5-Methyl-N′-[5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbonyl]-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbohydrazide, C22H22N8O2
-
Obaid Fahad Aldosari
and Gamal A. El-Hiti
Abstract
C22H22N8O2, monoclinic, P21/c (no. 14), a = 15.5175(5) Å, b = 7.9715(3) Å, c = 17.3941(5) Å, β = 90.005(3)°, V = 2151.61(12) Å3, Z = 4, Rgt(F) = 0.0592, wRref(F2) = 0.1780, T = 293(2) K.
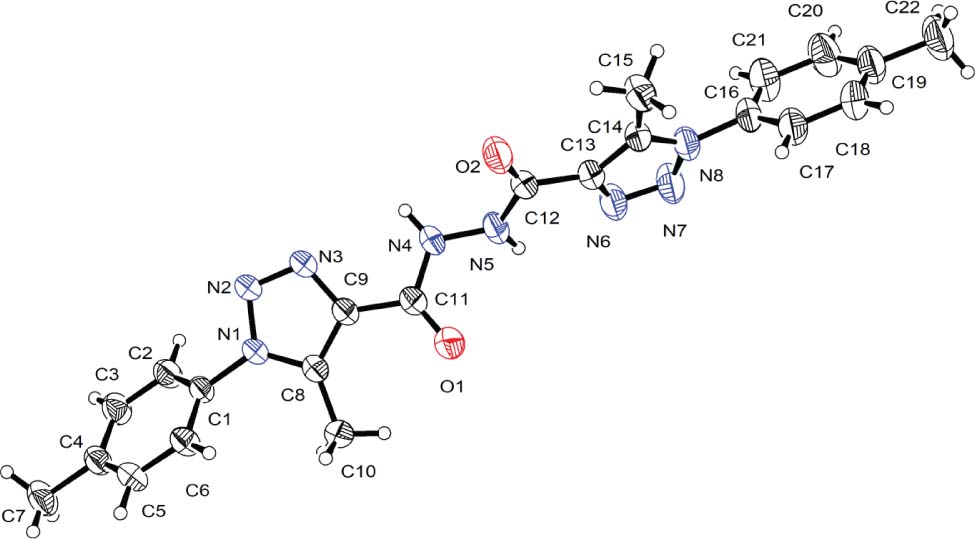
Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless needle |
| Size: | 0.38 × 0.20 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | SuperNova, Atlas, ω |
| θmax, completeness: | 29.9°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 19956, 5492, 0.027 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3531 |
| N(param)refined: | 293 |
| Programs: | CrysAlisPRO [1], SHELX [2], [3], WinGX/ORTEP [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.96790(12) | 0.0586(2) | 0.79433(10) | 0.0437(4) |
| C2 | 0.89047(14) | −0.0239(3) | 0.80137(12) | 0.0547(5) |
| H2 | 0.866434 | −0.079612 | 0.759562 | 0.066* |
| C3 | 0.84903(15) | −0.0227(3) | 0.87167(13) | 0.0602(6) |
| H3 | 0.796371 | −0.077359 | 0.876566 | 0.072* |
| C4 | 0.88377(14) | 0.0576(3) | 0.93492(11) | 0.0518(5) |
| C5 | 0.96172(14) | 0.1376(3) | 0.92605(11) | 0.0529(5) |
| H5 | 0.986290 | 0.191577 | 0.968075 | 0.063* |
| C6 | 1.00460(14) | 0.1398(3) | 0.85619(11) | 0.0505(5) |
| H6 | 1.057095 | 0.194996 | 0.851124 | 0.061* |
| C7 | 0.83736(18) | 0.0535(4) | 1.01108(13) | 0.0710(7) |
| H7A | 0.834672 | −0.059948 | 1.029431 | 0.106* |
| H7B | 0.779980 | 0.096337 | 1.004684 | 0.106* |
| H7C | 0.867871 | 0.121508 | 1.047607 | 0.106* |
| C8 | 1.09266(12) | 0.0225(2) | 0.70254(10) | 0.0442(4) |
| C9 | 1.09452(13) | 0.0445(3) | 0.62392(11) | 0.0469(4) |
| C10 | 1.15761(14) | −0.0406(3) | 0.75754(12) | 0.0553(5) |
| H10A | 1.177426 | 0.050192 | 0.789216 | 0.083* |
| H10B | 1.205401 | −0.087267 | 0.729791 | 0.083* |
| H10C | 1.132189 | −0.125651 | 0.789347 | 0.083* |
| C11 | 1.16846(14) | 0.0198(3) | 0.57203(11) | 0.0514(5) |
| C12 | 1.23475(13) | 0.0625(3) | 0.38549(11) | 0.0517(5) |
| C13 | 1.29432(13) | −0.0268(3) | 0.33374(12) | 0.0527(5) |
| C14 | 1.33624(13) | 0.0293(3) | 0.26919(12) | 0.0520(5) |
| C15 | 1.34021(17) | 0.1947(3) | 0.23121(14) | 0.0688(7) |
| H15A | 1.396466 | 0.242328 | 0.238353 | 0.103* |
| H15B | 1.297701 | 0.267686 | 0.253327 | 0.103* |
| H15C | 1.329043 | 0.181677 | 0.177255 | 0.103* |
| C16 | 1.43024(14) | −0.1322(3) | 0.17649(13) | 0.0599(6) |
| C17 | 1.50621(15) | −0.0466(4) | 0.17030(15) | 0.0695(7) |
| H17 | 1.522426 | 0.029961 | 0.207951 | 0.083* |
| C18 | 1.55879(16) | −0.0753(4) | 0.10730(16) | 0.0755(7) |
| H18 | 1.610103 | −0.015771 | 0.102806 | 0.091* |
| C19 | 1.53726(18) | −0.1886(4) | 0.05159(15) | 0.0756(7) |
| C20 | 1.4600(2) | −0.2720(4) | 0.05912(17) | 0.0926(10) |
| H20 | 1.443611 | −0.348057 | 0.021283 | 0.111* |
| C21 | 1.40587(18) | −0.2460(4) | 0.12147(17) | 0.0831(8) |
| H21 | 1.354226 | −0.304452 | 0.125872 | 0.100* |
| C22 | 1.5959(2) | −0.2185(5) | −0.01578(19) | 0.1049(11) |
| H22A | 1.609256 | −0.113447 | −0.039952 | 0.157* |
| H22B | 1.567777 | −0.290794 | −0.052111 | 0.157* |
| H22C | 1.648162 | −0.270555 | 0.001692 | 0.157* |
| N1 | 1.01030(10) | 0.0598(2) | 0.72083(8) | 0.0457(4) |
| N2 | 0.96332(11) | 0.1015(2) | 0.65742(9) | 0.0553(5) |
| N3 | 1.01520(12) | 0.0900(2) | 0.59885(9) | 0.0554(5) |
| N4 | 1.14820(12) | 0.0166(3) | 0.49665(10) | 0.0616(5) |
| H4 | 1.097628 | 0.045055 | 0.481164 | 0.074* |
| N5 | 1.21055(12) | −0.0333(3) | 0.44502(10) | 0.0617(5) |
| H5A | 1.234788 | −0.129357 | 0.451240 | 0.074* |
| N6 | 1.31080(14) | −0.1927(3) | 0.34385(12) | 0.0720(6) |
| N7 | 1.36070(15) | −0.2444(3) | 0.28864(14) | 0.0797(7) |
| N8 | 1.37675(12) | −0.1092(3) | 0.24288(11) | 0.0604(5) |
| O1 | 1.24163(11) | 0.0029(3) | 0.59494(9) | 0.0775(5) |
| O2 | 1.20913(12) | 0.2028(2) | 0.37390(9) | 0.0722(5) |
Source of material
The title compound was synthesized as previously reported [5] from reaction of ethyl 2-cyano-3-ethoxyacrylate and 5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbohydrazide in boiling ethanol for 2 h. The solid obtained was collected by filtration, washed with ethanol, dried and recrystallized from dimethylformamide to give colourless crystals in 73% yield (Mp. 296–297 °C; lit. Mp. 296–297 °C [5]).
Experimental details
All H atoms were placed in calculated positions and refined using a riding model. For the methyl groups, C—H bonds were fixed at 0.96 Å and Uiso(H) set to 1.5Ueq(C) with free rotation around the C—C bond (HFIX 137 in SHELX [3]). For the rest of the hydrogens, Uiso(H) was set to 1.2Ueq(C, N) with aromatic C—H and N—H distances of 0.93 and 0.86 Å respectively.
Comment
Compounds containing the triazole moiety show various biological activities [6], [7], [8], [9]. Therefore, various efficient synthetic processes have been developed for the production of 1,2,3-triazoles [10], [11], [12]. Recently, the crystal structures for related compounds have been published [13], [14].
The asymmetric unit consists of one molecule of the title compound. The molecule consists of two tolyl rings [A (C1—C7) and D (C16–C22)] and two triazolyl rings [B (N1—N3, C8—C10) and C (N6–N8, C13–C15)]. The twist angles between pairs of rings are 50.6(1)°, 59.2(1)° and 61.0(1)° for A/B, B/C and C/D respectively. The torsion angle (C11—N4—N5—C12) for the formyl dihydrazide group is −124.5(2)°.
The molecules are aligned roughly parallel to [−101] in the crystal. Interactions of π-π type occur between identical tolyl groups (D) of neighbouring molecules with a centroid-to-centroid distance of 4.8 Å. Tolyl (A) and triazolyl (B) rings also interact with a centroid-to-centroid distance of 4.0 Å. Weak C—H⋯X interactions occur with O and N atoms acting as acceptors and C⋯O and C⋯N distances in the range 3.14 Å to 3.46 Å.
Acknowledgements
The authors thank Majmaah and Cardiff Universities for their continuous support.
References
1. Rigaku Oxford Diffraction. CrysAlis PRO. Rigaku Oxford Diffraction, Yarnton, England (2015).Search in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
4. Farrugia, L. J.: WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 45 (2012) 849–854.10.1107/S0021889812029111Search in Google Scholar
5. Abdel-Wahab, B. F.; Alotaibi, M. H.; El-Hiti, G. A.: Synthesis of new symmetrical N,N′-diacylhydrazines and 2-(1,2,3-triazol-4-yl)-1,3,4-oxadiazoles. Lett. Org. Chem. 14 (2017) 591–596.10.2174/1570178614666170524130223Search in Google Scholar
6. Bonandi, E.; Christodoulou, M. S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D.: The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discovery Today 22 (2017) 1572–1581.10.1016/j.drudis.2017.05.014Search in Google Scholar PubMed
7. Ferreira, S. B.; Sodero, A. C.; Cardoso, M. F.; Lima, E. S.; Kaiser, C. R.; Silva, F. P.; Ferreira, V. F.: Synthesis, biological activity, and molecular modeling studies of 1H-1,2,3-triazole derivatives of carbohydrates as alpha-glucosidases inhibitors. J. Med. Chem. 53 (2010) 2364–2375.10.1021/jm901265hSearch in Google Scholar PubMed
8. Slámová, K.; Marhol, P.; Bezouska, K.; Lindkvist, L.; Hansen, S. G.; Kren, V.; Jensen, H. H.: Synthesis and biological activity of glycosyl-1H-1,2,3-triazoles. Bioorg. Med. Chem. Lett. 20 (2010) 4263–4265.10.1016/j.bmcl.2010.04.151Search in Google Scholar PubMed
9. Xu, J.; Cao, Y.; Zhang, J.; Yu, S.; Zou, Y.; Chai, X.; Wu, Q.; Zhang, D.; Jiang, Y.; Sun, Q.: Design, synthesis and antifungal activities of novel 1,2,4-triazole derivatives. Eur. J. Med. Chem. 46 (2011) 3142–3148.10.1016/j.ejmech.2011.02.042Search in Google Scholar PubMed
10. Fletcher, J. T.; Christensen, J. A.; Villa, E. M.: Tandem synthesis of 1-formyl-1,2,3-triazoles. Tetrahedron Lett. 58 (2017) 4450–4454.10.1016/j.tetlet.2017.10.023Search in Google Scholar PubMed PubMed Central
11. Smith, C. D.; Greaney, M. F.: Zinc mediated azide–alkyne ligation to 1,5-and 1,4,5-substituted 1,2,3-triazoles. Org. Lett. 15 (2013) 4826–4829.10.1021/ol402225dSearch in Google Scholar PubMed PubMed Central
12. Totobenazara, J.; Burke, A. J.: New click-chemistry methods for 1,2,3-triazoles synthesis: recent advances and applications. Tetrahedron Lett. 56 (2015) 2853–2859.10.1016/j.tetlet.2015.03.136Search in Google Scholar
13. Abu El-Enin, M. A. B.; Abdel-Wahab, B. F.; Baashen, M.; Ghabbour, H. A.; El-Hiti, G. A.: (E)-1-[5-Methyl-1-(p-tolyl)-1H-1,2,3-triazol-4-yl]-3-{3-[5-methyl-1-(p-tolyl)-1H-1,2,3-triazol-4-yl]-1-phenyl-1H-pyrazol-4-yl}prop-2-en-1-one. IUCrData 2 (2017) x171729.10.1107/S2414314617017291Search in Google Scholar
14. El-Hiti, G. A.; Abdel-Wahab, B. F.; Mostafa, M. S.; Khidre, R. E.; Hegazy, A. S.; Kariuki, B. M.: 5-Methyl-N′-(5-methyl-1-phenyl-1H-1,2,3-triazole-4-carbonyl)-1-phenyl-1H-1,2,3-triazole-4-carbohydrazide. IUCrData 3 (2018) x180424.10.1107/S2414314618004248Search in Google Scholar
©2019 Obaid Fahad Aldosari et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Crystal structure of poly[diaqua-(μ8-1,1′:2′,1′′-terphenyl-3,3′′,4′,5′-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)dicopper(II)], C22H14Cu2O10
- Crystal structure of 2-((1H-benzo[d]imidazol-2-ylimino)methyl)-4,6-di-tert-butylphenol, C22H27N3O
- Crystal structure of (4-ethoxynaphthalen-1-yl)(furan-2-yl)methanone, C17H14O3
- Crystal structure of 1-nonylpyridazin-1-ium iodide, C13H23N2I
- Crystal structure of bis[diaqua(1,10-phenanthroline-κ2N, N′)-copper(II)]diphenylphosphopentamolybdate dihydrate, C36H38Cu2Mo5N4O27P2
- The crystal structure of tetrakis(imidazole)-copper(I) hexafluorophosphate, C12H16CuF6PN8
- The crystal structure of dimethyl ((3,5-di-tert-butyl-4-hydroxyphenyl)(phenyl)methyl)phosphonate, C23H33O4P
- Crystal structure of diaqua-bis(1,10-phenanthroline κ2N,N′)nickel(II) trifluoroacetate- trifluoroacetic acid (1/1), C30H21F9N4NiO8
- Crystal structure of 2-(naphthalen-2-yl)-1,8-naphthyridine, C18H12N2
- Synthesis and crystal structure of a new polymorph of diisopropylammonium trichloroacetate, C8H16Cl3NO2
- Crystal structure of dimethanol-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)cadmium(II) C34H34CdN12O2S2
- Crystal structure of ethyl 2,2-difluoro-2-(7-methoxy-2-oxo-2H-chromen-3-yl)acetate, C14H12F2O5
- The crystal structure of bis[μ2-(N,N-diethylcarbamodithioato-κS:κS,κS′)] bis[1′-(diphenylphosphino-κP)-1-cyanoferrocene]disilver(I), C56H56Ag2Fe2N4P2S4
- Crystal structure of bis(di-n-butylammonium) tetrachloridodiphenylstannate(IV), C28H50Cl4N2Sn
- The crystal structure of poly[(μ5-2-((5-bromo-3-formyl-2-hydroxybenzylidene)amino)benzenesulfonato-κ6O:O:O,O′:O′:O′′)sodium(I)], C13H9O4NSBrNa
- Crystal structure of catena-{poly[bis(O,O′-diethyldithiophosphato-S)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)-zinc(II)] di-acetonitrile solvate}, {C20H30N4O4P2S4Zn ⋅ 2 C2H3N}n
- Halogen and hydrogen bonding in the layered crystal structure of 2-iodoanilinium triiodide, C6H7I4N
- Crystal structure of cyclohexane-1,4-diammonium 2-[(2-carboxylatophenyl)disulfanyl]benzoate — dimethylformamide — monohydrate (1/1/1), [C6H16N2][C14H8O4S2] ⋅ C3H7NO⋅H2O
- The synthesis and crystal structure of isobutyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C16H13Cl2F6N3O3S
- Isolation and crystal structure of bufotalinin — methanol (1/1), C25H34O7
- Crystal structure of benzylbis(1,3-diphenylpropane-1,3-dionato-κ2O,O′) chloridotin(IV), C37H29ClO4Sn
- Crystal structure of Bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imidazol)}diiodidocadmium(II), [Cd(C11H11N5)2I2], C22H22N10I2Cd
- Crystal structure of 4-isobutoxybenzaldehyde oxime, C11H15NO2
- The crystal structure of bis(acetato-κ1O)-bis(N′-hydroxypyrimidine-2-carboximidamide-κ2N,N′)manganese(II) — methanol (1/2), C14H18MnN8O6, 2(CH3OH)′
- Crystal structure of poly[bis(μ2-bis(4-(1H-imidazol-1-yl)phenyl)amine-κ2N:N′)-bis(nitrato-κO)cadmium(II)], C36H30CdN12O6
- Crystal structure and optical properties of 1,6-bis(methylthio)pyrene, C18H14S2
- The crystal structure of hexaquamagnesium(II) bis(3,4-dinitropyrazol-1-ide), C6H14MgN8O14
- Halogen bonds in the crystal structure of 4,3:5,4-terpyridine – 1,4-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure and photochromic properties of a novel photochromic perfluordiarylethene containing a triazole bridged pyridine group moiety, C24H18F6N4S2
- Crystal structure of bis[(μ3-oxido)-(μ2-(N,N-diisopropylthiocarbamoylthio) acetato-κ2O,O′)-((N,N-diisopropylthiocarbamoylthio)acetato-κO)-bis(di-4-methylbenzyl-tin(IV))], C100H136N4O10S8Sn4
- Crystal structure of dibromidobis(4-bromobenzyl)tin(IV), C14H12Br4Sn
- The crystal structure of (4Z)-2-[(E)-(1-ethyl-3,3-dimethyl-1,3-dihydro-2H-indol-2-ylidene)methyl]-4-[(1-ethyl-3,3-dimethyl-3H-indolium-2-yl)methylidene]-3-oxocyclobut-1-en-1-olate, C30H32N2O2
- The crystal structure of (E)-3-(4-(dimethylamino)styryl)-5,5-dimethylcyclohex-2-en-1-one, C18H23NO
- Crystal structure of dihydrazinium 1H-pyrazole-3,5-dicarboxylate, C5H12N6O4
- Crystal structure of poly[μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4-sulfidobenzoate-κ2O:S)cobalt(II)] dihydrate, C42H44Co2N8O7S2
- Crystal structure of 8-(3,4-dimethylbenzylidene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C17H18O4
- Crystal structure of 4-(2-bromo-4-(6-morpholino-3-phenyl-3H-benzo[f]chromen-3-yl) cyclohexa-2,5-dien-1-yl)morpholine, C33H31BrN2O
- Synthesis and crystal structure of 2-((1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)methylene)-2,3-dihydro-1H-inden-1-one, C23H16N2OS
- Crystal structure of poly[(μ2-1,1′-(oxybis(4,1-phenylene)bis(1H-imidazole)-κ2N,N′)(μ2-1,3-benzenecarboxylato-κ3O,O′:O′′)zinc(II)] dihydrate, C26H22N4O7Zn
- Crystal structure of diaqua-bis(cinnamato-κ2O,O′)zinc(II), C18H18ZnO6
- Crystal structure of 2-(prop-2-yn-1-yloxy)-1-naphthaldehyde, C14H10O2
- Crystal structure and photochromic properties of 1-(2-methyl-5-phenyl-3-thienyl)-2-{2-methyl-5-[4-(9-fluorenone hydrazone)-phenyl]-3-thienyl}perfluorocyclopentene, C41H26F6N2S2
- Hydrothermal synthesis and crystal structure of cylo[tetraaqua-bis(μ2-1,4-bis(1H-benzo[d]imidazol-1-yl)but-2-ene-κ2N:N′)-bis(μ2-4-nitro-phthalate-κ2O,O′)dinickel(II)], C26H23N5O8Ni
- Crystal structure of 3-[methyl(phenyl)amino]-1-phenylthiourea, C14H15N3S
- Crystal structure of 1-(4-chlorophenyl)-3-[methyl(phenyl)amino]thiourea, C14H14ClN3S
- Crystal structure of 2-tert-butyl-1H-imidazo[4,5-b]pyridine, C10H13N3
- Crystal structure of 5-carboxy-2-(2-carboxyphenyl)-1H-imidazol-3-ium-4-carboxylate dihydrate, C12H8N2O6⋅2(H2O)
- The crystal structure of dichlorido-μ2-dichlorido-(η2-1,4-bis(4-vinylbenzyl)-1,4-diazabicyclo[2.2.2]octane-1,4-diium)dicopper(I), C24H30N2Cu2Cl4
- Crystal structure of 4-bromobenzyl (Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C22H29BrN2OS
- Crystal structure of (4S,4aS,6aR,6bR,12aS,12bR,14aS,14bR)-3,3,6a,6b,9,9,12a-heptamethyloctadecahydro-1H,3H-4,14b-ethanophenanthro[1,2-h]isochromene-1(6bH)-one, C30H48O2
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C30H33F6N3S
- The crystal structure of 3-methoxyphenanthridin-6(5H)-one, C14H11NO2
- Crystal structure of 4-(5,5-difluoro-1,3,7,9-tetramethyl-3H,5H-5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)pyridin-1-ium tetraiodidoferrate(III), C18H19BF2FeI4N3
- Crystal structure of 2-(3-methoxyphenyl)-3-((phenylsulfonyl)methyl)imidazo[1,2-a]pyridine, C21H18N2O3S
- Crystal structure of [(2-(2-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) perchlorate, C29H50Cl2N4NiO8
- Crystal structure of (Z)-6-(dimethylamino)-3,3-bis(4-(dimethylamino)phenyl)-2-(2-(quinoxalin-2-ylmethylene)hydrazinyl)-2,3-dihydroinden-1-one, C35H35N7O
- 5-Methyl-N′-[5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbonyl]-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbohydrazide, C22H22N8O2
- Crystal structure of 2,3-dichloro-6-methoxyquinoxaline, C9H6Cl2N2O
- Synthesis and crystal structure of 7-chloro-2-(ethylsulfinyl)-6-fluoro-3-(1H-pyrazole-1-yl)-4H-thiochromen-4-one, C13H10FN3OS2
- Crystal structure of 4-ethylpiperazine-1-carbothioic dithioperoxyanhydride, C14H26N4S4
- Crystal structure of 2-(2-(6-methylpyridin-2-yl)naphthalen-1-yl)pyrimidine, C20H15N3
- The crystal structure of N′-((1E,2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-ylidene)-3-methylbenzohydrazide, C23H22N2O4
- Crystal structure of catena-poly[(μ2-isophthalato-κ2O:O′)-(2,5-di(pyrazin-2-yl)-4,4′-bipyridine-κ3N,N′,N′′)zinc(II)] — water (2/5), C26H21N6O6.5Zn
- Crystal structure of (3E,5E)-3,5-bis(3-nitrobenzylidene)-1-((4-(trifluoromethyl)phenyl)sulfonyl)piperidin-4-one — dichloromethane (2/1), C53H38Cl2F6N6O14S2
- Crystal structure of (μ2-oxido)-bis(N,N′-o-phenylenebis(salicylideneiminato))diiron(III) — N,N′-dimethylformamide, C47H43Fe2N4O9
- Crystal structure of N1,N3-bis(2-hydroxyethyl)-N1, N1,N3,N3-tetramethylpropane-1,3-diaminium dibromide, C11H28Br2N2O2
- Crystal structure of (E)-N-(4-chlorophenyl)-1-(pyridin-2-yl)methanimine, C12H9ClN2
- Crystal structure of 8-bromo-6-oxo-2-phenyl-6H-pyrrolo[3,2,1-ij]quinoline-5-carbaldehyde, C18H11BrNO2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride trihydrate, C8H18N8Cl2 ⋅ 3 H2O
- Crystal structure of (E)-4-bromo-N-(pyridin-2-ylmethylene)aniline, C12H9BrN2
- Crystal structure of bis[(2-(3-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ-O)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Br2N4NiO8
- The crystal structure of (1E,2E)-2-methyl-4-((7-oxo-7H-furo[3,2-g]chromen-9-yl)oxy)but-2-enal O-isonicotinoyl oxime–trichloromethane (3/1), C67H49Cl3N6O18
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-methyl-1H-imidazol-3-ium hexafluoridophosphate(V), C8H13F6N2O2P
- Crystal structure of bis[(2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κO)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) hemihydrate C42H65Br2N4NiO8.5
- The crystal structure of N-(7-(4-fluorobenzylidene)-3-(4-fluorophenyl)-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbonothioyl)benzamide, C28H23F2N3OS
- The crystal structure of N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide, C18H20N4O2
- Crystal structure of (E)-2-(3,6-bis(ethylamino)-2,7-dimethyl-9H-xanthen-9-yl)-N′-((6-methylpyridin-2-yl)methylene)benzohydrazide – methanol (1/1), C34H37N5O3
- Crystal structure of 2-oxo-1-(pyrimidin-5-ylmethyl)-3-(3-(trifluoromethyl)phenyl)-1,2-dihydro-5l4-pyrido[1,2-a]pyrimidin-4-olate, C20H13F3N4O2
- Crystal structure of poly[(μ3-9H-carbazole-3,6-dicarboxylato-κ3O1: O2: O3)(μ2-4-(pyridin-4-yl)pyridine-κ2N1:N1′)zinc(II)], C19H11N2O4Zn
- Crystal structure of (E)-N′-((1,8-dihydropyren-1-yl)-methylene)picolinohydrazide, C23H15N3O
- Crystal structure of catena-poly{[μ2-1,2-bis(diphenylphosphino)ethane]dichloridocadmium(II)}, C26H24CdCl2P2
- Crystal structure of the 1:2 co-crystal between N,N′-bis(4-pyridylmethyl)oxalamide and acetic acid as a dihydrate, C14H14N4O2⋅2 C2H4O2⋅2 H2O
- Crystal structure of the co-crystal N,N′-bis(3-pyridylmethyl)oxalamide acetic acid (1/2), C14H14N4O2⋅2C2H4O2
- Crystal structure of the co-crystal N,N′-bis(4-pyridylmethyl)oxalamide and 2,3,5,6-tetrafluoro-1,4-di-iodobenzene (1/1), C14H14N4O2⋅C6F4I2
- Crystal structure of the co-crystal 4-[(4-carboxyphenyl)disulfanyl]benzoic acid–(1E,4E)-1-N,4-N-bis(pyridin-4-ylmethylidene)cyclohexane-1,4-diamine (1/1), C14H10O4S2⋅C18H20N4
- Crystal structure of hexacarbonyl-bis(μ2-di-n-propyldithiocarbamato-κ3S,S′:S;κ3S:S:S′)-di-rhenium(I), C20H28N2O6Re2S4
- Crystal structure of fac-tricarbonyl-morpholine-κN-(morpholinocarbamodithioato-κ2S,S′)rhenium(I), C12H17N2O5ReS2
Articles in the same Issue
- Frontmatter
- Crystal structure of poly[diaqua-(μ8-1,1′:2′,1′′-terphenyl-3,3′′,4′,5′-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)dicopper(II)], C22H14Cu2O10
- Crystal structure of 2-((1H-benzo[d]imidazol-2-ylimino)methyl)-4,6-di-tert-butylphenol, C22H27N3O
- Crystal structure of (4-ethoxynaphthalen-1-yl)(furan-2-yl)methanone, C17H14O3
- Crystal structure of 1-nonylpyridazin-1-ium iodide, C13H23N2I
- Crystal structure of bis[diaqua(1,10-phenanthroline-κ2N, N′)-copper(II)]diphenylphosphopentamolybdate dihydrate, C36H38Cu2Mo5N4O27P2
- The crystal structure of tetrakis(imidazole)-copper(I) hexafluorophosphate, C12H16CuF6PN8
- The crystal structure of dimethyl ((3,5-di-tert-butyl-4-hydroxyphenyl)(phenyl)methyl)phosphonate, C23H33O4P
- Crystal structure of diaqua-bis(1,10-phenanthroline κ2N,N′)nickel(II) trifluoroacetate- trifluoroacetic acid (1/1), C30H21F9N4NiO8
- Crystal structure of 2-(naphthalen-2-yl)-1,8-naphthyridine, C18H12N2
- Synthesis and crystal structure of a new polymorph of diisopropylammonium trichloroacetate, C8H16Cl3NO2
- Crystal structure of dimethanol-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)cadmium(II) C34H34CdN12O2S2
- Crystal structure of ethyl 2,2-difluoro-2-(7-methoxy-2-oxo-2H-chromen-3-yl)acetate, C14H12F2O5
- The crystal structure of bis[μ2-(N,N-diethylcarbamodithioato-κS:κS,κS′)] bis[1′-(diphenylphosphino-κP)-1-cyanoferrocene]disilver(I), C56H56Ag2Fe2N4P2S4
- Crystal structure of bis(di-n-butylammonium) tetrachloridodiphenylstannate(IV), C28H50Cl4N2Sn
- The crystal structure of poly[(μ5-2-((5-bromo-3-formyl-2-hydroxybenzylidene)amino)benzenesulfonato-κ6O:O:O,O′:O′:O′′)sodium(I)], C13H9O4NSBrNa
- Crystal structure of catena-{poly[bis(O,O′-diethyldithiophosphato-S)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)-zinc(II)] di-acetonitrile solvate}, {C20H30N4O4P2S4Zn ⋅ 2 C2H3N}n
- Halogen and hydrogen bonding in the layered crystal structure of 2-iodoanilinium triiodide, C6H7I4N
- Crystal structure of cyclohexane-1,4-diammonium 2-[(2-carboxylatophenyl)disulfanyl]benzoate — dimethylformamide — monohydrate (1/1/1), [C6H16N2][C14H8O4S2] ⋅ C3H7NO⋅H2O
- The synthesis and crystal structure of isobutyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C16H13Cl2F6N3O3S
- Isolation and crystal structure of bufotalinin — methanol (1/1), C25H34O7
- Crystal structure of benzylbis(1,3-diphenylpropane-1,3-dionato-κ2O,O′) chloridotin(IV), C37H29ClO4Sn
- Crystal structure of Bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imidazol)}diiodidocadmium(II), [Cd(C11H11N5)2I2], C22H22N10I2Cd
- Crystal structure of 4-isobutoxybenzaldehyde oxime, C11H15NO2
- The crystal structure of bis(acetato-κ1O)-bis(N′-hydroxypyrimidine-2-carboximidamide-κ2N,N′)manganese(II) — methanol (1/2), C14H18MnN8O6, 2(CH3OH)′
- Crystal structure of poly[bis(μ2-bis(4-(1H-imidazol-1-yl)phenyl)amine-κ2N:N′)-bis(nitrato-κO)cadmium(II)], C36H30CdN12O6
- Crystal structure and optical properties of 1,6-bis(methylthio)pyrene, C18H14S2
- The crystal structure of hexaquamagnesium(II) bis(3,4-dinitropyrazol-1-ide), C6H14MgN8O14
- Halogen bonds in the crystal structure of 4,3:5,4-terpyridine – 1,4-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure and photochromic properties of a novel photochromic perfluordiarylethene containing a triazole bridged pyridine group moiety, C24H18F6N4S2
- Crystal structure of bis[(μ3-oxido)-(μ2-(N,N-diisopropylthiocarbamoylthio) acetato-κ2O,O′)-((N,N-diisopropylthiocarbamoylthio)acetato-κO)-bis(di-4-methylbenzyl-tin(IV))], C100H136N4O10S8Sn4
- Crystal structure of dibromidobis(4-bromobenzyl)tin(IV), C14H12Br4Sn
- The crystal structure of (4Z)-2-[(E)-(1-ethyl-3,3-dimethyl-1,3-dihydro-2H-indol-2-ylidene)methyl]-4-[(1-ethyl-3,3-dimethyl-3H-indolium-2-yl)methylidene]-3-oxocyclobut-1-en-1-olate, C30H32N2O2
- The crystal structure of (E)-3-(4-(dimethylamino)styryl)-5,5-dimethylcyclohex-2-en-1-one, C18H23NO
- Crystal structure of dihydrazinium 1H-pyrazole-3,5-dicarboxylate, C5H12N6O4
- Crystal structure of poly[μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4-sulfidobenzoate-κ2O:S)cobalt(II)] dihydrate, C42H44Co2N8O7S2
- Crystal structure of 8-(3,4-dimethylbenzylidene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C17H18O4
- Crystal structure of 4-(2-bromo-4-(6-morpholino-3-phenyl-3H-benzo[f]chromen-3-yl) cyclohexa-2,5-dien-1-yl)morpholine, C33H31BrN2O
- Synthesis and crystal structure of 2-((1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)methylene)-2,3-dihydro-1H-inden-1-one, C23H16N2OS
- Crystal structure of poly[(μ2-1,1′-(oxybis(4,1-phenylene)bis(1H-imidazole)-κ2N,N′)(μ2-1,3-benzenecarboxylato-κ3O,O′:O′′)zinc(II)] dihydrate, C26H22N4O7Zn
- Crystal structure of diaqua-bis(cinnamato-κ2O,O′)zinc(II), C18H18ZnO6
- Crystal structure of 2-(prop-2-yn-1-yloxy)-1-naphthaldehyde, C14H10O2
- Crystal structure and photochromic properties of 1-(2-methyl-5-phenyl-3-thienyl)-2-{2-methyl-5-[4-(9-fluorenone hydrazone)-phenyl]-3-thienyl}perfluorocyclopentene, C41H26F6N2S2
- Hydrothermal synthesis and crystal structure of cylo[tetraaqua-bis(μ2-1,4-bis(1H-benzo[d]imidazol-1-yl)but-2-ene-κ2N:N′)-bis(μ2-4-nitro-phthalate-κ2O,O′)dinickel(II)], C26H23N5O8Ni
- Crystal structure of 3-[methyl(phenyl)amino]-1-phenylthiourea, C14H15N3S
- Crystal structure of 1-(4-chlorophenyl)-3-[methyl(phenyl)amino]thiourea, C14H14ClN3S
- Crystal structure of 2-tert-butyl-1H-imidazo[4,5-b]pyridine, C10H13N3
- Crystal structure of 5-carboxy-2-(2-carboxyphenyl)-1H-imidazol-3-ium-4-carboxylate dihydrate, C12H8N2O6⋅2(H2O)
- The crystal structure of dichlorido-μ2-dichlorido-(η2-1,4-bis(4-vinylbenzyl)-1,4-diazabicyclo[2.2.2]octane-1,4-diium)dicopper(I), C24H30N2Cu2Cl4
- Crystal structure of 4-bromobenzyl (Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C22H29BrN2OS
- Crystal structure of (4S,4aS,6aR,6bR,12aS,12bR,14aS,14bR)-3,3,6a,6b,9,9,12a-heptamethyloctadecahydro-1H,3H-4,14b-ethanophenanthro[1,2-h]isochromene-1(6bH)-one, C30H48O2
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C30H33F6N3S
- The crystal structure of 3-methoxyphenanthridin-6(5H)-one, C14H11NO2
- Crystal structure of 4-(5,5-difluoro-1,3,7,9-tetramethyl-3H,5H-5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)pyridin-1-ium tetraiodidoferrate(III), C18H19BF2FeI4N3
- Crystal structure of 2-(3-methoxyphenyl)-3-((phenylsulfonyl)methyl)imidazo[1,2-a]pyridine, C21H18N2O3S
- Crystal structure of [(2-(2-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) perchlorate, C29H50Cl2N4NiO8
- Crystal structure of (Z)-6-(dimethylamino)-3,3-bis(4-(dimethylamino)phenyl)-2-(2-(quinoxalin-2-ylmethylene)hydrazinyl)-2,3-dihydroinden-1-one, C35H35N7O
- 5-Methyl-N′-[5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbonyl]-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbohydrazide, C22H22N8O2
- Crystal structure of 2,3-dichloro-6-methoxyquinoxaline, C9H6Cl2N2O
- Synthesis and crystal structure of 7-chloro-2-(ethylsulfinyl)-6-fluoro-3-(1H-pyrazole-1-yl)-4H-thiochromen-4-one, C13H10FN3OS2
- Crystal structure of 4-ethylpiperazine-1-carbothioic dithioperoxyanhydride, C14H26N4S4
- Crystal structure of 2-(2-(6-methylpyridin-2-yl)naphthalen-1-yl)pyrimidine, C20H15N3
- The crystal structure of N′-((1E,2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-ylidene)-3-methylbenzohydrazide, C23H22N2O4
- Crystal structure of catena-poly[(μ2-isophthalato-κ2O:O′)-(2,5-di(pyrazin-2-yl)-4,4′-bipyridine-κ3N,N′,N′′)zinc(II)] — water (2/5), C26H21N6O6.5Zn
- Crystal structure of (3E,5E)-3,5-bis(3-nitrobenzylidene)-1-((4-(trifluoromethyl)phenyl)sulfonyl)piperidin-4-one — dichloromethane (2/1), C53H38Cl2F6N6O14S2
- Crystal structure of (μ2-oxido)-bis(N,N′-o-phenylenebis(salicylideneiminato))diiron(III) — N,N′-dimethylformamide, C47H43Fe2N4O9
- Crystal structure of N1,N3-bis(2-hydroxyethyl)-N1, N1,N3,N3-tetramethylpropane-1,3-diaminium dibromide, C11H28Br2N2O2
- Crystal structure of (E)-N-(4-chlorophenyl)-1-(pyridin-2-yl)methanimine, C12H9ClN2
- Crystal structure of 8-bromo-6-oxo-2-phenyl-6H-pyrrolo[3,2,1-ij]quinoline-5-carbaldehyde, C18H11BrNO2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride trihydrate, C8H18N8Cl2 ⋅ 3 H2O
- Crystal structure of (E)-4-bromo-N-(pyridin-2-ylmethylene)aniline, C12H9BrN2
- Crystal structure of bis[(2-(3-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ-O)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Br2N4NiO8
- The crystal structure of (1E,2E)-2-methyl-4-((7-oxo-7H-furo[3,2-g]chromen-9-yl)oxy)but-2-enal O-isonicotinoyl oxime–trichloromethane (3/1), C67H49Cl3N6O18
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-methyl-1H-imidazol-3-ium hexafluoridophosphate(V), C8H13F6N2O2P
- Crystal structure of bis[(2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κO)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) hemihydrate C42H65Br2N4NiO8.5
- The crystal structure of N-(7-(4-fluorobenzylidene)-3-(4-fluorophenyl)-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbonothioyl)benzamide, C28H23F2N3OS
- The crystal structure of N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide, C18H20N4O2
- Crystal structure of (E)-2-(3,6-bis(ethylamino)-2,7-dimethyl-9H-xanthen-9-yl)-N′-((6-methylpyridin-2-yl)methylene)benzohydrazide – methanol (1/1), C34H37N5O3
- Crystal structure of 2-oxo-1-(pyrimidin-5-ylmethyl)-3-(3-(trifluoromethyl)phenyl)-1,2-dihydro-5l4-pyrido[1,2-a]pyrimidin-4-olate, C20H13F3N4O2
- Crystal structure of poly[(μ3-9H-carbazole-3,6-dicarboxylato-κ3O1: O2: O3)(μ2-4-(pyridin-4-yl)pyridine-κ2N1:N1′)zinc(II)], C19H11N2O4Zn
- Crystal structure of (E)-N′-((1,8-dihydropyren-1-yl)-methylene)picolinohydrazide, C23H15N3O
- Crystal structure of catena-poly{[μ2-1,2-bis(diphenylphosphino)ethane]dichloridocadmium(II)}, C26H24CdCl2P2
- Crystal structure of the 1:2 co-crystal between N,N′-bis(4-pyridylmethyl)oxalamide and acetic acid as a dihydrate, C14H14N4O2⋅2 C2H4O2⋅2 H2O
- Crystal structure of the co-crystal N,N′-bis(3-pyridylmethyl)oxalamide acetic acid (1/2), C14H14N4O2⋅2C2H4O2
- Crystal structure of the co-crystal N,N′-bis(4-pyridylmethyl)oxalamide and 2,3,5,6-tetrafluoro-1,4-di-iodobenzene (1/1), C14H14N4O2⋅C6F4I2
- Crystal structure of the co-crystal 4-[(4-carboxyphenyl)disulfanyl]benzoic acid–(1E,4E)-1-N,4-N-bis(pyridin-4-ylmethylidene)cyclohexane-1,4-diamine (1/1), C14H10O4S2⋅C18H20N4
- Crystal structure of hexacarbonyl-bis(μ2-di-n-propyldithiocarbamato-κ3S,S′:S;κ3S:S:S′)-di-rhenium(I), C20H28N2O6Re2S4
- Crystal structure of fac-tricarbonyl-morpholine-κN-(morpholinocarbamodithioato-κ2S,S′)rhenium(I), C12H17N2O5ReS2

