Abstract
The continuous process for the production of insoluble sulfur (IS) has been developed to overcome the disadvantages associated with the traditional batch process, such as low automation, discontinuous production, and high consumption of sulfur and CS2. The consumption of CS2 in the continuous process is lower than that in the batch process. The recovery of solvent facilitates the recycling of CS2 and N2 in addition to the closed circulation of the entire system. The key process and recovery parameters of the IS synthesis were simulated using Aspen Plus. Sulfur was completely recovered after high-temperature pyrolysis, and the utilization ratio of sulfur atoms was increased from approximately 50% to more than 95%. Tail gas was comprehensively utilized through compression and cryogenic green treatment. Based on the developed green synthesis process and simulated process parameters, the continuous production of IS was evaluated on an industrial scale of 10,000 metric tonnes. Owing to the continuous characteristics of the production process, the air tightness of the production process was significantly improved, and the quality of the product was more stable than that of the product obtained by the current domestic batch process. The unconverted sulfur could be recycled after recovery, without low-grade sulfur as a by-product. The amount of sulfur used as raw material per unit product was reduced; the comprehensive conversion rate of sulfur to IS exceeded 95%, which significantly improved the utilization rate of sulfur atoms. Through the compression and condensation of the tail gas, the recovery efficiency of CS2 was considerably improved at the same temperature, and compressed N2 could be simultaneously obtained. The liquefaction rate of CS2 approached 91.78%, which was characterized by obvious greening.
1 Introduction
Insoluble sulfur (IS), a high-performance rubber auxiliary material that can replace the use of ordinary sulfur, is a large molecule of sulfur polymerization, named after insoluble in CS2. It is slow to migrate in rubber and can effectively prevent rubber frosting and improve the heat and wear resistance of tires in tire production. Therefore, IS is an essential and important raw material in tire production [1,2,3].
Currently, there are three methods to produce IS: one-step melting method, high-temperature water method, and continuous method. The first two methods are batch reaction processes (collectively referred to as batch processes). High contents of IS can be obtained after separation, washing, and drying. In the one-step melting method, ordinary sulfur is melted at 130–150°C. Subsequently, the temperature of the mixture is increased to 300–400°C for a certain period; ordinary sulfur is converted into IS, but the conversion efficiency is low [4,5]. IS is extracted after rapid cooling of the solvent, and the product is obtained after drying, crushing, oil filling, and screening. However, the IS produced by the molten one-step process is poorly dispersed and subsequently needs to be crushed. Moreover, the index of the product visibly fluctuates. In the high-temperature water method, the high-temperature sulfur vapour is introduced into an acidic water medium, and the sulfur is cooled to produce IS. The reactant is a mixture of soluble and IS. The mixture is washed, dried, crushed, and extracted to obtain IS. This method involves low production costs [4,6]. However, owing to the use of water for cooling and washing, a large quantity of wastewater is generated. Consequently, the market competitiveness is weak.
In the continuous method, common sulfur is melted and rapidly heated to produce superheated steam. Using its pressure, it is injected into the quench liquid at high speed and rapidly cooled. A plastic mixture of insoluble and soluble sulfur is obtained. Subsequently, CS2 is used to extract the soluble sulfur in the mixture [7–9]. The products of the continuous process exhibit stable quality and excellent properties. Additionally, the process involves low consumption of raw and auxiliary materials, high degree of automation, and unattended operation on-site.
In this study, the green synthesis process of IS by the continuous method was evaluated to efficiently improve the yield of synthesis, reduce the loss of extractant, and recycle sulfur completely. Aspen Plus software was used to simulate the key processes of the synthesis with the experimental conditions as the boundary, to obtain the optimized process parameters [10,11]. The results of the simulation are used to adjust the operating parameters of the actual production so as to maximize the benefits while meeting the operational targets.
2 Studies on the green synthesis process of IS
Two types of processes are mainly used to produce IS: batch process and continuous process. Currently, batch processes, such as high-temperature water and melting methods, are used for the production of IS in China. However, some drawbacks in the high-temperature water method alter the quality of thermal stability of IS. In contrast to the continuous production process, the melting method involves a lower capacity of equipment, higher consumption of CS2, and underlying risks in production. The IS produced in the continuous process can be recycled continuously. The simultaneous recycling of CS2 lowers its consumption below the average level, reduces the cost of the enterprise, and demonstrates a higher degree of automation [11,12]. The product index is stable, and its application performance in rubber is better than that of IS produced by the batch process.
2.1 Mechanism of IS generation
When sulfur is cracked by heating, its molecular structure changes very complicated: when the temperature IS lower than 159°C, ordinary sulfur becomes light yellow liquid sulfur with a ring structure consisting of eight sulfur atoms after melting by heating. When the temperature exceeds a certain temperature, ordinary sulfur S8 undergoes ring-opening cracking to form an unsaturated sulfur atom chain free radical monomer with electrons at both ends. This free radical monomer forms linear macromolecular chain sulfur through polymerization, which is the main body of IS. The polymerization reaction is reversible, and the degree of polymerization of the polymerization products varies [13]. First, the average molecular weight range of IS was investigated by ideal model iodometric titration, and the experimental results showed that its molecular weight was about 2,800–3,100. Then, the density simulation method based on molecular dynamics combined with experimental methods was used to study the number of sulfur atoms in the polymerized sulfur chain (Sn) of IS, and the results showed that n = 96 was appropriate. The following simulation study was based on S96 [14].
Gauss View is used to obtain chemical kinetic information and thermodynamic information. According to the Schrodinger equation and Hartree–Fock equation, combined with density functional theory, the equation is as follows.
Finally, combining its reaction machine with the existing physical and chemical data (Table 1), Aspen Plus was used to model the IS synthesis process, and sensitivity analysis was used to study the relationship between temperature and IS yield (Scheme 1).
Reaction thermodynamics and kinetic parameters
| Reaction heat (kJ·mol−1) | Rate constant (cm3·mol−1·s−1) | |
|---|---|---|
| Polymerization reaction | −80.1 | 7.58 × 10−8 |
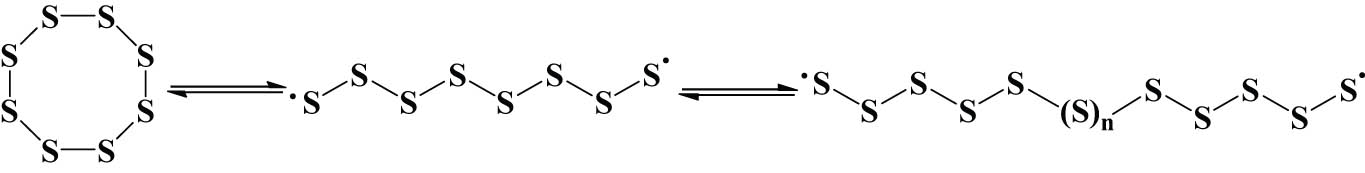
Synthetic route to IS.
2.2 Studies on the recycling process of by-product sulfur
The raw materials of IS are generally common sulfur from mining or recovered products from petroleum and gas desulfurization devices. However, the production of IS requires common sulfur of high purity to ensure that the quality of IS is unaffected. The purity requirements are generally greater than 99.95%. The conversion rate cannot always be increased because the polymerization is a reversible equilibrium reaction. Therefore, the IS products obtained by polymerization contain a large quantity of ordinary sulfur in addition to organic compounds mixed in the production process [15]. Therefore, the by-product sulfur cannot be used in the production of IS; the treated by-product generates a low price and subsequently leads to an increase in production costs. At present, the maximum conversion rate that can be achieved by the melt method in the laboratory stage is around 50–60%. The point of the continuous method compared to the melt method mainly lies in the finer particle size and better stability of the product obtained, and since the reaction is reversible, changing the method has little effect on the maximum conversion rate of the substance. In this process, the unreacted common sulfur in the sulfur polymerization reaction is recovered and re-introduced into the system after treatment to participate in the polymerization reaction.
As the cycle process continues, organic matter accumulates in the sulfur and therefore needs to be removed. The enrichment of alkanes in the by-product sulfur mainly consists of chain breaking and dehydrogenation reactions. The C–C bonds in alkanes are broken by temperature, and the main components of enriched organic matter contain alkanes, cyclic alkanes, aromatic hydrocarbons, and other hydrocarbons. After a complex chemical reaction of high-temperature cracking, the final products of organic matter in sulfur are H2S and coke particles.
2.3 Development of tail gas recycling process
The entire production process of IS is performed under N2 to avoid the influence of O2 on the product. However, the continuous addition of N2 to the system increases the system pressure. Therefore, the excess gas is discharged from the system. The production tail gas is a mixture of CS2 and atmospheric N2, which is the main component. In the tail gas treatment method, N2 is condensed and directly discharged into the atmosphere. Therefore, N2 is not recycled; this results in its underutilization and subsequently raises production costs. The separation and recovery of CS2 and N2 from the mixture are essential for environmental protection [16,17], and reduced material consumption and production costs. Additionally, this step is a technical challenge for IS production plants. In the conventional recovery of CS2, frozen water is recycled in the condenser, and CS2 is condensed into a liquid. However, the recovery rate of CS2 in tail gas by the frozen water method is inadequate owing to the high vapour pressure and volatile characteristics of CS2, and excess N2 in the tail gas. Thus, the consumption of CS2 is generally high. Tail gas compression and cryogenic design are implemented to reduce the consumption of solvent in the production of IS and facilitate green production. The compressed tail gas replaces pure N2 using pressure to supplement the production gas; this can reduce the overall use of N2 and the loss of CS2 [18].
3 Simulation and research of green recovery process of IS
The scale-up process from the laboratory stage to actual industrial production generally follows a comprehensive experimental cycle. For reaction systems, the interaction of various process parameters affects the scale-up to actual industrial production capacities. Since the 1990s, the chemical process simulation system has entered a period of extensive development, where the value of chemical process simulation technology has been recognized and widely used. It is a powerful auxiliary tool for design, research, and production. Chemical process simulations “reproduce” the actual production process on a computer. Therefore, they do not involve any changes in pipelines, equipment, and energy. The designer is provided a considerable degree of freedom to analyse different schemes and process conditions on the computer. Therefore, process simulation considerably saves time and operating costs; it is useful in analysing the planning, research, and development of chemical processes and their technical reliability. Aspen Plus is representative simulation software, which is widely used in chemical, oil refining, oil and gas processing, petrochemical, and other industries [19–22]. In this study, we simulate the green industrialization of IS based on Aspen Plus in order to obtain more suitable and green process parameters.
3.1 Aspen Plus model construction
With relevant experimental data obtained through a literature review as the boundary conditions. The sensitivity analysis module in Aspen Plus is used to select reliable physical properties, methods, and models. Users can use this tool to change one or more process variables and study the impact of their changes on other processes. Additionally, the module is used to analyse and verify whether the solution of the design specification is within the range of manipulated variables [23] to conduct a simulation study of the process. In this simulation, the physical property methods of PR-BM and RK-SOVE and the built-in physical property library of Aspen Plus are used in this article. In conjunction with the operational state, the Aspen Plus simulation makes the following assumptions.
The model shown in Figure 1 is established for the actual production process of IS. The whole system is closed with micro-positive pressure, in order to avoid leakage of materials or CS2 into the atmosphere. The RSTOIC module is used first for known chemical reactions. Ordinary sulfur S8 reacts with a small number of alkane impurities contained therein at high temperatures to generate small quantities of H2S and coke, which are subsequently separated by FLASH1 and FLASH2. The obtained pure sulfur undergoes a high-temperature cracking polymerization reaction in reactor R2 and is quenched to generate IS. Typically, the conversion rate of IS is 50–60%. In the extraction kettle, CS2 is used as the extractant at normal temperature, and a second extraction method is applied [24,25]. Moreover, some materials are transported by a pump during the actual production process. These materials are not considered during modelling and the steady-state flow is adopted by default. Therefore, there is a gap between the energy loss in the simulation and the actual process. However, the loss of materials is ignored and considered equal to the output of the actual process.
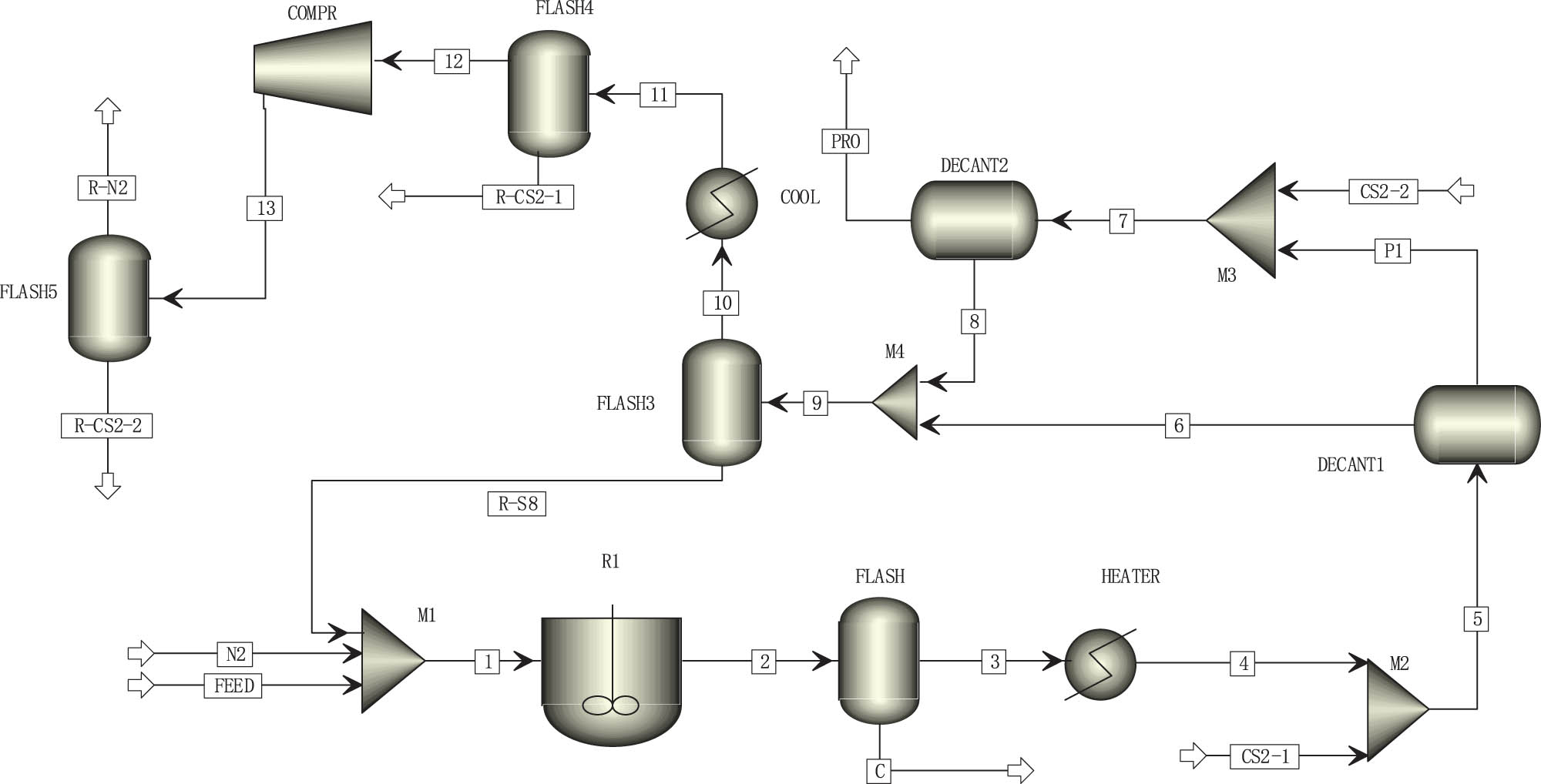
Process flowchart for IS production in Aspen Plus.
4 IS synthesis process Aspen Plus simulation
The investigation of a process generally involves time, concentration, temperature, pressure, and other process conditions. This process is mainly concerned with the effect of temperature on the production process, the effect of CS2 dose on the extraction effect of the product, and the temperature and pressure on the liquefaction rate of CS2 when the mixture of CS2 and N2 is separated. Therefore, in this process, the main module consists of the reactor and separator, supplemented by other modules; each module is built sequentially. Independent analysis and optimization are applied to the follow-up module. Subsequently, the most efficient process conditions are determined.
4.1 Production process of IS
In the production process of IS, common sulfur and by-product sulfur are mixed and fed into reactor R1, which reacts to produce IS after high-temperature action. During the reaction process, the temperature has a large influence on the product. Figure 2 shows the relationship between the polymerization temperature and the yield of IS. The polymerization temperature has a significant effect on the yield of IS. When the reaction temperature is lower than 400°C, the yield curve of IS increases rapidly and gradually decreases. When the temperature is in the range of 420–450°C, the reaction reaches an approximate maximum, and the reaction temperature is no longer the dominant factor in the reaction. As the temperature continues to increase, the yield of IS increases slowly and is stabilized near the maximum, probably because the temperature was too high, and some IS was cracked. In this study, the reaction temperature is 450°C; the overall reaction of IS yield is approximately 59.1%.
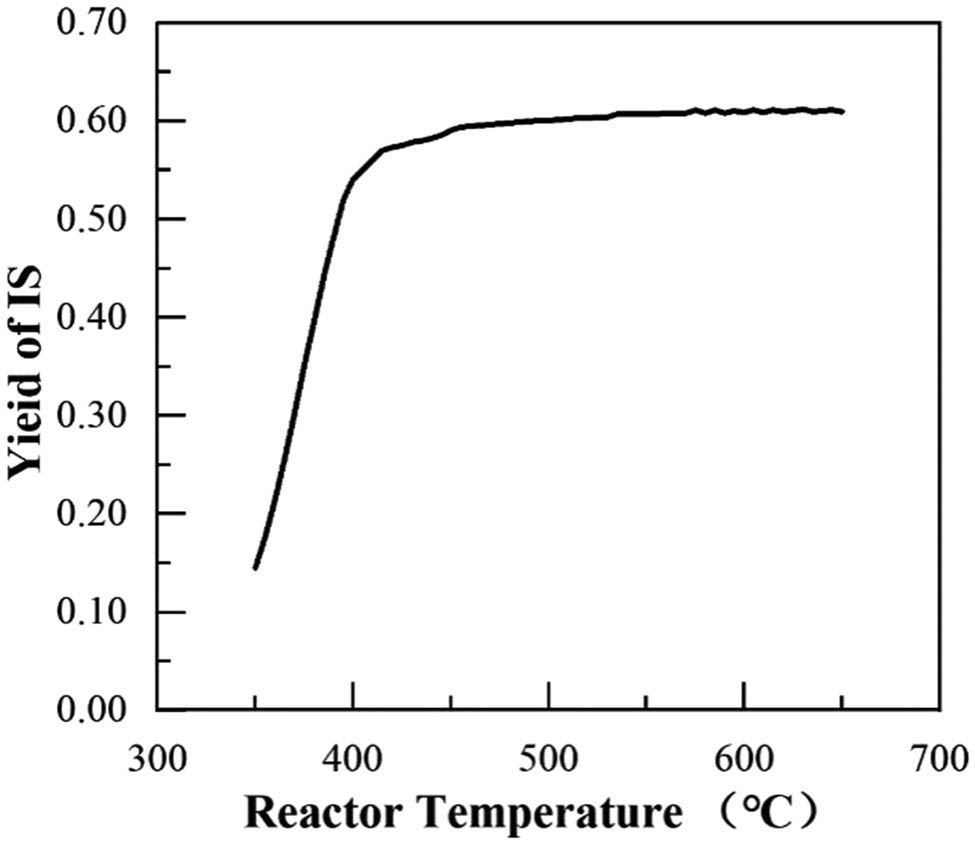
Relationship between the polymerization temperature and the IS yield.
4.2 Extraction process
The obtained reaction products were mixed with CS2, and since IS is insoluble in CS2, high-quality IS can be obtained by dissolving the by-product sulfur, but the efficiency of extraction is influenced by temperature and dose.
4.2.1 Effect of extraction temperature on the IS yield
Controlling the temperature significantly influences the yield of IS. The lower limit of the extraction temperature is set to 0°C; extremely low temperatures are not conducive to extraction efficiency and result in higher costs. The upper limit is set to 45°C; CS2 will vaporize at temperatures above 46.5°C. The effect of extraction temperature on the content of IS was studied by varying the temperature. Figure 3 shows that the efficiency of CS2 in the product increases with the increase in temperature. The extraction efficiency is 94.60% at 25°C and 95.01% at 30°C. In this study, the extraction temperature is 30°C, and the final mass fraction of IS is 95.01%.
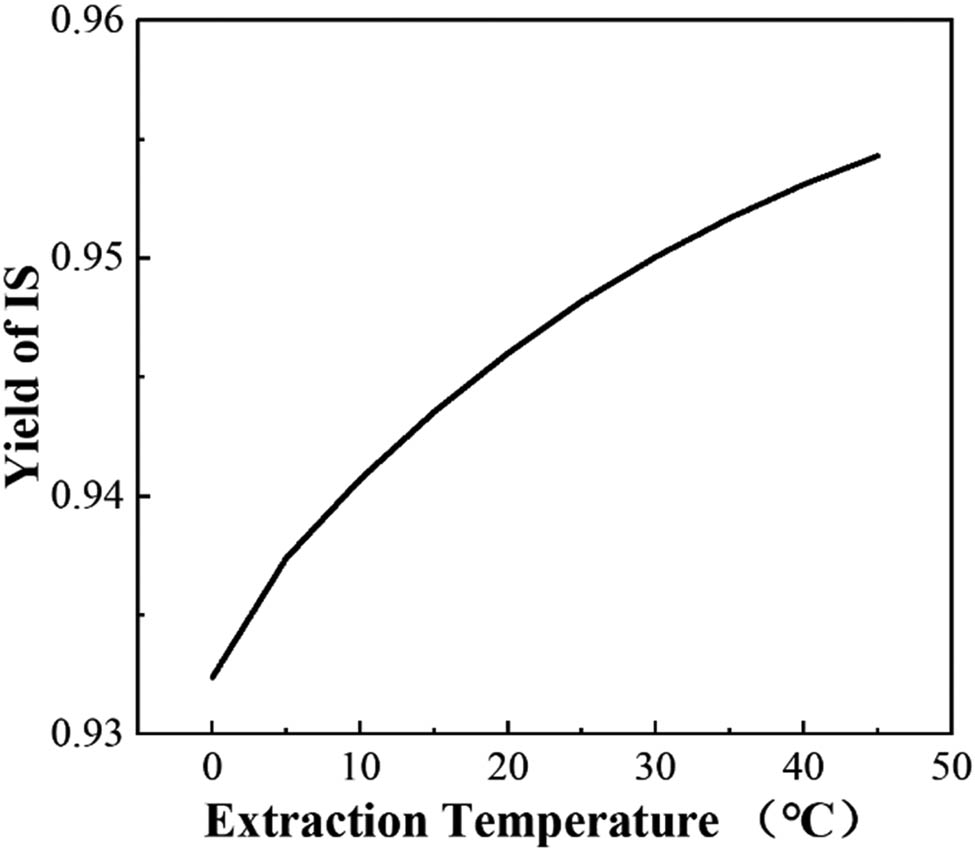
Relationship between the extraction temperature and the IS yield.
4.2.2 Effect of extractant dosage on extraction efficiency
Two-stage extraction was used to improve the extraction efficiency. Figure 4 shows that the mass fraction of IS increases with the increasing amount of extractant at the set temperature. The yield of IS was low, not reaching 90% when the feed ratio of CS2/S8 is less than 100. The extraction rate increases slowly when the feed ratio of CS2/S8 is greater than 175. Increasing the amount of CS2 would result in high production costs. The feed ratio of CS2/S8 was set at 150 to allow the collection of IS. This ratio offers a product of 95.01%, while avoiding the waste of raw materials.
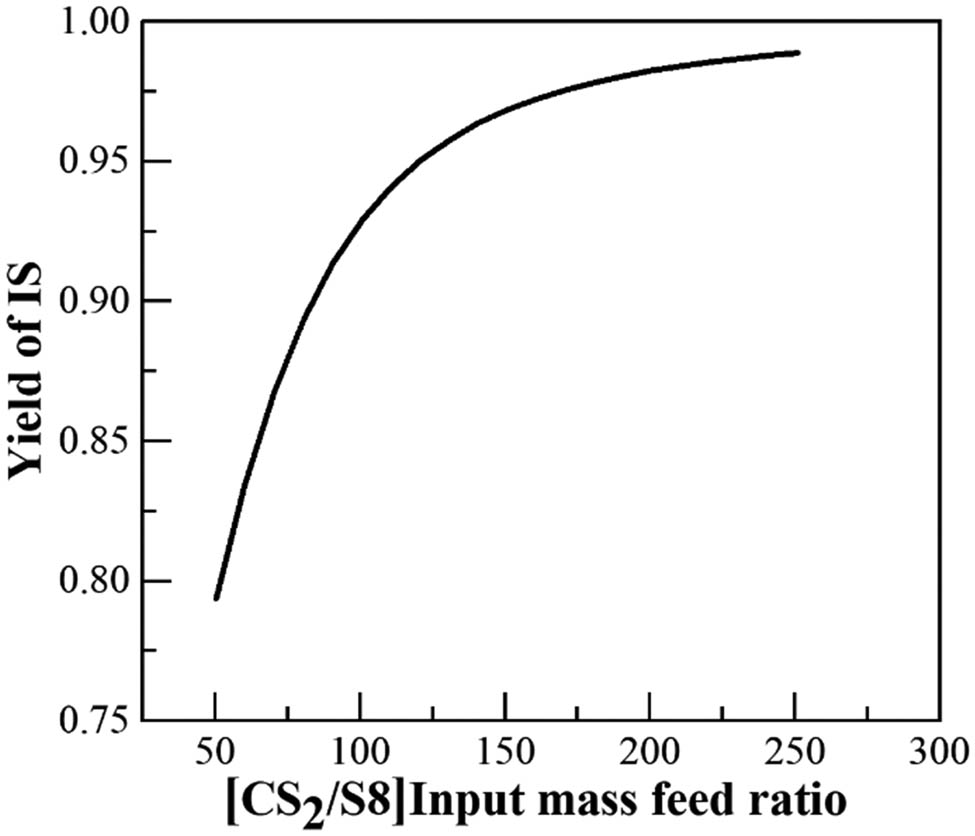
Relationship between the extraction dosage and the IS yield.
4.3 Tail gas recovery process
CS2 and N2 in the tail gas are compressed, but CS2, as a condensable gas, can be liquefied at the right temperature and pressure and will exist in a different phase from N2, so that the two can be separated. Therefore, the effect of temperature and pressure on the liquefaction rate of CS2 is investigated separately.
4.3.1 Effect of temperature on liquefaction rate of CS2
As shown in Figure 5, under the initial set pressure, the temperature control significantly influences the liquefaction rate of CS2. When the temperature is lower than 10°C, the liquefaction rate of CS2 is higher. With the increase in temperature, the liquefaction rate of CS2 begins to decrease significantly. Particularly, the increase in temperature greatly increases the molecular activity of CS2, which reduces its tendency to liquefy. Therefore, lower temperatures result in higher liquefaction yields. However, considering the actual industrial production, the temperature is controlled at 10–15°C to avoid significant waste of energy in the recovery process. The liquefaction rate of CS2 approaches values above 90%. In this study, 12°C is considered the separation temperature of CS2, which optimizes the liquefaction rate of CS2 in the entire separation process.
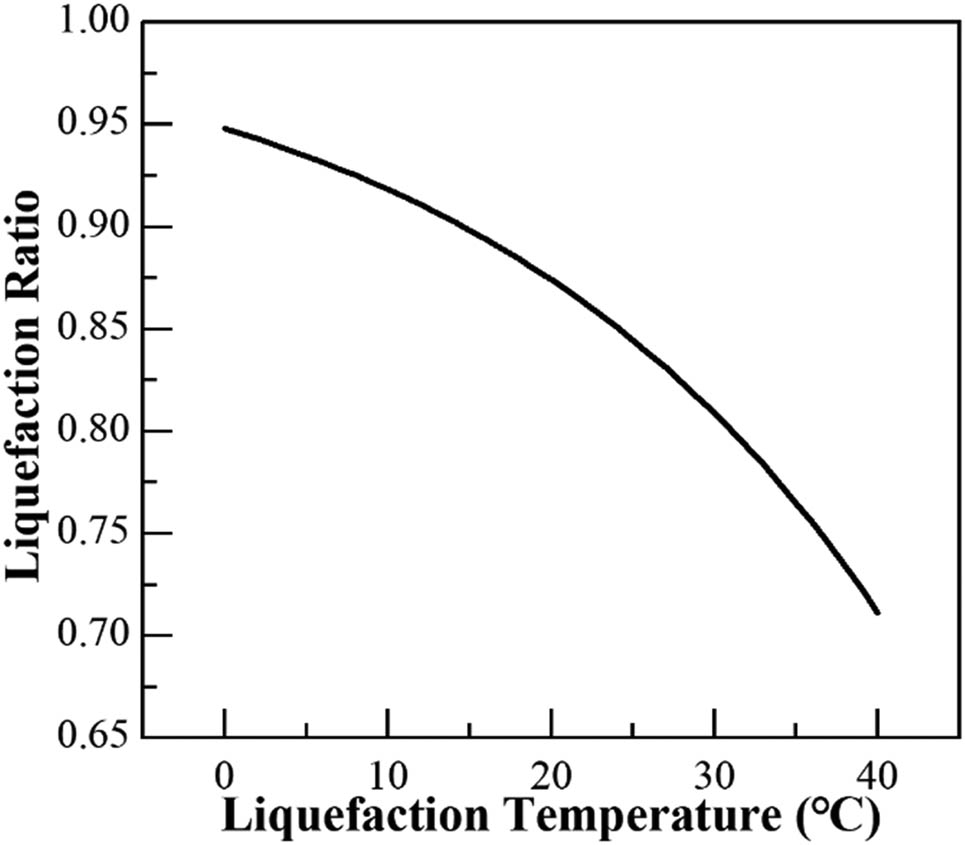
Relationship between the temperature and the liquefaction rate of CS2.
4.3.2 Effect of pressure on liquefaction rate of CS2
Figure 6 shows that the pressure considerably influences the liquefaction rate of CS2. At the set temperature, the liquefaction rate of CS2 increases with the increase in pressure. For pressures lower than 400 kPa, the liquefaction rate of CS2 increases rapidly with the increase in pressure; the rate decreases gradually when the pressure is higher than 600 kPa. In this study, the pressure of the entire cryogenic recovery unit is set to 450 kPa, and the liquefaction rate of CS2 in tail gas is approximately 91.78% using compression and cryogenic separation at 12°C.
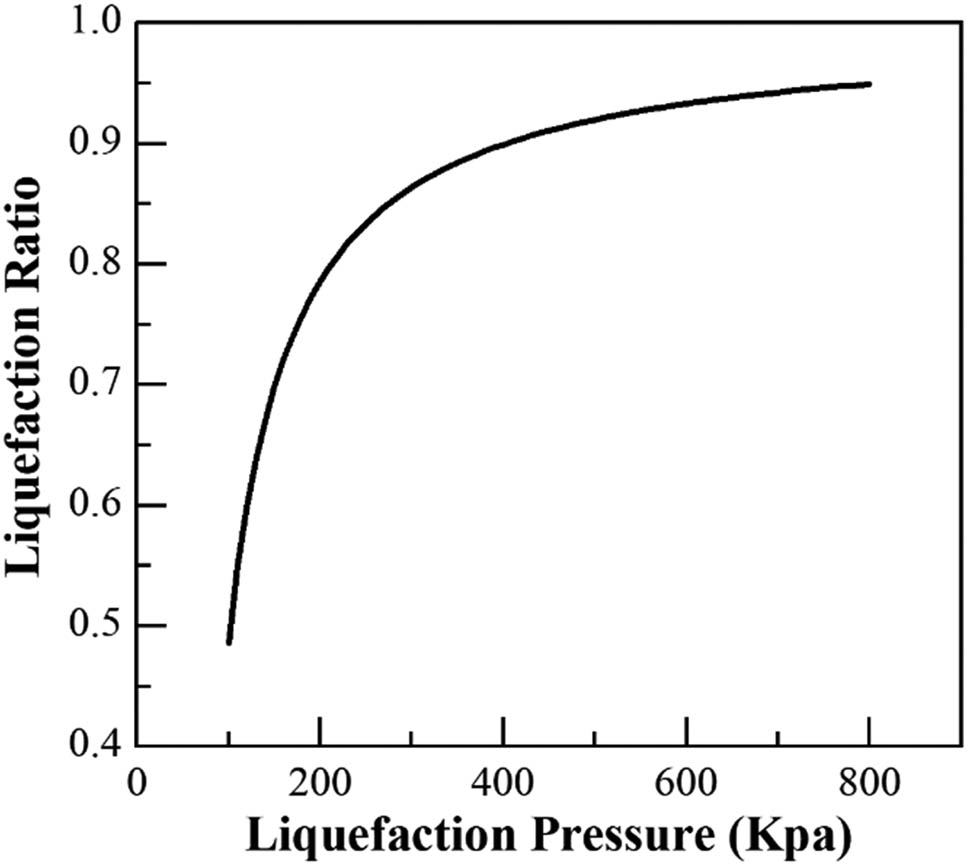
Relationship between the pressure and the liquefaction rate of CS2.
5 Green process cycle
Based on the Aspen Plus simulation of the IS production process, a continuous and green production process is developed. The by-product sulfur is completely recycled, as shown in Figure 7. The process mainly consists of reaction, extraction, compression, condensation, and other auxiliary systems. Liquid sulfur after high-temperature cracking and polymerization reaction forms IS. The extraction step uses CS2; relatively pure CS2 is obtained after separation. Subsequently, the product is obtained after washing and drying. The extracted common sulfur is recycled into the reaction system for participation in the next reaction cycle. After separation, most of the CS2 is in a liquefied form. The tail gas undergoes cryogenic compression, and liquefied CS2 is directed into the tank. Following the extraction step, supplementary N2 gas participates in the process cycle. The results of the simulation reduce the debugging interval and provide reference values for the actual operation parameters of IS production in Shandong Yanggu Huatai Chemical Co., Ltd., China.
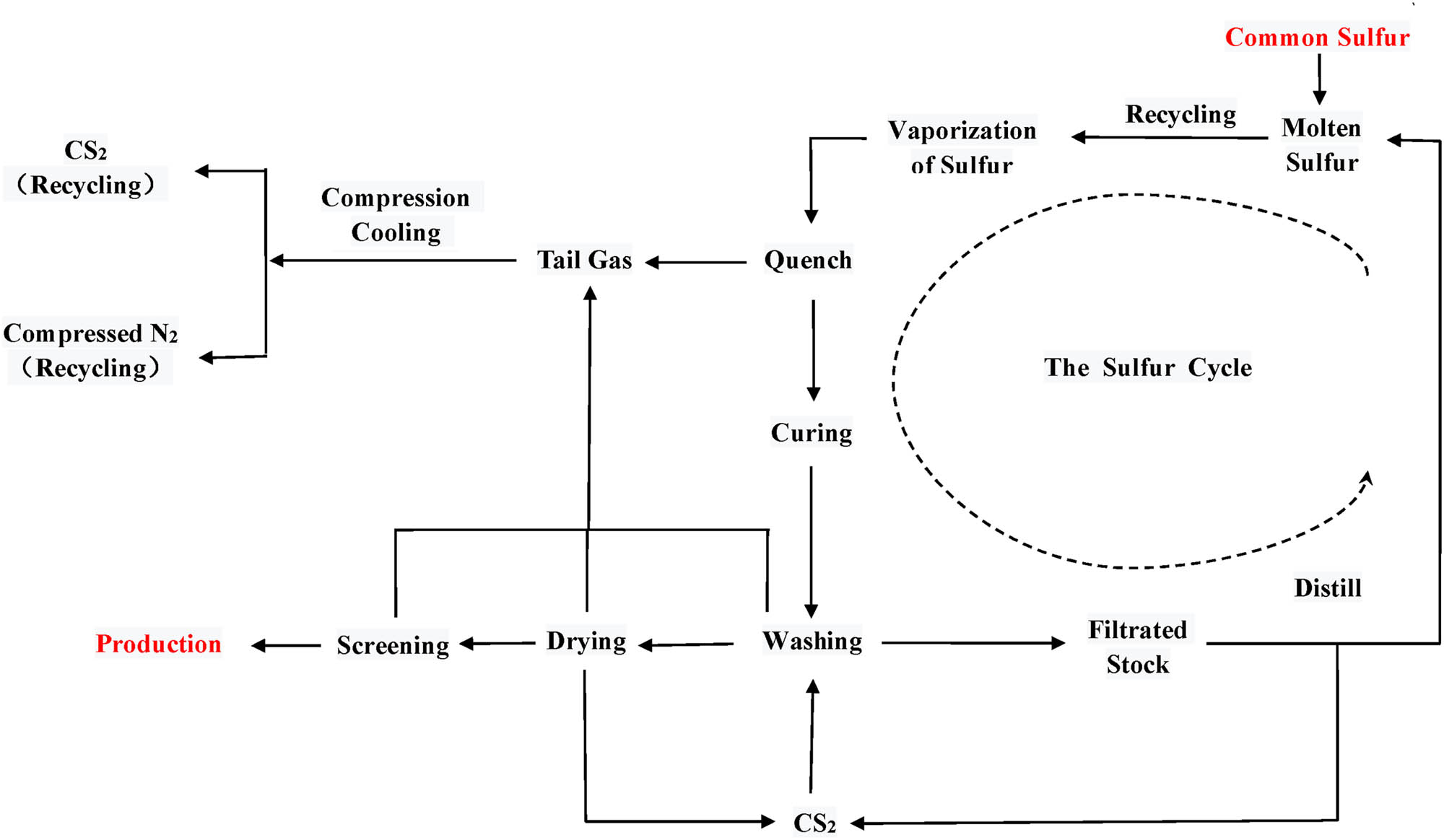
Diagram of IS production system process.
6 Conclusions
The actual process model of continuous production of IS is established using Aspen Plus. The simulation and optimization results provide a suitable reference for the actual operation. The model can reasonably predict the different working conditions of the IS production process.
The process of producing IS was studied by simulation with Aspen Plus software, from which the utilization rate of sulfur atoms was greatly improved from about 50% in the current intermittent method to more than 95%. Through the process of CS2 extraction of common sulfur, the extractant feed volume-to-mass ratio, temperature, and pressure analysis was optimized between the process modules by the control variable method, from the optimization results; it can be learned that the product with a mass of more than 95% can be obtained at a feed ratio of 150 for CS2/S8 and an extraction temperature of 30°C. By compressing and condensing the tail gas, the pressure of the deep cooling and compression device is controlled at 450–500 kPa, and the temperature is controlled at 8–10°C. The recovery efficiency of CS2 is greatly improved so that the liquefaction rate of CS2 reaches 91.78%, and the consumption of unit IS is greatly reduced through recycling. Moreover, while recovering CS2, compressed nitrogen can be obtained at the same time, which realizes the green recycling of tail gas.
Acknowledgments
The authors express their sincere appreciation to the Shandong Provincial Key Research and Development Program (Plan No. 2017CXGC1101 & Project No. 2019JZZY010513).
-
Funding information: The project was funded by the Shandong Provincial Key Research and Development Program (Plan No. 2017CXGC1101 and Project No. 2019JZZY010513).
-
Author contributions: Liang-liang Zhou: investigation, methodology, and writing – original draft; Xiao-lai Zhang: conceptualization, investigation, and supervision; Wen-bo Wang: data curation; Meng-cheng Du: formal analysis; De-long Ma: project administration.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: Available data are presented in the manuscript.
References
[1] Cataldo F. A study on the structure and properties of polymeric sulfur. Angew Makromol Chem. 1997;249:137–49. 10.1002/apmc.1997.052490109.Suche in Google Scholar
[2] Donohue J, Caron A. On structure of carbon disulfide-insoluble sulfur. J Polym Sci. 1961;50(153):S17–22. 10.1002/pol.1961.1205015333.Suche in Google Scholar
[3] Ouyang F, Hao T, Li Y, Yin C. Preparation of insoluble sulfur. Chem Ind Eng Pro (China). 2015;34(5):1389–394,1400.Suche in Google Scholar
[4] Terada N, Kouge K, Komaguchi K, Hayakawa S, Tsutsumi H. Thermal stability change of insoluble sulfur by a heat treatment and its mechanism study. Anal Sci. 2020;36(1):75–9.10.2116/analsci.19SAP05Suche in Google Scholar
[5] Wang C. Experimental study on the influencing factors of the stability of environmentally friendly insoluble sulphur. Fresenius Env Bull. 2020;29(9A):8600–9.Suche in Google Scholar
[6] Wang M, Guo Y, Shi P, Qi C, An H. The influence of heating mechanism for sulfur polymerization. Chin J Appl Chem. 2016;45(11):2190–2.Suche in Google Scholar
[7] Wang Y, Li F, Wang F, Pu Y, Zhao N, Xiao F. Effect of extractant on preparation of insoluble sulfur and optimization of operation conditions. Petrochem Technol (Beijing, China). 2017;46(8):1017–21.Suche in Google Scholar
[8] Mutlu H, Ceper EB, Li X, Yang J, Dong W, Ozmen MM, et al. Sulfur Chemistry in Polymer and Materials Science. Macromol Rapid Commun. 2019;40(1):1800650-1-1800650-51. 10.1002/marc.201800650.Suche in Google Scholar
[9] Jorg H, Christoph Z. Process for the production of polymeric sulfur: US, 2005143507; 2005-06-30.Suche in Google Scholar
[10] Wei L. Response surface methodology for optimizing production process parameters of polysulfur. Inorg Chem Ind. 2020;52(9):47–51.Suche in Google Scholar
[11] Zhang Y, Ji D, Ma S, Wang W, Dong R, Shi L, et al. Synthesis of 2, 2′-dibenzoylaminodiphenyl disulfide based on Aspen Plus simulation and the development of green synthesis processes. Green Process Synth. 2020;9(1):248–58. 10.1515/gps-2020-0026.Suche in Google Scholar
[12] Hou W, Su H, Hu Y, Chu J. Modeling simulation and optimization of a whole industrial catalytic naphtha reforming process on aspen plus platform. Chin J Chem Eng. 2006;14(5):584–91. 10.1016/s1004-9541(06)60119-5.Suche in Google Scholar
[13] Chung WJ, Griebel JJ, Kim ET, Yoon H, Simmonds AG, Ji HJ, et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat Chem. 2013;5(6):518–24.10.1038/nchem.1624Suche in Google Scholar PubMed
[14] Ma J, Zhao J, Wang R, Shen B. Theoretical study on structure of polymeric sulfur. Asian J Chem. 2015;27(12):4583–6.10.14233/ajchem.2015.19240Suche in Google Scholar
[15] Zhao J, Shu Y, Wang R, Shen B. A novel and easy way to improve the thermal stability of insoluble sulfur by curing process. Phosphorus Sulfur Silicon Relat Elem. 2017;192(4):431–6. 10.1080/10426507.2016.1247842.Suche in Google Scholar
[16] Ouyang F, Gu J, Zhang Y, Weng H. Study on new extractant for insoluble sulfur. Spec Petrochem (Tianjin, China). 2008; 25(3):7–12.Suche in Google Scholar
[17] Kansha Y, Kishimoto A, Nakagawa T, Tsutsumi A. A novel cryogenic air separation process based on self-heat recuperation. Sep Purif Technol. 2011;77(3):389–96. 10.1016/j.seppur.2011.01.012.Suche in Google Scholar
[18] Chen S. Comparison of nitrogen producing processes of pressure shift adsorption (PSA) and cryogenic separation. Mod Chem Ind. 2013;33(2):76–8.Suche in Google Scholar
[19] Ouyang FS, Gao P, Li B. Application of solubility parameter method in development of extractants for insoluble sulfur. J East China Univ Sci Technol Nat Sci Ed. 2010;36(1):25–30.Suche in Google Scholar
[20] Gursel IV, Hessel V, Wang Q, Noel T, Lang J. Window of opportunity – potential of increase in profitability using modular compact plants and micro-reactor based flow processing. Green Process Synth. 2012;1(4):315–36. 10.1515/gps-2012-0046.Suche in Google Scholar
[21] Agudelo Y, Barrera Zapata R. Use of advanced simulation software Aspen Plus as teaching tool in chemical reaction engineering. Rev Educ Ing. 2015;10(19):57–68.Suche in Google Scholar
[22] Ghasem N. Enhanced teaching and student learning through a simulator-based course in chemical unit operations design. Eur J Educ. 2016;41(4):455–67. 10.1080/03043797.2015.1095158.Suche in Google Scholar
[23] Tozzi PV, Wisniewski CM, Zalewski NJ, Savelski MJ, Slater CS, Richetti FA. Life cycle assessment of solvent extraction as a low-energy alternative to distillation for recovery of N-methyl-2-pyrrolidone from process waste. Green Process Synth. 2018;7(4):277–86. 10.1515/gps-2017-0030.Suche in Google Scholar
[24] Wang R, Shen B, Liu J, Zhao J. Optimization of insoluble sulfur extraction by response surface methodology. Chin J Appl Chem. 2018;47(7):1457–61.Suche in Google Scholar
[25] Ouyang FS, Bi YX, Li B, Sun Q, Weng HX. Effect of the molecular structures of extractants on separating insoluble sulfur. J East China Univ Sci Technol Nat Sci Ed. 2008;34(4):482–6.Suche in Google Scholar
© 2022 Liang-liang Zhou et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Artikel in diesem Heft
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal


