Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
-
Mohamed S. Kamar
, Huseyin Ozan Tekin
, Abdullah M. Alzahrai
Abstract
Variable single and/or swarms of post-granitic dikes are widespread at Gabal Serbal, Southwestern Sinai, Egypt. The present article aims to identify and discriminate these multiphase dikes through detailed geological, petrographical, and geochemical examinations. These dikes are classified into two subphases: (1) acidic dikes (porphyritic dacite, microgranite, granophyre, and alkaline granophyre dikes); and (2) basic dikes (basalt and dolerite dikes). They range from vertical or steeply inclined bodies, 0.5–15 m wide, pink to black color, and NE–SW to N–S directions. Acidic dikes with different mineralogical constituents have medium to high k-characters, originating from calc-alkaline magma and extruded in a volcanic arc environment. In contrast, basic dikes have medium k-characters, originating from tholeiitic magma and developing within a plate environment. Basic dikes are enriched with opaque minerals, where the basaltic dike contains iron oxides (magnetite and hematite), such as apatite in addition to copper minerals. Dolerite dike comprises magnetite, titanomagnetite, and pyrite.
1 Introduction
The basement rocks of Sinai represent the northwestern extremity of the Arabian-Nubian Shield (ANS) separated from the main block by the Gulf of Suez and the Gulf of Aqaba. These rocks were formed as a result of complex events of subduction, accretion, and extension during Pan African times [1,2]. The Gabal Serbal area is located in the southwestern part of Sinai Peninsula, between latitudes 28° 32′ and 28° 40′ 43˝N and longitudes 33° 34′ and 33° 41′ 30″E (Figure 1). The area under investigation covers about 233 km2 and is considered a part of the Arabian-Nubian Shield. The Pan-African dike swarms in southwestern Sinai were classified into two swarms: the first (oldest) strikes at 35° and exhibits calc-alkaline chemical properties while the second strikes at 135° and displays transitional to mildly alkaline properties [3]. Geology is considered one of the most important and best sciences in ancient and modern times. It helps discover some of the geological secrets hidden in deep burials and different rocks containing some minerals that have economic feasibility [4]. Dikes are economically significant because they are critical in the formation of world deposits such as tungsten and gold [5,6], and they structurally control the flow and thus the exploration of natural resources such as groundwater and geothermal energy [7,8]. On the other hand, radioactive mineralization linked with red, black, and jasperoid silica veins exhibits apparent brilliant yellow secondary uranium-bearing minerals [9,10,11,12]. Dikes may be straight (and parallel to rift zones), curved, or radiating, with the focal point of the swarm understood to designate the core of a magmatic edifice or mantle plume [13], and are often linked with crustal extension [14,15,16]. It has long been assumed that dike emplacement is somewhat regulated by the regional stress field based on field connections [17,18]. The present study aims to detect the petrography and geochemistry analyses in addition to determine some of the economic mineralization associated with the different dikes at the Gabal Serbal area, Southwestern Sinai, Egypt.
![Figure 1
Distribution map of dikes traversing the rock varieties in the G. Serbal area, Southwestern Sinai, Egypt (Kamar [19]).](/document/doi/10.1515/chem-2022-0136/asset/graphic/j_chem-2022-0136_fig_001.jpg)
Distribution map of dikes traversing the rock varieties in the G. Serbal area, Southwestern Sinai, Egypt (Kamar [19]).
2 Materials and methods
2.1 Geological setting of dikes
The post-granitic dikes of the study area occur either as single dikes or as swarms. These dikes are the product of the most recent episodes of magmatism. They have vertical or steeply sloped bodies that have been injected into all of the preceding strata. Their widths range from 0.5 to 15 m. The dike swarms are more resistant to weathering and erosion than their host. The contacts between dikes and their host rocks are sharp. These dikes are classified into acidic and basic dikes. Acidic dikes are represented by porphyritic dacite, microgranite, granophyre, and alkaline granophyre. Porphyritic dacite dikes are massive and fine-grained with a grayish color. These dikes strike at NE–SW, and N–S. Porphyritic dacite dikes have irregular shapes and their width varies between 0.8 and 2.5 m. They cut the gneisses and migmatites in Wadi Agela and the younger granites in Wadi Gebeiy. Microgranite dikes are massive, porphyritic, and fine-grained with pale red color. They exhibit porphyritic texture, where quartz, plagioclase, and K-feldspar are the phenocrysts. These dikes are vertical and strike at NNE–SSW and N–S. They range between 0.5 and 2.5 m in width and cut the younger granites in Wadi Gebeiy. Granophyre dikes are massive, porphyritic, and fine-grained with a greenish-gray color. Biotite, quartz, K-feldspar, and plagioclase phenocrysts are embedded in a quartz-feldspathic matrix. These dikes are of limited distribution and strike at NE–SW with their widths varying between 0.5 m and 5 m. They cut the gneisses in Wadi Agela and the younger granites in Wadi Abura. Alkaline granophyre dikes are massive, porphyritic, and fine-grained with red color. Quartz, K-feldspar, riebeckite, and plagioclase phenocrysts are embedded in the quartz-feldspathic matrix. These dikes are of limited distribution and strike at NE–SW to ENE–WSW and their widths vary between 1 and 15 m (Figure 2a). They cut the gneisses and migmatites and alkaline granite in Wadi Agela and also the alkali feldspar granite in Wadi Abura. Basic dikes are represented by basalt and dolerite dikes. Basalt dikes are hard, fine-grained with a dark green to grayish-green color. They occur as single or as swarms (Figure 2b). They are marked by negative topography and suffer exfoliation weathering forming onion structures. Basaltic dikes strike NNE–SSW, N–S, and NNW–SSE varying in width from 0.5 to 5 m. Also, the thicker dikes attain a well-pronounced chilled margin against the host rock. They are observed cutting the gneisses and migmatites at Wadis: Agela, Allyiat, Rim, and Um Takha also cutting metasediments–metavolcanic association at Wadi Wirqa. They cut older granites between Wadi Ramuz and Wadi Wirqa. These dikes cut the younger granite at Wadis Geba, Gebeiy, and Abura. Dolerite dikes are medium-grained with dark-green to reddish-brown color. They are marked by negative topography relative to their country rocks. These dikes strike at NNE-SSW and NE–SW. They vary in their width between 0.5 and 6 m. The thicker dikes attain a very distinctive chilled margin, where the margin of the dike is fine-grained, and the grain size increases inwards the dyke core. The latter is characterized by porphyritic texture with coarse-grained plagioclase phenocrysts. They cut the gneisses at Wadi Rim: the older granites between Wadi Ramuz and Wadi Wirqa and younger granites at Wadi Geba. One of them cuts the syenogranite at the mouth of Wadi Geba. It exhibits minerals alteration product at its periphery and the contact record high radioactive anomaly. This dike acts as a physical and chemical barrier for the accumulation of radioactive minerals. In the study area, there are about 142 major dikes, and totally, about 278.8 km length was traced and mapped (Figure 1). The directional trend analysis indicates that these dikes exhibit three main trends, namely, N (33–56°) E with tensional regime N (34–57°) W, N (57–76°) E with tensional regime N (14–33°) W, and N (12–32°) E with tensional regime N (58–78°) W regarding their number and length proportions.
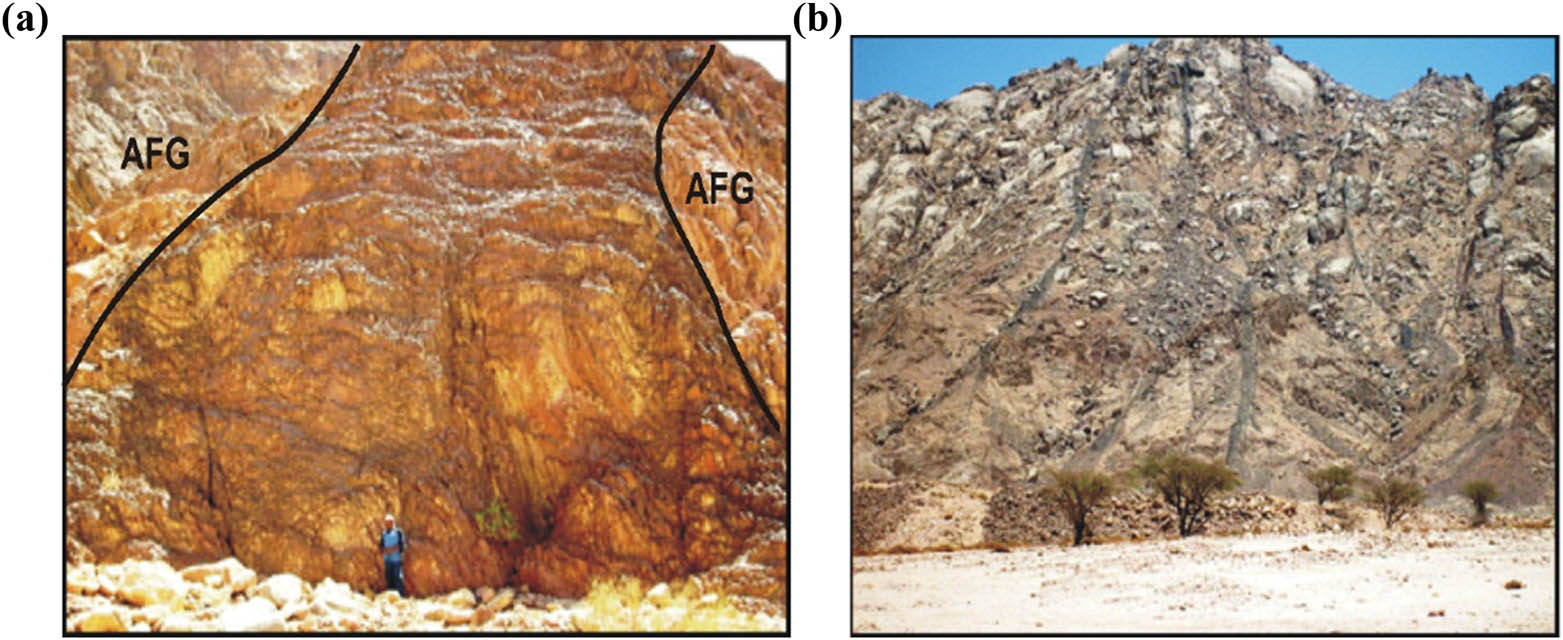
Field photographs showing (a) alkaline granophyre dike cutting alkali feldspar granite (AFG) at Wadi Abura; striking at ENE; and (b) dyke swarms of basalt cutting syenogranite at Wadi Geba; striking at N 65° E.
2.2 Methods and sample collection
A total of 36 samples were collected from the studied dikes in the assessed region. Chemical analysis was performed on 18 samples from various types of dikes. The samples (about 1 kg) were transferred to the laboratory for analysis. The acquired samples were dried and sieved through a fine mesh (1 mm) (0.256) mm sieve for homologation. To completely dry the samples, they were put in an oven and baked for a day at 100°C. After weighing the samples, they were transported to a 212.6 cm3 polyethylene measuring cylinder with a 9.5 cm diameter and a 3 cm height. Major oxides and trace elements were detected using the X-ray fluorescence technique. X-ray fluorescence analysis (XRF) is a sophisticated spectroscopic method used to determine a substance’s elemental makeup. Numerous elements, from beryllium (Be) through uranium, may be investigated using XRF (U). The X-ray powder diffraction technique collects and analyzes the spectrum produced when the test material is exposed to X-ray radiation. The voltage has been adjusted at 10 kV for light elements, 20–30 kV for medium elements, and 40–50 kV for heavy elements. Additionally, since the environment has a considerable effect on the spectrum when studying light elements, the sample chamber is either evacuated or helium-filled [20,21,22]. The spectrometer was used to determine the chemical element content of a variety of substances, including those that are solid, powdered, or liquid, as well as those that are deposited on the surface or on filters. Heavy minerals were separated using a heavy liquid (bromoform) separation technique, followed by magnetic fractionation using a Frantz isodynamic separator. The binocular microscope was used to collect the heavy minerals, which were then identified using the environmental scanning electron microscope (ESEM)-energy dispersive X-ray (EDX) approach. All studies were conducted at the Nuclear Materials Authority’s Laboratories (NMA).
3 Results
3.1 Petrography of dikes
More than 30 samples of post-granitic dikes were investigated using a polarizing microscope in order to detect their mineralogical and textural relationships. They can be classified into acidic and basic dikes as follows.
3.1.1 Acidic dikes
3.1.1.1 Porphyritic dacite
Porphyritic dacite is composed of plagioclase, quartz, and K-feldspar as essential felsic minerals, whereas biotite and hornblende are mafic minerals. Porphyritic dacite is marked by the porphyritic texture. Plagioclase crystals range in size from euhedral to subhedral (0.8 mm × 2.1 mm) and are composed of oligoclase and andesine (An17–36). It exhibits albite and pericline twinning and has been changed to saussurite (Figure 3a). Occasionally, normal zoning of plagioclase phenocrysts is seen. Quartz is found as 1.2 mm × 2.4 mm subhedral to anhedral phenocrysts and as a fine-grained groundmass. Phenocrysts exhibit excessive extinction as a result of the cataclastic effect. K-Feldspar appears as 1.1 mm × 2.3 mm subhedral phenocrysts and as anhedral fine-grained groundmass. Orthoclase and orthoclase microperthite are the minerals that comprise them. Kaolinization of K-feldspar occurs in stages ranging from partial to total. Biotite occurs in the form of subhedral phenocrysts with dimensions of 0.5 mm × 2.1 mm and as a fine-grained groundmass. It demonstrates partial transformation to chlorite and iron oxides at the crystal borders. Hornblende occurs as 0.5 mm × 1.9 mm subhedral to anhedral phenocrysts. Opaques appear as fine crystals ranging from subhedral to anhedral in conjunction with mafic minerals.
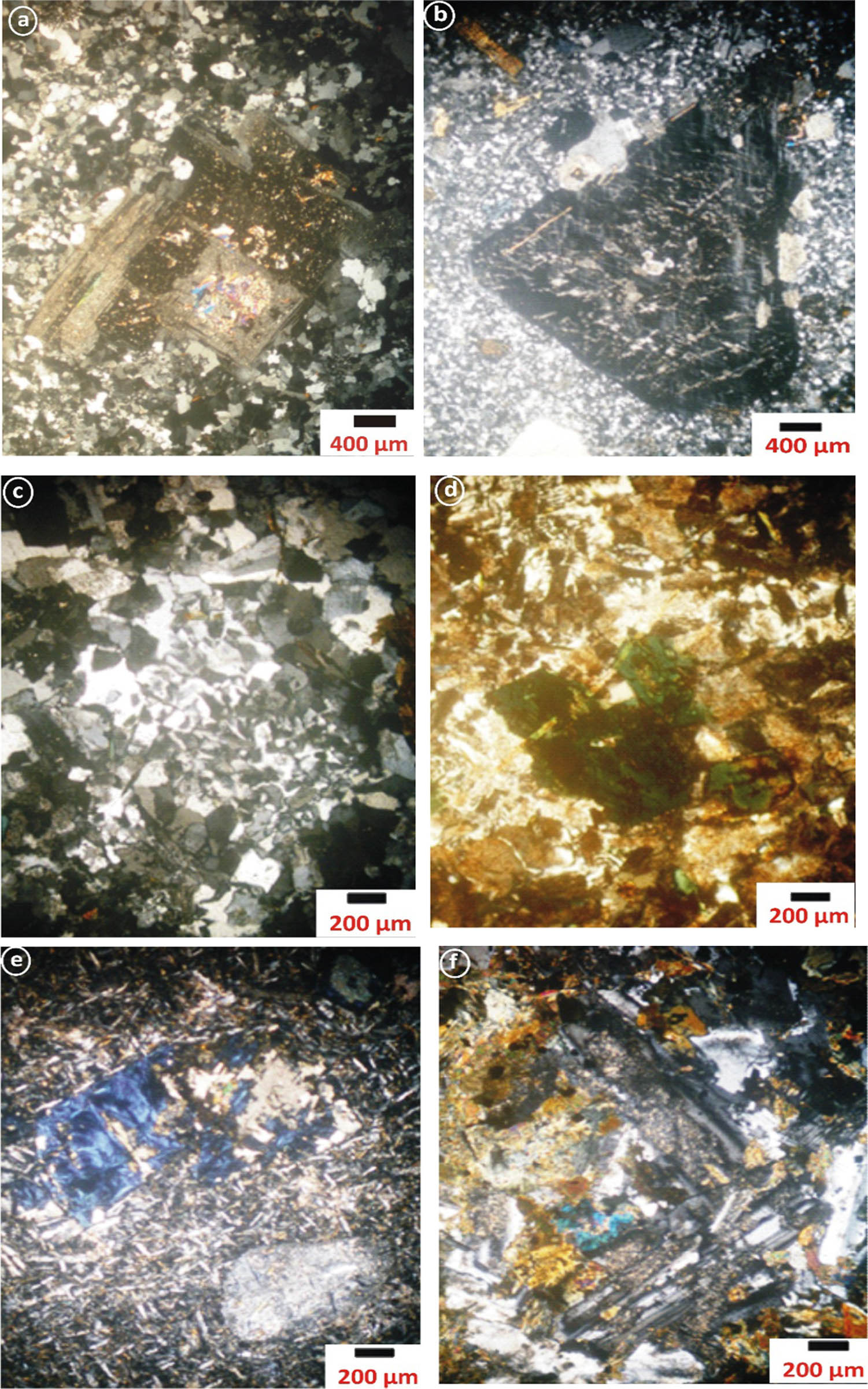
Photomicrographs of (a) porphyritic dacite dike showing pericline twinning in the phenocryst of plagioclase (C.N.); (b) microgranite dike showing phenocryst of microcline micro perthite (C.N.); (c) granophyre dike showing graphic texture (C.N.); (d) alkaline granophyre dike showing riebeckite crystals (C.N.); (e) basaltic dike showing blue pennite type of chlorite and fine-grained lathes plagioclase (C.N.); (f) dolerite dike showing characteristic dolerite and intergranular texture (C.N.).
3.1.1.2 Microgranite
Microgranite is composed essentially of quartz, K-feldspar, plagioclase, and biotite as essential minerals, while opaques are accessory minerals. Quartz occurs as euhedral to subhedral phenocrysts (1.1 mm × 2.1 mm) and as fine-grained groundmass. The phenocryst shows weak undulose extinction and corroded edges by the component of the groundmass. K-Feldspar exists as euhedral to subhedral phenocrysts (1.1 mm × 1.9 mm) and as fine-grained groundmass. K-Feldspar is represented by orthoclase and microcline microperthite (Figure 3b). It is sometimes altered to kaolinite and poikilitically quartz and plagioclase. Plagioclase exists as subhedral to anhedral phenocrysts (0.8 mm × 2.1 mm) embedded in fine-grained groundmass forming porphyritic texture. It is of albitic to oligoclase (An8–15) in composition. It shows albite/Carlsbad and pericline twinning and altered to saussurite. Normal zoning of phenocrysts is observed. Biotite occurs as small euhedral to subhedral flakes with the pleochroic formula: X = brown and Y = Z = dark brown. It is altered to chlorite, especially along its cleavage planes. Opaques are represented by fine-grained aggregates associated with biotite.
3.1.1.3 Granophyre dike
It is composed essentially of quartz, K-feldspar, plagioclase, and biotite, while opaques are accessories. Quartz forms subhedral to anhedral crystals and attains 0.3–2.1 mm in length and 0.1 to 0.6 mm in width. Granophyre dike is intergrown with K-feldspar to produce a graphic texture (Figure 3c). K-Feldspar occurs as subhedral to euhedral crystals, ranges from (0.4 mm × 2.50 mm) to (0.2 mm × 0.9 mm) in dimensions, and are represented by perthite and orthoclase perthite and partly altered to kaolinite. Plagioclase forms euhedral to subhedral crystals, and ranges 0.2 mm × 3.5 mm in phenocryst and 0.1 mm × 0.9 in the groundmass. It ranges in composition from oligoclase to andesine (An15–34) and shows zoning and lamellar twinning. Biotite occurs as subhedral to anhedral phenocryst flakes (0.3 × 3.4 mm) with the pleochroic formula X = yellowish-brown and Y = Z = dark brown. It is partly altered to chlorite and iron oxides. Opaques are rare and usually associated with biotite.
3.1.1.4 Alkaline granophyre
Alkaline granophyre is macroscopically hard, massive, and fine-grained with red color. The alkaline granophyre is composed of essentially quartz, K-feldspar, plagioclase, and riebeckite. Opaques are accessories. Quartz forms subhedral to anhedral crystals and attains 0.1–1.9 mm in length and 0.1–0.5 mm in width. Sometimes, quartz intergrow with K-feldspar forming a graphic texture. K-Feldspar occurs as subhedral to euhedral crystals and attains 2.1 mm × 5.5 mm across in phenocryst and 0.1 mm × 0.7 mm across in the groundmass. It is represented by perthite and orthoclase micro perthite and partly altered to kaolinite. Plagioclase is rare and forms euhedral to subhedral crystals 0.2 mm × 3.5 mm in phenocryst and 0.1 × 0.9 in the groundmass. It ranges in composition from oligoclase to andesine (An13–38) and shows zoning and partly altered to saussurite. Riebeckite occurs as subhedral phenocrysts and attains 0.2–3.1 mm in length and 0.3–1.2 mm in width. The pleochroic formula is X = dark blue, Y = light blue, and Z = greenish (Figure 3d). Opaques are rare and usually associated with riebeckite.
3.1.2 Basic dikes
3.1.2.1 Basaltic dike
Basaltic dike is composed of essentially plagioclase phenocrysts and groundmass, hornblende, and pyroxene. Apatite, epidote, chlorite, and opaques are accessory minerals. The intergrowth texture is well developed. Plagioclase occurs as subhedral crystals (0.3 mm × 3.5 mm), phenocrysts, and as groundmass (0.2 mm × 0.5 mm) of labradorite composition (An60–68). Plagioclase crystals show intergranular texture and are associated with calcite, chlorite, and epidote. Partial alteration to saussurite is occasionally present. Hornblende exists as fine-grained euhedral to subhedral crystals. It is altered to chlorite and iron oxides. Pyroxene occurs as subhedral to anhedral crystals and is sometimes altered to tremolite and actinolite. Pyroxene crystals are intergrown with plagioclase laths forming intergranular and subophitic textures. Apatite occurs as elongate euhedral to subhedral crystals, which occur either as independent crystals or incorporated within hornblende and plagioclase. Epidote occurs as an anhedral crystal associated with chlorite. Chlorite occurs as an anhedral phenocryst of pennate type (Figure 3e). Opaques occur as anhedral crystals and are associated with hornblende and pyroxene.
3.1.2.2 Dolerite dike
It composes of plagioclase, hornblende, and pyroxene in addition to trace amounts of biotite, opaques, and sphene. Occasionally, an intergranular texture develops. Plagioclase is composed of fine- to medium-grained subhedral to anhedral crystals (1.1 mm × 2.5 mm) of andesine to labradorite (An48–56)composition. It contains albite and albite/Carlsbad twinning, as well as saussurite and calcite. Plagioclase laths have an intergranular pyroxene texture. Hornblende crystals range in size from subhedral to anhedral, measuring 1.1 mm × 2.4 mm. It undergoes partial to full transformation into chlorite at the cleavage planes and crystal boundaries and sometimes displays simple merging. Pyroxene is represented by augite and occurs in crystals ranging in size from subhedral to anhedral to 1.1 mm × 2.5 mm. Occasionally, augite crystals develop between plagioclase laths, forming a Doleritic texture (Figure 3f). It demonstrates a diuretic-induced modification of tremolite and actinolite. Biotite occurs as subhedral crystals and undergoes a transformation into chlorite and iron oxides. Opaques form anhedral crystals and are seen in association with mafic elements. Sphene crystallizes in anhedral clusters with plagioclase and ferromagnesian minerals.
3.2 Geochemistry of dikes
Representative samples from the examined dikes were selected and analyzed for major (%) and trace (ppm) elements (Table 1).
Whole rock (Major and trace elements) analysis of post-granitic dikes of G. Serbal area, Southwestern Sinai, Egypt
| Basic dikes | Acidic dikes | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxides | Dolerite | Basalt | Porphyritic dacite | Microgranite | Granophyre | Alkaline granophyre | ||||||||||||
| 1 | 2 | 3 | 5 | 8 | 14 | 10 | 11 | 15 | 12 | 13 | 16 | 3 | 6 | 16 | 4 | 7 | 17 | |
| SiO2 | 49.9 | 48.9 | 49.4 | 49.85 | 50.10 | 49.97 | 68.9 | 69.20 | 69.05 | 71.52 | 72.10 | 71.81 | 69.97 | 70.10 | 70.03 | 71.86 | 70.50 | 71.18 |
| TiO2 | 1.86 | 1.80 | 1.83 | 1.50 | 1.40 | 1.45 | 0.55 | 0.50 | 0.525 | 0.36 | 0.26 | 0.31 | 0.12 | 0.10 | 0.11 | 0.25 | 0.25 | 0.25 |
| Al2O3 | 13.95 | 14.10 | 14.02 | 14.90 | 14.80 | 14.85 | 13.16 | 12.85 | 13.005 | 13.02 | 12.90 | 12.96 | 14.69 | 14.20 | 14.44 | 13.26 | 13.32 | 13.29 |
| Fe2O3 t | 9.86 | 9.95 | 9.905 | 9.70 | 9.60 | 9.65 | 3.01 | 2.80 | 2.905 | 3.52 | 3.10 | 3.31 | 2.80 | 2.50 | 2.65 | 2.80 | 2.90 | 2.85 |
| MgO | 8.30 | 8.65 | 8.475 | 8.83 | 8.60 | 8.715 | 2.75 | 2.70 | 2.725 | 1.12 | 1.02 | 1.07 | 1.28 | 1.10 | 1.19 | 0.43 | 0.52 | 0.475 |
| CaO | 10.15 | 10.20 | 10.17 | 8.48 | 8.10 | 8.29 | 4.54 | 4.20 | 4.37 | 1.68 | 1.40 | 1.54 | 2.52 | 2.30 | 2.41 | 2.24 | 2.36 | 2.3 |
| Na2O | 2.30 | 2.10 | 2.2 | 1.82 | 1.90 | 1.86 | 3.40 | 3.50 | 3.45 | 3.20 | 3.60 | 3.4 | 2.43 | 2.70 | 2.565 | 4.50 | 4.30 | 4.4 |
| K2O | 0.75 | 1.01 | 0.88 | 1.09 | 1.60 | 1.345 | 2.36 | 2.70 | 2.53 | 3.40 | 3.90 | 3.65 | 4.74 | 4.80 | 4.77 | 3.30 | 3.10 | 3.2 |
| P2O5 | 0.85 | 0.90 | 0.875 | 0.52 | 0.65 | 0.585 | 0.53 | 0.40 | 0.465 | 0.12 | 0.11 | 0.115 | 0.12 | 0.11 | 0.115 | 0.06 | 0.07 | 0.065 |
| L.O.I | 1.90 | 1.70 | 1.8 | 1.50 | 1.40 | 1.45 | 0.15 | 0.83 | 0.49 | 0.70 | 0.80 | 0.75 | 0.33 | 0.70 | 0.515 | 0.25 | 0.68 | 0.465 |
| Total | 99.82 | 99.31 | 99.56 | 98.07 | 98.15 | 98.11 | 99.35 | 99.68 | 99.515 | 98.64 | 99.19 | 98.915 | 99.0 | 98.61 | 98.80 | 98.93 | 98.0 | 98.46 |
| Trace elements (ppm) | ||||||||||||||||||
| Cr | 150 | 214 | 182 | 429 | 390 | 409.5 | 32 | 28 | 30 | 42 | 35 | 38.5 | 34 | 30 | 32 | 26 | 28 | 27 |
| Ni | 86 | 83 | 84.5 | 137 | 120 | 128.5 | 7 | 6 | 6.5 | 11 | 10 | 10.5 | 8 | 7 | 7.5 | 7 | 9 | 8 |
| Cu | 42 | 29 | 35.5 | 53 | 50 | 51.5 | 11 | 10 | 10.5 | 11 | 11 | 11 | 11 | 10 | 10.5 | 10 | 11 | 10.5 |
| Zn | 175 | 419 | 297 | 107 | 101 | 104 | 30 | 25 | 27.5 | 37 | 32 | 34.5 | 45 | 40 | 42.5 | 57 | 51 | 54 |
| Zr | 88 | 191 | 139.5 | 173 | 180 | 176.5 | 157 | 165 | 161 | 222 | 211 | 216.5 | 235 | 248 | 241.5 | 490 | 432 | 461 |
| Rb | 14 | 58 | 36 | 73 | 81 | 77 | 133 | 142 | 137.5 | 181 | 192 | 186.5 | 122 | 132 | 127 | 172 | 167 | 169.5 |
| Y | 16 | 17 | 16.5 | 20 | 21 | 20.5 | 24 | 27 | 25.5 | 32 | 38 | 35 | 18 | 20 | 19 | 36 | 30 | 33 |
| Ba | 280 | 428 | 354 | 586 | 508 | 547 | 178 | 108 | 143 | 103 | 88 | 95.5 | 161 | 98 | 129.5 | 62 | 70 | 66 |
| Pb | 80 | 91 | 85.5 | 72 | 60 | 66 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| Sr | 560 | 638 | 599 | 385 | 375 | 380 | 82 | 66 | 74 | 178 | 160 | 169 | 215 | 198 | 206.5 | 103 | 125 | 114 |
| Ga | 9 | 6 | 7.5 | 28 | 22 | 25 | 22 | 20 | 21 | 19 | 15 | 17 | 20 | 18 | 19 | 16 | 18 | 17 |
| V | 255 | 422 | 338.5 | 212 | 201 | 206.5 | 4 | 4 | 4 | 10 | 8 | 9 | 7 | 6 | 6.5 | 8 | 11 | 9.5 |
| Nb | <2 | <2 | <2 | <2 | <2 | <2 | 3 | 3 | 3 | 8 | 9 | 8.5 | 4 | 6 | 5 | 8 | 7 | 7.5 |
3.2.1 Geochemical classification and characteristics
The studied dikes are classified based on the total alkali–silica diagram (TAS), as suggested by Le Maitre [23] and Cox et al. [24] (Figure 4a and b). The diagrams clarify that the dolerite and basalt samples fall in the basalt field, while porphyritic dacite samples fall in the dacite field. The other acidic dikes (granophyre, alkaline granophyre, and microgranite) samples fall in the rhyolite field.
3.2.2 Magma type and tectonic setting
On the A–F–M ternary diagram (Figure 5), the studied dolerite and basalt samples fall within the tholeiitic field. On the other hand, porphyritic dacite, microgranite, granophyre, and alkaline granophyre samples fall within the calc-alkaline field [25]. To illustrate the potassic nature of the studied dikes, the analyses are plotted on the K2O vs SiO2 diagram (Figure 5b) as suggested by Peccerillo and Taylor [26]. It shows that the two samples of dolerite fall in the basalt and medium-k calc-alkaline fields, the majority of samples fall in the basalt field, and a few other samples fall in the medium to high calc-alkaline field. The porphyritic dacite, microgranite, and alkaline granophyre samples fall in dacite and rhyolite, medium-k calc-alkaline fields. The granophyre samples fall in dacite and rhyolite, high k-calc alkaline fields. Binary variation diagrams between MgO and other major oxides (SiO2, TiO2, CaO, Na2O + K2O, Fe2O3, CaO/Al2O3) are illustrated in (Figure 6) to evaluate the alteration effects on the major oxides’ compositions of the investigated dikes. These samples exhibit typical crystallization patterns as seen in these figures. This finding rules out the likelihood of these elements being mobilized and implies that these oxides represent magmatic characteristics. Thus, these main oxides may be classified. Zr can be utilized to determine the elemental mobility and to better comprehend magma differentiation. The binary diagrams of Zr–Nb and Zr–Rb (Figure 7) revealed magmatic tendencies with positive relationships between Nb–Zr and Rb–Zr. Thus, trace elements tend to be the least affected by postcrystallization processes and may thus reflect basic properties. Trace elements seem to be more discriminating than large oxides since they are very sensitive to crystal fractionation, rather than to changes in the fundamental composition. The ternary diagram Y + Zr–TiO2*100–Cr after Davies et al. [27] is constructed to show the original differentiation trend of a magmatic suite because Y and Zr are enriched progressively during fractionation like Na2O and K2O (Figure 8a). The TiO2–Zr diagram (Figure 8b), after Pearce [28], shows that basalt and dolerite fall within the plate field, while porphyritic dacite, microgranite, and granophyre fall in the volcanic arc field.
![Figure 5
(a) A–F–M diagram for different dikes. Fields, after Irvine and Baragar [25]; symbols as in Figure 4. (b) K2O vs SiO2 diagram for different dikes (Peccerillo and Taylor [26]). 1 = basalt, 2 = basalt andesite, 3 = andesite, 4 = dacite and rhyolite, I = arc-tholeiitic, II = calc-alkaline, III = high K-calc-alkaline, and IV = shoshonite; symbols as in Figure 4.](/document/doi/10.1515/chem-2022-0136/asset/graphic/j_chem-2022-0136_fig_005.jpg)
(a) A–F–M diagram for different dikes. Fields, after Irvine and Baragar [25]; symbols as in Figure 4. (b) K2O vs SiO2 diagram for different dikes (Peccerillo and Taylor [26]). 1 = basalt, 2 = basalt andesite, 3 = andesite, 4 = dacite and rhyolite, I = arc-tholeiitic, II = calc-alkaline, III = high K-calc-alkaline, and IV = shoshonite; symbols as in Figure 4.
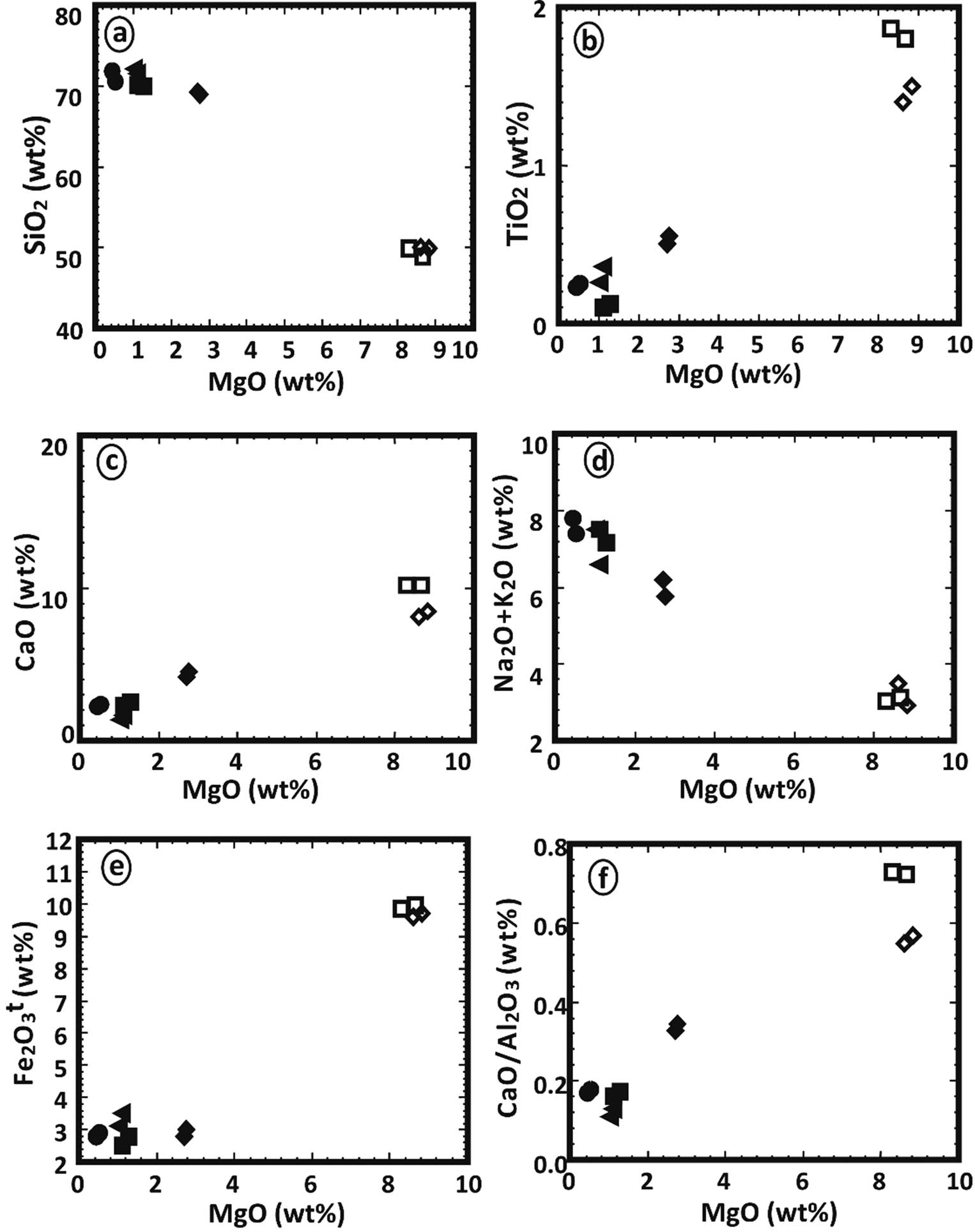
MgO vs (a) SiO2, (b) TiO2, (c) CaO, (d) Na2O + K2O, (e) Fe2O3, and (f) CaO/Al2O3 for different dikes; symbols as in Figure 4.
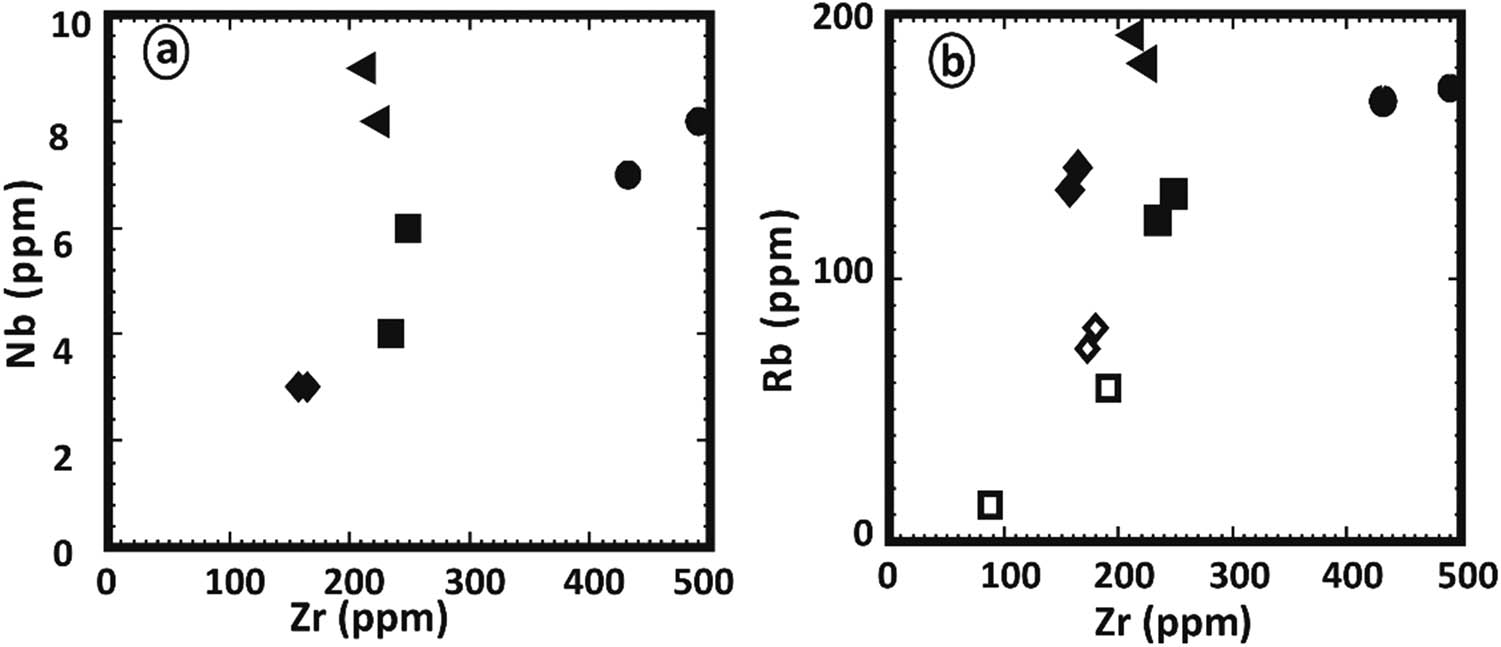
Zr vs (a) Nb, (b) Rb for different dikes; symbols as in Figure 4.
3.2.3 Petrogenesis
The correlation of the normalized trace elements of the studied dikes with the average of chondrite, after Wood et al. [29] (Figure 9), revealed a depletion in Cr, Ni, Zn, V (except in dolerite and basalt dikes), Cu, and Ga. The other elements Ba, Rb, Sr, Zr, Y, and V (dolerite and basalt dikes) show an enrichment relative to the average of chondrites. The studied dikes are also normalized relative to the primitive mantle values (Figure 10), after Taylor and McLennan [30]. The diagram shows strong enrichment in Ba, Rb, Sr, Zr, and Y, while it shows slight enrichment in Cu (in dolerite and basalt), V (in dolerite and basalt), Zn (in dolerite, basalt, and alkaline granophyre), and Ga. It shows a depletion in Cr, Ni, V (except in dolerite and basalt), and slight depletion in Cu (except dolerite and basalt) and Zn (except dolerite, basalt, and alkaline granophyre) relative to the average of primitive mantle values. Two samples were selected (one for basalt and one for dolerite) for studying the opaque minerals at Wadi Geba. The opaques in basalt are represented by magnetite hematite oxides, apatite, and copper minerals. Magnetite occurs as an anhedral crystal and black with a metallic luster in reflected light. Magnetite was confirmed by the ESEM-EDX technique and contains 60.42% Fe2O3 (Figure 11). It is partially or frequently oxidized to hematite (martite). Hematite is steel-gray black with metallic luster in reflected light, with a tendency to a marginal red. Hematite was confirmed by the ESEM-EDX technique and contains 83.95% Fe2O3 (Figure 12). Apatite is colorless in a thin section, usually found as minute six-sided prismatic crystals, moderate relief, and weak birefringence. It occurs as an anhedral crystal with a subrounded shape and was confirmed by the ESEM-EDX technique (Figure 13). It contains 25.3% P2O5, 29.68% SiO2, and 27.68% CaO. The copper mineral occurs as an anhedral crystal and is confirmed by the ESEM-EDX technique (Figure 14). It contains 30.12% CuO, 9.94% Fe2O3, and 35.50% SiO2. Opaques in dolerite are represented by oxide minerals (magnetite and titanomagnetite) and sulfide minerals (pyrite). Magnetite occurs as a euhedral to subhedral mineral and is confirmed by the ESEM-EDX technique (Figure 15). It contains 87.58% Fe2O3. Titanomagnetite is also occurring as euhedral to subhedral minerals. It is less abundant than magnetite and confirmed by the ESEM-EDX technique (Figure 16). It contains 58.40% Fe2O3 and 7.77% TiO2. Sulfide minerals are represented by pyrite, which occurs as cubes and crystalline in the cubic system. It is brass-yellow with a metallic luster in reflected light. It was confirmed by the ESEM-EDX technique (Figure 17) and containing 54.55% SO3 and 45.45% Fe2O3.
![Figure 10
Distribution pattern of trace elements of different dikes normalized to average chondrite (Wood et al. [29]).](/document/doi/10.1515/chem-2022-0136/asset/graphic/j_chem-2022-0136_fig_010.jpg)
Distribution pattern of trace elements of different dikes normalized to average chondrite (Wood et al. [29]).
![Figure 11
Distribution pattern of trace elements of different dikes normalized to average primitive mantle (Taylor and McLennan [30]).](/document/doi/10.1515/chem-2022-0136/asset/graphic/j_chem-2022-0136_fig_011.jpg)
Distribution pattern of trace elements of different dikes normalized to average primitive mantle (Taylor and McLennan [30]).
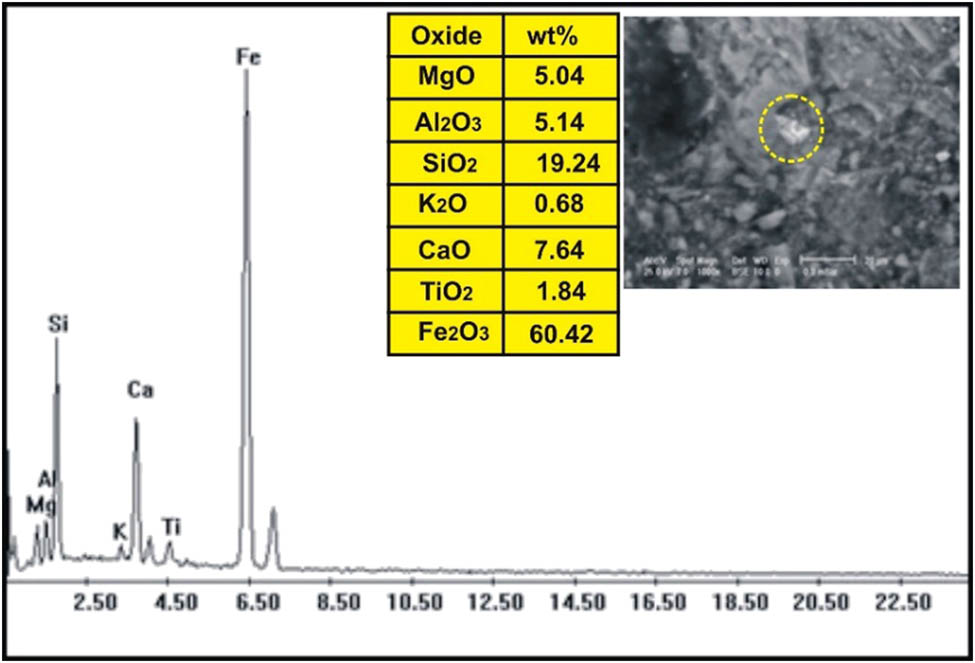
ESEM-EDX analysis of magnetite in basalt dike at Wadi Geba.
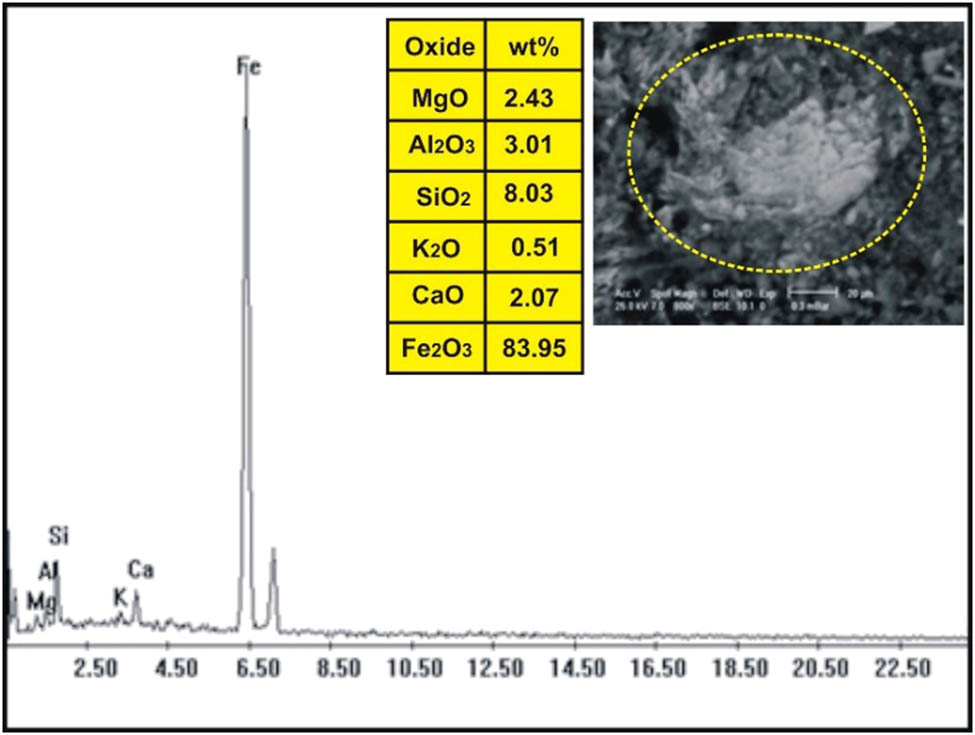
ESEM-EDX analysis of magnetite partially oxidized to hematite in basalt dike at Wadi Geba.
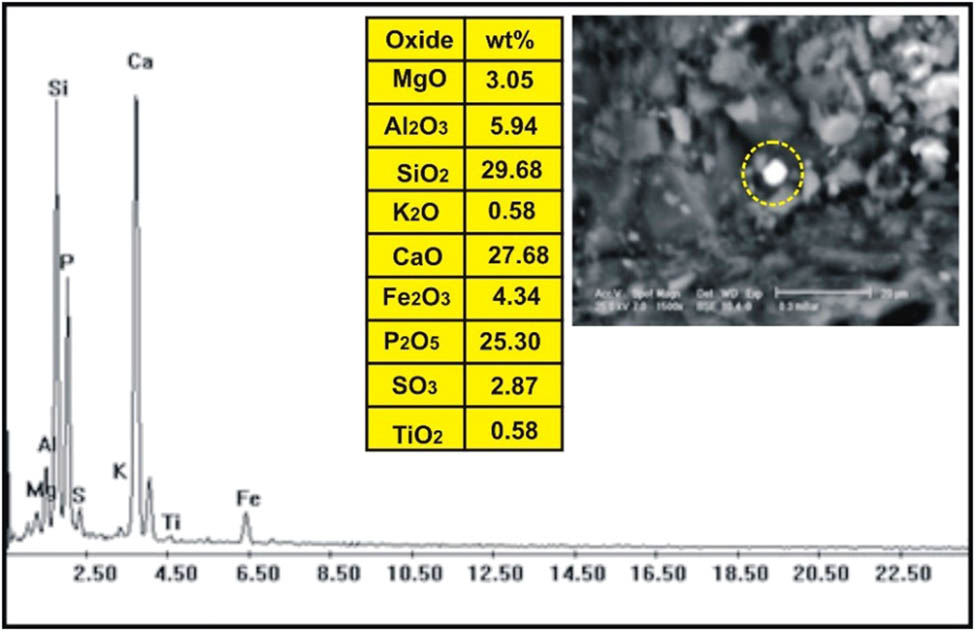
ESEM-EDX analysis of apatite in basalt dike at Wadi Geba.
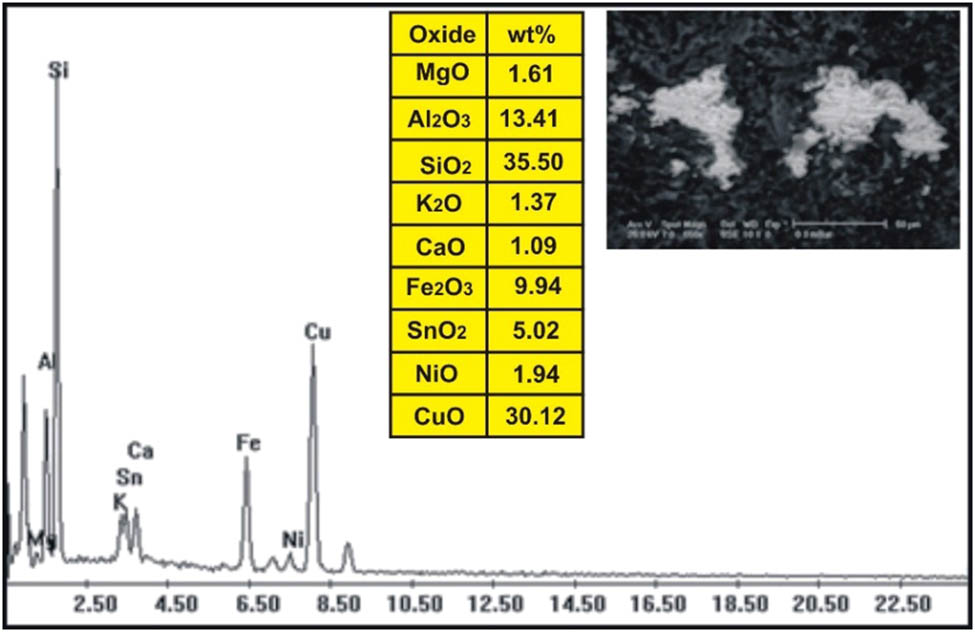
ESEM-EDX analysis of copper minerals in basalt dike at Wadi Geba.
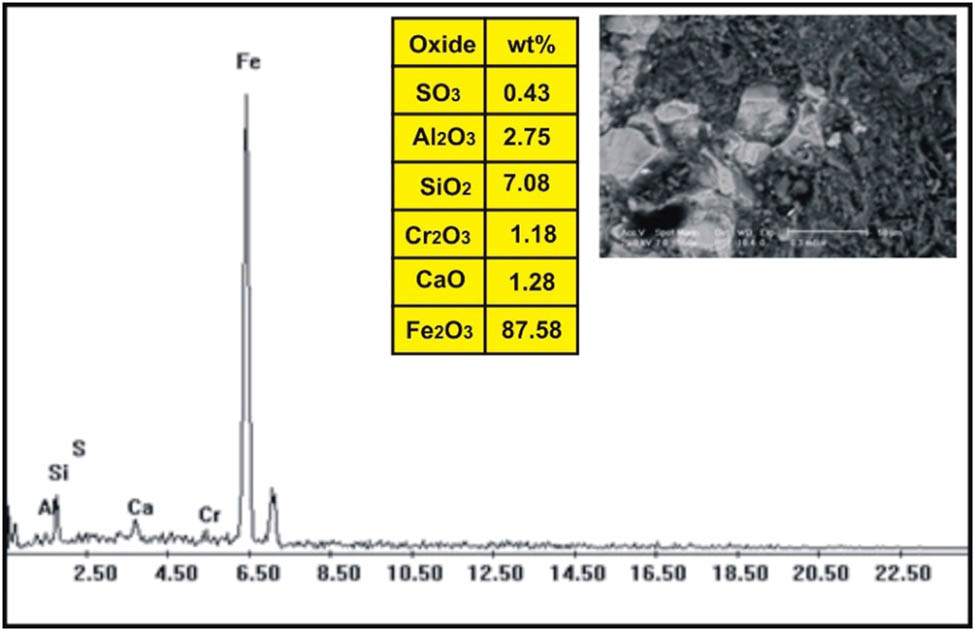
ESEM-EDX analysis of magnetite in dolerite dike at Wadi Geba.
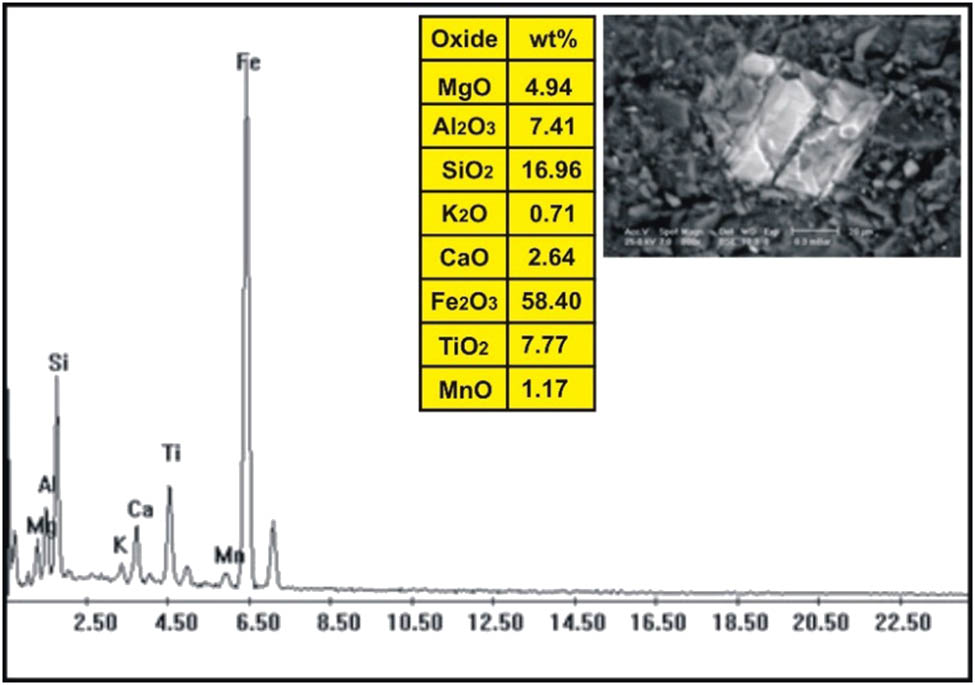
ESEM-EDX analysis of titanomagnetite in dolerite dike at Wadi Geba.
Goethite was formed after due to oxidation of pyrite according to the equation
It is yellow to brown in color, greasy to earthy luster, yellowish-brown streak, nonmagnetic unless heated, perfect cleavage in one direction, and partially soluble in hydrochloric acid. Goethite was confirmed by the ESEM-EDX technique (Figure 18) and contains 88.13% Fe2O3 and 6.45% SO3 (Figure 19).
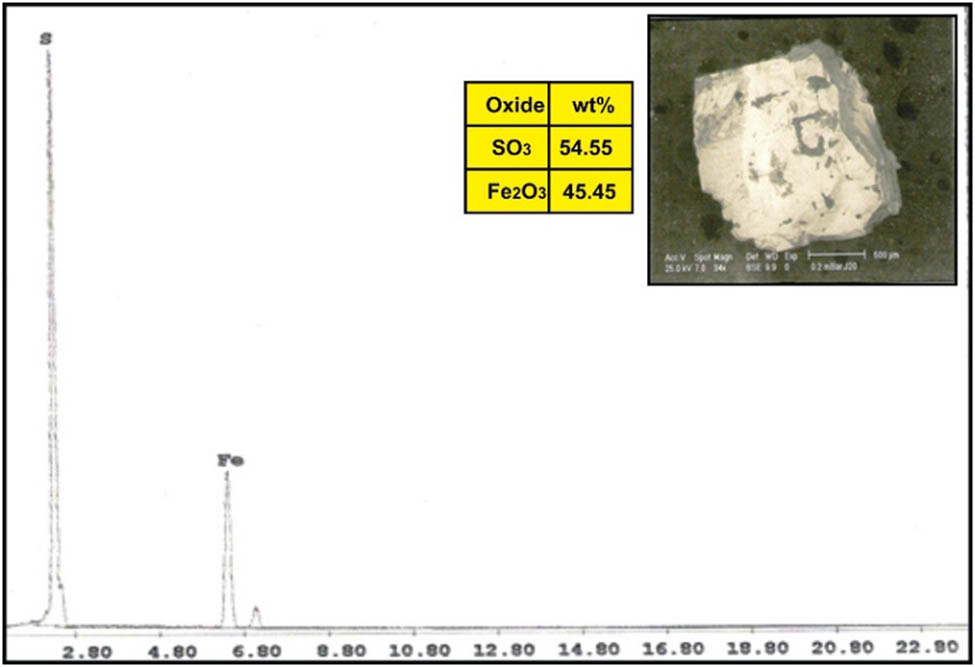
ESEM-EDX analysis of pyrite in dolerite dike at Wadi Geba.
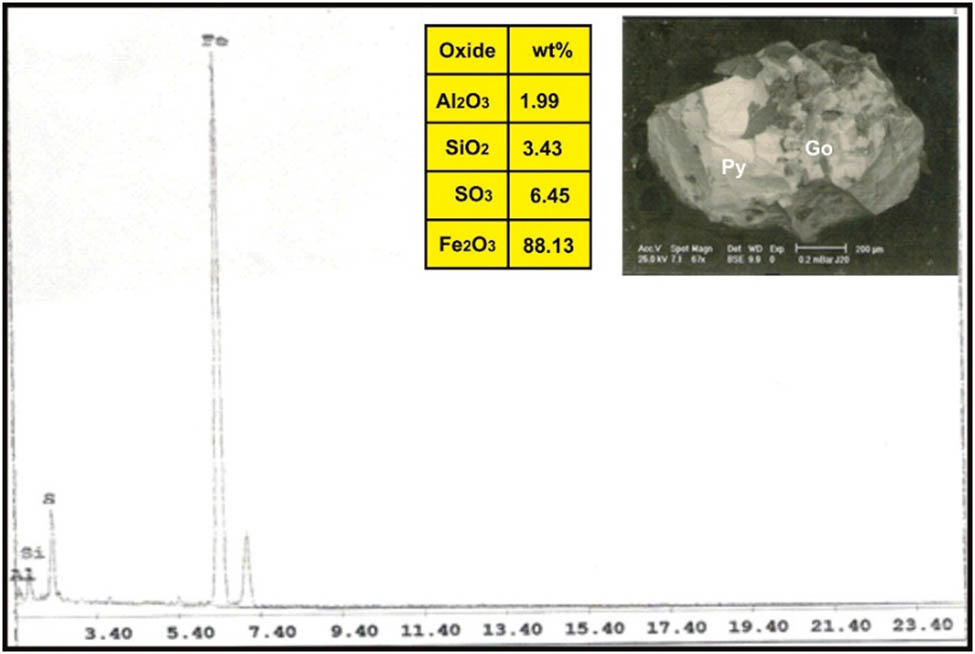
ESEM-EDX analysis of pyrite (Py) replaced by goethite (Go) in dolerite dike at Wadi Geba.
4 Conclusion
The examined area is split by prominent clusters of post-granitic dikes, which create separate and lengthy subparallel dike swarms. The directional trend study of post-granitic dikes reveals three principal trends in terms of quantity and length proportions: N (33–56) E with tensional regime N (34–57) W, N (57–76) E with tensional regime N (14–33) W, and N (12–32) E with tensional regime N (58–78) W. These dikes are classified into acidic (porphyritic dacite, microgranite, granophyre, alkaline granophyre) and basic (basalt and dolerite) dikes. The acidic dikes show quite different contents in plagioclase, quartz, alkali felspar, and biotite. On the other hand, basic dikes are represented by pyroxene, hornblende, plagioclase, and iron oxides as well as the opaque minerals relatively more abundant in the basalt dike. The basic dikes have medium k-characters, originating from tholeiitic magma and extruded within a plate environment. The acidic dikes have medium to high k-characters, originating from calc-alkaline magma and extruded in a volcanic arc environment. The opaques in basalt dikes are represented by magnetite, hematite, apatite, and copper minerals, while dolerite dikes include magnetite, titanomagnetite, pyrite, and goethite minerals.
Acknowledgements
Hamdy A. Awad is funded by a scholarship under the Joint Executive Program between Egypt and Russian Federation.
-
Funding information: None.
-
Author contributions: All the authors contributed to writing data curation writing and reviewing a manuscript.
-
Conflict of interest: The authors declare that they have no conflict of interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Lasheen ESR, Saleh GM, Khaleal FM, Alwetaishi M. Petrogenesis of neoproterozoic ultramafic rocks, Wadi Ibib–Wadi Shani, South Eastern Desert, Egypt: constraints from whole rock and mineral chemistry. Appl Sci. 2021;11(22):10524.10.3390/app112210524Suche in Google Scholar
[2] Saleh GM, Khaleal FM, Lasheen ESR. Geochemistry and paleoweathering of metasediments and pyrite-bearing quartzite during the Neoproterozoic Era, Wadi Ibib-Wadi Suwawrib, South Eastern Desert, Egypt. Arab J Geosci. 2022;15:1–27.10.1007/s12517-021-09141-5Suche in Google Scholar
[3] Friz-Töpfer A. Geochemical characterization of Pan-African dyke swarms in southern Sinai: from continental margin to intraplate magmatism. Precambrian Res. 1991;49:281–300.10.1016/0301-9268(91)90038-CSuche in Google Scholar
[4] Awad HAM, Nastavkin AV. Some mechanical and physical studies of granitic rocks in Um Taghir, Eastern Desert, Egypt. J Phys Conf Ser IOP Publishing. 2021; p. 12012.10.1088/1742-6596/1945/1/012012Suche in Google Scholar
[5] Rasmussen KL, Lentz DR, Falck H, Pattison DRM. Felsic magmatic phases and the role of late-stage aplitic dykes in the formation of the world-class Cantung Tungsten skarn deposit, Northwest Territories, Canada, Ore. Ore Geol Rev. 2011;41:75–111.10.1016/j.oregeorev.2011.06.011Suche in Google Scholar
[6] Li L, Li S-R, Santosh M, Li Q, Gu Y, Lü WJ, et al. Dyke swarms and their role in the genesis of world-class gold deposits: insights from the Jiaodong peninsula, China. J Asian Earth Sci. 2016;130:2–22.10.1016/j.jseaes.2016.06.015Suche in Google Scholar
[7] Babiker M, Gudmundsson A. Geometry, structure and emplacement of mafic dykes in the Red Sea Hills, Sudan. J African Earth Sci. 2004;38:279–92.10.1016/j.jafrearsci.2004.01.003Suche in Google Scholar
[8] Nukman M, Moeck I. Structural controls on a geothermal system in the Tarutung Basin, north central Sumatra. J Asian Earth Sci. 2013;74:86–96.10.1016/j.jseaes.2013.06.012Suche in Google Scholar
[9] Awad HA, Zakaly HMH, Nastavkin AV, El Tohamy AM, El-Taher A. Radioactive mineralizations on granitic rocks and silica veins on shear zone of El-Missikat area, Central Eastern Desert, Egypt. Appl Radiat Isot. 2020:109493.10.1016/j.apradiso.2020.109493Suche in Google Scholar PubMed
[10] El Mezayen AM, Heikal MA, El-Feky MG, Shahin HA, Zeid IKA, Lasheen SR. Petrology, geochemistry, radioactivity, and M–W type rare earth element tetrads of El Sela altered granites, south eastern desert, Egypt. Acta Geochim. 2019;38:95–119.10.1007/s11631-018-0274-7Suche in Google Scholar
[11] Zakaly HMH, Uosif MAM, Issa SAM, Tekin HO, Madkour H, Tammam M, et al. An extended assessment of natural radioactivity in the sediments of the mid-region of the Egyptian Red Sea coast. Mar Pollut Bull. 2021;171:112658.10.1016/j.marpolbul.2021.112658Suche in Google Scholar
[12] Zakaly HM, Uosif MA, Madkour H, Tammam M, Issa S, Elsaman R, et al. Assessment of natural radionuclides and heavy metal concentrations in marine sediments in view of tourism activities in Hurghada city, northern Red Sea. Egypt J Phys Sci. 2019;3:21–47. 10.21315/JPS2019.30.3.3. Suche in Google Scholar
[13] Ernst RE, Buchan KL. Giant radiating dyke swarms: their use in identifying pre-Mesozoic large igneous provinces and mantle plumes. Geophys Monogr. 1997;100:297–334.10.1029/GM100p0297Suche in Google Scholar
[14] Marinoni LB. Crustal extension from exposed sheet intrusions: review and method proposal. J Volcanol Geotherm Res. 2001;107:27–46.10.1016/S0377-0273(00)00318-8Suche in Google Scholar
[15] Zhang CL, Zou HB. Permian A-type granites in Tarim and western part of Central Asian Orogenic Belt (CAOB): genetically related to a common Permian mantle plume? Lithos. 2013;172:47–60.10.1016/j.lithos.2013.04.001Suche in Google Scholar
[16] Chen N, Dong J, Chen J, Dong C, Shen Z. Geometry and emplacement of the Late Cretaceous mafic dyke swarms on the islands in Zhejiang Province, Southeast China: insights from high-resolution satellite images. J Asian Earth Sci. 2014;79:302–11.10.1016/j.jseaes.2013.10.001Suche in Google Scholar
[17] Sibson RH. Brittle-failure controls on maximum sustainable overpressure in different tectonic regimes. Am Assoc Pet Geol Bull. 2003;87:901–8.10.1306/01290300181Suche in Google Scholar
[18] Takada A. Variations in magma supply and magma partitioning: the role of tectonic settings. J Volcanol Geotherm Res. 1999;93:93–110.10.1016/S0377-0273(99)00082-7Suche in Google Scholar
[19] Khaleal FM, Kamar MS, El-Sherif AM. Geology, geochemistry and radioactivity of the monzogranite Rocks, North Wadi Ghadir, South Eastern Desert, Egypt. Nucl Sci Sci J. 2017;6:71–91. 10.21608/NSSJ.2017.30774.Suche in Google Scholar
[20] Dar MA, Uosif MA, Mohamedein LI, Madkour AG, Zakaly HMH. Radiation hazards and the cancer risk assessments in the sediments of Timsah Lake, Egypt. J King Abdulaziz Univ Mar Sci. 2020;30:1–16. 10.4197/Mar.30-1.1.Suche in Google Scholar
[21] El-Taher A, Zakaly HMH, Elsaman R. Environmental implications and spatial distribution of natural radionuclides and heavy metals in sediments from four harbours in the Egyptian Red Sea coast. Appl Radiat Isot. 2018;131:13–22. 10.1016/j.apradiso.2017.09.024.Suche in Google Scholar
[22] Mostafa MYA, Zakaly HMH, Uosif MAM, Issa SAM, Madkour H, Tammam M. Sediment natural radioactivity and heavy metals assessment from the beaches of Ras-gharib, Red Sea, Egypt. AIP Conference of Proceedings. AIP Publishing LLC AIP Publishing; 2020. p. 020011. 10.1063/5.0032164.Suche in Google Scholar
[23] Le Maitre RW. A proposal by the IUGS Subcommission on the Systematics of Igneous Rocks for a chemical classification of volcanic rocks based on the total alkali silica (TAS) diagram: (on behalf of the IUGS Subcommission on the Systematics of Igneous Rocks). Aust J Earth Sci. 1984;31:243–55.10.1080/08120098408729295Suche in Google Scholar
[24] Cox KG, Bell JD, Pankhurst RJ. The Interpretation of Igneous Rocks. London: London University; 1979. p. 450.10.1007/978-94-017-3373-1Suche in Google Scholar
[25] Irvine TNJ, Baragar WRA. A guide to the chemical classification of the common volcanic rocks. Can J Earth Sci. 1971;8:523–48.10.1139/e71-055Suche in Google Scholar
[26] Peccerillo A, Taylor SR. Geochemistry of Eocene calc-alkaline volcanic rocks from the Kastamonu area, northern Turkey. Contrib Mineral Petrol. 1976;58:63–81.10.1007/BF00384745Suche in Google Scholar
[27] Davies JF, Grant RWE, Whitehead RES. Immobile trace elements and Archean volcanic stratigraphy in the Timmins mining area, Ontario. Can J Earth Sci. 1979;16:305–11.10.1139/e79-029Suche in Google Scholar
[28] Pearce JA. Geochemical evidence for the genesis and eruptive setting of lavas from Tethyan ophiolites. Proceedings International Ophiolite Symposium, Cyprus 1979. Cyprus: Ministry of Agriculture and Natural Resources; 1980. p. 261–72.Suche in Google Scholar
[29] Wood DA, Tarney J, Varet J, Saunders AD, Bougault H, Joron JL, et al. Geochemistry of basalts drilled in the North Atlantic by IPOD Leg 49: implications for mantle heterogeneity. Earth Planet Sci Lett. 1979;42:77–97.10.1016/0012-821X(79)90192-4Suche in Google Scholar
[30] Taylor SR, McLennan SM. The continental crust: its composition and evolution. Oxford: Blackwell; 1985. p. 1–312.Suche in Google Scholar
© 2022 Mohamed S. Kamar et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop
Artikel in diesem Heft
- Regular Articles
- Photocatalytic degradation of Rhodamine B in aqueous phase by bimetallic metal-organic framework M/Fe-MOF (M = Co, Cu, and Mg)
- Assessment of using electronic portal imaging device for analysing bolus material utilised in radiation therapy
- A detailed investigation on highly dense CuZr bulk metallic glasses for shielding purposes
- Simulation of gamma-ray shielding properties for materials of medical interest
- Environmental impact assesment regulation applications and their analysis in Turkey
- Sample age effect on parameters of dynamic nuclear polarization in certain difluorobenzen isomers/MC800 asphaltene suspensions
- Passenger demand forecasting for railway systems
- Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach
- Gamma, neutron, and heavy charged ion shielding properties of Er3+-doped and Sm3+-doped zinc borate glasses
- Bridging chiral de-tert-butylcalix[4]arenes: Optical resolution based on column chromatography and structural characterization
- Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt
- Comparison of the yield and purity of plasma exosomes extracted by ultracentrifugation, precipitation, and membrane-based approaches
- Bioactive triterpenoids from Indonesian medicinal plant Syzygium aqueum
- Investigation of the effects of machining parameters on surface integrity in micromachining
- The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion
- Gamma-ray shielding properties of Nd2O3-added iron–boron–phosphate-based composites
- Numerical investigation on perforated sheet metals under tension loading
- Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt
- Two new polypodane-type bicyclic triterpenoids from mastic
- Structural, physical, and mechanical properties of the TiO2 added hydroxyapatite composites
- Tribological properties and characterization of borided Co–Mg alloys
- Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies
- Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system
- Cyclic connectivity index of bipolar fuzzy incidence graph
- The role of passage numbers of donor cells in the development of Arabian Oryx – Cow interspecific somatic cell nuclear transfer embryos
- Mechanical property evaluation of tellurite–germanate glasses and comparison of their radiation-shielding characteristics using EPICS2017 to other glass systems
- Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation
- Microwave-assisted preparation of Ag/Fe magnetic biochar from clivia leaves for adsorbing daptomycin antibiotics
- Iminodisuccinic acid enhances antioxidant and mineral element accumulation in young leaves of Ziziphus jujuba
- Cytotoxic activity of guaiane-type sesquiterpene lactone (deoxycynaropicrin) isolated from the leaves of Centaurothamnus maximus
- Effects of welding parameters on the angular distortion of welded steel plates
- Simulation of a reactor considering the Stamicarbon, Snamprogetti, and Toyo patents for obtaining urea
- Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils
- Impact of a live bacterial-based direct-fed microbial (DFM) postpartum and weaning system on performance, mortality, and health of Najdi lambs
- Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models
- Physicochemical properties and some mineral concentration of milk samples from different animals and altitudes
- Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies
- Diagnostic and therapeutic radioisotopes in nuclear medicine: Determination of gamma-ray transmission factors and safety competencies of high-dense and transparent glassy shields
- Calculation of NaI(Tl) detector efficiency using 226Ra, 232Th, and 40K radioisotopes: Three-phase Monte Carlo simulation study
- Isolation and identification of unstable components from Caesalpinia sappan by high-speed counter-current chromatography combined with preparative high-performance liquid chromatography
- Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique
- Characterization of the elastic modulus of ceramic–metal composites with physical and mechanical properties by ultrasonic technique
- GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro
- Texturing of nanocoatings for surface acoustic wave-based sensors for volatile organic compounds
- Insights into the molecular basis of some chalcone analogues as potential inhibitors of Leishmania donovani: An integrated in silico and in vitro study
- (1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 4-amino-3-phenylbutanoate hydrochloride: Synthesis and anticonvulsant activity
- On the relative extraction rates of colour compounds and caffeine during brewing, an investigation of tea over time and temperature
- Characterization of egg shell powder-doped ceramic–metal composites
- Rapeseed oil-based hippurate amide nanocomposite coating material for anticorrosive and antibacterial applications
- Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation
- Efficiency analysis of photovoltaic systems installed in different geographical locations
- Risk prioritization model driven by success factor in the light of multicriteria decision making
- Theoretical investigations on the excited-state intramolecular proton transfer in the solvated 2-hydroxy-1-naphthaldehyde carbohydrazone
- Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system
- Machine learning-based forecasting of potability of drinking water through adaptive boosting model
- The potential effect of the Rumex vesicarius water seeds extract treatment on mice before and during pregnancy on the serum enzymes and the histology of kidney and liver
- Impact of benzimidazole functional groups on the n-doping properties of benzimidazole derivatives
- Extraction of red pigment from Chinese jujube peel and the antioxidant activity of the pigment extracts
- Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires
- A focusing study on radioprotective and antioxidant effects of Annona muricata leaf extract in the circulation and liver tissue: Clinical and experimental studies
- Clinical comprehensive and experimental assessment of the radioprotective effect of Annona muricata leaf extract to prevent cellular damage in the ileum tissue
- Effect of WC content on ultrasonic properties, thermal and electrical conductivity of WC–Co–Ni–Cr composites
- Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface
- The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution
- Study on the influence of physical interlayers on the remaining oil production under different development modes
- Optimized linear regression control of DC motor under various disturbances
- Influence of different sample preparation strategies on hypothesis-driven shotgun proteomic analysis of human saliva
- Determination of flow distance of the fluid metal due to fluidity in ductile iron casting by artificial neural networks approach
- Investigation of mechanical activation effect on high-volume natural pozzolanic cements
- In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite
- Determination of oil composition of cowpea (Vigna unguiculata L.) seeds under influence of organic fertilizer forms
- Activated partial thromboplastin time maybe associated with the prognosis of papillary thyroid carcinoma
- Treatment of rat brain ischemia model by NSCs-polymer scaffold transplantation
- Lead and cadmium removal with native yeast from coastal wetlands
- Characterization of electroless Ni-coated Fe–Co composite using powder metallurgy
- Ferrate synthesis using NaOCl and its application for dye removal
- Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches
- Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense
- Experimental study on turning machine with permanent magnetic cutting tool
- Numerical simulation and mathematical modeling of the casting process for pearlitic spheroidal graphite cast iron
- Design, synthesis, and cytotoxicity evaluation of novel thiophene, pyrimidine, pyridazine, and pyridine: Griseofulvin heterocyclic extension derivatives
- Isolation and identification of promising antibiotic-producing bacteria
- Ultrasonic-induced reversible blood–brain barrier opening: Safety evaluation into the cellular level
- Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan
- Effect of calcium lactate in standard diet on selected markers of oxidative stress and inflammation in ovariectomized rats
- Identification of crucial salivary proteins/genes and pathways involved in pathogenesis of temporomandibular disorders
- Zirconium-modified attapulgite was used for removing of Cr(vi) in aqueous solution
- The stress distribution of different types of restorative materials in primary molar
- Reducing surface heat loss in steam boilers
- Deformation behavior and formability of friction stir processed DP600 steel
- Synthesis and characterization of bismuth oxide/commercial activated carbon composite for battery anode
- Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics
- Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs
- Separation and evaluation of potential antioxidant, analgesic, and anti-inflammatory activities of limonene-rich essential oils from Citrus sinensis (L.)
- Bioactivity of a polyhydroxy gorgostane steroid from Xenia umbellata
- BiCAM-based automated scoring system for digital logic circuit diagrams
- Analysis of standard systems with solar monitoring systems
- Structural and spectroscopic properties of voriconazole and fluconazole – Experimental and theoretical studies
- New plant resistance inducers based on polyamines
- Experimental investigation of single-lap bolted and bolted/bonded (hybrid) joints of polymeric plates
- Investigation of inlet air pressure and evaporative cooling of four different cogeneration cycles
- Review Articles
- Comprehensive review on synthesis, physicochemical properties, and application of activated carbon from the Arecaceae plants for enhanced wastewater treatment
- Research progress on speciation analysis of arsenic in traditional Chinese medicine
- Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review
- An insight on Vietnamese bio-waste materials as activated carbon precursors for multiple applications in environmental protection
- Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review
- Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review
- Sonodynamic therapy for breast cancer: A literature review
- Recent progress of amino acid transporters as a novel antitumor target
- Aconitum coreanum Rapaics: Botany, traditional uses, phytochemistry, pharmacology, and toxicology
- Corrigendum
- Corrigendum to “Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt”
- Corrigendum to “Design of a Robust sliding mode controller for bioreactor cultures in overflow metabolism via an interdisciplinary approach”
- Corrigendum to “Statistical analysis on the radiological assessment and geochemical studies of granite rocks in the north of Um Taghir area, Eastern Desert, Egypt”
- Corrigendum to “Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry”
- Corrigendum to “Mechanical properties, elastic moduli, transmission factors, and gamma-ray-shielding performances of Bi2O3–P2O5–B2O3–V2O5 quaternary glass system”
- Erratum
- Erratum to “Copper(ii) complexes supported by modified azo-based ligands: Nucleic acid binding and molecular docking studies”
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil
- Human health risk assessment and distribution of VOCs in a chemical site, Weinan, China
- Preparation and characterization of Sparassis latifolia β-glucan microcapsules
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Improving the thermal performance of existing buildings in light of the requirements of the EU directive 2010/31/EU in Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Study of plant resources with ethnomedicinal relevance from district Bagh, Azad Jammu and Kashmir, Pakistan
- Studies on the chemical composition of plants used in traditional medicine in Congo
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Strip spraying technology for precise herbicide application in carrot fields
- Special Issue on Pharmacology and Metabolomics of Ethnobotanical and Herbal Medicine
- Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas
- Antioxidant and antimicrobial properties of polyphenolics from Withania adpressa (Coss.) Batt. against selected drug-resistant bacterial strains
- Integrating network pharmacology and molecular docking to explore the potential mechanism of Xinguan No. 3 in the treatment of COVID-19
- Chemical composition and in vitro and in vivo biological assortment of fixed oil extracted from Ficus benghalensis L.
- A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases
- Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye)
- Protective effects of asperuloside against cyclophosphamide-induced urotoxicity and hematotoxicity in rats
- Special Issue on Essential Oil, Extraction, Phytochemistry, Advances, and Application
- Identification of volatile compounds and antioxidant, antibacterial, and antifungal properties against drug-resistant microbes of essential oils from the leaves of Mentha rotundifolia var. apodysa Briq. (Lamiaceae)
- Phenolic contents, anticancer, antioxidant, and antimicrobial capacities of MeOH extract from the aerial parts of Trema orientalis plant
- Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and Rosmarinus officinalis against multidrug-resistant microbes and their acute toxicity study
- Special Issue on Marine Environmental Sciences and Significance of the Multidisciplinary Approaches
- An insightful overview of the distribution pattern of polycyclic aromatic hydrocarbon in the marine sediments of the Red Sea
- Antifungal–antiproliferative norcycloartane-type triterpenes from the Red Sea green alga Tydemania expeditionis
- Solvent effect, dipole moment, and DFT studies of multi donor–acceptor type pyridine derivative
- An extensive assessment on the distribution pattern of organic contaminants in the aerosols samples in the Middle East
- Special Issue on 4th IC3PE
- Energetics of carboxylic acid–pyridine heterosynthon revisited: A computational study of intermolecular hydrogen bond domination on phenylacetic acid–nicotinamide cocrystals
- A review: Silver–zinc oxide nanoparticles – organoclay-reinforced chitosan bionanocomposites for food packaging
- Green synthesis of magnetic activated carbon from peanut shells functionalized with TiO2 photocatalyst for Batik liquid waste treatment
- Coagulation activity of liquid extraction of Leucaena leucocephala and Sesbania grandiflora on the removal of turbidity
- Hydrocracking optimization of palm oil over NiMoO4/activated carbon catalyst to produce biogasoline and kerosine
- Special Issue on Pharmacology and metabolomics of ethnobotanical and herbal medicine
- Cynarin inhibits PDGF-BB-induced proliferation and activation in hepatic stellate cells through PPARγ
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension
- Topical Issue on phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Phytochemical analysis of leaves and stems of Physalis alkekengi L. (Solanaceae)
- Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects
- Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain
- Protective effect of Allium atroviolaceum-synthesized SeNPs on aluminum-induced brain damage in mice
- Mechanism study of Cordyceps sinensis alleviates renal ischemia–reperfusion injury
- Plant-derived bisbenzylisoquinoline alkaloid tetrandrine prevents human podocyte injury by regulating the miR-150-5p/NPHS1 axis
- Network pharmacology combined with molecular docking to explore the anti-osteoporosis mechanisms of β-ecdysone derived from medicinal plants
- Chinese medicinal plant Polygonum cuspidatum ameliorates silicosis via suppressing the Wnt/β-catenin pathway
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part I
- Investigation of improved optical and conductivity properties of poly(methyl methacrylate)–MXenes (PMMA–MXenes) nanocomposite thin films for optoelectronic applications
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2022)
- Model predictive control for precision irrigation of a Quinoa crop

![Figure 4
(a) Na2O + K2O vs SiO2 (TAS) diagram for different dikes, after Le Maitre [23]. (b) Na2O + K2O vs SiO2 for the studied dikes (Cox et al. [24]).](/document/doi/10.1515/chem-2022-0136/asset/graphic/j_chem-2022-0136_fig_004.jpg)
![Figure 8
Y + Zr-TiO2*100–Cr ternary diagram for different dikes, after Davies et al. [27]; symbols as in Figure 4.](/document/doi/10.1515/chem-2022-0136/asset/graphic/j_chem-2022-0136_fig_008.jpg)
![Figure 9
TiO2 vs Zr diagram for different dikes, after Pearce [28]; symbols as in Figure 4.](/document/doi/10.1515/chem-2022-0136/asset/graphic/j_chem-2022-0136_fig_009.jpg)
