Synthesis and characterization of surfactants derived from phenolphthalein: In vivo and in silico studies of their antihyperlipidemic effect
-
Hicham Zgueni
, Amine Azzane
, Ashwag S. Alanazi
, Mohamed El Ghozlani
Abstract
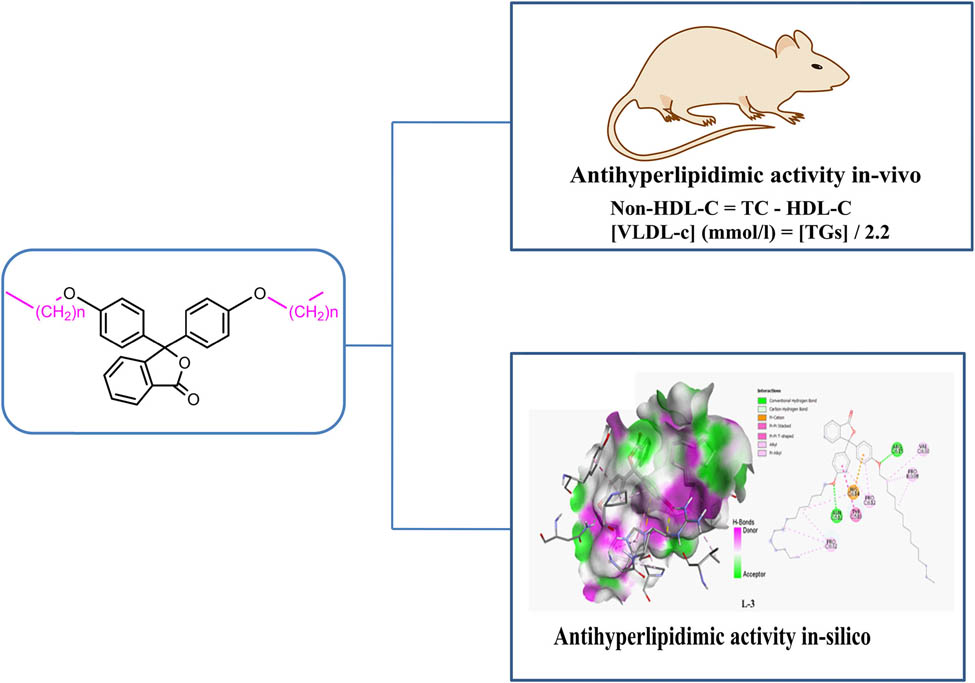
New phenolphthalein-based surfactants were synthesized, and their antihyperlipidemic activity was evaluated through both in vivo and in silico investigations. The target compounds were synthesized using the O-alkylation reaction between phenolphthalein and different alkyl halides, i.e., decyl bromide (L-1), undecyl bromide (L-2), and dodecyl bromide (L-3). The structures of the synthesized surfactants were established through spectroscopic techniques such as IR, 1H-NMR, and 13C-NMR, as well as confirmed by mass spectrometry. In vivo experiments were conducted on rats with Triton-induced hyperlipidemia, and the results demonstrate a significant reduction in serum triglycerides, total cholesterol (TC), and glycerol (Gly), as well as an improvement in the high-density lipoprotein-cholesterol/TC ratio in hyperlipidemic rats treated with a dose of 100 mg/kg (L-1, L-2, and L-3) per body weight when compared with anti-hyperlipidemic drug. The new phenolphthalein surfactants showed an effective antihyperlipidemic activity, similar to that of the marketed drug. In addition, in silico molecular docking studies were performed to assess the interaction that can occur between the synthesized surfactants and the target SARS-CoV-2 N protein (5KKN) involved in lipid metabolism. The achieved outcomes showed that the investigated surfactant derivatives had a strong affinity for the target proteins, supporting their in vivo antihyperlipidemic activity. The quantum chemical parameters of the molecules studied were calculated by calculations made at the HF level at the 6-31++G(d,p) level in gas and water phases.
Graphical abstract
List of abbreviations
- 13C-NMR
-
Nuclear magnetic resonance of carbon
- 1H-NMR
-
Nuclear magnetic resonance of protons
- ADT
-
AutoDockTools
- Gly
-
Glycerol
- HDL-C
-
High-density lipoprotein cholesterol
- HF
-
Hartree–Fock
- HOMO
-
Highest occupied molecular orbital
- HSAB
-
Hard and soft acid -bases
- IR
-
Infraredouge
- IS
-
Ionizsation spray
- LDL
-
Low- density lipoprotein
- LUMO
-
Lowest unoccupied molecular orbital
- MS
-
Mass spectra
- PCM
-
Polariszation continuum model
- PMH
-
Maximum hardness principle
- PTC
-
Phase transfer catalyst
- SD
-
Standard deviation
- TC
-
Total cholesterol
- TG
-
Triglyceriderudes
- TLC
-
Thin film chromatography
- TMS
-
Tetramethylsilane
- V-LDL
-
very low- density lipoprotein
1 Introduction
Hyperlipidemia is a medical condition characterized by high levels of lipids (fats) in the blood [1,2]. Lipids include cholesterol, triglycerides (TGs), and lipoproteins (high-density lipoprotein cholesterol [HDL-c], low-density lipoprotein cholesterol [LDL-c], and very low-density lipoprotein [VLDL]). Lipids are essential for normal body functions [3,4,5,6,7,8], but abnormal levels can lead to serious health problems such as cardiovascular disease [4,9], atherosclerosis [10], high blood pressure, and diabetes [11,12]. These abnormalities can be caused by genetic or acquired factors such as an unhealthy diet, physical inactivity, smoking, obesity, diabetes, and other medical conditions. Treatment strategies include lifestyle changes, such as healthy eating and exercise, along with drugs like statins, fibrates, and ion-exchange resins [13,14,15]. Several studies have shown that long-term use of these drugs can lead to toxic side effects [16,17,18,19]. For example, statins can have adverse effects on the nervous system, metabolism, and skeletal muscle [20,21]. Therefore, it is crucial to identify new, powerful, and innovative therapies that could offer an effective alternative for preventing disease progression and reducing associated morbidity and mortality rates.
For many years, heterocycles have been widely used in the research and development of new biologically active molecules [22,23,24]. Heterocyclic compounds make up the majority of molecules used in the pharmaceutical industry [25]. These compounds are the basis of many drugs, including hypolipidemic agents [26]. Therefore, heterocyclic chemistry has been booming in recent years and is attracting great interest worldwide, which results in continued exploration of these compounds. Benzofuranone has an essential component in heterocyclic systems. Indeed, it is the basic skeleton for a wide variety of potential applications in medicine, chemistry, and other fields. Several benzofuranone derivatives have been reported that have powerful biological activities, such as antibacterial [27], antioxidant, and anti-inflammatory [28].
Surfactants are compounds that reduce the surface tension between different phases, facilitating processes such as cleaning, emulsification, and dispersion [29]. They are essential in detergents, cosmetics, paints, and advanced industrial technologies [30].
Molecular modeling and quantum chemistry provide powerful tools for understanding the mechanisms of chemical reactions and predicting the physical and chemical properties of substances [31]. In this context, gas and liquid phase calculations are of great importance for studying the behavior of a molecule or chemical system under different environmental conditions [32].
Gas-phase calculations are performed to understand how a molecule behaves on its own, independent of any environmental influences [33]. These calculations usually provide reference data by simulating ideal conditions and are used in molecular geometry optimization, electronic energy calculations, and understanding reaction mechanisms.
On the other hand, liquid-phase calculations investigate how a molecule behaves under the influence of solvents [34]. Most chemical reactions, biochemical processes, and pharmaceutical applications occur in liquid media. Therefore, considering solvent effects is a critical requirement to obtain more accurate and realistic results. Polarizable continuum model or similar methods are usually used to model solvent effects [34].
The objective of this study focused on the modification of the phenolphthalein molecule that belongs to the benzofuranone family by the synthesis of novel surfactants with a polar head phenolphthalein using an O-alkylation reaction. These molecules offer the ability to reduce surface or interfacial tension between two phases, such as water and oil, air and water, or two immiscible liquids. This property is due to their unique molecular structure, which includes a hydrophilic (phenolphtalein) and a hydrophobic (alkyl chain) part, as they can be found in many industrial applications, including cleaning products, cosmetics, food, paints, and coatings. In addition, in this work, the synthesized surfactants were tested in vivo to evaluate their antihyperlipidemic activity. In silico studies have also been conducted to confirm and reinforce experimental results.
2 Materials and methods
2.1 Chemistry
All solvents used were of laboratory grade and were purified and dried following the standard procedures. Thin-film chromatography was used to monitor eluents. The IR spectra were obtained using an IS50 FT-IR spectrometer, covering the range from 4,000 to 400 cm−1 using KBr disks. Nuclear magnetic resonance (NMR) spectra were recorded on a JNM-ECZ500R/S1 FT NMR System (JEOL) using TMS as internal reference. Mass spectra (MS) are obtained using an Ultimate 3000 (Dionex) coupled with a Thermo Scientific Exactive Plus mass spectrometer. The samples are first dissolved in a dichloromethane solution and then ionized in positive mode using the ionization spray technique. The melting points were obtained using a bank Koffler device.
2.2 Synthesis and characterization data of products
Phenolphthalein (0.5 g, 1.57 mmol) was dissolved in 12 ml of acetonitrile. Then, 0.52 g of potassium carbonate and tetrabutylammonium bromide were added to the solution. The mixture is magnetically stirred until completely dissolved; after 45 min, the alkylhalide (0.976 g, 3.91 mmol) is added to the reaction mixture drop by drop under stirring, and after complete addition, the reaction mixture is refluxed for 8 h. Acetonitrile was evaporated in vacuum, and the water-washed reaction mixture was decanted with dichloromethane. The solvent was evaporated, and the resulting product was purified by recrystallization in ethanol.
2.3 Biology
2.3.1 Animals
Adult Wistar rats, weighing between 100 and 200 g, were housed in polyethylene cages under controlled environmental and standard conditions. The rats had ad libitum access to food and were maintained with free access to water and a standard laboratory diet, provided ad libitum. The diet consisted of a minimum of 12% crude protein, a minimum of 2% crude fat, a maximum of 14% crude fiber, a maximum of 10% minerals, and vitamins. This study was conducted under carefully controlled and standardized conditions and diets for rats throughout the experiment. The goal was to prevent external variables from altering their lipid profiles significantly. In normal rats, no significant change was noticed in the control group after 48 h of oral administration. All of the tests were carried out following local ethics guidelines (FSTE/2015) after a 3-week acclimation period to alleviate shipping stress.
2.3.2 Experimental design of antihyperlipidemic activity
The antihyperlipidemic activity was carried out as described in previous studies [29,30,31,32,33,34]. Rats were randomly divided into five groups (n = 5). The first group consisted of rats that were treated with distilled water (10 ml/kg body weight) orally, injected with saline (1 ml/kg bw) intraperitoneally, and served as the normal control group. In the second group, rats were treated with distilled water, injected with Triton solution (200 mg/kg bw) intraperitoneally, and served as the hyperlipidemic group. The third group consisted of rats treated with atorvastatin (10 mg/kg), which were injected intraperitoneally with Triton solution. The fourth, fifth, and sixth groups were composed of rats treated with L-1, L-2, and L-3 at a dose of 100 mg/kg bw. This dose was selected following a series of tests carried out with different doses (50, 80, 90, and 100 mg/kg) to determine the appropriate dose to be used. Each dose was administered to three rats, and 100 mg/kg was identified as the minimum dose that induced significant antihyperlipidemic activity. Injected with Triton solution induced hyperlipidemia in rats that were used in several studies to demonstrate hypolipidemic and dyslipidemic effects of many plants and products [35,36,37]. The last group consisted of rats that were treated with simvastatin at 20 mg/kg bw, injected with Triton solution, and served as the simvastatin treatment group.
2.3.3 Biochemical lipid profile
Twenty-four hours after Triton injection, rats were slightly anesthetized with ether, blood was collected from the retro-orbital sinus using heparinized capillaries and centrifuged at 5,000 rpm for 10 min to obtain plasma. Then the plasma was stored at −20°C until the day the lipid parameters were measured. TG, total cholesterol (TC), and HDL-C levels were determined using Test Kits (SGM Italia). The non-HDL-C value was determined using the following formula [38,39]:
2.4 Statistical analysis
Results are expressed as mean ± standard deviation (SD). Data were analyzed with “GraphPad Prism 9” software, using one-way ANOVA (Tukey’s test). The difference in p-value less than 0.05 was considered statistically significant [35,40].
2.5 Method of docking study
The crystal structure of the target COVID-19 protease protein was obtained from the Protein Data Bank (www.rcsb.org) prior to conducting docking simulations using the Autodock Vina program and the AutoDockTools (ADT) version 1.5.6 graphical interface [41]. The docking simulation aimed to explore the binding of substituted derivatives to the single-domain antibody B6 protein and the RNA-binding domain of the SARS-CoV-2 N protein (5KKN). Before docking, the 5KKN protein was prepared by removing water molecules and adding Kollman and Gasteiger charges via ADT. A grid map was created with dimensions of 60 Å in the X, Y, and Z directions, with the center of the grid box positioned at 14.229, −58.381, and −7.841 Å relative to the ligand’s location in the protein. The docked molecules and their hydrogen bond interactions were visualized using Discovery Studio Visualizer and PyMol software, which provided insights into the potential binding mechanisms of the derivatives and their anti-COVID properties.
To ensure the reliability of the molecular docking procedure, we examined the crystal structure of the 5KKN protein, which includes the co-crystallized ligand ND-646. The presence of this ligand confirms that the binding pocket is well-defined and experimentally validated. Although a formal re-docking of ND-646 was not performed, the docking grid was precisely centered on its original position, ensuring accurate targeting of the active site. The docking parameters used in AutoDock Vina, including grid dimensions, spacing, and exhaustiveness, were carefully selected based on established literature protocols to maximize accuracy and reproducibility. Furthermore, the binding affinity values obtained for our ligands are within the expected range for stable protein–ligand interactions, supporting the robustness of the docking methodology applied in this study.
Additionally, molecular docking of the targeted products was performed with the 5KKN protein, a key enzyme involved in lipid regulation and dyslipidemia. This analysis identified the preferred conformations of ligands binding to the protein, offering valuable insights into molecular interactions and inhibition potentials.
3 Results and discussion
3.1 Synthesis of new phenolphthalein derivatives
Phenolphthalein-based surfactant compounds were synthesized using the phase transfer catalyst technique [42], and the method is represented in Scheme 1. This process involved the successful preparation of three distinct compounds, designated as L-1, L-2, and L-3. These compounds were synthesized through the O-alkylation reaction of the phenolphthalein molecule with various alkyl bromides: decyl bromide L-1, undecyl bromide L-2, and dodecyl bromide L-3. The reaction was carried out in acetonitrile, with potassium carbonate serving as a base to deprotonate phenolphthalein, thereby increasing its reactivity toward alkyl bromides. The compounds were obtained in yields of over 80%.
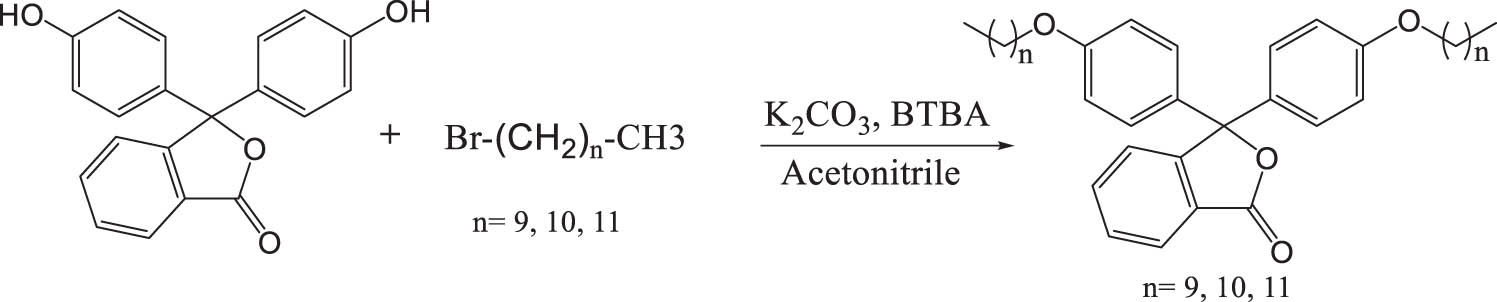
Synthesis of new surfactants derived from phenolphthalein.
The structure of the newly synthesized compounds was characterized by the standard spectroscopic techniques, namely infrared spectroscopy (IR) and proton nuclear magnetic resonance spectroscopy (1H NMR, 13C NMR), as well as confirmed by mass spectrometry (MS). The results obtained from these techniques validated the proposed structural elucidations and confirmed the successful chemical synthesis of the new compounds.
In the IR spectra, the absorption band observed in the region of 1,754–1,756 cm−1 was attributed to the (C═O) functional group. Additionally, the disappearance of the broad band characteristic of the OH group at around 3,400 cm−1 was noted [43].
In the ¹H NMR spectra, the absence of a proton signal for the OH group at 9.36 ppm, along with the presence of a triplet at 0.861 ppm corresponding to the protons of the two terminal CH₃ groups of the aliphatic chains, and the multiplets in the region 1.252–1.769 ppm originating from the CH₂ groups of the alkyl chains, confirms the formation of the desired compounds. Furthermore, the ¹³C NMR spectra reveal the presence of signals between 14.208 and 68.121 ppm, attributed to the CH₂ carbons of the alkyl chains. The MS of the analyzed products show the presence of the molecular ion peak corresponding to the exact mass of the chemical formula of the proposed structures, thereby confirming the formation of the desired products.
3.2 Anti-hyperlipidemic activity
The findings of the antihyperlipidemic activity of L-1, L-2, and L-3 are represented in Table 1. Treatment with Triton increased the TC and TG levels in treated rats compared with the control group. Oral administration of L-1, L-2, and L-3 led to a notable improvement in the lipid profile of triton-hyperlipidemic rats. Indeed, treating rats with L-1, L-2, and L-3 at a dose of 100 mg/kg bw significantly lowered the levels of TC (p < 0.05) compared to the Triton-hyperlipidemic group. Moreover, a significant reduction in TG concentrations was registered in the same groups after L-1, L-2, and L-3 oral administration (p < 0.0001). The treatment of rats with atorvastatin remarkably decreased the TC levels (p < 0.05) and TG levels (p < 0.0001) in hyperlipidemic rats. In contrast, oral administration of the three products (L-1, L-2, and L-3) did not have any significant variation in the HDL-c levels at the end of the study in hyperlipidemic rats.
Effects of L-1, L-2, and L-3 (100 mg/kg) on TC, TG, HDL-c, VLDL, and non-HDL-c levels in Tyloxapol (Triton WR-1339)-induced hyperlipidemic rats
| Groups | TC (mmol/L) | TGs (mmol/L) | HDL-cholesterol (mmol/L) | VLDL (mmol/L) | Non-HDL-cholesterol (mmol/L) |
|---|---|---|---|---|---|
| Normal group | 2.148 ± 0.28 | 0.69 ± 0.102 | 1.59 ± 0.25 | 0.31 ± 0.10 | 0.55 ± 0.07 |
| Hyperlipidemic control | 8.53 ± 2.31 | 14.82 ± 2.31 | 1.56 ± 1.08 | 6.74 ± 2.38 | 6.97 ± 1.55 |
| Hyperlipidemic + atorvastatin (10 mg/kg) | 4.042 ± 1.29* 52.61% | 0.698 ± 0.102**** 95.29% | 1.27 ± 0.46 18.59% | 0.32 ± 0.09** 95.25% | 2.76 ± 1.57 60.40% |
| L1 (100 mg/kg) | 3.7 ± 0.41* 56.62% | 1.46 ± 0.15**** 90.15% | 0.91 ± 0.08 41.67% | 0.66 ± 0.15** 90.21% | 2.79 ± 0.49 59.97% |
| L2 (100 mg/kg) | 2.54 ± 0.23* 70.22% | 1.077 ± 0.35**** 92.73% | 1.235 ± 0.17 20.83% | 0.48 ± 0.36** 92.88% | 1.306 ± 0.06* 81.26% |
| L3 (100 mg/kg) | 2.68 ± 1.59* 68.58% | 2.13 ± 1.05**** 85.63% | 1.601 ± 1.010 −2.63% | 0.969 ± 1.08** 85.62% | 1.08 ± 0.58* 84.51% |
N = 5 animals per group. All values are expressed as mean ± SEM. *p < 0.05, **p < 0.01, and ****p < 0.0001 compared to the hyperlipidemic group.
Interestingly, the oral administration of L-1, L-2, L-3 (100 mg/kg), and atorvastatin in hyperlipidemic rats notably reduced the value of VLDL-c (p < 0.01). In contrast, no significant change in the HDL-c levels was observed after the administration of three products at a dose of 100 mg/kg and after atorvastatin oral administration.
3.3 Molecular docking
Molecular docking is a computational modeling technique used to predict the preferred conformation of one molecule when it binds to another, forming a stable complex [44,45]. This method is crucial in drug design and the study of protein-ligand interactions, and the understanding of biological mechanisms. It involves several steps, including the preparation of protein and ligand structures, definition of the binding site [46,47,48], generation of possible ligand conformations, and evaluation of the best conformation based on affinity scores. By studying how a small molecule (ligand) binds to a large target molecule (protein), molecular docking helps identify potential new drugs and understand interactions at the molecular level [49].
In this section, molecular docking of the products shown in Figure 1 is carried out with the 5KKN protein, a key enzyme involved in lipid regulation and responsible for hyperlipidemia [50,51]. The 5KKN protein, corresponding to the RNA-binding domain of the SARS-CoV-2 nucleocapsid, was selected for docking simulations in this study due to its well-characterized structure and availability in the Protein Data Bank. Although 5KKN is not directly linked to lipid metabolism, it was used as a general model to explore the binding capacity of the synthesized compounds in silico. This approach provides a preliminary understanding of molecular interactions and pharmacophoric behavior. However, future investigations will aim to explore interactions with more biologically relevant targets involved in lipid regulation, such as acetyl-CoA carboxylase, HMG-CoA reductase, or PPAR receptors, to better align with the antihyperlipidemic profile of the compounds. The molecular docking process identifies the preferred conformations of ligands when they bind to this protein, providing valuable information on molecular interactions and inhibition potentials. The affinities of these products, assessed by binding scores, are presented in Table 3, allowing comparison of the efficiency of each ligand in binding to the 5KKN protein. In addition, the interactions between the ligands and proteins are detailed in 2D representations, showing hydrogen interactions and hydrophobic contacts, and in 3D, offering a spatial view of the binding sites and conformations adopted. These representations are illustrated in Figure 2, providing a visual understanding of interaction mechanisms and facilitating the identification of potential candidates for the development of treatments against hyperlipidemia [52]. To strengthen the reliability of our in silico results, additional quantum chemical calculations were carried out using the DFT/B3LYP method with the 6-311G(d,p) basis set, offering greater accuracy than the initial HF method. Moreover, molecular dynamics (MD) simulations were performed for 100 ns using GROMACS to evaluate the stability of the ligand–protein complexes under aqueous conditions. The results confirmed the stability of all complexes, especially L-3, and supported the docking outcomes with consistent binding energies obtained through MM-PBSA analysis.
Structure of the three ligands.

2D and 3D visualization of the formed complexes (L-1, L-2, and L-3) by using molecular docking.
3.4 In silico ADME/toxicity analysis
To complement the pharmacological evaluation of the synthesized surfactants L-1, L-2, and L-3, in silico ADME and toxicity predictions were performed using SwissADME and ProTox-II web tools. The results indicate that all three compounds exhibit favorable pharmacokinetic properties. Specifically, the surfactants show good gastrointestinal (GI) absorption, low blood–brain barrier permeability, and no inhibition of major cytochrome P450 enzymes (CYP1A2, CYP2C19, CYP2C9, CYP2D6, and CYP3A4), suggesting a low risk of metabolic interactions. Additionally, Lipinski’s Rule of Five was satisfied for all compounds, indicating drug-likeness. Regarding toxicity, ProTox-II predictions classified L-1, L-2, and L-3 as Class IV (300 < LD₅₀ ≤ 2,000 mg/kg), indicating they are relatively non-toxic. No mutagenic, carcinogenic, hepatotoxic, or immunotoxic effects were predicted. These results suggest that the synthesized compounds have a favorable safety profile, supporting their potential as antihyperlipidemic agents.
The molecular docking results indicate that L-3 exhibits the best binding affinity (−6.7 kcal/mol) to the 5KKN protein, which plays a key role in lipid regulation. This higher affinity suggests that L-3 may form stronger and more stable interactions with the active residues of the protein, potentially leading to more effective inhibition. In vivo, this enhanced binding affinity is reflected by a significant improvement in lipid profiles, particularly the reduction in TC and TG levels in hyperlipidemic rats. These results imply that L-3, with its superior binding affinity, may exert a stronger therapeutic effect in lowering lipid levels compared to L-1 and L-2, which show slightly lower binding affinities (−6.5 kcal/mol). Therefore, the stronger binding interactions of L-3 may directly contribute to its better efficacy in vivo, supporting its potential as a more effective treatment for hyperlipidemia.
Table 2 shows the affinities of the three ligands (L-1, L-2, and L-3) toward the 5KKN protein, expressed in kilocalories per mole (kcal/mol). Ligands L-1 and L-2 exhibit an identical affinity of −6.5 kcal/mol, suggesting a similar binding capacity to the target protein; in contrast, ligand L-3 demonstrates a slightly higher affinity of −6.7 kcal/mol, indicating stronger binding. Although the difference in the binding affinity between L-3 (−6.7 kcal/mol) and L-1/L-2 (−6.5 kcal/mol) appears minimal, even small variations in docking scores can indicate meaningful differences in molecular interactions. In drug discovery, an increase in affinity, even by 0.2 kcal/mol, can enhance binding stability and interaction specificity, potentially leading to improved inhibitory effects [41,53,54,55]. Additionally, this difference could stem from stronger hydrogen bonding, enhanced hydrophobic contacts, or additional π–π interactions with key residues. This difference in affinity could be attributed to more optimal interactions between the L-3 ligand and active residues of the 5KKN protein. For example, L-3 could form stronger hydrogen bonds, more stable hydrophobic interactions, or even additional salt bridges compared to ligands L-1 and L-2. These enhanced interactions may increase the stability of the ligand–protein complex, thereby reducing the free energy of binding. The proximity of the affinity values of the three ligands indicates that minor structural modifications of the L-1 and L-2 ligands could potentially improve their affinity, reaching or surpassing that of L-3. This suggests that optimization of these ligands could be a viable strategy for enhancing their therapeutic potential. For instance, the addition or modification of certain functional groups could enhance interactions with the protein, thereby increasing the binding affinity. In conclusion, although ligands L-1 and L-2 show promising affinity, L-3 emerges as a slightly superior candidate for further development due to its marginally better affinity. However, further studies, including detailed structural analyses and biological assays, will be needed to confirm these results and to explore the possibilities for ligand optimization to develop effective inhibitors against the 5KKN protein involved in hyperlipidemia.
Affinity of the three ligands under study
| Ligands | Affinity (kcal/mol) |
|---|---|
| L-1 | −6.5 |
| L-2 | −6.5 |
| L-3 | −6.7 |
Figure 2 shows a molecular docking simulation, illustrating in detail the interaction between a ligand and target protein. It shows a 3D view of the protein binding pocket with the docked ligand inside. Key interactions (Table 3), such as hydrogen bonds and hydrophobic interactions, are highlighted by dotted lines and precise annotations, allowing the critical contact zones between the ligand and protein residues to be visualized. Simulation results indicate a high binding affinity, suggested by low (negative) docking scores, testifying then the stability and thermodynamic favorability of the complex formed. The critical protein residues involved in ligand binding are clearly identified and annotated in the figure. These residues play an essential role in the interaction with the ligand, forming hydrogen bonds or participating in hydrophobic or π–π interactions. For example, amino acids such as tyrosine, phenylalanine and histidine may be involved in π–π interactions, while residues such as serine or threonine may form hydrogen bonds with the ligand.
Key interactions between ligands and active site residues
| Compound | Interacting residue(s) | Type of interaction | Binding site region |
|---|---|---|---|
| L-1 | HIS41, CYS145 | Hydrogen bonds and π–π stacking | Catalytic dyad |
| L-2 | GLU166, MET165 | Hydrogen bonds, van der Waals interactions | Substrate-binding pocket |
| L-3 | HIS41, GLY143, SER144 | Hydrogen bonds and hydrophobic interactions | Catalytic site |
Figure 2 also provides information on the conformation of the ligand in the binding pocket, showing how the ligand adjusts spatially to maximize its interactions with protein residues. This information is crucial for the design of future inhibitors or modulators, as it allows the identification of specific contact points that can be modified to improve the affinity and specificity of the ligand for the therapeutic target. In conclusion, the three ligands may be drug treatments for hyperlipidemia.
3.5 Gaussian software
When theoretical simulations are used to determine the activities and active regions of a molecule, this is one of the simplest and quickest methods available. Molecular active site identification, activity comparisons, and differences in activity with relation to various biological materials are all topics that are addressed by these computational tools. When all of the programs are utilized in the computations, the Gaussian package is the one that is utilized the most frequently. This instrument is used to compute a wide variety of quantum chemical properties, and the numerical values of these properties are compared [56]. By using these comparisons, we are able to make a judgment on the molecular activity. With these characteristics, the lowest unoccupied molecule orbital (LUMO) and the highest occupied molecular orbital (HOMO) are the most well-known and commonly used. The most well-known and commonly used of these features are the LUMO and the HOMO. Both of these orbitals occur in the molecular structures. The numerical values of these properties are altered in order to employ them for comparing the activities of the molecules [57]. The ability of the molecules to donate electrons is demonstrated by the numerical value of their HOMO parameter, while the ability of the molecules to absorb electrons is represented by the numerical value of their LUMO parameter. When the HOMO parameter of a molecule has the highest positive numerical value, it is generally acknowledged that the molecule will work more actively than other molecules [58]. In spite of this, it is common knowledge that, out of all the compounds [59], the molecule that possesses the highest activity has the largest negative numerical value for the LUMO parameter. The parameters are presented in Table 4 and Figures 3–5, respectively.
Calculated quantum chemical parameters of the synthesized molecules
| E HOMO | E LUMO | I | A | ΔE | H | μ | χ | PA | ω | ε | Dipole | Energy | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HF/6-31++g(d,p) LEVEL gas phase | |||||||||||||
| L-1 | −8.4323 | 0.9516 | 8.4323 | −0.9516 | 9.3839 | 4.6920 | 0.2131 | 3.7404 | −3.7404 | 1.4909 | 0.6707 | 6.041 | −50204.4115 |
| L-2 | −8.4318 | 0.9521 | 8.4318 | −0.9521 | 9.3839 | 4.6920 | 0.2131 | 3.7398 | −3.7398 | 1.4905 | 0.6709 | 6.043 | −52327.4573 |
| L-3 | −8.3352 | 1.0071 | 8.3352 | −1.0071 | 9.3423 | 4.6711 | 0.2141 | 3.6640 | −3.6640 | 1.4370 | 0.6959 | 4.603 | −54450.5271 |
| HF/6-31++g(d,p) LEVEL water phase | |||||||||||||
| L-1 | −8.9039 | 1.0893 | 8.9039 | −1.0893 | 9.9932 | 4.9966 | 0.2001 | 3.9073 | −3.9073 | 1.5278 | 0.6546 | 10.144 | −50204.9622 |
| L-2 | −8.9034 | 1.0896 | 8.9034 | −1.0896 | 9.9929 | 4.9965 | 0.2001 | 3.9069 | −3.9069 | 1.5275 | 0.6547 | 10.022 | −52328.0353 |
| L-3 | −8.5311 | 1.0925 | 8.5311 | −1.0925 | 9.6237 | 4.8118 | 0.2078 | 3.7193 | −3.7193 | 1.4374 | 0.6957 | 6.105 | −54451.1055 |
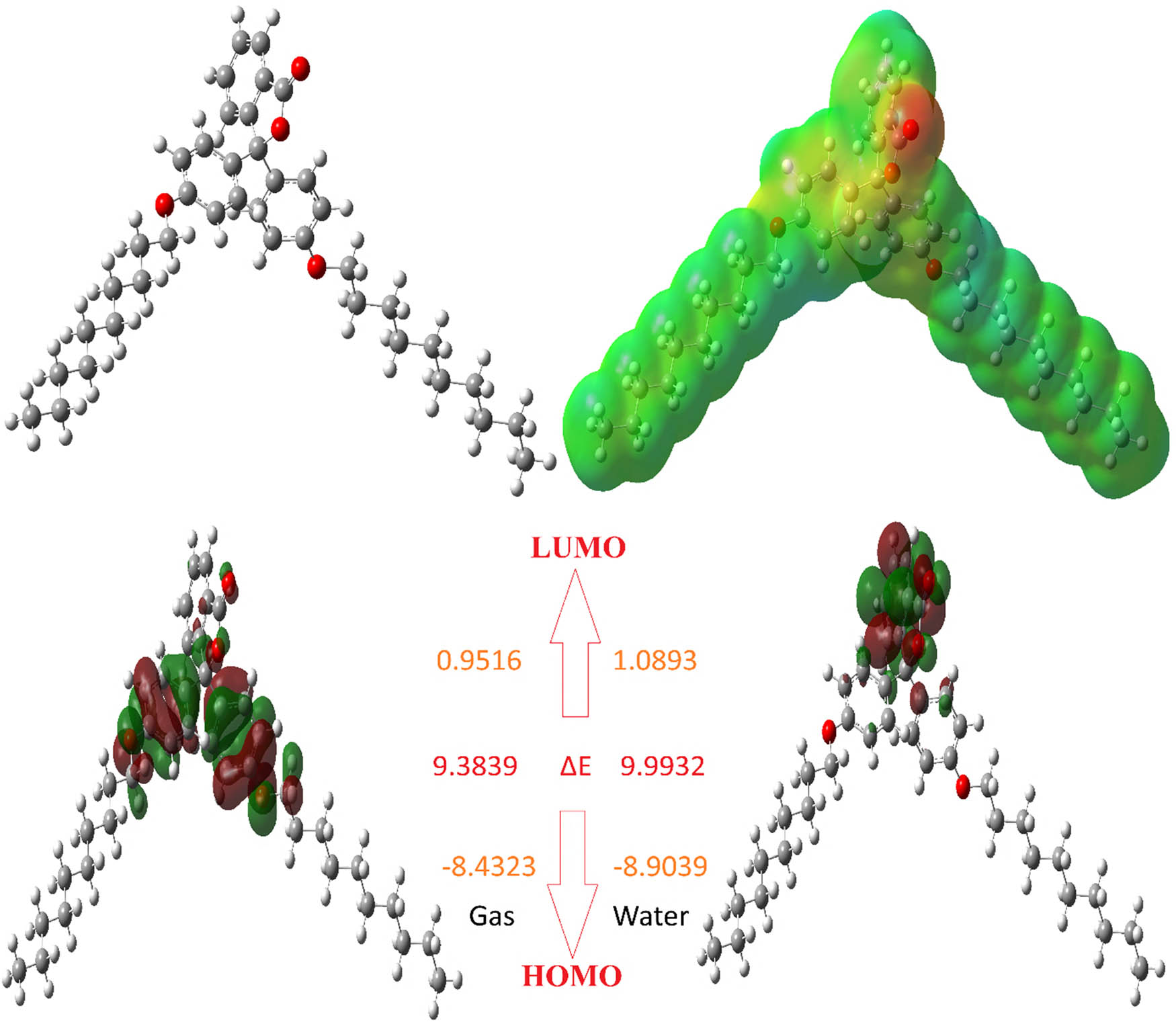
Representations of the optimized structure, HOMO, LUMO, and ESP of molecule L-1.

Representations of the optimized structure, HOMO, LUMO, and ESP of molecule L-2.
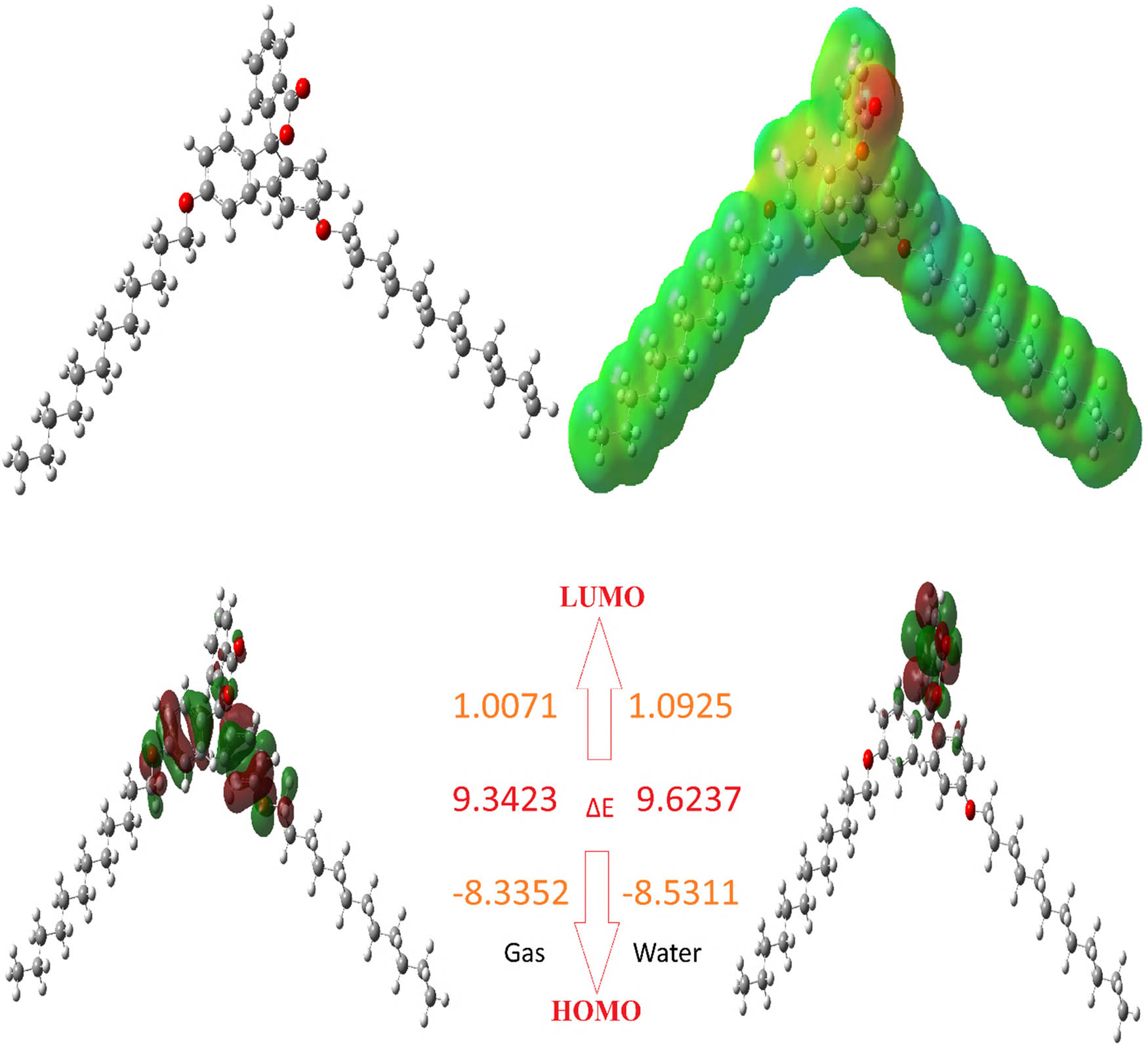
Representations of the optimized structure, HOMO, LUMO, and ESP of molecule L-3.
Numerous quantum chemical metrics, such as ΔE, EHOMO, ELUMO, chemical hardness, softness, electronegativity, and chemical potential, are frequently employed to compare the activities of the molecules. These are related to a chemical species’ capacity to donate and receive electrons. For a chemical molecule, Koopman’s theorem [60] offers an alternate approach based on boundary orbital energies to calculate the ionization energy and electron affinity. In 1963, Pearson created the “chemical hardness” metric [61] to quantify a chemical species’ resistance to deformation or electron cloud polarization. Based on the chemical hardness, the hard and soft acid–bases (HSABs) [62] and maximum hardness principles [63] are taken into account in both theoretical and applied research. Lewis acids and bases are categorized as hard or soft using the HSAB principle, which states that soft acids prefer to coordinate with soft bases and hard acids prefer to coordinate with hard bases. In molecules, polarizable chemical species are represented by the soft notion, whereas non-polarizable chemical species are represented by the hard notion. Non-polarizable (hard) molecules have difficulty in transferring electrons to other molecules, whereas polarizable (soft) chemical species do so readily [62]. The ΔE energy gap and chemical hardness are connected, as per Koopman’s theorem. The HOMO–LUMO gap is larger in hard molecules and smaller in soft ones. Essential characteristics that gauge stability and reactivity are chemical hardness and softness. The polarizability of a molecule is measured by softness (1/n), which is the opposite of chemical hardness. Soft molecules are more reactive than hard molecules because they donate electrons to an acceptor more easily [64].
Electrostatic potential (ESP) maps obtained with Gaussian software are important tools used to visualize the electrical charge distribution on the surface of a molecule. These maps provide important information in understanding the reactive sites, intermolecular interactions, and bonding properties of a molecule [64].
ESP represents the magnitude and direction of the electrical force that a virtual test charge would experience in the vicinity of a molecule. Gaussian software calculates this potential based on quantum chemical calculations and projects it onto the surface of the molecule [65].
The electron density of the molecules is calculated using the optimized geometry. This density represents the negative charge distribution in different regions of the molecule. Gaussian calculates the ESP on the surface of the molecule using this electron density and creates a color-coded map. In the map, red usually represents electron-rich (negative potential), blue represents electron-poor (positive potential), and green represents neutral regions [66].
ESP maps are used in many areas such as reactivity analysis, drug design, investigation of hydrogen-bond interactions, determination of molecular interactions, and catalyst design. Additionally, ESP maps can be used to understand interactions with biomolecules and identify binding sites [67].
3.6 Comparison study
The products presented (Table 5) show variable antihyperlipidemic activity depending on the model used and the concentration tested. Coumarin bisindole (10 mg/kg) and xanthene derivatives (50 mg/kg) show significant efficacy at low doses, probably through inhibition of HMG-CoA reductase and improvement in LDL clearance. Benzocoumarin derivatives (100 mg/kg) and benzofurans (250 mg/kg) showed moderate activity, while phenolphthalein-based surfactants (100 mg/kg) showed interesting potential in the Triton model. Extracts of plants such as Salvia tingitana, Artemisia arborescens, and Moricandia suffruticosa reduced plasma lipids in the diabetic rat model, suggesting an antioxidant effect and regulation of lipid metabolism.
Comparison of the efficacy of our antihyperlipidemic agents with the literature
| Antihyperlipidemic agent | Model | Concentration (mg/kg) | References |
|---|---|---|---|
| Coumarin bisindole | Hamster model | 10 | [68] |
| Benzocoumarin derivatives | Triton model | 100 | [69] |
| N-(acetylphenyl)-1-benzofuran-2-carboxamides | Triton model | 250 | [70] |
| Xanthene derivatives | Triton model | 50 | [71] |
| Salvia tingitana | Streptozotocin-induced diabetic rats | 60 and 80 | [72] |
| Artemisia arborescens | Streptozotocin-induced diabetic rats | 40 and 80 | [73] |
| Moricandia suffruticosa | Streptozotocin-induced diabetic rats | 100 and 140 | [74] |
| Hertia maroccana | Triton-induced hyperlipidemia in rats | 500 | [75] |
| Ammodaucus leucotrichus | Triton-induced hyperlipidemia in rats | 50 and 250 | [76] |
| New phenolphthalein-based surfactants | Triton model | 100 | This study |
3.7 Conclusion
This study allowed the successful synthesis of new surfactants based on phenolphthalein with high yields. In vivo studies have shown that these surfactants have significant antihyperlipidemic effects, while in silico studies have revealed strong binding interactions and high binding affinities with the key receptor residues. As a result of the Gaussian software calculations made with the effectiveness in environmental pollution removal, the quantum chemical parameters of the molecules were compared. In this comparison, when the numerical values of the HOMO, LUMO, energy gap, and electronegativity parameters of the molecules were examined, it was observed that molecule L-3 had higher activity than the other molecules. Both gas- and water-phase calculations of the molecules were made., The calculations in the water phase after the gas phase did not change the comparison results but caused the numerical value to increase. The most important reason for this is that a molecule with a high dielectric constant, such as water, as a result of the interaction with the working molecules, increases the dipole moment values and the numerical values of the quantum chemical parameters of the molecules. Overall, these promising results can provide valuable information for the development of new surfactants based on phenolphthalein as therapeutic agents’ potential against hyperlipidemia.
Acknowledgments
The authors thank the National Center for Technical and Scientific Research (CNRST) for the support on characterization techniques. The authors extend their appreciation to Princess Nourah bint Abdulrahman University researcher supporting project number (PNURSP2025R342), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia, for supporting this work.
-
Funding information: This work is supported by Princess Nourah bint Abdulrahman University researcher supporting project number (PNURSP2025R342), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
-
Author contributions: Hicham Zgueni, Amine Azzane, and Mohammed El Mesky: writing – original draft, methodology, formal analysis, and conceptualization. Mohammed Chalkha and Mohamed El Ghozlani: visualization and validation. Burak Tüzün: software, writing – original draft, and methodology. Mohamed Hefnawy and Ashwag S. Alanazi: software, review, and editing. El Houssine Mabrouk, Mohamed Eddouks, and Driss Chebabe: validation, supervision, project administration, and formal analysis.
-
Conflict of interest: The authors declare no conflicts of interest, financial or otherwise.
-
Ethical approval: All experiments were performed according to the local ethical committee, Faculty of Science and Techniques Errachidia, Morocco.
-
Data availability statement: All data generated in this work will be made available upon a reasonable request from the corresponding author.
References
[1] Onwe PE, Folawiyo MA, Anyigor-Ogah CS, Umahi G, Okorocha AE, Afoke AO. Hyperlipidemia: etiology and possible control. IOSR J Dent Med Sci. 2015;14(10):93–100.Search in Google Scholar
[2] Stewart J, McCallin T, Martinez J, Chacko S, Yusuf S. Hyperlipidemia. Pediatr Rev. 2020;41(8):393–402.10.1542/pir.2019-0053Search in Google Scholar PubMed
[3] Havel RJ. Lipoproteins and lipid transport. In: Kritchevsky D, Paoletti R, Holmes WL, editors. Lipids, lipoproteins, and drugs. Boston, MA: Springer US. p. 37–59. Advances in Experimental Medicine and Biology. 1975;63. 10.1007/978-1-4684-3258-9_3.Search in Google Scholar PubMed
[4] Stein EA, Myers GL. Lipids, lipoproteins and apolipoproteins. Fundam Clin Chem. 1987;3:4789.Search in Google Scholar
[5] Ginsberg HN. Lipoprotein physiology. Endocrinol Metab Clin. 1998;27(3):503–19.10.1016/S0889-8529(05)70023-2Search in Google Scholar
[6] Bali S, Utaal MS. Serum lipids and lipoproteins: a brief review of the composition, transport and physiological functions. Int J Sci Rep. 2019;5:309.10.18203/issn.2454-2156.IntJSciRep20194253Search in Google Scholar
[7] Jovandaric MZ, Milenkovic SJ. Significance of lipid and lipoprotein in organism. Apolipoproteins, triglycerides and cholesterol. IntechOpen; 2020. https://www.intechopen.com/chapters/71384.10.5772/intechopen.91407Search in Google Scholar
[8] Boren J, Taskinen MR, Björnson E, Packard CJ. Metabolism of triglyceride-rich lipoproteins in health and dyslipidaemia. Nat Rev Cardiol. 2022;19(9):577–92.10.1038/s41569-022-00676-ySearch in Google Scholar PubMed
[9] Rana JS, Nieuwdorp M, Jukema JW, Kastelein JJP. Cardiovascular metabolic syndrome – an interplay of, obesity, inflammation, diabetes and coronary heart disease. Diabetes Obes Metab. 2007;9(3):218–32.10.1111/j.1463-1326.2006.00594.xSearch in Google Scholar PubMed
[10] Stocker R, Keaney JF. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84(4):1381–478.10.1152/physrev.00047.2003Search in Google Scholar PubMed
[11] Epstein M, Sowers JR. Diabetes mellitus and hypertension. Hypertension. 1992;19(5):403–18.10.1161/01.HYP.19.5.403Search in Google Scholar PubMed
[12] Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75(2):285–92.10.1161/HYPERTENSIONAHA.119.14240Search in Google Scholar PubMed PubMed Central
[13] Gianazza E, Brioschi M, Iezzi A, Paglia G, Banfi C. Pharmacometabolomics for the study of lipid-lowering therapies: opportunities and challenges. Int J Mol Sci. 2023;24(4):3291.10.3390/ijms24043291Search in Google Scholar PubMed PubMed Central
[14] Jian-Jun LI, Shui-Ping Z, Dong Z, Guo-Ping LU, Dao-Quan P, Jing LIU, et al. China guidelines for lipid management. J Geriatr Cardiol. 2023;20(9):621.10.26599/1671-5411.2023.09.008Search in Google Scholar PubMed PubMed Central
[15] McCune DF, Piascik MT, Hadley RW. Treatment of hyperlipidemias and atherosclerotic cardiovascular disease. Brody's human pharmacology: Mechanism-based therapeutics, 6th edn.; 2024. p. 367.Search in Google Scholar
[16] Holman RR, Paul SK, Bethel MA, Neil HAW, Matthews DR. Long-Term Follow-up after Tight Control of Blood Pressure in Type 2 Diabetes. N Engl J Med. 2008;359(15):1565–76.10.1056/NEJMoa0806359Search in Google Scholar PubMed
[17] Mancini GJ, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: proceedings of a Canadian Working Group Consensus Conference. Can J Cardiol. 2011;27(5):635–62.10.1016/j.cjca.2011.05.007Search in Google Scholar PubMed
[18] Ho CKM, Walker SW. Statins and their interactions with other lipid-modifying medications: safety issues in the elderly. Ther Adv Drug Saf. 2012;3(1):35–46.10.1177/2042098611428486Search in Google Scholar PubMed PubMed Central
[19] Newman CB. Safety of statins and nonstatins for treatment of dyslipidemia. Endocrinol Metab Clin. 2022;51(3):655–79.10.1016/j.ecl.2022.01.004Search in Google Scholar PubMed
[20] Bouitbir J, Sanvee GM, Panajatovic MV, Singh F, Krähenbühl S. Mechanisms of statin-associated skeletal muscle-associated symptoms. Pharmacol Res. 2020;154:104201.10.1016/j.phrs.2019.03.010Search in Google Scholar PubMed
[21] Mollazadeh H, Tavana E, Fanni G, Bo S, Banach M, Pirro M, et al. Effects of statins on mitochondrial pathways. J Cachexia Sarcopenia Muscle. 2021;12(2):237–51.10.1002/jcsm.12654Search in Google Scholar PubMed PubMed Central
[22] Baranwal J, Kushwaha S, Singh S, Jyoti A. A review on the synthesis and pharmacological activity of heterocyclic compounds. Curr Phys Chem. 2023;13(1):2–19.10.2174/1877946813666221021144829Search in Google Scholar
[23] Kazemi M, Karezani N. Research on biological and bioactive molecules containing pyrrole scaffolds. Biol Mol Chem. 2023;1(1):15–26.Search in Google Scholar
[24] Sadek KU, Mekheimer RA, Abd-Elmonem M, Abo-Elsoud FA, Hayallah AM, Mostafa SM, et al. Recent developments in the synthesis of hybrid heterocycles, a promising approach to develop multi-target antibacterial agents. J Mol Struct. 2023;1286:135616.10.1016/j.molstruc.2023.135616Search in Google Scholar
[25] Kumar A, Mishra A. Role of heterocyclic compounds in pharmaceuticals and medicines. J Exp Zool India. 2023;26(1)26:1–9.10.51470/jez.2023.26.1.1Search in Google Scholar
[26] Ashton MJ, Ashford A, Loveless AH, Riddell D, Salmon J, Stevenson GVW. Heterocyclic analogs of chlorcyclizine with potent hypolipidemic activity. J Med Chem. 1984;27(10):1245–53.10.1021/jm00376a002Search in Google Scholar PubMed
[27] Pires JR, Saito C, Gomes SL, Giesbrecht AM, Amaral do AT. Investigation of 5-Nitrofuran Derivatives: Synthesis, Antibacterial Activity, and Quantitative Structure−Activity Relationships. J Med Chem. 2001;44(22):3673–81.10.1021/jm0101693Search in Google Scholar PubMed
[28] Closse A, Haefliger W, Hauser D, Gubler HU, Dewald B, Baggiolini M. 2,3-Dihydrobenzofuran-2-ones: a new class of highly potent antiinflammatory agents. J Med Chem. 1981;24(12):1465–71.10.1021/jm00144a019Search in Google Scholar PubMed
[29] De S, Malik S, Ghosh A, Saha R, Saha B. A review on natural surfactants. RSC Adv. 2015;5(81):65757–67.10.1039/C5RA11101CSearch in Google Scholar
[30] Kumar N, Tyagi R. Industrial applications of dimeric surfactants: a review. J Dispers Sci Technol. 2014;35(2):205–14.10.1080/01932691.2013.780243Search in Google Scholar
[31] Myroslava O, Poustforoosh A, Inna B, Parchenko V, Tüzün B, Gutyj B. Molecular descriptors and in silico studies of 4-((5-(decylthio)-4-methyl-4n-1, 2, 4-triazol-3-yl) methyl) morpholine as a potential drug for the treatment of fungal pathologies. Comput Biol Chem. 2024;113:108206.10.1016/j.compbiolchem.2024.108206Search in Google Scholar PubMed
[32] Barghady N, Assou SA, Er-Rajy M, Boujdi K, Arzine A, Rhazi Y, et al. Design, synthesis, characterization, and theoretical calculations, along with in silico and in vitro antimicrobial properties of new isoxazole-amide conjugates. Open Chem. 2024;22(1):20240109.10.1515/chem-2024-0109Search in Google Scholar
[33] Goswami AK, Aboul-Soud MA, Gogoi N, El-Shazly M, Giesy JP, Tüzün B, et al. Integrative in silico evaluation of the antiviral potential of terpenoids and its metal complexes derived from Homalomena aromatica based on main protease of SARS-CoV-2. Open Chem. 2024;22(1):20240085.10.1515/chem-2024-0085Search in Google Scholar
[34] Karimi S, Mafton-Azad L, Behnajady B, Tüzün B. Response surface methodology (RSM) design to optimize the cathode of Li-ions batteries recycling in deep eutectic solvent and DFT simulation. Korean J Chem Eng. 2024;1:21.10.21203/rs.3.rs-4548121/v1Search in Google Scholar
[35] Azzane A, Azzaoui B, Akdad M, Bouadid I, Eddouks M. Effect of calamintha officinalis on vascular contractility and angiotensinconverting enzyme-2. Cardiovasc Hematol Agents Med Chem Former. 2022;20(3):219–36.10.2174/1871525720666220302125242Search in Google Scholar PubMed
[36] Azzane A, Amssayef A, El-Haidani A, Eddouks M. Effect of pulicaria mauritanica on glucose metabolism and glycogen content in streptozotocin-induced diabetic rats. Cardiovasc Hematol Agents Med Chem Former Curr Med Chem-Cardiovasc Hematol Agents. 2022;20(3):197–211.10.2174/1871525720666220510204624Search in Google Scholar PubMed
[37] Azzane A, Eddouks M. Antihyperglycemic, antihyperlipidemic, and antioxidant effects of salvia tingitana in streptozotocin-induced diabetic rats. Cardiovasc Hematol Disord Drug Targets. 2022;22(2):118–27.Search in Google Scholar
[38] Ikewuchi JC, Ikewuchi CC, Ifeanacho MO. Attenuation of salt-loading induced cardiomegaly and dyslipidemia in Wistar rats by aqueous leaf extract of Chromolaena odorata. Pharmacol Amp Pharm. 2014;5(2):160–70.10.4236/pp.2014.52022Search in Google Scholar
[39] Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.10.1093/clinchem/18.6.499Search in Google Scholar
[40] Azzane A, Amssayef A, Eddouks M. Salvia aucheri exhibits antihypertensive activity in hypertensive rats. Cardiovasc Hematol Agents Med Chem Former. 2023;21(3):167–76.10.2174/1871525721666221221163432Search in Google Scholar PubMed
[41] Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–61.10.1002/jcc.21334Search in Google Scholar PubMed PubMed Central
[42] Chebabe D, Dermaj A, El Abidine Ait Chikh Z, Hajjaji N, Rico‐Lattes I, Lattes A. Synthesis of new surfactants mono and bipolar derived from 1,2,4‐triazole‐5‐thione. Synth Commun. 2004;34(22):4189–98.10.1081/SCC-200036625Search in Google Scholar
[43] Zhang B, Wang Z, Zhang X. Synthesis and properties of a series of cyanate resins based on phenolphthalein and its derivatives. Polymer. 2009;50(3):817–24.10.1016/j.polymer.2008.12.006Search in Google Scholar
[44] Agarwal S, Mehrotra R. An overview of molecular docking. JSM Chem. 2016;4(2):1024–8.Search in Google Scholar
[45] Khamis MA, Gomaa W, Ahmed WF. Machine learning in computational docking. Artif Intell Med. 2015;63(3):135–52.10.1016/j.artmed.2015.02.002Search in Google Scholar PubMed
[46] Grinter SZ, Zou X. Challenges, applications, and recent advances of protein-ligand docking in structure-based drug design. Molecules. 2014;19(7):10150–76.10.3390/molecules190710150Search in Google Scholar PubMed PubMed Central
[47] Du X, Li Y, Xia YL, Ai SM, Liang J, Sang P, et al. Insights into protein–ligand interactions: mechanisms, models, and methods. Int J Mol Sci. 2016;17(2):144.10.3390/ijms17020144Search in Google Scholar PubMed PubMed Central
[48] Antunes DA, Devaurs D, Kavraki LE. Understanding the challenges of protein flexibility in drug design. Expert Opin Drug Discovery. 2015;10(12):1301–13.10.1517/17460441.2015.1094458Search in Google Scholar PubMed
[49] Pinzi L, Rastelli G. Molecular docking: shifting paradigms in drug discovery. Int J Mol Sci. 2019;20(18):4331.10.3390/ijms20184331Search in Google Scholar PubMed PubMed Central
[50] Anton L. Oligomeric structures of metabolic protein assemblies-Structure-function analysis of acetyl-CoA carboxylase and urease. University_of_Basel; 2020. Disponible sur https://edoc.unibas.ch/79024/.Search in Google Scholar
[51] Wu X, Huang T. Recent development in acetyl-CoA carboxylase inhibitors and their potential as novel drugs. Future Med Chem. 2020;12(6):533–61.10.4155/fmc-2019-0312Search in Google Scholar PubMed
[52] Pagadala NS, Syed K, Tuszynski J. Software for molecular docking: a review. Biophys Rev. 2017;9(2):91–102.10.1007/s12551-016-0247-1Search in Google Scholar PubMed PubMed Central
[53] Homeyer N, Gohlke H. Free energy calculations by the molecular mechanics Poisson− Boltzmann surface area method. Mol Inf. 2012;31(2):114–22.10.1002/minf.201100135Search in Google Scholar PubMed
[54] Genheden S, Ryde U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discovery. 2015;10(5):449–61.10.1517/17460441.2015.1032936Search in Google Scholar PubMed PubMed Central
[55] Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30(16):2785–91.10.1002/jcc.21256Search in Google Scholar PubMed PubMed Central
[56] Tapera M, Kekeçmuhammed H, Tüzün B, Daştan SD, Çelik MS, Taslimi P, et al. Novel 1, 2, 4-triazole-maleamic acid derivatives: synthesis and evaluation as anticancer agents with carbonic anhydrase inhibitory activity. J Mol Struct. 2024;1313:138680.10.1016/j.molstruc.2024.138680Search in Google Scholar
[57] Khalilov AN, Cisterna J, Cárdenas A, Tuzun B, Erkan S, Gurbanov AV, et al. Synthesis, crystal structure, Hirshfeld surface analyses, and DFT studies of (S)-2-(3, 5-di‑tert‑butyl‑4-hydroxyphenyl)-3, 3-diethoxy-1-phenylpropan-1-one. J Mol Struct. 2024;1313:138652.10.1016/j.molstruc.2024.138652Search in Google Scholar
[58] Belkheiri A, Dahmani K, Mzioud K, Rbaa M, Galai M, Hmada A, et al. Advanced evaluation of novel quinoline derivatives for corrosion inhibition of mild steel in acidic environments: A comprehensive electrochemical, computational, and surface study. Int J Electrochem Sci. 2024;19(10):100772.10.1016/j.ijoes.2024.100772Search in Google Scholar
[59] Çelik MS, Kütük N, Yenidünya AF, Çetinkaya S, Tüzün B. Removal of safranin O from wastewater using Streptomyces griseobrunneus dead biomass and in silico calculations. Biomass Convers Biorefinery. 2024;14(20):25873–84.10.1007/s13399-023-04558-2Search in Google Scholar
[60] Majumdar D, Chatterjee A, Feizi-Dehnayebi M, Kiran NS, Tuzun B, Mishra D. 8-Aminoquinoline derived two Schiff base platforms: Synthesis, characterization, DFT insights, corrosion inhibitor, molecular docking, and pH-dependent antibacterial study. Heliyon. 2024;10(15):e35591.10.1016/j.heliyon.2024.e35591Search in Google Scholar PubMed PubMed Central
[61] Schrader T, Khanifaev J, Perlt E. Koopmans’ theorem for acidic protons. Chem Commun. 2023;59(93):13839–42.10.1039/D3CC04304ESearch in Google Scholar PubMed
[62] Pearson RG. Hard and soft acids and bases. J Am Chem Soc. 1963;85(22):3533–9.10.1021/ja00905a001Search in Google Scholar
[63] Parr RG, Chattaraj PK. Principle of maximum hardness. J Am Chem Soc. 1991;113(5):1854–5.10.1021/ja00005a072Search in Google Scholar
[64] Ayers PW. An elementary derivation of the hard/soft-acid/base principle. J Chem Phys. 2005;122:141102.10.1063/1.1897374Search in Google Scholar PubMed
[65] Phillips JC. Generalized Koopmans’ theorem. Phys Rev. 1961;123(2):420.10.1103/PhysRev.123.420Search in Google Scholar
[66] Bhat MA, Tüzün B, Koyuncu I, Temiz E, Taslimi P, Naglah AM, et al. Synthesis, molecular modelling and choline esterase enzyme inhibitory activity of novel enaminone derivatives of sulfonamides. Bull Chem Soc Ethiop. 2024;38(5):1351–68.10.4314/bcse.v38i5.13Search in Google Scholar
[67] Zahirović A, Fočak M, Fetahović S, Tüzün B, Višnjevac A, Muzika V, et al. Hydrazone-flavonol based oxidovanadium (V) complexes: Synthesis, characterization and antihyperglycemic activity of chloro derivative in vivo. J Inorg Biochem. 2024;258:112637.10.1016/j.jinorgbio.2024.112637Search in Google Scholar PubMed
[68] Sashidhara KV, Kumar A, Kumar M, Srivastava A, Puri A. Synthesis and antihyperlipidemic activity of novel coumarin bisindole derivatives. Bioorg Med Chem Lett. 2010;20(22):6504–7.10.1016/j.bmcl.2010.09.055Search in Google Scholar PubMed
[69] Sashidhara KV, Kumar A, Kumar M, Sonkar R, Bhatia G, Khanna AK. Novel coumarin derivatives as potential antidyslipidemic agents. Bioorg Med Chem Lett. 2010;20(14):4248–51.10.1016/j.bmcl.2010.05.023Search in Google Scholar PubMed
[70] Al‐Qirim T, Shattat G, Sweidan K, El‐Huneidi W, Sheikha GA, Khalaf RA, et al. In Vivo Antihyperlipidemic activity of a new series of N‐(Benzoylphenyl) and N‐(Acetylphenyl)‐1‐benzofuran‐2‐carboxamides in rats. Arch Pharm. 2012;345(5):401–6.10.1002/ardp.201100225Search in Google Scholar PubMed
[71] El Mesky M, Zgueni H, Rhazi Y, El-Guourrami O, Abchir O, Jabha M, et al. Prediction by DFT and synthesis of new xanthene derivatives: Evaluation of their toxicity and antihyperlipidemic properties in-vivo and in-silico. J Mol Struct. 2024;1313:138705.10.1016/j.molstruc.2024.138705Search in Google Scholar
[72] Azzane A, Eddouks M. Antihyperglycemic, antihyperlipidemic, and antioxidant effects of salvia tingitana in streptozotocin-induced diabetic rats. Cardiovasc Haematol Disord-Drug Targetsrug Targets-Cardiovasc Hematol Disord. 2022;22(2):118–27.10.2174/1871529X22666220806122012Search in Google Scholar PubMed
[73] Azzane A, Farid O, Eddouks M. Antihyperglycemic and antidyslipidemic effects of Artemisia arborescens aqueous extract on streptozotocin-induced diabetic rats. Cardiovasc Hematol Agents Med Chem Former. 2023;21(2):120–38.10.2174/1871525720666220425094135Search in Google Scholar PubMed
[74] Azzane A, Amssayef A, Eddouks M. Antihyperglycemic and antidyslipidemic effect of moricandia suffruticosa in normal and streptozotocin-induced diabetic rats. Cardiovasc Haematol Disord-Drug Targetsrug Targets-Cardiovasc Hematol Disord. 2022;22(1):58–66.10.2174/1871529X22666220513124452Search in Google Scholar PubMed
[75] Elbouny H, Benjamaa R, Ouahzizi B, Azzane A, Bakali AH, Bammou M, et al. Bioactivity of Hertia maroccana and Teucrium malenconianum: antioxidant, antibacterial, and anti-hyperlipidemic effects. Not Sci Biol. 2024;16(4):12095.10.55779/nsb16412095Search in Google Scholar
[76] Amssayef A, Soulaimani B, Abbad I, Ousaaid D, Azzane A, Eddouks M. Study of the antihyperlipidemic effect of Ammodaucus leucotrichus essential oil in rats. Comp Clin Pathol. 2025;1:9.10.1007/s00580-025-03634-5Search in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Phytochemical investigation and evaluation of antioxidant and antidiabetic activities in aqueous extracts of Cedrus atlantica
- Influence of B4C addition on the tribological properties of bronze matrix brake pad materials
- Discovery of the bacterial HslV protease activators as lead molecules with novel mode of action
- Characterization of volatile flavor compounds of cigar with different aging conditions by headspace–gas chromatography–ion mobility spectrometry
- Effective remediation of organic pollutant using Musa acuminata peel extract-assisted iron oxide nanoparticles
- Analysis and health risk assessment of toxic elements in traditional herbal tea infusions
- Cadmium exposure in marine crabs from Jiaxing City, China: Insights into health risk assessment
- Green-synthesized silver nanoparticles of Cinnamomum zeylanicum and their biological activities
- Tetraclinis articulata (Vahl) Mast., Mentha pulegium L., and Thymus zygis L. essential oils: Chemical composition, antioxidant and antifungal properties against postharvest fungal diseases of apple, and in vitro, in vivo, and in silico investigation
- Exploration of plant alkaloids as potential inhibitors of HIV–CD4 binding: Insight into comprehensive in silico approaches
- Recovery of phenylethyl alcohol from aqueous solution by batch adsorption
- Electrochemical approach for monitoring the catalytic action of immobilized catalase
- Green synthesis of ZIF-8 for selective adsorption of dyes in water purification
- Optimization of the conditions for the preparation of povidone iodine using the response surface methodology
- A case study on the influence of soil amendment on ginger oil’s physicochemical properties, mineral contents, microbial load, and HPLC determination of its vitamin level
- Removal of antiviral favipiravir from wastewater using biochar produced from hazelnut shells
- Effect of biochar and soil amendment on bacterial community composition in the root soil and fruit of tomato under greenhouse conditions
- Bioremediation of malachite green dye using Sargassum wightii seaweed and its biological and physicochemical characterization
- Evaluation of natural compounds as folate biosynthesis inhibitors in Mycobacterium leprae using docking, ADMET analysis, and molecular dynamics simulation
- Novel insecticidal properties of bioactive zoochemicals extracted from sea urchin Salmacis virgulata
- Elevational gradients shape total phenolic content and bioactive potential of sweet marjoram (Origanum majorana L.): A comparative study across altitudinal zones
- Study on the CO2 absorption performance of deep eutectic solvents formed by superbase DBN and weak acid diethylene glycol
- Preparation and wastewater treatment performance of zeolite-modified ecological concrete
- Multifunctional chitosan nanoparticles: Zn2+ adsorption, antimicrobial activity, and promotion of aquatic health
- Comparative analysis of nutritional composition and bioactive properties of Chlorella vulgaris and Arthrospira platensis: Implications for functional foods and dietary supplements
- Growth kinetics and mechanical characterization of boride layers formed on Ti6Al4V
- Enhancement of water absorption properties of potassium polyacrylate-based hydrogels in CaCl2-rich soils using potassium di- and tri-carboxylate salts
- Electrochemical and microbiological effects of dumpsite leachates on soil and air quality
- Modeling benzene physicochemical properties using Zagreb upsilon indices
- Characterization and ecological risk assessment of toxic metals in mangrove sediments near Langen Village in Tieshan Bay of Beibu Gulf, China
- Protective effect of Helicteres isora, an efficient candidate on hepatorenal toxicity and management of diabetes in animal models
- Valorization of Juglans regia L. (Walnut) green husk from Jordan: Analysis of fatty acids, phenolics, antioxidant, and cytotoxic activities
- Molecular docking and dynamics simulations of bioactive terpenes from Catharanthus roseus essential oil targeting breast cancer
- Selection of a dam site by using AHP and VIKOR: The Sakarya Basin
- Characterization and modeling of kidney bean shell biochar as adsorbent for caffeine removal from aquatic environments
- The effects of short-term and long-term 2100 MHz radiofrequency radiation on adult rat auditory brainstem response
- Biochemical insights into the anthelmintic and anti-inflammatory potential of sea cucumber extract: In vitro and in silico approaches
- Resveratrol-derived MDM2 inhibitors: Synthesis, characterization, and biological evaluation against MDM2 and HCT-116 cells
- Phytochemical constituents, in vitro antibacterial activity, and computational studies of Sudanese Musa acuminate Colla fruit peel hydro-ethanol extract
- Chemical composition of essential oils reviewed from the height of Cajuput (Melaleuca leucadendron) plantations in Buru Island and Seram Island, Maluku, Indonesia
- Phytochemical analysis and antioxidant activity of Azadirachta indica A. Juss from the Republic of Chad: in vitro and in silico studies
- Stability studies of titanium–carboxylate complexes: A multi-method computational approach
- Efficient adsorption performance of an alginate-based dental material for uranium(vi) removal
- Synthesis and characterization of the Co(ii), Ni(ii), and Cu(ii) complexes with a 1,2,4-triazine derivative ligand
- Evaluation of the impact of music on antioxidant mechanisms and survival in salt-stressed goldfish
- Optimization and validation of UPLC method for dapagliflozin and candesartan cilexetil in an on-demand formulation: Analytical quality by design approach
- Biomass-based cellulose hydroxyapatite nanocomposites for the efficient sequestration of dyes: Kinetics, response surface methodology optimization, and reusability
- Multifunctional nitrogen and boron co-doped carbon dots: A fluorescent probe for Hg2+ and biothiol detection with bioimaging and antifungal applications
- Separation of sulphonamides on a C12-diol mixed-mode HPLC column and investigation of their retention mechanism
- Characterization and antioxidant activity of pectin from lemon peels
- Fast PFAS determination in honey by direct probe electrospray ionization tandem mass spectrometry: A health risk assessment insight
- Correlation study between GC–MS analysis of cigarette aroma compounds and sensory evaluation
- Synthesis, biological evaluation, and molecular docking studies of substituted chromone-2-carboxamide derivatives as anti-breast cancer agents
- The influence of feed space velocity and pressure on the cold flow properties of diesel fuel
- Acid etching behavior and mechanism in acid solution of iron components in basalt fibers
- Protective effect of green synthesized nanoceria on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rat
- Evaluation of the antianxiety activity of green zinc nanoparticles mediated by Boswellia thurifera in albino mice by following the plus maze and light and dark exploration tests
- Yeast as an efficient and eco-friendly bifunctional porogen for biomass-derived nitrogen-doped carbon catalysts in the oxygen reduction reaction
- Novel descriptors for the prediction of molecular properties
- Synthesis and characterization of surfactants derived from phenolphthalein: In vivo and in silico studies of their antihyperlipidemic effect
- Turmeric oil-fortified nutraceutical-SNEDDS: An approach to boost therapeutic effectiveness of dapagliflozin during treatment of diabetic patients
- Analysis and study on volatile flavor compounds of three Yunnan cultivated cigars based on headspace-gas chromatography-ion mobility spectrometry
- Near-infrared IR780 dye-loaded poloxamer 407 micelles: Preparation and in vitro assessment of anticancer activity
- Special Issue on Advancing Sustainable Chemistry for a Greener Future
- One-pot fabrication of highly porous morphology of ferric oxide-ferric oxychloride/poly-O-chloroaniline nanocomposite seeded on poly-1H pyrrole: Photocathode for green hydrogen generation from natural and artificial seawater
- High-efficiency photocathode for green hydrogen generation from sanitation water using bismuthyl chloride/poly-o-chlorobenzeneamine nanocomposite
- Innovative synthesis of cobalt-based catalysts using ionic liquids and deep eutectic solvents: A minireview on electrocatalytic water splitting
- Special Issue on Phytochemicals, Biological and Toxicological Analysis of Plants
- Comparative analysis of fruit quality parameters and volatile compounds in commercially grown citrus cultivars
- Total phenolic, flavonoid, flavonol, and tannin contents as well as antioxidant and antiparasitic activities of aqueous methanol extract of Alhagi graecorum plant used in traditional medicine: Collected in Riyadh, Saudi Arabia
- Study on the pharmacological effects and active compounds of Apocynum venetum L.
- Chemical profile of Senna italica and Senna velutina seed and their pharmacological properties
- Essential oils from Brazilian plants: A literature analysis of anti-inflammatory and antimalarial properties and in silico validation
- Toxicological effects of green tea catechin extract on rat liver: Delineating safe and harmful doses
- Unlocking the potential of Trigonella foenum-graecum L. plant leaf extracts against diabetes-associated hypertension: A proof of concept by in silico studies
Articles in the same Issue
- Research Articles
- Phytochemical investigation and evaluation of antioxidant and antidiabetic activities in aqueous extracts of Cedrus atlantica
- Influence of B4C addition on the tribological properties of bronze matrix brake pad materials
- Discovery of the bacterial HslV protease activators as lead molecules with novel mode of action
- Characterization of volatile flavor compounds of cigar with different aging conditions by headspace–gas chromatography–ion mobility spectrometry
- Effective remediation of organic pollutant using Musa acuminata peel extract-assisted iron oxide nanoparticles
- Analysis and health risk assessment of toxic elements in traditional herbal tea infusions
- Cadmium exposure in marine crabs from Jiaxing City, China: Insights into health risk assessment
- Green-synthesized silver nanoparticles of Cinnamomum zeylanicum and their biological activities
- Tetraclinis articulata (Vahl) Mast., Mentha pulegium L., and Thymus zygis L. essential oils: Chemical composition, antioxidant and antifungal properties against postharvest fungal diseases of apple, and in vitro, in vivo, and in silico investigation
- Exploration of plant alkaloids as potential inhibitors of HIV–CD4 binding: Insight into comprehensive in silico approaches
- Recovery of phenylethyl alcohol from aqueous solution by batch adsorption
- Electrochemical approach for monitoring the catalytic action of immobilized catalase
- Green synthesis of ZIF-8 for selective adsorption of dyes in water purification
- Optimization of the conditions for the preparation of povidone iodine using the response surface methodology
- A case study on the influence of soil amendment on ginger oil’s physicochemical properties, mineral contents, microbial load, and HPLC determination of its vitamin level
- Removal of antiviral favipiravir from wastewater using biochar produced from hazelnut shells
- Effect of biochar and soil amendment on bacterial community composition in the root soil and fruit of tomato under greenhouse conditions
- Bioremediation of malachite green dye using Sargassum wightii seaweed and its biological and physicochemical characterization
- Evaluation of natural compounds as folate biosynthesis inhibitors in Mycobacterium leprae using docking, ADMET analysis, and molecular dynamics simulation
- Novel insecticidal properties of bioactive zoochemicals extracted from sea urchin Salmacis virgulata
- Elevational gradients shape total phenolic content and bioactive potential of sweet marjoram (Origanum majorana L.): A comparative study across altitudinal zones
- Study on the CO2 absorption performance of deep eutectic solvents formed by superbase DBN and weak acid diethylene glycol
- Preparation and wastewater treatment performance of zeolite-modified ecological concrete
- Multifunctional chitosan nanoparticles: Zn2+ adsorption, antimicrobial activity, and promotion of aquatic health
- Comparative analysis of nutritional composition and bioactive properties of Chlorella vulgaris and Arthrospira platensis: Implications for functional foods and dietary supplements
- Growth kinetics and mechanical characterization of boride layers formed on Ti6Al4V
- Enhancement of water absorption properties of potassium polyacrylate-based hydrogels in CaCl2-rich soils using potassium di- and tri-carboxylate salts
- Electrochemical and microbiological effects of dumpsite leachates on soil and air quality
- Modeling benzene physicochemical properties using Zagreb upsilon indices
- Characterization and ecological risk assessment of toxic metals in mangrove sediments near Langen Village in Tieshan Bay of Beibu Gulf, China
- Protective effect of Helicteres isora, an efficient candidate on hepatorenal toxicity and management of diabetes in animal models
- Valorization of Juglans regia L. (Walnut) green husk from Jordan: Analysis of fatty acids, phenolics, antioxidant, and cytotoxic activities
- Molecular docking and dynamics simulations of bioactive terpenes from Catharanthus roseus essential oil targeting breast cancer
- Selection of a dam site by using AHP and VIKOR: The Sakarya Basin
- Characterization and modeling of kidney bean shell biochar as adsorbent for caffeine removal from aquatic environments
- The effects of short-term and long-term 2100 MHz radiofrequency radiation on adult rat auditory brainstem response
- Biochemical insights into the anthelmintic and anti-inflammatory potential of sea cucumber extract: In vitro and in silico approaches
- Resveratrol-derived MDM2 inhibitors: Synthesis, characterization, and biological evaluation against MDM2 and HCT-116 cells
- Phytochemical constituents, in vitro antibacterial activity, and computational studies of Sudanese Musa acuminate Colla fruit peel hydro-ethanol extract
- Chemical composition of essential oils reviewed from the height of Cajuput (Melaleuca leucadendron) plantations in Buru Island and Seram Island, Maluku, Indonesia
- Phytochemical analysis and antioxidant activity of Azadirachta indica A. Juss from the Republic of Chad: in vitro and in silico studies
- Stability studies of titanium–carboxylate complexes: A multi-method computational approach
- Efficient adsorption performance of an alginate-based dental material for uranium(vi) removal
- Synthesis and characterization of the Co(ii), Ni(ii), and Cu(ii) complexes with a 1,2,4-triazine derivative ligand
- Evaluation of the impact of music on antioxidant mechanisms and survival in salt-stressed goldfish
- Optimization and validation of UPLC method for dapagliflozin and candesartan cilexetil in an on-demand formulation: Analytical quality by design approach
- Biomass-based cellulose hydroxyapatite nanocomposites for the efficient sequestration of dyes: Kinetics, response surface methodology optimization, and reusability
- Multifunctional nitrogen and boron co-doped carbon dots: A fluorescent probe for Hg2+ and biothiol detection with bioimaging and antifungal applications
- Separation of sulphonamides on a C12-diol mixed-mode HPLC column and investigation of their retention mechanism
- Characterization and antioxidant activity of pectin from lemon peels
- Fast PFAS determination in honey by direct probe electrospray ionization tandem mass spectrometry: A health risk assessment insight
- Correlation study between GC–MS analysis of cigarette aroma compounds and sensory evaluation
- Synthesis, biological evaluation, and molecular docking studies of substituted chromone-2-carboxamide derivatives as anti-breast cancer agents
- The influence of feed space velocity and pressure on the cold flow properties of diesel fuel
- Acid etching behavior and mechanism in acid solution of iron components in basalt fibers
- Protective effect of green synthesized nanoceria on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rat
- Evaluation of the antianxiety activity of green zinc nanoparticles mediated by Boswellia thurifera in albino mice by following the plus maze and light and dark exploration tests
- Yeast as an efficient and eco-friendly bifunctional porogen for biomass-derived nitrogen-doped carbon catalysts in the oxygen reduction reaction
- Novel descriptors for the prediction of molecular properties
- Synthesis and characterization of surfactants derived from phenolphthalein: In vivo and in silico studies of their antihyperlipidemic effect
- Turmeric oil-fortified nutraceutical-SNEDDS: An approach to boost therapeutic effectiveness of dapagliflozin during treatment of diabetic patients
- Analysis and study on volatile flavor compounds of three Yunnan cultivated cigars based on headspace-gas chromatography-ion mobility spectrometry
- Near-infrared IR780 dye-loaded poloxamer 407 micelles: Preparation and in vitro assessment of anticancer activity
- Special Issue on Advancing Sustainable Chemistry for a Greener Future
- One-pot fabrication of highly porous morphology of ferric oxide-ferric oxychloride/poly-O-chloroaniline nanocomposite seeded on poly-1H pyrrole: Photocathode for green hydrogen generation from natural and artificial seawater
- High-efficiency photocathode for green hydrogen generation from sanitation water using bismuthyl chloride/poly-o-chlorobenzeneamine nanocomposite
- Innovative synthesis of cobalt-based catalysts using ionic liquids and deep eutectic solvents: A minireview on electrocatalytic water splitting
- Special Issue on Phytochemicals, Biological and Toxicological Analysis of Plants
- Comparative analysis of fruit quality parameters and volatile compounds in commercially grown citrus cultivars
- Total phenolic, flavonoid, flavonol, and tannin contents as well as antioxidant and antiparasitic activities of aqueous methanol extract of Alhagi graecorum plant used in traditional medicine: Collected in Riyadh, Saudi Arabia
- Study on the pharmacological effects and active compounds of Apocynum venetum L.
- Chemical profile of Senna italica and Senna velutina seed and their pharmacological properties
- Essential oils from Brazilian plants: A literature analysis of anti-inflammatory and antimalarial properties and in silico validation
- Toxicological effects of green tea catechin extract on rat liver: Delineating safe and harmful doses
- Unlocking the potential of Trigonella foenum-graecum L. plant leaf extracts against diabetes-associated hypertension: A proof of concept by in silico studies

