Abstract
C100H136N4O10S8Sn4, triclinic, P1̄ (no. 2), a = 13.7756(4) Å, b = 13.9663(5) Å, c = 16.6392(5) Å, α = 71.501(2)°, β = 73.952(2)°, γ = 63.180(2)°, V = 2675.49(16) Å3, Z = 1, Rgt(F) = 0.0383, wRref(F2) = 0.1209, T = 296(2) K.
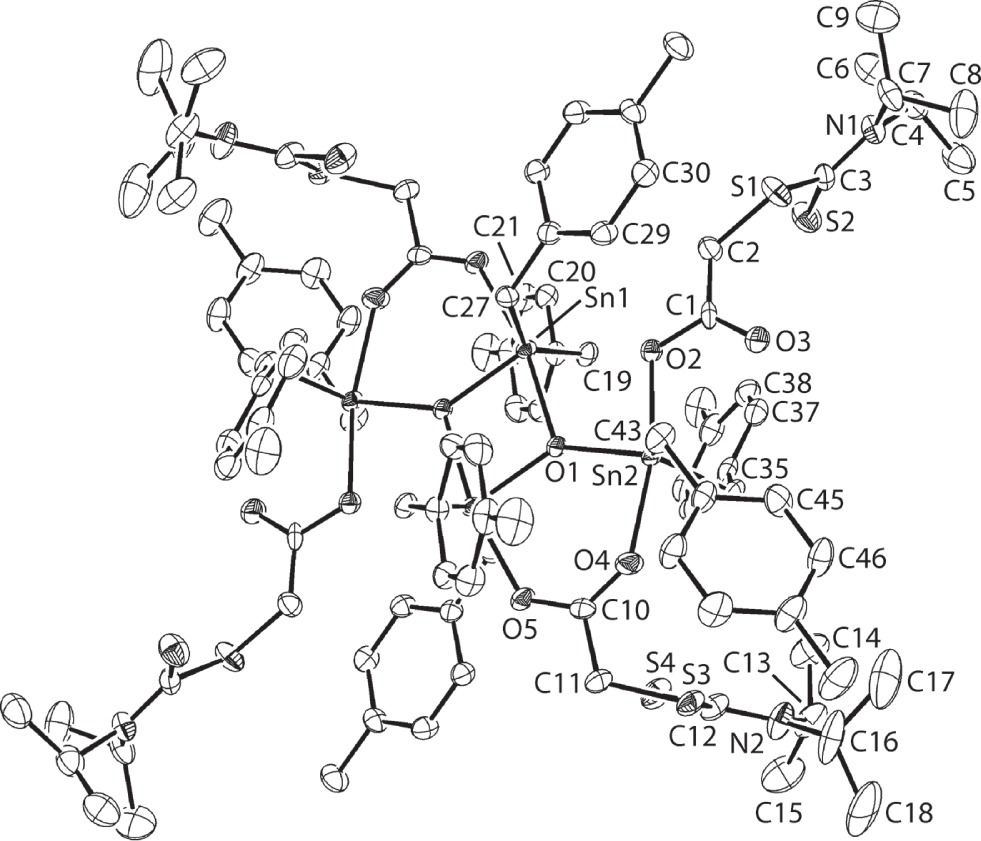
The molecular structure is shown in the figure (hydrogen atoms are omitted for clarity). Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless prism |
| Size: | 0.30 × 0.20 × 0.06 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.13 mm−1 |
| Diffractometer, scan mode: | CCD, φ and ω |
| θmax, completeness: | 28.3°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 25986, 13129, 0.033 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 10218 |
| N(param)refined: | 580 |
| Programs: | Bruker [1], SHELX [2], [3], [4], WinGX/ORTEP [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Sn1 | 0.06743(2) | 0.58266(2) | 0.45775(2) | 0.01558(7) |
| Sn2 | 0.15015(2) | 0.36975(2) | 0.34440(2) | 0.01747(7) |
| S1 | 0.42834(8) | 0.59921(9) | 0.17472(7) | 0.0328(2) |
| S2 | 0.26061(8) | 0.78921(9) | 0.07176(7) | 0.0365(2) |
| S3 | 0.03115(8) | 0.09307(7) | 0.33558(6) | 0.02718(19) |
| S4 | −0.16901(8) | 0.30322(9) | 0.31429(7) | 0.0360(2) |
| O1 | 0.05008(18) | 0.44303(18) | 0.44150(14) | 0.0181(5) |
| O2 | 0.19911(18) | 0.50238(19) | 0.32557(15) | 0.0203(5) |
| O3 | 0.3275(2) | 0.4344(2) | 0.22008(17) | 0.0320(6) |
| O4 | 0.0680(2) | 0.2578(2) | 0.38506(18) | 0.0297(6) |
| O5 | −0.0586(2) | 0.2745(2) | 0.50262(16) | 0.0236(5) |
| N1 | 0.4801(2) | 0.7224(3) | 0.02918(19) | 0.0278(7) |
| N2 | −0.0466(3) | 0.1949(3) | 0.1902(2) | 0.0387(8) |
| C1 | 0.2792(3) | 0.5079(3) | 0.2607(2) | 0.0214(7) |
| C2 | 0.2993(3) | 0.6088(3) | 0.2414(2) | 0.0257(7) |
| H2A | 0.297168 | 0.624248 | 0.294855 | 0.031* |
| H2B | 0.240496 | 0.670124 | 0.213068 | 0.031* |
| C3 | 0.3925(3) | 0.7092(3) | 0.0837(2) | 0.0244(7) |
| C4 | 0.4710(3) | 0.8154(4) | −0.0479(3) | 0.0363(9) |
| H4 | 0.546978 | 0.804128 | −0.074974 | 0.044* |
| C5 | 0.4174(4) | 0.8129(4) | −0.1156(3) | 0.0470(12) |
| H5A | 0.455285 | 0.742366 | −0.130359 | 0.071* |
| H5B | 0.421516 | 0.869466 | −0.165831 | 0.071* |
| H5C | 0.341579 | 0.825255 | −0.093244 | 0.071* |
| C6 | 0.4218(4) | 0.9262(4) | −0.0239(3) | 0.0431(11) |
| H6A | 0.345675 | 0.943736 | 0.000702 | 0.065* |
| H6B | 0.427534 | 0.981909 | −0.074382 | 0.065* |
| H6C | 0.461102 | 0.922835 | 0.017059 | 0.065* |
| C7 | 0.5963(3) | 0.6458(3) | 0.0413(2) | 0.0320(9) |
| H7 | 0.592182 | 0.590591 | 0.094576 | 0.038* |
| C8 | 0.6515(4) | 0.5849(5) | −0.0301(3) | 0.0551(14) |
| H8A | 0.610328 | 0.545296 | −0.031057 | 0.083* |
| H8B | 0.724958 | 0.533998 | −0.020643 | 0.083* |
| H8C | 0.654391 | 0.636536 | −0.083933 | 0.083* |
| C9 | 0.6587(4) | 0.7049(5) | 0.0541(4) | 0.0548(13) |
| H9A | 0.672227 | 0.753785 | 0.001042 | 0.082* |
| H9B | 0.727629 | 0.651701 | 0.072009 | 0.082* |
| H9C | 0.615653 | 0.746377 | 0.097228 | 0.082* |
| C10 | −0.0019(3) | 0.2298(3) | 0.4408(2) | 0.0199(7) |
| C11 | −0.0161(3) | 0.1306(3) | 0.4375(2) | 0.0267(7) |
| H11A | −0.093701 | 0.144209 | 0.453912 | 0.032* |
| H11B | 0.022869 | 0.068377 | 0.479896 | 0.032* |
| C12 | −0.0658(3) | 0.2026(3) | 0.2714(3) | 0.0288(8) |
| C13 | −0.1116(4) | 0.2826(4) | 0.1233(3) | 0.0440(11) |
| H13 | −0.079378 | 0.255229 | 0.070405 | 0.053* |
| C14 | −0.0954(5) | 0.3886(4) | 0.1040(3) | 0.0526(13) |
| H14A | −0.137608 | 0.427029 | 0.149119 | 0.079* |
| H14B | −0.119514 | 0.434147 | 0.050496 | 0.079* |
| H14C | −0.018788 | 0.371765 | 0.100293 | 0.079* |
| C15 | −0.2307(4) | 0.2978(5) | 0.1402(4) | 0.0631(15) |
| H15A | −0.235145 | 0.227269 | 0.161622 | 0.095* |
| H15B | −0.261102 | 0.333994 | 0.087830 | 0.095* |
| H15C | −0.271560 | 0.341978 | 0.181954 | 0.095* |
| C16 | 0.0427(5) | 0.1001(4) | 0.1572(3) | 0.0601(16) |
| H16 | 0.077435 | 0.047404 | 0.206563 | 0.072* |
| C17 | 0.1312(5) | 0.1337(6) | 0.0943(4) | 0.080(2) |
| H17A | 0.101694 | 0.183376 | 0.043673 | 0.119* |
| H17B | 0.192248 | 0.069402 | 0.079162 | 0.119* |
| H17C | 0.155740 | 0.169417 | 0.120375 | 0.119* |
| C18 | 0.0000(6) | 0.0414(5) | 0.1230(4) | 0.083(2) |
| H18A | −0.057465 | 0.024947 | 0.164866 | 0.124* |
| H18B | 0.058865 | −0.025889 | 0.111039 | 0.124* |
| H18C | −0.028785 | 0.087533 | 0.071155 | 0.124* |
| C19 | −0.0052(3) | 0.6997(3) | 0.3498(2) | 0.0219(7) |
| H19A | 0.050772 | 0.723579 | 0.311100 | 0.026* |
| H19B | −0.022505 | 0.661147 | 0.320068 | 0.026* |
| C20 | −0.1074(3) | 0.8019(3) | 0.3631(2) | 0.0204(7) |
| C21 | −0.0998(3) | 0.8989(3) | 0.3590(2) | 0.0240(7) |
| H21 | −0.030880 | 0.899272 | 0.353335 | 0.029* |
| C22 | −0.1938(3) | 0.9961(3) | 0.3634(2) | 0.0285(8) |
| H22 | −0.186507 | 1.060318 | 0.360261 | 0.034* |
| C23 | −0.2981(3) | 0.9982(3) | 0.3724(2) | 0.0297(8) |
| C24 | −0.3055(3) | 0.9013(3) | 0.3778(2) | 0.0319(8) |
| H24 | −0.374710 | 0.900862 | 0.385022 | 0.038* |
| C25 | −0.2125(3) | 0.8038(3) | 0.3729(2) | 0.0252(7) |
| H25 | −0.220287 | 0.739835 | 0.376170 | 0.030* |
| C26 | −0.3998(4) | 1.1045(4) | 0.3771(3) | 0.0475(12) |
| H26A | −0.462087 | 1.096027 | 0.369836 | 0.071* |
| H26B | −0.388025 | 1.163411 | 0.332436 | 0.071* |
| H26C | −0.413611 | 1.121115 | 0.431909 | 0.071* |
| C27 | 0.2161(3) | 0.4893(3) | 0.5129(2) | 0.0215(7) |
| H27A | 0.200636 | 0.501921 | 0.570031 | 0.026* |
| H27B | 0.234015 | 0.411858 | 0.519487 | 0.026* |
| C28 | 0.3165(3) | 0.5108(3) | 0.4661(2) | 0.0216(7) |
| C29 | 0.4042(3) | 0.4359(3) | 0.4217(2) | 0.0244(7) |
| H29 | 0.400849 | 0.370730 | 0.422148 | 0.029* |
| C30 | 0.4958(3) | 0.4572(3) | 0.3772(2) | 0.0261(7) |
| H30 | 0.552788 | 0.406344 | 0.347780 | 0.031* |
| C31 | 0.5042(3) | 0.5531(3) | 0.3756(2) | 0.0245(7) |
| C32 | 0.4193(3) | 0.6256(3) | 0.4220(2) | 0.0262(7) |
| H32 | 0.424730 | 0.688937 | 0.423619 | 0.031* |
| C33 | 0.3261(3) | 0.6058(3) | 0.4663(2) | 0.0236(7) |
| H33 | 0.269733 | 0.656358 | 0.496266 | 0.028* |
| C34 | 0.6023(3) | 0.5793(4) | 0.3252(3) | 0.0338(9) |
| H34A | 0.667105 | 0.512118 | 0.324875 | 0.051* |
| H34B | 0.612363 | 0.625563 | 0.351491 | 0.051* |
| H34C | 0.589740 | 0.616719 | 0.267361 | 0.051* |
| C35 | 0.0938(3) | 0.4180(3) | 0.2241(2) | 0.0253(7) |
| H35A | 0.157492 | 0.399247 | 0.179796 | 0.030* |
| H35B | 0.052988 | 0.375255 | 0.227274 | 0.030* |
| C36 | 0.0219(3) | 0.5382(3) | 0.1976(2) | 0.0249(7) |
| C37 | 0.0662(3) | 0.6143(3) | 0.1495(2) | 0.0276(8) |
| H37 | 0.141704 | 0.590471 | 0.130369 | 0.033* |
| C38 | −0.0006(3) | 0.7255(3) | 0.1297(2) | 0.0318(8) |
| H38 | 0.031229 | 0.775083 | 0.097883 | 0.038* |
| C39 | −0.1138(3) | 0.7649(3) | 0.1560(3) | 0.0341(9) |
| C40 | −0.1582(3) | 0.6886(4) | 0.2019(3) | 0.0344(9) |
| H40 | −0.234062 | 0.712510 | 0.219008 | 0.041* |
| C41 | −0.0917(3) | 0.5761(3) | 0.2231(2) | 0.0292(8) |
| H41 | −0.123594 | 0.526435 | 0.254481 | 0.035* |
| C42 | −0.1848(4) | 0.8859(4) | 0.1388(3) | 0.0505(12) |
| H42A | −0.255132 | 0.897749 | 0.128725 | 0.076* |
| H42B | −0.149809 | 0.923386 | 0.089199 | 0.076* |
| H42C | −0.194958 | 0.914038 | 0.187564 | 0.076* |
| C43 | 0.2986(3) | 0.2319(3) | 0.3784(3) | 0.0279(8) |
| H43A | 0.360903 | 0.252456 | 0.354138 | 0.033* |
| H43B | 0.294110 | 0.210778 | 0.440337 | 0.033* |
| C44 | 0.3154(3) | 0.1361(3) | 0.3456(2) | 0.0257(7) |
| C45 | 0.3446(3) | 0.1391(3) | 0.2577(3) | 0.0326(9) |
| H45 | 0.356714 | 0.199449 | 0.219999 | 0.039* |
| C46 | 0.3557(4) | 0.0538(3) | 0.2257(3) | 0.0374(10) |
| H46 | 0.375480 | 0.057846 | 0.166963 | 0.045* |
| C47 | 0.3380(3) | −0.0371(3) | 0.2791(3) | 0.0352(9) |
| C48 | 0.3114(4) | −0.0413(3) | 0.3668(3) | 0.0363(9) |
| H48 | 0.300875 | −0.102408 | 0.404493 | 0.044* |
| C49 | 0.3004(3) | 0.0441(3) | 0.3993(3) | 0.0325(9) |
| H49 | 0.282499 | 0.039028 | 0.458175 | 0.039* |
| C50 | 0.3471(5) | −0.1277(4) | 0.2428(3) | 0.0539(13) |
| H50A | 0.322138 | −0.096562 | 0.188139 | 0.081* |
| H50B | 0.302517 | −0.165096 | 0.281434 | 0.081* |
| H50C | 0.422555 | −0.179160 | 0.235740 | 0.081* |
Source of material
The melting point (uncorrected) of the compound was measured on an electrothermal digital melting point apparatus. The elemental analysis was performed on a Perkin-Elmer EA2400 CHN analyser. The IR spectrum was recorded using a Perkin-Elmer RX1 spectrometer in a Nujol mull between KBr plates.
Di(4-methylbenzyl)tin dichloride was synthesized by the direct reaction of 4-methylbenzyl chloride (Sigma-Aldrich) and metallic tin powder (Sigma-Aldrich) in toluene according to a literature procedure [6]. The base hydrolysis of di(4-methylbenzyl)tin dichloride using 10% sodium hydroxide solution (Merck) afforded the di(4-methybenzyl)tin oxide. Diisopropyldithiocarbomylacetic acid was synthesized from diisopropylamine (Merck), carbon disulfide (Merck) and chloroacetic acid (Sigma-Aldrich) according to a literature procedure [7]. Di(4-methylbenzyl)tin oxide (0.75 g, 2.0 mmol) and diisopropyldithiocarbomylacetic acid (0.94 g, 2.0 mmol) were heated in 95% ethanol (100 mL) for 1 h until the oxide dissolved. After filtration, the filtrate was evaporated slowly until colourless crystals were formed. Yield: 0.75 g (20%). M. pt: 424−426 K. Calcd. for C100H136O10N4S8Sn4: C 51.19; H 6.00; N 2.45%. Found: C 50.91; H 5.88; N 2.66%. IR (cm−1) 503 (m) ν(Sn—O), 639 (m) ν(Sn—O—Sn), 1411, 1378 (s) νsym(COO), 1662, 1597 (s) νasym(COO).
Experimental details
The C-bound H atoms were geometrically placed (C—H = 0.93−0.98 Å) and refined as riding with Uiso(H) = 1.2−1.5Ueq(C). Owing to poor agreement, five reflections, i.e. (0 0 1), (16 7 1), (1 1 1), (17 7 2) and (15 7 0), were omitted from the final cycles of refinement.
Comment
Organotin(IV) carboxylates have been widely investigated because of their structural diversity [8] and their potential biological properties, in particular anti-tumour potential [9], [10]. In addition, organotin compounds containing dithiocarbamate anions have also received much attention due to their promising anti-fungal, anti-bacterial and anti-tumour activities [11], [12]. In continuation of our efforts in exploring the coordination chemistry and anti-proliferative activities of organotin carboxylates, herein the synthesis and structural features of an organotin compound with a carboxylate ligand containing a dithiocarbamate fragment are described.
The title compound is a centrosymmetric, tetra-nuclear species shown in the figure (50% displacement ellipsoids; unlabelled atoms are related by the symmetry operation (i): −x, 1 − y, 1 − z). The molecule is constructed about a central Sn2O2 core with Sn1—O1, O1i bond lengths of 2.173(2) and 2.050(2) Å, respectively. Connected to this ring is the exocyclic Sn2 atom [Sn2—O1 = 2.026(2) Å]. The additional link between the endocyclic-Sn1 and exocyclic-Sn2 atoms is provided by an almost symmetrically bridging carboxylate ligand [Sn2—O4 = 2.164(2) Å and Sn1—O5 = 2.237(2) Å]. The exocyclic-Sn2 atom is also coordinated by a monodentate carboxylate ligand [Sn2—O2 = 2.151(2) Å] with the Sn2⋯O3 separation of 3.012(3) Å considered too long for a significant bonding interaction. There is some evidence for the O2 atom semi-bridging the Sn1 atom as the Sn1⋯O2 separation is 2.628(2) Å. The five-coordinate geometries for each of the Sn1 and Sn2 atoms is completed by two carbon atoms derived from the benzyl substituents. To a first approximation, the C2O3 donor sets define distorted trigonal bipyramidal geometries with O1—Sn1—O5 [168.41(9)°] and O2—Sn2—O4 [165.91(9)°] axial angles. Each of the carboxylate ligands is twisted as seen in the dihedral angles between the CO2 and CS2 residues of 70.0(4) and 81.3(3)° for the O2- and O4-carboxylate ligands, respectively.
Molecules stack in columns along the a axis being sustained by tolyl-Me—C—H⋯S(thione) [C34—H34a⋯S4ii: H34a⋯S4ii = 2.79 Å, C34⋯S4ii = 3.741(5) Å with an angle at H34a = 169° for symmetry operation (ii) 1 + x, y, z] and π⋯π interactions between centrosymmetrically related tolyl moieties [inter-centroid Cg(C28—C33)⋯Cg(C28—C33)iii separation = 3.709(2) Å for symmetry operation (iii): 1 − x, 1 − y, 1 − z]. The chains assemble in the three-dimensional architecture without directional interactions between them.
Tetranuclear clusters of the general formula {[R2SnX]2O}2 are common hydrolysis products of diorganotin species [13]. In keeping with this observation, there are at least five structures of this type containing dithiocarbamate-functionalized carboxylates, i.e. R′2NC(=S)SCH2CO2−. The three structures with R = n-Bu and R′ = Et [14], R′2 = (CH2)4 [15] and R′2 = (CH2)5 [16] adopt the same structural motif as the title structure. The two structures with R = n-Bu and R′ = Me [17] and R = n-Oct and R′2 = (CH2CH2)2O [18] adopt essentially the same motif but the carboxylate bridge involves one oxygen atom only. Both motifs have ample precedents in the crystallographic literature of the diorganotin bis(carboxylates) [8].
Acknowledgements
Sunway University is thanked for supporting studies in organotin chemistry.
References
1. Bruker. APEX2 and SAINT. Bruker AXS Inc., Madison, WI, U.S.A. (2008).Search in Google Scholar
2. Sheldrick, G. M.: SADABS. University of Göttingen, Germany (1996).Search in Google Scholar
3. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
4. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
5. Farrugia, L. J.: WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 45 (2012) 849–854.10.1107/S0021889812029111Search in Google Scholar
6. Sisido, K.; Takeda, Y.; Kinugawa, Z.: Direct synthesis of organotin compounds I. di- and tribenzyltin chlorides. J. Am. Chem. Soc. 83 (1961) 538–541.10.1021/ja01464a008Search in Google Scholar
7. Nachmias, G.: Sur quelques nouveaux derives de lacide dithiocarbamique N-disubstitues. C. r. hebd. séances Acad. Sci. 232 (1951) 1118–1119.Search in Google Scholar
8. Tiekink, E. R. T.: Structural chemistry of organotin carboxylates: a review of the crystallographic literature. Appl. Organomet. Chem. 5 (1991) 1–23.10.1002/aoc.590050102Search in Google Scholar
9. Gielen, M.: Organotin compounds and their therapeutic potential: a report from the Organometallic Chemistry Department of the Free University of Brussels. Appl. Organomet. Chem. 16 (2002) 481–494.10.1002/aoc.331Search in Google Scholar
10. Anasamy, T.; Thy, C. K.; Lo, K. M.; Chee, C. F.; Yeap, S. K.; Kamalidehghan, B.; Chung, L. Y.: Tribenzyltin carboxylates as anticancer drug candidates: effect on the cytotoxicity, motility and invasiveness of breast cancer cell lines. Eur. J. Med. Chem. 125 (2017) 770–783.10.1016/j.ejmech.2016.09.061Search in Google Scholar PubMed
11. Tiekink, E. R. T.: Tin dithiocarbamates: applications and structures. Appl. Organomet. Chem. 22 (2008) 533–550.10.1002/aoc.1441Search in Google Scholar
12. Khan, N.; Farina, Y.; Lo, K. M.; Rajab, N. F.; Awang, N.: Synthesis, spectral characterization, X-ray studies and in vitro cytotoxic activities of triorganotin(IV) derivatives of p-substituted N-methylbenzylaminedithiocarbamates. J. Mol. Struct. 1076 (2014) 403–410.10.1016/j.molstruc.2014.08.015Search in Google Scholar
13. Dakternieks, D.; Jurkschat, K.; van Dreumel, S.; Tiekink, E. R. T.: Molecular dynamics within diorganotin systems: solution and solid state studies of new mixed distannoxane dimers [tBu2(Cl)SnOSn(Cl)R2]2. Inorg. Chem. 36 (1997) 2023–2029.10.1021/ic9611608Search in Google Scholar PubMed
14. Ng, S. W.; Kumar Das, V. G.: Tetrabutylbis[(N,N-diethylthiocarbamoylthio)acetato] distannoxane dimer. Acta Crystallogr. C51 (1995) 1774–1776.10.1107/S0108270195003143Search in Google Scholar
15. Yin, H.-D.; Xue, S.-C.; Wang, Q.-B.: Synthesis, characterization and crystal structure of the dimeric organotin compound: {[n-Bu2Sn(O2CCH2CS2NC4H8)]2O}2. Chin. J. Inorg. Chem. 20 (2004) 421–425.Search in Google Scholar
16. Yin, H.-D.; Xue, S.-C.; Wang, Q.-B.: Synthesis, characterization of di-n-butyltin compounds with (thiocarbamoylthio) acetic acid and crystal structure of {[n-Bu2Sn(O2CCH2CS2NC5H10)]2O}2⋅2 H2O and n-Bu2Sn(O2CCH2CS2NC5H10)2. Indian J. Chem., Sect. B 44 (2005) 1040–1045.Search in Google Scholar
17. Yin, H.-D.; Xue, S.-C.; Liu, G.-F.: Synthesis and crystal structure of bis{oxo-bis[N,N-dimethylthiocarbamoylthioacetatodi-n-butyltin(IV)]} and bis{oxo-bis[N,N-diethylthiocarbamoylthioacetato di-n-butyltin(IV)]}. Acta Chim. Sinica 62 (2004) 603–609.Search in Google Scholar
18. Yin, H.-D.; Gao, Z.-J.; Li, G.; Xu, H.-L.; Hong, M.: Synthesis and crystal structure of the dimeric organotin complex {[(n-C8H17)2Sn(O2CCH2CS2NC4H8O)]2O}2. Chin. J. Inorg. Chem. 22 (2006) 157–160.Search in Google Scholar
©2019 See Mun Lee et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Crystal structure of poly[diaqua-(μ8-1,1′:2′,1′′-terphenyl-3,3′′,4′,5′-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)dicopper(II)], C22H14Cu2O10
- Crystal structure of 2-((1H-benzo[d]imidazol-2-ylimino)methyl)-4,6-di-tert-butylphenol, C22H27N3O
- Crystal structure of (4-ethoxynaphthalen-1-yl)(furan-2-yl)methanone, C17H14O3
- Crystal structure of 1-nonylpyridazin-1-ium iodide, C13H23N2I
- Crystal structure of bis[diaqua(1,10-phenanthroline-κ2N, N′)-copper(II)]diphenylphosphopentamolybdate dihydrate, C36H38Cu2Mo5N4O27P2
- The crystal structure of tetrakis(imidazole)-copper(I) hexafluorophosphate, C12H16CuF6PN8
- The crystal structure of dimethyl ((3,5-di-tert-butyl-4-hydroxyphenyl)(phenyl)methyl)phosphonate, C23H33O4P
- Crystal structure of diaqua-bis(1,10-phenanthroline κ2N,N′)nickel(II) trifluoroacetate- trifluoroacetic acid (1/1), C30H21F9N4NiO8
- Crystal structure of 2-(naphthalen-2-yl)-1,8-naphthyridine, C18H12N2
- Synthesis and crystal structure of a new polymorph of diisopropylammonium trichloroacetate, C8H16Cl3NO2
- Crystal structure of dimethanol-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)cadmium(II) C34H34CdN12O2S2
- Crystal structure of ethyl 2,2-difluoro-2-(7-methoxy-2-oxo-2H-chromen-3-yl)acetate, C14H12F2O5
- The crystal structure of bis[μ2-(N,N-diethylcarbamodithioato-κS:κS,κS′)] bis[1′-(diphenylphosphino-κP)-1-cyanoferrocene]disilver(I), C56H56Ag2Fe2N4P2S4
- Crystal structure of bis(di-n-butylammonium) tetrachloridodiphenylstannate(IV), C28H50Cl4N2Sn
- The crystal structure of poly[(μ5-2-((5-bromo-3-formyl-2-hydroxybenzylidene)amino)benzenesulfonato-κ6O:O:O,O′:O′:O′′)sodium(I)], C13H9O4NSBrNa
- Crystal structure of catena-{poly[bis(O,O′-diethyldithiophosphato-S)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)-zinc(II)] di-acetonitrile solvate}, {C20H30N4O4P2S4Zn ⋅ 2 C2H3N}n
- Halogen and hydrogen bonding in the layered crystal structure of 2-iodoanilinium triiodide, C6H7I4N
- Crystal structure of cyclohexane-1,4-diammonium 2-[(2-carboxylatophenyl)disulfanyl]benzoate — dimethylformamide — monohydrate (1/1/1), [C6H16N2][C14H8O4S2] ⋅ C3H7NO⋅H2O
- The synthesis and crystal structure of isobutyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C16H13Cl2F6N3O3S
- Isolation and crystal structure of bufotalinin — methanol (1/1), C25H34O7
- Crystal structure of benzylbis(1,3-diphenylpropane-1,3-dionato-κ2O,O′) chloridotin(IV), C37H29ClO4Sn
- Crystal structure of Bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imidazol)}diiodidocadmium(II), [Cd(C11H11N5)2I2], C22H22N10I2Cd
- Crystal structure of 4-isobutoxybenzaldehyde oxime, C11H15NO2
- The crystal structure of bis(acetato-κ1O)-bis(N′-hydroxypyrimidine-2-carboximidamide-κ2N,N′)manganese(II) — methanol (1/2), C14H18MnN8O6, 2(CH3OH)′
- Crystal structure of poly[bis(μ2-bis(4-(1H-imidazol-1-yl)phenyl)amine-κ2N:N′)-bis(nitrato-κO)cadmium(II)], C36H30CdN12O6
- Crystal structure and optical properties of 1,6-bis(methylthio)pyrene, C18H14S2
- The crystal structure of hexaquamagnesium(II) bis(3,4-dinitropyrazol-1-ide), C6H14MgN8O14
- Halogen bonds in the crystal structure of 4,3:5,4-terpyridine – 1,4-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure and photochromic properties of a novel photochromic perfluordiarylethene containing a triazole bridged pyridine group moiety, C24H18F6N4S2
- Crystal structure of bis[(μ3-oxido)-(μ2-(N,N-diisopropylthiocarbamoylthio) acetato-κ2O,O′)-((N,N-diisopropylthiocarbamoylthio)acetato-κO)-bis(di-4-methylbenzyl-tin(IV))], C100H136N4O10S8Sn4
- Crystal structure of dibromidobis(4-bromobenzyl)tin(IV), C14H12Br4Sn
- The crystal structure of (4Z)-2-[(E)-(1-ethyl-3,3-dimethyl-1,3-dihydro-2H-indol-2-ylidene)methyl]-4-[(1-ethyl-3,3-dimethyl-3H-indolium-2-yl)methylidene]-3-oxocyclobut-1-en-1-olate, C30H32N2O2
- The crystal structure of (E)-3-(4-(dimethylamino)styryl)-5,5-dimethylcyclohex-2-en-1-one, C18H23NO
- Crystal structure of dihydrazinium 1H-pyrazole-3,5-dicarboxylate, C5H12N6O4
- Crystal structure of poly[μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4-sulfidobenzoate-κ2O:S)cobalt(II)] dihydrate, C42H44Co2N8O7S2
- Crystal structure of 8-(3,4-dimethylbenzylidene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C17H18O4
- Crystal structure of 4-(2-bromo-4-(6-morpholino-3-phenyl-3H-benzo[f]chromen-3-yl) cyclohexa-2,5-dien-1-yl)morpholine, C33H31BrN2O
- Synthesis and crystal structure of 2-((1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)methylene)-2,3-dihydro-1H-inden-1-one, C23H16N2OS
- Crystal structure of poly[(μ2-1,1′-(oxybis(4,1-phenylene)bis(1H-imidazole)-κ2N,N′)(μ2-1,3-benzenecarboxylato-κ3O,O′:O′′)zinc(II)] dihydrate, C26H22N4O7Zn
- Crystal structure of diaqua-bis(cinnamato-κ2O,O′)zinc(II), C18H18ZnO6
- Crystal structure of 2-(prop-2-yn-1-yloxy)-1-naphthaldehyde, C14H10O2
- Crystal structure and photochromic properties of 1-(2-methyl-5-phenyl-3-thienyl)-2-{2-methyl-5-[4-(9-fluorenone hydrazone)-phenyl]-3-thienyl}perfluorocyclopentene, C41H26F6N2S2
- Hydrothermal synthesis and crystal structure of cylo[tetraaqua-bis(μ2-1,4-bis(1H-benzo[d]imidazol-1-yl)but-2-ene-κ2N:N′)-bis(μ2-4-nitro-phthalate-κ2O,O′)dinickel(II)], C26H23N5O8Ni
- Crystal structure of 3-[methyl(phenyl)amino]-1-phenylthiourea, C14H15N3S
- Crystal structure of 1-(4-chlorophenyl)-3-[methyl(phenyl)amino]thiourea, C14H14ClN3S
- Crystal structure of 2-tert-butyl-1H-imidazo[4,5-b]pyridine, C10H13N3
- Crystal structure of 5-carboxy-2-(2-carboxyphenyl)-1H-imidazol-3-ium-4-carboxylate dihydrate, C12H8N2O6⋅2(H2O)
- The crystal structure of dichlorido-μ2-dichlorido-(η2-1,4-bis(4-vinylbenzyl)-1,4-diazabicyclo[2.2.2]octane-1,4-diium)dicopper(I), C24H30N2Cu2Cl4
- Crystal structure of 4-bromobenzyl (Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C22H29BrN2OS
- Crystal structure of (4S,4aS,6aR,6bR,12aS,12bR,14aS,14bR)-3,3,6a,6b,9,9,12a-heptamethyloctadecahydro-1H,3H-4,14b-ethanophenanthro[1,2-h]isochromene-1(6bH)-one, C30H48O2
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C30H33F6N3S
- The crystal structure of 3-methoxyphenanthridin-6(5H)-one, C14H11NO2
- Crystal structure of 4-(5,5-difluoro-1,3,7,9-tetramethyl-3H,5H-5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)pyridin-1-ium tetraiodidoferrate(III), C18H19BF2FeI4N3
- Crystal structure of 2-(3-methoxyphenyl)-3-((phenylsulfonyl)methyl)imidazo[1,2-a]pyridine, C21H18N2O3S
- Crystal structure of [(2-(2-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) perchlorate, C29H50Cl2N4NiO8
- Crystal structure of (Z)-6-(dimethylamino)-3,3-bis(4-(dimethylamino)phenyl)-2-(2-(quinoxalin-2-ylmethylene)hydrazinyl)-2,3-dihydroinden-1-one, C35H35N7O
- 5-Methyl-N′-[5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbonyl]-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbohydrazide, C22H22N8O2
- Crystal structure of 2,3-dichloro-6-methoxyquinoxaline, C9H6Cl2N2O
- Synthesis and crystal structure of 7-chloro-2-(ethylsulfinyl)-6-fluoro-3-(1H-pyrazole-1-yl)-4H-thiochromen-4-one, C13H10FN3OS2
- Crystal structure of 4-ethylpiperazine-1-carbothioic dithioperoxyanhydride, C14H26N4S4
- Crystal structure of 2-(2-(6-methylpyridin-2-yl)naphthalen-1-yl)pyrimidine, C20H15N3
- The crystal structure of N′-((1E,2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-ylidene)-3-methylbenzohydrazide, C23H22N2O4
- Crystal structure of catena-poly[(μ2-isophthalato-κ2O:O′)-(2,5-di(pyrazin-2-yl)-4,4′-bipyridine-κ3N,N′,N′′)zinc(II)] — water (2/5), C26H21N6O6.5Zn
- Crystal structure of (3E,5E)-3,5-bis(3-nitrobenzylidene)-1-((4-(trifluoromethyl)phenyl)sulfonyl)piperidin-4-one — dichloromethane (2/1), C53H38Cl2F6N6O14S2
- Crystal structure of (μ2-oxido)-bis(N,N′-o-phenylenebis(salicylideneiminato))diiron(III) — N,N′-dimethylformamide, C47H43Fe2N4O9
- Crystal structure of N1,N3-bis(2-hydroxyethyl)-N1, N1,N3,N3-tetramethylpropane-1,3-diaminium dibromide, C11H28Br2N2O2
- Crystal structure of (E)-N-(4-chlorophenyl)-1-(pyridin-2-yl)methanimine, C12H9ClN2
- Crystal structure of 8-bromo-6-oxo-2-phenyl-6H-pyrrolo[3,2,1-ij]quinoline-5-carbaldehyde, C18H11BrNO2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride trihydrate, C8H18N8Cl2 ⋅ 3 H2O
- Crystal structure of (E)-4-bromo-N-(pyridin-2-ylmethylene)aniline, C12H9BrN2
- Crystal structure of bis[(2-(3-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ-O)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Br2N4NiO8
- The crystal structure of (1E,2E)-2-methyl-4-((7-oxo-7H-furo[3,2-g]chromen-9-yl)oxy)but-2-enal O-isonicotinoyl oxime–trichloromethane (3/1), C67H49Cl3N6O18
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-methyl-1H-imidazol-3-ium hexafluoridophosphate(V), C8H13F6N2O2P
- Crystal structure of bis[(2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κO)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) hemihydrate C42H65Br2N4NiO8.5
- The crystal structure of N-(7-(4-fluorobenzylidene)-3-(4-fluorophenyl)-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbonothioyl)benzamide, C28H23F2N3OS
- The crystal structure of N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide, C18H20N4O2
- Crystal structure of (E)-2-(3,6-bis(ethylamino)-2,7-dimethyl-9H-xanthen-9-yl)-N′-((6-methylpyridin-2-yl)methylene)benzohydrazide – methanol (1/1), C34H37N5O3
- Crystal structure of 2-oxo-1-(pyrimidin-5-ylmethyl)-3-(3-(trifluoromethyl)phenyl)-1,2-dihydro-5l4-pyrido[1,2-a]pyrimidin-4-olate, C20H13F3N4O2
- Crystal structure of poly[(μ3-9H-carbazole-3,6-dicarboxylato-κ3O1: O2: O3)(μ2-4-(pyridin-4-yl)pyridine-κ2N1:N1′)zinc(II)], C19H11N2O4Zn
- Crystal structure of (E)-N′-((1,8-dihydropyren-1-yl)-methylene)picolinohydrazide, C23H15N3O
- Crystal structure of catena-poly{[μ2-1,2-bis(diphenylphosphino)ethane]dichloridocadmium(II)}, C26H24CdCl2P2
- Crystal structure of the 1:2 co-crystal between N,N′-bis(4-pyridylmethyl)oxalamide and acetic acid as a dihydrate, C14H14N4O2⋅2 C2H4O2⋅2 H2O
- Crystal structure of the co-crystal N,N′-bis(3-pyridylmethyl)oxalamide acetic acid (1/2), C14H14N4O2⋅2C2H4O2
- Crystal structure of the co-crystal N,N′-bis(4-pyridylmethyl)oxalamide and 2,3,5,6-tetrafluoro-1,4-di-iodobenzene (1/1), C14H14N4O2⋅C6F4I2
- Crystal structure of the co-crystal 4-[(4-carboxyphenyl)disulfanyl]benzoic acid–(1E,4E)-1-N,4-N-bis(pyridin-4-ylmethylidene)cyclohexane-1,4-diamine (1/1), C14H10O4S2⋅C18H20N4
- Crystal structure of hexacarbonyl-bis(μ2-di-n-propyldithiocarbamato-κ3S,S′:S;κ3S:S:S′)-di-rhenium(I), C20H28N2O6Re2S4
- Crystal structure of fac-tricarbonyl-morpholine-κN-(morpholinocarbamodithioato-κ2S,S′)rhenium(I), C12H17N2O5ReS2
Articles in the same Issue
- Frontmatter
- Crystal structure of poly[diaqua-(μ8-1,1′:2′,1′′-terphenyl-3,3′′,4′,5′-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)dicopper(II)], C22H14Cu2O10
- Crystal structure of 2-((1H-benzo[d]imidazol-2-ylimino)methyl)-4,6-di-tert-butylphenol, C22H27N3O
- Crystal structure of (4-ethoxynaphthalen-1-yl)(furan-2-yl)methanone, C17H14O3
- Crystal structure of 1-nonylpyridazin-1-ium iodide, C13H23N2I
- Crystal structure of bis[diaqua(1,10-phenanthroline-κ2N, N′)-copper(II)]diphenylphosphopentamolybdate dihydrate, C36H38Cu2Mo5N4O27P2
- The crystal structure of tetrakis(imidazole)-copper(I) hexafluorophosphate, C12H16CuF6PN8
- The crystal structure of dimethyl ((3,5-di-tert-butyl-4-hydroxyphenyl)(phenyl)methyl)phosphonate, C23H33O4P
- Crystal structure of diaqua-bis(1,10-phenanthroline κ2N,N′)nickel(II) trifluoroacetate- trifluoroacetic acid (1/1), C30H21F9N4NiO8
- Crystal structure of 2-(naphthalen-2-yl)-1,8-naphthyridine, C18H12N2
- Synthesis and crystal structure of a new polymorph of diisopropylammonium trichloroacetate, C8H16Cl3NO2
- Crystal structure of dimethanol-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)cadmium(II) C34H34CdN12O2S2
- Crystal structure of ethyl 2,2-difluoro-2-(7-methoxy-2-oxo-2H-chromen-3-yl)acetate, C14H12F2O5
- The crystal structure of bis[μ2-(N,N-diethylcarbamodithioato-κS:κS,κS′)] bis[1′-(diphenylphosphino-κP)-1-cyanoferrocene]disilver(I), C56H56Ag2Fe2N4P2S4
- Crystal structure of bis(di-n-butylammonium) tetrachloridodiphenylstannate(IV), C28H50Cl4N2Sn
- The crystal structure of poly[(μ5-2-((5-bromo-3-formyl-2-hydroxybenzylidene)amino)benzenesulfonato-κ6O:O:O,O′:O′:O′′)sodium(I)], C13H9O4NSBrNa
- Crystal structure of catena-{poly[bis(O,O′-diethyldithiophosphato-S)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)-zinc(II)] di-acetonitrile solvate}, {C20H30N4O4P2S4Zn ⋅ 2 C2H3N}n
- Halogen and hydrogen bonding in the layered crystal structure of 2-iodoanilinium triiodide, C6H7I4N
- Crystal structure of cyclohexane-1,4-diammonium 2-[(2-carboxylatophenyl)disulfanyl]benzoate — dimethylformamide — monohydrate (1/1/1), [C6H16N2][C14H8O4S2] ⋅ C3H7NO⋅H2O
- The synthesis and crystal structure of isobutyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C16H13Cl2F6N3O3S
- Isolation and crystal structure of bufotalinin — methanol (1/1), C25H34O7
- Crystal structure of benzylbis(1,3-diphenylpropane-1,3-dionato-κ2O,O′) chloridotin(IV), C37H29ClO4Sn
- Crystal structure of Bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imidazol)}diiodidocadmium(II), [Cd(C11H11N5)2I2], C22H22N10I2Cd
- Crystal structure of 4-isobutoxybenzaldehyde oxime, C11H15NO2
- The crystal structure of bis(acetato-κ1O)-bis(N′-hydroxypyrimidine-2-carboximidamide-κ2N,N′)manganese(II) — methanol (1/2), C14H18MnN8O6, 2(CH3OH)′
- Crystal structure of poly[bis(μ2-bis(4-(1H-imidazol-1-yl)phenyl)amine-κ2N:N′)-bis(nitrato-κO)cadmium(II)], C36H30CdN12O6
- Crystal structure and optical properties of 1,6-bis(methylthio)pyrene, C18H14S2
- The crystal structure of hexaquamagnesium(II) bis(3,4-dinitropyrazol-1-ide), C6H14MgN8O14
- Halogen bonds in the crystal structure of 4,3:5,4-terpyridine – 1,4-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure and photochromic properties of a novel photochromic perfluordiarylethene containing a triazole bridged pyridine group moiety, C24H18F6N4S2
- Crystal structure of bis[(μ3-oxido)-(μ2-(N,N-diisopropylthiocarbamoylthio) acetato-κ2O,O′)-((N,N-diisopropylthiocarbamoylthio)acetato-κO)-bis(di-4-methylbenzyl-tin(IV))], C100H136N4O10S8Sn4
- Crystal structure of dibromidobis(4-bromobenzyl)tin(IV), C14H12Br4Sn
- The crystal structure of (4Z)-2-[(E)-(1-ethyl-3,3-dimethyl-1,3-dihydro-2H-indol-2-ylidene)methyl]-4-[(1-ethyl-3,3-dimethyl-3H-indolium-2-yl)methylidene]-3-oxocyclobut-1-en-1-olate, C30H32N2O2
- The crystal structure of (E)-3-(4-(dimethylamino)styryl)-5,5-dimethylcyclohex-2-en-1-one, C18H23NO
- Crystal structure of dihydrazinium 1H-pyrazole-3,5-dicarboxylate, C5H12N6O4
- Crystal structure of poly[μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4-sulfidobenzoate-κ2O:S)cobalt(II)] dihydrate, C42H44Co2N8O7S2
- Crystal structure of 8-(3,4-dimethylbenzylidene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C17H18O4
- Crystal structure of 4-(2-bromo-4-(6-morpholino-3-phenyl-3H-benzo[f]chromen-3-yl) cyclohexa-2,5-dien-1-yl)morpholine, C33H31BrN2O
- Synthesis and crystal structure of 2-((1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)methylene)-2,3-dihydro-1H-inden-1-one, C23H16N2OS
- Crystal structure of poly[(μ2-1,1′-(oxybis(4,1-phenylene)bis(1H-imidazole)-κ2N,N′)(μ2-1,3-benzenecarboxylato-κ3O,O′:O′′)zinc(II)] dihydrate, C26H22N4O7Zn
- Crystal structure of diaqua-bis(cinnamato-κ2O,O′)zinc(II), C18H18ZnO6
- Crystal structure of 2-(prop-2-yn-1-yloxy)-1-naphthaldehyde, C14H10O2
- Crystal structure and photochromic properties of 1-(2-methyl-5-phenyl-3-thienyl)-2-{2-methyl-5-[4-(9-fluorenone hydrazone)-phenyl]-3-thienyl}perfluorocyclopentene, C41H26F6N2S2
- Hydrothermal synthesis and crystal structure of cylo[tetraaqua-bis(μ2-1,4-bis(1H-benzo[d]imidazol-1-yl)but-2-ene-κ2N:N′)-bis(μ2-4-nitro-phthalate-κ2O,O′)dinickel(II)], C26H23N5O8Ni
- Crystal structure of 3-[methyl(phenyl)amino]-1-phenylthiourea, C14H15N3S
- Crystal structure of 1-(4-chlorophenyl)-3-[methyl(phenyl)amino]thiourea, C14H14ClN3S
- Crystal structure of 2-tert-butyl-1H-imidazo[4,5-b]pyridine, C10H13N3
- Crystal structure of 5-carboxy-2-(2-carboxyphenyl)-1H-imidazol-3-ium-4-carboxylate dihydrate, C12H8N2O6⋅2(H2O)
- The crystal structure of dichlorido-μ2-dichlorido-(η2-1,4-bis(4-vinylbenzyl)-1,4-diazabicyclo[2.2.2]octane-1,4-diium)dicopper(I), C24H30N2Cu2Cl4
- Crystal structure of 4-bromobenzyl (Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C22H29BrN2OS
- Crystal structure of (4S,4aS,6aR,6bR,12aS,12bR,14aS,14bR)-3,3,6a,6b,9,9,12a-heptamethyloctadecahydro-1H,3H-4,14b-ethanophenanthro[1,2-h]isochromene-1(6bH)-one, C30H48O2
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C30H33F6N3S
- The crystal structure of 3-methoxyphenanthridin-6(5H)-one, C14H11NO2
- Crystal structure of 4-(5,5-difluoro-1,3,7,9-tetramethyl-3H,5H-5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)pyridin-1-ium tetraiodidoferrate(III), C18H19BF2FeI4N3
- Crystal structure of 2-(3-methoxyphenyl)-3-((phenylsulfonyl)methyl)imidazo[1,2-a]pyridine, C21H18N2O3S
- Crystal structure of [(2-(2-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) perchlorate, C29H50Cl2N4NiO8
- Crystal structure of (Z)-6-(dimethylamino)-3,3-bis(4-(dimethylamino)phenyl)-2-(2-(quinoxalin-2-ylmethylene)hydrazinyl)-2,3-dihydroinden-1-one, C35H35N7O
- 5-Methyl-N′-[5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbonyl]-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbohydrazide, C22H22N8O2
- Crystal structure of 2,3-dichloro-6-methoxyquinoxaline, C9H6Cl2N2O
- Synthesis and crystal structure of 7-chloro-2-(ethylsulfinyl)-6-fluoro-3-(1H-pyrazole-1-yl)-4H-thiochromen-4-one, C13H10FN3OS2
- Crystal structure of 4-ethylpiperazine-1-carbothioic dithioperoxyanhydride, C14H26N4S4
- Crystal structure of 2-(2-(6-methylpyridin-2-yl)naphthalen-1-yl)pyrimidine, C20H15N3
- The crystal structure of N′-((1E,2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-ylidene)-3-methylbenzohydrazide, C23H22N2O4
- Crystal structure of catena-poly[(μ2-isophthalato-κ2O:O′)-(2,5-di(pyrazin-2-yl)-4,4′-bipyridine-κ3N,N′,N′′)zinc(II)] — water (2/5), C26H21N6O6.5Zn
- Crystal structure of (3E,5E)-3,5-bis(3-nitrobenzylidene)-1-((4-(trifluoromethyl)phenyl)sulfonyl)piperidin-4-one — dichloromethane (2/1), C53H38Cl2F6N6O14S2
- Crystal structure of (μ2-oxido)-bis(N,N′-o-phenylenebis(salicylideneiminato))diiron(III) — N,N′-dimethylformamide, C47H43Fe2N4O9
- Crystal structure of N1,N3-bis(2-hydroxyethyl)-N1, N1,N3,N3-tetramethylpropane-1,3-diaminium dibromide, C11H28Br2N2O2
- Crystal structure of (E)-N-(4-chlorophenyl)-1-(pyridin-2-yl)methanimine, C12H9ClN2
- Crystal structure of 8-bromo-6-oxo-2-phenyl-6H-pyrrolo[3,2,1-ij]quinoline-5-carbaldehyde, C18H11BrNO2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride trihydrate, C8H18N8Cl2 ⋅ 3 H2O
- Crystal structure of (E)-4-bromo-N-(pyridin-2-ylmethylene)aniline, C12H9BrN2
- Crystal structure of bis[(2-(3-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ-O)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Br2N4NiO8
- The crystal structure of (1E,2E)-2-methyl-4-((7-oxo-7H-furo[3,2-g]chromen-9-yl)oxy)but-2-enal O-isonicotinoyl oxime–trichloromethane (3/1), C67H49Cl3N6O18
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-methyl-1H-imidazol-3-ium hexafluoridophosphate(V), C8H13F6N2O2P
- Crystal structure of bis[(2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κO)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) hemihydrate C42H65Br2N4NiO8.5
- The crystal structure of N-(7-(4-fluorobenzylidene)-3-(4-fluorophenyl)-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbonothioyl)benzamide, C28H23F2N3OS
- The crystal structure of N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide, C18H20N4O2
- Crystal structure of (E)-2-(3,6-bis(ethylamino)-2,7-dimethyl-9H-xanthen-9-yl)-N′-((6-methylpyridin-2-yl)methylene)benzohydrazide – methanol (1/1), C34H37N5O3
- Crystal structure of 2-oxo-1-(pyrimidin-5-ylmethyl)-3-(3-(trifluoromethyl)phenyl)-1,2-dihydro-5l4-pyrido[1,2-a]pyrimidin-4-olate, C20H13F3N4O2
- Crystal structure of poly[(μ3-9H-carbazole-3,6-dicarboxylato-κ3O1: O2: O3)(μ2-4-(pyridin-4-yl)pyridine-κ2N1:N1′)zinc(II)], C19H11N2O4Zn
- Crystal structure of (E)-N′-((1,8-dihydropyren-1-yl)-methylene)picolinohydrazide, C23H15N3O
- Crystal structure of catena-poly{[μ2-1,2-bis(diphenylphosphino)ethane]dichloridocadmium(II)}, C26H24CdCl2P2
- Crystal structure of the 1:2 co-crystal between N,N′-bis(4-pyridylmethyl)oxalamide and acetic acid as a dihydrate, C14H14N4O2⋅2 C2H4O2⋅2 H2O
- Crystal structure of the co-crystal N,N′-bis(3-pyridylmethyl)oxalamide acetic acid (1/2), C14H14N4O2⋅2C2H4O2
- Crystal structure of the co-crystal N,N′-bis(4-pyridylmethyl)oxalamide and 2,3,5,6-tetrafluoro-1,4-di-iodobenzene (1/1), C14H14N4O2⋅C6F4I2
- Crystal structure of the co-crystal 4-[(4-carboxyphenyl)disulfanyl]benzoic acid–(1E,4E)-1-N,4-N-bis(pyridin-4-ylmethylidene)cyclohexane-1,4-diamine (1/1), C14H10O4S2⋅C18H20N4
- Crystal structure of hexacarbonyl-bis(μ2-di-n-propyldithiocarbamato-κ3S,S′:S;κ3S:S:S′)-di-rhenium(I), C20H28N2O6Re2S4
- Crystal structure of fac-tricarbonyl-morpholine-κN-(morpholinocarbamodithioato-κ2S,S′)rhenium(I), C12H17N2O5ReS2

