Abstract
C16H13Cl2F6N3O3S, monoclinic, P21/n (no. 14), a = 5.8650(10) Å, b = 30.196(5) Å, c = 11.777(2) Å, β = 96.619(2)°, V = 2071.8(6) Å3, Z = 4, Rgt(F) = 0.0521, wRref(F2) = 0.1211, T = 173 K.
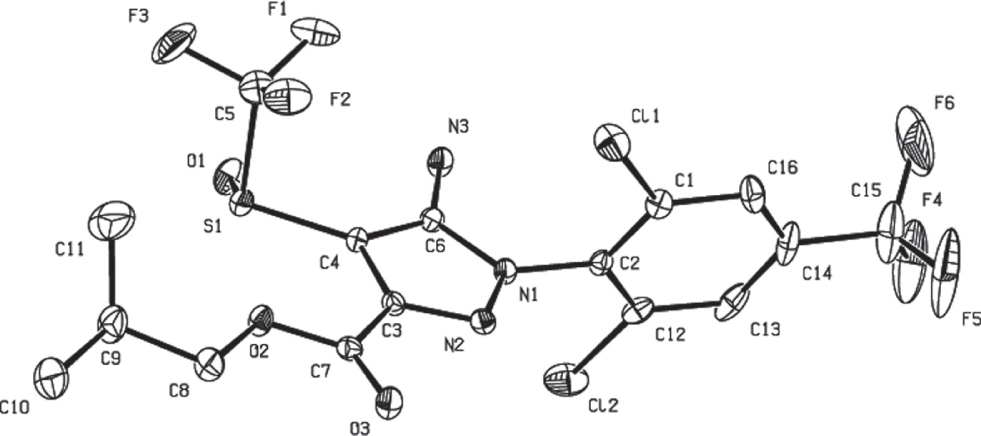
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.12 × 0.10 × 0.07 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.49 mm−1 |
| Diffractometer, scan mode: | CCD, φ and ω-scans |
| θmax, completeness: | 26.4°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 15720, 4233, 0.041 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3370 |
| N(param)refined: | 282 |
| Programs: | Bruker [1], SHELX [2], [3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Cl1 | 0.04011(14) | 0.77877(3) | 0.63055(7) | 0.0316(2) |
| Cl2 | 0.70487(13) | 0.80621(3) | 0.36042(7) | 0.0363(2) |
| S1 | 0.25821(12) | 0.63290(2) | 0.37233(6) | 0.01874(17) |
| F1 | −0.1676(3) | 0.66337(7) | 0.36723(18) | 0.0433(5) |
| F2 | −0.0452(3) | 0.62899(7) | 0.52224(16) | 0.0417(5) |
| F3 | −0.1378(4) | 0.59258(8) | 0.3664(2) | 0.0566(6) |
| F4 | 0.3827(7) | 0.96016(8) | 0.4564(2) | 0.0917(11) |
| F5 | 0.3646(7) | 0.95215(8) | 0.6342(2) | 0.1048(13) |
| F6 | 0.0659(7) | 0.94870(9) | 0.5147(3) | 0.1043(12) |
| O1 | 0.2263(4) | 0.64386(7) | 0.24872(16) | 0.0268(5) |
| O2 | 0.4919(3) | 0.61221(6) | 0.61135(16) | 0.0216(4) |
| O3 | 0.6658(3) | 0.66245(7) | 0.73301(16) | 0.0235(5) |
| N1 | 0.3989(4) | 0.75131(7) | 0.49104(18) | 0.0154(5) |
| N2 | 0.5007(4) | 0.72835(8) | 0.58411(18) | 0.0165(5) |
| N3 | 0.1727(4) | 0.74014(8) | 0.31342(19) | 0.0208(5) |
| H3A | 0.161985 | 0.768221 | 0.302337 | 0.025* |
| H3B | 0.106985 | 0.722121 | 0.263337 | 0.025* |
| C1 | 0.2111(5) | 0.81497(10) | 0.5649(2) | 0.0194(6) |
| C2 | 0.3750(4) | 0.79788(9) | 0.5002(2) | 0.0158(6) |
| C3 | 0.4624(4) | 0.68636(9) | 0.5581(2) | 0.0152(5) |
| C4 | 0.3317(4) | 0.68134(9) | 0.4490(2) | 0.0154(6) |
| C5 | −0.0389(6) | 0.63015(11) | 0.4100(3) | 0.0306(7) |
| C6 | 0.2918(4) | 0.72429(9) | 0.4091(2) | 0.0160(6) |
| C7 | 0.5530(5) | 0.65337(9) | 0.6443(2) | 0.0168(6) |
| C8 | 0.5814(5) | 0.57675(10) | 0.6884(3) | 0.0260(7) |
| H8A | 0.528995 | 0.580884 | 0.764661 | 0.031* |
| H8B | 0.751323 | 0.577059 | 0.697354 | 0.031* |
| C9 | 0.4942(6) | 0.53363(10) | 0.6377(3) | 0.0325(8) |
| H9 | 0.535656 | 0.532141 | 0.557767 | 0.039* |
| C10 | 0.6128(7) | 0.49519(11) | 0.7045(3) | 0.0429(9) |
| H10A | 0.779295 | 0.497710 | 0.704012 | 0.064* |
| H10B | 0.559522 | 0.467142 | 0.668981 | 0.064* |
| H10C | 0.575912 | 0.496021 | 0.783572 | 0.064* |
| C11 | 0.2373(7) | 0.52951(14) | 0.6321(5) | 0.0660(14) |
| H11A | 0.191410 | 0.532069 | 0.709273 | 0.099* |
| H11B | 0.188995 | 0.500652 | 0.599703 | 0.099* |
| H11C | 0.164258 | 0.553130 | 0.583744 | 0.099* |
| C12 | 0.5094(5) | 0.82674(10) | 0.4451(2) | 0.0216(6) |
| C13 | 0.4847(6) | 0.87218(10) | 0.4564(3) | 0.0294(7) |
| H13 | 0.578475 | 0.892076 | 0.419955 | 0.035* |
| C14 | 0.3209(6) | 0.88800(10) | 0.5219(3) | 0.0308(8) |
| C15 | 0.2889(10) | 0.93731(13) | 0.5339(3) | 0.0560(12) |
| C16 | 0.1830(6) | 0.85995(10) | 0.5763(2) | 0.0260(7) |
| H16 | 0.071064 | 0.871411 | 0.620638 | 0.031* |
Source of material
All chemical reagents and solvents including fipronil were of analytical grade quality, which were obtained from commercial suppliers and used directly without further purification (Wuhan Guoyao Chemical Reagent Co., Ltd.). The synthesis process of the target product involves the following steps by an improved Pinner reaction. Firstly, to a 250 mL three-necked bottle, added 0.01 mol (4.37 g) fipronil and then dissolved by 30 mL isobutyl alcohol, 0.02 mol of anhydrous ferric chloride in batches was added as a catalyst, ultrasonic stirring for 0.5 h, the reaction mixture was reacted at 100 °C for 9 h. The resulting solution was then cooled to room temperature, and removed excess solvent to give a tan cream. Secondly, 100 mL of distilled water and 50 mL of ethyl acetate were added to the tan cream one by one, and extracted three times. The organic phases were combined and washed with saturated sodium carbonate solution (30 mL), distilled water and brine, respectively, and then dried over MgSO4. The filtrate was concentrated, swirled and adsorbed onto 6 g of activated silica gel. Finally, the crude product was obtained by column chromatography on silica gel with Vethyl acetate/Vpetroleum ether (1:4) the eluent, which was dried under vacuum to give the title compound. Yield: 91.2%, 1H NMR (CDCl3, 400 MHz, ppm) δ 7.79 (s, 2H, Ar—H), 5.17 (s, 2H, C—NH2), 4.15 (d, J = 8.0 Hz, 2H, C—CH2), 2.08 (td, J = 20.0, 12.0 Hz, 1H, C—CH), 0.99 (dd, J = 8.0, 8.0 Hz, 6H, C—CH3). IR (KBr, ν, cm−1): 3451, 3328 (N—H), 2970 (Ar—H), 1728 (C=O), 1618 (—C=N), 1497 (benzene ring skeleton vibration), 1326 (C—F), 1144, 1065 (C—O—C), 875, 816 (C—H), 657 (aromatic ring C—H). MS (FAB): m/e, 512 (M+).
After allowing the Vethyl acetate/Vpetroleum ether (1:5) to stand in air for 8 days, transparent colorless block crystals of the title compound formed by slow evaporation of the solvent. The crystals were isolated, washed with light petroleum and dried in vacuum (yield 89.6%).
Experimental details
All H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms with C—H distances in the range 0.93–0.98 Å, and with Uiso(H) = 1.2 Ueq for aryl and nitrogen H atoms and 1.5 Ueq for the methyl H atoms. The special instructions are as follows:
Fixed Uiso,
At 1.2 times of: All C(H) groups, All C(H, H) groups, All N(H, H) groups
At 1.5 times of: All C(H, H, H) groups
Riding coordinates: N3(H3A, H3B)
Ternary CH refined with riding coordinates: C9(H9)
Secondary CH2 refined with riding coordinates: C8(H8A, H8B)
Aromatic/amide H refined with riding coordinates: C13(H13), C16(H16)
Idealised Me refined as rotating group: C10(H10A, H10B, H10C), C11(H11A, H11B, H11C).
Comment
Phenylpyrazole derivatives have attracted increasing attention owing to their excellent biological activities [5]. In the field of pesticides, they play a fundamental role in the rapid development of insecticides, and in the field of medicine, their derivatives can reduce analgesia and inflammation [6]. In the agricultural field, phenylpyrazole compounds are mainly used to deal with soil insects such as gold nematodes, aquatic insects such as rice, and control of aphids [7]. In addition, phenylpyrazole heterocycle derivatives are useful inter-mediates and ligands, from which many macrocyclic compounds with high fluorescence quantum yield and supermolecular structures with extended conjugated aromatic system can be constructed. Heterocyclic ester derivatives and its complexes are important chemical reagents, which can act as a foundational role of a wide range of applications in the fields of catalysis, biochemistry, functional materials, medicine and other research areas [8]. However, to our best knowledge, only a few phenylpyrazole ester compounds have been reported. Considering on the advantages of high stability, long duration of action, good solubility and small side effects for phenylpyrazole ester derivatives, the title compound has been synthesized by an improved Pinner reaction using FeCl3 as green catalyst, and isobutyl alcohol as solvent and reactant [9].
The molecular structure consists of a pyrazole ring, including a trifluoromethanesulfinyl group and carboxylic acid isobutyl ester group linked on the peripheral part, and a phenyl ring, which is bridged with C—N bond 1.418(3) Å formed by N1—C2 single bond to the 2,6-dichloro-4-trifluoromethylphenyl moiety. The S1—C4 bond distance is 1.747(3) Å, significantly longer than the double bond length of S1—O1 (1.484(2) Å), which is the single bond connecting 5-membered heterocyclic pyrazole ring. The 2,6-dichloro-4-(trifluoromethyl)phenyl moiety is twisted out of the plane of pyrazole ring and the two heterocyclic rings makes a dihedral angle of 73.38(3)° [10]. The isobutylate group is coplanar to the plane of pyrazole ring due to the carboxyl planar structure. However the CF3 group in the trifluoromethanesulfinyl moiety is almost perpendicular to the pyrazole plane and the angle of C4—S1—C5 is 95.56(14)° [11]. The N2—C3 bond distance is 1.318(4) Å, which is the shortest carbon-nitrogen double bond. The C5—F1, C5—F2 and C5—F3 bond distance (CF3 group connecting with phenyl moiety) are 1.320(4) Å, 1.327(4) Å and 1.349(4) Å, respectively and their average bond distance is slightly larger than the average value of the other CF3 group in the trifluoromethanesulfinyl moiety. Generally the geometric parameters are similar to fibronil [12].
There are some non-classical hydrogen bonds in the packing crystal structure of the title compound, which is partially facilitated by F—H interactions between neighboring molecules. The two most prominent F—H interactions are F2—H11C (bond distance 2.660(2) Å) and F3—H11B (bond distance 2.8640(25) Å), which acts in centrosymmetric pairs between adjacent molecules, connecting these molecules to chains along the b axis of the unit cell. The other hydrogen bond F4—H10C has 2.8787(29) Å bond distance along the c axis and connects these chains with each other. The F—H interactions F1—H3B (bond distance 2.7735(21) Å) is along the a axis, working with other three kinds of hydrogen bonds and building up a perfect two-dimensional structure.
The bioactivities of the title compound and model phenylpyrazole insecticide (fipronil) against the 3rd instar larvae of Plutella xylostella were investigated by the leaf disc-dipping assay. Leaves of Chinese cabbage grown in the greenhouse were collected, and discs (5 cm diameter) were punched from each leaf. Two kinds of compounds were dissolved in organic solvent (acetone) and suspended in distilled water containing Triton X-100. Leaf discs were dipped in each test solution for 35 s and allowed to dry for 2.5 h. The treated leaf discs were placed into 10 cm diameter Petri dishes. Then, ten Plutella xylostella larvae were introduced into each dish. Doubly distilled water containing acetone-Triton X-100 solution was used as the control. Petri dishes were kept in incubator at 24 °C and 80% relative humidity under a photoperiod of 16:8 h light: dark. All treatments were replicated three times. Mortalities were determined 24 h after treatment. The death rate of each treatment group was determined. LC50 value was calculated by the SPSS. Bioactivity result exhibited that the activities of the title compound against Plutella xylostella after 24 h is 15.26 mg⋅L−1, better than that of fipronil 27.24 mg⋅L−1. These researches propose a novel insight to provide some novel phenylpyrazole insecticide by an improved pinner reaction.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 20702064
Award Identifier / Grant number: 21177161
Award Identifier / Grant number: 31402137
Funding statement: The authors thank the Natural Science Foundation of Hubei province for Distinguished Young Scholars (No. 2013CFA034); National Natural Science Foundation of China (Grant Nos. 20702064, 21177161 and 31402137); the Program for Excellent Talents in Hubei Province (RCJH15001); the Opening Project of Key Laboratory of Green Catalysis of Sichuan Institutes of High Education (LYZ1107) and the Fundamental Research Funds for the Central University, South-Central University for Nationalities (CZP17077).
References
1. BRUKER. SAINT, APEX2 and SADABS. Bruker AXS Inc., Madison, WI, USA (2009).Search in Google Scholar
2. Sheldrick, G. M.: SHELXT – integrated space-group and crystal-structure determination. Acta Crystallogr. A71 (2015) 3–8.10.1107/S2053273314026370Search in Google Scholar
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar
4. Brandenburg, K.: DIAMOND. Visual Crystal Structure Information System. Ver. 4.0. Crystal Impact, Bonn, Germany (2015).Search in Google Scholar
5. Hainzl, D.; Cole, L. M.; Casida, J. E.: Mechanisms for selective toxicity of fipronil insecticide and its sulfone metabolite and desulfinyl photoproduct. Chem. Res. Toxicol. 11 (1998) 1529–1535.10.1021/tx980157tSearch in Google Scholar
6. Smolen, J. M.; Stone, A. T.: Divalent metal ion-catalyzed hydrolysis of phosphorothionate ester pesticides and their corresponding oxonates. Environ. Sci. Technol. 6 (1997) 1664–1673.10.1021/es960499qSearch in Google Scholar
7. Young, B. L.; Yang, M. G.; Youn, Y. L.; Jae, K. L.: Conversion of α-aminonitriles to amides by a new pinner type reaction. Tetrahedron Lett. 8 (1990) 1169–1170.10.1016/S0040-4039(00)88755-9Search in Google Scholar
8. Luzyanin, K. V.; Kukushkin, V. Y.; Kuznetsov, M. L.; Garnovskii, D. A.; Haukka, M.; Pombeiro, A. J. L.: Novel reactivity mode of hydroxamic acids: a metalla-pinner reaction. Inorg. Chem. 41 (2002) 2981–2986.10.1021/ic025554cSearch in Google Scholar PubMed
9. Gavin, D. J.; Mojiaca, C. A.: Optimization of an imidate hydrolysis reaction: use of DOE and mathematical modeling to deliver yield and productivity improvements. Org. Process Res. Dev. 5 (2001) 659–664.10.1021/op010225+Search in Google Scholar
10. Li, X. C.; Lim, W. T.; Kim, S. H.; Son, Y. A.: Crystal structure of 4-formylphenyl-diphenylamine, C19H15NO. Z. Kristallogr. NCS 224 (2009) 459–460.10.1524/ncrs.2009.0200Search in Google Scholar
11. Qi, Y. Y.; Zhang, S. X.: Crystal structure of 4,4′-bipyridine-chloroacetic acid (1:2), C10H8N2⋅2 C2H3ClO2, C14H14Cl2N2O4. Z. Kristallogr. NCS 228 (2013) 341–342.10.1524/ncrs.2013.0167Search in Google Scholar
12. Park, H.; Kim, J.; Kwon, E.; Kim, T. H.: Crystal structure of fipronil. Acta Crystallogr. E73 (2017) 1472–1474.10.1107/S205698901701310XSearch in Google Scholar PubMed PubMed Central
©2019 Lianqing Chen et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Crystal structure of poly[diaqua-(μ8-1,1′:2′,1′′-terphenyl-3,3′′,4′,5′-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)dicopper(II)], C22H14Cu2O10
- Crystal structure of 2-((1H-benzo[d]imidazol-2-ylimino)methyl)-4,6-di-tert-butylphenol, C22H27N3O
- Crystal structure of (4-ethoxynaphthalen-1-yl)(furan-2-yl)methanone, C17H14O3
- Crystal structure of 1-nonylpyridazin-1-ium iodide, C13H23N2I
- Crystal structure of bis[diaqua(1,10-phenanthroline-κ2N, N′)-copper(II)]diphenylphosphopentamolybdate dihydrate, C36H38Cu2Mo5N4O27P2
- The crystal structure of tetrakis(imidazole)-copper(I) hexafluorophosphate, C12H16CuF6PN8
- The crystal structure of dimethyl ((3,5-di-tert-butyl-4-hydroxyphenyl)(phenyl)methyl)phosphonate, C23H33O4P
- Crystal structure of diaqua-bis(1,10-phenanthroline κ2N,N′)nickel(II) trifluoroacetate- trifluoroacetic acid (1/1), C30H21F9N4NiO8
- Crystal structure of 2-(naphthalen-2-yl)-1,8-naphthyridine, C18H12N2
- Synthesis and crystal structure of a new polymorph of diisopropylammonium trichloroacetate, C8H16Cl3NO2
- Crystal structure of dimethanol-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)cadmium(II) C34H34CdN12O2S2
- Crystal structure of ethyl 2,2-difluoro-2-(7-methoxy-2-oxo-2H-chromen-3-yl)acetate, C14H12F2O5
- The crystal structure of bis[μ2-(N,N-diethylcarbamodithioato-κS:κS,κS′)] bis[1′-(diphenylphosphino-κP)-1-cyanoferrocene]disilver(I), C56H56Ag2Fe2N4P2S4
- Crystal structure of bis(di-n-butylammonium) tetrachloridodiphenylstannate(IV), C28H50Cl4N2Sn
- The crystal structure of poly[(μ5-2-((5-bromo-3-formyl-2-hydroxybenzylidene)amino)benzenesulfonato-κ6O:O:O,O′:O′:O′′)sodium(I)], C13H9O4NSBrNa
- Crystal structure of catena-{poly[bis(O,O′-diethyldithiophosphato-S)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)-zinc(II)] di-acetonitrile solvate}, {C20H30N4O4P2S4Zn ⋅ 2 C2H3N}n
- Halogen and hydrogen bonding in the layered crystal structure of 2-iodoanilinium triiodide, C6H7I4N
- Crystal structure of cyclohexane-1,4-diammonium 2-[(2-carboxylatophenyl)disulfanyl]benzoate — dimethylformamide — monohydrate (1/1/1), [C6H16N2][C14H8O4S2] ⋅ C3H7NO⋅H2O
- The synthesis and crystal structure of isobutyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C16H13Cl2F6N3O3S
- Isolation and crystal structure of bufotalinin — methanol (1/1), C25H34O7
- Crystal structure of benzylbis(1,3-diphenylpropane-1,3-dionato-κ2O,O′) chloridotin(IV), C37H29ClO4Sn
- Crystal structure of Bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imidazol)}diiodidocadmium(II), [Cd(C11H11N5)2I2], C22H22N10I2Cd
- Crystal structure of 4-isobutoxybenzaldehyde oxime, C11H15NO2
- The crystal structure of bis(acetato-κ1O)-bis(N′-hydroxypyrimidine-2-carboximidamide-κ2N,N′)manganese(II) — methanol (1/2), C14H18MnN8O6, 2(CH3OH)′
- Crystal structure of poly[bis(μ2-bis(4-(1H-imidazol-1-yl)phenyl)amine-κ2N:N′)-bis(nitrato-κO)cadmium(II)], C36H30CdN12O6
- Crystal structure and optical properties of 1,6-bis(methylthio)pyrene, C18H14S2
- The crystal structure of hexaquamagnesium(II) bis(3,4-dinitropyrazol-1-ide), C6H14MgN8O14
- Halogen bonds in the crystal structure of 4,3:5,4-terpyridine – 1,4-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure and photochromic properties of a novel photochromic perfluordiarylethene containing a triazole bridged pyridine group moiety, C24H18F6N4S2
- Crystal structure of bis[(μ3-oxido)-(μ2-(N,N-diisopropylthiocarbamoylthio) acetato-κ2O,O′)-((N,N-diisopropylthiocarbamoylthio)acetato-κO)-bis(di-4-methylbenzyl-tin(IV))], C100H136N4O10S8Sn4
- Crystal structure of dibromidobis(4-bromobenzyl)tin(IV), C14H12Br4Sn
- The crystal structure of (4Z)-2-[(E)-(1-ethyl-3,3-dimethyl-1,3-dihydro-2H-indol-2-ylidene)methyl]-4-[(1-ethyl-3,3-dimethyl-3H-indolium-2-yl)methylidene]-3-oxocyclobut-1-en-1-olate, C30H32N2O2
- The crystal structure of (E)-3-(4-(dimethylamino)styryl)-5,5-dimethylcyclohex-2-en-1-one, C18H23NO
- Crystal structure of dihydrazinium 1H-pyrazole-3,5-dicarboxylate, C5H12N6O4
- Crystal structure of poly[μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4-sulfidobenzoate-κ2O:S)cobalt(II)] dihydrate, C42H44Co2N8O7S2
- Crystal structure of 8-(3,4-dimethylbenzylidene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C17H18O4
- Crystal structure of 4-(2-bromo-4-(6-morpholino-3-phenyl-3H-benzo[f]chromen-3-yl) cyclohexa-2,5-dien-1-yl)morpholine, C33H31BrN2O
- Synthesis and crystal structure of 2-((1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)methylene)-2,3-dihydro-1H-inden-1-one, C23H16N2OS
- Crystal structure of poly[(μ2-1,1′-(oxybis(4,1-phenylene)bis(1H-imidazole)-κ2N,N′)(μ2-1,3-benzenecarboxylato-κ3O,O′:O′′)zinc(II)] dihydrate, C26H22N4O7Zn
- Crystal structure of diaqua-bis(cinnamato-κ2O,O′)zinc(II), C18H18ZnO6
- Crystal structure of 2-(prop-2-yn-1-yloxy)-1-naphthaldehyde, C14H10O2
- Crystal structure and photochromic properties of 1-(2-methyl-5-phenyl-3-thienyl)-2-{2-methyl-5-[4-(9-fluorenone hydrazone)-phenyl]-3-thienyl}perfluorocyclopentene, C41H26F6N2S2
- Hydrothermal synthesis and crystal structure of cylo[tetraaqua-bis(μ2-1,4-bis(1H-benzo[d]imidazol-1-yl)but-2-ene-κ2N:N′)-bis(μ2-4-nitro-phthalate-κ2O,O′)dinickel(II)], C26H23N5O8Ni
- Crystal structure of 3-[methyl(phenyl)amino]-1-phenylthiourea, C14H15N3S
- Crystal structure of 1-(4-chlorophenyl)-3-[methyl(phenyl)amino]thiourea, C14H14ClN3S
- Crystal structure of 2-tert-butyl-1H-imidazo[4,5-b]pyridine, C10H13N3
- Crystal structure of 5-carboxy-2-(2-carboxyphenyl)-1H-imidazol-3-ium-4-carboxylate dihydrate, C12H8N2O6⋅2(H2O)
- The crystal structure of dichlorido-μ2-dichlorido-(η2-1,4-bis(4-vinylbenzyl)-1,4-diazabicyclo[2.2.2]octane-1,4-diium)dicopper(I), C24H30N2Cu2Cl4
- Crystal structure of 4-bromobenzyl (Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C22H29BrN2OS
- Crystal structure of (4S,4aS,6aR,6bR,12aS,12bR,14aS,14bR)-3,3,6a,6b,9,9,12a-heptamethyloctadecahydro-1H,3H-4,14b-ethanophenanthro[1,2-h]isochromene-1(6bH)-one, C30H48O2
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C30H33F6N3S
- The crystal structure of 3-methoxyphenanthridin-6(5H)-one, C14H11NO2
- Crystal structure of 4-(5,5-difluoro-1,3,7,9-tetramethyl-3H,5H-5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)pyridin-1-ium tetraiodidoferrate(III), C18H19BF2FeI4N3
- Crystal structure of 2-(3-methoxyphenyl)-3-((phenylsulfonyl)methyl)imidazo[1,2-a]pyridine, C21H18N2O3S
- Crystal structure of [(2-(2-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) perchlorate, C29H50Cl2N4NiO8
- Crystal structure of (Z)-6-(dimethylamino)-3,3-bis(4-(dimethylamino)phenyl)-2-(2-(quinoxalin-2-ylmethylene)hydrazinyl)-2,3-dihydroinden-1-one, C35H35N7O
- 5-Methyl-N′-[5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbonyl]-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbohydrazide, C22H22N8O2
- Crystal structure of 2,3-dichloro-6-methoxyquinoxaline, C9H6Cl2N2O
- Synthesis and crystal structure of 7-chloro-2-(ethylsulfinyl)-6-fluoro-3-(1H-pyrazole-1-yl)-4H-thiochromen-4-one, C13H10FN3OS2
- Crystal structure of 4-ethylpiperazine-1-carbothioic dithioperoxyanhydride, C14H26N4S4
- Crystal structure of 2-(2-(6-methylpyridin-2-yl)naphthalen-1-yl)pyrimidine, C20H15N3
- The crystal structure of N′-((1E,2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-ylidene)-3-methylbenzohydrazide, C23H22N2O4
- Crystal structure of catena-poly[(μ2-isophthalato-κ2O:O′)-(2,5-di(pyrazin-2-yl)-4,4′-bipyridine-κ3N,N′,N′′)zinc(II)] — water (2/5), C26H21N6O6.5Zn
- Crystal structure of (3E,5E)-3,5-bis(3-nitrobenzylidene)-1-((4-(trifluoromethyl)phenyl)sulfonyl)piperidin-4-one — dichloromethane (2/1), C53H38Cl2F6N6O14S2
- Crystal structure of (μ2-oxido)-bis(N,N′-o-phenylenebis(salicylideneiminato))diiron(III) — N,N′-dimethylformamide, C47H43Fe2N4O9
- Crystal structure of N1,N3-bis(2-hydroxyethyl)-N1, N1,N3,N3-tetramethylpropane-1,3-diaminium dibromide, C11H28Br2N2O2
- Crystal structure of (E)-N-(4-chlorophenyl)-1-(pyridin-2-yl)methanimine, C12H9ClN2
- Crystal structure of 8-bromo-6-oxo-2-phenyl-6H-pyrrolo[3,2,1-ij]quinoline-5-carbaldehyde, C18H11BrNO2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride trihydrate, C8H18N8Cl2 ⋅ 3 H2O
- Crystal structure of (E)-4-bromo-N-(pyridin-2-ylmethylene)aniline, C12H9BrN2
- Crystal structure of bis[(2-(3-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ-O)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Br2N4NiO8
- The crystal structure of (1E,2E)-2-methyl-4-((7-oxo-7H-furo[3,2-g]chromen-9-yl)oxy)but-2-enal O-isonicotinoyl oxime–trichloromethane (3/1), C67H49Cl3N6O18
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-methyl-1H-imidazol-3-ium hexafluoridophosphate(V), C8H13F6N2O2P
- Crystal structure of bis[(2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κO)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) hemihydrate C42H65Br2N4NiO8.5
- The crystal structure of N-(7-(4-fluorobenzylidene)-3-(4-fluorophenyl)-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbonothioyl)benzamide, C28H23F2N3OS
- The crystal structure of N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide, C18H20N4O2
- Crystal structure of (E)-2-(3,6-bis(ethylamino)-2,7-dimethyl-9H-xanthen-9-yl)-N′-((6-methylpyridin-2-yl)methylene)benzohydrazide – methanol (1/1), C34H37N5O3
- Crystal structure of 2-oxo-1-(pyrimidin-5-ylmethyl)-3-(3-(trifluoromethyl)phenyl)-1,2-dihydro-5l4-pyrido[1,2-a]pyrimidin-4-olate, C20H13F3N4O2
- Crystal structure of poly[(μ3-9H-carbazole-3,6-dicarboxylato-κ3O1: O2: O3)(μ2-4-(pyridin-4-yl)pyridine-κ2N1:N1′)zinc(II)], C19H11N2O4Zn
- Crystal structure of (E)-N′-((1,8-dihydropyren-1-yl)-methylene)picolinohydrazide, C23H15N3O
- Crystal structure of catena-poly{[μ2-1,2-bis(diphenylphosphino)ethane]dichloridocadmium(II)}, C26H24CdCl2P2
- Crystal structure of the 1:2 co-crystal between N,N′-bis(4-pyridylmethyl)oxalamide and acetic acid as a dihydrate, C14H14N4O2⋅2 C2H4O2⋅2 H2O
- Crystal structure of the co-crystal N,N′-bis(3-pyridylmethyl)oxalamide acetic acid (1/2), C14H14N4O2⋅2C2H4O2
- Crystal structure of the co-crystal N,N′-bis(4-pyridylmethyl)oxalamide and 2,3,5,6-tetrafluoro-1,4-di-iodobenzene (1/1), C14H14N4O2⋅C6F4I2
- Crystal structure of the co-crystal 4-[(4-carboxyphenyl)disulfanyl]benzoic acid–(1E,4E)-1-N,4-N-bis(pyridin-4-ylmethylidene)cyclohexane-1,4-diamine (1/1), C14H10O4S2⋅C18H20N4
- Crystal structure of hexacarbonyl-bis(μ2-di-n-propyldithiocarbamato-κ3S,S′:S;κ3S:S:S′)-di-rhenium(I), C20H28N2O6Re2S4
- Crystal structure of fac-tricarbonyl-morpholine-κN-(morpholinocarbamodithioato-κ2S,S′)rhenium(I), C12H17N2O5ReS2
Articles in the same Issue
- Frontmatter
- Crystal structure of poly[diaqua-(μ8-1,1′:2′,1′′-terphenyl-3,3′′,4′,5′-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)dicopper(II)], C22H14Cu2O10
- Crystal structure of 2-((1H-benzo[d]imidazol-2-ylimino)methyl)-4,6-di-tert-butylphenol, C22H27N3O
- Crystal structure of (4-ethoxynaphthalen-1-yl)(furan-2-yl)methanone, C17H14O3
- Crystal structure of 1-nonylpyridazin-1-ium iodide, C13H23N2I
- Crystal structure of bis[diaqua(1,10-phenanthroline-κ2N, N′)-copper(II)]diphenylphosphopentamolybdate dihydrate, C36H38Cu2Mo5N4O27P2
- The crystal structure of tetrakis(imidazole)-copper(I) hexafluorophosphate, C12H16CuF6PN8
- The crystal structure of dimethyl ((3,5-di-tert-butyl-4-hydroxyphenyl)(phenyl)methyl)phosphonate, C23H33O4P
- Crystal structure of diaqua-bis(1,10-phenanthroline κ2N,N′)nickel(II) trifluoroacetate- trifluoroacetic acid (1/1), C30H21F9N4NiO8
- Crystal structure of 2-(naphthalen-2-yl)-1,8-naphthyridine, C18H12N2
- Synthesis and crystal structure of a new polymorph of diisopropylammonium trichloroacetate, C8H16Cl3NO2
- Crystal structure of dimethanol-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)cadmium(II) C34H34CdN12O2S2
- Crystal structure of ethyl 2,2-difluoro-2-(7-methoxy-2-oxo-2H-chromen-3-yl)acetate, C14H12F2O5
- The crystal structure of bis[μ2-(N,N-diethylcarbamodithioato-κS:κS,κS′)] bis[1′-(diphenylphosphino-κP)-1-cyanoferrocene]disilver(I), C56H56Ag2Fe2N4P2S4
- Crystal structure of bis(di-n-butylammonium) tetrachloridodiphenylstannate(IV), C28H50Cl4N2Sn
- The crystal structure of poly[(μ5-2-((5-bromo-3-formyl-2-hydroxybenzylidene)amino)benzenesulfonato-κ6O:O:O,O′:O′:O′′)sodium(I)], C13H9O4NSBrNa
- Crystal structure of catena-{poly[bis(O,O′-diethyldithiophosphato-S)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)-zinc(II)] di-acetonitrile solvate}, {C20H30N4O4P2S4Zn ⋅ 2 C2H3N}n
- Halogen and hydrogen bonding in the layered crystal structure of 2-iodoanilinium triiodide, C6H7I4N
- Crystal structure of cyclohexane-1,4-diammonium 2-[(2-carboxylatophenyl)disulfanyl]benzoate — dimethylformamide — monohydrate (1/1/1), [C6H16N2][C14H8O4S2] ⋅ C3H7NO⋅H2O
- The synthesis and crystal structure of isobutyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C16H13Cl2F6N3O3S
- Isolation and crystal structure of bufotalinin — methanol (1/1), C25H34O7
- Crystal structure of benzylbis(1,3-diphenylpropane-1,3-dionato-κ2O,O′) chloridotin(IV), C37H29ClO4Sn
- Crystal structure of Bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imidazol)}diiodidocadmium(II), [Cd(C11H11N5)2I2], C22H22N10I2Cd
- Crystal structure of 4-isobutoxybenzaldehyde oxime, C11H15NO2
- The crystal structure of bis(acetato-κ1O)-bis(N′-hydroxypyrimidine-2-carboximidamide-κ2N,N′)manganese(II) — methanol (1/2), C14H18MnN8O6, 2(CH3OH)′
- Crystal structure of poly[bis(μ2-bis(4-(1H-imidazol-1-yl)phenyl)amine-κ2N:N′)-bis(nitrato-κO)cadmium(II)], C36H30CdN12O6
- Crystal structure and optical properties of 1,6-bis(methylthio)pyrene, C18H14S2
- The crystal structure of hexaquamagnesium(II) bis(3,4-dinitropyrazol-1-ide), C6H14MgN8O14
- Halogen bonds in the crystal structure of 4,3:5,4-terpyridine – 1,4-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure and photochromic properties of a novel photochromic perfluordiarylethene containing a triazole bridged pyridine group moiety, C24H18F6N4S2
- Crystal structure of bis[(μ3-oxido)-(μ2-(N,N-diisopropylthiocarbamoylthio) acetato-κ2O,O′)-((N,N-diisopropylthiocarbamoylthio)acetato-κO)-bis(di-4-methylbenzyl-tin(IV))], C100H136N4O10S8Sn4
- Crystal structure of dibromidobis(4-bromobenzyl)tin(IV), C14H12Br4Sn
- The crystal structure of (4Z)-2-[(E)-(1-ethyl-3,3-dimethyl-1,3-dihydro-2H-indol-2-ylidene)methyl]-4-[(1-ethyl-3,3-dimethyl-3H-indolium-2-yl)methylidene]-3-oxocyclobut-1-en-1-olate, C30H32N2O2
- The crystal structure of (E)-3-(4-(dimethylamino)styryl)-5,5-dimethylcyclohex-2-en-1-one, C18H23NO
- Crystal structure of dihydrazinium 1H-pyrazole-3,5-dicarboxylate, C5H12N6O4
- Crystal structure of poly[μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4-sulfidobenzoate-κ2O:S)cobalt(II)] dihydrate, C42H44Co2N8O7S2
- Crystal structure of 8-(3,4-dimethylbenzylidene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C17H18O4
- Crystal structure of 4-(2-bromo-4-(6-morpholino-3-phenyl-3H-benzo[f]chromen-3-yl) cyclohexa-2,5-dien-1-yl)morpholine, C33H31BrN2O
- Synthesis and crystal structure of 2-((1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)methylene)-2,3-dihydro-1H-inden-1-one, C23H16N2OS
- Crystal structure of poly[(μ2-1,1′-(oxybis(4,1-phenylene)bis(1H-imidazole)-κ2N,N′)(μ2-1,3-benzenecarboxylato-κ3O,O′:O′′)zinc(II)] dihydrate, C26H22N4O7Zn
- Crystal structure of diaqua-bis(cinnamato-κ2O,O′)zinc(II), C18H18ZnO6
- Crystal structure of 2-(prop-2-yn-1-yloxy)-1-naphthaldehyde, C14H10O2
- Crystal structure and photochromic properties of 1-(2-methyl-5-phenyl-3-thienyl)-2-{2-methyl-5-[4-(9-fluorenone hydrazone)-phenyl]-3-thienyl}perfluorocyclopentene, C41H26F6N2S2
- Hydrothermal synthesis and crystal structure of cylo[tetraaqua-bis(μ2-1,4-bis(1H-benzo[d]imidazol-1-yl)but-2-ene-κ2N:N′)-bis(μ2-4-nitro-phthalate-κ2O,O′)dinickel(II)], C26H23N5O8Ni
- Crystal structure of 3-[methyl(phenyl)amino]-1-phenylthiourea, C14H15N3S
- Crystal structure of 1-(4-chlorophenyl)-3-[methyl(phenyl)amino]thiourea, C14H14ClN3S
- Crystal structure of 2-tert-butyl-1H-imidazo[4,5-b]pyridine, C10H13N3
- Crystal structure of 5-carboxy-2-(2-carboxyphenyl)-1H-imidazol-3-ium-4-carboxylate dihydrate, C12H8N2O6⋅2(H2O)
- The crystal structure of dichlorido-μ2-dichlorido-(η2-1,4-bis(4-vinylbenzyl)-1,4-diazabicyclo[2.2.2]octane-1,4-diium)dicopper(I), C24H30N2Cu2Cl4
- Crystal structure of 4-bromobenzyl (Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C22H29BrN2OS
- Crystal structure of (4S,4aS,6aR,6bR,12aS,12bR,14aS,14bR)-3,3,6a,6b,9,9,12a-heptamethyloctadecahydro-1H,3H-4,14b-ethanophenanthro[1,2-h]isochromene-1(6bH)-one, C30H48O2
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C30H33F6N3S
- The crystal structure of 3-methoxyphenanthridin-6(5H)-one, C14H11NO2
- Crystal structure of 4-(5,5-difluoro-1,3,7,9-tetramethyl-3H,5H-5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)pyridin-1-ium tetraiodidoferrate(III), C18H19BF2FeI4N3
- Crystal structure of 2-(3-methoxyphenyl)-3-((phenylsulfonyl)methyl)imidazo[1,2-a]pyridine, C21H18N2O3S
- Crystal structure of [(2-(2-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) perchlorate, C29H50Cl2N4NiO8
- Crystal structure of (Z)-6-(dimethylamino)-3,3-bis(4-(dimethylamino)phenyl)-2-(2-(quinoxalin-2-ylmethylene)hydrazinyl)-2,3-dihydroinden-1-one, C35H35N7O
- 5-Methyl-N′-[5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbonyl]-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbohydrazide, C22H22N8O2
- Crystal structure of 2,3-dichloro-6-methoxyquinoxaline, C9H6Cl2N2O
- Synthesis and crystal structure of 7-chloro-2-(ethylsulfinyl)-6-fluoro-3-(1H-pyrazole-1-yl)-4H-thiochromen-4-one, C13H10FN3OS2
- Crystal structure of 4-ethylpiperazine-1-carbothioic dithioperoxyanhydride, C14H26N4S4
- Crystal structure of 2-(2-(6-methylpyridin-2-yl)naphthalen-1-yl)pyrimidine, C20H15N3
- The crystal structure of N′-((1E,2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-ylidene)-3-methylbenzohydrazide, C23H22N2O4
- Crystal structure of catena-poly[(μ2-isophthalato-κ2O:O′)-(2,5-di(pyrazin-2-yl)-4,4′-bipyridine-κ3N,N′,N′′)zinc(II)] — water (2/5), C26H21N6O6.5Zn
- Crystal structure of (3E,5E)-3,5-bis(3-nitrobenzylidene)-1-((4-(trifluoromethyl)phenyl)sulfonyl)piperidin-4-one — dichloromethane (2/1), C53H38Cl2F6N6O14S2
- Crystal structure of (μ2-oxido)-bis(N,N′-o-phenylenebis(salicylideneiminato))diiron(III) — N,N′-dimethylformamide, C47H43Fe2N4O9
- Crystal structure of N1,N3-bis(2-hydroxyethyl)-N1, N1,N3,N3-tetramethylpropane-1,3-diaminium dibromide, C11H28Br2N2O2
- Crystal structure of (E)-N-(4-chlorophenyl)-1-(pyridin-2-yl)methanimine, C12H9ClN2
- Crystal structure of 8-bromo-6-oxo-2-phenyl-6H-pyrrolo[3,2,1-ij]quinoline-5-carbaldehyde, C18H11BrNO2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride trihydrate, C8H18N8Cl2 ⋅ 3 H2O
- Crystal structure of (E)-4-bromo-N-(pyridin-2-ylmethylene)aniline, C12H9BrN2
- Crystal structure of bis[(2-(3-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ-O)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Br2N4NiO8
- The crystal structure of (1E,2E)-2-methyl-4-((7-oxo-7H-furo[3,2-g]chromen-9-yl)oxy)but-2-enal O-isonicotinoyl oxime–trichloromethane (3/1), C67H49Cl3N6O18
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-methyl-1H-imidazol-3-ium hexafluoridophosphate(V), C8H13F6N2O2P
- Crystal structure of bis[(2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κO)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) hemihydrate C42H65Br2N4NiO8.5
- The crystal structure of N-(7-(4-fluorobenzylidene)-3-(4-fluorophenyl)-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbonothioyl)benzamide, C28H23F2N3OS
- The crystal structure of N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide, C18H20N4O2
- Crystal structure of (E)-2-(3,6-bis(ethylamino)-2,7-dimethyl-9H-xanthen-9-yl)-N′-((6-methylpyridin-2-yl)methylene)benzohydrazide – methanol (1/1), C34H37N5O3
- Crystal structure of 2-oxo-1-(pyrimidin-5-ylmethyl)-3-(3-(trifluoromethyl)phenyl)-1,2-dihydro-5l4-pyrido[1,2-a]pyrimidin-4-olate, C20H13F3N4O2
- Crystal structure of poly[(μ3-9H-carbazole-3,6-dicarboxylato-κ3O1: O2: O3)(μ2-4-(pyridin-4-yl)pyridine-κ2N1:N1′)zinc(II)], C19H11N2O4Zn
- Crystal structure of (E)-N′-((1,8-dihydropyren-1-yl)-methylene)picolinohydrazide, C23H15N3O
- Crystal structure of catena-poly{[μ2-1,2-bis(diphenylphosphino)ethane]dichloridocadmium(II)}, C26H24CdCl2P2
- Crystal structure of the 1:2 co-crystal between N,N′-bis(4-pyridylmethyl)oxalamide and acetic acid as a dihydrate, C14H14N4O2⋅2 C2H4O2⋅2 H2O
- Crystal structure of the co-crystal N,N′-bis(3-pyridylmethyl)oxalamide acetic acid (1/2), C14H14N4O2⋅2C2H4O2
- Crystal structure of the co-crystal N,N′-bis(4-pyridylmethyl)oxalamide and 2,3,5,6-tetrafluoro-1,4-di-iodobenzene (1/1), C14H14N4O2⋅C6F4I2
- Crystal structure of the co-crystal 4-[(4-carboxyphenyl)disulfanyl]benzoic acid–(1E,4E)-1-N,4-N-bis(pyridin-4-ylmethylidene)cyclohexane-1,4-diamine (1/1), C14H10O4S2⋅C18H20N4
- Crystal structure of hexacarbonyl-bis(μ2-di-n-propyldithiocarbamato-κ3S,S′:S;κ3S:S:S′)-di-rhenium(I), C20H28N2O6Re2S4
- Crystal structure of fac-tricarbonyl-morpholine-κN-(morpholinocarbamodithioato-κ2S,S′)rhenium(I), C12H17N2O5ReS2

