Abstract
This article experiments wetland-based biochar as an effective adsorbent for phosphorus removal. In this experiment, four common wetland plants, canna (C), umbrella palm (U), bamboo reed (B), and Thalia dealbata (T), were used as the main raw materials. Twelve kinds of biochar (C300, C500, C700, U300, U500, U700, B300, B500, B700, T300, T500, and T700) were obtained at three pyrolysis temperatures (300°C, 500°C, and 700°C). The results show that canna (C) and umbrella palm (U) are more suitable as raw materials for phosphorus removal using biochar. If bamboo reed (B) and Thalia dealbata (T) are used as raw materials for phosphorus removal using biochar, there is a greater risk of phosphorus release. With the increase of pyrolysis temperature (700°C > 500°C > 300°C), there is an increasing trend of phosphorus adsorption effect. The theoretical maximum adsorption capacity of C700, U700, and C500 was 39.24, 7.08, and 7.26 mg P·g−1 at an initial concentration of 50 mg·L−1 phosphorus, respectively. The theoretical adsorption capacity of C700 (Q max = 39.24 mg P·g−1) was much higher than that of the general modified adsorption materials. It also has a larger tolerance range to pH (3–11). The results of kinetic model fitting showed that the adsorption mechanism of C700, U700, and C500 on phosphorus can be better simulated by intra-particle diffusion and Elovich model, and the adsorption mechanism includes surface adsorption and intra-particle diffusion. The fitting of isothermal adsorption model showed that Langmuir–Freundlich equation is more suitable for the description of adsorption characteristics of C700, U700, and C500, and the fitting coefficient R 2 is 0.9928, 0.9949, and 0.9897, respectively. It indicates that the adsorption of phosphorus on C700, U700, and C500 has a balance of uniform and nonuniform surface, and monolayer and multilayer adsorption could occur. The results from this work demonstrated that the biochar obtained from canna at 700°C has good adsorption and phosphorus removal potential without modification, and it can be used as the preferred biochar for phosphorus removal of high concentration with large pH changes. In the final validation experiment, the phosphorus removal rate of C700 was up to 77.4% on the treatment of actual phosphorus containing wastewater.
1 Introduction
Eutrophication has become one of the most serious water environment problems in the world. According to Liebig’s least factor law, phosphorus is often considered as one of the main inducing factors of eutrophication in water bodies. It is generally considered internationally that the concentration of phosphorus exceeds 0.02 mg·L−1; that is, it meets the standard of eutrophication in water [1]. Therefore, phosphorus removal from water environment is an important concern. At present, some methods, such as constructed wetland, crystallization, adsorption, and other methods, are used to treat phosphorus wastewater [2]. In the adsorption method, the selection and preparation of adsorbent is the key adsorption technology for its successful application. At present, biomass adsorbent or its derivatives have attracted much attention due to their rich sources, with simple preparation and good adsorption effect [3]. The most common adsorbent is activated carbon, but the high cost limits its widespread use. Therefore, alternative sorbents are urgently needed. In recent years, biochar has attracted extensive attention due to its large specific surface area, multiphase pore structure, abundant surface functional groups, and abundant inherent minerals, which indicate that biochar can be used as an excellent adsorbent. Biochar is a carbon-rich product derived from the pyrolysis of waste biomass under anaerobic conditions [4,5]. As a multifunctional environment friendly material, it also has good adsorption characteristics and can effectively remove pollutants in wastewater [6]. At present, the raw materials for biochar preparation mainly focus on agricultural straw, wood chips, fruit shells, sludge, and livestock and poultry feces, but systematic studies on wetland plants are rare. In 2021, China took constructed wetlands as an important measure to improve the ecological environment of rivers and lakes [7]. With the increase of wetland scale, the yield of wetland plants also increases, and it will cause secondary pollution if not harvested in time. In today’s situation of increasingly prominent resources and energy problems, the utilization of wetland plant resources can not only avoid the secondary pollution caused by incineration and landfill, but also alleviate the situation of resource shortage. Study has shown that biochar was a kind of adsorbent with negative charge on the surface and has weak adsorption capacity for inorganic phosphate and other anions in water [8]. Besides, biochar itself contains phosphorus, which may have a tendency to release phosphorus in the process of use [9]. Previous studies have shown that adding unmodified biochar to wetland substrates can improve the removal efficiency of nitrogen and phosphorus. Studies have found that biochar generated from different plant bases at the same pyrolysis temperature contains different functional groups [10], which may result in different adsorption effects of different plant-based biochar on various target pollutants. Therefore, in this study, four common wetland plants in Southwest China were used as raw materials for biochar, and 12 kinds of biochar were obtained at three different pyrolysis temperatures, and to study the phosphorus removal ability of these biochars, providing another feasible way for eutrophication water purification and reuse of wetland plant resources.
2 Materials and methods
2.1 Raw material preparation
Macrophytes (canna, umbrella palm, bamboo reed, and Thalia dealbata) were collected from a wetland park in Guiyang, Guizhou Province, China. Impurities on macrophyte were washed off with distilled water, and the macrophyte was dried in an oven at 80°C. Later, the dried macrophyte was crushed and passed through a 100-mesh sieve.
2.2 Preparation of wetland plant-based biochar
The dried macrophyte powder was then placed in a quartz crucible, and then the crucible was put in a muffle furnace. The pyrolysis temperature was increased to target temperature (300°C, 500°C, and 700°C) at a heating rate of 5°C·min−1 and held for 2 h. After cooling to room temperature, the biochar was removed and was washed with distilled water until the elute was nearly neutral. Canna (C), umbrella palm (U), bamboo reed (B), and Thalia dealbata (T) were pyrolyzed at three temperatures (300°C, 500°C, and 700°C). A total of 12 kinds of biochar can be obtained: C300, C500, C700; U300, U500, U700; B300, B500, B700; T300, T500, and T700.
2.3 Adsorption experiments
2.3.1 Dynamic adsorption experiment
About 0.200 g of the biochar was accurately weighed and placed in the sample bottle. About 30 mL of KH2PO4 (potassium dihydrogen phosphate) solution (pH = 7) with an initial concentration of 50 mg·L−1 was added. Set a series of time points (5, 10, 15, 30, 60, 90, 120, 180, 240, 480, 780, 1,440, and 2,160 min) and repeat three times for each point. The sample bottles were placed in a thermostatic oscillator and oscillated at a speed of 160 rpm·min−1 at 28°C. After balancing, the liquid was filtered and the concentration of phosphorus was determined by molybdenum antimony anti-spectrophotometry. The adsorption capacity Q t (mg·g−1) of biochar at different times can be calculated by the following formula:
where Q t is the capacity for biochar adsorption on biochar at time t (mg⋅g−1); C 0 is the initial concentration of P, mg⋅L−1; C t is filtrate phosphorus concentration after adsorption, mg⋅L−1; V is the volume of solution, L; m is the mass of carbon, g. Pseudo-first-order, pseudo-second-order, intra-particle diffusion, and Elovich dynamic model equations were used for simulation.
Pseudo-first-order:
Pseudo-second-order:
Intra-particle diffusion:
Elovich dynamic model equations:
where q t is the adsorption capacity of adsorbent at t (mg·g−1); q e is the adsorption capacity of adsorbent at equilibrium time (mg·g−1); k 1 is adsorption rate constant of pseudo-first-order, mg·L−1·min−1; k 2 is adsorption rate constant of pseudo-second-order, mg·g−1·min−1. a 1 is the kinetic constant, mg·L−1; k 3 is the diffusion rate constant, mg·g−1·min−1. a 2 is the kinetic constant, mg·L−1; k 4 is the rate constant of Elovich model, mg·g−1·min−1.
2.3.2 Isothermal adsorption experiment
About 0.200 g of the sample was accurately weighed and placed in the sample bottle, and 30 mL of potassium dihydrogen phosphate solution with the initial concentration of 0, 1, 2, 5, 8, 10, 30, 50, 100, and 200 mg·L−1 was added, respectively, and repeated three times for each concentration. The sample bottle was placed in a thermostatic oscillator at a speed of 160 rpm·min−1 and oscillated at 28°C for 24 h. After balancing, the filtrate was filtered and the concentration of phosphorus was determined by molybdenum antimony anti-spectrophotometry:
where Q e is the adsorption capacity of biochar to be measured at equilibrium, mg·g−1; C 0 is the initial concentration of phosphorus, mg·L−1; C t is the concentration of filtrate phosphorus at adsorption equilibrium, mg·L−1; V is the volume of solution, L; m is the mass of carbon, g. Langmuir, Freundlich, and Langmuir–Freundlich isothermal adsorption equations were used to fit the experimental data, respectively.
Langmuir model (Eq. 7):
Freundlich model (Eq. 8):
Langmuir–Freundlich model (Eq. 9): based on the Langmuir equation, introducing the Freundlich equation in exponential form, where n is a parameter of inhomogeneity. When n is 1, the adsorbent surface is ideal, and the Langmuir–Freundlich equation becomes the similar Langmuir equation. When n < l, it means more uneven.
where q e is the adsorption quantity at adsorption equilibrium, mg·g−1; q m is the saturated adsorption capacity, mg·g−1; C e is the solution concentration at equilibrium, mg·L−1; K L is adsorption equilibrium constant, L·mg−1; K F is the constant of adsorption strength; n is a constant and is related to the adsorption system; K LF is the adsorption equilibrium constant, L·mg−1.
2.3.3 Influence of pH value
In the experiment, 0.5 mol·L−1 H2SO4 and NaOH solution were used to modulate the initial pH value of 50 mg·L−1 potassium dihydrogen phosphate solution, and the initial pH was set to 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, and 11.0. About 0.200 g biochar was accurately weighed into the sample bottle, and 30 mL of solutions with different initial pH values were added, and each pH point was repeated three times. The sample bottle was placed in a thermostatic oscillator at a speed of 160 rpm·min−1 and oscillated at 28°C for 24 h. After balancing, the filtrate was filtered and the phosphorus concentration was determined by molybdenum antimony anti-spectrophotometry. The adsorption capacity of biochar at equilibrium, Q t (mg·g−1), can be calculated from the following equation:
2.4 Data analysis
Excel 2007 was used for data analysis and data processing. Origin 9.0 was used for graph drawing and model fitting.
3 Results and discussion
3.1 Adsorption kinetics
Phosphorus adsorption kinetics of 12 wetland plant-based biochars are shown in Figure 1. The phosphorus adsorption capacity of the 12 biochars is significantly different. The adsorption capacity of phosphorus for C700 and U700 reached equilibrium basically at 750 min. When the adsorption time is 1,440 min, the phosphorus removal of C700, U700, and C500 biochars is the highest, reaching 98.6%, 20.5%, and 11.3%, respectively. It indicates that the different wetland plants as biochar raw materials using phosphorus adsorption are significantly different. Different pyrolysis temperatures also have great influence on the phosphorus adsorption capacity of biochar derived from the same wetland plant. When the adsorption time is 2,160 min, the adsorption capacity of C700 and U700 is positive, while for the other ten kinds of biochar it is negative, indicating that canna and umbrella palm of the four wetland plants are more suitable as biochar raw materials for phosphorus adsorption. In this experiment, canna showed a greater potential for phosphorus adsorption. The actual adsorption capacity is as high as 7.62 mg·g−1, which is significantly higher than the cotton stalk biochar loaded with iron oxide (0.963 mg P·g−1) [11] and ginkgo shell Fe/C composite (1.62 mg·g−1) [12]. Although the adsorption capacity of C700 on phosphorus is lower than that of nano-silver studied by Tuyen [13], Table 1 comprehensively shows that the adsorption capacity of C700 on phosphorus is greater than that of adsorbents prepared by many other materials.

Adsorption kinetics adsorption on 12 kinds of biochar.
Comparison of maximum phosphorus adsorption capacity of some adsorbents
| Adsorbents | T (℃) | pH | Adsorbent added (g·L−1) | q m (mg·g−1) |
|---|---|---|---|---|
| Magnetic nanoparticles | 25 | 3 | 1 | 9.72 [15] |
| Canna biochar | 28 | 3–11 | 6 | 7.62 (this study) |
| H2SO4-modified activated carbon | 35 | 3–10 | 4 | 7.26 [16] |
| ZnCl2-modified activated carbon | 35 | 6–10 | 6 | 5.1 [17] |
| Iron-supported water hyacinth biochar | 25 | 3–9 | 5 | 5.07 [18] |
| Modified attapulgite clay | 25 | 4–6 | 1 | 4 [19] |
| Alum sludge | 20 | 4–5 | 5–6 | 3.20 [20] |
| Orange peel magnetic biochar | 25 | — | 6.25 | 0.22–1.2 [21] |
In this experiment, pseudo-first-order, pseudo-second-order, intra-particle diffusion, and Elovich kinetic model are used to fit the experimental data, and the influence mechanism of 12 kinds of biochar on phosphorus adsorption is analyzed from the perspective of kinetics. The error between fitting results and experimental values is expressed by correlation coefficient R 2, and the larger R 2 value is, the closer the description of the adsorption process by the model. As shown in Table 2, it is found that the adsorption of phosphorus by C700, C300, and B300 accords with the characteristics of pseudo-first-order, with R 2 values of 0.889, 0.925, and 0.911, respectively, and the adsorption of phosphorus by 12 carbons did not accord with the pseudo-second-order kinetics simulation. The C700 and U700 biochars with the best adsorption effect were more consistent with the intra-particle diffusion and Elovich model, and their fitting correlation coefficients are 0.874, 0.861 and 0.947, 0.939, respectively. The Elovich fitting effect is the best, with R 2 > 0.9. This indicates that the adsorption process of phosphorus on C700 and U700 mainly includes surface adsorption, internal particle diffusion, and external liquid film diffusion [14]. In the Elovich equation, K 4 is an indicator of how fast the adsorption rate changes over time, and the larger its value is, the faster the adsorption rate decreases. As shown in Table 3, the adsorption rate of U700 (K 4 = 0.406) decreases over time than that of biochar C700 (K 4 = 0.804) slowly; that is, although the phosphorus adsorption capacity of U700 was lower than that of C700, however, U700 has better continuous phosphorus removal effect.
Pseudo first-order and pseudo second-order kinetic fitting parameters
| Pseudo first-order | Pseudo second-order | |||||
|---|---|---|---|---|---|---|
| k 1 (min−1) | q e (mg·g−1) | R 2 | k 2 (min−1) | q e (mg·g−1) | R 2 | |
| U300 | 0.127 | −3.00 | 0.793 | −1.049 | −2.78 | −1.43 × 104 |
| U500 | 0.057 | −1.47 | 0.676 | 3.49 × 104 | −1.26 | −6.656 |
| U700 | 0.002 | 1.71 | 0.889 | −2.546 | 0.5 | −5.979 |
| C300 | 0.269 | −8.75 | 0.925 | −2.925 | −8.52 | −1.001 |
| C500 | −1.119 | 1.41 | −2.342 | 9.35 × 104 | −1.15 | −4.381 |
| C700 | 0.06 | 5.98 | 0.383 | 2.05 × 104 | 5.23 | −1.913 |
| B300 | 0.092 | −2.36 | 0.911 | −2.429 | −2.12 | −1.609 |
| B500 | 0.002 | −0.93 | 0.756 | 2.78 × 104 | −0.41 | −2.02 × 104 |
| B700 | 3.053 | −0.73 | −5.773 | 3.39 × 104 | −0.73 | −8.418 |
| T300 | 0.465 | −3.59 | 0.063 | −7.749 | −3.56 | −6.292 |
| T500 | 0.289 | −1.79 | 0.445 | 6.73 × 104 | −1.75 | −1.91 × 104 |
| T700 | 0.372 | −2.49 | 0.252 | 1.34 × 104 | −2.46 | −4.6 |
Intra-particle diffusion and Elovich kinetic fitting parameters
| Intra-particle diffusion | Elovich | |||||
|---|---|---|---|---|---|---|
| a 1 | k 3 (min−1) | R 2 | a 2 | k 4 (min−1) | R 2 | |
| U300 | −2.394 | −0.024 | 0.437 | −1.678 | −0.232 | 0.727 |
| U500 | −0.904 | −0.022 | 0.686 | −0.353 | −0.191 | 0.909 |
| U700 | −0.312 | 0.051 | 0.874 | −1.290 | 0.406 | 0.947 |
| C300 | −8.168 | −0.022 | 0.253 | −7.388 | −0.238 | 0.534 |
| C500 | −1.931 | 0.049 | 0.781 | −2.767 | 0.339 | 0.694 |
| C700 | 3.534 | 0.106 | 0.861 | 1.610 | 0.804 | 0.939 |
| B300 | −1.808 | −0.019 | 0.290 | −1.133 | −0.207 | 0.595 |
| B500 | −0.06 | −0.021 | 0.835 | 0.336 | −0.156 | 0.791 |
| B700 | −0.936 | 0.012 | 0.500 | −1.174 | 0.092 | 0.490 |
| T300 | −3.284 | −0.017 | 0.511 | −2.969 | −0.124 | 0.479 |
| T500 | −1.632 | −0.007 | 0.469 | −1.46 | −0.062 | 0.565 |
| T700 | −2.448 | −8.264 | 0.003 | −2.297 | −0.034 | 0.101 |
3.2 Isothermal adsorption
The isothermal adsorption of phosphorus by 12 wetland plant-based biochars is shown in Figure 2. There is significant difference among these 12 biochars in adsorption capacity of phosphorus. C700, U700, and C500 have an adsorption effect on phosphorus, while the other nine kinds of biochar were negative on phosphorus removal. With the increase of phosphorus concentration, the adsorption capacity of C700, U700, and C500 increases gradually, indicating that this unmodified carbon was more suitable for high-concentration phosphorus removal. In addition, the adsorption capacity of C700 on phosphorus is significantly better than that of U700 and C500. Langmuir, Freundlich, and Langmuir–Freundlich equations are used to simulate the isothermal adsorption of phosphorus on 12 biochars.
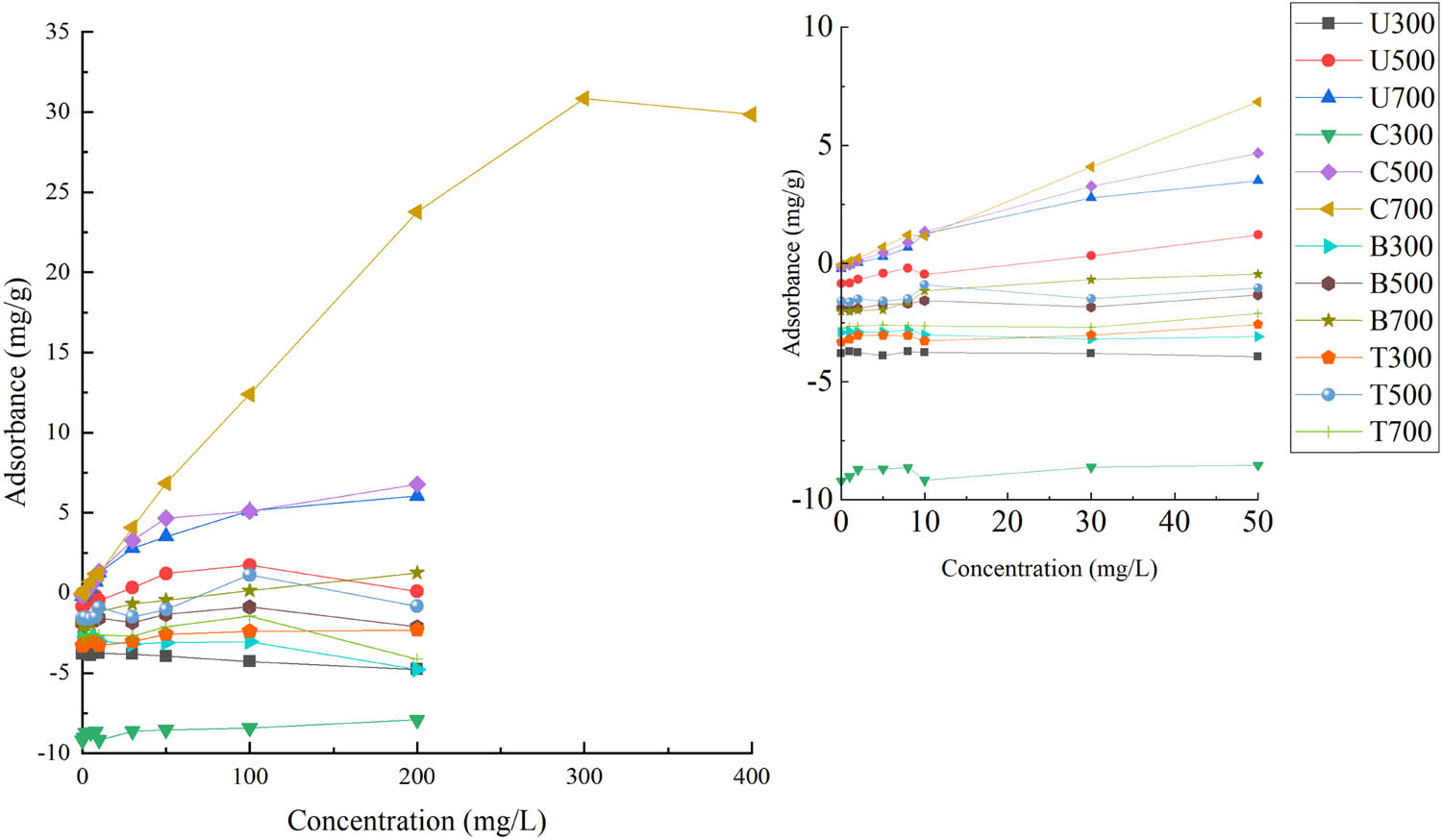
Isothermal adsorption on 12 kinds of biochar.
The best fitting process is determined by comparing R 2. The results showed (Table 4) that the adsorption of phosphorus by C700, U700, and C500 biochars is the combination of Langmuir and Freundlich models; that is, the Langmuir–Freundlich equation had the highest fitting degree for C700, U700, and C500 biochars, and R 2 is 0.9928, 0.9949, and 0.9897, which is described as equilibrium on uniform and nonuniform surfaces, applied to low and high adsorbent concentrations.
Isothermal adsorption fitting parameters
| Langmuir | Freundlich | Langmuir–Freundlich | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k L | q m | R 2 | k F | n | R 2 | k LF | q m | n | R 2 | |
| U300 | 7.433 | −4.08 | 1.917 | −3.55 | 0.04 | −14.037 | 0.001 | −245.60 | 0.04 | −14.037 |
| U500 | 0.0194 | 1.159 | 0.6023 | 0.03 | 0.61 | 0.214 | −2.478 | 0.82 | 4.18 | 0.444 |
| U700 | 0.0159 | 8.074 | 0.9917 | 0.36 | 0.54 | 0.955 | 0.0103 | 7.08 | 1.19 | 0.9949 |
| C300 | −16.776 | −8.522 | −63.69 | −9.04 | −0.01 | −63.445 | −1.659 | −3.59 | 0.01 | −63.45 |
| C500 | 0.0199 | 8.354 | 0.9865 | 0.467 | 0.51 | 0.946 | 0.0119 | 7.26 | 1.23 | 0.9897 |
| C700 | −5.511 | 10.10 | 0.0590 | 0.499 | 0.703 | 0.973 | 3.6191 | 39.24 | 0.02 | 0.9928 |
| B300 | 0.0015 | −3.179 | −2.7808 | −2.56 | 0.07 | −2.321 | −5.843 | −3.261 | 77.0 | −3.117 |
| B500 | −4.675 | −1.581 | −2.902 | −1.88 | −0.04 | −2.920 | −3.985 | −1.419 | 0.44 | −2.883 |
| B700 | 0.000 | −0.947 | −0.273 | 6.68 × 10−1 | 4.02 | −0.856 | −1.028 | −0.065 | 0.019 | 0.1704 |
| T300 | −6.248 | −2.772 | −8.786 | −3.33 | −0.05 | −8.410 | −1.081 | −0.250 | 0.005 | −8.438 |
| T500 | 1.87 × 104 | −1.044 | −0.359 | −1.88 | −0.24 | −0.035 | −4.121 | −1.570 | 273.49 | −0.313 |
| T700 | −49.812 | −2.612 | −1.905 | −2.51 | 0.01 | −1.895 | −44.29 | −2.614 | 1.542 | −1.905 |
The theoretical maximum adsorption capacity of C700, U700, and C500 is 39.24, 7.08, and 7.26 mg P·g−1, respectively. These theoretical adsorption amounts are close to the real adsorption amounts (Figure 2), which also indicates that the Langmuir–Freundlich equation is suitable for simulating the phosphorus adsorption process of these three carbons. The theoretical adsorption capacity of C700 on phosphorus (Q max = 39.24 mg P·g−1) is much higher than that of previous studies. For example, sesame stalk biochar was modified with ZnCl2 and MgO (8.67–9.68 mg P·g−1) [22], Orange peel biochar was modified with Fe2O3 (0.22–1.24 mg P·g−1) [23], and cotton straw biochar was modified with Fe2O3 (0.96 mg P·g−1) [11]. Therefore, canna, a kind of wetland plant, is an excellent biochar adsorbent raw material for phosphorous removal.
3.3 pH effect
As can be seen from Figure 3, C700, U700, C500, and U500 have adsorption effect on phosphorus when pH is in the range of 3–11, while the removal rates of the other eight kinds of biochar are all negative, showing no effect on phosphorus removal. pH has different effects on phosphorus adsorption by different biochars. U500 showed a rising trend with the increase of pH, indicating that alkaline environment can promote absorption of phosphorus of U500. U700 decreased with the increase of pH, indicating that U700 is favorable for phosphorus adsorption in acidic environment. The influence of pH on C700 is small. When pH = 7, the adsorption capacity of the biochar to phosphorus is slightly lower; however, the adsorption capacity of C700 is up to 7.0 mg·g−1. It showed that C700 can be used to treat high-concentration phosphorus of industrial wastewater with a wide range of pH tolerance.

Effect of pH on adsorption capacity of 12 kinds of biochar.
3.4 Effect of biochar on removal of actual phosphorus containing wastewater
In order to verify the performance of the biochar (C700) with the best phosphorus removal effect screened in the batch experiment in practical application, we used C700 as the adsorbent to remove phosphorus in the actual wastewater. After 24 h of oscillation, the total phosphorus reduction amount in the wastewater reached 126.3 mg·L−1, with an average removal rate of 77.4% (Table 5).
Effect of biochar (C700) on the treatment of real wastewater containing phosphorus
| C700 | |
|---|---|
| Initial phosphorus concentration (mg·L−1) | 163.2 |
| Post-treatment phosphorus concentration (mg·L−1) | 36.9 ± 3.3 |
| Removal rate of phosphorus (%) | 77.4 ± 2.0 |
4 Conclusion
The adsorption kinetics model fitting results showed that the adsorption mechanism of C700 and U700 on phosphorus can be better simulated by intra-particle diffusion and Elovich model. The adsorption mechanism includes surface adsorption and intra-particle diffusion. The Langmuir–Freundlich equation is more suitable to describe the adsorption characteristics of C700 and U700, which indicated that the adsorption of C700 and U700 has a balance of uniform and nonuniform surface, and both single and multilayer adsorption can occur.
In the range of pH 3–11, C700, U700, C500, and U500 have adsorption effect on phosphorus, and pH has different effects on them. Therefore, in the practical application of biochar, more attention should be paid to the pH of the target wastewater, and biochar with a wide range of pH tolerance should be selected. The C700 in this experiment has a wide range of pH adaptation, which can be used as the preferred biochar for the treatment of high-concentration phosphorous wastewater with a wide range of pH variation.
It is found that the adsorption and desorption capacity of the biochar produced by different wetland plant-based biochar and produced by the same plants at different pyrolysis temperatures are significantly different. From the perspective of plant species, the adsorption and desorption capacities of the four wetland plant-based biochar, canna (C), umbrella palm (U), bamboo reed (B), and Thalia dealbata (T) show significant differences. Bamboo reed and Thalia dealbata did not have the ability to adsorb phosphorus, indicating that if unmodified biochar of B and T were directly used for phosphorus removal, there would be a greater risk of exceeding the standard of phosphorus due to phosphorus desorption. C and U are more suitable as the raw material for biochar using phosphorus adsorption. In terms of pyrolysis temperature, taking C, U as an example, it shows that with the increase of pyrolysis temperature (700°C > 500°C > 300°C), the increasing trend of phosphorus adsorption effect. The results showed that the biochar obtained under 700°C condition is more conducive to phosphorus adsorption. When the pyrolysis temperature of canna is 700°C, the biochar C700 has the best phosphorus removal capacity, and the maximum theoretical adsorption capacity is up to 39.24 mg P·g−1, which is higher than the maximum theoretical adsorption capacity of many modified biochar. In addition, the phosphorus removal rate of C700 was up to 77.4% through a validation experiment on the treatment of actual phosphorus containing wastewater. Therefore, it can be used as an alternative adsorbent with greater adsorption potential.
Acknowledgment
The authors thank all reviewers for their valuable comments, which greatly helped in improving the quality of the final manuscript.
-
Funding information: This research received Guizhou Provincial Support Program for Top Talents in Science and Technology in Colleges (QIANJIAOHEKY[2016]097), Guizhou Provincial Science and Technology Plan Project (QIANJIAOHELHZI[2016]7283), and Anshun College Ph.D. Fund Project (asubsjj 2016) No. 07.
-
Author contributions: Qiaoling Xu: writing – original draft; Li Wang, Minxia Tan, Xiaolei Wang, Jiajie Li, Hejun Geng: methodology, data curation.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article. These datasets are also available from the corresponding author on reasonable request.
References
[1] Lin Y, He Z, Yang Y. Nitrogen versus phosphorus limitation of phytoplankton growth in Ten Mile Creek, Florida, USA. Hydrobiologia. 2008;605(1):247–58.10.1007/s10750-008-9360-xSearch in Google Scholar
[2] Yadav D, Kapur M, Kumar P. Adsorptive removal of phosphate from aqueous solution using rice husk and fruit juice residue. Process Saf Environ Prot. 2015;94:402–9.10.1016/j.psep.2014.09.005Search in Google Scholar
[3] Mei YL, Xu J, Zhang Y, Li B, Fan SS, Xu HC. Effect of Fe–N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution. Bioresour Technol. 2021;325:124732.10.1016/j.biortech.2021.124732Search in Google Scholar
[4] Gupta P, Ann TW, Lee SM. Use of biochar to enhance constructed wetland performance in wastewater reclamation. Environ Eng Res. 2015;21.10.4491/eer.2015.067Search in Google Scholar
[5] Hou J, Huang L, Yang ZM, Zhao YQ, Deng CR, Chen YC, et al. Adsorption of ammonium on biochar prepared from giant reed. Environ Sci Pollut Res. 2016;23(19):1–9.10.1007/s11356-016-7084-4Search in Google Scholar
[6] Deng C, Huang L, Liang Y, Xiang H, Jiang J, Wang Q, et al. Response of microbes to biochar strengthen nitrogen removal in subsurface of constructed wetlands: microbial community structure and metabolite characteristics. Sci Total Environ. 2019;694:133687.10.1016/j.scitotenv.2019.133687Search in Google Scholar
[7] Ministry of Ecology and Environment. Technical guide for water purification of constructed wetland; 2021.Search in Google Scholar
[8] Micháleková-Richveisová B, Frišták V, Pipíška M, Ďuriška L, Moreno-Jimenez E, Soja G. Iron-impregnated biochars as effective phosphate sorption materials. Env Sci Pollut Res Int. 2017;24(1):463–75.10.1007/s11356-016-7820-9Search in Google Scholar
[9] Wang YJ, Wang RF, Wang WF, Sheng Y, Liu AZ. Comparation of phosphorus removal effect of biochar and concrete slagin constructed wetland. Chin J Environ Eng. 2021;15(1):136–42.Search in Google Scholar
[10] Cui XQ. Phosphorus removal from aqueous solution using wetland-based 2biochar: Batch experiment. ZhengJiang: Zhejiang University; 2018.Search in Google Scholar
[11] Ren J, Li N, Li L, An JK, Zhao L, Ren NQ. Granulation and ferric oxides loading enable biochar derived from cotton stalk to remove phosphate from water. Bioresour Technol. 2015;178:119–25.10.1016/j.biortech.2014.09.071Search in Google Scholar
[12] Liu J, Zhu ZQ, Zhu YN, Yan QM, He H, Zhang LH, et al. Adsorption characteristics of phosphate in water by the porous biomorph-genetic composite of Fe/C with Ginkgo shell template. Res Environ Sci. 2019;32(7):1239–49.Search in Google Scholar
[13] Mohan S, Nair VV. Phosphorus removal from aqueous solution by adsorption using silver nanoparticles: batch experiment. J Hazardous Toxic Radioactive Waste. 2020;24(4):4020031–8.10.1061/(ASCE)HZ.2153-5515.0000520Search in Google Scholar
[14] Jiang YH, Liu JX. Experimental study on phosphorus removal by fly ash brickbat particle. Chin J Environ Eng. 2011;5(7):1532–7.Search in Google Scholar
[15] Daou TJ, Begin-Colin S, Greneehe JM. Phosphate adsorption properties of magnetite-based nanoparticles. Chem Mater. 2007;19(18):4494–505.10.1021/cm071046vSearch in Google Scholar
[16] Kumar P, Sudha S, Chand S. Phosphate removal from aqueous solution using coir-pith activated carbon. Sep Sci Technol. 2010;45(10):1463–70.10.1080/01496395.2010.485604Search in Google Scholar
[17] Namasivayam C, Sangeetha D. Equilibrium and kinetic studies of adsorption of phosphate onto ZnCl2 activated coir pith carbon. J Colloid Interface Sci. 2004;280(2):359–65.10.1016/j.jcis.2004.08.015Search in Google Scholar PubMed
[18] Cai R. Capture and reuse of phosphorus in eutrophic water by iron-impregnated biochar. Hunan: Hunan Normal University; 2017.Search in Google Scholar
[19] Ye H, Chen F, Sheng Y. Adsorption of phosphate from aqueous solution onto modified palygorskites. Sep Purif Technol. 2006;50(3):283–90.10.1016/j.seppur.2005.12.004Search in Google Scholar
[20] Jiang H, Liao LB, Wang SP. Phosphate and fluoride adsorption on to hydroxy-Fe-montmorillonite complex. Geochimica. 2003;32(6):573–81.Search in Google Scholar
[21] Chen B, Chen Z. Sorpfion of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere. 2009;76(1):127–33.10.1016/j.chemosphere.2009.02.004Search in Google Scholar PubMed
[22] Chen B, Chen Z, Lv S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour Technol. 2011;102(2):716–23.10.1016/j.biortech.2010.08.067Search in Google Scholar PubMed
[23] Park JH, Ok YS, Kim SH, Cho JS, Heo JS, Delaune RD, et al. Evaluation of phosphorus adsorption capacity of sesame straw biochar on aqueous solution: influence of activation methods and pyrolysis temperatures. Environ Geochem Health. 2015;37(6):969–83.10.1007/s10653-015-9709-9Search in Google Scholar PubMed
© 2022 QiaoLing Xu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

