcohols and phenols). The bottom spectrum of Figure 3 shows the FT-IR bands of green Ag-NPs and identifies the possible functional groups in the suspension. The absorpion peaks located at 2931.21, 1731.86, 1428.84, 1248.00, and 1039.00 cm−1 were suggested to indicate the presence of an amide group or proteins. These results indicate that the carbonyl group of proteins adsorbed strongly to metals, indicating that proteins could also have formed a layer along with the bio-organics, securing interactions with phytosynthesized NPs; they also show that their secondary structure was not affected during the reaction with Ag ions or after binding with Ag-NPs [26]. In addition, biomolecules in the extract have a strong ability to bind metal, suggesting the formation of a layer covering metal NPs and acting as a capping agent to prevent agglomeration and providing stability in the medium [26,27].
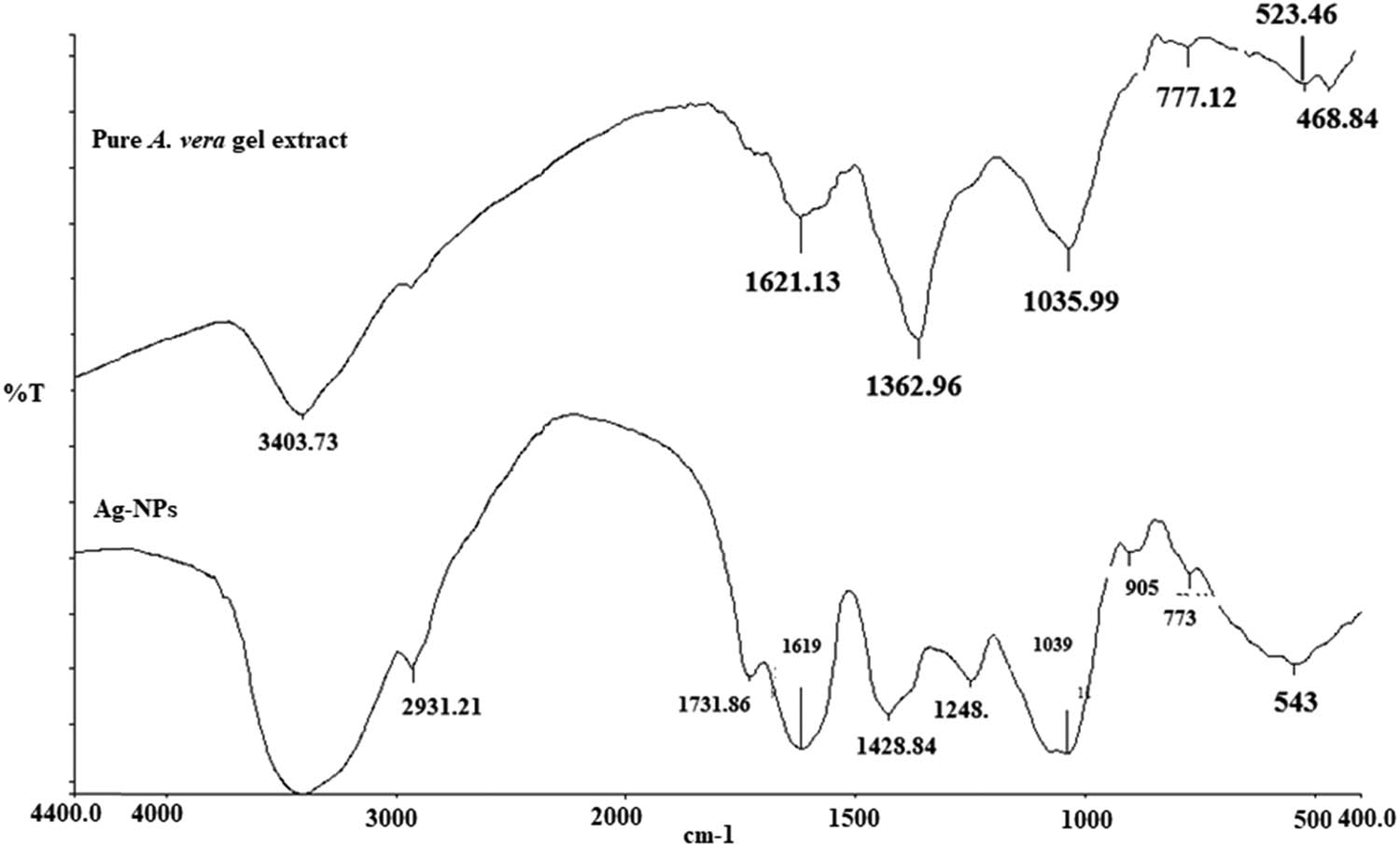
FTIR spectra of pure A. vera gel extract and the phytosynthesized Ag-NPs.
3.3 DLS measurements analysis
DLS is one of the most multifunctional techniques used for identifying the size and size distribution of NPs. The DLS measurements of the average size of Ag-NPs are depicted in Figure 4. The average size of the formed Ag-NPs was about 82 nm, while the PdI value was 0.134. The PdI value represents the monodispersity of the NPs. DLS technique determines the hydrodynamics of nanoparticle size and is measured in an aqueous suspension containing metallic core, ions, and biological biomolecules attached to the surface of NPs [28,29].
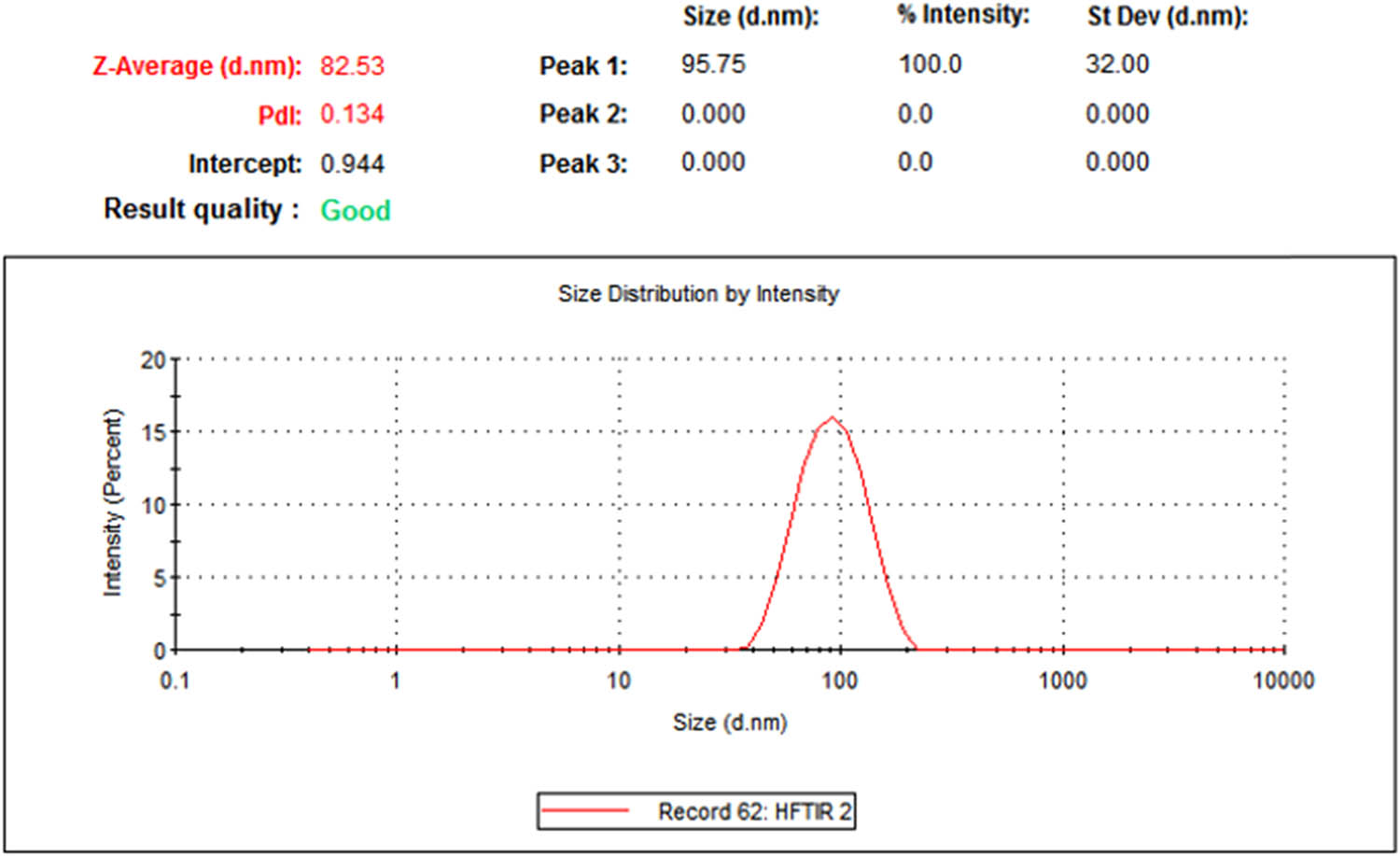
DLS measurement of the average size of phytosynthesized Ag-NPs.
3.4 TEM images of Ag-NPs
The size and morphology of the Ag-NPs were examined using TEM. TEM images of the phytosynthesized Ag-NPs are shown in Figure 5. The diameter of the particles was determined to be in the range of 5–10 nm, which was smaller than the result obtained by DLS. TEM images presented monodisperse Ag-NPs with a mostly spherical shape. These results are in agreement with previous studies by Mohamed et al. and Tippayawat et al. who reported spherical Ag-NPs synthesized using Aloe vera extract between 70 and 190 nm in size [30,31].
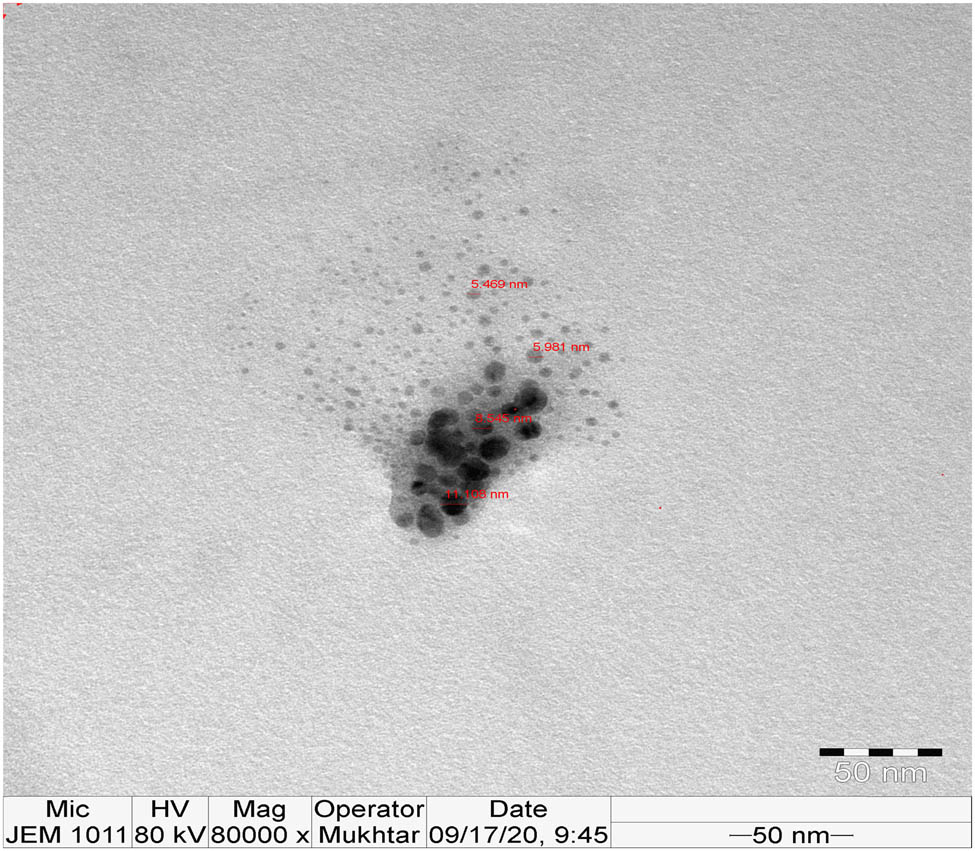
TEM image of phytosynthesized Ag-NPs.
3.5 Cytotoxic effects
The effect of different concentrations (3.125, 6.25, 12.5, 25, 50, and 100 µg mL−1) of Ag NPs on the B16F10 cell line has been assessed using an in-vitro MTT assay. The results suggested that phytosynthesized Ag-NPs showed marked cytotoxic activity against the B16F10 cancer cell line. The cytotoxic effect of the synthesized Ag-NPs at the highest concentration (100 µg mL−1) was more profound on B16F10 cells as compared to the standard anti-cancer drug, NSAID used as a positive control (Figure 6).
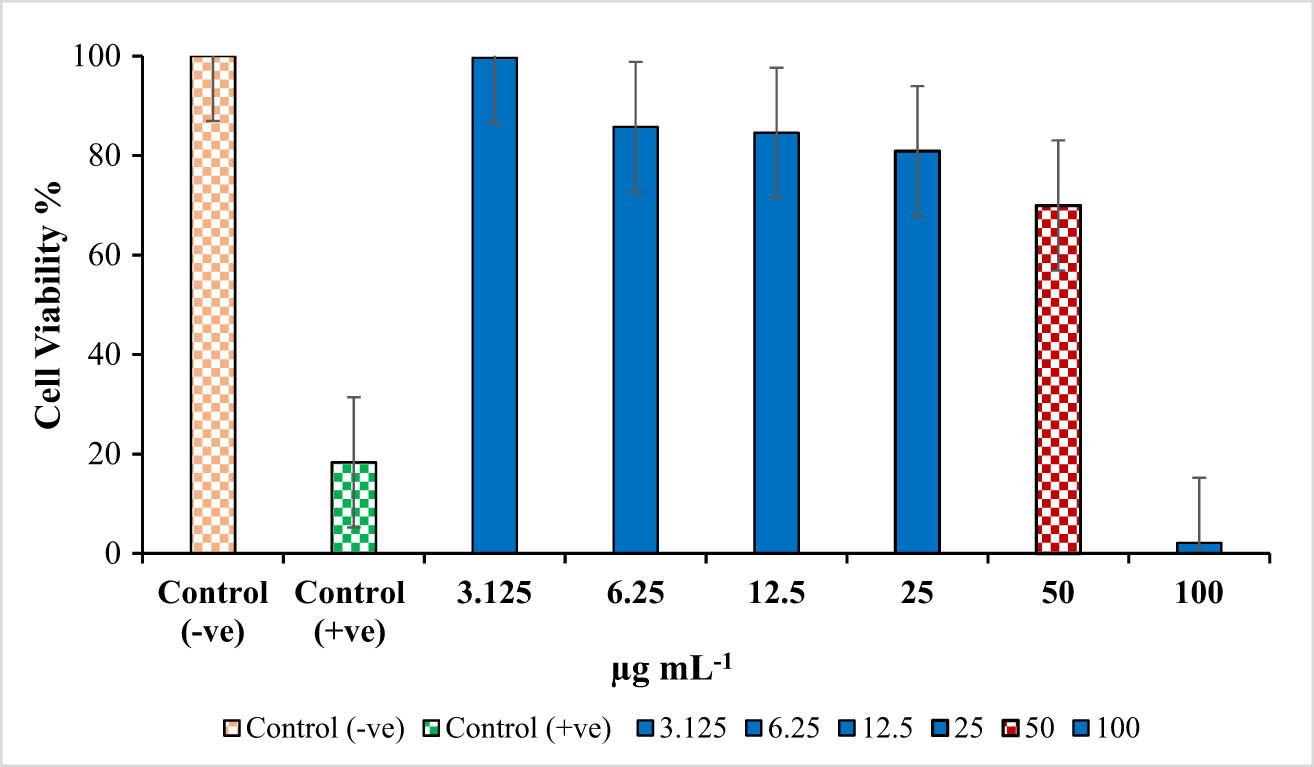
In vitro cytotoxic activity of Ag-NPs synthesized using Aloe vera gel extract against B16F10 cell line.
Thus, the cell viability of the cancer cell line decreased with the increasing concentration of synthesized Ag-NPs. At low concentration (3.125 µg mL−1), approximately 100% cell viability was established, while at 50 µg mL−1 (IC50), cell viability was 69.94%, and at 100 µg mL−1 concentration, the cell viability was reduced to 2.14% against 18.5% with the standard drug (positive control) (Figure 6). To the best of our knowledge, this is the first study that evaluated the cytotoxicity of Ag-NPs synthesized with A. vera gel extract on B16F10 cancer cell line. However, the cytotoxic effect of AgNPs synthesized with A. vera leaf extract has been previously reported against different cancer cell lines. For instance, Mohamed et al. [30] reported the antitumor activity of Ag-NPs against human MCF7 breast cancer cell line and found that at a high concentration of 200 μg mL−1, the cell viability was 28.7%. Tippayawat et al. [31] determined the cytotoxicity of Ag-NPs@ A. vera on PBMCs human cells. The % survival of the cells in less than 0.0025 mg mL−1 of both NPs was significantly higher than 50%, which confirms that these AgNPs@AVs were non-toxic to human PBMCs and had a promising future in therapeutics.
Many studies have reported that the cytotoxic effect of Ag-NPs could be caused by intracellular oxidative stress, which causes damage to cellular components such as lipids, DNA, and proteins, ultimately leading to cell death [18,32]. On the other hand, the anticancer activity of Ag-NPs may be due to Ag+ ions, which suggests that a significant amount of Ag+ ions will inhibit the sarcoplasmic reticulum which leads to cell death [33].
3.6 Antibacterial activity
The disc-diffusion method has been utilized to screen the antimicrobial activity of green synthesized Ag-NPs against a different strain of microbes: G+ bacteria (Enterococcus faecalis, Staphylococcus aureus, Bacillus cereus) G− bacteria (Escherichia coli).
Aloe vera extract did not show any inhibition in the bacterial growth in all the strains of bacteria evaluated. Figure 7 shows the inhibition zones (mm) around the disk containing green synthesized Ag-NPs at different concentrations (5 and 10 μg mL−1). At a higher concentration of 10 μg mL−1, synthesized Ag-NPs were more effective than the lower concentration (5 μg mL−1). The synthesized Ag-NPs showed the maximum zone inhibition (9.1 ± 0.100 mm) against P. aeruginosa and followed by E. coli (7.0 ± 0.000 mm), S. aureus (4.1 ± 0.100 mm), and B. cereus (2.03 ± 0.0577 mm) at a concentration of 100 μg mL−1. The synthesized Ag-NPs at a concentration of 100 μg mL−1 exhibited a significant (p ≥ 0.05) anti-bacterial effect on P. aeruginosa as compared to the other strains. The antibacterial activity of synthesized Ag-NPs increased in a dose-dependent manner. Similar results were reported in a study by Anju and Tippayawat [18,31] which showed that Ag-NPs synthesized using Aloe vera leaves extract showed similar antibacterial activity against various bacterial strains.
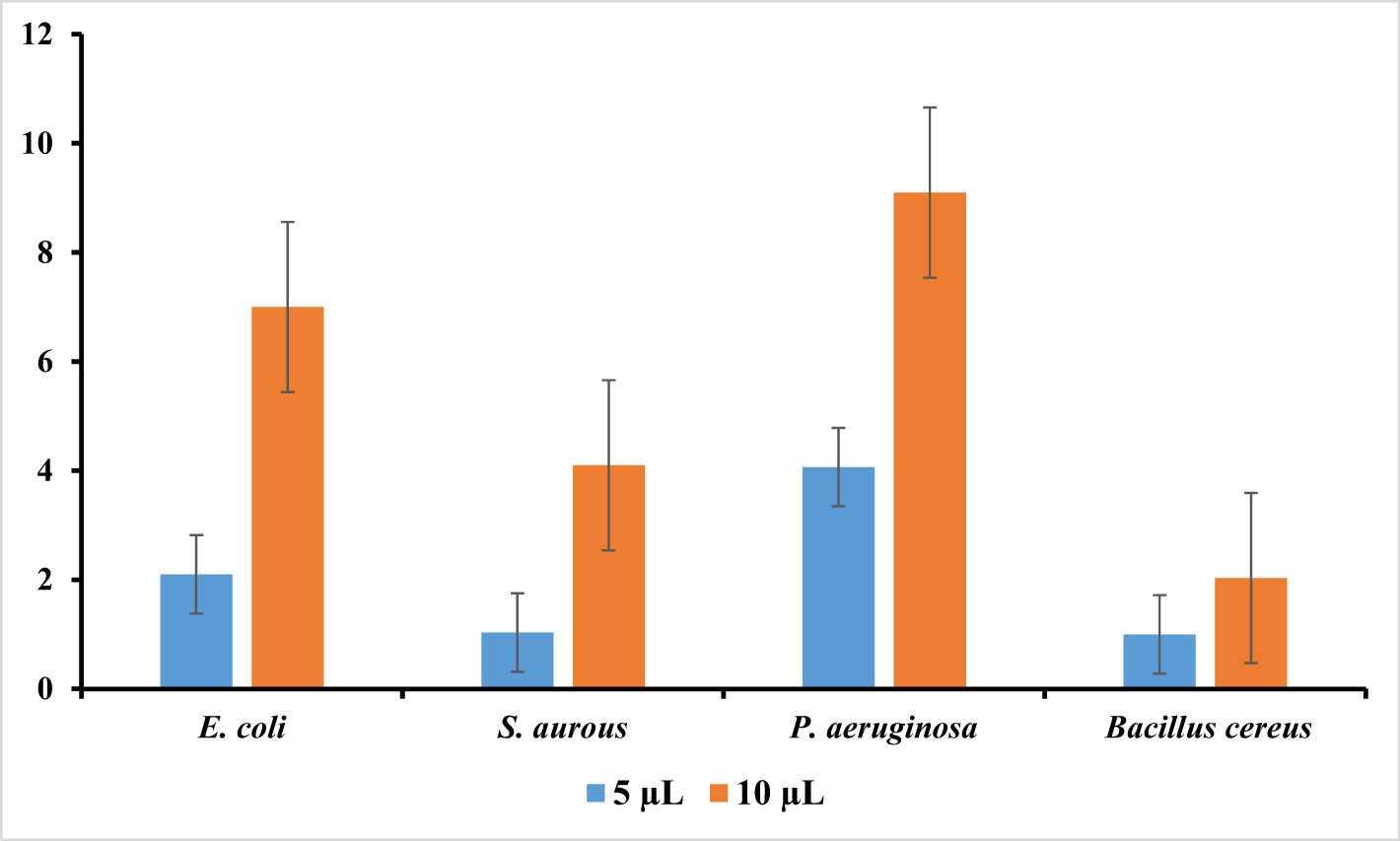
The sizes of inhibition zones (mm) representing antibacterial activity of aqueous extract of Aloe vera (blue columns) and the synthesized Ag-NPs of Aloe vera (orange columns): E. coli, S. aureus, Pseudomonas aeruginosa, and B. cereus. Different letters within a particular concentration show the statistical difference (p ≥ 0.05).
Various mechanisms have been proposed for the antibacterial activity of Ag-NPs in previous studies [34]. Some studies reported that Ag-NPs exhibited antimicrobial effects against bacterial cells via (a) membrane disruption due to the association/interaction of Ag-NPs with DNA and other biomolecules, subsequently causing inhibition of cell multiplication, and (b) formation of reactive oxygen species through interaction with enzymes and/or biomolecules, leading to cellular damage [35].
Gram-positive bacteria possess a thick cell wall of the peptidoglycan layer composed of linear polysaccharide chains, cross-linked by short peptides, which is a rigid structure that hinders the penetration of AgNPs into the bacterial cell wall, compared to Gram-negative bacteria where the cell wall consists of a thinner peptidoglycan layer [36,37,38].
The findings of the anti-fungal activity test show that the prepared AgNPs have marked antifungal activity against Bipolar heterothallic, Fusarium oxysporum, and Macrophomina as shown in Figure 8. The maximum fungal growth inhibition by the prepared AgNPs was observed for B. heterothallic (4 mm), followed by F. oxysporum and Macrophomina (6 and 7 mm, respectively). Taken together, for all the fungal strains, the synthesized AgNPs showed a significant (p ≥ 0.05) anti-fungal activity compared to the A. vera extract and control without treatment that exhibited complete fungal growth as shown in Figure 8. This result can be attributed to the lack of any active antimicrobial compounds in the extracellular extract. It has been recognized that there are several factors that contribute to the antimicrobial activity of Ag-NPs, including their shape, size, and surface charge, the tolerance to Ag-NPs, species-sensitivity, the type, genus, and species of the bacteria, the concentration of the NPs, and the duration of exposure to the pathogens [39]. The observed results showed the capability of AgNPs to prevent the fungal growth in the plates.
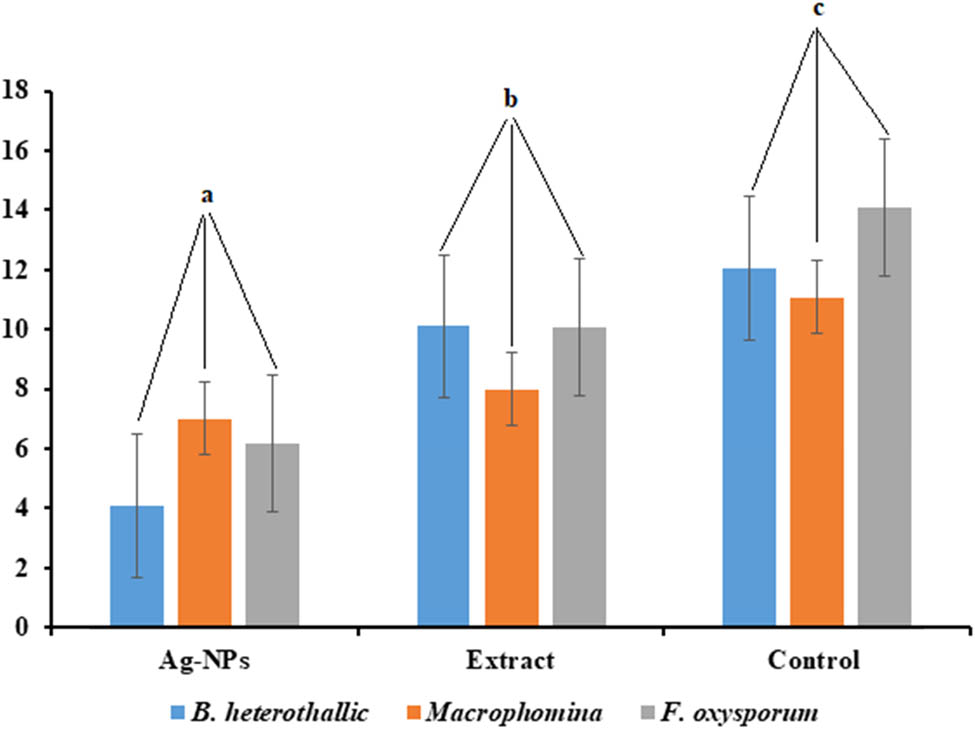
Antifungal activity of pure extract of A. vera (a), Ag-NPs produced using A. vera (b), and control (c). Different letters show a significant difference (p ≥ 0.05) between the treatments and control.
Whereas the mechanism for AgNPs fungicidal activity is unknown, it has been proposed that AgNPs inhibit the budding process by forming pores on the fungal cell membrane, which can lead to cell death [40]. It has been proposed that AgNP’s antibacterial activity is mediated by the formation of free radicals, membrane damage, and the formation of pits on the surface of the bacterial cell wall membrane. Furthermore, the production of free radicals can alter the chemical structure of DNA and proteins [40].
4 Conclusion
The present study is significant in the field of nanotherapeutics in terms of the phytosynthesis of Ag-NPs using A. vera gel extract which is an ecofriendly procedure that is relatively inexpensive, rapid, facile, and does not require any toxic chemicals. The synthesized NPs have been characterized using UV-Vis, DLS, FTIR, and TEM. The green synthesized NPs were spherical in shape, with an average size of 82 nm in diameter. Additionally, these NPs exhibited marked antimicrobial activity against different bacterial and fungal strains. Further, the findings also showed the AgNPs’ potential cytotoxicity against viable cells from the B16-F10 melanoma cell line. These findings open up new possibilities to utilize the NPs synthesized from A. vera gel extract for a wide range of applications including, pharmaceutical, cosmetic, biomedical, and nanomedical fields.
Acknowledgments
The authors extend their appreciation to the Researchers Support Project (number RSP-2021/173) of King Saud University.
-
Funding information: The authors extend their appreciation to the Researchers Support Project (number RSP-2021/173) of King Saud University, Riyadh, Saudi Arabia, for payment of the charge for publishing this manuscript.
-
Author contributions: Mona S. Alwhibi: funding acquisition, validation, supervision, formal analysis, project administration; Dina A. Soliman: visualization, resources, data curation, formal analysis, methodology, writing – original draft; Manal A. Awad: writing – review and editing, resources, formal analysis; Asma B. Alangery: data curation; Horiah Al Dehaish: resources; Yasmeen A. Alwasel: data curation.
-
Conflict of interest: The authors declare no competing financial interests.
Reference
[1] Siddiquee MA, Ud din Parray M, Mehdi SH, Alzahrani KA, Alshehri AA, Malik MA, et al. Green synthesis of silver nanoparticles from Delonix regia leaf extracts: in-vitro cytotoxicity and interaction studies with bovine serum albumin. Mater Chem Phys. 2020;242:122493.10.1016/j.matchemphys.2019.122493Search in Google Scholar
[2] Tade RS, Nangare NS, Patil PO. Agro-industrial waste-mediated green synthesis of silver nanoparticles and evaluation of its antibacterial activity. Nano Biomed Eng. 2020;12(1):57–66.10.5101/nbe.v12i1.p57-66Search in Google Scholar
[3] Smith BR, Gambhir SS. Nanomaterials for in vivo imaging. Chem Rev. 2017;117(3):901–86.10.1021/acs.chemrev.6b00073Search in Google Scholar PubMed
[4] Kumar V, Singh S, Srivastava B, Bhadouria R, Singh R. J Environ Chem Eng. 2019;7(3):103094.10.1016/j.jece.2019.103094Search in Google Scholar
[5] Jabir MS, Hussien AA, Sulaiman GM, Yaseen NY, Dewir YH, Alwahibi MS, et al. Green synthesis of silver nanoparticles from Eriobotrya japonica extract: a promising approach against cancer cells proliferation, inflammation, allergic disorders and phagocytosis induction. Artif Cells Nanomed Biotechnol. 2021;49(1):48–60.10.1080/21691401.2020.1867152Search in Google Scholar PubMed
[6] Gul AR, Shaheen F, Rafique R, Bal J, Waseem S, Park TJ. Grass-mediated biogenic synthesis of silver nanoparticles and their drug delivery evaluation: a biocompatible anti-cancer therapy. Chem Eng J. 2021;407:127202.10.1016/j.cej.2020.127202Search in Google Scholar
[7] Nouri AF, Yaraki MT, Lajevardi AD, Rezaei ZE, Ghorbanpour MA, Tanzifi M. Ultrasonic-assisted green synthesis of silver nanoparticles using Mentha aquatica leaf extract for enhanced antibacterial properties and catalytic activity. Colloids Interface Sci Commun. 2020;35:100252.10.1016/j.colcom.2020.100252Search in Google Scholar
[8] Das G, Shin HS, Kumar A, Vishnuprasad CN, Patra JK. Photo-mediated optimized synthesis of silver nanoparticles using the extracts of outer shell fiber of Cocos nucifera L. fruit and detection of its antioxidant, cytotoxicity and antibacterial potential. Saudi J Biol Sci. 2021;28(1):980–7.10.1016/j.sjbs.2020.11.022Search in Google Scholar PubMed PubMed Central
[9] Mohammed SS, Lawrance AV, Sampath S, Sunderam V, Madhavan Y. Facile green synthesis of silver nanoparticles from sprouted Zingiberaceae species: spectral characterization and its potential biological applications. Mater Tech. 2021;35:1–4.10.1080/10667857.2020.1863571Search in Google Scholar
[10] Gul AR, Shaheen F, Rafique R, Bal J, Waseem S, Park TJ. Grass-mediated biogenic synthesis of silver nanoparticles and their drug delivery evaluation: a biocompatible anti-cancer therapy. Chem Eng J. 2020;407:127202.10.1016/j.cej.2020.127202Search in Google Scholar
[11] Nasrollahzadeh M, Sajjadi M, Maham M, Sajadi SM, Barzinjy AA. Biosynthesis of the palladium/sodium borosilicate nanocomposite using Euphorbia milii extract and evaluation of its catalytic activity in the reduction of chromium(vi), nitro compounds and organic dyes. Mater Res Bull. 2018;102:24–35.10.1016/j.materresbull.2018.01.032Search in Google Scholar
[12] Mahdiani M, Soofivand F, Ansari F, Salavati-Niasari M. Grafting of CuFe12O19 nanoparticles on CNT and graphene: eco-friendly synthesis, characterization and photocatalytic activity. J Clean Prod. 2018;176:1185–97.10.1016/j.jclepro.2017.11.177Search in Google Scholar
[13] Rasli NI, Basri H, Harun Z. Zinc oxide from aloe vera extract: two-level factorial screening of biosynthesis parameters. Heliyon. 2020;6(1):e03156.10.1016/j.heliyon.2020.e03156Search in Google Scholar
[14] Mankodi H. Studies on different type of sutures using aloe vera gel coating. Int J Text Fashion Technol. 2013;4:11–6.Search in Google Scholar
[15] Nandal U, Bhardwaj R. Aloe vera: a valuable wonder plant for food, medicine and cosmetic use – a review. Int J Pharm Sci Rev Res. 2012;1:59–67.Search in Google Scholar
[16] Chow JT, Williamson DA, Yates KM, Goux WJ. Chemical characterization of the immunomodulating polysaccharide of Aloe vera L. Carbohydr Res. 2005;340(6):113142.10.1016/j.carres.2005.02.016Search in Google Scholar
[17] Reynolds T, Dweck AC. Aloe vera leaf gel: a review update. J Ethnopharmacol. 1999;68:3–37. 10.1016/s0378-8741(99)00085-9.Search in Google Scholar
[18] Anju TR, Parvathy S, Veettil MV, Rosemary J, Ansalna TH, Shahzabanu MM, et al. Green synthesis of silver nanoparticles from Aloe vera leaf extract and its antimicrobial activity. Mater Today Proc. 2021 Mar 12;43:3956–60.10.1016/j.matpr.2021.02.665Search in Google Scholar
[19] Zaidan MR, Noor A, Badrul AR, Adlin A, Norazah A, Zakiah I. In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Trop Biomed. 2005;22(2):165–70.Search in Google Scholar
[20] Noginov MA, Zhu G, Bahoura M, Adegoke J, Small C, Ritzo BA, et al. The effect of gain and absorption on surface plasmons in metal nanoparticles. Appl Phys B. 2007;86:455–60. 10.1007/s00340-006-2401-0.Search in Google Scholar
[21] Vidhu VK, Aromal SA, Philip D. Green synthesis of silver nanoparticles using Macrotyloma uniflorum. Spectrochim Acta A Mol Biomol Spectros. 2011;83(1):392–7.10.1016/j.saa.2011.08.051Search in Google Scholar PubMed
[22] Boken J, Khurana P, Thatai S, Kumar D, Prasad S. Plasmonic nanoparticles and their analytical applications: a review. Appl Spectrosc Rev. 2017;52(9):774–820.10.1080/05704928.2017.1312427Search in Google Scholar
[23] Rajkumar T, Sapi A, Das G, Debnath T, Ansari A, Patra JK. Biosynthesis of silver nanoparticle using extract of Zea mays (corn flour) and investigation of its cytotoxicity effect and radical scavenging potential. JPPBEG. 2019;193:1–7.10.1016/j.jphotobiol.2019.01.008Search in Google Scholar PubMed
[24] Medda S, Hajra A, Dey U, Bose P, Mondal NK. Biosynthesis of silver nanoparticles from Aloe vera leaf extract and antifungal activity against Rhizopus sp. and Aspergillus sp. Appl Nanosci. 2015;5(7):875–80.10.1007/s13204-014-0387-1Search in Google Scholar
[25] Awad AM, Salem NM, Abdeen AO. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int J Ind Chem. 2013;4(1):1–6.10.1186/2228-5547-4-29Search in Google Scholar
[26] Aslany S, Tafvizi F, Naseh V. Characterization and evaluation of cytotoxic and apoptotic effects of green synthesis of silver nanoparticles using Artemisia Ciniformis on human gastric adenocarcinoma. Mater Today Commun. 2020;24:101011.10.1016/j.mtcomm.2020.101011Search in Google Scholar
[27] Gupta A, Koirala AR, Gupta B, Parajuli N. Improved method for separation of silver nanoparticles synthesized using the Nyctanthes arbor-tristis shrub. ACMY. 2019;3(1):35–42.10.2478/acmy-2019-0005Search in Google Scholar
[28] Moteriya P, Chanda S. Green synthesis of silver nanoparticles from Caesalpinia pulcherrima leaf extract and evaluation of their antimicrobial, cytotoxic and genotoxic potential (3-in-1 system). J Inorg Organomet Polym. 2020;30:3920–32. 10.1007/s10904020-01532-7.Search in Google Scholar
[29] Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6(8):1794–807.10.1021/nl061025kSearch in Google Scholar PubMed
[30] Mohamed N, El-Masry HM. Aloe Vera gel extract and sunlight mediated synthesis of silver nanoparticles with highly effective antibacterial and anticancer activity. J Nanoanal. 2020;7(1):73–82.Search in Google Scholar
[31] Tippayawat P, Phromviyo N, Boueroy P, Chompoosor A. Green synthesis of silver nanoparticles in aloe vera plant extract prepared by a hydrothermal method and their synergistic antibacterial activity. Peer J. 2016;4:e2589.10.7717/peerj.2589Search in Google Scholar PubMed PubMed Central
[32] Khan Y, Numan M, Ali M, Khali AT, Ali T, Abbas N, et al. Bio-synthesized silver nanoparticles using different plant extracts as anti-cancer agent. J Nanomed Biother Discovery. 2017;7(154):2.Search in Google Scholar
[33] Panda MK, Dhal NK, Kumar M, Mishra PM, Behera RK. Green synthesis of silver nanoparticles and its potential effect on phytopathogens. Mater Today Proc. 2021;35:233–8.10.1016/j.matpr.2020.05.188Search in Google Scholar
[34] Panda MK, Singh YD, Behera RK, Dhal NK. Biosynthesis of nanoparticles and their potential application in food and agricultural sector. Green nanoparticles. Cham: Springer; 2020. p. 213–25.10.1007/978-3-030-39246-8_10Search in Google Scholar
[35] Alwahibi MS, Soliman DA, Alonaizan A, Marraiki NA, El-Zaidy M, Al Subeie MS. Green biosynthesis of silver nanoparticle using Commiphora myrrh extract and evaluation of their antimicrobial activity and colon cancer cells viability. J King Saud Univ Sci. 2020;32(8):3372–9.10.1016/j.jksus.2020.09.024Search in Google Scholar
[36] Azizian-Shermeh O, Valizadeh M, Taherizadeh M, Beigomi M. Phytochemical investigation and phytosynthesis of eco-friendly stable bioactive gold and silver nan oparticles using petal extract of saffron (Crocus sativus L.) and study of their antimicrobial activities. Appl Nanosci. 2019;10:1–4.10.1007/s13204-019-01059-5Search in Google Scholar
[37] Arshad H, Muhammad AS, Saima S, Umer H. Salvadora persica mediated synthesis of silver nanoparticles and their antimicrobial efficacy. Sci Rep. 2021;11(1):1–11.10.1038/s41598-021-85584-wSearch in Google Scholar PubMed PubMed Central
[38] Qais FA, Khan Mohd SA, Ahmed I, Althubiani AS. Potential of nanoparticles in combating Candida infections. Lett Drug Des Discov. 2019;16(5):478–91.10.2174/1570180815666181015145224Search in Google Scholar
[39] Ghojavand S, Madani M, Karimi J. Green synthesis, characterization and antifungal activity of silver nanoparticles using stems and flowers of felty germander. J Inorg Organomet Polym Mater. 2020;30(8):2987–97.10.1007/s10904-020-01449-1Search in Google Scholar
[40] Paul A, Roychoudhury A. Go green to protect plants: repurposing the antimicrobial activity of biosynthesized silver nanoparticles to combat phytopathogens. Nanotechnol Environ Eng. 2021;6(1):1–22.10.1007/s41204-021-00103-6Search in Google Scholar
© 2021 Mona S. Alwhibi et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis
Articles in the same Issue
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis

