Abstract
C6H7I4N, monoclinic, P21/m (no. 11), a = 9.2818(2) Å, b = 6.55289(16) Å, c = 11.0561(3) Å, β = 114.051(3)°, V = 614.08(3) Å3, Z = 2, Rgt(F) = 0.0180, wRref(F2) = 0.0367, T = 109(2) K.
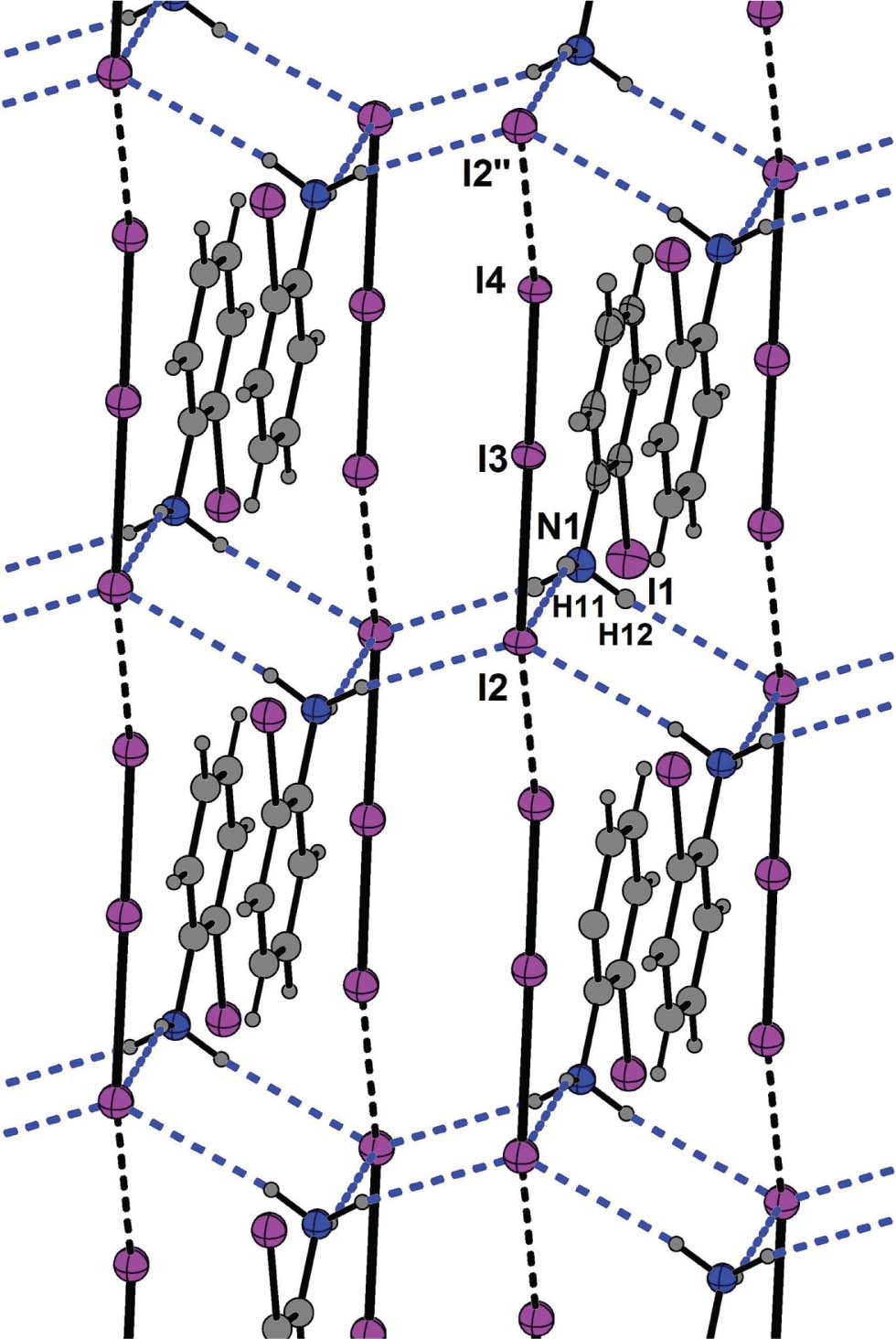
A part of the layered title crystal structure is shown in the figure. Tables 1 and 2 contain details on the crystal structure as well as measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Orange plate |
| Size: | 0.25 × 0.13 × 0.01 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 10.1 mm−1 |
| Diffractometer, scan mode: | Xcalibur, Eos, φ and ω |
| θmax, completeness: | 27.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 6606, 1466, 0.034 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1296 |
| N(param)refined: | 76 |
| Programs: | Diamond [1], CrysAlisPRO [2], SHELX [3], [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| I1 | 0.88099(4) | 0.250000 | 0.03135(3) | 0.02161(10) |
| N1 | 0.8563(5) | 0.250000 | 0.3175(4) | 0.0163(9) |
| H11 | 0.849(4) | 0.250000 | 0.396(3) | 0.034(10)* |
| H12 | 0.911(4) | 0.138(5) | 0.315(3) | 0.034(10)* |
| C1 | 0.6981(6) | 0.250000 | 0.2069(5) | 0.0155(11) |
| C2 | 0.6836(6) | 0.250000 | 0.0786(5) | 0.0138(10) |
| C3 | 0.5335(6) | 0.250000 | −0.0243(5) | 0.0165(11) |
| H3 | 0.522156 | 0.250000 | −0.111808 | 0.020* |
| C4 | 0.4018(6) | 0.250000 | 0.0040(5) | 0.0195(11) |
| H4 | 0.301644 | 0.250000 | −0.064539 | 0.023* |
| C5 | 0.4188(6) | 0.250000 | 0.1348(5) | 0.0215(12) |
| H5 | 0.329780 | 0.250000 | 0.153573 | 0.026* |
| C6 | 0.5663(6) | 0.250000 | 0.2365(5) | 0.0163(11) |
| H6 | 0.577935 | 0.250000 | 0.324080 | 0.020* |
| I2 | 0.95898(4) | 0.250000 | 0.67290(3) | 0.01430(9) |
| I3 | 0.60419(3) | 0.250000 | 0.62212(3) | 0.01337(8) |
| I4 | 0.28733(4) | 0.250000 | 0.58194(3) | 0.01551(9) |
Source of material
All chemicals were obtained from commercial sources and used as purchased. The Raman spectra were measured using a Bruker MULTIRAM spectrometer (Nd: YAG-laser at 1064 nm; InGaAsdetector) with an apodized resolution of 4 cm−1 in the region of 4000−70 cm−1. The title compound was synthesized by dissolving 0.22 g (1 mmol) 2-iodoaniline and 0.25 g (1 mmol) diiodine in 1 mL of 57% aqueous hydroiodic acid. Heating to 300 K for a few minutes yielded a dark colored solution. Evaporation at room temerature gave dark orange plate crystals of the title compound (systematic name: 2-iodobenzenaminium triiodide).
Experimental details
A single crystal of the title compound was directly selected from the mother liquor and rapidly transferred into the cold gas-stream (T = 100 K) of the Xcalibur four-circle diffractometer equipped with an EOS detector [2]. An absorption correction (Gaussian method) was applied [2]. The structure solution and the refinement were carried out using the SHELX program system [3], [4], [5]. Atomic coordinates of hydrogen atoms involved in hydrogen bonds were refined using distance restraints. All other hydrogen atoms were added using a riding model with fixed Uiso parameters. The maximum residual peak of 0.63 e Å−3 is found 1.03 Å from I1 and the deepest hole of −0.84 e Å−3 is found 1.01 Å from I3.
Comment
Today, polyiodides (in the 19th and the beginning of the 20th century periodides [6]) are defined as the anionic parts of salts that fulfill the general formula In-2m-n(n = 2−5, m = integer). Even polyiodides with a complex topology are constructed of basic units: I−, I3− and I2. Thus, the triiodide anion has been considered as the simplest polyiodide species. These ions and the I2 molecule tend to form extended aggregates by means of halogen bonds [7], [8], [9]. Especially for the I3− anion many polymeric structures that contain triiodide anions only are reported [9], [10]. Some of them are outstretched [11] and others show more complicated topologies [12]. Extended theoretical studies on the phenomenon of halogen bonding led to a deeper understanding of this type of non-covalent interaction [13]. Polyiodides are of interest not only because of their unique structures, but also because of their applications. Short chain polyiodides are key ions in the charge transfer processes of the classical dye-sensitised solar cells [14], [15]. Moreover, polyiodide species may be used as ambipolar zinc electrolytes [16] and contributed to developments in the field of lithium–iodine redox batteries [17]. However, there is still an academic interest in new polyiodide-containing salts based on organic cations as the lengths and shapes of cations influence the topology of the polyiodide anions [12], [18], [19], [20], [21]. We have already shown that heterocyclic cations like pyridinium derivatives [22], [23] or naturally occurring bases like caffeine [24] are excellent educts for the synthesis of polyiodide containing salts. In particular, there is profound interest in the competition between hydrogen and halogen bonding in haloanilinium halogenides [25], [26]. This contribution is part of a project, which focuses on polyiodides trapped in hydrogen-bonded surroundings [27], [28], [29].
All non-hydrogen atoms are located on the mirror plane in the centrosymmetric space group P21/m. Bond lengths and angles in the cation are within the expected range [26]. Each cation donates three hydrogen bonds to three adjacent triiodide anions (N1-H11⋯I2: 3.643(4) Å, N1-H12⋯I2′: 3.677(2) Å; ′ = 2 − x, −y, 1 − z). These geometric descriptors indicate charge-supported N-H⋯I hydrogen bonds [21].
The I–I distances in the formal triiodide anion of the title structure are: I2–I3: 3.1069(4) Å and I3–I4 2.7895(4) Å. These measures indicate a serious asymmetry, which is comparable to the situation in the structure of 1,8-diammoniooctane hexaiodide [21] (I–I distances: 2.7739(4) Å, 3.1778(4) Å). The more covalent part of the title anion (I3–I4) is only halogen bonded to the I2′′ atom of a neighboring triiodide anion (I4–I2′′: 3.5752(4) Å, ′′ = −1 + x, y, z). This secondary I⋯I bonding interaction is weak, but significantly shorter than any van der Waals distances in various scales [30]. Further significant I⋯I interactions can be ruled out as the corresponding iodine to iodine distances (>4.1 Å) are longer than the van der Waals sum.
The more ionic atom I2 solely forms the already mentioned hydrogen bonds. Consequently, the halogen bond and the three hydrogen bonds on one side of the triiodide anion, and only one halogen bonding interaction on the other side, can be made responsible for the observed asymmetry (cf. the figure).
The hydrogen bonds (blue dashed bonds in the figure) connect cations and triiodide anions resulting in a zig-zag ladder sub structure along the [010] direction. This substructure consists of ring motifs formed by two aminium groups and two triiodide anions (cf. the figure). This arrangement, which can be classified using the graph set descriptors
Within the Raman spectrum of the title compound the lines which are characteristic for an asymmetric triiodide anion [21], [33] are found at 109 cm−1(vs) and 155 cm−1 (vs). A general overview on the spectroscopy of triiodide species is given by Deplano et al. in 1999 [34], but the topic is still a matter of intense research activity [35], [36], [37]. Salts which are based on iodoanilines and structurally related compounds are an interesting system to study halogen bonds in competition with other forces that influence the packing schemes of the corresponding crystal structures [26], [38], [39].
Acknowledgements
I thank E. Hammes for technical support. I gratefully acknowledge support by the Ministry of Innovation, Science and Research of North-Rhine Westphalia and the German Research Foundation (DFG) for financial support (Xcalibur diffractometer; INST 208/533-1, project no. 162659349). Funding by the open access fund of the Heinrich-Heine-Universität Düsseldorf is gratefully acknowledged.
References
1. Brandenburg, K.: DIAMOND. Visual Crystal Structure Information System. Ver. 4.5.2. Crystal Impact, Bonn, Germany (2018).Search in Google Scholar
2. Oxford Diffraction: CrysAlisPRO, (version 1.171.33.42). Oxford Diffraction Ltd., Oxford, UK (2009).Search in Google Scholar
3. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
4. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
5. Hübschle, C. B.; Sheldrick, G. M.; Dittrich, B.: ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 44 (2011) 1281–1284.10.1107/S0021889811043202Search in Google Scholar PubMed PubMed Central
6. Tilden, W. A.: On the periodides of some of the organic bases. J. Chem. Soc. 18 (1865) 99–105.10.1039/JS8651800099Search in Google Scholar
7. Bartashevich, E.; Yushina, I.; Kropotina, K.; Muhitdinova, S.; Tsirelson, V.: Testing the tools for revealing and characterizing the iodine-iodine halogen bond in crystals. Acta Crystallogr. B73 (2017) 217–226.10.1107/S2052520617002931Search in Google Scholar PubMed
8. Blake, A. J.; Devillanova, F. A.; Gould, R. O.; Li, W. S.; Lippolis, V.; Parsons, S.; Radek, C.; Schröder, M.: Template self-assembly of polyiodide networks. Chem. Soc. Rev. 27 (1998) 195–205.10.1039/a827195zSearch in Google Scholar
9. Svensson, P. H.; Kloo, L.: Synthesis, structure, and bonding in polyiodide and metal iodide-iodine systems. Chem. Rev. 103 (2003) 1649–1684.10.1021/cr0204101Search in Google Scholar PubMed
10. Bof de Oliveira, A.; Beck, J.; Daniels, J.: Synthesis, crystal structure and Hirshfeld analysis of a new crystalline modification of the radical ion salt octamethylenetetrathiafulvalenium triiodide (OMTTF)I3. Acta Crystallogr. E74 (2018) 1547–1552.10.1107/S2056989018013907Search in Google Scholar PubMed PubMed Central
11. Denker, M.; Breunig, H. J.; Ebert, K. H.; Behrens, U.: Iodketten in (Me4Sb)3I8 und isolierte Triiodid-Ionen in Me4AsI3. Angew. Chem. 106 (1994) 1023–1024.10.1002/ange.19941060911Search in Google Scholar
12. Reiss, G. J.; van Megen, M.: Two new polyiodides in the 4,4′-bipyridinium diiodide/iodine system. Z. Naturforsch. B67 (2012) 5–10 and references cited.10.5560/ZNB.2012.67b0005Search in Google Scholar
13. Thirman, J.; Engelage, E.; Huber, S. M.; Head-Gordon, M.: Characterizing the interplay of Pauli repulsion, electrostatics, dispersion and charge transfer in halogen bonding with energy decomposition analysis. Phys. Chem. Chem. Phys. 20 (2018) 905–915.10.1039/C7CP06959FSearch in Google Scholar PubMed
14. O’Regan, B.; Grätzel, M.: A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353 (1991) 737–739.10.1038/353737a0Search in Google Scholar
15. Correa-Baena, J.-P.; Abate, A.; Saliba, M.; Tress, W.; Jesper Jacobsson, T.; Grätzel, M.; Hagfeldt, A.: The rapid evolution of highly efficient perovskite solar cells. Energy Environ. Sci. 10 (2017) 710–727.10.1039/C6EE03397KSearch in Google Scholar
16. Li, B.; Nie, Z.; Vijayakumar, M.; Li, G.; Liu, J.; Sprenkle, V.; Wang, W.: Ambipolar zinc-polyiodide electrolyte for a high-energy density aqueous redox flow battery. Nature Commun. 6 (2015) article no. 6303, 8 pages.10.1038/ncomms7303Search in Google Scholar PubMed PubMed Central
17. Zhao, Y.; Hong, M.; Bonnet Mercier, N.; Yu, G.; Choi, H. C.; Byon, H. R.: A 3.5 V lithium–iodine hybrid redox battery with vertically aligned carbon nanotube current collector. Nano Lett. 14 (2014) 1085–1092.10.1021/nl404784dSearch in Google Scholar PubMed
18. Walbaum, C.; Pantenburg, I.; Meyer, G.: Penta-, Hepta- und Oktaiodid-Anionen in Salzen mit Erdalkalimetall-Kronenether-Kationen. Z. Naturforsch. B65 (2010) 1077–1083.10.1515/znb-2010-0904Search in Google Scholar
19. Walbaum, C.; Pantenburg, I.; Junk, P.; Deacon, G.; Meyer, G.: Bulky cations and four different polyiodide anions in [Lu(Db18c6)(H2O)3(thf)6]4(I3)2(I5)6(I8)(I12). Z. Anorg. Allgem. Chem. 636 (2010) 1444–1446.10.1002/zaac.201000112Search in Google Scholar
20. Peuronen, A.; Rinta, H.; Lahtinen, M.: N⋯I halogen bonding supported stabilization of a discrete pseudo-linear [I12]2− polyiodide. CrystEngComm 17 (2015) 1736–1740 and references cited therein.10.1039/C4CE01866DSearch in Google Scholar
21. van Megen, M.; Reiss, G. J.: I62− Anion composed of two asymmetric triiodide moieties: a competition between halogen and hydrogen bond. Inorganics 1 (2013) 3–13.10.3390/inorganics1010003Search in Google Scholar
22. Reiss, G. J.; Leske, P. B.: The twinned crystal structure of bis(4-aminopyridin-1-ium) iodide triiodide, C20H28I8N8. Z. Kristallogr. NCS 229 (2014) 452–454 and references cited.10.1515/ncrs-2014-0193Search in Google Scholar
23. Reiss, G. J.; Leske, P. B.: 2-Aminopyridin-1-ium triiodide. Acta Crystallogr. E69 (2013) o1060–o1061.10.1107/S1600536813015389Search in Google Scholar PubMed PubMed Central
24. Merkelbach, J.; Majewski, M. A.; Reiss, G. J.: Crystal structure of caffeinium triiodide – caffeine (1/1), C16H21I3N8O4. Z. Kristallogr. NCS 233 (2018) 941–944.10.1515/ncrs-2018-0125Search in Google Scholar
25. Attrell, R. J.; Widdifield, C. M.; Korobkov, I.; Bryce, D. L.: Weak halogen bonding in solid haloanilinium halides probed directly via chlorine-35, bromine-81, and iodine-127 NMR spectroscopy. Cryst. Growth Des. 12 (2012) 1641–1653.10.1021/cg201683pSearch in Google Scholar
26. Gray, L.; Jones, P., G.: Secondary bonding interactions in some haloanilinium halides. Z. Naturforsch. B57 (2002) 61–72.10.1515/znb-2002-0108Search in Google Scholar
27. Reiss, G. J.: I5– polymers with a layered arrangement: synthesis, spectroscopy, and structure of a new polyiodide salt in the nicotine/HI/I2 system. Z. Naturforsch. B70 (2015) 735–739.10.1515/znb-2015-0092Search in Google Scholar
28. Reiss, G. J.: Two iodine-rich (dimethylphosphoryl)methanaminium iodides. Z. Kristallogr. CM 232 (2017) 789–795.10.1515/zkri-2017-2071Search in Google Scholar
29. Reiss, G. J.: A cyclic I102− anion in the layered crystal structure of theophyllinium pentaiodide, C7H9I5N4O2. Z. Kristallogr. NCS (NCRS_2019_0082) accepted.10.1515/ncrs-2019-0082Search in Google Scholar
30. Hu, S.-Z.; Zhou, Z.-H.; Xie, Z.-X.; Robertson, B. E.: A comparative study of crystallographic van der Waals radii. Z. Kristallogr. CM 229 (2014) 517–523.10.1515/zkri-2014-1726Search in Google Scholar
31. Grell, J.; Bernstein, J.; Tinhofer, G.: Investigation of hydrogen bond patterns: a review of mathematical tools for the graph set approach. Crystallogr. Rev. 8 (2002) 1–56.10.1080/08893110211936Search in Google Scholar
32. Reiss, G. J.; van Megen, M.: Synthesis, structure and spectroscopy of a new polyiodide in the α,ω-diazaniumalkane iodide/iodine system. Z. Naturforsch. 67B (2012) 447–451.10.5560/znb.2012-0079Search in Google Scholar
33. Marks, T. J.; Kalina, D. W.: Highly conductive halogenated low-dimensional materials. In: Extended linear chain compounds, Volume 1 (Ed. Miller, J. S.), p. 197–331. Plenum Press, New York, NY, USA (1982).10.1007/978-1-4613-3249-7_6Search in Google Scholar
34. Deplano, P.; Ferraro, J. R.; Mercuri, M. L.; Trogu, E. F.: Structural and Raman spectroscopic studies as complementary tools in elucidating the nature of the bonding in polyiodides and in donor I2 adduct. Coord. Chem. Rev. 188 (1999) 71–95.10.1016/S0010-8545(98)00238-0Search in Google Scholar
35. Yushina, I. D.; Batalov, V. I.; Bartashevich, E. V.; Davydov, A. O.; Zelenovskiy, P. S.; Masunov, A. E.: Raman spectroscopy and theoretic study of hyperpolarizability effect in diiodobutenyl-bis-thioquinolinium triiodide at low temperature. J. Raman Spectrosc. 48 (2017) 1411–1413.10.1002/jrs.5159Search in Google Scholar
36. Abe, H.; Tokita, T.; Iwata, K.; Ozawa, S.: Lithium-triggered spontaneous formation of polyiodides in room-temperature ionic liquid-alcohol solutions. Spectrochim. Acta A 212 (2019) 255–261.10.1016/j.saa.2018.12.049Search in Google Scholar PubMed
37. Shestimerova, T. A.; Yelavik, N. A.; Mironov, A. V.; Kuznetsov, A. N.; Bykov, M. A.; Grigorieva, A. V.; Utochnikova, V. V.; Lepnev, L. S.; Shevelkov, A. V.: From isolated anions to polymer structures through linking with I2: synthesis, structure, and properties of two complex bismuth(III) iodine iodides. Inorg. Chem. 57 (2018) 4077–4087.10.1021/acs.inorgchem.8b00265Search in Google Scholar PubMed
38. Kassl, C. J.; Swenson, D. C.; Pigge, F. C.: Charge-assisted halogen bonding in bromo- and iodophenylpyridinium chlorides. Cryst. Growth Des. 15 (2015) 4571–4580.10.1021/acs.cgd.5b00835Search in Google Scholar
39. Raatikainen, K.; Cametti, M.; Rissanen, K.: The subtle balance of weak supramolecular interactions: the hierarchy of halogen and hydrogen bonds in haloanilinium and halopyridinium salts Beilstein J. Org. Chem. 4 (2010) 6 (13 pages).10.3762/bjoc.6.4Search in Google Scholar PubMed PubMed Central
©2019 Guido J. Reiss, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Crystal structure of poly[diaqua-(μ8-1,1′:2′,1′′-terphenyl-3,3′′,4′,5′-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)dicopper(II)], C22H14Cu2O10
- Crystal structure of 2-((1H-benzo[d]imidazol-2-ylimino)methyl)-4,6-di-tert-butylphenol, C22H27N3O
- Crystal structure of (4-ethoxynaphthalen-1-yl)(furan-2-yl)methanone, C17H14O3
- Crystal structure of 1-nonylpyridazin-1-ium iodide, C13H23N2I
- Crystal structure of bis[diaqua(1,10-phenanthroline-κ2N, N′)-copper(II)]diphenylphosphopentamolybdate dihydrate, C36H38Cu2Mo5N4O27P2
- The crystal structure of tetrakis(imidazole)-copper(I) hexafluorophosphate, C12H16CuF6PN8
- The crystal structure of dimethyl ((3,5-di-tert-butyl-4-hydroxyphenyl)(phenyl)methyl)phosphonate, C23H33O4P
- Crystal structure of diaqua-bis(1,10-phenanthroline κ2N,N′)nickel(II) trifluoroacetate- trifluoroacetic acid (1/1), C30H21F9N4NiO8
- Crystal structure of 2-(naphthalen-2-yl)-1,8-naphthyridine, C18H12N2
- Synthesis and crystal structure of a new polymorph of diisopropylammonium trichloroacetate, C8H16Cl3NO2
- Crystal structure of dimethanol-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)cadmium(II) C34H34CdN12O2S2
- Crystal structure of ethyl 2,2-difluoro-2-(7-methoxy-2-oxo-2H-chromen-3-yl)acetate, C14H12F2O5
- The crystal structure of bis[μ2-(N,N-diethylcarbamodithioato-κS:κS,κS′)] bis[1′-(diphenylphosphino-κP)-1-cyanoferrocene]disilver(I), C56H56Ag2Fe2N4P2S4
- Crystal structure of bis(di-n-butylammonium) tetrachloridodiphenylstannate(IV), C28H50Cl4N2Sn
- The crystal structure of poly[(μ5-2-((5-bromo-3-formyl-2-hydroxybenzylidene)amino)benzenesulfonato-κ6O:O:O,O′:O′:O′′)sodium(I)], C13H9O4NSBrNa
- Crystal structure of catena-{poly[bis(O,O′-diethyldithiophosphato-S)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)-zinc(II)] di-acetonitrile solvate}, {C20H30N4O4P2S4Zn ⋅ 2 C2H3N}n
- Halogen and hydrogen bonding in the layered crystal structure of 2-iodoanilinium triiodide, C6H7I4N
- Crystal structure of cyclohexane-1,4-diammonium 2-[(2-carboxylatophenyl)disulfanyl]benzoate — dimethylformamide — monohydrate (1/1/1), [C6H16N2][C14H8O4S2] ⋅ C3H7NO⋅H2O
- The synthesis and crystal structure of isobutyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C16H13Cl2F6N3O3S
- Isolation and crystal structure of bufotalinin — methanol (1/1), C25H34O7
- Crystal structure of benzylbis(1,3-diphenylpropane-1,3-dionato-κ2O,O′) chloridotin(IV), C37H29ClO4Sn
- Crystal structure of Bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imidazol)}diiodidocadmium(II), [Cd(C11H11N5)2I2], C22H22N10I2Cd
- Crystal structure of 4-isobutoxybenzaldehyde oxime, C11H15NO2
- The crystal structure of bis(acetato-κ1O)-bis(N′-hydroxypyrimidine-2-carboximidamide-κ2N,N′)manganese(II) — methanol (1/2), C14H18MnN8O6, 2(CH3OH)′
- Crystal structure of poly[bis(μ2-bis(4-(1H-imidazol-1-yl)phenyl)amine-κ2N:N′)-bis(nitrato-κO)cadmium(II)], C36H30CdN12O6
- Crystal structure and optical properties of 1,6-bis(methylthio)pyrene, C18H14S2
- The crystal structure of hexaquamagnesium(II) bis(3,4-dinitropyrazol-1-ide), C6H14MgN8O14
- Halogen bonds in the crystal structure of 4,3:5,4-terpyridine – 1,4-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure and photochromic properties of a novel photochromic perfluordiarylethene containing a triazole bridged pyridine group moiety, C24H18F6N4S2
- Crystal structure of bis[(μ3-oxido)-(μ2-(N,N-diisopropylthiocarbamoylthio) acetato-κ2O,O′)-((N,N-diisopropylthiocarbamoylthio)acetato-κO)-bis(di-4-methylbenzyl-tin(IV))], C100H136N4O10S8Sn4
- Crystal structure of dibromidobis(4-bromobenzyl)tin(IV), C14H12Br4Sn
- The crystal structure of (4Z)-2-[(E)-(1-ethyl-3,3-dimethyl-1,3-dihydro-2H-indol-2-ylidene)methyl]-4-[(1-ethyl-3,3-dimethyl-3H-indolium-2-yl)methylidene]-3-oxocyclobut-1-en-1-olate, C30H32N2O2
- The crystal structure of (E)-3-(4-(dimethylamino)styryl)-5,5-dimethylcyclohex-2-en-1-one, C18H23NO
- Crystal structure of dihydrazinium 1H-pyrazole-3,5-dicarboxylate, C5H12N6O4
- Crystal structure of poly[μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4-sulfidobenzoate-κ2O:S)cobalt(II)] dihydrate, C42H44Co2N8O7S2
- Crystal structure of 8-(3,4-dimethylbenzylidene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C17H18O4
- Crystal structure of 4-(2-bromo-4-(6-morpholino-3-phenyl-3H-benzo[f]chromen-3-yl) cyclohexa-2,5-dien-1-yl)morpholine, C33H31BrN2O
- Synthesis and crystal structure of 2-((1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)methylene)-2,3-dihydro-1H-inden-1-one, C23H16N2OS
- Crystal structure of poly[(μ2-1,1′-(oxybis(4,1-phenylene)bis(1H-imidazole)-κ2N,N′)(μ2-1,3-benzenecarboxylato-κ3O,O′:O′′)zinc(II)] dihydrate, C26H22N4O7Zn
- Crystal structure of diaqua-bis(cinnamato-κ2O,O′)zinc(II), C18H18ZnO6
- Crystal structure of 2-(prop-2-yn-1-yloxy)-1-naphthaldehyde, C14H10O2
- Crystal structure and photochromic properties of 1-(2-methyl-5-phenyl-3-thienyl)-2-{2-methyl-5-[4-(9-fluorenone hydrazone)-phenyl]-3-thienyl}perfluorocyclopentene, C41H26F6N2S2
- Hydrothermal synthesis and crystal structure of cylo[tetraaqua-bis(μ2-1,4-bis(1H-benzo[d]imidazol-1-yl)but-2-ene-κ2N:N′)-bis(μ2-4-nitro-phthalate-κ2O,O′)dinickel(II)], C26H23N5O8Ni
- Crystal structure of 3-[methyl(phenyl)amino]-1-phenylthiourea, C14H15N3S
- Crystal structure of 1-(4-chlorophenyl)-3-[methyl(phenyl)amino]thiourea, C14H14ClN3S
- Crystal structure of 2-tert-butyl-1H-imidazo[4,5-b]pyridine, C10H13N3
- Crystal structure of 5-carboxy-2-(2-carboxyphenyl)-1H-imidazol-3-ium-4-carboxylate dihydrate, C12H8N2O6⋅2(H2O)
- The crystal structure of dichlorido-μ2-dichlorido-(η2-1,4-bis(4-vinylbenzyl)-1,4-diazabicyclo[2.2.2]octane-1,4-diium)dicopper(I), C24H30N2Cu2Cl4
- Crystal structure of 4-bromobenzyl (Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C22H29BrN2OS
- Crystal structure of (4S,4aS,6aR,6bR,12aS,12bR,14aS,14bR)-3,3,6a,6b,9,9,12a-heptamethyloctadecahydro-1H,3H-4,14b-ethanophenanthro[1,2-h]isochromene-1(6bH)-one, C30H48O2
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C30H33F6N3S
- The crystal structure of 3-methoxyphenanthridin-6(5H)-one, C14H11NO2
- Crystal structure of 4-(5,5-difluoro-1,3,7,9-tetramethyl-3H,5H-5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)pyridin-1-ium tetraiodidoferrate(III), C18H19BF2FeI4N3
- Crystal structure of 2-(3-methoxyphenyl)-3-((phenylsulfonyl)methyl)imidazo[1,2-a]pyridine, C21H18N2O3S
- Crystal structure of [(2-(2-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) perchlorate, C29H50Cl2N4NiO8
- Crystal structure of (Z)-6-(dimethylamino)-3,3-bis(4-(dimethylamino)phenyl)-2-(2-(quinoxalin-2-ylmethylene)hydrazinyl)-2,3-dihydroinden-1-one, C35H35N7O
- 5-Methyl-N′-[5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbonyl]-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbohydrazide, C22H22N8O2
- Crystal structure of 2,3-dichloro-6-methoxyquinoxaline, C9H6Cl2N2O
- Synthesis and crystal structure of 7-chloro-2-(ethylsulfinyl)-6-fluoro-3-(1H-pyrazole-1-yl)-4H-thiochromen-4-one, C13H10FN3OS2
- Crystal structure of 4-ethylpiperazine-1-carbothioic dithioperoxyanhydride, C14H26N4S4
- Crystal structure of 2-(2-(6-methylpyridin-2-yl)naphthalen-1-yl)pyrimidine, C20H15N3
- The crystal structure of N′-((1E,2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-ylidene)-3-methylbenzohydrazide, C23H22N2O4
- Crystal structure of catena-poly[(μ2-isophthalato-κ2O:O′)-(2,5-di(pyrazin-2-yl)-4,4′-bipyridine-κ3N,N′,N′′)zinc(II)] — water (2/5), C26H21N6O6.5Zn
- Crystal structure of (3E,5E)-3,5-bis(3-nitrobenzylidene)-1-((4-(trifluoromethyl)phenyl)sulfonyl)piperidin-4-one — dichloromethane (2/1), C53H38Cl2F6N6O14S2
- Crystal structure of (μ2-oxido)-bis(N,N′-o-phenylenebis(salicylideneiminato))diiron(III) — N,N′-dimethylformamide, C47H43Fe2N4O9
- Crystal structure of N1,N3-bis(2-hydroxyethyl)-N1, N1,N3,N3-tetramethylpropane-1,3-diaminium dibromide, C11H28Br2N2O2
- Crystal structure of (E)-N-(4-chlorophenyl)-1-(pyridin-2-yl)methanimine, C12H9ClN2
- Crystal structure of 8-bromo-6-oxo-2-phenyl-6H-pyrrolo[3,2,1-ij]quinoline-5-carbaldehyde, C18H11BrNO2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride trihydrate, C8H18N8Cl2 ⋅ 3 H2O
- Crystal structure of (E)-4-bromo-N-(pyridin-2-ylmethylene)aniline, C12H9BrN2
- Crystal structure of bis[(2-(3-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ-O)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Br2N4NiO8
- The crystal structure of (1E,2E)-2-methyl-4-((7-oxo-7H-furo[3,2-g]chromen-9-yl)oxy)but-2-enal O-isonicotinoyl oxime–trichloromethane (3/1), C67H49Cl3N6O18
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-methyl-1H-imidazol-3-ium hexafluoridophosphate(V), C8H13F6N2O2P
- Crystal structure of bis[(2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κO)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) hemihydrate C42H65Br2N4NiO8.5
- The crystal structure of N-(7-(4-fluorobenzylidene)-3-(4-fluorophenyl)-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbonothioyl)benzamide, C28H23F2N3OS
- The crystal structure of N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide, C18H20N4O2
- Crystal structure of (E)-2-(3,6-bis(ethylamino)-2,7-dimethyl-9H-xanthen-9-yl)-N′-((6-methylpyridin-2-yl)methylene)benzohydrazide – methanol (1/1), C34H37N5O3
- Crystal structure of 2-oxo-1-(pyrimidin-5-ylmethyl)-3-(3-(trifluoromethyl)phenyl)-1,2-dihydro-5l4-pyrido[1,2-a]pyrimidin-4-olate, C20H13F3N4O2
- Crystal structure of poly[(μ3-9H-carbazole-3,6-dicarboxylato-κ3O1: O2: O3)(μ2-4-(pyridin-4-yl)pyridine-κ2N1:N1′)zinc(II)], C19H11N2O4Zn
- Crystal structure of (E)-N′-((1,8-dihydropyren-1-yl)-methylene)picolinohydrazide, C23H15N3O
- Crystal structure of catena-poly{[μ2-1,2-bis(diphenylphosphino)ethane]dichloridocadmium(II)}, C26H24CdCl2P2
- Crystal structure of the 1:2 co-crystal between N,N′-bis(4-pyridylmethyl)oxalamide and acetic acid as a dihydrate, C14H14N4O2⋅2 C2H4O2⋅2 H2O
- Crystal structure of the co-crystal N,N′-bis(3-pyridylmethyl)oxalamide acetic acid (1/2), C14H14N4O2⋅2C2H4O2
- Crystal structure of the co-crystal N,N′-bis(4-pyridylmethyl)oxalamide and 2,3,5,6-tetrafluoro-1,4-di-iodobenzene (1/1), C14H14N4O2⋅C6F4I2
- Crystal structure of the co-crystal 4-[(4-carboxyphenyl)disulfanyl]benzoic acid–(1E,4E)-1-N,4-N-bis(pyridin-4-ylmethylidene)cyclohexane-1,4-diamine (1/1), C14H10O4S2⋅C18H20N4
- Crystal structure of hexacarbonyl-bis(μ2-di-n-propyldithiocarbamato-κ3S,S′:S;κ3S:S:S′)-di-rhenium(I), C20H28N2O6Re2S4
- Crystal structure of fac-tricarbonyl-morpholine-κN-(morpholinocarbamodithioato-κ2S,S′)rhenium(I), C12H17N2O5ReS2
Articles in the same Issue
- Frontmatter
- Crystal structure of poly[diaqua-(μ8-1,1′:2′,1′′-terphenyl-3,3′′,4′,5′-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)dicopper(II)], C22H14Cu2O10
- Crystal structure of 2-((1H-benzo[d]imidazol-2-ylimino)methyl)-4,6-di-tert-butylphenol, C22H27N3O
- Crystal structure of (4-ethoxynaphthalen-1-yl)(furan-2-yl)methanone, C17H14O3
- Crystal structure of 1-nonylpyridazin-1-ium iodide, C13H23N2I
- Crystal structure of bis[diaqua(1,10-phenanthroline-κ2N, N′)-copper(II)]diphenylphosphopentamolybdate dihydrate, C36H38Cu2Mo5N4O27P2
- The crystal structure of tetrakis(imidazole)-copper(I) hexafluorophosphate, C12H16CuF6PN8
- The crystal structure of dimethyl ((3,5-di-tert-butyl-4-hydroxyphenyl)(phenyl)methyl)phosphonate, C23H33O4P
- Crystal structure of diaqua-bis(1,10-phenanthroline κ2N,N′)nickel(II) trifluoroacetate- trifluoroacetic acid (1/1), C30H21F9N4NiO8
- Crystal structure of 2-(naphthalen-2-yl)-1,8-naphthyridine, C18H12N2
- Synthesis and crystal structure of a new polymorph of diisopropylammonium trichloroacetate, C8H16Cl3NO2
- Crystal structure of dimethanol-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)cadmium(II) C34H34CdN12O2S2
- Crystal structure of ethyl 2,2-difluoro-2-(7-methoxy-2-oxo-2H-chromen-3-yl)acetate, C14H12F2O5
- The crystal structure of bis[μ2-(N,N-diethylcarbamodithioato-κS:κS,κS′)] bis[1′-(diphenylphosphino-κP)-1-cyanoferrocene]disilver(I), C56H56Ag2Fe2N4P2S4
- Crystal structure of bis(di-n-butylammonium) tetrachloridodiphenylstannate(IV), C28H50Cl4N2Sn
- The crystal structure of poly[(μ5-2-((5-bromo-3-formyl-2-hydroxybenzylidene)amino)benzenesulfonato-κ6O:O:O,O′:O′:O′′)sodium(I)], C13H9O4NSBrNa
- Crystal structure of catena-{poly[bis(O,O′-diethyldithiophosphato-S)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)-zinc(II)] di-acetonitrile solvate}, {C20H30N4O4P2S4Zn ⋅ 2 C2H3N}n
- Halogen and hydrogen bonding in the layered crystal structure of 2-iodoanilinium triiodide, C6H7I4N
- Crystal structure of cyclohexane-1,4-diammonium 2-[(2-carboxylatophenyl)disulfanyl]benzoate — dimethylformamide — monohydrate (1/1/1), [C6H16N2][C14H8O4S2] ⋅ C3H7NO⋅H2O
- The synthesis and crystal structure of isobutyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C16H13Cl2F6N3O3S
- Isolation and crystal structure of bufotalinin — methanol (1/1), C25H34O7
- Crystal structure of benzylbis(1,3-diphenylpropane-1,3-dionato-κ2O,O′) chloridotin(IV), C37H29ClO4Sn
- Crystal structure of Bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imidazol)}diiodidocadmium(II), [Cd(C11H11N5)2I2], C22H22N10I2Cd
- Crystal structure of 4-isobutoxybenzaldehyde oxime, C11H15NO2
- The crystal structure of bis(acetato-κ1O)-bis(N′-hydroxypyrimidine-2-carboximidamide-κ2N,N′)manganese(II) — methanol (1/2), C14H18MnN8O6, 2(CH3OH)′
- Crystal structure of poly[bis(μ2-bis(4-(1H-imidazol-1-yl)phenyl)amine-κ2N:N′)-bis(nitrato-κO)cadmium(II)], C36H30CdN12O6
- Crystal structure and optical properties of 1,6-bis(methylthio)pyrene, C18H14S2
- The crystal structure of hexaquamagnesium(II) bis(3,4-dinitropyrazol-1-ide), C6H14MgN8O14
- Halogen bonds in the crystal structure of 4,3:5,4-terpyridine – 1,4-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure and photochromic properties of a novel photochromic perfluordiarylethene containing a triazole bridged pyridine group moiety, C24H18F6N4S2
- Crystal structure of bis[(μ3-oxido)-(μ2-(N,N-diisopropylthiocarbamoylthio) acetato-κ2O,O′)-((N,N-diisopropylthiocarbamoylthio)acetato-κO)-bis(di-4-methylbenzyl-tin(IV))], C100H136N4O10S8Sn4
- Crystal structure of dibromidobis(4-bromobenzyl)tin(IV), C14H12Br4Sn
- The crystal structure of (4Z)-2-[(E)-(1-ethyl-3,3-dimethyl-1,3-dihydro-2H-indol-2-ylidene)methyl]-4-[(1-ethyl-3,3-dimethyl-3H-indolium-2-yl)methylidene]-3-oxocyclobut-1-en-1-olate, C30H32N2O2
- The crystal structure of (E)-3-(4-(dimethylamino)styryl)-5,5-dimethylcyclohex-2-en-1-one, C18H23NO
- Crystal structure of dihydrazinium 1H-pyrazole-3,5-dicarboxylate, C5H12N6O4
- Crystal structure of poly[μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4-sulfidobenzoate-κ2O:S)cobalt(II)] dihydrate, C42H44Co2N8O7S2
- Crystal structure of 8-(3,4-dimethylbenzylidene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C17H18O4
- Crystal structure of 4-(2-bromo-4-(6-morpholino-3-phenyl-3H-benzo[f]chromen-3-yl) cyclohexa-2,5-dien-1-yl)morpholine, C33H31BrN2O
- Synthesis and crystal structure of 2-((1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)methylene)-2,3-dihydro-1H-inden-1-one, C23H16N2OS
- Crystal structure of poly[(μ2-1,1′-(oxybis(4,1-phenylene)bis(1H-imidazole)-κ2N,N′)(μ2-1,3-benzenecarboxylato-κ3O,O′:O′′)zinc(II)] dihydrate, C26H22N4O7Zn
- Crystal structure of diaqua-bis(cinnamato-κ2O,O′)zinc(II), C18H18ZnO6
- Crystal structure of 2-(prop-2-yn-1-yloxy)-1-naphthaldehyde, C14H10O2
- Crystal structure and photochromic properties of 1-(2-methyl-5-phenyl-3-thienyl)-2-{2-methyl-5-[4-(9-fluorenone hydrazone)-phenyl]-3-thienyl}perfluorocyclopentene, C41H26F6N2S2
- Hydrothermal synthesis and crystal structure of cylo[tetraaqua-bis(μ2-1,4-bis(1H-benzo[d]imidazol-1-yl)but-2-ene-κ2N:N′)-bis(μ2-4-nitro-phthalate-κ2O,O′)dinickel(II)], C26H23N5O8Ni
- Crystal structure of 3-[methyl(phenyl)amino]-1-phenylthiourea, C14H15N3S
- Crystal structure of 1-(4-chlorophenyl)-3-[methyl(phenyl)amino]thiourea, C14H14ClN3S
- Crystal structure of 2-tert-butyl-1H-imidazo[4,5-b]pyridine, C10H13N3
- Crystal structure of 5-carboxy-2-(2-carboxyphenyl)-1H-imidazol-3-ium-4-carboxylate dihydrate, C12H8N2O6⋅2(H2O)
- The crystal structure of dichlorido-μ2-dichlorido-(η2-1,4-bis(4-vinylbenzyl)-1,4-diazabicyclo[2.2.2]octane-1,4-diium)dicopper(I), C24H30N2Cu2Cl4
- Crystal structure of 4-bromobenzyl (Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C22H29BrN2OS
- Crystal structure of (4S,4aS,6aR,6bR,12aS,12bR,14aS,14bR)-3,3,6a,6b,9,9,12a-heptamethyloctadecahydro-1H,3H-4,14b-ethanophenanthro[1,2-h]isochromene-1(6bH)-one, C30H48O2
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C30H33F6N3S
- The crystal structure of 3-methoxyphenanthridin-6(5H)-one, C14H11NO2
- Crystal structure of 4-(5,5-difluoro-1,3,7,9-tetramethyl-3H,5H-5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)pyridin-1-ium tetraiodidoferrate(III), C18H19BF2FeI4N3
- Crystal structure of 2-(3-methoxyphenyl)-3-((phenylsulfonyl)methyl)imidazo[1,2-a]pyridine, C21H18N2O3S
- Crystal structure of [(2-(2-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) perchlorate, C29H50Cl2N4NiO8
- Crystal structure of (Z)-6-(dimethylamino)-3,3-bis(4-(dimethylamino)phenyl)-2-(2-(quinoxalin-2-ylmethylene)hydrazinyl)-2,3-dihydroinden-1-one, C35H35N7O
- 5-Methyl-N′-[5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbonyl]-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbohydrazide, C22H22N8O2
- Crystal structure of 2,3-dichloro-6-methoxyquinoxaline, C9H6Cl2N2O
- Synthesis and crystal structure of 7-chloro-2-(ethylsulfinyl)-6-fluoro-3-(1H-pyrazole-1-yl)-4H-thiochromen-4-one, C13H10FN3OS2
- Crystal structure of 4-ethylpiperazine-1-carbothioic dithioperoxyanhydride, C14H26N4S4
- Crystal structure of 2-(2-(6-methylpyridin-2-yl)naphthalen-1-yl)pyrimidine, C20H15N3
- The crystal structure of N′-((1E,2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-ylidene)-3-methylbenzohydrazide, C23H22N2O4
- Crystal structure of catena-poly[(μ2-isophthalato-κ2O:O′)-(2,5-di(pyrazin-2-yl)-4,4′-bipyridine-κ3N,N′,N′′)zinc(II)] — water (2/5), C26H21N6O6.5Zn
- Crystal structure of (3E,5E)-3,5-bis(3-nitrobenzylidene)-1-((4-(trifluoromethyl)phenyl)sulfonyl)piperidin-4-one — dichloromethane (2/1), C53H38Cl2F6N6O14S2
- Crystal structure of (μ2-oxido)-bis(N,N′-o-phenylenebis(salicylideneiminato))diiron(III) — N,N′-dimethylformamide, C47H43Fe2N4O9
- Crystal structure of N1,N3-bis(2-hydroxyethyl)-N1, N1,N3,N3-tetramethylpropane-1,3-diaminium dibromide, C11H28Br2N2O2
- Crystal structure of (E)-N-(4-chlorophenyl)-1-(pyridin-2-yl)methanimine, C12H9ClN2
- Crystal structure of 8-bromo-6-oxo-2-phenyl-6H-pyrrolo[3,2,1-ij]quinoline-5-carbaldehyde, C18H11BrNO2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride trihydrate, C8H18N8Cl2 ⋅ 3 H2O
- Crystal structure of (E)-4-bromo-N-(pyridin-2-ylmethylene)aniline, C12H9BrN2
- Crystal structure of bis[(2-(3-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ-O)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Br2N4NiO8
- The crystal structure of (1E,2E)-2-methyl-4-((7-oxo-7H-furo[3,2-g]chromen-9-yl)oxy)but-2-enal O-isonicotinoyl oxime–trichloromethane (3/1), C67H49Cl3N6O18
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-methyl-1H-imidazol-3-ium hexafluoridophosphate(V), C8H13F6N2O2P
- Crystal structure of bis[(2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κO)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) hemihydrate C42H65Br2N4NiO8.5
- The crystal structure of N-(7-(4-fluorobenzylidene)-3-(4-fluorophenyl)-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbonothioyl)benzamide, C28H23F2N3OS
- The crystal structure of N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide, C18H20N4O2
- Crystal structure of (E)-2-(3,6-bis(ethylamino)-2,7-dimethyl-9H-xanthen-9-yl)-N′-((6-methylpyridin-2-yl)methylene)benzohydrazide – methanol (1/1), C34H37N5O3
- Crystal structure of 2-oxo-1-(pyrimidin-5-ylmethyl)-3-(3-(trifluoromethyl)phenyl)-1,2-dihydro-5l4-pyrido[1,2-a]pyrimidin-4-olate, C20H13F3N4O2
- Crystal structure of poly[(μ3-9H-carbazole-3,6-dicarboxylato-κ3O1: O2: O3)(μ2-4-(pyridin-4-yl)pyridine-κ2N1:N1′)zinc(II)], C19H11N2O4Zn
- Crystal structure of (E)-N′-((1,8-dihydropyren-1-yl)-methylene)picolinohydrazide, C23H15N3O
- Crystal structure of catena-poly{[μ2-1,2-bis(diphenylphosphino)ethane]dichloridocadmium(II)}, C26H24CdCl2P2
- Crystal structure of the 1:2 co-crystal between N,N′-bis(4-pyridylmethyl)oxalamide and acetic acid as a dihydrate, C14H14N4O2⋅2 C2H4O2⋅2 H2O
- Crystal structure of the co-crystal N,N′-bis(3-pyridylmethyl)oxalamide acetic acid (1/2), C14H14N4O2⋅2C2H4O2
- Crystal structure of the co-crystal N,N′-bis(4-pyridylmethyl)oxalamide and 2,3,5,6-tetrafluoro-1,4-di-iodobenzene (1/1), C14H14N4O2⋅C6F4I2
- Crystal structure of the co-crystal 4-[(4-carboxyphenyl)disulfanyl]benzoic acid–(1E,4E)-1-N,4-N-bis(pyridin-4-ylmethylidene)cyclohexane-1,4-diamine (1/1), C14H10O4S2⋅C18H20N4
- Crystal structure of hexacarbonyl-bis(μ2-di-n-propyldithiocarbamato-κ3S,S′:S;κ3S:S:S′)-di-rhenium(I), C20H28N2O6Re2S4
- Crystal structure of fac-tricarbonyl-morpholine-κN-(morpholinocarbamodithioato-κ2S,S′)rhenium(I), C12H17N2O5ReS2

