Abstract
C47H43Fe2N4O9, orthorhombic, Pbca (no. 61), a = 24.6961(8) Å, b = 14.0952(5) Å, c = 24.696(3) Å, V = 8596.6(4) Å3, Z = 8, Rgt(F) = 0.0374, wRref(F2) = 0.1209, T = 296(2) K [1–3].
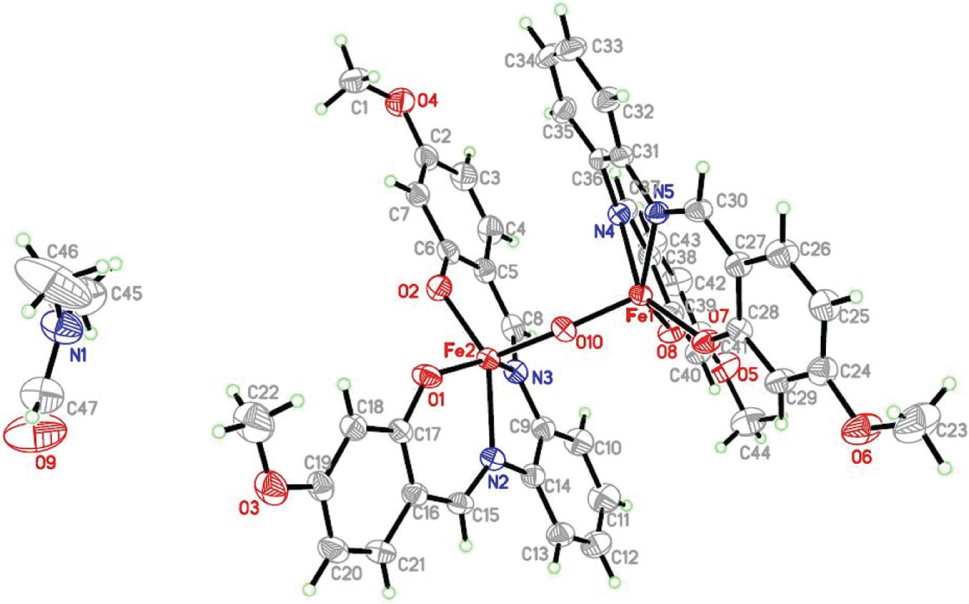
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Red block |
| Size: | 0.10 × 0.08 × 0.06 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.74 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 25.0°, 98% |
| N(hkl)measured, N(hkl)unique, Rint: | 95920, 7442, 0.061 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5491 |
| N(param)refined: | 583 |
| Programs: | Bruker [1], SHELX [2], [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Fe1 | 0.52550(2) | 0.71504(3) | 0.63533(2) | 0.03486(13) |
| N1 | 0.79736(13) | 0.0119(2) | 0.73154(15) | 0.0792(9) |
| O1 | 0.58499(8) | 0.38875(14) | 0.63622(8) | 0.0514(5) |
| C1 | 0.53504(17) | 0.4742(3) | 0.92416(16) | 0.0771(11) |
| H1A | 0.5340 | 0.4081 | 0.9150 | 0.116* |
| H1B | 0.5396 | 0.4810 | 0.9626 | 0.116* |
| H1C | 0.5648 | 0.5040 | 0.9058 | 0.116* |
| Fe2 | 0.52293(2) | 0.47247(3) | 0.64541(2) | 0.03739(13) |
| N2 | 0.49206(9) | 0.41138(15) | 0.57417(10) | 0.0410(6) |
| O2 | 0.52168(8) | 0.45185(14) | 0.72238(8) | 0.0475(5) |
| C2 | 0.47301(14) | 0.5166(2) | 0.85461(13) | 0.0558(9) |
| O3 | 0.70929(10) | 0.15478(16) | 0.57262(10) | 0.0695(7) |
| N3 | 0.43797(9) | 0.49383(16) | 0.65052(10) | 0.0415(6) |
| C3 | 0.42165(15) | 0.5531(2) | 0.84272(15) | 0.0642(9) |
| H3 | 0.3998 | 0.5774 | 0.8701 | 0.077* |
| C4 | 0.40438(14) | 0.5527(2) | 0.79114(15) | 0.0585(9) |
| H4 | 0.3699 | 0.5756 | 0.7837 | 0.070* |
| O4 | 0.48615(11) | 0.51777(18) | 0.90816(10) | 0.0739(7) |
| N4 | 0.50100(9) | 0.74156(15) | 0.71605(9) | 0.0379(5) |
| C5 | 0.43618(12) | 0.51898(19) | 0.74768(13) | 0.0453(7) |
| O5 | 0.25958(9) | 0.7883(2) | 0.61751(11) | 0.0802(8) |
| N5 | 0.59207(9) | 0.78663(15) | 0.67104(9) | 0.0379(5) |
| O6 | 0.64832(11) | 0.8924(2) | 0.42401(9) | 0.0795(8) |
| C6 | 0.48881(12) | 0.48484(19) | 0.75972(12) | 0.0435(7) |
| O7 | 0.54892(8) | 0.76792(14) | 0.56725(8) | 0.0482(5) |
| C7 | 0.50578(13) | 0.4821(2) | 0.81398(13) | 0.0479(7) |
| H7 | 0.5395 | 0.4569 | 0.8226 | 0.057* |
| C8 | 0.41333(12) | 0.5175(2) | 0.69504(14) | 0.0487(8) |
| H8 | 0.3772 | 0.5352 | 0.6921 | 0.058* |
| O8 | 0.45020(8) | 0.73485(14) | 0.61633(8) | 0.0461(5) |
| O9 | 0.74998(15) | −0.0952(3) | 0.68331(15) | 0.1446(16) |
| C9 | 0.41154(11) | 0.4919(2) | 0.60013(13) | 0.0457(7) |
| C10 | 0.36052(13) | 0.5292(2) | 0.58843(15) | 0.0624(9) |
| H10 | 0.3399 | 0.5568 | 0.6157 | 0.075* |
| O10 | 0.54291(8) | 0.59225(12) | 0.63298(8) | 0.0441(5) |
| C11 | 0.34081(15) | 0.5248(3) | 0.53633(17) | 0.0766(11) |
| H11 | 0.3067 | 0.5495 | 0.5288 | 0.092* |
| C12 | 0.37063(15) | 0.4847(3) | 0.49538(17) | 0.0715(10) |
| H12 | 0.3569 | 0.4834 | 0.4603 | 0.086* |
| C15 | 0.51341(11) | 0.3430(2) | 0.54649(12) | 0.0450(7) |
| H15 | 0.4941 | 0.3216 | 0.5166 | 0.054* |
| C14 | 0.44105(11) | 0.4492(2) | 0.55852(13) | 0.0456(7) |
| C13 | 0.42053(13) | 0.4464(2) | 0.50598(14) | 0.0562(8) |
| H13 | 0.4406 | 0.4186 | 0.4783 | 0.067* |
| C16 | 0.56339(12) | 0.29780(19) | 0.55735(12) | 0.0429(7) |
| C17 | 0.59797(11) | 0.32341(19) | 0.60063(12) | 0.0411(7) |
| C18 | 0.64806(12) | 0.2758(2) | 0.60565(13) | 0.0475(7) |
| H18 | 0.6720 | 0.2930 | 0.6330 | 0.057* |
| C19 | 0.66184(13) | 0.2040(2) | 0.57022(13) | 0.0505(8) |
| C20 | 0.62657(14) | 0.1755(2) | 0.52940(13) | 0.0568(8) |
| H20 | 0.6355 | 0.1251 | 0.5068 | 0.068* |
| C21 | 0.57875(13) | 0.2225(2) | 0.52302(13) | 0.0534(8) |
| H21 | 0.5556 | 0.2044 | 0.4952 | 0.064* |
| C22 | 0.74769(18) | 0.1793(3) | 0.6129(2) | 0.1029(16) |
| H22A | 0.7563 | 0.2456 | 0.6100 | 0.154* |
| H22B | 0.7800 | 0.1425 | 0.6079 | 0.154* |
| H22C | 0.7328 | 0.1666 | 0.6481 | 0.154* |
| C23 | 0.6877(2) | 0.9532(4) | 0.40174(17) | 0.1119(18) |
| H23A | 0.6846 | 1.0149 | 0.4179 | 0.168* |
| H23B | 0.6823 | 0.9581 | 0.3633 | 0.168* |
| H23C | 0.7231 | 0.9280 | 0.4088 | 0.168* |
| C24 | 0.64485(12) | 0.8825(2) | 0.47888(13) | 0.0519(8) |
| C25 | 0.68134(12) | 0.9213(2) | 0.51472(14) | 0.0554(8) |
| H25 | 0.7102 | 0.9578 | 0.5025 | 0.066* |
| C26 | 0.67402(12) | 0.9048(2) | 0.56866(14) | 0.0565(8) |
| H26 | 0.6986 | 0.9307 | 0.5930 | 0.068* |
| C27 | 0.63106(11) | 0.8507(2) | 0.58882(12) | 0.0418(7) |
| C28 | 0.59245(11) | 0.81400(19) | 0.55163(12) | 0.0400(7) |
| C29 | 0.60103(12) | 0.8308(2) | 0.49690(12) | 0.0529(8) |
| H29 | 0.5766 | 0.8067 | 0.4718 | 0.064* |
| C30 | 0.62794(11) | 0.8370(2) | 0.64560(12) | 0.0452(7) |
| H30 | 0.6541 | 0.8669 | 0.6666 | 0.054* |
| C31 | 0.59404(11) | 0.77841(19) | 0.72797(12) | 0.0405(7) |
| C32 | 0.63990(13) | 0.7940(2) | 0.75999(13) | 0.0530(8) |
| H32 | 0.6723 | 0.8129 | 0.7441 | 0.064* |
| C33 | 0.63676(15) | 0.7814(2) | 0.81518(14) | 0.0615(9) |
| H33 | 0.6671 | 0.7922 | 0.8366 | 0.074* |
| C34 | 0.58849(15) | 0.7524(2) | 0.83900(14) | 0.0597(9) |
| H34 | 0.5869 | 0.7433 | 0.8763 | 0.072* |
| C35 | 0.54300(14) | 0.7371(2) | 0.80775(13) | 0.0511(8) |
| H35 | 0.5109 | 0.7174 | 0.8238 | 0.061* |
| C36 | 0.54543(12) | 0.75119(19) | 0.75225(12) | 0.0414(7) |
| C37 | 0.45241(12) | 0.75649(19) | 0.73349(12) | 0.0426(7) |
| H37 | 0.4481 | 0.7670 | 0.7704 | 0.051* |
| C38 | 0.40505(11) | 0.75817(19) | 0.70095(12) | 0.0417(7) |
| C39 | 0.40598(11) | 0.7498(2) | 0.64355(12) | 0.0408(7) |
| C40 | 0.35661(11) | 0.7607(2) | 0.61482(13) | 0.0490(8) |
| H40 | 0.3566 | 0.7567 | 0.5772 | 0.059* |
| C41 | 0.30874(12) | 0.7773(2) | 0.64180(14) | 0.0566(9) |
| C42 | 0.30764(13) | 0.7833(3) | 0.69814(15) | 0.0664(10) |
| H42 | 0.2751 | 0.7929 | 0.7162 | 0.080* |
| C43 | 0.35528(13) | 0.7749(2) | 0.72670(14) | 0.0582(9) |
| H43 | 0.3545 | 0.7805 | 0.7642 | 0.070* |
| C44 | 0.25620(15) | 0.7867(3) | 0.56023(16) | 0.0826(12) |
| H44A | 0.2775 | 0.8375 | 0.5455 | 0.124* |
| H44B | 0.2191 | 0.7943 | 0.5494 | 0.124* |
| H44C | 0.2697 | 0.7271 | 0.5470 | 0.124* |
| C45 | 0.7528(2) | 0.0294(5) | 0.7667(2) | 0.146(3) |
| H45A | 0.7196 | 0.0146 | 0.7484 | 0.220* |
| H45B | 0.7527 | 0.0951 | 0.7771 | 0.220* |
| H45C | 0.7561 | −0.0095 | 0.7984 | 0.220* |
| C46 | 0.8515(2) | 0.0449(5) | 0.7431(3) | 0.191(4) |
| H46A | 0.8769 | 0.0117 | 0.7206 | 0.287* |
| H46B | 0.8598 | 0.0333 | 0.7805 | 0.287* |
| H46C | 0.8537 | 0.1117 | 0.7359 | 0.287* |
| C47 | 0.79054(18) | −0.0497(3) | 0.69243(19) | 0.0873(13) |
| H47 | 0.8198 | −0.0594 | 0.6694 | 0.105* |
Source of material
Schiff base ligand was purchased from Acros Ltd. Company and used without further purification, the other reagents were commercially available and used as purchased. The title compound was synthesized by the reaction of FeCl3 (64.88 mg, 0.4 mmol) and 6,6′-((1,2-phenylenebis(azanylylidene))bis(methanylylidene))bis(3-methoxyphenol) (49.64 mg, 0.4 mmol) in DMF (10 mL). The mixture was stirred for 6 h, then a colourless solution formed. The resulting solution was filtered. The filtrate was allowed to stand for a few days at room temperature until light-pink crystals were obtained. Single crystals suitable for X-ray diffraction analysis were obtained by slow evaporation of a DMF solution. (25%, m.p. 320–325 K).
Experimental details
Hydrogen atoms were added with geometric restraints.
Comment
Organic molecules containing imine groups, commonly known as Schiff bases, are of interest to inorganic chemists as these are widely used in designing molecular ferromagnets, in catalysis, in biological modeling applications and in preparing liquid crystals [4], [5], [6], [7], [8]. Schiff bases also play a key role as chelating ligands in main group and transition metal coordination chemistry, due to their ease of synthesis, stability under a variety of oxidative and reductive conditions, and their structural versatility, which is associated with their applications [9], [10], [11]. Undoubtedly, salen type ligands are receiving the most attention among all the Schiff bases. Ligands categorized under this class consist of two imine nitrogen and two phenolic oxygen donors that usually coordinate in the basal plane of the metal ion, salen-type ligands are significant because of the ability of the phenoxo oxygen atoms to form 2-bridges, thus affording high-nuclearity compounds, which could act as promising candidates to offer valuable insight into various natural electron-transfer events [12]. Now, we synthesized the new dinuclear Fe compounds with N,N′-bis(salicylidene)phenylediamine ligands. Some similar compounds have been reported [13], [14], [15].
As shown in the figure, the title compound contains a dinuclear Fe(III) complex. The compound shows a distorted octahedral geometry involving N2O2 donors as the basal plane. Both the Fe centers have a five coordinated square-pyramicdal geometry, in which N2O2 donors from the ligand and the axial position is occupied by a bridging oxygen atom.
Acknowledgements
The authors gratefully acknowledge the financial support by Young Talent fund of University Association for Science and Technology in Shaanxi, China, Educational Commission of Shaanxi Province of China (16 J K1393) and Science Foundation of Shaanxi Province of China (2016JQ2001).
References
1. Bruker. SMART and SAINT for Windows NT Software Reference Manuals, Version 5.0. Bruker Analytical X-Ray Systems, Madison, WI (1997).Search in Google Scholar
2. Sheldrick, G. M.: SADABS, a software for empirical absorption correction. University of Göttingen, Göttingen, Germany (1997).Search in Google Scholar
3. SHELXL. Reference Manual, version 5.1; Bruker Analytical X-Ray Systems, Madison, WI (1997).Search in Google Scholar
4. de Barbarin, C. O. R.; Bailey, N. A.; Fenton, D. E.; He, Q. Y.: Zinc (II) complexes derived from potentially hexadentate (N4O2) acyclic ligands containing pyridinyl andphenolic groups. J. Chem. Soc. Dalton Trans. 2 (1997) 161–166.10.1039/a605706cSearch in Google Scholar
5. Larson, E. J.; Pecoraro, V. L.: The peroxide-dependent μ2-O bond formation of manganese complex [Mn(IV)SALPN(O)]2. J. Am. Chem. Soc. 113 (1991) 3810–3818.10.1021/ja00010a025Search in Google Scholar
6. Khan, M. M. T.; Srinivas, D.; Kureshy, R. I.; Khan, N. H.: Synthesis, characterization, and EPR studies of stable ruthenium (III) Schiff base chloro and carbonyl complexes. Inorg. Chem. 29 (1990) 2320–2326.10.1021/ic00337a026Search in Google Scholar
7. Miyasaka, H.; Ieda, H.; Mastumoto, N.; Crescenzi, R.; Floriani, C.: Assembling bi-, tri-and pentanuclear complexes into extended structures using a desolvation reaction: synthesis, structure, and magnetic properties of manganese(III) Schiff-Base hexacyanoferrate polymeric compounds and their derived extended structures. Inorg. Chem. 37 (1998) 255–263.10.1021/ic9709640Search in Google Scholar
8. Ramade, I.; Kahn, O.; Jeannin, Y.; Robert, F.: Design and magnetic properties of a magnetically isolated GdIIICuII pair. crystal structures of [Gd(hfa)3Cu(salen)], [Y(hfa)3Cu(salen)], [Gd(hfa)3Cu(salen)(Meim)], and [La(hfa)3(H2O)Cu(salen)] [hfa = Hexafluoroacetylacetonato, salen = N,N′-ethylenebis (salicylideneaminato), Meim = 1-Methylimidazole]. Inorg. Chem. 36 (1997) 930–936.10.1021/ic9607595Search in Google Scholar
9. Ziessel, R.: Schiff-based bipyridine ligands. unusual coordination features and mesomorphic behaviour. Coord. Chem. Rev. 216 (2001) 195–223.10.1016/S0010-8545(00)00410-0Search in Google Scholar
10. Cozzi, P. G.: Metal-salen Schiff base complexes in catalysis: practical aspects. Chem. Soc. Rev. 64 (2004) 410–421.10.1039/B307853CSearch in Google Scholar
11. Gupta, K. C.; Sutar, A. K.: Catalytic activities of Schiff base transition metal complexes. Coord. Chem. Rev. 252 (2008) 1420–1450.10.1016/j.ccr.2007.09.005Search in Google Scholar
12. Katsuki, T.: Catalytic asymmetric oxidations using optically active (salen)manganese(III) complexes as catalysts. Coord. Chem. Rev. 140 (1995) 189–214.10.1016/0010-8545(94)01124-TSearch in Google Scholar
13. Jana, S.; Chatterjee, S.; Chattopadhyay, S.: Syntheses, characterization and X-ray crystal structures of hexa-coordinated monomeric and oxo-bridged dimeric Fe(III) compounds with salen-type Schiff bases. Polyhedron 48 (2012) 189–198.10.1016/j.poly.2012.08.085Search in Google Scholar
14. Meng, Q.; Clegg, J. K.; Brock, A. J.; Jolliffe, K. A.; Lindoy, L. F.; Wei, G.: Mono-and dinucleating Ni(II), Cu(II), Zn(II) and Fe(III) complexes of symmetric and unsymmetric Schiff bases incorporating salicylimine functions–synthetic and structural studies. Polyhedron 74 (2014) 113–121.10.1016/j.poly.2014.03.002Search in Google Scholar
15. Oyaizu, K.; Dewi, E. L.; Tsuchida, E. A.: μ-Oxo diiron(III) complex with a short Fe-Fe distance: crystal structure of (μ-oxo) bis[N, N′-o-phenylenebis(salicylideneiminato)iron(III)]. Inorg. Chim. Acta 321 (2001) 205–208.10.1016/S0020-1693(01)00532-1Search in Google Scholar
©2019 Li Ya et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Crystal structure of poly[diaqua-(μ8-1,1′:2′,1′′-terphenyl-3,3′′,4′,5′-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)dicopper(II)], C22H14Cu2O10
- Crystal structure of 2-((1H-benzo[d]imidazol-2-ylimino)methyl)-4,6-di-tert-butylphenol, C22H27N3O
- Crystal structure of (4-ethoxynaphthalen-1-yl)(furan-2-yl)methanone, C17H14O3
- Crystal structure of 1-nonylpyridazin-1-ium iodide, C13H23N2I
- Crystal structure of bis[diaqua(1,10-phenanthroline-κ2N, N′)-copper(II)]diphenylphosphopentamolybdate dihydrate, C36H38Cu2Mo5N4O27P2
- The crystal structure of tetrakis(imidazole)-copper(I) hexafluorophosphate, C12H16CuF6PN8
- The crystal structure of dimethyl ((3,5-di-tert-butyl-4-hydroxyphenyl)(phenyl)methyl)phosphonate, C23H33O4P
- Crystal structure of diaqua-bis(1,10-phenanthroline κ2N,N′)nickel(II) trifluoroacetate- trifluoroacetic acid (1/1), C30H21F9N4NiO8
- Crystal structure of 2-(naphthalen-2-yl)-1,8-naphthyridine, C18H12N2
- Synthesis and crystal structure of a new polymorph of diisopropylammonium trichloroacetate, C8H16Cl3NO2
- Crystal structure of dimethanol-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)cadmium(II) C34H34CdN12O2S2
- Crystal structure of ethyl 2,2-difluoro-2-(7-methoxy-2-oxo-2H-chromen-3-yl)acetate, C14H12F2O5
- The crystal structure of bis[μ2-(N,N-diethylcarbamodithioato-κS:κS,κS′)] bis[1′-(diphenylphosphino-κP)-1-cyanoferrocene]disilver(I), C56H56Ag2Fe2N4P2S4
- Crystal structure of bis(di-n-butylammonium) tetrachloridodiphenylstannate(IV), C28H50Cl4N2Sn
- The crystal structure of poly[(μ5-2-((5-bromo-3-formyl-2-hydroxybenzylidene)amino)benzenesulfonato-κ6O:O:O,O′:O′:O′′)sodium(I)], C13H9O4NSBrNa
- Crystal structure of catena-{poly[bis(O,O′-diethyldithiophosphato-S)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)-zinc(II)] di-acetonitrile solvate}, {C20H30N4O4P2S4Zn ⋅ 2 C2H3N}n
- Halogen and hydrogen bonding in the layered crystal structure of 2-iodoanilinium triiodide, C6H7I4N
- Crystal structure of cyclohexane-1,4-diammonium 2-[(2-carboxylatophenyl)disulfanyl]benzoate — dimethylformamide — monohydrate (1/1/1), [C6H16N2][C14H8O4S2] ⋅ C3H7NO⋅H2O
- The synthesis and crystal structure of isobutyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C16H13Cl2F6N3O3S
- Isolation and crystal structure of bufotalinin — methanol (1/1), C25H34O7
- Crystal structure of benzylbis(1,3-diphenylpropane-1,3-dionato-κ2O,O′) chloridotin(IV), C37H29ClO4Sn
- Crystal structure of Bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imidazol)}diiodidocadmium(II), [Cd(C11H11N5)2I2], C22H22N10I2Cd
- Crystal structure of 4-isobutoxybenzaldehyde oxime, C11H15NO2
- The crystal structure of bis(acetato-κ1O)-bis(N′-hydroxypyrimidine-2-carboximidamide-κ2N,N′)manganese(II) — methanol (1/2), C14H18MnN8O6, 2(CH3OH)′
- Crystal structure of poly[bis(μ2-bis(4-(1H-imidazol-1-yl)phenyl)amine-κ2N:N′)-bis(nitrato-κO)cadmium(II)], C36H30CdN12O6
- Crystal structure and optical properties of 1,6-bis(methylthio)pyrene, C18H14S2
- The crystal structure of hexaquamagnesium(II) bis(3,4-dinitropyrazol-1-ide), C6H14MgN8O14
- Halogen bonds in the crystal structure of 4,3:5,4-terpyridine – 1,4-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure and photochromic properties of a novel photochromic perfluordiarylethene containing a triazole bridged pyridine group moiety, C24H18F6N4S2
- Crystal structure of bis[(μ3-oxido)-(μ2-(N,N-diisopropylthiocarbamoylthio) acetato-κ2O,O′)-((N,N-diisopropylthiocarbamoylthio)acetato-κO)-bis(di-4-methylbenzyl-tin(IV))], C100H136N4O10S8Sn4
- Crystal structure of dibromidobis(4-bromobenzyl)tin(IV), C14H12Br4Sn
- The crystal structure of (4Z)-2-[(E)-(1-ethyl-3,3-dimethyl-1,3-dihydro-2H-indol-2-ylidene)methyl]-4-[(1-ethyl-3,3-dimethyl-3H-indolium-2-yl)methylidene]-3-oxocyclobut-1-en-1-olate, C30H32N2O2
- The crystal structure of (E)-3-(4-(dimethylamino)styryl)-5,5-dimethylcyclohex-2-en-1-one, C18H23NO
- Crystal structure of dihydrazinium 1H-pyrazole-3,5-dicarboxylate, C5H12N6O4
- Crystal structure of poly[μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4-sulfidobenzoate-κ2O:S)cobalt(II)] dihydrate, C42H44Co2N8O7S2
- Crystal structure of 8-(3,4-dimethylbenzylidene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C17H18O4
- Crystal structure of 4-(2-bromo-4-(6-morpholino-3-phenyl-3H-benzo[f]chromen-3-yl) cyclohexa-2,5-dien-1-yl)morpholine, C33H31BrN2O
- Synthesis and crystal structure of 2-((1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)methylene)-2,3-dihydro-1H-inden-1-one, C23H16N2OS
- Crystal structure of poly[(μ2-1,1′-(oxybis(4,1-phenylene)bis(1H-imidazole)-κ2N,N′)(μ2-1,3-benzenecarboxylato-κ3O,O′:O′′)zinc(II)] dihydrate, C26H22N4O7Zn
- Crystal structure of diaqua-bis(cinnamato-κ2O,O′)zinc(II), C18H18ZnO6
- Crystal structure of 2-(prop-2-yn-1-yloxy)-1-naphthaldehyde, C14H10O2
- Crystal structure and photochromic properties of 1-(2-methyl-5-phenyl-3-thienyl)-2-{2-methyl-5-[4-(9-fluorenone hydrazone)-phenyl]-3-thienyl}perfluorocyclopentene, C41H26F6N2S2
- Hydrothermal synthesis and crystal structure of cylo[tetraaqua-bis(μ2-1,4-bis(1H-benzo[d]imidazol-1-yl)but-2-ene-κ2N:N′)-bis(μ2-4-nitro-phthalate-κ2O,O′)dinickel(II)], C26H23N5O8Ni
- Crystal structure of 3-[methyl(phenyl)amino]-1-phenylthiourea, C14H15N3S
- Crystal structure of 1-(4-chlorophenyl)-3-[methyl(phenyl)amino]thiourea, C14H14ClN3S
- Crystal structure of 2-tert-butyl-1H-imidazo[4,5-b]pyridine, C10H13N3
- Crystal structure of 5-carboxy-2-(2-carboxyphenyl)-1H-imidazol-3-ium-4-carboxylate dihydrate, C12H8N2O6⋅2(H2O)
- The crystal structure of dichlorido-μ2-dichlorido-(η2-1,4-bis(4-vinylbenzyl)-1,4-diazabicyclo[2.2.2]octane-1,4-diium)dicopper(I), C24H30N2Cu2Cl4
- Crystal structure of 4-bromobenzyl (Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C22H29BrN2OS
- Crystal structure of (4S,4aS,6aR,6bR,12aS,12bR,14aS,14bR)-3,3,6a,6b,9,9,12a-heptamethyloctadecahydro-1H,3H-4,14b-ethanophenanthro[1,2-h]isochromene-1(6bH)-one, C30H48O2
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C30H33F6N3S
- The crystal structure of 3-methoxyphenanthridin-6(5H)-one, C14H11NO2
- Crystal structure of 4-(5,5-difluoro-1,3,7,9-tetramethyl-3H,5H-5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)pyridin-1-ium tetraiodidoferrate(III), C18H19BF2FeI4N3
- Crystal structure of 2-(3-methoxyphenyl)-3-((phenylsulfonyl)methyl)imidazo[1,2-a]pyridine, C21H18N2O3S
- Crystal structure of [(2-(2-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) perchlorate, C29H50Cl2N4NiO8
- Crystal structure of (Z)-6-(dimethylamino)-3,3-bis(4-(dimethylamino)phenyl)-2-(2-(quinoxalin-2-ylmethylene)hydrazinyl)-2,3-dihydroinden-1-one, C35H35N7O
- 5-Methyl-N′-[5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbonyl]-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbohydrazide, C22H22N8O2
- Crystal structure of 2,3-dichloro-6-methoxyquinoxaline, C9H6Cl2N2O
- Synthesis and crystal structure of 7-chloro-2-(ethylsulfinyl)-6-fluoro-3-(1H-pyrazole-1-yl)-4H-thiochromen-4-one, C13H10FN3OS2
- Crystal structure of 4-ethylpiperazine-1-carbothioic dithioperoxyanhydride, C14H26N4S4
- Crystal structure of 2-(2-(6-methylpyridin-2-yl)naphthalen-1-yl)pyrimidine, C20H15N3
- The crystal structure of N′-((1E,2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-ylidene)-3-methylbenzohydrazide, C23H22N2O4
- Crystal structure of catena-poly[(μ2-isophthalato-κ2O:O′)-(2,5-di(pyrazin-2-yl)-4,4′-bipyridine-κ3N,N′,N′′)zinc(II)] — water (2/5), C26H21N6O6.5Zn
- Crystal structure of (3E,5E)-3,5-bis(3-nitrobenzylidene)-1-((4-(trifluoromethyl)phenyl)sulfonyl)piperidin-4-one — dichloromethane (2/1), C53H38Cl2F6N6O14S2
- Crystal structure of (μ2-oxido)-bis(N,N′-o-phenylenebis(salicylideneiminato))diiron(III) — N,N′-dimethylformamide, C47H43Fe2N4O9
- Crystal structure of N1,N3-bis(2-hydroxyethyl)-N1, N1,N3,N3-tetramethylpropane-1,3-diaminium dibromide, C11H28Br2N2O2
- Crystal structure of (E)-N-(4-chlorophenyl)-1-(pyridin-2-yl)methanimine, C12H9ClN2
- Crystal structure of 8-bromo-6-oxo-2-phenyl-6H-pyrrolo[3,2,1-ij]quinoline-5-carbaldehyde, C18H11BrNO2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride trihydrate, C8H18N8Cl2 ⋅ 3 H2O
- Crystal structure of (E)-4-bromo-N-(pyridin-2-ylmethylene)aniline, C12H9BrN2
- Crystal structure of bis[(2-(3-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ-O)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Br2N4NiO8
- The crystal structure of (1E,2E)-2-methyl-4-((7-oxo-7H-furo[3,2-g]chromen-9-yl)oxy)but-2-enal O-isonicotinoyl oxime–trichloromethane (3/1), C67H49Cl3N6O18
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-methyl-1H-imidazol-3-ium hexafluoridophosphate(V), C8H13F6N2O2P
- Crystal structure of bis[(2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κO)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) hemihydrate C42H65Br2N4NiO8.5
- The crystal structure of N-(7-(4-fluorobenzylidene)-3-(4-fluorophenyl)-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbonothioyl)benzamide, C28H23F2N3OS
- The crystal structure of N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide, C18H20N4O2
- Crystal structure of (E)-2-(3,6-bis(ethylamino)-2,7-dimethyl-9H-xanthen-9-yl)-N′-((6-methylpyridin-2-yl)methylene)benzohydrazide – methanol (1/1), C34H37N5O3
- Crystal structure of 2-oxo-1-(pyrimidin-5-ylmethyl)-3-(3-(trifluoromethyl)phenyl)-1,2-dihydro-5l4-pyrido[1,2-a]pyrimidin-4-olate, C20H13F3N4O2
- Crystal structure of poly[(μ3-9H-carbazole-3,6-dicarboxylato-κ3O1: O2: O3)(μ2-4-(pyridin-4-yl)pyridine-κ2N1:N1′)zinc(II)], C19H11N2O4Zn
- Crystal structure of (E)-N′-((1,8-dihydropyren-1-yl)-methylene)picolinohydrazide, C23H15N3O
- Crystal structure of catena-poly{[μ2-1,2-bis(diphenylphosphino)ethane]dichloridocadmium(II)}, C26H24CdCl2P2
- Crystal structure of the 1:2 co-crystal between N,N′-bis(4-pyridylmethyl)oxalamide and acetic acid as a dihydrate, C14H14N4O2⋅2 C2H4O2⋅2 H2O
- Crystal structure of the co-crystal N,N′-bis(3-pyridylmethyl)oxalamide acetic acid (1/2), C14H14N4O2⋅2C2H4O2
- Crystal structure of the co-crystal N,N′-bis(4-pyridylmethyl)oxalamide and 2,3,5,6-tetrafluoro-1,4-di-iodobenzene (1/1), C14H14N4O2⋅C6F4I2
- Crystal structure of the co-crystal 4-[(4-carboxyphenyl)disulfanyl]benzoic acid–(1E,4E)-1-N,4-N-bis(pyridin-4-ylmethylidene)cyclohexane-1,4-diamine (1/1), C14H10O4S2⋅C18H20N4
- Crystal structure of hexacarbonyl-bis(μ2-di-n-propyldithiocarbamato-κ3S,S′:S;κ3S:S:S′)-di-rhenium(I), C20H28N2O6Re2S4
- Crystal structure of fac-tricarbonyl-morpholine-κN-(morpholinocarbamodithioato-κ2S,S′)rhenium(I), C12H17N2O5ReS2
Articles in the same Issue
- Frontmatter
- Crystal structure of poly[diaqua-(μ8-1,1′:2′,1′′-terphenyl-3,3′′,4′,5′-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)dicopper(II)], C22H14Cu2O10
- Crystal structure of 2-((1H-benzo[d]imidazol-2-ylimino)methyl)-4,6-di-tert-butylphenol, C22H27N3O
- Crystal structure of (4-ethoxynaphthalen-1-yl)(furan-2-yl)methanone, C17H14O3
- Crystal structure of 1-nonylpyridazin-1-ium iodide, C13H23N2I
- Crystal structure of bis[diaqua(1,10-phenanthroline-κ2N, N′)-copper(II)]diphenylphosphopentamolybdate dihydrate, C36H38Cu2Mo5N4O27P2
- The crystal structure of tetrakis(imidazole)-copper(I) hexafluorophosphate, C12H16CuF6PN8
- The crystal structure of dimethyl ((3,5-di-tert-butyl-4-hydroxyphenyl)(phenyl)methyl)phosphonate, C23H33O4P
- Crystal structure of diaqua-bis(1,10-phenanthroline κ2N,N′)nickel(II) trifluoroacetate- trifluoroacetic acid (1/1), C30H21F9N4NiO8
- Crystal structure of 2-(naphthalen-2-yl)-1,8-naphthyridine, C18H12N2
- Synthesis and crystal structure of a new polymorph of diisopropylammonium trichloroacetate, C8H16Cl3NO2
- Crystal structure of dimethanol-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)cadmium(II) C34H34CdN12O2S2
- Crystal structure of ethyl 2,2-difluoro-2-(7-methoxy-2-oxo-2H-chromen-3-yl)acetate, C14H12F2O5
- The crystal structure of bis[μ2-(N,N-diethylcarbamodithioato-κS:κS,κS′)] bis[1′-(diphenylphosphino-κP)-1-cyanoferrocene]disilver(I), C56H56Ag2Fe2N4P2S4
- Crystal structure of bis(di-n-butylammonium) tetrachloridodiphenylstannate(IV), C28H50Cl4N2Sn
- The crystal structure of poly[(μ5-2-((5-bromo-3-formyl-2-hydroxybenzylidene)amino)benzenesulfonato-κ6O:O:O,O′:O′:O′′)sodium(I)], C13H9O4NSBrNa
- Crystal structure of catena-{poly[bis(O,O′-diethyldithiophosphato-S)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)-zinc(II)] di-acetonitrile solvate}, {C20H30N4O4P2S4Zn ⋅ 2 C2H3N}n
- Halogen and hydrogen bonding in the layered crystal structure of 2-iodoanilinium triiodide, C6H7I4N
- Crystal structure of cyclohexane-1,4-diammonium 2-[(2-carboxylatophenyl)disulfanyl]benzoate — dimethylformamide — monohydrate (1/1/1), [C6H16N2][C14H8O4S2] ⋅ C3H7NO⋅H2O
- The synthesis and crystal structure of isobutyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C16H13Cl2F6N3O3S
- Isolation and crystal structure of bufotalinin — methanol (1/1), C25H34O7
- Crystal structure of benzylbis(1,3-diphenylpropane-1,3-dionato-κ2O,O′) chloridotin(IV), C37H29ClO4Sn
- Crystal structure of Bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imidazol)}diiodidocadmium(II), [Cd(C11H11N5)2I2], C22H22N10I2Cd
- Crystal structure of 4-isobutoxybenzaldehyde oxime, C11H15NO2
- The crystal structure of bis(acetato-κ1O)-bis(N′-hydroxypyrimidine-2-carboximidamide-κ2N,N′)manganese(II) — methanol (1/2), C14H18MnN8O6, 2(CH3OH)′
- Crystal structure of poly[bis(μ2-bis(4-(1H-imidazol-1-yl)phenyl)amine-κ2N:N′)-bis(nitrato-κO)cadmium(II)], C36H30CdN12O6
- Crystal structure and optical properties of 1,6-bis(methylthio)pyrene, C18H14S2
- The crystal structure of hexaquamagnesium(II) bis(3,4-dinitropyrazol-1-ide), C6H14MgN8O14
- Halogen bonds in the crystal structure of 4,3:5,4-terpyridine – 1,4-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure and photochromic properties of a novel photochromic perfluordiarylethene containing a triazole bridged pyridine group moiety, C24H18F6N4S2
- Crystal structure of bis[(μ3-oxido)-(μ2-(N,N-diisopropylthiocarbamoylthio) acetato-κ2O,O′)-((N,N-diisopropylthiocarbamoylthio)acetato-κO)-bis(di-4-methylbenzyl-tin(IV))], C100H136N4O10S8Sn4
- Crystal structure of dibromidobis(4-bromobenzyl)tin(IV), C14H12Br4Sn
- The crystal structure of (4Z)-2-[(E)-(1-ethyl-3,3-dimethyl-1,3-dihydro-2H-indol-2-ylidene)methyl]-4-[(1-ethyl-3,3-dimethyl-3H-indolium-2-yl)methylidene]-3-oxocyclobut-1-en-1-olate, C30H32N2O2
- The crystal structure of (E)-3-(4-(dimethylamino)styryl)-5,5-dimethylcyclohex-2-en-1-one, C18H23NO
- Crystal structure of dihydrazinium 1H-pyrazole-3,5-dicarboxylate, C5H12N6O4
- Crystal structure of poly[μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4-sulfidobenzoate-κ2O:S)cobalt(II)] dihydrate, C42H44Co2N8O7S2
- Crystal structure of 8-(3,4-dimethylbenzylidene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C17H18O4
- Crystal structure of 4-(2-bromo-4-(6-morpholino-3-phenyl-3H-benzo[f]chromen-3-yl) cyclohexa-2,5-dien-1-yl)morpholine, C33H31BrN2O
- Synthesis and crystal structure of 2-((1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)methylene)-2,3-dihydro-1H-inden-1-one, C23H16N2OS
- Crystal structure of poly[(μ2-1,1′-(oxybis(4,1-phenylene)bis(1H-imidazole)-κ2N,N′)(μ2-1,3-benzenecarboxylato-κ3O,O′:O′′)zinc(II)] dihydrate, C26H22N4O7Zn
- Crystal structure of diaqua-bis(cinnamato-κ2O,O′)zinc(II), C18H18ZnO6
- Crystal structure of 2-(prop-2-yn-1-yloxy)-1-naphthaldehyde, C14H10O2
- Crystal structure and photochromic properties of 1-(2-methyl-5-phenyl-3-thienyl)-2-{2-methyl-5-[4-(9-fluorenone hydrazone)-phenyl]-3-thienyl}perfluorocyclopentene, C41H26F6N2S2
- Hydrothermal synthesis and crystal structure of cylo[tetraaqua-bis(μ2-1,4-bis(1H-benzo[d]imidazol-1-yl)but-2-ene-κ2N:N′)-bis(μ2-4-nitro-phthalate-κ2O,O′)dinickel(II)], C26H23N5O8Ni
- Crystal structure of 3-[methyl(phenyl)amino]-1-phenylthiourea, C14H15N3S
- Crystal structure of 1-(4-chlorophenyl)-3-[methyl(phenyl)amino]thiourea, C14H14ClN3S
- Crystal structure of 2-tert-butyl-1H-imidazo[4,5-b]pyridine, C10H13N3
- Crystal structure of 5-carboxy-2-(2-carboxyphenyl)-1H-imidazol-3-ium-4-carboxylate dihydrate, C12H8N2O6⋅2(H2O)
- The crystal structure of dichlorido-μ2-dichlorido-(η2-1,4-bis(4-vinylbenzyl)-1,4-diazabicyclo[2.2.2]octane-1,4-diium)dicopper(I), C24H30N2Cu2Cl4
- Crystal structure of 4-bromobenzyl (Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C22H29BrN2OS
- Crystal structure of (4S,4aS,6aR,6bR,12aS,12bR,14aS,14bR)-3,3,6a,6b,9,9,12a-heptamethyloctadecahydro-1H,3H-4,14b-ethanophenanthro[1,2-h]isochromene-1(6bH)-one, C30H48O2
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C30H33F6N3S
- The crystal structure of 3-methoxyphenanthridin-6(5H)-one, C14H11NO2
- Crystal structure of 4-(5,5-difluoro-1,3,7,9-tetramethyl-3H,5H-5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)pyridin-1-ium tetraiodidoferrate(III), C18H19BF2FeI4N3
- Crystal structure of 2-(3-methoxyphenyl)-3-((phenylsulfonyl)methyl)imidazo[1,2-a]pyridine, C21H18N2O3S
- Crystal structure of [(2-(2-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) perchlorate, C29H50Cl2N4NiO8
- Crystal structure of (Z)-6-(dimethylamino)-3,3-bis(4-(dimethylamino)phenyl)-2-(2-(quinoxalin-2-ylmethylene)hydrazinyl)-2,3-dihydroinden-1-one, C35H35N7O
- 5-Methyl-N′-[5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbonyl]-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbohydrazide, C22H22N8O2
- Crystal structure of 2,3-dichloro-6-methoxyquinoxaline, C9H6Cl2N2O
- Synthesis and crystal structure of 7-chloro-2-(ethylsulfinyl)-6-fluoro-3-(1H-pyrazole-1-yl)-4H-thiochromen-4-one, C13H10FN3OS2
- Crystal structure of 4-ethylpiperazine-1-carbothioic dithioperoxyanhydride, C14H26N4S4
- Crystal structure of 2-(2-(6-methylpyridin-2-yl)naphthalen-1-yl)pyrimidine, C20H15N3
- The crystal structure of N′-((1E,2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-ylidene)-3-methylbenzohydrazide, C23H22N2O4
- Crystal structure of catena-poly[(μ2-isophthalato-κ2O:O′)-(2,5-di(pyrazin-2-yl)-4,4′-bipyridine-κ3N,N′,N′′)zinc(II)] — water (2/5), C26H21N6O6.5Zn
- Crystal structure of (3E,5E)-3,5-bis(3-nitrobenzylidene)-1-((4-(trifluoromethyl)phenyl)sulfonyl)piperidin-4-one — dichloromethane (2/1), C53H38Cl2F6N6O14S2
- Crystal structure of (μ2-oxido)-bis(N,N′-o-phenylenebis(salicylideneiminato))diiron(III) — N,N′-dimethylformamide, C47H43Fe2N4O9
- Crystal structure of N1,N3-bis(2-hydroxyethyl)-N1, N1,N3,N3-tetramethylpropane-1,3-diaminium dibromide, C11H28Br2N2O2
- Crystal structure of (E)-N-(4-chlorophenyl)-1-(pyridin-2-yl)methanimine, C12H9ClN2
- Crystal structure of 8-bromo-6-oxo-2-phenyl-6H-pyrrolo[3,2,1-ij]quinoline-5-carbaldehyde, C18H11BrNO2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride trihydrate, C8H18N8Cl2 ⋅ 3 H2O
- Crystal structure of (E)-4-bromo-N-(pyridin-2-ylmethylene)aniline, C12H9BrN2
- Crystal structure of bis[(2-(3-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ-O)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Br2N4NiO8
- The crystal structure of (1E,2E)-2-methyl-4-((7-oxo-7H-furo[3,2-g]chromen-9-yl)oxy)but-2-enal O-isonicotinoyl oxime–trichloromethane (3/1), C67H49Cl3N6O18
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-methyl-1H-imidazol-3-ium hexafluoridophosphate(V), C8H13F6N2O2P
- Crystal structure of bis[(2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κO)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) hemihydrate C42H65Br2N4NiO8.5
- The crystal structure of N-(7-(4-fluorobenzylidene)-3-(4-fluorophenyl)-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbonothioyl)benzamide, C28H23F2N3OS
- The crystal structure of N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide, C18H20N4O2
- Crystal structure of (E)-2-(3,6-bis(ethylamino)-2,7-dimethyl-9H-xanthen-9-yl)-N′-((6-methylpyridin-2-yl)methylene)benzohydrazide – methanol (1/1), C34H37N5O3
- Crystal structure of 2-oxo-1-(pyrimidin-5-ylmethyl)-3-(3-(trifluoromethyl)phenyl)-1,2-dihydro-5l4-pyrido[1,2-a]pyrimidin-4-olate, C20H13F3N4O2
- Crystal structure of poly[(μ3-9H-carbazole-3,6-dicarboxylato-κ3O1: O2: O3)(μ2-4-(pyridin-4-yl)pyridine-κ2N1:N1′)zinc(II)], C19H11N2O4Zn
- Crystal structure of (E)-N′-((1,8-dihydropyren-1-yl)-methylene)picolinohydrazide, C23H15N3O
- Crystal structure of catena-poly{[μ2-1,2-bis(diphenylphosphino)ethane]dichloridocadmium(II)}, C26H24CdCl2P2
- Crystal structure of the 1:2 co-crystal between N,N′-bis(4-pyridylmethyl)oxalamide and acetic acid as a dihydrate, C14H14N4O2⋅2 C2H4O2⋅2 H2O
- Crystal structure of the co-crystal N,N′-bis(3-pyridylmethyl)oxalamide acetic acid (1/2), C14H14N4O2⋅2C2H4O2
- Crystal structure of the co-crystal N,N′-bis(4-pyridylmethyl)oxalamide and 2,3,5,6-tetrafluoro-1,4-di-iodobenzene (1/1), C14H14N4O2⋅C6F4I2
- Crystal structure of the co-crystal 4-[(4-carboxyphenyl)disulfanyl]benzoic acid–(1E,4E)-1-N,4-N-bis(pyridin-4-ylmethylidene)cyclohexane-1,4-diamine (1/1), C14H10O4S2⋅C18H20N4
- Crystal structure of hexacarbonyl-bis(μ2-di-n-propyldithiocarbamato-κ3S,S′:S;κ3S:S:S′)-di-rhenium(I), C20H28N2O6Re2S4
- Crystal structure of fac-tricarbonyl-morpholine-κN-(morpholinocarbamodithioato-κ2S,S′)rhenium(I), C12H17N2O5ReS2

