Abstract
C30H21F9N4NiO8, monoclinic, P21/c (no. 13), a = 10.0630(11) Å, b = 9.625(1) Å, c = 18.196(2) Å, β = 116.10(3)°, V = 1582.7(5) Å3, Z = 2, Rgt(F) = 0.0511, wRref(F2) = 0.1261, T = 293(2) K.
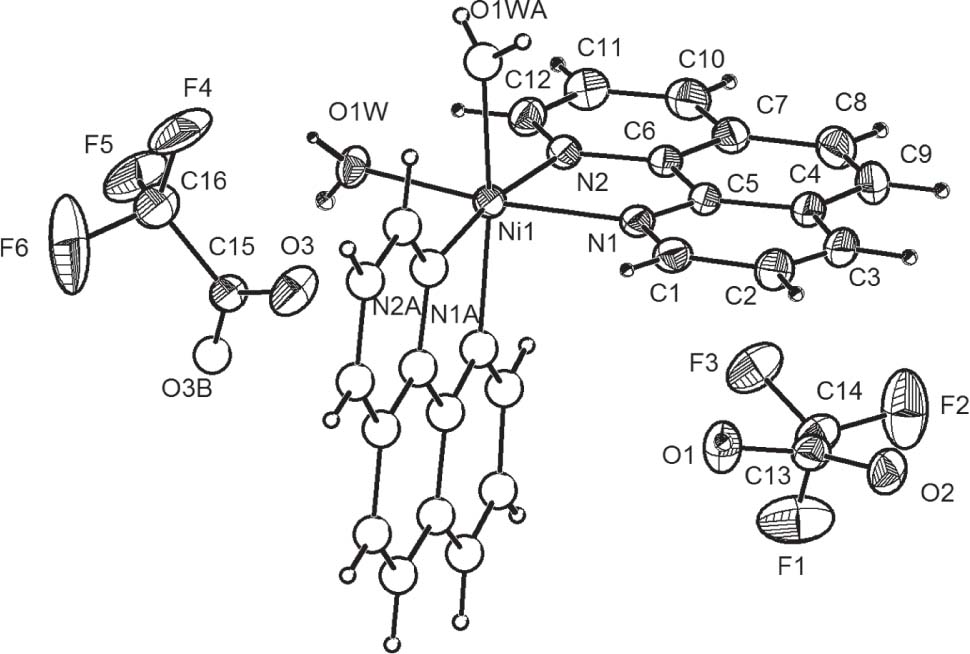
The asymmetric unit of the title crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.29 × 0.22 × 0.17 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.72 mm−1 |
| Diffractometer, scan mode: | Bruker D8 Venture, φ and ω-scans |
| θmax, completeness: | 25°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 7825, 2789, 0.055 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1773 |
| N(param)refined: | 259 |
| Programs: | Bruker programs [1], SHELX [2], [3], OLEX2 [4], DIAMOND [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Ni1 | 0.000000 | 0.57723(6) | 0.250000 | 0.0480(3) |
| O1W | 0.1279(4) | 0.7328(3) | 0.2369(2) | 0.0606(8) |
| O1 | 0.1119(4) | 0.1083(4) | 0.3130(2) | 0.0885(10) |
| H1Aa | 0.023886 | 0.114020 | 0.280980 | 0.133* |
| O2 | 0.0422(3) | −0.0619(3) | 0.37090(18) | 0.0787(9) |
| O3 | 0.3863(3) | 0.6494(3) | 0.2445(2) | 0.0981(12) |
| N1 | −0.1107(4) | 0.4271(3) | 0.2840(2) | 0.0515(8) |
| N2 | 0.1397(3) | 0.5616(3) | 0.37401(19) | 0.0522(8) |
| F1 | 0.3736(3) | −0.0366(4) | 0.4255(2) | 0.1435(14) |
| F2 | 0.2844(4) | −0.0143(5) | 0.5081(2) | 0.1569(16) |
| F3 | 0.3297(3) | 0.1613(3) | 0.45731(19) | 0.1113(10) |
| F4a | 0.3993(18) | 0.9190(10) | 0.2599(16) | 0.165(6) |
| F5a | 0.6276(15) | 0.9073(11) | 0.3027(9) | 0.142(4) |
| H1WA | 0.084(4) | 0.800(4) | 0.210(2) | 0.053(13)* |
| H1WB | 0.223(7) | 0.720(5) | 0.242(3) | 0.14(2)* |
| F6a | 0.490(2) | 0.8949(14) | 0.1755(8) | 0.236(6) |
| C1 | −0.2357(5) | 0.3615(4) | 0.2387(3) | 0.0642(11) |
| H1 | −0.287219 | 0.386553 | 0.183916 | 0.077* |
| C2 | −0.2940(6) | 0.2573(4) | 0.2685(4) | 0.0816(15) |
| H2 | −0.381973 | 0.213276 | 0.234370 | 0.098* |
| C3 | −0.2208(6) | 0.2210(4) | 0.3480(4) | 0.0795(15) |
| H3 | −0.258298 | 0.150944 | 0.368796 | 0.095* |
| C4 | −0.0880(5) | 0.2883(4) | 0.3999(3) | 0.0638(12) |
| C5 | −0.0384(5) | 0.3902(4) | 0.3635(3) | 0.0541(10) |
| C6 | 0.0966(5) | 0.4634(4) | 0.4123(3) | 0.0544(10) |
| C7 | 0.1772(5) | 0.4319(4) | 0.4957(3) | 0.0629(11) |
| C8 | 0.1214(6) | 0.3271(5) | 0.5298(3) | 0.0794(14) |
| H8 | 0.173133 | 0.305640 | 0.585088 | 0.095* |
| C9 | −0.0034(7) | 0.2591(5) | 0.4844(4) | 0.0813(15) |
| H9 | −0.035864 | 0.190554 | 0.508684 | 0.098* |
| C10 | 0.3063(6) | 0.5063(5) | 0.5395(3) | 0.0759(13) |
| H10 | 0.363176 | 0.488102 | 0.594868 | 0.091* |
| C11 | 0.3497(5) | 0.6057(5) | 0.5016(3) | 0.0724(12) |
| H11 | 0.435571 | 0.656628 | 0.530729 | 0.087* |
| C12 | 0.2643(5) | 0.6303(4) | 0.4189(3) | 0.0618(11) |
| H12 | 0.295560 | 0.698035 | 0.393497 | 0.074* |
| C13 | 0.1280(5) | 0.0243(4) | 0.3693(3) | 0.0608(11) |
| C14 | 0.2773(5) | 0.0352(5) | 0.4412(3) | 0.0732(13) |
| C15 | 0.500000 | 0.7027(6) | 0.250000 | 0.0568(15) |
| C16 | 0.500000 | 0.8594(7) | 0.250000 | 0.088(2) |
aOccupancy: 0.5.
Source of materials
All reagents were of analytical grade and used as purchased without further purification. A mixture of nickel oxide (0.0027 mmol, 0.2049 g), trifluoroacetic acid (HTFA) (0.0054 mol, 0.6140 g) and H2O (60 mL) were added to the round bottom flask in turn. After refluxing for 2 h, 1,10-phenanthroline (Phen) (0.0014 mmol, 0.2681 g) was added. Continue heating reflux for 6 h, and then the solution was cooled, filtered and recrystallized. Green block-shaped crystals of the title compound were obtained after 3 days.
Experimental details
Absorption corrections were applied by using multi-scan program [1]. All of the non-hydrogen atoms were refined anisotropically. Hydrogen atoms bound to carbon atoms were generated geometrically and refined isotropically with a riding model except for those bound to water molecules which were located from the difference Fourier synthesis. Significantly larger ellipsoids for the fluorine atoms of the TFA anions point to a positional disorder.
Comment
The synthesis and applications of fluorinated compounds have recently received significant attention due to their excellent performance in pharmaceuticals, agricultural chemistry and materials science [6], [7], [8], [9], [10], [11], [12]. Trifluoromethyl is widely used in the design of drug molecular synthesis because it can significantly improve the metabolic stability, lipophilic property and bioavailability of drug molecules [13], [14], [15]. Particularly, the F atom of trifluoroacetic acid can form weak F⋯F and F⋯H interactions, which can reinforce the crystal packing and construct more flexible structures. 1,10-Phenanthroline is a multidentate ligand with a rigid structure, which may adopt diverse binding modes to construct novel functional complexes [16]. However, as far as we know, trifluoroacetic coordination compounds, especially compounds with strong hydrogen bonding interactions between transition metal complexes and guest molecules are rarely reported.
The basic structural unit of the title compound [Ni(Phen)2(H2O)2] (TFA)2⋅HTFA consists of one diaqua-bis(1,10-phenanthroline) with nickel [Ni(Phen)2(H2O)2]2+ cation, two trifluoroacetate anions and one independent trifluoroacetic acid molecule (see the figure). The central Ni ion exhibits a distorted octahedral coordination, defined by four nitrogen atoms from two different Phen ligands and two oxygen atoms from two individual water molecules. The HTFA molecule and TFA anions remain uncoordinated. The Ni—O bond length is 2.055(3) Å, and two Ni—N bond lengths are 2.073(3) and 2.079(3) Å. The N—Ni—N bite angles are all 79.67(13)°. The angle of OW—Ni—OW is 86.48(18)°. The bond angles of OW—Ni—N are in the range of 91.13(12)° and 170.41(13)°. The fluorine atoms of the TFA anions of the HTFA molecule of the title compound are disordered over two positions. The water molecules in the structure of the title compound participate in the formation of intermolecular hydrogen bonds: (a) hydrogen bonds (O1W—H1WB⋯O3: 2.666(4) Å and 165(5)°) with TFA anions, which connect the mononuclear units to form a chain structure along the a axis; (b) hydrogen bonds (O1W—H1WA⋯O23: 2.780(4) Å and 170(4)°: − x, y + 1, − z + 1/2) link the uncoordinated HTFA molecule. Furthermore, there are intermolecular hydrogen bonds between adjacent HTFA molecules (O1—H1A⋯O11: 2.404(7) Å and 151.0°: −x, +y, 1/2 − z). The molecular structure and packing are stabilized by intermolecular O—H⋯O hydrogen bond interactions. Geometric parameters are within the expectations [17].
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 51279073
Funding statement: This work was supported by the National Natural Science Foundation of China (no. 51279073), the Project of Science and Technology in Henan Province (no. 152102310108).
References
1. Bruker. SAINT. Bruker AXS Inc., Madison, WI, USA (2015).Suche in Google Scholar
2. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42 (2009) 339–341.10.1107/S0021889808042726Suche in Google Scholar
5. Brandenburg, K.: DIAMOND. Visual Crystal Structure Information System. Ver. 4.0. Crystal Impact, Bonn, Germany (2015).Suche in Google Scholar
6. Guo, S.; Cong, F.; Guo, R.; Wang, L.; Tang, P. P.: Asymmetric silver-catalysed intermolecular bromotrifl uoromethoxylation of alkenes with a new trifluoromethoxylation reagent. Nat. Chem. 9 (2017) 546–551.10.1038/nchem.2711Suche in Google Scholar PubMed
7. Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J.: Organic fluorine compounds: a great opportunity for enhanced materials properties. Chem. Soc. Rev. 40 (2011) 3496–3508.10.1039/c0cs00221fSuche in Google Scholar PubMed
8. Jian, F. F.; Zhao, P. S.; Zhang, L.; Zheng, J.: Synthesis, structure and quantum chemical calculations on p-trifluoromethylphenyl thioacid amide. J. Fluorine Chem. 127 (2006) 63–67.10.1016/j.jfluchem.2005.10.003Suche in Google Scholar
9. Tagaya, A.; Koike, K.; Koike, Y.; Okamoto, Y.: Effect of trifluoromethyl substituents on birefringence of polystyrene. Polym. Adv. Technol. 28 (2017) 994–999.10.1002/pat.3825Suche in Google Scholar
10. Nemes, A.; Mérész, E.; Jalsovszky, I.; Szabó, D.; Böcskei, Z.; Rábai, József.: Unusual reactivity of trifluoromethyl groups in meso-tetrakis(trifluoromethyl)porphyrin. J. Fluorine Chem. 203 (2017) 75–80.10.1016/j.jfluchem.2017.05.009Suche in Google Scholar
11. Gakh, A. A.; Shermolovich, Y.: Trifluoromethylated heterocycles. Curr. Top. Med. Chem. 14 (2014) 952–965.10.2174/1568026614666140202210424Suche in Google Scholar PubMed
12. Zhu, W.; Wang, J.; Wang, S. N.; Gu, Z. N.; Aceña, J. L.; Izawa, K.; Liu, H.; Soloshonok, V. A.: Recent advances in the trifluoromethylation methology and new CF3-containing drugs. J. Fluorine Chem. 167 (2014) 37–54.10.1016/j.jfluchem.2014.06.026Suche in Google Scholar
13. Isanbor, C.; O’Hagan, D.: Fluorine in medicinal chemistry: a review of anticancer agents. J. Fluorine Chem. 127 (2006) 303–319.10.1016/j.jfluchem.2006.01.011Suche in Google Scholar
14. Pinard, E.; Alanine, A.; Alberati, D.; Bender, M.; Borroni, E.; Bourdeaux, P.; Brom, V.; Burner, S.; Fischer, H.; Hainzl, D.; Halm, R.; Hauser, N.; Jolidon, S.; Lengyel, J.; Marty, H.; Meyer, T.; Moreau, J.; Mory, R.; Narquizian, R.; Nettekoven, M.; Norcross, R. D.; Puellmann, B.; Schmid, P.; Schmitt, S.; Stalder, H.; Wermuth, R.; Wettstein, J. G.; Zimmerli, D.: Selective GlyT1 inhibitors: discovery of [4-(3-fluoro-5-trifluoromethylpyridin-2-yl)piperazin-1-yl][5-methanesulfonyl-2-((S)-2,2,2-trifluoro-1-methylethoxy)phenyl]methanone (RG1678), a promising novel medicine to treat schizophrenia. J. Med. Chem. 53 (2010) 4603–4614.10.1021/jm100210pSuche in Google Scholar PubMed
15. Feraldi-Xypolia, A.; Pardo, D. G.; Cossy, J.: Synthesis of α-(trifluoromethyl)pyridazine derivatives. Eur. J. Org. Chem. 27 (2018) 3541–3553.10.1002/ejoc.201701412Suche in Google Scholar
16. Kang, H. X.; Fu, Y. Q.; Ju, F. Y.; Li, G. L.; Li, X. L.; Liu, G. Z.: Syntheses, structures, and magnetic properties of two Mn(II) coordination polymers based on 4-fluorocinnamic acid and 1,10-phenanthroline. Russ. J. Coord. Chem. 44 (2018) 340–346.10.1134/S1070328418050020Suche in Google Scholar
17. Nikiforov, A. A.; Eremin, A. V.; Gurzhii, V. V.; Misharev, A. D.; Medvedskii, N. L.; Ponyaev, A. I.; Belyaev, A. N.: Syntheses and structural studies of the nickel(II) octahedral complexes Ni(N∩N) x L2 with nitrogen-containing and carboxylate ligands. Russ. J. Coord. Chem. 43 (2017) 269–277.10.1134/S1070328417050050Suche in Google Scholar
©2019 Lan Zhang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Frontmatter
- Crystal structure of poly[diaqua-(μ8-1,1′:2′,1′′-terphenyl-3,3′′,4′,5′-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)dicopper(II)], C22H14Cu2O10
- Crystal structure of 2-((1H-benzo[d]imidazol-2-ylimino)methyl)-4,6-di-tert-butylphenol, C22H27N3O
- Crystal structure of (4-ethoxynaphthalen-1-yl)(furan-2-yl)methanone, C17H14O3
- Crystal structure of 1-nonylpyridazin-1-ium iodide, C13H23N2I
- Crystal structure of bis[diaqua(1,10-phenanthroline-κ2N, N′)-copper(II)]diphenylphosphopentamolybdate dihydrate, C36H38Cu2Mo5N4O27P2
- The crystal structure of tetrakis(imidazole)-copper(I) hexafluorophosphate, C12H16CuF6PN8
- The crystal structure of dimethyl ((3,5-di-tert-butyl-4-hydroxyphenyl)(phenyl)methyl)phosphonate, C23H33O4P
- Crystal structure of diaqua-bis(1,10-phenanthroline κ2N,N′)nickel(II) trifluoroacetate- trifluoroacetic acid (1/1), C30H21F9N4NiO8
- Crystal structure of 2-(naphthalen-2-yl)-1,8-naphthyridine, C18H12N2
- Synthesis and crystal structure of a new polymorph of diisopropylammonium trichloroacetate, C8H16Cl3NO2
- Crystal structure of dimethanol-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)cadmium(II) C34H34CdN12O2S2
- Crystal structure of ethyl 2,2-difluoro-2-(7-methoxy-2-oxo-2H-chromen-3-yl)acetate, C14H12F2O5
- The crystal structure of bis[μ2-(N,N-diethylcarbamodithioato-κS:κS,κS′)] bis[1′-(diphenylphosphino-κP)-1-cyanoferrocene]disilver(I), C56H56Ag2Fe2N4P2S4
- Crystal structure of bis(di-n-butylammonium) tetrachloridodiphenylstannate(IV), C28H50Cl4N2Sn
- The crystal structure of poly[(μ5-2-((5-bromo-3-formyl-2-hydroxybenzylidene)amino)benzenesulfonato-κ6O:O:O,O′:O′:O′′)sodium(I)], C13H9O4NSBrNa
- Crystal structure of catena-{poly[bis(O,O′-diethyldithiophosphato-S)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)-zinc(II)] di-acetonitrile solvate}, {C20H30N4O4P2S4Zn ⋅ 2 C2H3N}n
- Halogen and hydrogen bonding in the layered crystal structure of 2-iodoanilinium triiodide, C6H7I4N
- Crystal structure of cyclohexane-1,4-diammonium 2-[(2-carboxylatophenyl)disulfanyl]benzoate — dimethylformamide — monohydrate (1/1/1), [C6H16N2][C14H8O4S2] ⋅ C3H7NO⋅H2O
- The synthesis and crystal structure of isobutyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C16H13Cl2F6N3O3S
- Isolation and crystal structure of bufotalinin — methanol (1/1), C25H34O7
- Crystal structure of benzylbis(1,3-diphenylpropane-1,3-dionato-κ2O,O′) chloridotin(IV), C37H29ClO4Sn
- Crystal structure of Bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imidazol)}diiodidocadmium(II), [Cd(C11H11N5)2I2], C22H22N10I2Cd
- Crystal structure of 4-isobutoxybenzaldehyde oxime, C11H15NO2
- The crystal structure of bis(acetato-κ1O)-bis(N′-hydroxypyrimidine-2-carboximidamide-κ2N,N′)manganese(II) — methanol (1/2), C14H18MnN8O6, 2(CH3OH)′
- Crystal structure of poly[bis(μ2-bis(4-(1H-imidazol-1-yl)phenyl)amine-κ2N:N′)-bis(nitrato-κO)cadmium(II)], C36H30CdN12O6
- Crystal structure and optical properties of 1,6-bis(methylthio)pyrene, C18H14S2
- The crystal structure of hexaquamagnesium(II) bis(3,4-dinitropyrazol-1-ide), C6H14MgN8O14
- Halogen bonds in the crystal structure of 4,3:5,4-terpyridine – 1,4-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure and photochromic properties of a novel photochromic perfluordiarylethene containing a triazole bridged pyridine group moiety, C24H18F6N4S2
- Crystal structure of bis[(μ3-oxido)-(μ2-(N,N-diisopropylthiocarbamoylthio) acetato-κ2O,O′)-((N,N-diisopropylthiocarbamoylthio)acetato-κO)-bis(di-4-methylbenzyl-tin(IV))], C100H136N4O10S8Sn4
- Crystal structure of dibromidobis(4-bromobenzyl)tin(IV), C14H12Br4Sn
- The crystal structure of (4Z)-2-[(E)-(1-ethyl-3,3-dimethyl-1,3-dihydro-2H-indol-2-ylidene)methyl]-4-[(1-ethyl-3,3-dimethyl-3H-indolium-2-yl)methylidene]-3-oxocyclobut-1-en-1-olate, C30H32N2O2
- The crystal structure of (E)-3-(4-(dimethylamino)styryl)-5,5-dimethylcyclohex-2-en-1-one, C18H23NO
- Crystal structure of dihydrazinium 1H-pyrazole-3,5-dicarboxylate, C5H12N6O4
- Crystal structure of poly[μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4-sulfidobenzoate-κ2O:S)cobalt(II)] dihydrate, C42H44Co2N8O7S2
- Crystal structure of 8-(3,4-dimethylbenzylidene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C17H18O4
- Crystal structure of 4-(2-bromo-4-(6-morpholino-3-phenyl-3H-benzo[f]chromen-3-yl) cyclohexa-2,5-dien-1-yl)morpholine, C33H31BrN2O
- Synthesis and crystal structure of 2-((1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)methylene)-2,3-dihydro-1H-inden-1-one, C23H16N2OS
- Crystal structure of poly[(μ2-1,1′-(oxybis(4,1-phenylene)bis(1H-imidazole)-κ2N,N′)(μ2-1,3-benzenecarboxylato-κ3O,O′:O′′)zinc(II)] dihydrate, C26H22N4O7Zn
- Crystal structure of diaqua-bis(cinnamato-κ2O,O′)zinc(II), C18H18ZnO6
- Crystal structure of 2-(prop-2-yn-1-yloxy)-1-naphthaldehyde, C14H10O2
- Crystal structure and photochromic properties of 1-(2-methyl-5-phenyl-3-thienyl)-2-{2-methyl-5-[4-(9-fluorenone hydrazone)-phenyl]-3-thienyl}perfluorocyclopentene, C41H26F6N2S2
- Hydrothermal synthesis and crystal structure of cylo[tetraaqua-bis(μ2-1,4-bis(1H-benzo[d]imidazol-1-yl)but-2-ene-κ2N:N′)-bis(μ2-4-nitro-phthalate-κ2O,O′)dinickel(II)], C26H23N5O8Ni
- Crystal structure of 3-[methyl(phenyl)amino]-1-phenylthiourea, C14H15N3S
- Crystal structure of 1-(4-chlorophenyl)-3-[methyl(phenyl)amino]thiourea, C14H14ClN3S
- Crystal structure of 2-tert-butyl-1H-imidazo[4,5-b]pyridine, C10H13N3
- Crystal structure of 5-carboxy-2-(2-carboxyphenyl)-1H-imidazol-3-ium-4-carboxylate dihydrate, C12H8N2O6⋅2(H2O)
- The crystal structure of dichlorido-μ2-dichlorido-(η2-1,4-bis(4-vinylbenzyl)-1,4-diazabicyclo[2.2.2]octane-1,4-diium)dicopper(I), C24H30N2Cu2Cl4
- Crystal structure of 4-bromobenzyl (Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C22H29BrN2OS
- Crystal structure of (4S,4aS,6aR,6bR,12aS,12bR,14aS,14bR)-3,3,6a,6b,9,9,12a-heptamethyloctadecahydro-1H,3H-4,14b-ethanophenanthro[1,2-h]isochromene-1(6bH)-one, C30H48O2
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C30H33F6N3S
- The crystal structure of 3-methoxyphenanthridin-6(5H)-one, C14H11NO2
- Crystal structure of 4-(5,5-difluoro-1,3,7,9-tetramethyl-3H,5H-5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)pyridin-1-ium tetraiodidoferrate(III), C18H19BF2FeI4N3
- Crystal structure of 2-(3-methoxyphenyl)-3-((phenylsulfonyl)methyl)imidazo[1,2-a]pyridine, C21H18N2O3S
- Crystal structure of [(2-(2-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) perchlorate, C29H50Cl2N4NiO8
- Crystal structure of (Z)-6-(dimethylamino)-3,3-bis(4-(dimethylamino)phenyl)-2-(2-(quinoxalin-2-ylmethylene)hydrazinyl)-2,3-dihydroinden-1-one, C35H35N7O
- 5-Methyl-N′-[5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbonyl]-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbohydrazide, C22H22N8O2
- Crystal structure of 2,3-dichloro-6-methoxyquinoxaline, C9H6Cl2N2O
- Synthesis and crystal structure of 7-chloro-2-(ethylsulfinyl)-6-fluoro-3-(1H-pyrazole-1-yl)-4H-thiochromen-4-one, C13H10FN3OS2
- Crystal structure of 4-ethylpiperazine-1-carbothioic dithioperoxyanhydride, C14H26N4S4
- Crystal structure of 2-(2-(6-methylpyridin-2-yl)naphthalen-1-yl)pyrimidine, C20H15N3
- The crystal structure of N′-((1E,2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-ylidene)-3-methylbenzohydrazide, C23H22N2O4
- Crystal structure of catena-poly[(μ2-isophthalato-κ2O:O′)-(2,5-di(pyrazin-2-yl)-4,4′-bipyridine-κ3N,N′,N′′)zinc(II)] — water (2/5), C26H21N6O6.5Zn
- Crystal structure of (3E,5E)-3,5-bis(3-nitrobenzylidene)-1-((4-(trifluoromethyl)phenyl)sulfonyl)piperidin-4-one — dichloromethane (2/1), C53H38Cl2F6N6O14S2
- Crystal structure of (μ2-oxido)-bis(N,N′-o-phenylenebis(salicylideneiminato))diiron(III) — N,N′-dimethylformamide, C47H43Fe2N4O9
- Crystal structure of N1,N3-bis(2-hydroxyethyl)-N1, N1,N3,N3-tetramethylpropane-1,3-diaminium dibromide, C11H28Br2N2O2
- Crystal structure of (E)-N-(4-chlorophenyl)-1-(pyridin-2-yl)methanimine, C12H9ClN2
- Crystal structure of 8-bromo-6-oxo-2-phenyl-6H-pyrrolo[3,2,1-ij]quinoline-5-carbaldehyde, C18H11BrNO2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride trihydrate, C8H18N8Cl2 ⋅ 3 H2O
- Crystal structure of (E)-4-bromo-N-(pyridin-2-ylmethylene)aniline, C12H9BrN2
- Crystal structure of bis[(2-(3-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ-O)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Br2N4NiO8
- The crystal structure of (1E,2E)-2-methyl-4-((7-oxo-7H-furo[3,2-g]chromen-9-yl)oxy)but-2-enal O-isonicotinoyl oxime–trichloromethane (3/1), C67H49Cl3N6O18
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-methyl-1H-imidazol-3-ium hexafluoridophosphate(V), C8H13F6N2O2P
- Crystal structure of bis[(2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κO)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) hemihydrate C42H65Br2N4NiO8.5
- The crystal structure of N-(7-(4-fluorobenzylidene)-3-(4-fluorophenyl)-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbonothioyl)benzamide, C28H23F2N3OS
- The crystal structure of N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide, C18H20N4O2
- Crystal structure of (E)-2-(3,6-bis(ethylamino)-2,7-dimethyl-9H-xanthen-9-yl)-N′-((6-methylpyridin-2-yl)methylene)benzohydrazide – methanol (1/1), C34H37N5O3
- Crystal structure of 2-oxo-1-(pyrimidin-5-ylmethyl)-3-(3-(trifluoromethyl)phenyl)-1,2-dihydro-5l4-pyrido[1,2-a]pyrimidin-4-olate, C20H13F3N4O2
- Crystal structure of poly[(μ3-9H-carbazole-3,6-dicarboxylato-κ3O1: O2: O3)(μ2-4-(pyridin-4-yl)pyridine-κ2N1:N1′)zinc(II)], C19H11N2O4Zn
- Crystal structure of (E)-N′-((1,8-dihydropyren-1-yl)-methylene)picolinohydrazide, C23H15N3O
- Crystal structure of catena-poly{[μ2-1,2-bis(diphenylphosphino)ethane]dichloridocadmium(II)}, C26H24CdCl2P2
- Crystal structure of the 1:2 co-crystal between N,N′-bis(4-pyridylmethyl)oxalamide and acetic acid as a dihydrate, C14H14N4O2⋅2 C2H4O2⋅2 H2O
- Crystal structure of the co-crystal N,N′-bis(3-pyridylmethyl)oxalamide acetic acid (1/2), C14H14N4O2⋅2C2H4O2
- Crystal structure of the co-crystal N,N′-bis(4-pyridylmethyl)oxalamide and 2,3,5,6-tetrafluoro-1,4-di-iodobenzene (1/1), C14H14N4O2⋅C6F4I2
- Crystal structure of the co-crystal 4-[(4-carboxyphenyl)disulfanyl]benzoic acid–(1E,4E)-1-N,4-N-bis(pyridin-4-ylmethylidene)cyclohexane-1,4-diamine (1/1), C14H10O4S2⋅C18H20N4
- Crystal structure of hexacarbonyl-bis(μ2-di-n-propyldithiocarbamato-κ3S,S′:S;κ3S:S:S′)-di-rhenium(I), C20H28N2O6Re2S4
- Crystal structure of fac-tricarbonyl-morpholine-κN-(morpholinocarbamodithioato-κ2S,S′)rhenium(I), C12H17N2O5ReS2
Artikel in diesem Heft
- Frontmatter
- Crystal structure of poly[diaqua-(μ8-1,1′:2′,1′′-terphenyl-3,3′′,4′,5′-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)dicopper(II)], C22H14Cu2O10
- Crystal structure of 2-((1H-benzo[d]imidazol-2-ylimino)methyl)-4,6-di-tert-butylphenol, C22H27N3O
- Crystal structure of (4-ethoxynaphthalen-1-yl)(furan-2-yl)methanone, C17H14O3
- Crystal structure of 1-nonylpyridazin-1-ium iodide, C13H23N2I
- Crystal structure of bis[diaqua(1,10-phenanthroline-κ2N, N′)-copper(II)]diphenylphosphopentamolybdate dihydrate, C36H38Cu2Mo5N4O27P2
- The crystal structure of tetrakis(imidazole)-copper(I) hexafluorophosphate, C12H16CuF6PN8
- The crystal structure of dimethyl ((3,5-di-tert-butyl-4-hydroxyphenyl)(phenyl)methyl)phosphonate, C23H33O4P
- Crystal structure of diaqua-bis(1,10-phenanthroline κ2N,N′)nickel(II) trifluoroacetate- trifluoroacetic acid (1/1), C30H21F9N4NiO8
- Crystal structure of 2-(naphthalen-2-yl)-1,8-naphthyridine, C18H12N2
- Synthesis and crystal structure of a new polymorph of diisopropylammonium trichloroacetate, C8H16Cl3NO2
- Crystal structure of dimethanol-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)cadmium(II) C34H34CdN12O2S2
- Crystal structure of ethyl 2,2-difluoro-2-(7-methoxy-2-oxo-2H-chromen-3-yl)acetate, C14H12F2O5
- The crystal structure of bis[μ2-(N,N-diethylcarbamodithioato-κS:κS,κS′)] bis[1′-(diphenylphosphino-κP)-1-cyanoferrocene]disilver(I), C56H56Ag2Fe2N4P2S4
- Crystal structure of bis(di-n-butylammonium) tetrachloridodiphenylstannate(IV), C28H50Cl4N2Sn
- The crystal structure of poly[(μ5-2-((5-bromo-3-formyl-2-hydroxybenzylidene)amino)benzenesulfonato-κ6O:O:O,O′:O′:O′′)sodium(I)], C13H9O4NSBrNa
- Crystal structure of catena-{poly[bis(O,O′-diethyldithiophosphato-S)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)-zinc(II)] di-acetonitrile solvate}, {C20H30N4O4P2S4Zn ⋅ 2 C2H3N}n
- Halogen and hydrogen bonding in the layered crystal structure of 2-iodoanilinium triiodide, C6H7I4N
- Crystal structure of cyclohexane-1,4-diammonium 2-[(2-carboxylatophenyl)disulfanyl]benzoate — dimethylformamide — monohydrate (1/1/1), [C6H16N2][C14H8O4S2] ⋅ C3H7NO⋅H2O
- The synthesis and crystal structure of isobutyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C16H13Cl2F6N3O3S
- Isolation and crystal structure of bufotalinin — methanol (1/1), C25H34O7
- Crystal structure of benzylbis(1,3-diphenylpropane-1,3-dionato-κ2O,O′) chloridotin(IV), C37H29ClO4Sn
- Crystal structure of Bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imidazol)}diiodidocadmium(II), [Cd(C11H11N5)2I2], C22H22N10I2Cd
- Crystal structure of 4-isobutoxybenzaldehyde oxime, C11H15NO2
- The crystal structure of bis(acetato-κ1O)-bis(N′-hydroxypyrimidine-2-carboximidamide-κ2N,N′)manganese(II) — methanol (1/2), C14H18MnN8O6, 2(CH3OH)′
- Crystal structure of poly[bis(μ2-bis(4-(1H-imidazol-1-yl)phenyl)amine-κ2N:N′)-bis(nitrato-κO)cadmium(II)], C36H30CdN12O6
- Crystal structure and optical properties of 1,6-bis(methylthio)pyrene, C18H14S2
- The crystal structure of hexaquamagnesium(II) bis(3,4-dinitropyrazol-1-ide), C6H14MgN8O14
- Halogen bonds in the crystal structure of 4,3:5,4-terpyridine – 1,4-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure and photochromic properties of a novel photochromic perfluordiarylethene containing a triazole bridged pyridine group moiety, C24H18F6N4S2
- Crystal structure of bis[(μ3-oxido)-(μ2-(N,N-diisopropylthiocarbamoylthio) acetato-κ2O,O′)-((N,N-diisopropylthiocarbamoylthio)acetato-κO)-bis(di-4-methylbenzyl-tin(IV))], C100H136N4O10S8Sn4
- Crystal structure of dibromidobis(4-bromobenzyl)tin(IV), C14H12Br4Sn
- The crystal structure of (4Z)-2-[(E)-(1-ethyl-3,3-dimethyl-1,3-dihydro-2H-indol-2-ylidene)methyl]-4-[(1-ethyl-3,3-dimethyl-3H-indolium-2-yl)methylidene]-3-oxocyclobut-1-en-1-olate, C30H32N2O2
- The crystal structure of (E)-3-(4-(dimethylamino)styryl)-5,5-dimethylcyclohex-2-en-1-one, C18H23NO
- Crystal structure of dihydrazinium 1H-pyrazole-3,5-dicarboxylate, C5H12N6O4
- Crystal structure of poly[μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4-sulfidobenzoate-κ2O:S)cobalt(II)] dihydrate, C42H44Co2N8O7S2
- Crystal structure of 8-(3,4-dimethylbenzylidene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C17H18O4
- Crystal structure of 4-(2-bromo-4-(6-morpholino-3-phenyl-3H-benzo[f]chromen-3-yl) cyclohexa-2,5-dien-1-yl)morpholine, C33H31BrN2O
- Synthesis and crystal structure of 2-((1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)methylene)-2,3-dihydro-1H-inden-1-one, C23H16N2OS
- Crystal structure of poly[(μ2-1,1′-(oxybis(4,1-phenylene)bis(1H-imidazole)-κ2N,N′)(μ2-1,3-benzenecarboxylato-κ3O,O′:O′′)zinc(II)] dihydrate, C26H22N4O7Zn
- Crystal structure of diaqua-bis(cinnamato-κ2O,O′)zinc(II), C18H18ZnO6
- Crystal structure of 2-(prop-2-yn-1-yloxy)-1-naphthaldehyde, C14H10O2
- Crystal structure and photochromic properties of 1-(2-methyl-5-phenyl-3-thienyl)-2-{2-methyl-5-[4-(9-fluorenone hydrazone)-phenyl]-3-thienyl}perfluorocyclopentene, C41H26F6N2S2
- Hydrothermal synthesis and crystal structure of cylo[tetraaqua-bis(μ2-1,4-bis(1H-benzo[d]imidazol-1-yl)but-2-ene-κ2N:N′)-bis(μ2-4-nitro-phthalate-κ2O,O′)dinickel(II)], C26H23N5O8Ni
- Crystal structure of 3-[methyl(phenyl)amino]-1-phenylthiourea, C14H15N3S
- Crystal structure of 1-(4-chlorophenyl)-3-[methyl(phenyl)amino]thiourea, C14H14ClN3S
- Crystal structure of 2-tert-butyl-1H-imidazo[4,5-b]pyridine, C10H13N3
- Crystal structure of 5-carboxy-2-(2-carboxyphenyl)-1H-imidazol-3-ium-4-carboxylate dihydrate, C12H8N2O6⋅2(H2O)
- The crystal structure of dichlorido-μ2-dichlorido-(η2-1,4-bis(4-vinylbenzyl)-1,4-diazabicyclo[2.2.2]octane-1,4-diium)dicopper(I), C24H30N2Cu2Cl4
- Crystal structure of 4-bromobenzyl (Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C22H29BrN2OS
- Crystal structure of (4S,4aS,6aR,6bR,12aS,12bR,14aS,14bR)-3,3,6a,6b,9,9,12a-heptamethyloctadecahydro-1H,3H-4,14b-ethanophenanthro[1,2-h]isochromene-1(6bH)-one, C30H48O2
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C30H33F6N3S
- The crystal structure of 3-methoxyphenanthridin-6(5H)-one, C14H11NO2
- Crystal structure of 4-(5,5-difluoro-1,3,7,9-tetramethyl-3H,5H-5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)pyridin-1-ium tetraiodidoferrate(III), C18H19BF2FeI4N3
- Crystal structure of 2-(3-methoxyphenyl)-3-((phenylsulfonyl)methyl)imidazo[1,2-a]pyridine, C21H18N2O3S
- Crystal structure of [(2-(2-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) perchlorate, C29H50Cl2N4NiO8
- Crystal structure of (Z)-6-(dimethylamino)-3,3-bis(4-(dimethylamino)phenyl)-2-(2-(quinoxalin-2-ylmethylene)hydrazinyl)-2,3-dihydroinden-1-one, C35H35N7O
- 5-Methyl-N′-[5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbonyl]-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbohydrazide, C22H22N8O2
- Crystal structure of 2,3-dichloro-6-methoxyquinoxaline, C9H6Cl2N2O
- Synthesis and crystal structure of 7-chloro-2-(ethylsulfinyl)-6-fluoro-3-(1H-pyrazole-1-yl)-4H-thiochromen-4-one, C13H10FN3OS2
- Crystal structure of 4-ethylpiperazine-1-carbothioic dithioperoxyanhydride, C14H26N4S4
- Crystal structure of 2-(2-(6-methylpyridin-2-yl)naphthalen-1-yl)pyrimidine, C20H15N3
- The crystal structure of N′-((1E,2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-ylidene)-3-methylbenzohydrazide, C23H22N2O4
- Crystal structure of catena-poly[(μ2-isophthalato-κ2O:O′)-(2,5-di(pyrazin-2-yl)-4,4′-bipyridine-κ3N,N′,N′′)zinc(II)] — water (2/5), C26H21N6O6.5Zn
- Crystal structure of (3E,5E)-3,5-bis(3-nitrobenzylidene)-1-((4-(trifluoromethyl)phenyl)sulfonyl)piperidin-4-one — dichloromethane (2/1), C53H38Cl2F6N6O14S2
- Crystal structure of (μ2-oxido)-bis(N,N′-o-phenylenebis(salicylideneiminato))diiron(III) — N,N′-dimethylformamide, C47H43Fe2N4O9
- Crystal structure of N1,N3-bis(2-hydroxyethyl)-N1, N1,N3,N3-tetramethylpropane-1,3-diaminium dibromide, C11H28Br2N2O2
- Crystal structure of (E)-N-(4-chlorophenyl)-1-(pyridin-2-yl)methanimine, C12H9ClN2
- Crystal structure of 8-bromo-6-oxo-2-phenyl-6H-pyrrolo[3,2,1-ij]quinoline-5-carbaldehyde, C18H11BrNO2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride trihydrate, C8H18N8Cl2 ⋅ 3 H2O
- Crystal structure of (E)-4-bromo-N-(pyridin-2-ylmethylene)aniline, C12H9BrN2
- Crystal structure of bis[(2-(3-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ-O)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Br2N4NiO8
- The crystal structure of (1E,2E)-2-methyl-4-((7-oxo-7H-furo[3,2-g]chromen-9-yl)oxy)but-2-enal O-isonicotinoyl oxime–trichloromethane (3/1), C67H49Cl3N6O18
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-methyl-1H-imidazol-3-ium hexafluoridophosphate(V), C8H13F6N2O2P
- Crystal structure of bis[(2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κO)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) hemihydrate C42H65Br2N4NiO8.5
- The crystal structure of N-(7-(4-fluorobenzylidene)-3-(4-fluorophenyl)-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbonothioyl)benzamide, C28H23F2N3OS
- The crystal structure of N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide, C18H20N4O2
- Crystal structure of (E)-2-(3,6-bis(ethylamino)-2,7-dimethyl-9H-xanthen-9-yl)-N′-((6-methylpyridin-2-yl)methylene)benzohydrazide – methanol (1/1), C34H37N5O3
- Crystal structure of 2-oxo-1-(pyrimidin-5-ylmethyl)-3-(3-(trifluoromethyl)phenyl)-1,2-dihydro-5l4-pyrido[1,2-a]pyrimidin-4-olate, C20H13F3N4O2
- Crystal structure of poly[(μ3-9H-carbazole-3,6-dicarboxylato-κ3O1: O2: O3)(μ2-4-(pyridin-4-yl)pyridine-κ2N1:N1′)zinc(II)], C19H11N2O4Zn
- Crystal structure of (E)-N′-((1,8-dihydropyren-1-yl)-methylene)picolinohydrazide, C23H15N3O
- Crystal structure of catena-poly{[μ2-1,2-bis(diphenylphosphino)ethane]dichloridocadmium(II)}, C26H24CdCl2P2
- Crystal structure of the 1:2 co-crystal between N,N′-bis(4-pyridylmethyl)oxalamide and acetic acid as a dihydrate, C14H14N4O2⋅2 C2H4O2⋅2 H2O
- Crystal structure of the co-crystal N,N′-bis(3-pyridylmethyl)oxalamide acetic acid (1/2), C14H14N4O2⋅2C2H4O2
- Crystal structure of the co-crystal N,N′-bis(4-pyridylmethyl)oxalamide and 2,3,5,6-tetrafluoro-1,4-di-iodobenzene (1/1), C14H14N4O2⋅C6F4I2
- Crystal structure of the co-crystal 4-[(4-carboxyphenyl)disulfanyl]benzoic acid–(1E,4E)-1-N,4-N-bis(pyridin-4-ylmethylidene)cyclohexane-1,4-diamine (1/1), C14H10O4S2⋅C18H20N4
- Crystal structure of hexacarbonyl-bis(μ2-di-n-propyldithiocarbamato-κ3S,S′:S;κ3S:S:S′)-di-rhenium(I), C20H28N2O6Re2S4
- Crystal structure of fac-tricarbonyl-morpholine-κN-(morpholinocarbamodithioato-κ2S,S′)rhenium(I), C12H17N2O5ReS2

