Abstract
Introduction
Denture cleaners should not be harmful to dental prostheses elements, but immersions in cleaner solutions during a long time of using prosthesis may cause changes on Co–Cr alloy surfaces. There are five classes of denture cleaners: alkaline hypochlorites, alkaline peroxides, disinfectants, diluted acids, and enzymes. The aim of this work was to evaluate the influence of denture cleaners solutions on the surface properties of Co–Cr alloy.
Materials and method
Specimens cast from cobalt–chromium alloy were divided into eight groups: 1 – dry; 2 – ultrapure water; 3 – 20% wt/wt sodium; 4 – 20% chlorhexidine digluconate; 5 – Correga Tabs BioFormula; 6 – 20% wt/wt citric acid; 7 – 0.5% NaOCl; and 8 – 5.0% NaOCl. After immersion in 200 mL of cleaning agent solution at 45°C in 3 years, simulation of use, surface roughness, contact angle, surface free energy (SFE), and microscopic observation was performed.
Results
For citric acid and NaOCl, roughness (R a) raised clearly. These cleaning agents also caused R q, R v, R p, and R Sm to increase the most. The observed water contact angle after using denture cleaners, especially citric acid, and NaOCl decreases, and the values of SFE increase. Under a digital microscope, the harmful effect of citric acid and solutions of NaOCl was visible.
Conclusions
Diluted acids and alkaline hypochlorites in presented concentrations influence Co–Cr surface parameters like roughness and wettability. Other classes of denture cleaning agents do not affect surface roughness parameters which make them safer for the metallic components of removable partial dentures.
1 Introduction
Dental prostheses are made of various metal alloys. Precious metal alloys such as platinum, gold, and silver have good chemical resistance and biocompatibility; unfortunately, they have a high price and lower strength. Base metal alloys such as chromium and cobalt characterized with higher hardness have better mechanical strength and other properties. Easy-to-process materials with better melting and ductility properties are also preferable for making prostheses. In the oral cavity environment, they must be highly resistant to corrosion. Resistance to oxidation and corrosion makes Co–Cr alloys suitable for use in the oral environment [1]. One of the best ways to clean dentures is a combination of mechanical and chemical cleaning like brushing or using ultrasonic cleaners, or soaking in dental cleaners solutions [2]. One challenge for these alloys is the chemical resistance to cleaning agents used for a prosthesis. Regular contact with harmful substances from prosthesis cleaners may affect alloy surface properties. The removable partial denture (RPD) frameworks can corrode or stain in contact with oxide or chlorine present in cleaners [3]. The ideal denture cleaner should not cause harmful effects to the cobalt–chromium alloy and other parts of the denture like surface pitting or surface discoloration (tarnish) [4]. It should also act quickly, be non-toxic, easy to use, cheap, have antimicrobial properties, reduce odors and discoloration [5]. There are five classes of denture cleaners: alkaline hypochlorites, alkaline peroxides, diluted acids, enzymes, and disinfectants [3]. They are available in different forms: creams, pastes, gels, solutions, tabs, or soluble powders. Denture users prefer the chemical method for cleaning because it is easier and more comfortable than brushing [6,7]. The most common denture cleaners are alkaline peroxides such as sodium perborate, potassium monopersulfate, sodium bicarbonate, and others. Due to the alkaline pH of over 8.5 and generation of active oxide in contact with water, contaminations dissolve easily [2,5,8]. Alkaline hypochlorites are very effective against biofilm compared to peroxides or enzymes. It is worth remembering that hypochlorite concentration should be below 0.5% because higher concentrations adversely affect materials such as acrylics and alloys used in the manufacture of prostheses [9]. NaOCl directly reduces biofilm and removes discoloration, food debris, or tartar [2,10]. Diluted acid denture cleaners contain at least one acid and have a pH below 7. These can be organic or inorganic acids or substances that generate acid during use. They are a good disinfectant and also good at reducing mineral contamination. The most common acid used in this cleaning agent class is citric acid. The second most popular compound is acetic acid. The decrease in the pH of the solution during cleaning kills or stops the growth of microorganisms [11]. To disinfectants as denture cleaning agents class belong to chlorhexidine acetate or digluconate, salicylates, chloroform, ethanol, isopropyl alcohol, or 2% glutaric aldehyde [2,8]. The fifth class of denture cleaning agents is enzymes. They reduce food residues, bacteria plaque, and fungi. In example mutanase or protease act on glycoproteins, polysaccharides, or mucoproteins present in biofilm and have higher effectiveness than alkaline peroxides. One of the advantages of this group is that they are mild to the denture materials [7,12,13].
The average time of use of an RPD is 5 years. During this time, improper cleaning of the RPD can lead to degradation or corrosion of the prosthetic materials, especially in contact with chlorine or oxygen ions. Besides corrosion or color change, cleaners also affect the mechanical strength of Co–Cr, surface roughness and reflectance, lower hardness, loss of retention, and undesirable ion release. Side effects of this harmful acting of immersion-type cleaners may cause biofilm growth and better adhesion to prosthesis [3,14,15,16,17,18,19,20,21,22].
The study aims to compare the effect of five classes of denture cleaning agents on selected properties of the Co–Cr alloy used for RPDs. The tests assessed changes in surface roughness, wettability, and microscopic evaluation of the surface.
2 Methods
Samples of Co–Cr alloy (Wironit extrahart, BEGO, Germany) were cast according to manufacturer instructions. In the next step, they were wet ground with SiC papers (grit P180, P360, P1200, and P2400) and polished with diamond paste (grit, 6 μm). Ultrasonically cleaned isopropyl alcohol samples were divided into eight groups (Table 1).
Description of the solution for Co–Cr immersion
| Group number | Class of denture cleaning solution | Solution | Manufacturer |
|---|---|---|---|
| 1 | — | Negative control – dry; storage in air | — |
| 2 | — | Ultrapure water (Millipore Direct Q3) | Merck, Poland |
| 3 | Alkaline peroxides | 20% Sodium percarbonate p.a. | Biomus, Poland |
| 4 | Disinfectants | 20% Chlorhexidine digluconate | Amara, Poland |
| 5 | Enzymes | Correga Tabs BioFormula | gsk, United Kingdom |
| 6 | Diluted acids | 20% Citric acid p.a. | Stanlab, Poland |
| 7 | Alkaline hypochlorites | 0.5% Sodium hypochlorite p.a. | Chempur, Poland |
| 8 | Alkaline hypochlorites | Positive control −5.0% sodium hypochlorite p.a. | Chempur, Poland |
Dry samples were chosen as a negative control group in order to eliminate water which may cause a corrosive environment for cobalt–chromium alloy. Ultrapure water does not act as (strong as) an electrolyte solution. 20% Chlorhexidine digluconate is a commercially used disinfectant. Corega Tabs BioFormula is a commercial denture cleaner that contains the enzyme – subtilisin (protease); 20% concentration of citric acid and sodium perborate corresponds to concentrations of these substances in commercially used denture cleaners. However, we wanted to eliminate possible reactions of other substances added to market products. Commonly used denture cleaner’s concentration is 0.5% sodium hypochlorite but it is worth knowing that minimal antibacterial concentration is 0.05% NaOCl; 5.0% concentration of sodium hypochlorite was used as a positive group because it tends to be corrosive. After preparing cleaning solutions, samples were stored in 200 mL of each cleaning agent at a temperature of 45°C for 92 h. Immersion time corresponds to 1,095 cycles of cleaning, assuming the patient put a denture into a cleaner solution once a day for 5 min during 3 years period. A five roughness parameters (μm): R a – arithmetic average height defined as the arithmetic mean of the absolute values of the evaluation profile deviations from the mean line, R q – root mean square roughness, which is the square root of the arithmetic mean of the squares of the deviations from the mean line to the evaluation profile, R v – maximum depth of valleys, when dividing the evaluation profile into segments based on the sampling length. Then for each segment, the distance of the lowest point (R vi) from the mean lines obtained. R is the mean of the R vi values that we obtained from the segments. R v (ANSI) is defined as the maximum floor depth over the evaluation length, and R p is the maximum height of peaks calculated as divided by the evaluation profile into segments based on the sampling length. Then, for each segment, the distance of the highest point (R pi) from the mean line is obtained. R p is the mean of the R pi values that were obtained from the segments, and R Sm is the mean spacing at a mean line. Portions of the evaluation profile that project upward are called profile element mountains, and portions of the profile that project downwards are called profile element valleys. A mountain followed by a valley is called a “profile element.” The value of this parameter is the arithmetic mean of the width (Xs) of each profile element. Each parameter was measured with a contact profilometer (Surftest SJ- 410, Mitutoyo, Japan). On each sample, nine measurements were made. The Owens-Wendt method was used for calculating surface free energy (SFE). According to the theory, SFE is a work needed to separate two phases from the equilibrium state to create a new surface. SFE γ is a sum of dispersive γ D and polar component γ P (equation (1))
Dispersive component is calculated according to equation (2):
and polar component, as taken below (equation (3)):
where
The sessile drop method was used to measure water and diiodomethane (Sigma Aldrich, USA) contact angle. Captured images of drops were analyzed by ImageJ software with Contact Angle plug-in (National Institutes of Health, Bethesda, USA). A surface topography (2D and 3D analyses) was assessed by using a digital microscope (VHX, Keyence, Belgium) under ×1,500 magnification. To make more detailed surface observations, samples were observed under scanning electron microscope-energy-dispersive spectroscopy (SEM–EDS) microscope Hitachi S-4700. The images were made at a magnification of 2,000×, 5,000×, and 10,000×.
Analyzed numerical traits were depicted by their arithmetical weighted mean, standard deviation, 95% confidence interval, and minimum-to-maximum values. The normality of distribution was appraised by using the W Shapiro–Wilk test. The homogeneity of variances was tested by using Levene’s test. The Kruskal–Wallis one-way analysis of variance was fitted in order to assess the statistical significance of differences in the roughness of investigated surfaces treated with selected agents. Multiple comparisons were carried out a posteriori in search of specific pairs showing statistically meaningful dissimilarities.
A level of p < 0.05 was deemed statistically significant. All the statistical procedures were performed by using IBM® SPSS® Statistics, version 28 (Armonk, New York, USA).
3 Results
3.1 Roughness
Nine measurements per sample were made with a contact profilometer. A Gaussian filter was used to separate roughness from waviness and surface errors. Six roughness parameters were calculated: R a, R q, R p, R v, R Sm, and R mr (Tables 2–7). After immersion in different denture cleaners, all samples have a smooth surface with R a under 0.5 µm [23]. In the case of immersion in citric acid or sodium hypochlorite, the environment increases the surface roughness. The R a parameter is around twice higher compared with other groups (Table 2). R q parameter changes also show the highest value after storage Co–Cr alloy in a citric acid or NaOCl environment, and the difference is about twice higher (Table 3). Using citric acid or sodium hypochlorite causes a slight increase in R v value (Table 5). Also, about three times higher peaks R p can be observed on sample surfaces after immersion in the solutions mentioned earlier (Table 4). In 0.05% NaOCl solution, R Sm increased more than three times, compared with the negative control group (Table 6). We do not observe any significant changes in parameters R Lo and R mr (25%) (Table 7).
Descriptive statistics for R a measurements outcome
| No. | R a (µm) | Statistical parameter | p-Value | |||
|---|---|---|---|---|---|---|
| M | SD | 95% CI | Min. – max. | |||
| 1 | Dry | 0.016 | 0.001 | 0.015–0.017 | 0.014–0.018 | <0.001 |
| 2 | Ultrapure water | 0.015 | 0.001 | 0.013–0.016 | 0.013–0.016 | |
| 3 | 20% Sodium percarbonate | 0.014 | 0.001 | 0.013–0.015 | 0.012–0.016 | |
| 4 | 20% Chlorhexidine digluconate | 0.017 | 0.001 | 0.016–0.018 | 0.015–0.019 | |
| 5 | Correga Tabs BioFormula | 0.019 | 0.001 | 0.018–0.019 | 0.017–0.020 | |
| 6 | 20% Citric acid | 0.024 | 0.001 | 0.016–0.032 | 0.017–0.054 | |
| 7 | 0.5% Sodium hypochlorite | 0.029 | 0.003 | 0.027–0.031 | 0.023–0.031 | |
| 8 | 5.0% Sodium hypochlorite | 0.037 | 0.009 | 0.030–0.040 | 0.028–0.057 | |
Post-hoc multiple comparisons: (1) versus (6)–(8) at p < 0.001; (2) versus (6)–(8) at p < 0.001; (3) versus (6)–(8) at p < 0.001; (4) versus (6)–(8) at p < 0.001; (5) versus (6)–(8) at p < 0.001; (6) versus (1)–(5) at p < 0.001; (7) versus (1)–(5) at p < 0.001; (8) versus (1)–(5) at p < 0.001.
Descriptive statistics for R q measurements outcome
| No. | R q (µm) | Statistical parameter | p-Value | |||
|---|---|---|---|---|---|---|
| M | SD | 95% CI | Min. – max. | |||
| 1 | Dry | 0.020 | 0.002 | 0.019–0.021 | 0.017–0.023 | <0.001 |
| 2 | Ultrapure water | 0.020 | 0.002 | 0.018–0.021 | 0.017–0.023 | |
| 3 | 20% Sodium percarbonate | 0.018 | 0.001 | 0.016–0.019 | 0.016–0.020 | |
| 4 | 20% Chlorhexidine digluconate | 0.022 | 0.002 | 0.020–0.023 | 0.019–0.024 | |
| 5 | Correga Tabs BioFormula | 0.025 | 0.003 | 0.022–0.027 | 0.021–0.032 | |
| 6 | 20% Citric acid | 0.051 | 0.024 | 0.032–0.070 | 0.024–0.091 | |
| 7 | 0.5% Sodium hypochlorite | 0.045 | 0.004 | 0.042–0.048 | 0.036–0.050 | |
| 8 | 5.0% sodium hypochlorite | 0.054 | 0.012 | 0.044–0.064 | 0.041–0.079 | |
Post-hoc multiple comparisons: (1) versus (6)–(8) at p < 0.001; (2) versus (6)–(8) at p < 0.001; (3) versus (6)–(8) at p < 0.001; (4) versus (6)–(8) at p < 0.001; (5) versus (6)–(8) at p < 0.001; (6) versus (1)–(5) at p < 0.001; (7) versus (1)–(5) at p < 0.001; and (8) versus (1)–(5) at p < 0.001.
Descriptive statistics for R p measurements outcome
| No. | R p (µm) | Statistical parameter | p-Value | |||
|---|---|---|---|---|---|---|
| M | SD | 95% CI | Min. – max. | |||
| 1 | Dry | 0.070 | 0.008 | 0.064–0.077 | 0.060–0.082 | <0.001 |
| 2 | Ultrapure water | 0.077 | 0.011 | 0.069–0.086 | 0.063–0.088 | |
| 3 | 20% Sodium percarbonate | 0.068 | 0.007 | 0.062–0.074 | 0.060–0.081 | |
| 4 | 20% Chlorhexidine digluconate | 0.077 | 0.021 | 0.061–0.093 | 0.024–0.091 | |
| 5 | Correga Tabs BioFormula | 0.088 | 0.006 | 0.084–0.093 | 0.078–0.095 | |
| 6 | 20% Citric acid | 0.164 | 0.050 | 0.123–0.206 | 0.095–0.267 | |
| 7 | 0.5% Sodium hypochlorite | 0.250 | 0.043 | 0.218–0.283 | 0.183–0.317 | |
| 8 | 5.0% Sodium hypochlorite | 0.272 | 0.057 | 0.229–0.317 | 0.213–0.359 | |
Post-hoc multiple comparisons: (1) versus (6)–(8) at p < 0.001; (2) versus (6)–(8) at p < 0.001; (3) versus (6)–(8) at p < 0.001; (4) versus (6)–(8) at p < 0.001; (5) versus (6)–(8) at p < 0.001; (6) versus (1), (2), (3), (4), (5), (6), and (7) at p < 0.001; (7) versus (1), (2), (3), (4, (5), and (6) at p < 0.001; and (8) versus (1), (2), (3), (4), (5), and (6) at p < 0.001.
Descriptive statistics for R v measurements outcome
| No. | R v (µm) | Statistical parameter | p-Value | |||
|---|---|---|---|---|---|---|
| M | SD | 95% CI | Min. – max. | |||
| 1 | Dry | 0.063 | 0.010 | 0.055–0.070 | 0.050–0.085 | <0.001 |
| 2 | Ultrapure water | 0.083 | 0.016 | 0.066–0.100 | 0.062–0.110 | |
| 3 | 20% Sodium percarbonate | 0.067 | 0.011 | 0.058–0.075 | 0.049–0.082 | |
| 4 | 20% Chlorhexidine digluconate | 0.067 | 0.013 | 0.057-0.076 | 0.042–0.085 | |
| 5 | Correga Tabs BioFormula | 0.091 | 0.037 | 0.062–0.120 | 0.067–0.189 | |
| 6 | 20% Citric acid | 0.122 | 0.055 | 0.076–0.169 | 0.070–0.225 | |
| 7 | 0.5% Sodium hypochlorite | 0.084 | 0.021 | 0.068–0.101 | 0.064–0.121 | |
| 8 | 5.0% Sodium hypochlorite | 0.096 | 0.023 | 0.078–0114 | 0.072–0.143 | |
Post-hoc multiple comparisons: (1) versus (5) and (6) at p < 0.001; (2) versus (6) at p < 0.001; (3) versus (6) at p < 0.001; (4) versus (6) at p < 0.001; (5) versus (1) and (6) at p = 0.030; (6) versus (1)–(5) and (7) at p < 0.001; (7) versus (6) at p = 0.006; and (8) versus (1), (3), and (4) at p = 0.027.
Descriptive statistics for R Sm measurements outcome
| No. | R Sm (µm) | Statistical parameter | p-Value | |||
|---|---|---|---|---|---|---|
| M | SD | 95% CI | Min. – max. | |||
| 1 | Dry | 27.4 | 9.0 | 20.5–34.3 | 20.6–50.0 | <0.001 |
| 2 | Ultrapure water | 26.1 | 5.3 | 21.2–31.0 | 19.3–35.7 | |
| 3 | 20% Sodium percarbonate | 29.1 | 10.9 | 20.7–37.5 | 19.5–49.7 | |
| 4 | 20% Chlorhexidine digluconate | 22.5 | 3.1 | 20.1–24.9 | 18.3–28.6 | |
| 5 | Correga Tabs BioFormula | 33.2 | 22.0 | 16.2–50.1 | 21.4–90.4 | |
| 6 | 20% Citric acid | 58.2 | 29.0 | 27.7–88.7 | 28.2–100.9 | |
| 7 | 0.5% Sodium hypochlorite | 99.3 | 34.5 | 72.8–125.9 | 59.3–178.7 | |
| 8 | 5.0% Sodium hypochlorite | 62.6 | 14.5 | 51.5073.8 | 49.1–90.9 | |
Post-hoc multiple comparisons: (1) versus (6)–(8) at p < 0.001; (2) versus (6)–(8) at p < 0.001; (3) versus (6)–(8) at p < 0.001; (4) versus (6)–(8) at p = 0.015; (5) versus (6)–(8) at p = 0.015; (6) versus (1), (2), (3), (4), (5) and (7) at p = 0.005; (7) versus (1)–(6) and (8) at p < 0.001; and (8) versus (1)–(5) and (7) at p < 0.001.
Descriptive statistics for R mr (25%) measurements outcome
| No. | R mr (25%) | Statistical parameter | p-Value | |||
|---|---|---|---|---|---|---|
| M | SD | 95% CI | Min. – max. | |||
| 1 | Dry | 99.9 | 0.1 | 99.8–100.0 | 99.7–100.0 | =0.002 |
| 2 | Ultrapure water | 99.3 | 1.1 | 98.4–100.1 | 97.2–100.0 | |
| 3 | 20% Sodium percarbonate | 99.9 | 0.1 | 99.9–100.0 | 99.8–100.0 | |
| 4 | 20% Chlorhexidine digluconate | 100.0 | 0.04 | 99.9–100.0 | 99.9–100.0 | |
| 5 | Correga Tabs BioFormula | 99.8 | 0.3 | 99.6–100.0 | 99.3–100.0 | |
| 6 | 20% Citric acid | 97.0 | 2.8 | 94.8–99.1 | 91.9–100.0 | |
| 7 | 0.5% Sodium hypochlorite | 99.8 | 0.2 | 99.6–100.0 | 99.4–100.0 | |
| 8 | 5.0% Sodium hypochlorite | 99.6 | 0.4 | 99.3–99.9 | 99.0–100.0 | |
Post-hoc multiple comparisons: (6) versus all the remaining compounds at p < 0.001.
3.1.1 Contact angle and SFE analysis
Table 8 shows the influence of different cleaning solutions on contact angle changes on Co–Cr samples. In both cases, when using water or diiodomethane, the contact angle between the solid and fluid phases decreases. For water, it drops from 75.5° for the dry group to 13.5° for the group stored in 5.0% sodium hypochlorite. As with changes in surface roughness, the use of a weak acid such as, for example, citric acid or a hypochlorite solution causes greater changes in the amount of SFE than with the other groups of cleaning agents. The same relationship can be observed to diiodomethane contact angle changes – it varies from 68.0° for the dry group to 14.6° for the positive control group. According to measured values, all samples have hydrophilic surfaces.
Contact angle of water and diodomethane (mean with standard deviation) on Co–Cr surface after conditioning in dental cleaners
| Group | Water contact angle, θ (°) | Diiodomethane contact angle, θ (°) |
|---|---|---|
| Dry (negative control) | 75.7 ± 6.7 | 68.0 ± 9.4 |
| Water | 63.3 ± 6.7 | 56.7 ± 3.3 |
| 20% Sodium percarbonate | 48.5 ± 10.8 | 40.1 ± 3.3 |
| 20% Chlorhexidine digluconate | 40.0 ± 1.8 | 38.3 ± 3.6 |
| Corega Tabs Bio Formula | 28.5 ± 6.2 | 42.4 ± 8.2 |
| 20% Citric acid | 28.9 ± 2.9 | 40.0 ± 9.6 |
| 0.5% Sodium hypochlorite | 18.1 ± 2.8 | 24.5 ± 3.8 |
| 5.0% Sodium hypochlorite (positive control) | 13.5 ± 2.4 | 14.6 ± 2.4 |
Calculated SFE values for each group are shown in Table 9. The SFE is the highest for the group cleaned with 5.0% NaOCl. Every denture cleaning solution causes an increase in dispersive and polar components alike. However, in the case of Corega Tabs Bio Formula (enzyme), 20% citric acid (diluted acid) and 0.5% NaOCl (alkaline hypochlorite) polar components rise more than three times. This tendency also corresponds with the increased value of roughness parameters (especially R Sm) in the same groups.
SFE and its components of Co–Cr alloy after immersion in different denture cleaners
| Group | SFE (mJ/m2) | γ d (mJ/m2) | γ p (mJ/m2) |
|---|---|---|---|
| Dry (negative control) | 30.775 | 17.948 | 12.827 |
| Water | 40.732 | 18.525 | 22.206 |
| 20% Sodium percarbonate | 53.371 | 28.675 | 24.696 |
| 20% Chlorhexidine digluconate | 58.651 | 28.174 | 30.477 |
| Corega Tabs Bio Formula | 64.580 | 24.654 | 39.926 |
| 20% citric acid | 64.621 | 25.870 | 38.752 |
| 0.5% Sodium hypochlorite | 70.742 | 31.359 | 39.384 |
| 5.0% Sodium hypochlorite (positive control) | 72.781 | 38.937 | 33.844 |
The increasing value of SFE testifies to the increasing wettability of Co–Cr alloys after cleaning dentures, especially in weak acids and alkaline hypochlorites.
3.1.2 Microstructure
The Co–Cr alloy surface topography after storing in different denture cleaners is shown in Figure 1. Except for samples stored in NaOCl solutions, microstructures look similar. Some stains and surface discoloration on Co–Cr alloy stored in sodium hypochlorite solutions can be observed (Figure 1(7)). With increasing concentrations of NaOCl, dark structures occur in bigger areas (Figure 1(8)).
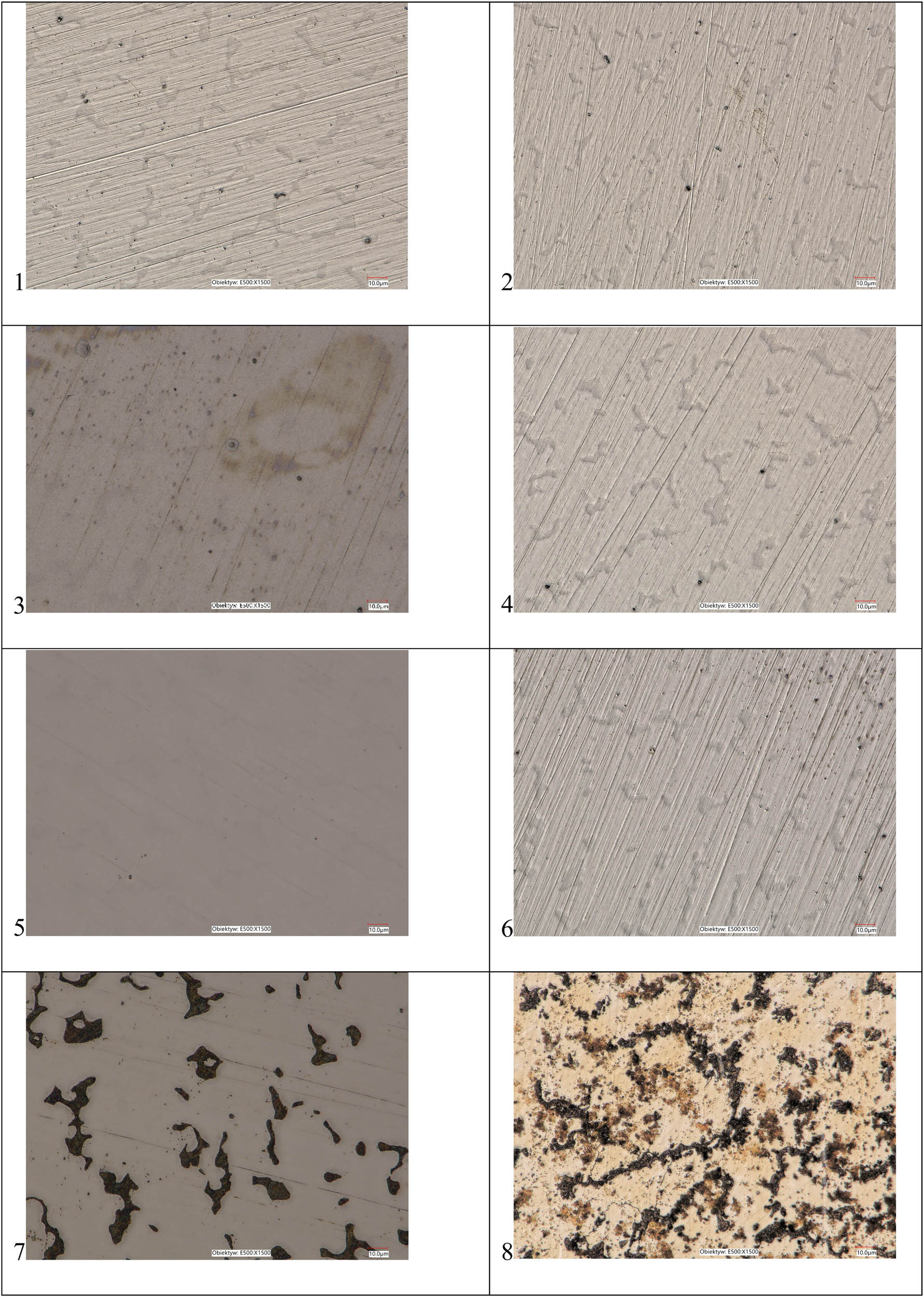
Digital microscopy images of Co–Cr alloy after storage: (1) dry, (2) in water, (3) 20% sodium percarbonate, (4) 20% chlorhexidine digluconate, (5) Corega Tabs Bio Formula, (6) 20% citric acid, (7) 0.5% NaOCl, and (8) 5.0% NaOCl. Magnification 1,500×.
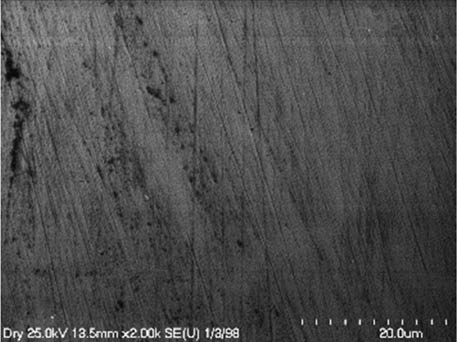
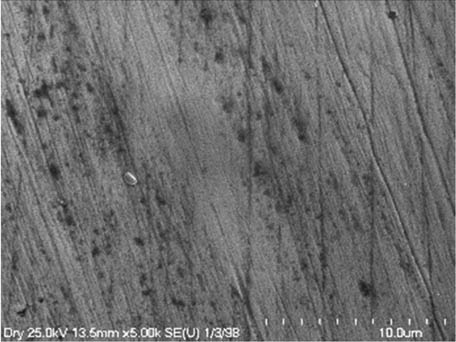
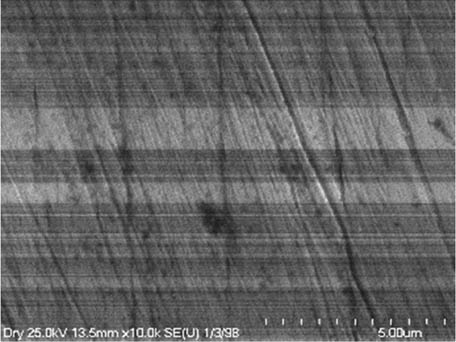
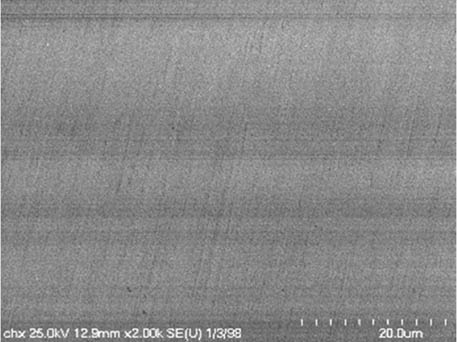
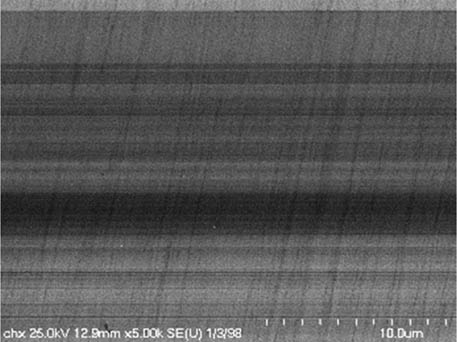
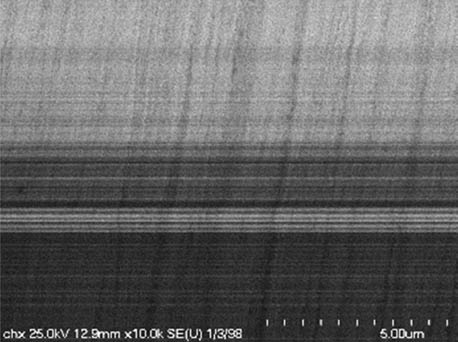
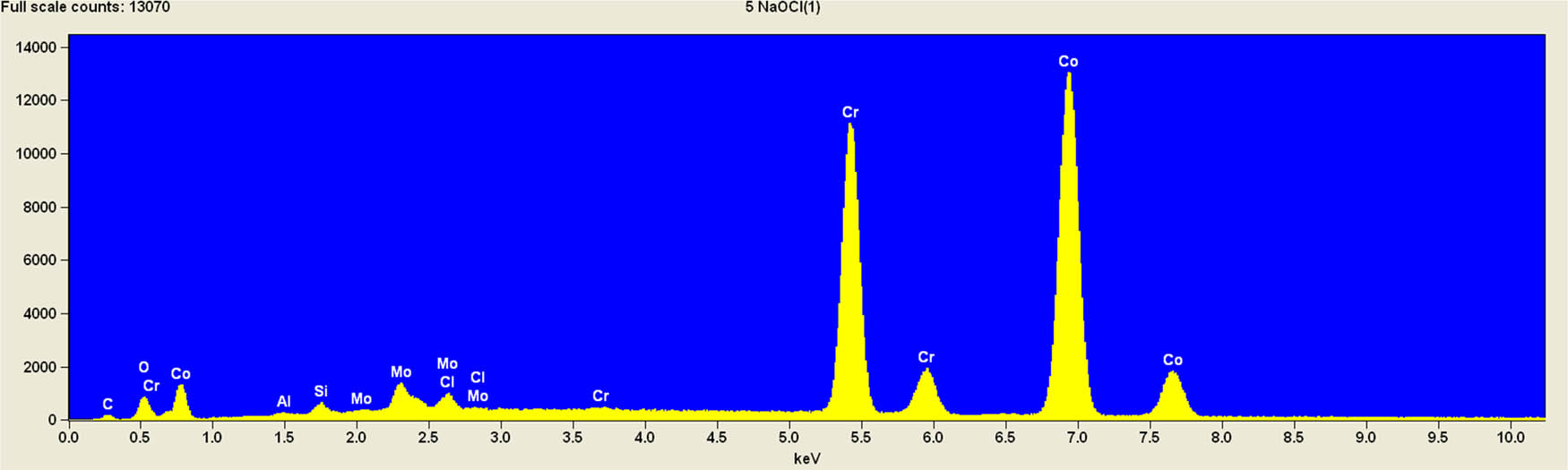
It is worth mentioning that SEM–EDS analysis (Table 10) shows that samples’ surfaces are without visible signs of the corrosive effect of denture cleaners. The exception is for samples stored in the 5.0% sodium hypochlorite solution. However, this is a positive control because of the high concentration of NaOCl which should act harmful for Co–Cr alloy. Some stains are visible in samples stored in 20% sodium percarbonate and 20% citric acid solution; however, EDS analysis did not show any changes in elements composition. Only in the positive control group, oxygen and chlorine occurred at EDS spectrums (Figure 2). We did not observe these elements even in a group with 0.5% NaOCl (Figure 3) or in the negative control group stored in a dry environment (Figure 4). This observation suggests that all classes of denture cleaning agents are safe for Co–Cr alloy during the simulation of 3 years using RPDs.
SEM analysis of Co–Cr samples stored in different solutions at magnifications 2,000×, 5,000×, 10,000×
| Magnification 2,000× | Magnification 5,000× | Magnification 10,000× | |
|---|---|---|---|
| Dry |
|
|
|
| Water |
|
|
|
| 20% Sodium percarbonate |

|
|

|
| 20% Chlorhexidine digluconate |
|
|
|
| Corega Tabs Bio Formula |

|
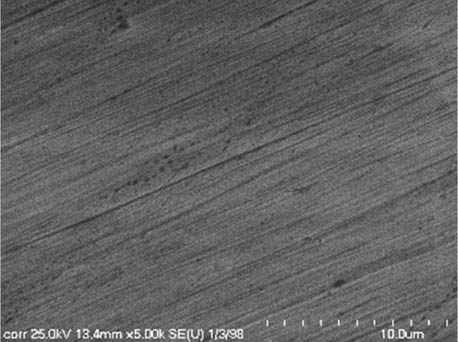
|

|
| 20% Citric acid |
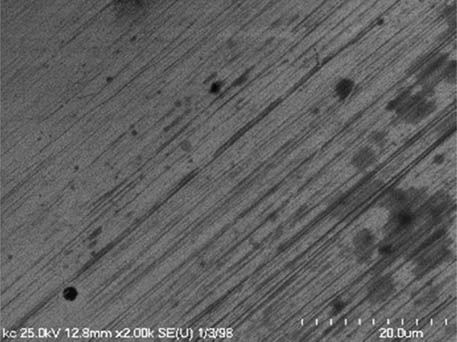
|
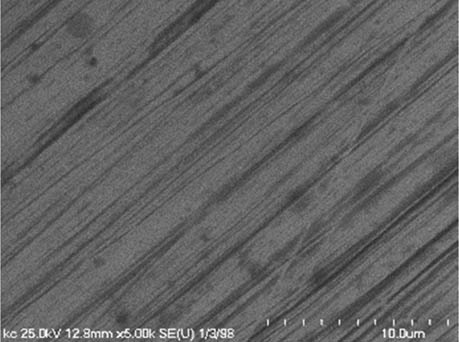
|
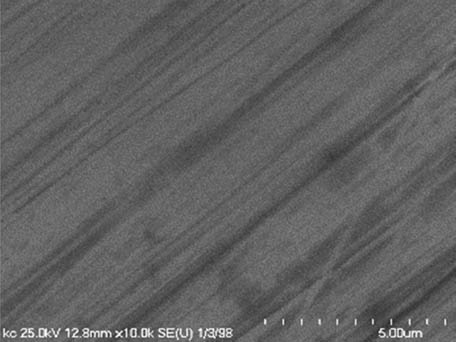
|
| 0.5% NaOCl |

|
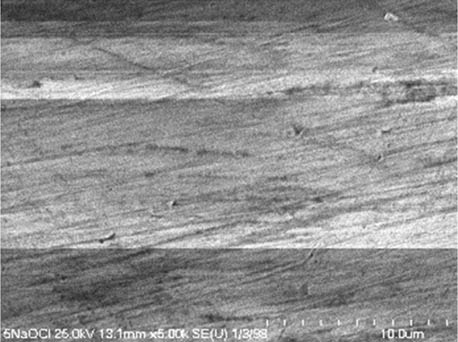
|

|
| 5.0% NaOCl |
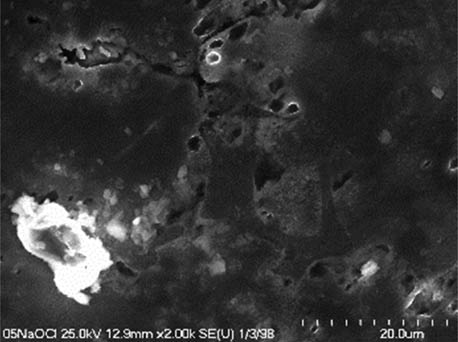
|
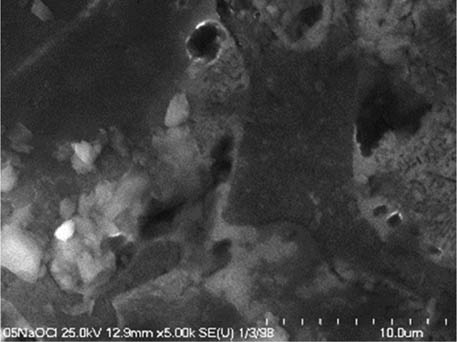
|

|
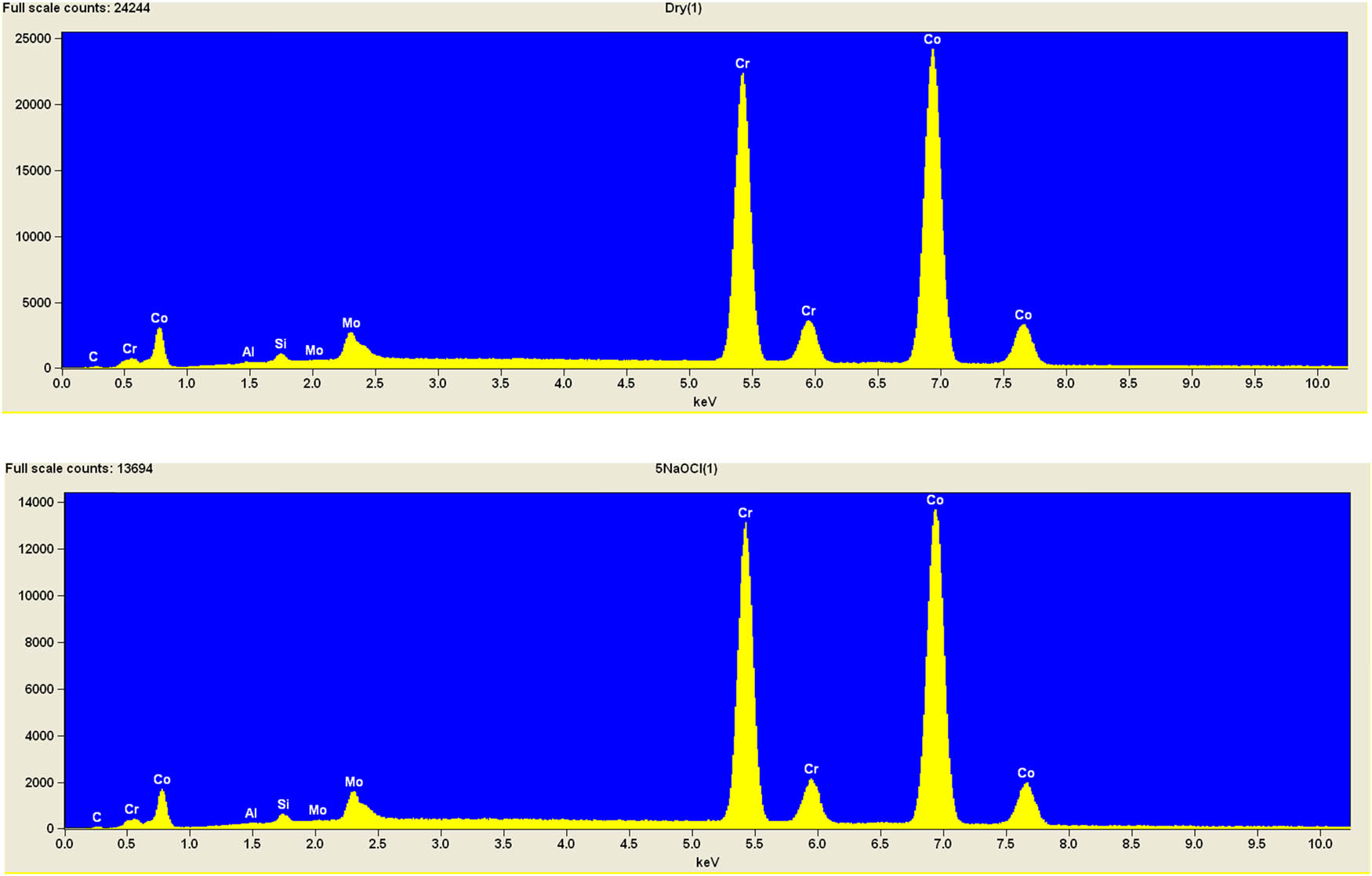
EDS analysis for a positive control group stored in 5.0% NaOCl.
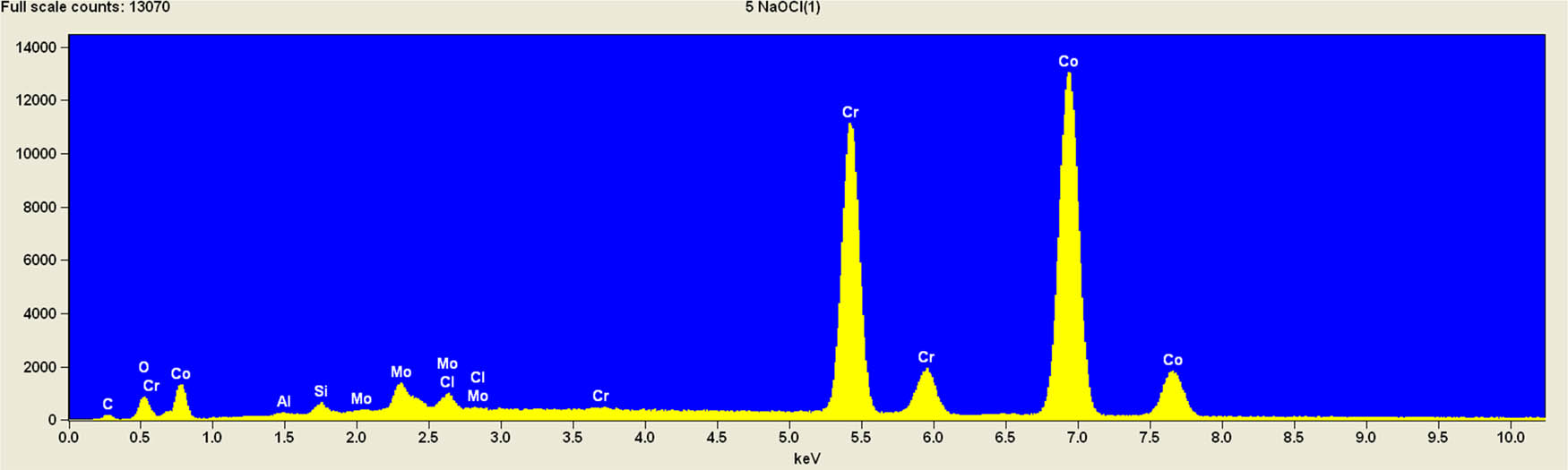
EDS analysis for the samples stored in 0.5% NaOCl.
EDS analysis for the negative control group (dry).
4 Discussion
Based on the obtained test results, it can be concluded that cleaning agents like water, alkaline peroxides, or disinfectants and their mixtures with enzymes and weak acids do not significantly affect denture surfaces cast from Co–Cr. After conditioning in the solutions of cleaning agents, the surface of the alloy did not develop to any significant degree during the time corresponding to the use of the prosthesis for 3 years. According to Curylofo et al. [18], using 20% citric acid solution or solutions of sodium hypochlorite in a concentration higher than 0.5% is harmful to Co–Cr alloy because R a increases over 0.2 μm, which cannot be clinically acceptable, due to promoting a bacteria adhesion. According to research carried out by Felipucci et al. [3] the citric acid solution and NaOCl solutions with concentrations higher than 0.05% may lead to a corrosion effect. The negative effect of using these cleaners is also discoloration and remaining stains. pH Changes (decreased value) also affect the corrosion resistance of the alloy, which may cause a roughness increase. The results from our tests for citric acid and NaOCl confirm that a more aggressive environment is harmful to metallic denture elements. According to Puscar et al.’s report [24] with increasing acidity of solution, corrosion resistance of cobalt–chromium alloy decreases. Morphological changes on the surface may be affected by Co-release. Rylska noted that in solutions with low pH values, the chemical passivation decreases susceptibility to electrochemical corrosion of Co–Cr dental alloy [25]. It is worth noting that strong acids inactivate surfaces stronger than weak acids by forming a passive layer on Co–Cr alloy surface with an oxide layer [26]. Many studies are focused only on R a measurements [27]. Arithmetic average roughness height is a widely used parameter, but as we see R a value do not fully characterize features of the surface like sharp or soft peak and valleys. Also, Zecchino [28] stated that R a is a parameter that characterizes only in general a surface topography. Different surfaces with the same R a range can have different surface morphologies, so it is necessary to use other R parameters to characterize differences in surface roughness. It should be emphasized that diluted acids develop Co–Cr surface more than other cleaning agents. The valleys (R v) on the surface become deeper. Its values rise mostly in citric acid and both NaOCl solutions to around 0.09 μm compared to 0.07 μm for dry storage samples. R v is an important parameter in the corrosive behavior of cleaning agents and Co–Cr alloy, because of its influence on the diffusion of active ions during corrosion [29]. On the corroded surface, a predominance of deep valleys is visible, and as Zecchino stated it is a correlation between a bigger R v value and greater corrosion [28]. We see that citric acid or NaOCl differs from other cleaning solutions and the negative control group and their R v values increase significantly. These classes of denture cleaning agents may affect more aggressively Co–Cr surface, than others. Surface picks (R p) are significantly higher, they rise from around 0.07 μm for dry storage Co–Cr to 0.273 μm for alloy immersed in 5.0% NaOCl, and it may affect occurring pitting on Co–Cr surfaces. Not only vertical parameters characterize surface character. Also, horizontal and hybrid parameters are important to determine surface properties like wettability, corrosion, friction, wear, and others [30]. The mean width of the profile elements R Sm also raises three times after storage in 0.5% sodium hypochlorite. Change of surface roughness influence is also on the wettability of materials. As per Uhorchuk et al.’s statement [31] surface roughness influence is on wetting characteristics, which correlate with changes in static contact angle. This is coincident with the Cassie-Wenzel theory and Nishioka et al.’s [32] results. Also, our investigation confirmed this thesis. Contact angle decreases as Co–Cr alloy surface roughness increases. The high value of SFE is undesirable due to higher bacteria adhesion. Bollen et al. [33] note that plaque accumulation increases the risk of periodontal inflammation and caries, so it is needed to keep the surface of prosthesis materials as smooth as possible. The side effect of increasing surface energy makes citric acid and sodium hypochlorite to unfavorable denture cleaners [32]. Also, Sobolewska reports that increased wettability of Co–Cr alloy may be a reason why bacteria adherer stronger. One of the factors that modulate the adhesive behaviors of bacteria proteins is SFE. Bacteria adhere stronger to surfaces with higher surface energy. On the other hand, water contact angle under 40° (highly hydrophilic surface) or over 130° (highly hydrophobic surface) seems to inhibit bacterial adhesion. Because of inconclusive results for modifying wettability and surface roughness [34], it was necessary to carry out an investigation, like SEM-EDS, but despite the positive control group, any other changes was observed.
Researchers show surface changes under microscopic observation in the case of using sodium hypochlorite solutions [35]. Dong observed also yellowish brown changes on the surface of Co–Cr alloy after immersion in weak acid [26]. There are also publications which show that other cleaning agents are safe for metal elements of the prosthesis. Curylofo et al. [18] state that the use of disinfectants like cetylpyridinium chloride is safe for metallic elements of RPDs for a period of 5 years, and in SEM–EDS analysis, any deleterious effect or changes in surfaces chemical composition was observed. The visual analysis [3] eliminates sodium hypochlorite and citric acid as safe for Co–Cr denture cleaners because of occurring tarnishes and presented stains. These observations also correspond with our results obtained from the SEM images. These side effects suggest that corrosion occurred, like pitting, but EDS analysis has shown changes in elements composition only for samples stored in 5.0% NaOCl, where chlorine and oxygen occurred.
5 Conclusions
On the basis of our measurements and their limitations (used solution of active substances not commercial cleaners with the selected concentration of the active substance), it can be concluded that as a class of denture cleaning agents, alkaline hypochlorites and diluted acids could cause deleterious effects on Co–Cr alloy surfaces in RPDs. Immersion in alkaline hypochlorites and diluted acids leads to an increased all roughness parameters compared to dry storage samples (negative control group) and growth of SFE of Co–Cr especially polar compound of SFE, which may cause better biofilm adhesion even in 3 years of simulation of use. Also, some stains and discoloration are remaining on the Co–Cr alloy surfaces, when alkaline hypochlorite or diluted acid was used. Other classes of denture cleaning agents like alkaline peroxides, enzymes, and disinfectants do not change significantly the values of the surface parameters and the SFE value. Hence, it can be concluded that the above-mentioned cleaning agents do not have harmful effects on the metallic components of RPDs, so they are suitable for RPD cleaners.
-
Funding information: The authors state no funding is involved.
-
Author contributions: Joanna Nowak – conceptualization, data curation, formal analysis, investigation, methodology, resources, writing- original draft, writing – review and editing, Klaudia Steinberg – investigation formal analysis, visualization, writing original draft, Jerzy Sokołowski – project administration, funding acquisition, Kinga Bociong – conceptualization, investigation, methodology, project administration, supervision, validation, writing – review and editing
-
Conflict of interest: The authors state no conflict of interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Borowicz J, Modzelewska A, Katarzyna Sarna-Boś AR. Zastosowanie stopów metali nieszlachetnych w protetyce stomatologicznej. Nowoczesny Technik Dentystyczny; n.d. https://technik.elamed.pl/artykul/zastosowanie-stopow-metali-nieszlachetnych-w-protetyce-stomatologicznej/45615 (accessed March 15, 2022).Suche in Google Scholar
[2] Budtz-Jørgensen E. Materials and methods for cleaning dentures. J Prosthet Dent. 1979;42:619–23. 10.1016/0022-3913(79)90190-2.Suche in Google Scholar
[3] Felipucci DNB, Davi LR, Paranhos HFO, Bezzon OL, Silva RF, Pagnano VO. Effect of different cleansers on the surface of removable partial denture. Braz Dental J. 2011;22:392–7. 10.1590/s0103-64402011000500008.Suche in Google Scholar
[4] Backenstose WM, Wells JG. Side effects of immersion-type cleansers on the metal components of dentures. J Prosthet Dent. 1977;37:615–21. 10.1016/0022-3913(77)90211-6.Suche in Google Scholar
[5] Anthony DH, Gibbons P. The nature and behavior of denture cleansers. J Prosthet Dent. 1958;8:796–810. 10.1016/0022-3913(58)90100-8.Suche in Google Scholar
[6] Gajwani-Jain S, Magdum D, Karagir A, Pharane P. Denture cleansers: A review. IOSR J Dental Med Sci (IOSR-JDMS) e-ISSN. 2015;14:94–6. 10.9790/0853-14249496.Suche in Google Scholar
[7] Budtz‐Jörgensen E, Kelstrup J. Enzymes as denture cleansers. Scand J Dent Res. 1977;85:209–15. 10.1111/J.1600-0722.1977.TB00555.X.Suche in Google Scholar
[8] Karthikeyan S, Asharaf Ali S. Denture disinfectants used in prosthodontics-a review; n.d.Suche in Google Scholar
[9] Peracini A, Regis RR, Souza RF, Pagnano VO, Silva CHL, Paranhos HD. Alkaline peroxides versus sodium hypochlorite for removing denture biofilm: A crossover randomized trial. Braz Dental J. 2016;27:700–4. 10.1590/0103-6440201600913.Suche in Google Scholar
[10] Abelson DC. Denture plaque and denture cleansers. J Prosthet Dent. 1981;45:376–9. 10.1016/0022-3913(81)90094-9.Suche in Google Scholar
[11] Kwasowe środki czyszczące; n.d. https://www.chemiaibiznes.com.pl/artykuly/kwasowe-srodki-czyszczace (accessed March 15, 2022).Suche in Google Scholar
[12] Budtz‐Jörgensen E. A 3-months’ study of enzymes as denture cleansers. J Oral Rehabil. 1978;5:35–9. 10.1111/J.1365-2842.1978.TB00389.X.Suche in Google Scholar
[13] Nakamoto K, Tamamoto M, Hamada T. Evaluation of denture cleansers with and without enzymes against Candida albicans. J Prosthet Dent. 1991;66:792–5. 10.1016/0022-3913(91)90418-V.Suche in Google Scholar
[14] Faot F, Cavalcanti YW, Pinto LD, da Silva WJ, Del Bel Cury AA. Efficacy of citric acid denture cleanser on the Candida albicans biofilm formed on poly(methyl methacrylate): Effects on residual biofilm and recolonization process. BMC Oral Health. 2014;14(1):1–7. 10.1186/1472-6831-14-77.Suche in Google Scholar
[15] Felipucci DNB, Davi LR, Paranhos HFO, Bezzon OL, Silva RF, Barbosa F, et al. Effect of different cleansers on the weight and ion release of removable partial denture: An in vitro study. J Appl Oral Sci. 2011;19:483–7. 10.1590/S1678-77572011000500008.Suche in Google Scholar
[16] Vasconcelos GLL, Curylofo PA, Raile PN, Macedo AP, Paranhos HFO, Pagnano VO. Effect of alkaline peroxides on the surface of cobalt chrome alloy: An in vitro study. J Prosthodont. 2019;28:e337–41. 10.1111/JOPR.12789.Suche in Google Scholar
[17] Papadopoulos T, Polyzois G, Tapanli A, Frangou M. The effect of disinfecting solutions on bending properties and weight changes of Co-Cr and Ti-6Al-7Nb alloys for dentures. Odontology. 2011;99:77–82. 10.1007/S10266-010-0135-2.Suche in Google Scholar
[18] Curylofo PA, Raile PN, Vasconcellos GLL, Macedo AP, Pagnano VO. Effect of denture cleansers on cobalt–chromium alloy surface: A simulated period of 5 years’ use. J Prosthodont. 2020;29:142–50. 10.1111/JOPR.12996.Suche in Google Scholar
[19] McGowan MJ, Shimoda LM, Woolsey GD. Effects of sodium hypochlorite on denture base metals during immersion for short-term sterilization. J Prosthet Dent. 1988;60:212–8. 10.1016/0022-3913(88)90318-6.Suche in Google Scholar
[20] Keyf F, Güngör T. Comparison of effects of bleach and cleansing tablet on reflectance and surface changes of a dental alloy used for removable partial dentures. J Biomater Appl. 2003;18:5–14. 10.1177/0885328203018001001.Suche in Google Scholar PubMed
[21] Backenstose WM, Wells JG. Side effects of immersion-type cleansers on the metal components of dentures; n.d.Suche in Google Scholar
[22] Rodrigues Garcia RCMM, De Souza JA, Rached RN, Del Bel Cury AA. Effect of denture cleansers on the surface roughness and hardness of a microwave-cured acrylic resin and dental alloys. J Prosthodont. 2004;13:173–8. 10.1111/J.1532-849X.2004.04028.X.Suche in Google Scholar
[23] Kassapidou M, Hjalmarsson L, Johansson CB, Hammarström Johansson P, Morisbak E, Wennerberg A, et al. Cobalt–chromium alloys fabricated with four different techniques: Ion release, toxicity of released elements and surface roughness. Dental Mater. 2020;36:e352–63. 10.1016/J.DENTAL.2020.08.012.Suche in Google Scholar
[24] Puskar T, Jevremovic D, Williams RJ, Eggbeer D, Vukelic D, Budak I. A comparative analysis of the corrosive effect of artificial saliva of variable pH on DMLS and cast Co-Cr-Mo dental alloy. Materials. 2014;6:6486–501. 10.3390/ma7096486.Suche in Google Scholar PubMed PubMed Central
[25] Rylska D, Sokołowski G, Sokołowski J, Łukomska-Szymańska M. Chemical passivation as a method of improving the electrochemical corrosion resistance of Co-Cr-based dental alloy. Acta Bioeng Biomech Original Pap. 2017;19(2):73–8. 10.5277/ABB-00600-2016-04.Suche in Google Scholar
[26] Dong H, Nagamatsu Y, Chen KK, Tajima K, Kakigawa H, Shi S, et al. Corrosion behavior of dental alloys in various types of electrolyzed water. Dental Mater J. 2003;22:482–93. 10.4012/DMJ.22.482.Suche in Google Scholar
[27] Gadelmawla ES, Koura MM, Maksoud TMA, Elewa IM, Soliman HH. Roughness parameters. J Mater Process Technol. 2002;123:133–45. 10.1016/S0924-0136(02)00060-2.Suche in Google Scholar
[28] amp16103p025; n.d.Suche in Google Scholar
[29] Toloei A, Stoilov V, Northwood D. The relationship between surface roughness and corrosion. ASME International Mechanical Engineering Congress and Exposition, Proceedings (IMECE); 2014. p. 2B. 10.1115/IMECE2013-65498.Suche in Google Scholar
[30] Melentiev R, Kang C, Shen G, Fang F. Study on surface roughness generated by micro-blasting on Co-Cr-Mo bio-implant. Wear. 2019;428–429:111–26. 10.1016/J.WEAR.2019.03.005.Suche in Google Scholar
[31] Uhorchuk R, Umretiya R, Anderson M, Elward B, Leon SB, Rebak R, et al. Influence of surface roughness on wettability of accident tolerant fuels cladding. Trans Am Nucl Soc. 2019;121:1813–5. 10.13182/T30895.Suche in Google Scholar
[32] Nishioka M, Yamabe Y, Hisatsune K, Fujii H. Influence of polishing of denture base resin and metal surfaces on wettability with water and saliva. Dental Mater J. 2006;25:161–5. 10.4012/DMJ.25.161.Suche in Google Scholar
[33] Bollen CM, Lambrechts P, Quirynen M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent Mater. 1997;13:258–69. 10.1016/S0109-5641(97)80038-3.Suche in Google Scholar
[34] Sterzenbach T, Helbig R, Hannig C, Hannig M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin Oral Invest. 2020;24:4237–60. 10.1007/S00784-020-03646-1.Suche in Google Scholar PubMed PubMed Central
[35] Borsa PCC, Marquezan M, May LG, Braun KO. Surface change assessment of Co-Cr alloy subjected to immersion in denture cleansers. Braz J Oral Sci. 2016;15:196–200. 10.20396/BJOS.V15I3.8649980.Suche in Google Scholar
© 2022 Joanna Nowak et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop
Artikel in diesem Heft
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop

