Abstract
Industrial crops and products have proved to be an excellent alternative to petro-based chemicals. Vegetable oils are rich in functional groups that can be transformed into monomers and polymers with applications such as biodiesel, lubricants, inks, coatings, and paints. This study describes the synthesis of rapeseed oil (RO)-based esteramide for the first time. The reaction was carried out by amidation of RO, producing diol fatty amide (N,N-bis(2-hydroxyethyl) RO fatty amide), followed by its esterification reaction with hippuric acid, resulting in RO-based hippurate amide (ROHA). Fourier-transform infrared spectroscopy and nuclear magnetic resonance confirmed the introduction of amide and ester moieties in ROHA. ROHA was further reinforced with silver nanoparticles (SNPs) to develop corrosion-protective nanocomposite coatings. ROHA/SNP coatings were scratch-resistant, impact-resistant, and flexible and showed good corrosion resistance performance toward 3.5 w/w% NaCl medium, with adequate corrosion protection efficiency, and antimicrobial behavior against Staphylococcus aureus, Chromobacterium violaceum, Escherichia coli, Pseudomonas aeruginosa, and Candida albicans. ROHA/SNP coatings can be safely used up to 250°C.
1 Introduction
The hazards and expenses associated with petro-based chemicals have motivated researchers to substitute sustainable resource-based raw materials for the synthesis of monomers and polymers. Due to their rich functional attributes, vegetable oils (VOs), such as linseed, soybean, castor, and jatropha, among others, are utilized extensively as a sustainable alternative in the form of epoxies, polyesters, diols, polyols, polyurethanes, alkyds, and polyesteramide (PEA) resins [1,2,3,4,5]. Rapeseed oil (RO) is rich in oleic acid and can be transformed into epoxies, polyols, polyurethanes, and alkyds [6,7,8,9,10,11,12]. VO-based PEA resin is prepared by an esterification reaction between an amide diol (obtained by amidation of VO) and a carboxylic acid. While amide diols are derived from VO, a sustainable resource, carboxylic acids are generally synthetic in origin. Recently, PEA has been synthesized using lactic acid, tartaric acid, citric acid, gallic acid, and others that are obtained from natural resources [13,14,15,16,17].
Hippuric acid (HA; N-benzoyl glycine), a conjugation product of phenolic acid and glycine, is an important gut microbial co-metabolite found in urine [18]. HA is used as a biomarker for occupational exposure to toluene and styrene, which are widely used in plastics, rubbers, paints, glues, and other manufacturing units. HA is classified as a uremic toxin and a compound of pharmacological interest [19,20,21,22,23]. Methyl hippurate, an HA derivative, has been used as an antifungal compound [24]. In studies based on clinical data, another HA derivative, methenamine hippurate, has been shown to prevent recurrent urinary tract infections [25,26]. Some HA derivatives have shown promising antibacterial, antifungal, and antiretroviral potential [27,28].
In this study, novel esteramide resin has been prepared from RO-based diol and HA. RO esteramide resin was formulated into corrosion-protective nanocomposite coatings by reinforcement with biosynthesized silver nanoparticles (SNPs). The coatings were postcured by thermal polymerization via heat curing and later subjected to physicomechanical and anticorrosive performance evaluation. Our studies confirmed that these esteramide coatings performed well as protective coatings.
2 Materials and methods
RO, diethanolamine, sodium metal, methanol, sodium chloride (Winlab Limited, Berkshire, UK), and HA (Aldrich Chemical Company Inc., Milwaukee, USA) were used as received. SNPs were biosynthesized in an aqueous extract of Leucaena leucocephala leaves with 0.01 M silver nitrate precursor at room temperature, as reported in our previously published article [29].
2.1 Synthesis of N,N-bis(2-hydroxyethyl) RO fatty amide (HERA)
HERA was prepared according to our previously published article using RO, sodium methoxide, and diethanolamine as starting materials. After washing the final product with NaCl solution, pure HERA was obtained [29].
2.2 Synthesis of RO-based hippurate amide (ROHA) and ROHA/SNP nanocomposite
HERA (1 mol) and HA (1 mol) were taken in a four-necked, flat-bottomed conical flask equipped with a nitrogen inlet tube and thermometer. The contents were placed on a magnetic stirrer and vigorously stirred, followed by an increase in temperature up to 140°C and maintained for 6 h, after which the heating was turned off while stirring continued for an additional 30 min. The reaction was monitored by recording Fourier-transform infrared (FTIR) spectra and determining the acid value (AV) at regular intervals of time. The formation of ROHA was confirmed by the desired low AV and the appearance of characteristic absorption bands in the FTIR spectrum.
SNP (1%w/w) was added to ROHA, and the contents were thoroughly stirred for 60 min to obtain ROHA/SNP nanocomposite.
2.3 Preparation of ROHA and ROHA/SNP nanocomposite coatings
The mild steel/carbon steel panels of standard sizes (composition: Fe, 99.51%; Mn, 0.34%; C, 0.10%; and P, 0.05%) were first polished with silicon carbide papers of different grades, then washed with double distilled water, methanol, and acetone, degreased, and dried for application of coating material. ROHA and ROHA/SNP were diluted with toluene 40% (w/w) and were applied by brush on mild steel panels of standard sizes (70 mm × 25 mm × 1 mm and 25 mm × 25 mm × 1 mm) for evaluation of their physicomechanical performance, gloss measurements, and corrosion tests in 3.5% (w/w) NaCl medium. Another set of circular panels (diameter 1 cm and thickness 150 μm) was prepared for scanning electron microscopy (SEM) analysis. The coated panels were placed in a hot air oven at different temperatures and time periods to optimize the baking temperature and time, which was found to be 180°C for 4 h.
2.4 Characterization
The structural elucidation of ROHA and ROHA/SNP was carried out by FTIR (Spectrum 100 FTIR spectrophotometer; Perkin Elmer Cetus Instruments, Norwalk, CT, USA) and nuclear magnetic resonance (NMR; 1H NMR and 13C NMR; JEOL DPX400MHz, Japan) using deuterated chloroform and dimethyl sulfoxide as solvents and tetramethylsilane as internal standard. Thermal stability of ROHA and ROHA/SNP was assessed by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC; Mettler Toledo AG, Analytical CH-8603, Schwerzenbach, Switzerland). The morphology was studied by transmission electron microscopy (TEM; JEM-2100F; JEOL, Tozakishima, Japan), SEM (JSM-7600F; JEOL, Japan), and energy-dispersive X-ray spectroscopy (EDX; Oxford, UK; AV [ASTM D555-61]). To quantify the surface and functional groups of developed materials, an XPS spectrometer (JPS-9030; JEOL, Japan) was used with a MgKα (1253.6 eV) X-ray source at 10 mA and 12 kV in ultrahigh vacuum (<10−7 Pa). Thickness measurements (ASTM D1186-B) of coatings were taken with an Elcometer (Model 345; Elcometer Instrument Ltd, Manchester, UK). Scratch hardness (BS 3900), pencil hardness test (ASTM D3363-05), crosshatch (ASTM D3359-02), impact test (IS 101: Part 5: Section 1: 1988), bend test (ASTM D3281-84), gloss (Gloss meter, Model: KSJ MG6-F1; KSJ Photoelectrical Instruments Co., Ltd, Quanzhou, China), and contact angle measurements (CAM200 Attention goniometer) were performed by standard methods.
For the corrosion resistance test, the specimens were used as working electrodes. An exposed surface area of 1.0 cm2 was fixed by PortHoles electrochemical sample mask, with Pt electrode as a counter electrode and 3 M KCl-filled Ag electrode as a reference electrode (Autolab potentiostat/galvanostat, PGSTAT204-FRA32, with NOVA 2.1 software; Metrohm Autolab B.V., Kanaalweg 29-G, 3526KM, Utrecht, Switzerland).
Antimicrobial activity: Microbial strains: A total of five microbial strains were used in this study, viz., Staphylococcus aureus ATCC 29213 (Gram-positive), Chromobacterium violaceum ATCC 12472, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 (Gram-negative), and Candida albicans ATCC 10231 (fungi). C. albicans was cultured in potato dextrose broth, while all other bacteria were cultured in nutrient broth. The antibacterial activity was determined using an agar well diffusion assay on Mueller–Hinton Agar (MHA) plates. On MHA medium, 100 µL of 0.5 McFarland standardized bacterial inoculum was swabbed. The wells of 8 mm in diameter were then drilled into the medium using a sterile cork borer. A total of 100 µL of the test material were transferred into separate wells, and the plates were incubated at 37°C for 24 h. In the case of the film, a section of 1 cm2 was cut and placed on the MHA plates. After incubation, the plates were observed for the presence of a clear zone of inhibition around the well, indicating antibacterial activity, and zone size was measured in mm. The doxycycline (10 µg/disc) and nystatin (100 units/disc) served as positive controls for bacterial and fungal strains, respectively. The assay to determine the minimum inhibition concentration (MIC) was performed using micro-broth dilution with concentrations ranging from 256 to 0.0625 µg/ml, as described earlier [29].
3 Results and discussion
The hydroxyl groups of HERA reacted with the carboxylic groups of HA to form ROHA (Scheme 1), which was then reinforced with SNP to form the ROHA/SNP nanocomposite. ROHA and ROHA/SNP turned into a hard, flexibility retentive, and well-adhered coating after curing. The reaction was carried out without the use of any solvent, and the raw materials used, such as RO and HA, were biobased. The nanofiller SNP for reinforcement was biosynthesized by the green route [29].

Synthesis of RO-based hippurate amide (ROHA).
3.1 Spectral analysis
FTIR (υ, cm −1 ): FTIR spectra of RO and HERA have been reported earlier [35].
ROHA (Figure 1) shows absorption bands at: 3,324 (–OH str, broad); 3,062 (–C–H str aromatic ring: Ar); 3,006 (C═C); 2,854–2,925 (–CH3, –CH2 stretching); 1,738 (>C═O ester str); 1,642 (>C═O amide); 1,465–1,403 (–CH3, –CH2 bending); 1,297–1,115 (–(C═O)–O–C str); 1,578, 716, and 693 (Ar–C═C–H); 1,029–1,078 (–C–O str of –C–O–H); and 801–928 (–O–H bending vibrations), as typical for the functional groups present in VO-based PEA. The broadband for the hydroxyl group supports that some of the hydroxyl groups in ROHA are not undergoing esterification reaction with carboxylic groups of HA and occur as hydrogen-bonded –OH groups.
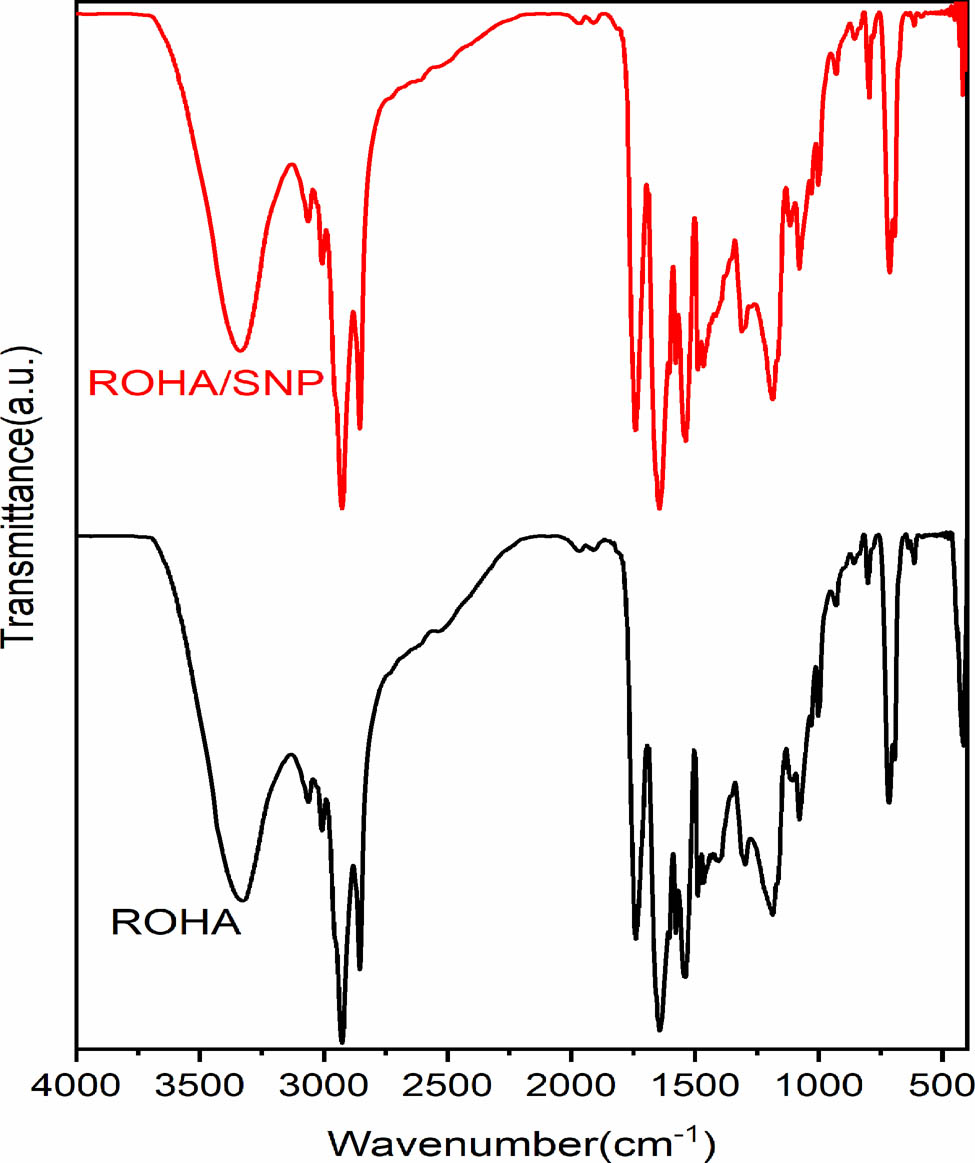
FTIR spectra of ROHA and ROHA/SNP nanocomposite.
ROHA/SNP (Figure 1) also reveals the presence of the aforementioned absorption bands, wherein some of the absorption bands exhibit a slight shift in values, such as –OH (3,335), 1,310–1,115 (–(C═O)–O–C str), and –O–H bending vibrations (795–930), due to interactions with SNP.
1 H NMR (DMSO- d 6 , δ , ppm)ROHA: 8.97–9.03 (–COOH); 8.48–8.59 (–NH–HA); 7.41–7.84 (aromatic ring protons); 5.86 (–OH); 5.25 (–CH═CH–); 4.06–4.27 (–CH 2–O–C(═O)–); 3.82–3.98 (–CH2HA); 3.56–3.62 (–N–CH 2–CH2–O–C(═O)–); 3.345–3.47 (–N–CH 2–CH2–OH); 3.05–3.09 (–N–CH2–CH 2–OH); 2.71 (–CH═CH–CH 2–CH═CH–); 2.31–2.45 (–CH 2–C(═O)–N–); 1.17–2.21(–CH 2–); and 0.79–0.86 (–CH 3) (Figure 2).
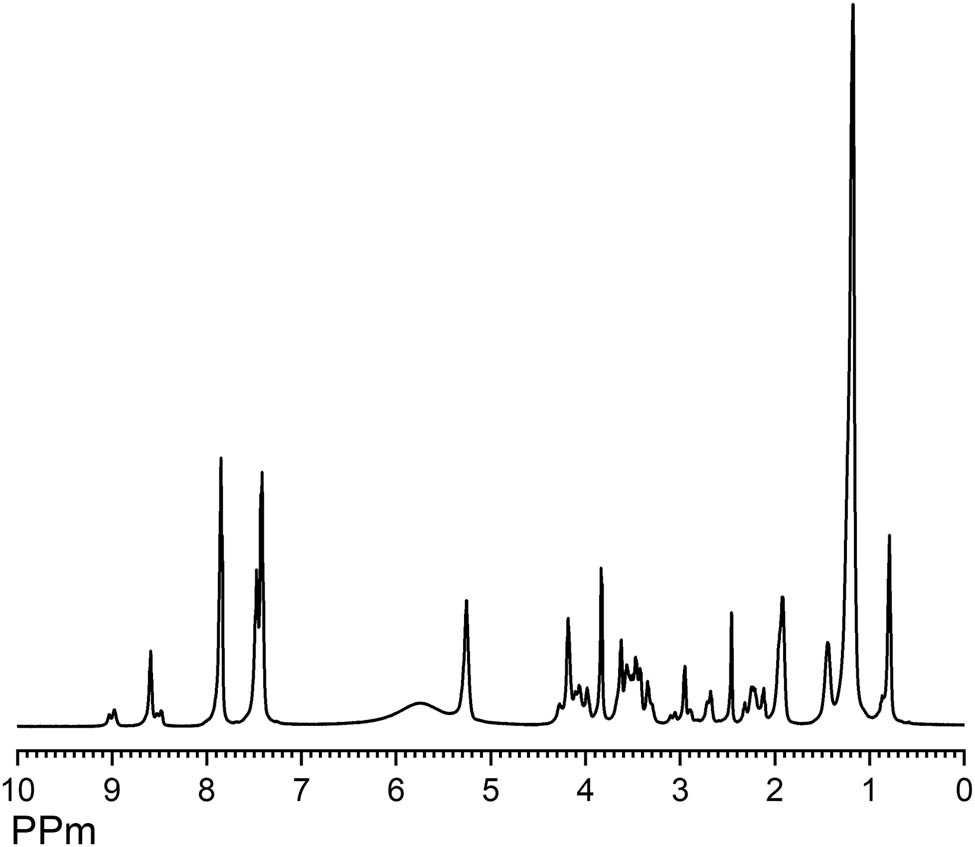
1H NMR spectra of ROHA.
13 C NMR (DMSO- d 6 , δ , ppm)ROHA: 174.67 (>N–C═O); 172.20–172.81 (>C═Oester); 166.23–166.77 (>NH–C═OHA); 127.28–127.97, 128.37, 131.29–131.61, and 133.66–134.25 (carbons ArHA); 41.03–42.24 (–CH2HA); 129.60–129.73 (–CH═CH–); 58.84–59.02 (>N–CH2 CH2–OH); 49.24–49.58 (>N–CH2CH2–OH); 22.13–33.93 (CH2chain); and 13.96 (–CH3) (Figure 3).
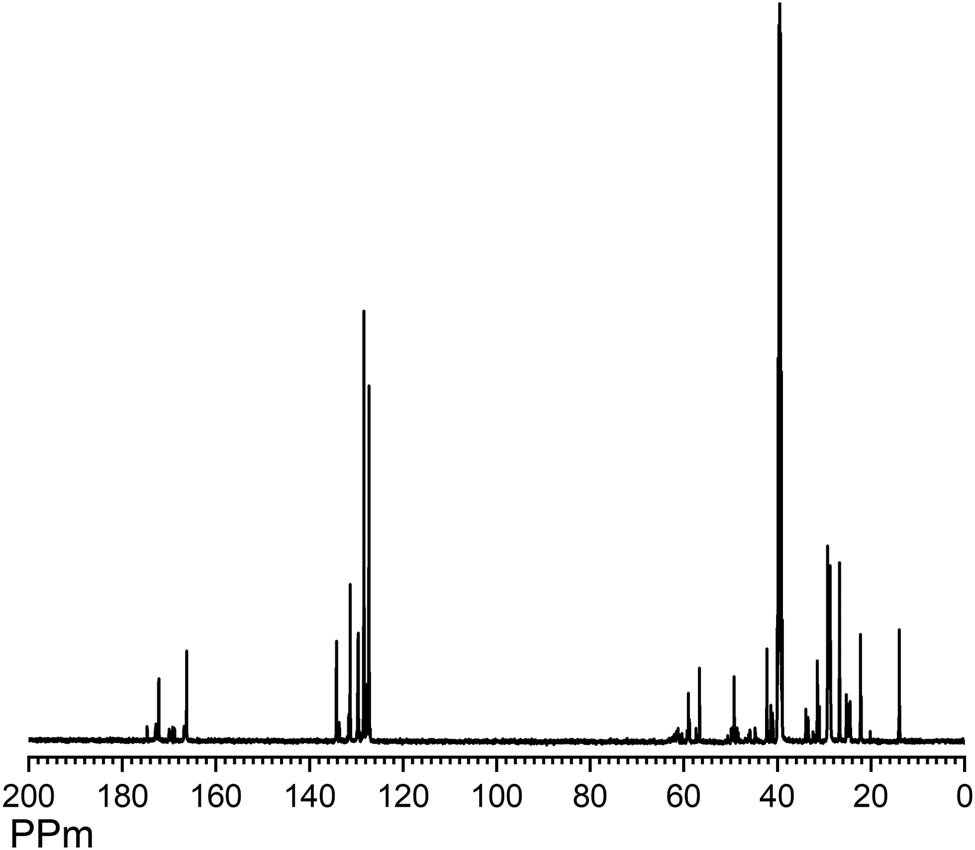
13C NMR spectra of ROHA.
The spectral analysis thus confirmed the reaction of HA with HERA, forming ROHA.
3.2 Morphology and hydrophobicity
TEM micrograph (Figure 4) of ROHA/SNP showed that SNPs were well-dispersed in ROHA; however, SNPs have been sheathed by the matrix. SEM micrograph (Figure 5a) of ROHA/SNP showed uniform distribution of SNP in ROHA matrix, with no pinholes or cracks. EDX (Figure 5b) peaks pertaining to Ag (1.19%), C (58.29%), N (21.31%), and O (19.21%) are evident and confirm the presence of Ag in ROHA/SNP.
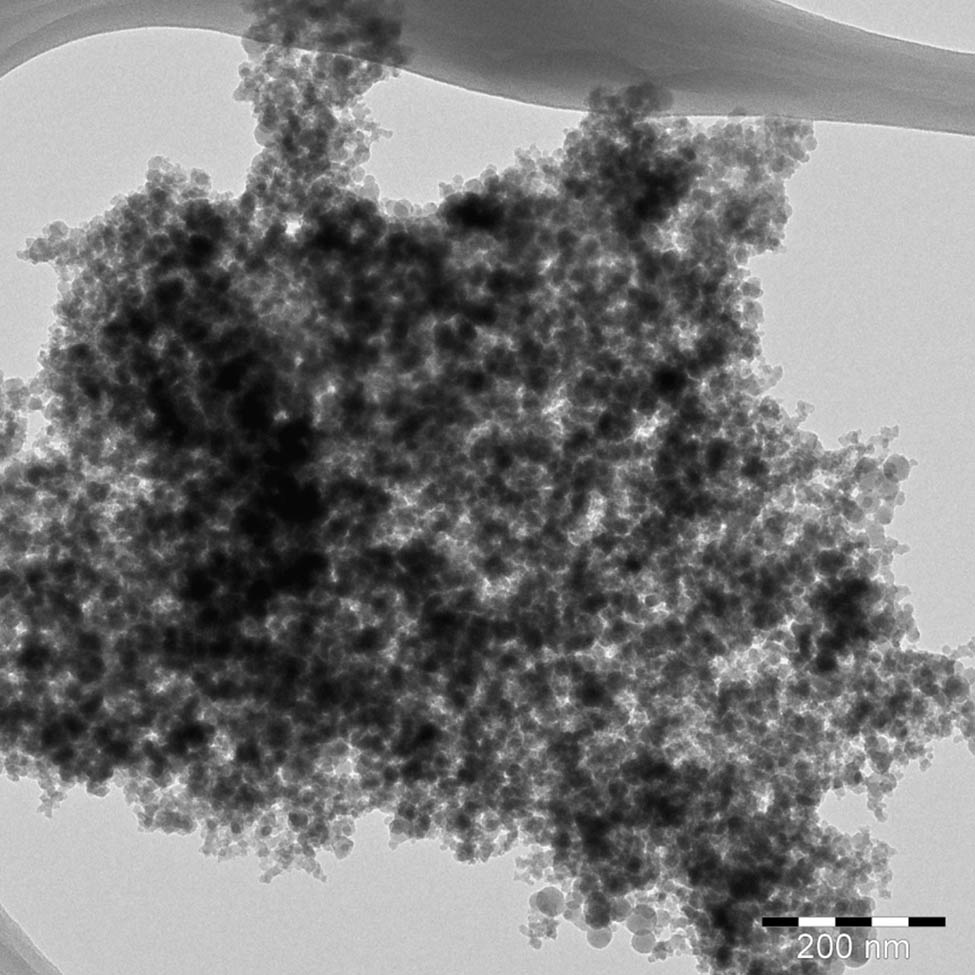
TEM micrograph of ROHA/SNP nanocomposite.
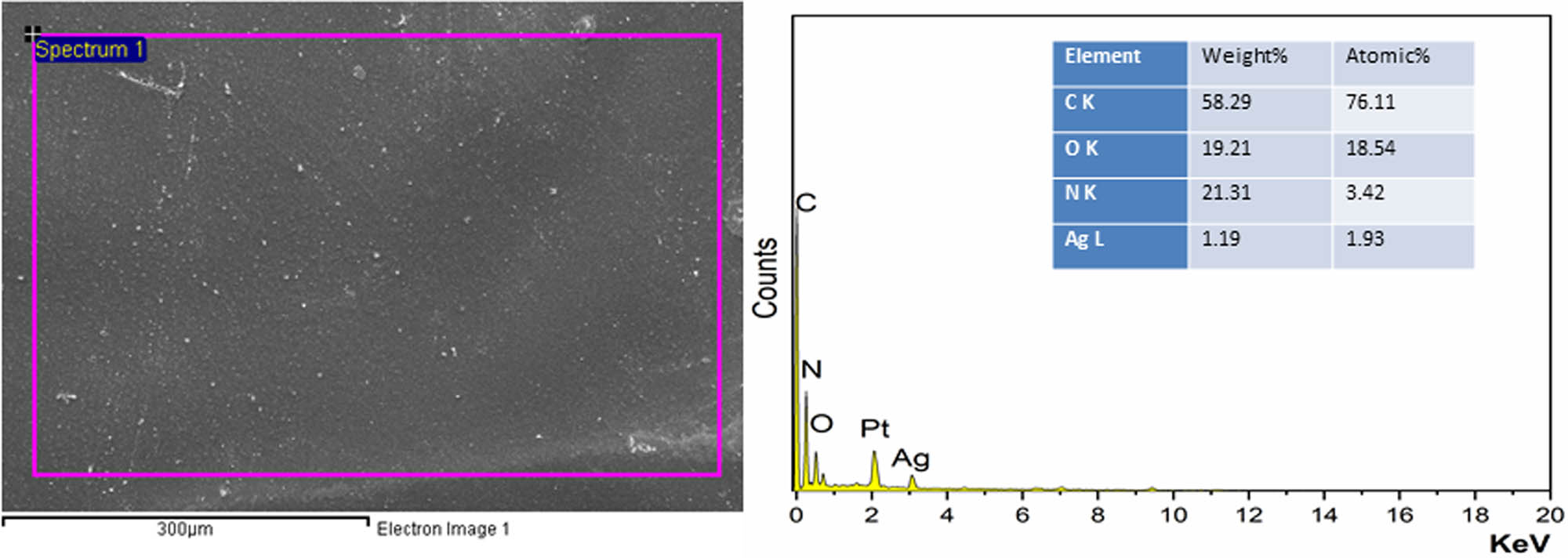
SEM–EDX micrograph of ROHA/SNP nanocomposite coating.
It is very important to evaluate the hydrophobicity of coatings as it plays a crucial role in determining their corrosion protection performance. The contact angle values (Figure 6) were found to be 80o and 86o for ROHA and ROHA/SNP, respectively. The contact angle value increased by the dispersion of SNP in ROHA, which supported the improved hydrophobicity of ROHA/SNP compared to the plain ROHA. Thus, the dispersion of SNP in the ROHA matrix improved the hydrophobicity of ROHA/SNP coating.
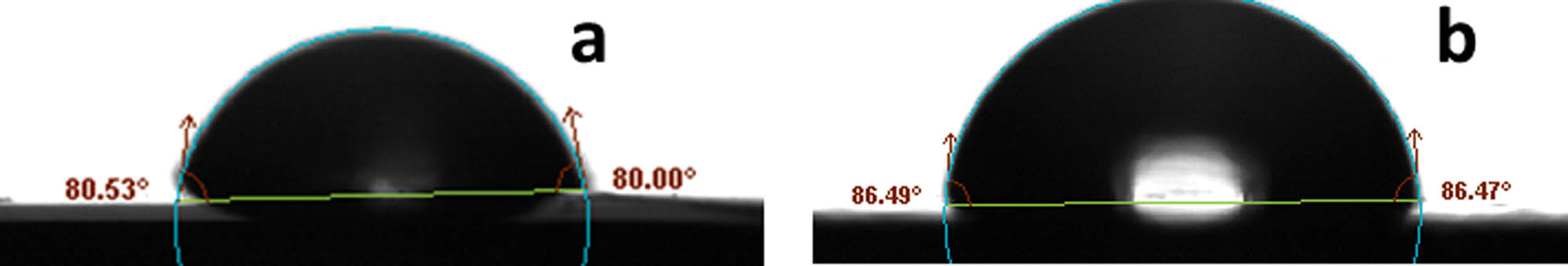
Hydrophobicity evaluation of ROHA (a) and ROHA/SNP nanocomposite (b) by contact angle measurements.
3.3 XPS analysis
XPS analysis was employed to elucidate the surface composition of the ROHA/SNP nanocomposite. The deconvoluted peaks of ROHA/SNP, C1s, N1s, O1s, and Ag3d are shown in Figure 7. The peaks for carbons (283.12 [C═C], 284.29 [C–C], 285.00 [C–N], 286.06 [C–O], and 287.68 [C═O]), nitrogens (399.13 [N–H] and 400.00 [N–C]), oxygens (531.40 [C═O] and 532.19 [C–O]), and silver (Ag3d5/2 [367.97] and Ag3d3/2 [374.20]) with respective binding energies supported the structure of ROHA/SNP nanocomposite [30,31].
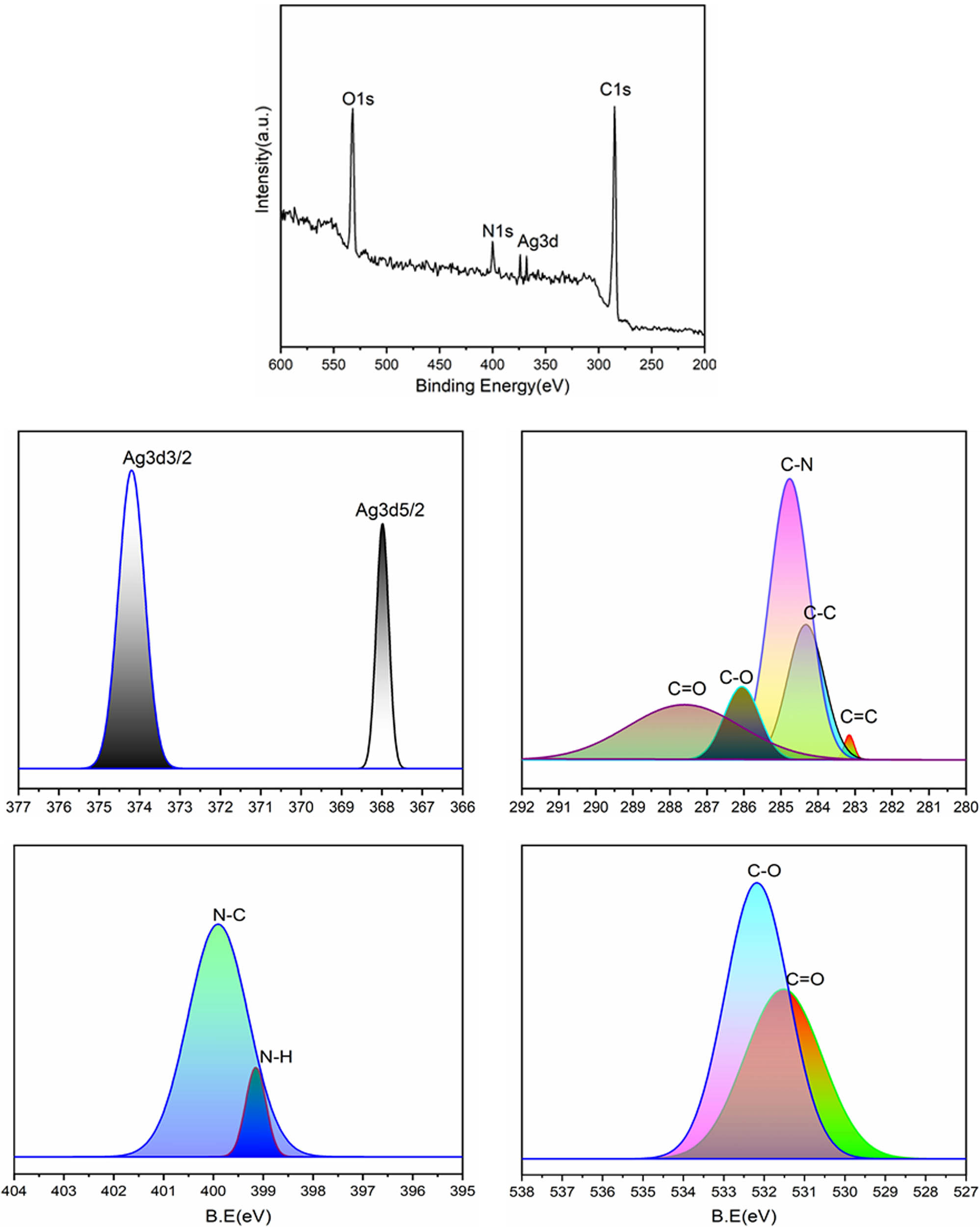
XPS of ROHA/SNP nanocomposite.
3.4 Coating properties
3.4.1 Physicomechanical properties
After curing at the required temperature and for a desirable period of time, hard and glossy coatings were obtained with a thickness of 125 microns. Both ROHA and ROHA/SNP coatings showed good scratch hardness, pencil hardness, and cross-hatch test results, as well as impact resistance and bend tests. The scratch hardness, cross hatch, pencil hardness, and gloss values increased with the dispersion of SNP in ROHA/SNP compared to ROHA (Table 1).
Physicomechanical properties of ROHA and ROHA/SNP nanocomposite coatings
| Properties | ROHA | ROHA/SNP |
|---|---|---|
| Scratch hardness (kg) | 2.8 | 3.1 |
| Cross hatch (%) | 95 | 100 |
| Pencil hardness | 2H | 3H |
| Gloss at 60o | 85 | 90 |
3.4.2 Corrosion resistance performance
3.4.2.1 Electrochemical impedance studies (EIS)
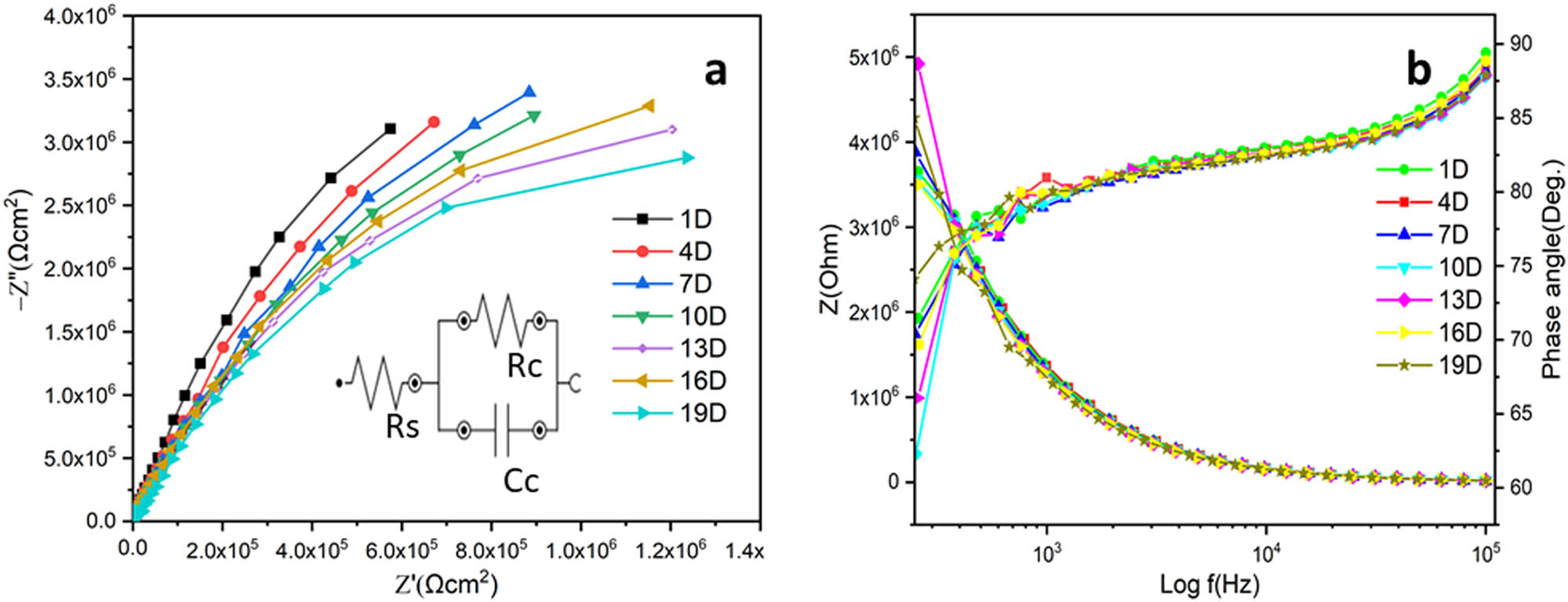
Nyquist plots of ROHA and ROHA/SNP were obtained at various immersion time periods (1, 4, 7, 10, 13, 16, and 19 days) in 3.5 wt% NaCl solution. As illustrated, the EIS plots of ROHA and ROHA/SNP nanocomposite coatings after the specific period of immersion displayed a one-time constant equivalent circuit model presented in Figure 8, displaying electrolyte resistance (R s), coating capacitance (C c), and coating resistance (R c). As can be seen in Table 2, the pure ROHA and ROHA/SNP coatings reveal that with an increased period of immersion, the solution resistance and coating resistance decrease because corrosive ions can gradually infiltrate via pores and capillary of the nanocomposite coating to adhere to the surface metal. Nyquist plots of ROHA and ROHA/SNP coatings showed similar behavior in terms of R s, R c, and C c. Furthermore, it could be seen that the arc frequencies decreased with time, which indicated an increase in corrosion. It can be due to the generation of pores in the nanocomposite coating or an increase in the exposed area of the existing pores.
EIS (a) and Bodetheta (b) spectra of ROHA coating.
EIS parameters of ROHA coatings in 3.5w/w% NaCl at room temperature
| Immersion times (days) | Solution resistance, R s (Ω) | Coating resistance, R c (MΩ) | Coating capacitance, C c (pF) | OCP (V) | χ 2 |
|---|---|---|---|---|---|
| 1 | 291 | 2.59 | 117 | –0.193 | 1.85 |
| 4 | 283 | 1.43 | 119 | –0.394 | 1.12 |
| 7 | 279 | 1.32 | 122 | –0.403 | 1.69 |
| 10 | 271 | 1.26 | 127 | –0.405 | 1.95 |
| 13 | 264 | 1.19 | 133 | –0.406 | 1.13 |
| 16 | 260 | 1.09 | 135 | –0.413 | 1.80 |
| 19 | 232 | 1.02 | 138 | –0.418 | 1.95 |
| EIS parameters of ROHA/SNP in 3.5w/w% NaCl at room temperature | |||||
|---|---|---|---|---|---|
| Immersion times (days) | Solution resistance, R s (Ω) | Coating resistance, R c (MΩ) | Coating capacitance, C c (pF) | OCP (V) | χ 2 |
| 1 | 281 | 9.13 | 109 | –0.554 | 1.67 |
| 4 | 278 | 8.69 | 112 | –0.550 | 1.91 |
| 7 | 277 | 8.54 | 115 | –0.544 | 1.65 |
| 10 | 261 | 8.30 | 120 | –0.528 | 1.59 |
| 13 | 247 | 8.08 | 121 | –0.464 | 1.73 |
| 16 | 233 | 7.56 | 126 | –0.454 | 1.82 |
| 19 | 220 | 7.06 | 130 | –0.083 | 1.39 |
Impedance responses of ROHA/SNP (Figure 9) show one capacitive arc found at the period of immersion, demonstrating an intact coating with a good barrier against water, oxygen, and corrosive ions. With increasing times of immersion, the resistance of the coating decreases. As shown in the figures, at initial exposure times, the resistance values of ROHA and ROHA/SNP coatings were 2.59 and 9.13 MΩ, correspondingly, which is approximately four times greater than pure ROHA coating. ROHA coating systems showed a one-time constant value even after 19 days of exposure time, which was characterized by a single capacitive loop that described the resistance of coatings. Consequently, the electrochemical behavior of ROHA/SNP coatings could be well inferred by the equivalent circuit model, shown in Figure 8, during all exposure periods. Since all observed impedance values for ROHA/SNP were higher than the plain ROHA coating, it seemed that ROHA/SNP coating showed higher barrier properties due to fine dispersion of SNP.
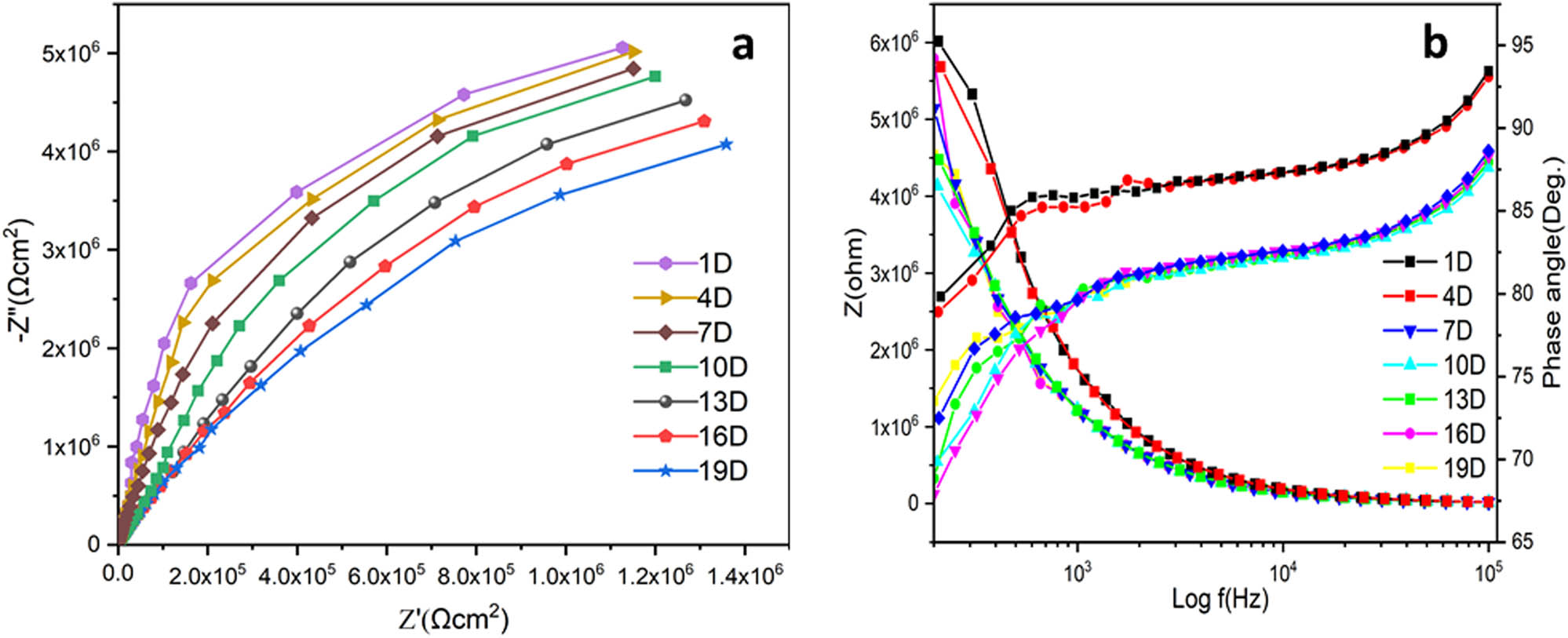
EIS (a) and Bodetheta (b) spectra of ROHA/SNP nanocomposite coating.
3.4.2.2 Coating resistance
As shown in Table 2, the incorporation of SNP into ROHA matrix generally improved Rc, irrespective of pure ROHA. This trend can be observed during all immersion periods in 3.5% (w/w) NaCl media. ROHA/SNP coating possesses Rc values (9.13 MΩ) four times greater than pure ROHA coating (2.59 MΩ) after 19 days of exposure time. Table 2 shows that Rc of ROHA/SNP decreased with increased immersion time and reached its lowest value after 19 days. Hence, the coating resistance, which is responsible for the presence of ions serving as charge carriers in the coating, decreased with increased immersion times due to the penetration of ions through pores and capillary channels of nanocomposite coating. It appeared that there were no pathways for corrosive ion transport when the coating resistance was high. The presence of SNP induced more hydrophobicity to the ROHA matrix, which had otherwise resulted in diminished coating performance due to water diffusion and ion transfer to the bare metal. Such nanosized silver particles occupy the superfine pores of the organic matrix, resulting in increased Rc. In addition, an amalgamation of SNP into ROHA improved the barrier properties of the resultant nanocomposite coating as these nanoparticles acted as hindrances against the diffusion of water and corrosive ions [32].
3.4.2.3 Open circuit potential (OCP)
OCP of ROHA and ROHA/SNP was observed during various periods (1, 4, 7, 10, 13, 16, and 19 days) of immersion in 3.5 wt% NaCl electrolyte (Table 2). As shown, both coatings had a negative charge when exposed to electrolyte during their respective periods of immersion. Negative OCP showed that the oxygen and water molecules could reach the metal substrate and then cathodic and anodic reactions happened, creating OH− and Fe2+ at the interface between nanocomposite coating and substrate. The organic coatings acted selectively to infuse ionic species; hence, the negative charge of ROHA and ROHA/SNP nanocomposite coatings designates these films as permeable to Fe2+ ions with reverse charge. The concentration of OH ions increased at the interface, which led to the creation of a passive layer on the substrate. As the time of exposure increased, Cl ions had a slower diffusion rate compared to OH ions that could reach the interface and repassivation happened [33]; then ROHA coating showed increase in the negative values of OCP with time. In the case of ROHA/SNP coating, nanosized silver particles occupied the pores present in the nanocomposite organic matrix and improved the crosslink density, resulting in a lower diffusion rate of corrosive ions and a lower value of OCP. OCP data were completely consistent with those observed for coating resistance. ROHA/SNP showed better anticorrosive performance as compared to ROHA.
3.5 TGA
DSC thermograms (Figure 10) of ROHA and ROHA/SNP showed two endothermic events at 150–200°C (centered at 175°C) and 300–350°C (centered at 325°C). TGA thermogram (Figure 11a) showed that 5 wt% loss occurred at 278 and 300°C in ROHA and ROHA/SNP, which supported the safe use of ROHA and ROHA/SNP up to 250°C. 25 wt%, 50 wt%, and 80% losses occurred at 328 and 328, 370 and 384, and 560 and 540°C in ROHA and ROHA/SNP, respectively. Until 500°C, both ROHA and ROHA/SNP showed variation in degradation pattern; however, beyond 500°C, the degradation ramp is the same in the case of both ROHA and ROHA/SNP. Differential thermogravimetric (DTG) thermograms (Figure 11b) of ROHA and ROHA/SNP exhibit endothermic events occurring from 250 to 500°C, corresponding to the degradation stages in TGA, which are distinct in DTG but appear somewhat merged with no clear-cut demarcation in TGA. The improved thermal stability of ROHA/SNP over ROHA is attributed to the dispersion of SNP in the matrix of the former [29].
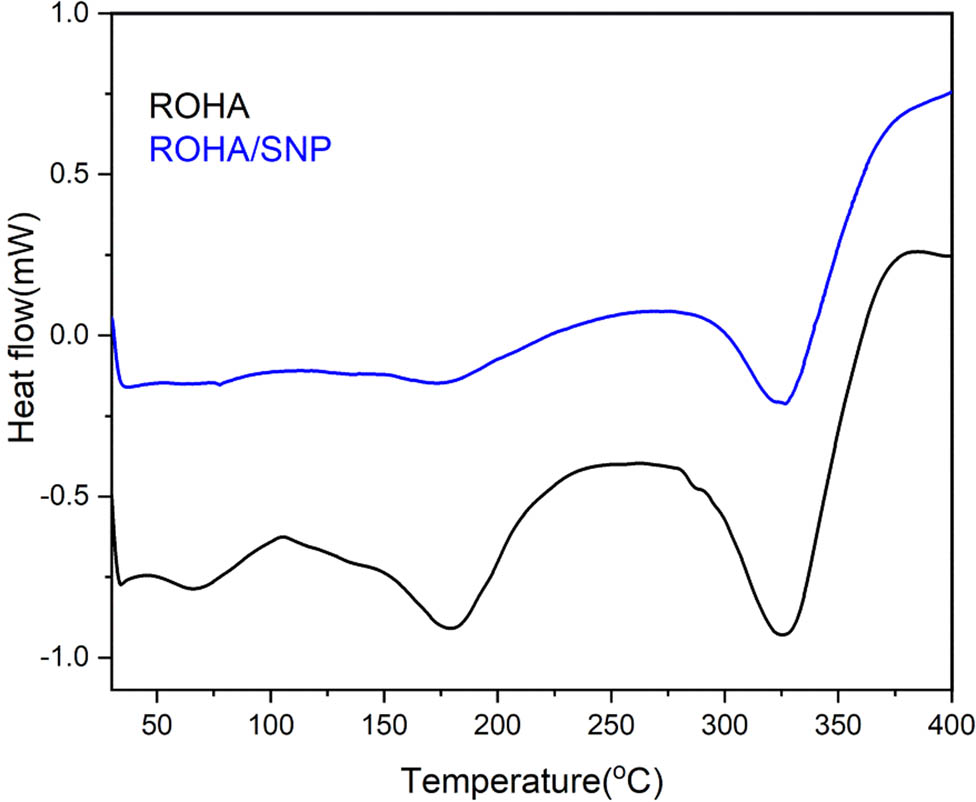
DSC thermogram of ROHA and ROHA/SNP nanocomposite.
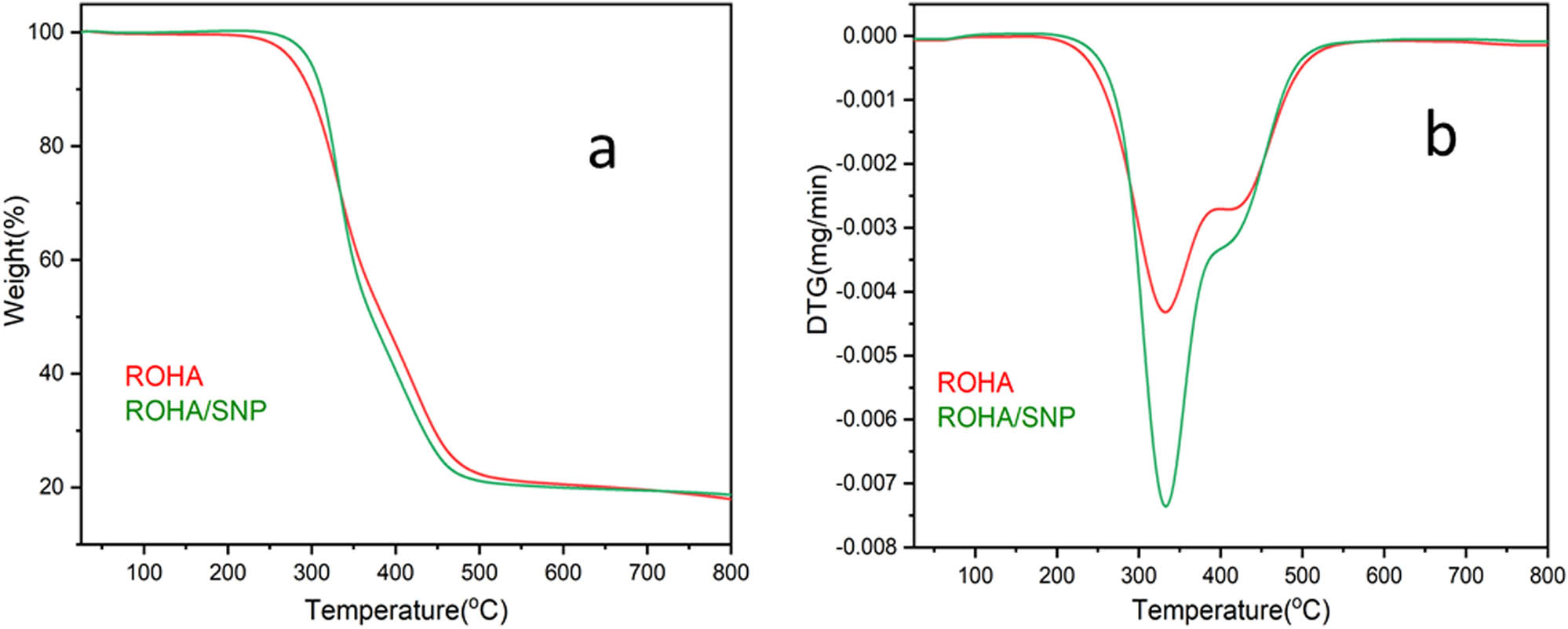
TGA (a)/DTG (b) thermogram of ROHA and ROHA/SNP nanocomposite.
3.6 Antimicrobial activity
The antibacterial activity of the synthesized test samples was determined against four bacterial strains and a solitary strain of C. albicans and recorded according to their respective zones of inhibition of antimicrobial activity and MIC values (Figure S1). SNP, a known metal with antimicrobial properties, demonstrated inhibition zones ranging from 13 to 16 mm (data not shown) against the test bacteria and fungi. Higher zones of inhibition were observed in the case of ROHA/SNP as compared to ROHA (Figure S1). ROHA demonstrated 15, 14, 16, 13, and 14 mm halo zones of inhibition against S. aureus, C. violaceum, E. coli, P. aeruginosa, and C. albicans, respectively. Furthermore, ROHA/SNP showed (Figure S2) comparatively enhanced antimicrobial activity against all the test pathogens as compared to ROHA (evident from zones of inhibition) with zone diameter ranging from 16 to 23 mm.
The pronounced antibacterial activity of the synthesized sample could be attributed to the large surface area possessed by nanosized SNP, which supports increased surface contact with pathogens. Furthermore, Ag is a known antimicrobial metal and may induce the production of intracellular ROS that overpowers the antioxidant system of the microbes, leading to oxidative stress and eventually cell death. We observed higher MIC values for S. aureus, which was found to be the least sensitive to the action of ROHA/SNP. This could plausibly be due to the difference in the make-up of the cell walls of Gram-positive and Gram-negative bacteria. The cell wall of Gram-positive bacteria (S. aureus) is composed of thickening peptidoglycan and possesses a negative charge on the surface due to the presence of teichoic acid, which is more sensitive to metal ions with a positive charge [34].
4 Conclusions
In this study, RO esteramide was synthesized without any organic solvent and reinforced with SNP. The esteramide resin was derived from biobased raw materials, i.e., RO and HA, and the SNP nanofiller was biosynthesized through green route using a plant extract. The coatings showed good physicomechanical and corrosion protection performance in saline medium and antimicrobial behavior against a number of microbes, such as S. aureus, C. violaceum, E. coli, P. aeruginosa, and C. albicans. With the dispersion of SNP, hydrophobicity, scratch resistance, and thermal stability of coatings were found to improve, as evident by higher contact angle (86o), scratch hardness (3.1 kg) values, and higher degradation temperatures of the nanocomposite relative to the plain esteramide coating. The coatings can be safely employed up to 250°C. The approach provides an excellent example of value-addition to an industrial crop for utilization as nanocomposite corrosion protective coatings.
Acknowledgment
The authors are grateful to the Researchers Supporting Project number (RSP-2021/113), King Saud University, Riyadh, Saudi Arabia for the support.
-
Funding information: This research was funded by Researchers Supporting Project number (RSP-2021/113).
-
Author contributions: Manawwer Alam: writing – original draft, methodology, project administration; resources, Mukhtar Ahmed: formal analysis, writing – review and editing, Mohammad Altaf: formal analysis, Fohad Mabood Husain: formal analysis, writing – review and editing.
-
Conflict of interest: The authors state no conflict of interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
References
[1] Sharmin E, Zafar F, Akram D, Alam M, Ahmad S. Recent advances in vegetable oils based environment friendly coatings: a review. Ind Crop Products. 2015;76:215–29.10.1016/j.indcrop.2015.06.022Suche in Google Scholar
[2] Alam M, Akram D, Sharmin E, Zafar F, Ahmad S. Vegetable oil based eco-friendly coating materials: A review article. Arab J Chem. 2014;7(4):469–79.10.1016/j.arabjc.2013.12.023Suche in Google Scholar
[3] Zafar F, Ghosal A, Sharmin E, Chaturvedi R, Nishat N. A review on cleaner production of polymeric and nanocomposite coatings based on waterborne polyurethane dispersions from seed oils. Prog Org Coat. 2019;131:259–75.10.1016/j.porgcoat.2019.02.014Suche in Google Scholar
[4] Yang F, Yu H, Deng Y, Xu X. Synthesis and characterization of different soybean oil-based polyols with fatty alcohol and aromatic alcohol. e-Polymers. 2021;21(1):491–9.10.1515/epoly-2021-0052Suche in Google Scholar
[5] Alam M, Sharmin E, Alandis NM, Ahmad N. Effect of organoclay on structure, morphology, thermal behavior and coating performance of Jatropha oil based polyesteramide. e-Polymers. 2017;17(6):491–500.10.1515/epoly-2017-0096Suche in Google Scholar
[6] Dworakowska S, Bogdal D, Prociak A. Microwave-assisted synthesis of polyols from rapeseed oil and properties of flexible polyurethane foams. Polymers. 2012;4:1462–77.10.3390/polym4031462Suche in Google Scholar
[7] Yesmine F, Hassen R, Bayramoglu G. A one step route synthesis of polyurethane network from epoxidized rapeseed oil. Prog Org Coat. 2017;105:48–55.10.1016/j.porgcoat.2016.12.021Suche in Google Scholar
[8] Su Y, Lin H, Zhang S, Yang Z, Yuan T. One-step synthesis of novel renewable vegetable oil-based acrylate prepolymers and their application in UV-Curable coatings. Polymers. 2020;12(5):1165.10.3390/polym12051165Suche in Google Scholar PubMed PubMed Central
[9] Patel VC, Varughese J, Krishnamoorthy PA, Jain RC, Singh AK, Ramamoorty M. Synthesis of alkyd resin from jatropha and rapeseed oils and their applications in electrical insulation. J Appl Polym Sci. 2008;107(3):1724–9.10.1002/app.27195Suche in Google Scholar
[10] Leszczyńska M, Malewska E, Ryszkowska J, Kurańska M, Gloc M, Leszczyński MK, et al. Vegetable fillers and rapeseed oil-based polyol as natural raw materials for the production of rigid polyurethane foams. Materials. 2021;14(7):1772.10.3390/ma14071772Suche in Google Scholar PubMed PubMed Central
[11] Tynek M, Pawłowicz R, Gromadzka J, Tylingo R, Wardencki W, Karlovits G. Virgin rapeseed oils obtained from different rape varieties by cold pressed method – their characteristics, properties, and differences. Eur J Lipid Sci Technol. 2012;114(3):357–66.10.1002/ejlt.201100296Suche in Google Scholar
[12] Kurańska M, Prociak A, Kirpluks M, Cabulis U. Polyurethane–polyisocyanurate foams modified with hydroxyl derivatives of rapeseed oil. Ind Crop Prod. 2015;74:849–57.10.1016/j.indcrop.2015.06.006Suche in Google Scholar
[13] Alam M, Altaf M, Ahmad N. Canola oil based poly(ester–ether–amide–urethane) nanocomposite and its anti-corrosive coatings. Polymers. 2021;13(19):3325.10.3390/polym13193325Suche in Google Scholar PubMed PubMed Central
[14] Alam M, Alandis N, Ahmad N, Husain F. Anticorrosive and antibacterial nanocomposite coating material from sustainable resource. Ind Crop Prod. 2020;158:112955.10.1016/j.indcrop.2020.112955Suche in Google Scholar
[15] Ahmad S, Ashraf SM, Sharmin E, Alam M. Ambient cured tartaric acid modified oil fatty amide anticorrosive coatings. J Macromol Sci Part A. 2005;42(6):751–64.10.1081/MA-200058648Suche in Google Scholar
[16] Bharathi NP, Khan NU, Alam M, Shreaz S, Hashmi AA. Edible oil-based metal-containing bioactive polymers: synthesis, characterization, physicochemical and biological studies. J Inorg Organomet Polym Mater. 2010;20(4):839–46.10.1007/s10904-010-9412-3Suche in Google Scholar
[17] Li X, Su Y, Chen Q, Lin Y, Tong Y. Synthesis and characterization of biodegradable hyperbranched poly(ester-amide)s based on natural material. Biomacromolecules. 2005;6:3181–8.10.1021/bm050531lSuche in Google Scholar PubMed
[18] Henning SM, Wang P, Abgaryan N, Vicinanza R, de Oliveira DM, Zhang Y, et al. Phenolic acid concentrations in plasma and urine from men consuming green or black tea and potential chemopreventive properties for colon cancer. Mol Nutr Food Res. 2013;57(3):483–93.10.1002/mnfr.201200646Suche in Google Scholar PubMed PubMed Central
[19] Decharat S. Hippuric Acid levels in paint workers at steel furniture manufacturers in Thailand. Saf Health Work. 2014;5(4):227–33.10.1016/j.shaw.2014.07.006Suche in Google Scholar PubMed PubMed Central
[20] Vitali L, Gonçalves S, Rodrigues V, Fávere VT, Micke GA. Development of a fast method for simultaneous determination of hippuric acid, mandelic acid, and creatinine in urine by capillary zone electrophoresis using polymer multilayer-coated capillary. Anal Bioanal Chem. 2017;409:1943–50, (1618–2650 (Electronic)).10.1007/s00216-016-0142-4Suche in Google Scholar PubMed
[21] Lees HJ, Swann JR, Wilson ID, Nicholson JK, Holmes E. Hippurate: the natural history of a mammalian–microbial cometabolite. J Proteome Res. 2013;12(4):1527–46.10.1021/pr300900bSuche in Google Scholar PubMed
[22] Chiu CH, Chen CT, Cheng MH, Pao LH, Wang C, Wan GH. Use of urinary hippuric acid and o-/p-/m-methyl hippuric acid to evaluate surgical smoke exposure in operating room healthcare personnel. Ecotoxicol Environ Saf. 2021 Jul 1;217:112231, (1090–2414 (Electronic)).10.1016/j.ecoenv.2021.112231Suche in Google Scholar PubMed
[23] Lu YC, Wu CC, Tsai IT, Hung WC, Lee TL, Hsuan CF, et al. Associations among total p-cresylsulfate, indoxyl sulfate and hippuric acid levels with hemodialysis quality indicators in maintenance hemodialysis patients. Clinica Chim Acta Int J Clin Chem. 2021 May;516:83–91, (1873–3492 (Electronic)).10.1016/j.cca.2021.01.015Suche in Google Scholar PubMed
[24] Maung CEH, Lee HG, Cho JY, Kim KA-O. Antifungal compound, methyl hippurate from Bacillus velezensis CE 100 and its inhibitory effect on growth of botrytis cinerea. World J Microbiol Biotechnol. 2021;37:159, (1573-0972 (Electronic)).10.1007/s11274-021-03046-xSuche in Google Scholar PubMed
[25] Hollyer I, Varias F, Ho B, Ison MA-O. Safety and efficacy of methenamine hippurate for the prevention of recurrent urinary tract infections in adult renal transplant recipients: a single center, retrospective study. Transpl Infect Dis. 2019;21:e13063, (1399–3062 (Electronic)).10.1111/tid.13063Suche in Google Scholar PubMed PubMed Central
[26] Bakhit M, Krzyzaniak N, Hilder J, Clark J, Scott AM, Mar CD. Use of methenamine hippurate to prevent urinary tract infections in community adult women: a systematic review and meta-analysis. Br J Gen Pract J R Coll Gen Pract. 2021;71(708):e528–37.10.3399/BJGP.2020.0833Suche in Google Scholar PubMed PubMed Central
[27] Tahir T, Ashfaq M, Asghar H, Shahzad IM, Tabassum R, Ashfaq A. Medicinal importance of azo and hippuric acid derivatives. Mini-Rev Med Chem. 2019;19(9):708–19.10.2174/1389557518666180727162018Suche in Google Scholar PubMed
[28] Abbas SY, El-Sharief MAMS, Basyouni WM, Fakhr IMI, El-Gammal EW. Thiourea derivatives incorporating a hippuric acid moiety: synthesis and evaluation of antibacterial and antifungal activities. Eur J Med Chem. 2013;64:111–20.10.1016/j.ejmech.2013.04.002Suche in Google Scholar PubMed
[29] Alam M, Alandis NM, Sharmin E, Ahmad N, Husain FM, Khan A. Mechanically strong, hydrophobic, antimicrobial, and corrosion protective polyesteramide nanocomposite coatings from Leucaena leucocephala oil: a sustainable resource. ACS Omega. 2020;5(47):30383–94.10.1021/acsomega.0c03333Suche in Google Scholar PubMed PubMed Central
[30] Zhu K, li X, Wang H, Li J, Fei G. Electrochemical and anti-corrosion behaviors of water dispersible graphene/acrylic modified alkyd resin latex composites coated carbon steel. J Appl Polym Sci. 2017;134(11):44445.10.1002/app.44445Suche in Google Scholar
[31] Ghosh T, Karak N. Mechanically robust hydrophobic interpenetrating polymer network-based nanocomposite of hyperbranched polyurethane and polystyrene as an effective anticorrosive coating. N J Chem. 2020;44(15):5980–94.10.1039/D0NJ00322KSuche in Google Scholar
[32] Cai M, Fan X, Yan H, Li Y, Song S, Li W, et al. In situ assemble Ti3C2Tx MXene@MgAl-LDH heterostructure towards anticorrosion and antiwear application. Chem Eng J. 2021;419:130050.10.1016/j.cej.2021.130050Suche in Google Scholar
[33] Dolatzadeh F, Moradian S, Jalili MM. Influence of various surface treated silica nanoparticles on the electrochemical properties of SiO2/polyurethane nanocoatings. Corros Sci. 2011;53(12):4248–57.10.1016/j.corsci.2011.08.036Suche in Google Scholar
[34] Lu K-T, Chang J-P. Synthesis and antimicrobial activity of metal-containing linseed oil-based waterborne urethane oil wood coatings. Polymers. 2020;12(3):663.10.3390/polym12030663Suche in Google Scholar PubMed PubMed Central
[35] Alam M, Altaf M, Ahmad N. Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties. e-Polymers. 2022;22:190–202.10.1515/epoly-2022-0021Suche in Google Scholar
© 2022 Manawwer Alam et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop
Artikel in diesem Heft
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop

