Abstract
Powdered Teucrium polium leaves (S1) were modified with zinc chloride (ZnCl2) (S2), a mixture of copper sulfide (CuS) and ZnCl2 (S3), and oxalic acid (H2C2O4) (S4). The porosity, surface area, and functional groups of these four samples, along with their ability to uptake KMnO4 from solutions, were inspected to identify the optimal adsorbent. For KMnO4 adsorption by the ideal adsorbent (S2), the pHZPC (pH value at which the adsorbent surface is uncharged), influences of experimental circumstances, and dynamic, isotherm, and thermodynamic parameters were examined. According to the results, the surface area, pore size, pore volume, and pHZPC of the optimum adsorbent (S2) are 3.689 m2/g, 570.20 Å, 0.01776 cm3/g, and 6.4, respectively. The optimal S2 dose, the ideal value of pH solution, and equilibrium time are 0.05 g, 5.5, and 192 min, respectively. The Langmuir and second-order models are appropriate for modeling this adsorption. Furthermore, increasing the temperature from 27 to 57°C increases the maximum adsorption capacity (q max) from 833.33 to 1000.00 mg/g. According to the thermodynamic data, this adsorption is both endothermic and spontaneous.
Graphical abstract
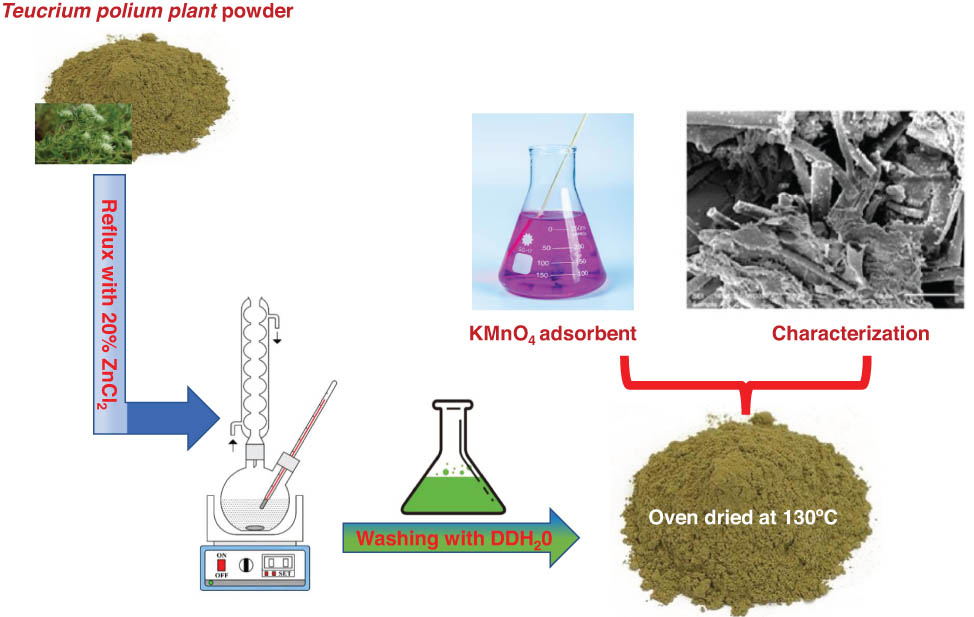
1 Introduction
Potassium permanganate (KMnO4) is mainly utilized as an oxidant agent to control plant growth, the taste, and odor of industrial products, and to disinfect industrial waste [1]. KMnO4 is also applied for the removal of cyanide, hazardous metals, dyes, phenols, and other organic contaminants from polluted wastewaters [2,3]. Furthermore, this chemical agent is used to oxidize and break organic molecules with longer chains [2,4]. KMnO4 is now widely employed as a potent oxidant for removing the solvents of chlorinated groundwater and soil [5] and preventing the growth of some aquatic organisms [1]. Unfortunately, the National Institute for Occupational Safety and Health (NIOSH) has designated KMnO4 as a toxic and harmful material to the health of humans. Whereas direct exposure to permanganate produces skin burns, shortness of breath, and eye pain, it may also cause nervous system damage since it causes shock and neurological collapse, while long-term exposure causes long-term harmful effects [6]. Because of these risks, technologies such as fluidized-bed crystallization [7] and adsorption [1,6] have been utilized to remove KMnO4 from industrial wastewaters before release. Adsorption has been claimed to be one of the best and most utilized methods due to its ease of use, high performance, nontoxicity, ecological friendliness, and a wide range of sorbents [1,8,9,10]. Therefore, the method of adsorption in this work was chosen. Adsorption performance of KMnO4 by activated carbon prepared from different natural raw materials was examined earlier [1,2,11,12]. Nanoparticles of zinc oxide (ZnO) [13], nickel ferrite [14], and copper sulfide (CuS) [15] were utilized for the elimination of KMnO4 and other pollutants from aqueous solutions. Although these nanoparticles have elevated adsorptive capacity, their applications are unwanted due to their expensive production costs. As a result, a variety of low-cost or no-cost materials have been used as effective adsorbents for removing some pollutants from water [16]. For example, modified palm oil fuel ash [17,18] and powdered Neem leaves [19,20,21] have been used to eliminate a variety of organic and inorganic contaminants from water. The modified leaf powder of Neem [22], Nitraria retusa [23], and Ocimum basilicum [24] have recently been utilized to remove KMnO4 from aqueous solutions. The adsorption performance of these low-cost adsorbents toward KMnO4 is significantly disparate. As a result, more research into the adsorption of KMnO4 by other sorbents at a cheap cost is needed.
Teucrium polium L., also known as germander, is a plant that has been used in traditional herbal remedies for more than 2,000 years for its antifungal, carminative, antispasmodic, antipyretic, anti-inflammatory, and antifungal qualities, as well as its capability to reduce high blood pressure [25]. This herbal plant is a member of the Lamiaceae family, which includes roughly 300 types, and blooms between June and August. These types can be found in abundance on hills and mountains all over the world, including the Mediterranean, Southwest Asia, Europe, and North Africa [26,27]. This plant, known in Saudi Arabia as Ja’adeh, is high in flavonoids [26].
T. polium L. has previously been employed for the synthesis of Fe2O3 nanoparticles, which are utilized as a catalyst for methyl orange degradation [26] and as a sorbent for eliminating As(iii) from solutions [28].
Despite its low cost and widespread availability in many countries, particularly in Saudi Arabia, no attempt has been made to date to employ T. polium L. leaf powder as a novel sorbent for the elimination of KMnO4 from polluted waters.
As a result, the goals of this study are to improve the adsorption ability of this herb by modifying its leaf powder with zinc chloride (ZnCl2), copper sulfide (CuS), and oxalic acid (H2C2O4) and to determine the adsorption effectiveness of KMnO4 using the prepared adsorbents. The produced samples will be characterized, and the performance of KMnO4 adsorption by these samples will be compared. In addition, the factors controlling this adsorption, as well as kinetics, isotherms, and thermodynamic constants, will be investigated in this study.
2 Methodology
2.1 Adsorbent preparation and modification
The T. polium leaves were obtained from a market for medicinal herbs in Tabuk, Kingdom of Saudi Arabia. These leaves were washed twice with distilled water, dehydrated in an oven overnight, ground into powder with an electric grinder, and labeled as S1. A specific amount of S1 (100 g) was mixed with 1,000 mL of ZnCl2 (20% w/w) and refluxed for 180 min at the boiling point, after which the mixture was allowed to cool at ambient temperature. After cooling, the mixture was filtered through a Buchner funnel connected to a vacuum bump. The residual solid was then treated with 250 mL of 2 M hydrochloric acid solution and heated for one and a half hours to eliminate any remaining ZnCl2. The solid part of the resultant mixture was then filtered out and washed several times with distilled water. The clean solid was then dehydrated in a 130°C oven for 30 h. In the end, the dry solid was ground and sieved to obtain similar particles, after which it was designated as S2.
The same weight of S1 (100 g) was mixed with copper sulfide (50 g). This mixture was also refluxed with 1,000 mL of ZnCl2 (20% w/w) using the same method and conditions mentioned earlier. In this case, the resulting adsorbent was designated as S3.
Using the same experimental settings and procedures as described earlier, the same quantity of S1 (100 g) was mixed and refluxed with 1,000 mL of H2C2O4 solution (20% w/w), and the resulting sample was labeled S4.
2.2 Adsorbent characterization
The groups that are chemically effective on the surface of the prepared four adsorbents (S1, S2, S3, and S4) were detected using a Fourier-transform infrared spectrometer (FT-IR; Nicolet iS5; Thermo Fisher Scientific, USA). These samples were also scanned by SEM apparatus at 10 kV accelerating power to determine the surface morphology of these adsorbents. Additionally, BET (NOVA-2200 Ver.6.11) technology was used for 22 h at 77.38 K to identify the textural characteristics (porosity and surface area) of these four adsorbents.
Moreover, five 0.05 M Na2CO3 solutions with varied pH starting values (2, 4, 6, 8, and 10) (pHi) were prepared. In a 150 mL plastic container, a given volume of each solution (40 mL) was combined with a fixed mass (0.1 g) of the optimum adsorbent (S2). The filled containers were sealed and shaken for 26 h at 27°C and 180 rpm in shaker incubators. After each solution was separated by filtering, the final pH (pHf) of each of these solutions was quantified by means of a pH meter. Finally, to determine the pHZPC value of this adsorbent, the values of pHi–pHf were assessed and graphed against pHi values.
2.3 Experiments of adsorption
2.3.1 Identifying the best adsorbent
In a 50 mL amber bottle, 30 mL of 200 mg/L KMnO4 solution was integrated separately with 0.03 g of each of the four samples developed in this work (S1, S2, S3, and S4) to find the best active adsorbent toward KMnO4. A shaker incubator was used to agitate the sealed amber bottles for 24 h at 27°C and 180 rpm. The mixtures were then filtered, and the residual concentration of KMnO4 in the filtrate was assessed at 525 nm using a Jenway 6800 UV-vis spectrophotometer. The percentage removal (%R) and amount of KMnO4 adsorbed (q e, mg/g) by each of these adsorbents were calculated using the following equations:
where V is the volume of KMnO4 solution (L); m is the adsorbent mass (g); C 0 is the adsorbate initial concentration; and C e is the adsorbate final concentration.
2.3.2 Effect of experimental parameters
The adsorption experiments of KMnO4 by the best effective adsorbent (S2) were carried out in a batch system to find out how the most important factors, such as dose of S2, KMnO4 concentration, temperature, contact time, and pH, influence the adsorption and to identify the optimal values of these factors. In all these experiments, 50 mL of KMnO4 solution and the required amount of S2 were added to the 100 mL amber bottles. The filled bottles were then placed in a shaker incubator at 180 rpm. The suspensions were filtered after the requested time for every experiment, and the residual concentrations of KMnO4 were measured as described earlier (Section 2.3.1). Table 1 contains a list of the experimental conditions that were used in this study.
Summary of the empirical conditions for adsorption of KMnO4 by S2
| Experiment | Adsorbate concentration (mg/g) | Time of adsorption | Adsorbent dose (g) | pH | Temperature (°C) |
|---|---|---|---|---|---|
| Dose impact | 60 | 24 h | 0.01–0.07 | 8.11 | 27 |
| pH impact | 500 | 24 h | 0.05 | 1.5–11.5 | 27 |
| Time impact | 100, 200, 300 | 0–320 min | 0.05 | 5.5 | 27 |
| Temp. and KMnO4 conc. impact | 10–1,400 | 360 min | 0.05 | 5.5 | 27–57 |
The %R and q e (KMnO4 adsorbed at time t, mg/g) were calculated using equations (1) and (2), respectively, and q t was calculated using the following equation:
where C t is the concentration of KMnO4 at time t.
2.3.3 Isotherm studies
The linear equations of the Temkin, Freundlich, and Langmuir isotherm models (Table 2) were used to analyze the outcomes of Section 2.3.2 for the adsorption of 10–1,400 (mg/L) KMnO4 solutions by S2 (0.05 g) at an interaction time of 360 min, pH 5.5, and temperatures ranging from 27 to 57°C. The values of R L (equilibrium parameter) associated with the Langmuir model’s essential characteristics were calculated using the following equation:
where C 0 is the highest concentration of KMnO4 and K L is the Langmuir constant.
Isotherm and kinetic models used in this work
| Model name | Linear equation | Plot | Constants |
|---|---|---|---|
| Isotherm models | |||
| Langmuir isotherm |
|
|
q max = (slope)−1 |
| K L = slope/intercept | |||
| Freundlich isotherm |
|
|
K F = exp(intercept) |
| n = (slope)−1 | |||
| Temkin isotherm |
|
|
B 1 = slope |
| K T = exp (intercept/slope) | |||
| Kinetic models | |||
| Pseudo first order |
|
|
k 1 = −slope |
| q e = exp(intercept) | |||
| Pseudo second order |
|
|
k 2 = (slope)2/intercept |
| q e = (slope)−1 | |||
| Intraparticle diffusion |
|
|
K dif = slope |
| C = intercept | |||
q max (mg/g): maximum adsorption capacity; K T, K F, and K L: constants of Temkin, Freundlich, and Langmuir, respectively; n: constants associated to intensity of adsorption; B 1: constants associated to the adsorption heat; q t and q e (mg/g): amount of CR adsorbed at time t and equilibrium (min), K 1 (1/min) and K 2 (g mg−1 min−1) rate constants of the kinetic models for first and second order, respectively; K dif (mg/g min−1)1/2: intra-particle diffusion rate constants; and C: another kinetic constant.
2.3.4 Kinetic studies
To determine the dynamic constants of this adsorption, the linear forms of the kinetic models listed in Table 2 were used to analyze the obtained data for the adsorption of 100, 200, and 300 mg/L of KMnO4 solutions by S2 at 27°C, pH 5.5, and different times (0–320 min) (Section 2.3.2). Hence, the kinetic constants can be used to determine the mechanism and rate of this adsorption.
2.3.5 Thermodynamic studies
The values of ∆G° (change in free energy), ∆S° (change in entropy), and ∆H° (change in enthalpy) for the adsorption of three KMnO4 solutions (50, 100, 200 mg/L) by S2 at the same empirical conditions of Section 2.3.3 were calculated by applying the following equations:
where T is the surrounding temperature (K) and R is the universal constant of gases.
3 Results and discussion
3.1 Structural characteristics of adsorbents
3.1.1 Analysis of FT-IR
The technique of FT-IR was used to reveal the influence of each chemical agent used in this work on the surface effective groups of T. polium L. (S1). The functional groups and corresponding FT-IR absorption bands for these four adsorbents (S1, S2, S3, and S4) are shown in Figure 1. This figure reveals that the T. polium L. (S1) has seven absorption bands at 3335.93, 2923.91, 2854.17, 1727.26, 1513.56, 1158.35, and 1025.46 (cm−1). These bands are related to stretching the hydrogen band of O–H, C–H (alkyl), C–H, C═O (aliphatic aldehydes), N–H (2°-amide), C–O, and C–H bending (in-plane), respectively. In the case of the modified samples (S2, S3, and S4), similar bands with minor shifts may be seen (Figure 1). Moreover, stretching of the N–H (1°-amide) II band at 1616.92 cm−1, C–H scissoring at 1454.89 cm−1, and C–H bending in out-of-plane at 780.67 cm−1 were detected only in the case of the sample modified by oxalic acid (S4). The outcomes of this part confirm that the chemical properties of the T. polium surface were slightly affected after modification by ZnCl2 as well as by a mixture of ZnCl2 and CuS, whereas these properties were significantly influenced by oxalic acid. Similar outcomes were reported in our previous research [29].

Spectra of FT-IR for the adsorbents prepared in this work, the raw powdered T. polium leaves (S1), raw powder modified by ZnCl2 (S2), raw powder modified by a mixture of CuS and ZnCl2 (S3), and raw powder modified by H2C2O4 (S4).
3.1.2 Analysis of SEM
The SEM pictures of the adsorbent materials (S1, S2, S3, and S4) are shown in Figure 2. This figure shows that the picture of S1 (T. polium before modification) differs considerably from that of the chemically revised samples (S2, S3, and S4), whereas most pleats in the new adsorbents collapsed and their construction was disrupted. Furthermore, many different pores and holes that are beneficial to the adsorption process appeared on the adsorbent surface after the chemical modification processes.

SEM images of the adsorbents prepared in this work.
3.1.3 Analysis of BET and pHZPC
The results of the BET surface analyzer obtained in this work indicate that the surface area of S2 (3.689 m2/g) is greater than that of S1 (0.381 m2/g), S3 (2.644 m2/g), and S4 (0.768 m2/g). The findings of this section also showed that the pore size and pore volume of S1, S2, S3, and S4 are 151.07 Å and 0.00147 cm3/g; 570.20 Å and 0.01776 cm3/g; 527.40 Å and 0.01332 cm3/g; and 157.28 Å and 0.00667 cm3/g, respectively. This also implies that the pore properties of S2 are higher than those of S1, S3, and S4. This demonstrates that the modifications of S1 with ZnCl2 are successful in increasing the surface area and porosity of this raw material.
According to the plot of pHi–pHf against pHi (Figure 3), the solution pH at which the net charge on the surface of the optimal adsorbent (S2) will be zero is 6.4.

pHZPC of the adsorbent of S2.
3.2 Adsorption results
3.2.1 Performance of adsorbents
The percentages of KMnO4 removed from the solution and the quantities of this adsorbate adsorbed by S1, S2, S3, and S4 are shown in Table 3. This table demonstrates that S2 is the best and most effective adsorbent due to its high porosity and larger surface area, as shown in Section 3.1.3. Therefore, S2 was selected for the adsorption of KMnO4 in this study.
Adsorption performance of the synthesized adsorbent
| Adsorbent | q e (mg/g) | % R |
|---|---|---|
| S1 | 15.60 | 7.80 |
| S2 | 141.85 | 70.92 |
| S3 | 111.78 | 55.89 |
| S4 | 122.15 | 61.07 |
3.2.2 Influence of the experimental circumstances
The values of % R (percentage of KMnO4 eliminated from solution) were plotted versus values of S2 doses (Figure 4a) to determine the optimum mass of S2 that can be used for KMnO4 adsorption. As demonstrated in Figure 4a, increasing the mass of S2 from 0.01 to 0.05 g increases % P values, which remain constant above 0.05 g. The augmenting of % R when the mass of S2 was raised in the first range of 0.01–0.05 g was due to increasing the adsorbent active sites [30]. The consistent % R values that were found when the mass of S2 increased over 0.05 g were due to the adsorbent particles forming a cluster assembly [31]. Thus, 0.05 g of S2 was chosen as the ideal dosage in this study.
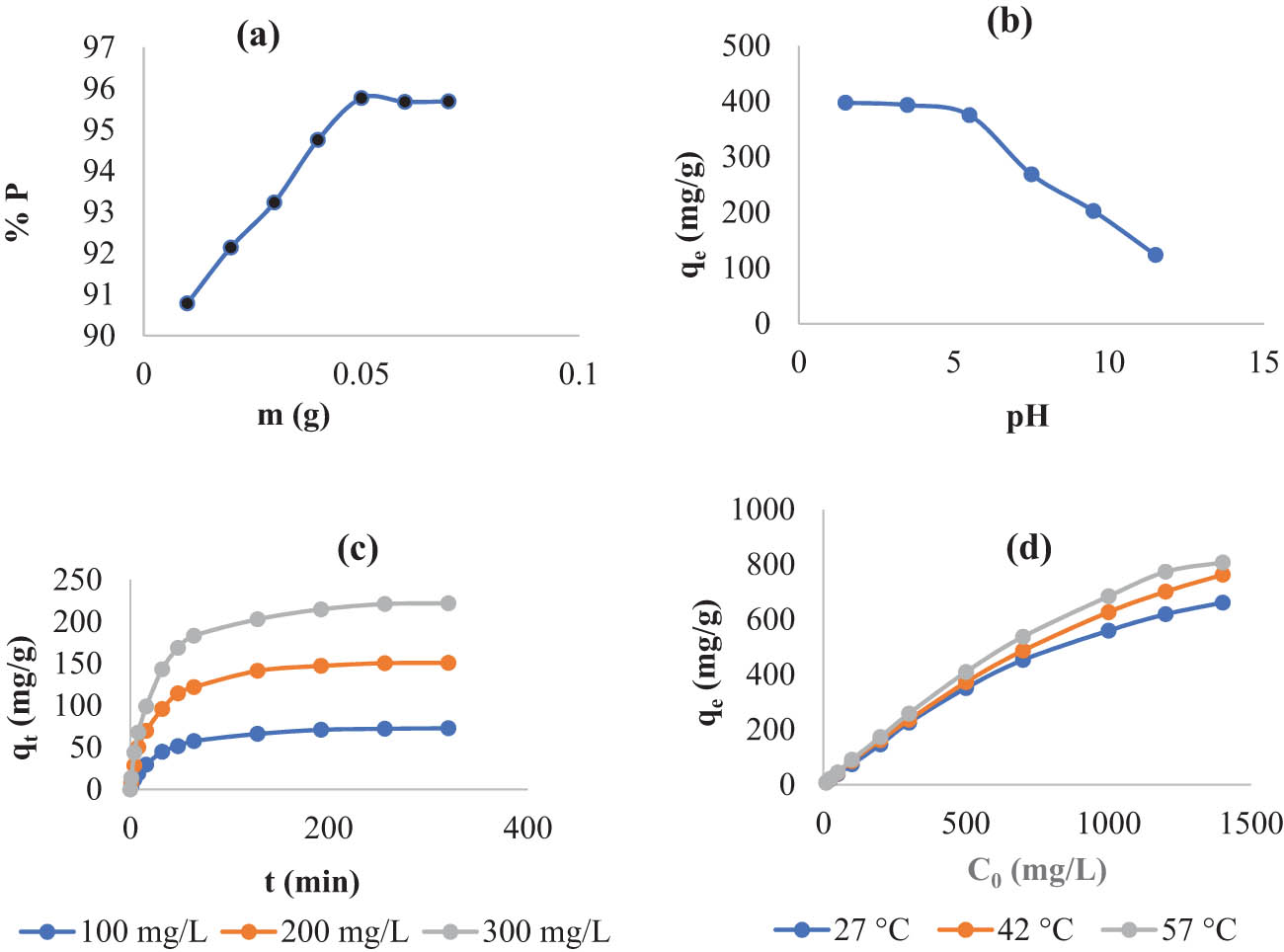
Impact of the experimental circumstances, adsorbent dose (a), solution pH (b), agitations time (c), and temperature and KMnO4 concentration (d).
According to Wu et al. [32], if the pH of the adsorbate solution is less than, equal to, or greater than the pHZPC of this adsorbent, then the adsorbent surface will be positively charged, uncharged, and negatively charged, respectively. This indicates that the pH of the adsorbate solution affects the adsorption process. As a result, the impact of this component has been investigated in this study. The experimental results associated with the influence of solution pH on this adsorption are represented in Figure 4b. As shown in this figure, the values of q e are somewhat and abruptly lowered when the pH of the KMnO4 solution is raised in the ranges of 1.5–5.5 and 5.5–11.5, respectively. The first lowering resulted from a decrease in the electrostatic attraction between the permanganate anions and the positive charges that appear on the surface of S2 (pHZPC > pH) and are reduced as pH increases in the first range (1.5–5.5) [32], whereas the rapid decrease in the q e values observed in the second range (5.5–11.5) was due to the electrostatic repulsion between the negatively charged S2 surface (pHZPC < pH) and the anions of permanganate [32]. Adsorption of KMnO4 by leaf powder of N. retusa [23] and O. basilicum [24] and copper sulfide nanoparticles [15] produced almost similar outcomes.
The influence of agitation time was examined for the adsorption of 100, 200, and 300 mg/L of KMnO4 solutions by 0.05 g of S2 under the experimental conditions listed in Table 1. Figure 4c depicts the results of this section. This figure shows that increasing the adsorption duration from 0 to 192 min steadily enhances the quantity of q
t
(amount of KMnO4 adsorbed at time t) for each solution, and no variation can be noted in the amount of q
t
beyond this time (192 min). This is because all of the effective adsorption sites were initially empty before gradually filling up with
Figure 3d represents the outcomes of the tests that were conducted to evaluate the influence of temperature and concentration on this adsorption. Based on this figure, the temperature has a positive influence on adsorption in this study, indicating that adsorption is an endothermic process. This may be expounded by the fact that when the temperature rises, the KMnO4 solution viscosity decreases, and the kinetic energy of the KMnO4 particles increases [33]. Figure 4d further shows that as the KMnO4 concentration is raised from 10 to 1,200 (mg/L), q e (amount of KMnO4 adsorbed at equilibrium) increases and becomes practically invariable at 1,200 mg/L. This could be explained by the fact that increasing the KMnO4 concentration in the range of 10–1,200 mg/g reduced the resistance to mass transfer of KMnO4 particles between the solid (S2 surface) and liquid (KMnO4 solution) [34], while there is no available operative site to adsorb additional molecules of KMnO4 when the concentration is augmented above 1,200 mg/L. In addition, following each of the adsorptions indicated earlier, the supernatant solutions of KMnO4 were crystal clear and no solid objects could be observed in the solution with the naked eye, demonstrating the stability of the synthesized adsorbent in KMnO4 solutions [35].
3.2.3 Isotherm constants
The empirical isotherm data were analyzed using the linear equations of the Temkin, Freundlich, and Langmuir isotherm models to describe the interaction between
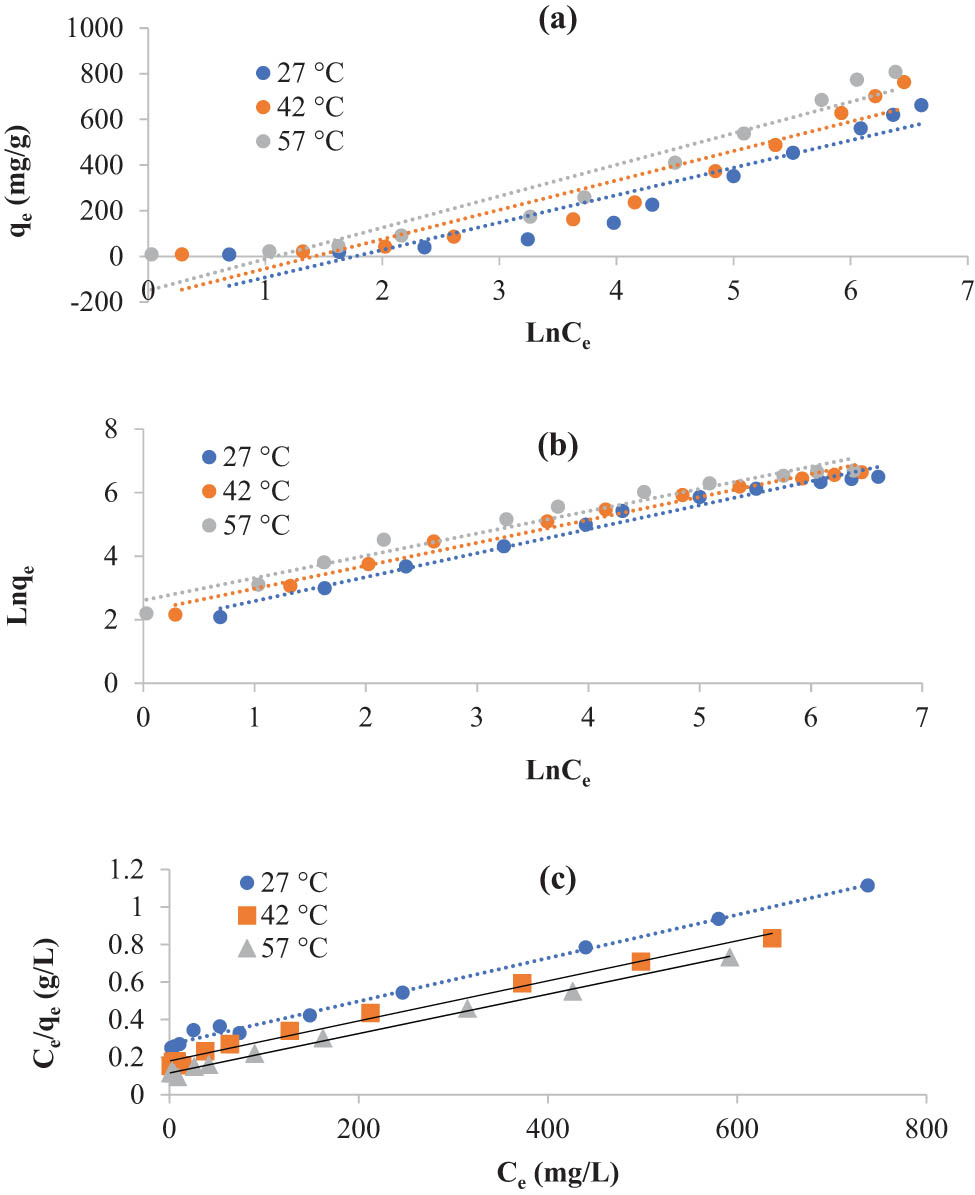
Temkin (a), Freundlich (b), and Langmuir (c) isotherm models for adsorption of KMnO4 by S2 (temperature = 27, 42, and 57°C; C 0 = 10–1,400 mg/L; m = 0.05 g; and time = 6 h).
Isotherm constants for adsorption of KMnO4 by S2
| Name of the isotherm model | Parameter | Temperature (°C) | Value |
|---|---|---|---|
| Temkin | K T (L/mg) | 27 | 0.171 |
| 42 | 0.243 | ||
| 57 | 0.339 | ||
| B 1 | 27 | 120.00 | |
| 42 | 128.85 | ||
| 57 | 137.64 | ||
| R 2 | 27 | 0.896 | |
| 42 | 0.894 | ||
| 57 | 0.922 | ||
| Freundlich | K F (mg/g) (L/mg)1/n | 27 | 6.256 |
| 42 | 9.574 | ||
| 57 | 13.615 | ||
| n | 27 | 1.326 | |
| 42 | 1.387 | ||
| 57 | 1.426 | ||
| 1/n | 27 | 0.754 | |
| 42 | 0.721 | ||
| 57 | 0.701 | ||
| R 2 | 27 | 0.980 | |
| 42 | 0.980 | ||
| 57 | 0.964 | ||
| Langmuir | q max (mg/g) | 27 | 833.33 |
| 42 | 909.09 | ||
| 57 | 1000.00 | ||
| K L (L/mg) | 27 | 0.0045 | |
| 42 | 0.0061 | ||
| 57 | 0.0087 | ||
| R L | 27 | 0.1370 | |
| 42 | 0.1044 | ||
| 57 | 0.0763 | ||
| R 2 | 27 | 0.994 | |
| 42 | 0.991 | ||
| 57 | 0.996 |
3.2.4 Kinetic modeling
The linearized forms of the kinetic models applied in this research are shown in Figure 6. The values of the dynamic factors given in Table 5 were computed using the intercepts and slopes of the curves of the first- (Figure 6a) and second-order (Figure 6b) kinetic models. Table 6 shows the dynamic constants of the intraparticle diffusion model (Figure 6c). Based on the kinetic constants of the first- and second-order models (Table 5), the model of second order described the whole experimental data extremely well, with R
2 values higher than that of the first order. The settlement between the empirical values of q
e and the values of q
e estimated by the second-order model (Table 5) is the second indicator that confirms that the second-order model adequately describes the empirical kinetic data [37]. This shows that a chemisorption mechanism, which involves electron sharing, electrostatic attraction between the permanganate anions and the cations that appear on the surface of S2, or exchange between the functional groups on the S2 surface and
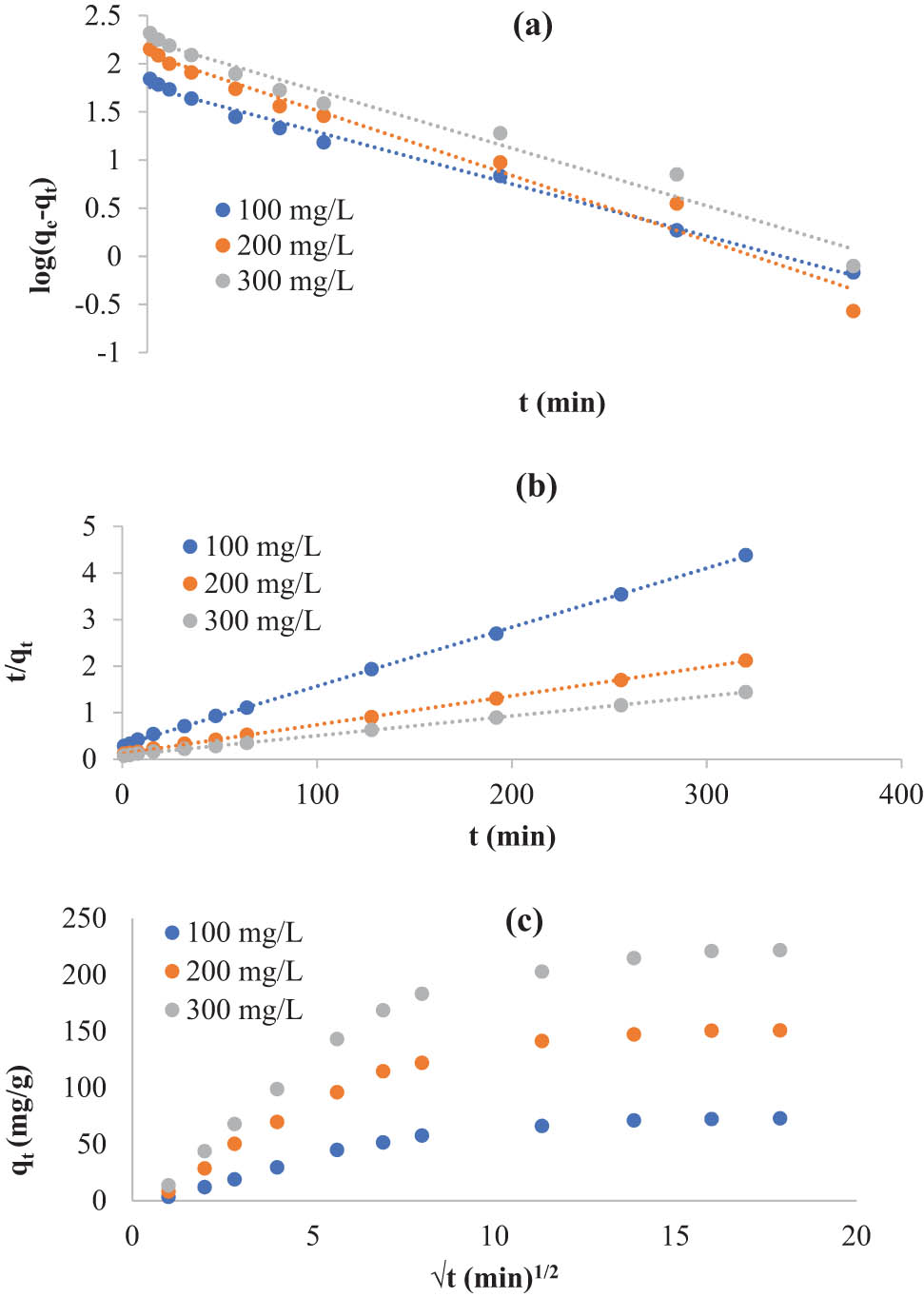
Kinetic models of the first order (a), second order (b), and intra-particle diffusion (c) for KMnO4 adsorption by S2 (temperature = 27°C; C 0 = 100, 200, and 300 mg/L; m = 0.05 g; and time = 0–320 min).
Parameters of the first- and second-order kinetic models for adsorption of KMnO4 by S2
| C 0 (mg/L) | q e,exp (mg/g) | Kinetic model | ||||||
|---|---|---|---|---|---|---|---|---|
| First order | Second order | |||||||
| q e1,cal (mg/g) | K 1 (h−1) | R 2 | q e2,cal (mg/g) | K 2 (g/mg h) | R 2 | Rate | ||
| 100 | 73.00 | 58.13 | 0.0177 | 0.992 | 78.74 | 0.00053 | 0.999 | 0.0417 |
| 200 | 150.83 | 126.44 | 0.0221 | 0.976 | 161.29 | 0.00032 | 0.999 | 0.0518 |
| 300 | 221.86 | 174.70 | 0.0196 | 0.973 | 238.10 | 0.00022 | 0.999 | 0.0516 |
Parameters of the intra-particle-diffusion kinetic model for adsorption of KMnO4 by S2
| C 0 (mg/L) | First region | Second region | ||||
|---|---|---|---|---|---|---|
| K dif (mg/h1/2g) | C | R 2 | K dif (mg/h1/2g) | C | R 2 | |
| 100 | 7.938 | –3.3252 | 0.992 | 1.0151 | 55.67 | 0.868 |
| 200 | 16.478 | –1.6496 | 0.982 | 1.4782 | 125.68 | 0.905 |
| 300 | 24.659 | –4.3888 | 0.991 | 2.9582 | 171.46 | 0.908 |
Figure 6c shows that the three plots are not linear over the entire time period; none of them passed from zero and have two linear regions with significant R
2 values (Table 6). This shows that there is some control over the boundary layer and that intraparticle diffusion is not the only rate-limiting process [38]. Identical results were found for
3.2.5 Outcomes of thermodynamic
The calculated ln(q e/C e) values for the adsorption of 50, 100, and 200 mg/L KMnO4 solutions by 0.05 g of S2 were plotted versus 1/T (equation 5) as illustrated in Figure 7. The values of ∆H° (change in enthalpy) and ∆S° (change in entropy) were estimated from the slopes and intercepts of the plots in this figure, respectively. The estimated ∆S° and ∆H° values (Table 7) were then inserted into equation (7) to get the ∆G° (change in free energy) values at each temperature (Table 7). The adsorption of KMnO4 is a chemical and exothermic process, based on the positive and high values of ∆H° (all >20.9 kJ mol−1) (Table 6) [40]. The adsorption of KMnO4 is a chemical and endothermic process, based on the positive and high values of ∆H° (all >20.9 kJ mol−1) (Table 7). The negative values of ∆G° (Table 7) prove that this adsorption is a spontaneous process [41]. Moreover, positive ∆S° values demonstrate that ∆S° rather than ∆H° is the driving factor for this adsorption [42]. The results discussed in this section are consistent with the temperature effect (Section 3.2.2) and kinetic results (Section 3.2.4).

Thermodynamic parameters for KMnO4 adsorption by S2 (temperature = 27, 42, and 57°C; C 0 = 50, 100, and 200 mg/L; m = 0.05 g; and time = 6 h).
Thermodynamic constants for KMnO4 adsorption by S2
| Initial concentration (mg/L) | ∆H° (kJ mol−1) | ∆S° (kJ mol−1) | ∆G° (kJ mol−1) | R 2 | ||
|---|---|---|---|---|---|---|
| 300 K | 315 K | 330 K | ||||
| 50 | 38.424 | 0.1381 | –3.0147 | –5.0866 | –7.1585 | 0.932 |
| 100 | 35.325 | 0.1269 | –2.7494 | –4.6531 | –6.5568 | 0.991 |
| 200 | 24.245 | 0.0891 | –2.5100 | –3.8478 | –5.1855 | 0.999 |
4 Comparative evaluation of low-cost adsorbents
Table 8 summarizes the uptake capabilities of T. polium leaf powder modified by ZnCl2 (S2) and the adsorbents earlier employed to eliminate KMnO4 from aqueous solutions. Based on this table, the adsorbent utilized in this study (S2) has the highest uptake capacity. The obtained uptake performance of this adsorbent, its stability, and its cheapness demonstrate that T. polium leaf powder modified by ZnCl2 will be given important consideration in the treatment of water and wastewater.
Adsorbents used for adsorption of KMnO4
| Adsorbents | q max (mg/g) | Sources | ||
|---|---|---|---|---|
| Modified T. polium L. (S2) | 833.33 | 27°C | This work | |
| 909.09 | 42°C | |||
| 1000.00 | 57°C | |||
| N. retusa leaf powder | 312.50 | 30°C | [23] | |
| 333.33 | 50°C | |||
| 344.83 | 60°C | |||
| O. basilicum leaf powder | 588.24 | 25°C | [24] | |
| 625.00 | 30°C | |||
| 666.67 | 45°C | |||
| 714.29 | 45°C | |||
| Modified Lamiaceae leaves | 333.33 | 25°C | [43] | |
| 384.62 | 35°C | |||
| 434.78 | 45°C | |||
| 476.19 | 60°C | |||
| Corn cob powder | 46.28 | [44] | ||
| Teak tree bark | 333.00 | [45] | ||
| Leaves of Neem | 60.60 | [46] | ||
| Sugar beet pulp | 250.02 | [47] | ||
| Orange peel modified by phosphoric acid | 307.64 | [48] | ||
| Sunflower seed husk | 45.26 | [49] | ||
| Mushroom waste as a substrate | 63.50 | [50] | ||
| Powder of mango leaves | 156.00 | [51] | ||
5 Conclusions
The highest percentage of KMnO4 removed from the solution (70.92%) and the maximum amount of this adsorbate adsorbed (141.85 mg/g) were obtained when the sample was modified by zinc chloride (S2). As a result, S2 was chosen as the unique adsorbent discovered in this study. It was found that increasing the mass of S2 from 0.01 to 0.05 g, the adsorption duration from 0 to 192 min, and the KMnO4 concentration from 10 to 1,200 mg/L, increases the adsorption amounts, which thereafter remain constant above 0.05 g, 192 min, and 1,200 mg/L, respectively. When the pH of the KMnO4 solution is elevated in the ranges of 1.5–5.5 and 5.5–11.5, in that sequence, the values of q e are slightly and suddenly dropped. Langmuir and second-order models are the most suitable for modeling the experimental data of this adsorption. Kinetics and thermodynamics data validated the endothermic, spontaneous, and chemical processes of this adsorption. The cheapness of T. polium leaves and ZnCl2, its stability in the chemical solutions, the easy and clean post-adsorption separation of this adsorbent from the aqueous solutions, and the high adsorption capacities (833.33, 909.09, and 1000.00 mg/g) obtained demonstrate that S2 will receive special attention in the field of treating contaminated water.
Acknowledgment
The authors are grateful to the Nanotechnology Research Unit, Faculty of Science, University of Tabuk, for providing us with the devices we needed to complete this research.
-
Funding information: The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
-
Author contributions: H.A.A. and N.A.A. designed the research plan and drafted, revised, and formatted the manuscript. H.A.A. performed the experimental work and data compilation. N.A.A. coordinated the work and discussed the results. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: The authors have no relevant financial or non-financial interests to disclose.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Verma RK, Kapoor R, Gupta SK, Chaudhari RR. An efficient technique for removal of K + and MnO4− ions through adsorption in aqueous solution by using activated charcoal. Pharm Chem J. 2014;1:20–5.Suche in Google Scholar
[2] Ordiales M, Fernández D, Verdeja L, Sancho J. Potassium permanganate as an alternative for gold mining wastewater treatment. J Min Met Mater Soc. 2015;67(9):1975–85.10.1007/s11837-015-1547-9Suche in Google Scholar
[3] Aprilliani F, Warsiki E, Iskandar A. Kinetic studies of potassium permanganate adsorption by activated carbon and its ability as ethylene oxidation material. Paper presented at the IOP Conference Series: Earth and Environmental Science; 2018. 10.1088/1755-1315/141/1/012003.Suche in Google Scholar
[4] Fayad PB, Zamyadi A, Broseus R, Prévost M, Sauvé S. Degradation of progestagens by oxidation with potassium permanganate in wastewater effluents. Chem Cent J. 2013 May;7(1):84.10.1186/1752-153X-7-84Suche in Google Scholar
[5] MacKinnon LK, Thomson NR. Laboratory-scale in situ chemical oxidation of a perchloroethylene pool using permanganate. J Contam Hydrol. 2002 May;56(1–2):49–74.10.1016/S0169-7722(01)00203-0Suche in Google Scholar
[6] Abdeen Z, Mohammad S, Mahmoud M. Adsorption of Mn (II) ion on polyvinyl alcohol/chitosan dry blending from aqueous solution. Env Nanotechnol Monit Manag. 2015;3:1–9.10.1016/j.enmm.2014.10.001Suche in Google Scholar
[7] Li GX, Huaug YH, Chen TC, Shih YJ, Zhang H. Reduction and immobilization of potassium permanganate on iron oxide catalyst by fluidized-bed crystallization technology. Appl Sci (Basel). 2012;2(1):166–74.10.3390/app2010166Suche in Google Scholar
[8] Bădescu IS, Bulgariu D, Ahmad I, Bulgariu L. Valorisation possibilities of exhausted biosorbents loaded with metal ions – A review. J Env Manage. 2018 Oct;224:288–97.10.1016/j.jenvman.2018.07.066Suche in Google Scholar PubMed
[9] Almasian A, Olya ME, Mahmoodi NM. Synthesis of polyacrylonitrile/polyamidoamine composite nanofibers using electrospinning technique and their dye removal capacity. J Taiwan Inst Chem Eng. 2015;49:119–28.10.1016/j.jtice.2014.11.027Suche in Google Scholar
[10] Mahmoodi NM, Hayati R, Arami M. Textile dye removal from single and ternary systems using date stones: Kinetic, isotherm, and thermodynamic studies. J Chem Eng Data. 2010;55(11):4638–49.10.1021/je1002384Suche in Google Scholar
[11] Ezeugo DJ, Anadebe C. Removal of potassium permanganate from aqueous solution by adsorption onto activated carbon prepared from animal bone and corn cob. Equatorial J Eng. 2018;14–21.Suche in Google Scholar
[12] Mahmoud ME, Yakout AA, Saad SR, Osman MM. Removal of potassium permanganate from water by modified carbonaceous materials. Desalination Water Treat. 2016;57(33):15559–69.10.1080/19443994.2015.1073180Suche in Google Scholar
[13] Rashad M, Al-Ghamdi SA, Alzahrani AOM, Al-Tabaa K, Al-Osemi S, Al-Atawi O, et al. Zinc oxide nanoparticles for adsorption of potassium permanganate from wastewater using shaking method. Desalination Water Treat. 2021;229:227–34.10.5004/dwt.2021.27387Suche in Google Scholar
[14] Mahmoodi NM. Nickel ferrite nanoparticle: Synthesis, modification by surfactant and dye removal ability. Water Air Soil Pollut. 2013;224(2):1419.10.1007/s11270-012-1419-7Suche in Google Scholar
[15] Aljohani MM, AL-Aoh HA. Adsorptive removal of permanganate anions from synthetic wastewater using copper sulfide nanoparticles. Mater Res Express. 2021;8(3):035012.10.1088/2053-1591/abef40Suche in Google Scholar
[16] Bulgariu L, Escudero LB, Bello OS, Iqbal M, Nisar J, Adegoke KA, et al. The utilization of leaf-based adsorbents for dyes removal: A review. J Mol Liq. 2019;276:728–47.10.1016/j.molliq.2018.12.001Suche in Google Scholar
[17] Al-aoh HA, Maah MJ, Ahmad A, Abas M. Isotherm and kinetic studies of 4-nitrophenol adsorption by NaOH-modified palm oil fuel ash. J Purit Util React Env. 2012;1:104–20.Suche in Google Scholar
[18] AL-Aoh HA, Maah MJ, Ahmad AA, Abas MR. Adsorption of 4-nitrophenol on palm oil fuel ash activated by amino silane coupling agent. Desalination Water Treat. 2012;40(1–3):159–67.10.1080/19443994.2012.671162Suche in Google Scholar
[19] Pandhare G, Trivedi N, Pathrabe R, Dawande S. Adsorption of cadmium (II) and lead (II) from a stock solution using neem leaves powder as a low-cost adsorbent. Int J Innov Res Sci Eng Technol. 2013;2(10):5752–61.Suche in Google Scholar
[20] Utkarsh M, Suresh G. Kinetic and equilibrium studies of Cr (VI) removal from aqueous solutions using activated neem bark. Res J Chem Env. 2011;15:2.Suche in Google Scholar
[21] Vinodhini V, Das N. Relevant approach to assess the performance of sawdust as adsorbent of chromium (VI) ions from aqueous solutions. Int J Env Sci Technol. 2010;7(1):85–92.10.1007/BF03326120Suche in Google Scholar
[22] Al-Aoh HA. Equilibrium, thermodynamic and kinetic study for potassium permanganate adsorption by Neem leaves powder. Desalination Water Treat. 2019;170:101–10.10.5004/dwt.2019.24905Suche in Google Scholar
[23] AL-Aoh HA. Adsorption of MnO4− from aqueous solution by Nitraria retusa leaves powder; kinetic, equilibrium and thermodynamic studies. Mater Res Express. 2019;6(11):115102.10.1088/2053-1591/ab4668Suche in Google Scholar
[24] Alamrani NA, Al-Aoh HA, Aljohani MMH, Bani-Atta SA, Sobhi M, Syed Khalid M, et al. Wastewater purification from permanganate ions by sorption on the Ocimum basilicum leaves powder modified by zinc chloride. J Chem. 2021;2021:2021.10.1155/2021/5561829Suche in Google Scholar
[25] Al-Shalabi E, Alkhaldi M, Sunoqrot S. Development and evaluation of polymeric nanocapsules for cirsiliol isolated from Jordanian Teucrium polium L. as a potential anticancer nanomedicine. J Drug Deliv Sci Technol. 2020;56:101544.10.1016/j.jddst.2020.101544Suche in Google Scholar
[26] Kouhbanani MA, Beheshtkhoo N, Taghizadeh S, Amani AM, Alimardani V. One-step green synthesis and characterization of iron oxide nanoparticles using aqueous leaf extract of Teucrium polium and their catalytic application in dye degradation. Adv Nat Sciences: Nanosci Nanotechnol. 2019;10(1):015007.10.1088/2043-6254/aafe74Suche in Google Scholar
[27] Khazaei M, Nematollahi-Mahani SN, Mokhtari T, Sheikhbahaei F. Review on Teucrium polium biological activities and medical characteristics against different pathologic situations. J Contemporary Med Sci. 2018;4(1):1–6.10.22317/jcms.03201801Suche in Google Scholar
[28] Karimi P, Javanshir S, Sayadi MH, Arabyarmohammadi H. Arsenic removal from mining effluents using plant-mediated, green-synthesized iron nanoparticles. Process (Basel). 2019;7(10):759.10.3390/pr7100759Suche in Google Scholar
[29] Alamrani NA, AL-Aoh HA. Elimination of congo red dye from industrial wastewater using Teucrium polium L. as a low-cost local adsorbent. Adsorpt Sci Technol. 2021;2021:2021.10.1155/2021/5728696Suche in Google Scholar
[30] Kuchekar SR, Patil MP, Gaikwad VB, Han SH. Synthesis and characterization of silver nanoparticles using Azadirachta indica (Neem) leaf extract. Int J Eng Sci Invent. 2017;6(4):47–55.Suche in Google Scholar
[31] Nekouei F, Nekouei S, Tyagi I, Gupta VK. Kinetic, thermodynamic and isotherm studies for acid blue 129 removal from liquids using copper oxide nanoparticle-modified activated carbon as a novel adsorbent. J Mol Liq. 2015;201:124–33.10.1016/j.molliq.2014.09.027Suche in Google Scholar
[32] Wu XL, Shi Y, Zhong S, Lin H, Chen JR. Facile synthesis of Fe3O4-graphene@ mesoporous SiO2 nanocomposites for efficient removal of Methylene Blue. Appl Surf Sci. 2016;378:80–6.10.1016/j.apsusc.2016.03.226Suche in Google Scholar
[33] Hameed BH, Ahmad AA. Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J Hazard Mater. 2009 May;164(2–3):870–5.10.1016/j.jhazmat.2008.08.084Suche in Google Scholar PubMed
[34] Kaya I, Yigit N, Benli M. Antimicrobial activity of various extracts of Ocimum basilicum L. and observation of the inhibition effect on bacterial cells by use of scanning electron microscopy. Afr J Tradit Complement Altern Med. 2008 Jun;5(4):363–9.10.4314/ajtcam.v5i4.31291Suche in Google Scholar PubMed PubMed Central
[35] Azha SF, Ismail S. Stability and durability studies of zwitterionic adsorbent coating for the removal of organic pollutants: chemical and thermal tolerance. IOP Conf Ser Mater Sci Eng. 2020;796(1):012054.10.1088/1757-899X/796/1/012054Suche in Google Scholar
[36] Darwish A, Rashad M, AL-Aoh HA. Methyl orange adsorption comparison on nanoparticles: Isotherm, kinetics, and thermodynamic studies. Dye Pigment. 2019;160:563–71.10.1016/j.dyepig.2018.08.045Suche in Google Scholar
[37] Zhang J, Ping Q, Niu M, Shi H, Li N. Kinetics and equilibrium studies from the methylene blue adsorption on diatomite treated with sodium hydroxide. Appl Clay Sci. 2013;83:12–6.10.1016/j.clay.2013.08.008Suche in Google Scholar
[38] Ho Y, McKay G. The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Can J Chem Eng. 1998;76(4):822–7.10.1002/cjce.5450760419Suche in Google Scholar
[39] Mahmoodi NM, Taghizadeh M, Taghizadeh A. Mesoporous activated carbons of low-cost agricultural bio-wastes with high adsorption capacity: preparation and artificial neural network modeling of dye removal from single and multicomponent (binary and ternary) systems. J Mol Liq. 2018;269:217–28.10.1016/j.molliq.2018.07.108Suche in Google Scholar
[40] Cegłowski M, Schroeder G. Removal of heavy metal ions with the use of chelating polymers obtained by grafting pyridine–pyrazole ligands onto polymethylhydrosiloxane. Chem Eng J. 2015;259:885–93.10.1016/j.cej.2014.08.058Suche in Google Scholar
[41] Zhou Y, Ge L, Fan N, Xia M. Adsorption of Congo red from aqueous solution onto shrimp shell powder. Adsorpt Sci Technol. 2018;36(5–6):1310–30.10.1177/0263617418768945Suche in Google Scholar
[42] Hou H, Zhou R, Wu P, Wu L. Removal of Congo red dye from aqueous solution with hydroxyapatite/chitosan composite. Chem Eng J. 2012;211:336–42.10.1016/j.cej.2012.09.100Suche in Google Scholar
[43] Aljohani MMH, Almizraq JMJ, Albalawi AM, Alshammari ASA, Albalawi NOS, Albalawi ANSA, et al. Efficient dye discoloration of modified Lamiaceae leaves. Mater Res Express. 2021;8(3):035503.10.1088/2053-1591/abeb8fSuche in Google Scholar
[44] Fatiha M, Belkacem B. Adsorption of methylene blue from aqueous solutions using natural clay. J Mater Env Sci. 2016;7(1):285–92.Suche in Google Scholar
[45] Patil S, Renukdas S, Patel N. Removal of methylene blue, a basic dye from aqueous solutions by adsorption using teak tree (Tectona grandis) bark powder. Int J Env Sci. 2011;1(5):711–26.Suche in Google Scholar
[46] Odoemelam SA, Emeh UN, Eddy NO. Experimental and computational chemistry studies on the removal of methylene blue and malachite green dyes from aqueous solution by neem (Azadirachta indica) leaves. J Taibah Univ Sci. 2018;12(3):255–65.10.1080/16583655.2018.1465725Suche in Google Scholar
[47] Li D, Yan J, Liu Z, Liu Z. Adsorption kinetic studies for removal of methylene blue using activated carbon prepared from sugar beet pulp. Int J Env Sci Technol. 2016;13(7):1815–22.10.1007/s13762-016-1012-5Suche in Google Scholar
[48] Guediri A, Bouguettoucha A, Chebli D, Chafai N, Amrane A. Molecular dynamic simulation and DFT computational studies on the adsorption performances of methylene blue in aqueous solutions by orange peel-modified phosphoric acid. J Mol Struct. 2020;1202:127290.10.1016/j.molstruc.2019.127290Suche in Google Scholar
[49] Ong ST, Keng PS, Lee SL, Leong MH, Hung YT. Equilibrium studies for the removal of basic dye by sunflower seed husk (Helianthus annuus). Int J Phys Sci. 2010;5(8):1270.Suche in Google Scholar
[50] Yan T, Wang L. Adsorptive removal of methylene blue from aqueous solution by spent mushroom substrate: equilibrium, kinetics, and thermodynamics. BioResources. 2013;8(3):4722–34.10.15376/biores.8.3.4722-4734Suche in Google Scholar
[51] Uddin MT, Rahman MA, Rukanuzzaman M, Islam MA. A potential low cost adsorbent for the removal of cationic dyes from aqueous solutions. Appl Water Sci. 2017;7(6):2831–42.10.1007/s13201-017-0542-4Suche in Google Scholar
© 2022 Hatem A. AL-Aoh and Nasser A. Alamrani, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop
Artikel in diesem Heft
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop

