Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
-
Qiuyun Zhang
, Binbin Yang
Abstract
In the present study, a facile solvothermal method was used for the synthesis of silicotungstic acid (HSiW) immobilized on Ce-based metal organic framework (Ce-BDC) and embedded in Zr-based metal-organic framework (UiO-66(Zr)) composite catalyst, namely, Ce-BDC@HSiW@UiO-66 for the production of biodiesel through green fatty acid esterification. The obtained hybrids were characterized by various characterization technologies, including Fourier transform infrared, X-ray diffraction, scanning electron microscopy, transmission electron microscopy, N2 physisorption, X-ray photoelectron spectroscopy, and temperature-programmed desorption of NH3 (NH3-TPD) analysis. The characterization analyses showed that the hybrids have been successfully synthesized. Also, the volume and pore size of UiO-66(Zr) were changed by introducing HSiW@Ce-BDC, and the resulting Ce-BDC@HSiW@UiO-66 possessed the mesoporous structure and relatively high surface area. Simultaneously, the NH3-TPD analysis of Ce-BDC@HSiW@UiO-66 reveals that the acid strength was increased in comparison with HSiW@Ce-BDC. In addition, the composite Ce-BDC@HSiW@UiO-66 demonstrated high catalytic activity, and the oleic acid esterification gave 81.5% conversion at optimum conditions of 0.2 g catalysts, 1:30 oleic acid to methanol molar ratio at 130°C for 4 h. More interestingly, after six recycling cycles, the reduction in the conversion rate was only 4.6%, indicating that Ce-BDC@HSiW@UiO-66 has excellent reusability. Our study provides an effective approach to synthesize multifunctional hybrids for green biofuel production.
1 Introduction
Recently, with the rapid growth of world population and the development of industrialization, the growing consumption of energy, especially fossil fuels, is increasing [1]. However, the depletion and the widespread use of fossil fuels bring a series of environmental pollution which has gotten much attention [2]. In the views of the above concerns, it is urged to develop the alternative efficient green fuels. Among the alternative fuels, biodiesel is considered to be a potential substitute fuel for its biodegradable, nontoxic, higher cetane index, and sulfur free properties [3]. Generally, biodiesel is able to be produced from fatty acid, various edible oils, and non-edible oils with small-chain alcohol via esterification/transesterification over acid/base catalyst [4].
The production of biodiesel by esterification process is an interesting choice via using low-cost feedstock with high fatty acid contents, which may be the best solution for cost reduction [5]. Currently, liquid inorganic acids (sulfuric acid, hydrochloric acid, phosphoric acid, etc.) are usually preferred as they present a high-level conversion rate of fatty acids in a shorter reaction time [6]. However, liquid acid catalysis suffers from the severe corrosion of the vessel with large amount of wastewater from neutralization steps, and the impossibility of reuse [7]. In contrast, many efforts have been devoted to study the different types of solid acid catalysts, such as heteropolyacids [8,9], zeolites [10], metal oxides [11], ionic liquid [12,13], and carbon-based acid catalyst [14], because it can reduce the problem of the reactor’s corrosion, and it can be easily separated and reused [15]. Heteropolyacids are certainly regarded as the most famous solid acid catalysts and catalyze a wide variety of reactions due to their stronger Brønsted acids and thermal stability [16]. Unfortunately, heteropolyacids have a low surface area (<10 m2·g−1) and can be easily soluble in polar solvent that lead to obtain a homogeneous catalysis [17]. Then, heteropolyacids may require an appropriate support with high surface area and chemical stability for corresponding impregnation or anchoring [18,19].
Lately, metal-organic framework (MOF) has the desirable structure and excellent properties, such as adjustable structure, high porosity, and high accessible surface area, that can be used as catalysts or extended by encapsulation of other functional species within MOF [20,21]. Up to now, UiO-66(Zr), Cu-BTC, MIL-100(Fe), and Ni-BDC have widespread and versatile applications as supported [22,23,24].
In this work, we prepared a novel Ce-BDC@HSiW@UiO-66 composite synthesized by the simple solvothermal method. In the preparation process, the synthesized Ce-BDC acted as the supporting material, and the silicotungstic acid was immobilized on Ce-BDC to form HSiW@Ce-BDC. Afterwards, the HSiW@Ce-BDC was embedded in UiO-66(Zr), forming the Ce-BDC@HSiW@UiO-66 hybrids. The physical and structural properties of the obtained hybrids were characterized by using Fourier transform infrared (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), N2 physisorption, X-ray photoelectron spectroscopy (XPS), and temperature-programmed desorption of NH3 (NH3-TPD). Meanwhile, the hybrids were used for esterification of green fatty acid. Furthermore, the recycling and leaching test were applied to study the stability and heterogeneous nature of the synthesized hybrids. This work presents an effective approach to obtain the multifunctional hybrids for green biofuel production.
2 Experimental methods
2.1 Chemicals and reagents
The chemicals were procured from the following sources: Absolute ethanol, oleic acid, stearic acid (98%), anhydrous methanol, and N,N-dimethylformamide (DMF) are obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Zirconium (IV) chloride (ZrCl4), cerium nitrate hexahydrate (Ce(NO3)3·6H2O), benzene-1,4-dicarboxylic acid (H2BDC), silicotungstic acid (HSiW, H4SiW12O40·nH2O), lauric acid (98%), methanol (>99%), myristic acid (98%), and palmitic acid (98%) were obtained from Shanghai Aladdin Industrial Inc. (Shanghai, China). All chemicals were of analytical grade and used without further purification, unless otherwise noted.
2.2 Catalyst preparation and characterization
The silicotungstic acid impregnation on Ce-BDC (denoted as HSiW@Ce-BDC) and embedded in UiO-66(Zr) was synthesized by solvothermal technique. Initially, Ce(NO3)3·6H2O (0.87 g) was dissolved in 40 mL of DMF and then commercial silicotungstic acid (0.8 g) and terephthalic acid (0.5 g) were added into the above solution under magnetic stirring for 1 h, and placed on an oil bath at 150°C and allowed to stir for 3 h. The product was then washed two times with DMF and deionized water to obtain the HSiW@Ce-BDC sample. After that, the HSiW@Ce-BDC was added to 30 mL of DMF, and ultrasonic reaction was carried out for 30 min, followed by the addition of ZrCl4 (0.51 g) and terephthalic acid (0.33 g), and the mixture was slowly stirred for 1 h and then placed in a 50 mL Teflon-lined stainless-steel autoclave at 120°C for 6 h. Finally, the attained product was centrifuged and washed with DMF and deionized water two times, dried at 80°C for 12 h in an oven. This sample was coded as Ce-BDC@HSiW@UiO-66. For comparison, the Ce-BDC and UiO-66(Zr) samples were also prepared following the same method.
FTIR spectra were analyzed on a PerkinElmer spectrum100 using KBr pellet technology (4,000–400 cm−1). XRD spectra was performed using D8 ADVANCE (Germany) with CuKĮ (1.5406 Å) radiation. Nitrogen adsorption–desorption isotherms were examined using a Quantachrome Quadrasorb EVO apparatus (Quantachrome Instruments, Boynton Beach, USA). XPS (Thermo ESCALAB 250XI) was used to measure the elemental compositions of the sample. The morphological features of the synthesized samples were observed by SEM (Hitachi S4800) and TEM (FEI Tecnai G2 20). The NH3-TPD (Micromeritics, AutoChem II 2920 instrument) was performed to measure the acid value of the sample.
2.3 Esterification experiments
Esterification experiments on conversion of fatty acid was carried out by a 50 mL high-pressure autoclave reactor, using the Ce-BDC@HSiW@UiO-66 catalyst. Initially, calculated amount of Ce-BDC@HSiW@UiO-66 catalyst was fed into the autoclave reactor and the reactants (fatty acid and methanol) were added. Then, the reactor was allowed to stir for an appropriate time via magnetically operated stirrer at 120–140°C on an oil bath. After the accomplishment of the esterification process, the catalyst was collected by centrifugation, and the residual methanol and by-products of water were rotary evaporated under reduced pressure. The acid value of the resulting product was measured according to the method described in ISO 660-2009 standard, and the fatty acid conversion was estimated as the reduction in acid value before and after the reaction [25].
3 Results and discussion
3.1 Characterization of the hybrids
The FTIR spectra of HSiW, Ce-BDC, and UiO-66(Zr) along with Ce-BDC@HSiW@UiO-66 are shown in Figure 1. For HSiW sample, the four peaks were observed at 980, 927, 884, and 804 cm−1, which corresponded to Keggin ion structure of HSiW [26]. In the infrared spectra of Ce-BDC, UiO-66(Zr) and Ce-BDC@HSiW@UiO-66 samples, the absorption bands attributed to the asymmetric and symmetric stretching of carboxyl (O═C–O) are observed at 1,583 and 1,397 cm−1, and the obvious bonds at 1,000–1,200 cm−1 are representing the stretching vibration of C–O [27,28]. Simultaneously, the absence of peak at 745 cm−1 was believed to be the O–H vibrations in the H2BDC ligand. These results indicate the presence of the organic ligands terephthalic acid in the synthesized samples. Notably, the obvious bonds in 553 cm−1 attributed to Zr–(OC) asymmetric stretch found in the UiO-66(Zr) and Ce-BDC@HSiW@UiO-66 samples [29]. Moreover, the bands’ absorption at 450–550 cm−1 were attributed to Ce–O vibration [30], indicating the existence of the bonds of Zr–O and Ce–O in the Ce-BDC@HSiW@UiO-66 sample. However, the intensity of the characteristic peaks of Ce-BDC@HSiW@UiO-66 showed to be weaker, which was caused likely by the interaction between HSiW, Ce-BDC, and UiO-66(Zr). Surprisingly, the peaks at 980 and 804 cm−1 are observed from Ce-BDC@HSiW@UiO-66 sample, which indicates that HSiW was embedded within the Ce-BDC and UiO-66(Zr) and the synthesis of the hybrids was successful.
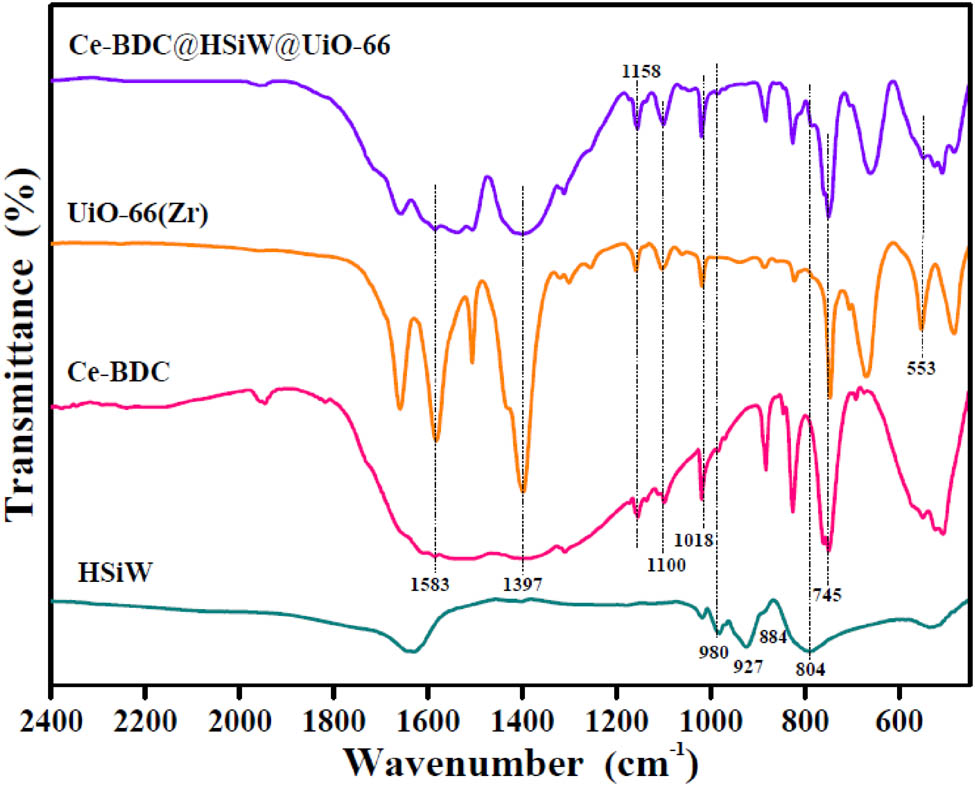
FTIR spectra of HSiW, Ce-BDC, UiO-66(Zr), and Ce-BDC@HSiW@UiO-66 hybrids.
The XRD diffraction patterns of the HSiW, Ce-BDC, HSiW@Ce-BDC, and UiO-66(Zr) along with Ce-BDC@HSiW@UiO-66 samples are depicted in Figure 2. Ce-BDC exhibits several major characteristic peaks at 2θ = 9.1°, 14.5°, 15.3°, 18.1°, 28.1°, 29.1°, and 30.0° [31], and similar patterns were observed for HSiW@Ce-BDC and Ce-BDC@HSiW@UiO-66 samples, indicating that the Ce-BDC is kept almost intact in the synthesis process. Additionally, the major characteristic peaks of HSiW could not be found from the XRD patterns of HSiW@Ce-BDC, which proves that HSiW is uniformly dispersed on the Ce-BDC, similar observations have been reported by Wang et al. [32]. When HSiW@Ce-BDC is embedded in UiO-66(Zr), some diffraction peaks of UiO-66(Zr) partially disappeared from Ce-BDC@HSiW@UiO-66 sample, and the intensity of the characteristic diffraction peaks of HSiW@Ce-BDC in Ce-BDC@HSiW@UiO-66 was weakened. This may be related to UiO-66(Zr)-encapsulated HSiW@Ce-BDC samples and the existence of the strong interaction between HSiW@Ce-BDC and UiO-66(Zr), which was also evident in the FTIR results.

XRD patterns of HSiW, Ce-BDC, HSiW@Ce-BDC, UiO-66(Zr), and Ce-BDC@HSiW@UiO-66 hybrids.
The surface morphology of HSiW, Ce-BDC, HSiW@Ce-BDC, UiO-66(Zr), and Ce-BDC@HSiW@UiO-66 hybrids were investigated through SEM imaging. As reported in Figure 3, the HSiW sample (Figure 3a) presents irregular sizes of massive structure, and Ce-BDC (Figure 3b) presents collectively packed irregular block-like particles. After the introduction of HSiW, the shape of HSiW@Ce-BDC was similar to those of Ce-BDC as shown in the SEM image in Figure 3c, but the irregular sizes became smaller, suggesting HSiW has successfully incorporated into Ce-BDC and the framework structure of Ce-BDC has good stability. UiO-66(Zr) sample (Figure 3d) exhibited relatively regular sizes of near-cubic particles. Obviously, the addition of HSiW@Ce-BDC provoked significant morphological changes in the structure of UiO-66(Zr) due to its electrostatic interaction with HSiW@Ce-BDC. Simultaneously, the Ce-BDC@HSiW@UiO-66 sample (Figure 3e) consists of blocky-shaped agglomerates with varying sizes, and the aggregated blocky-shaped particles have also formed many pores, suggesting that the porous structure is favorable to catalyze esterification reaction. The TEM characterization of Ce-BDC@HSiW@UiO-66 sample is also taken. As provided in Figure 4, Ce-BDC@HSiW@UiO-66 presents aggregated blocky-shaped particles, and its size is not uniform. Moreover, lots of mesopores are also clearly visible from the TEM images, which is consistent with SEM analysis.
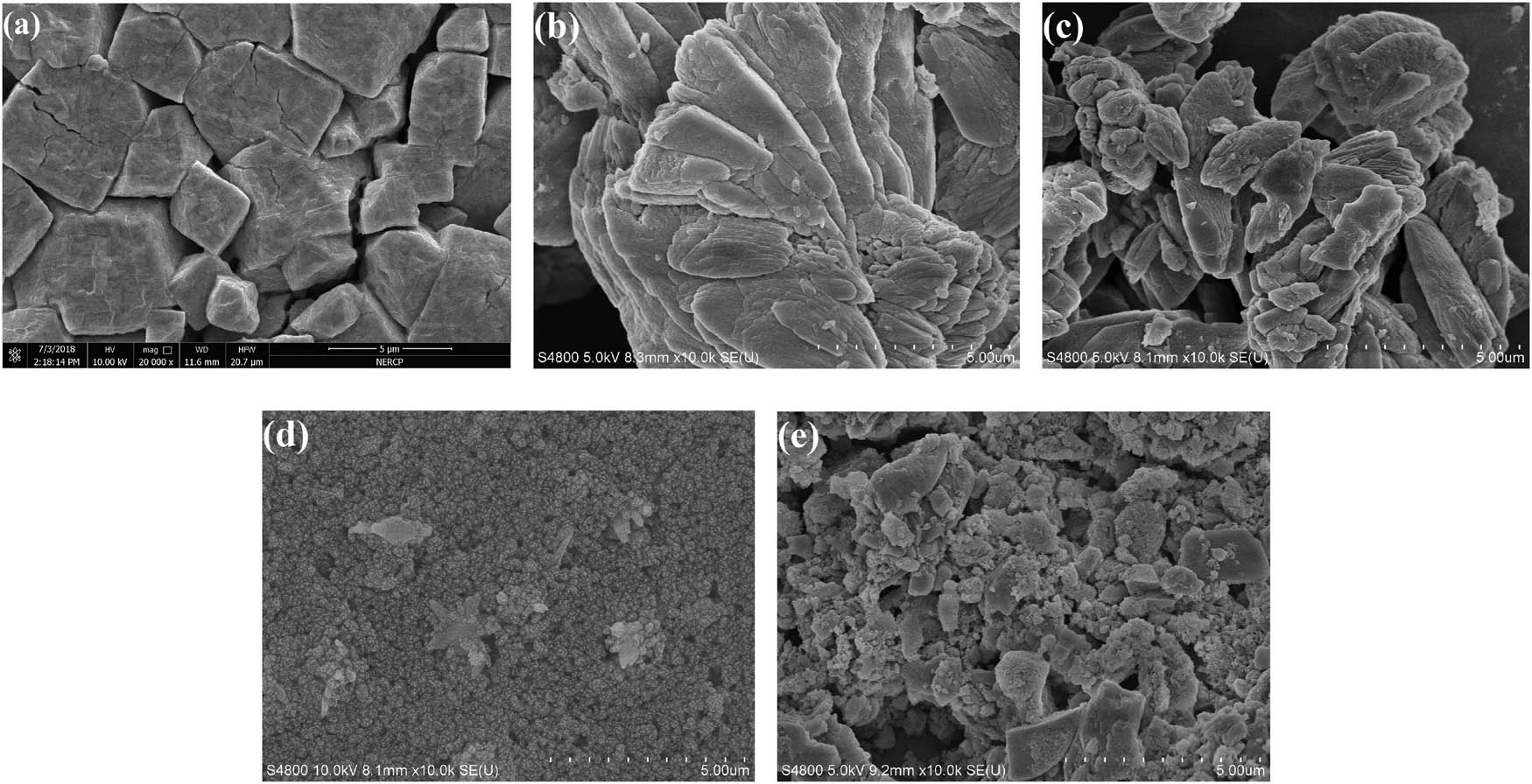
SEM images of (a) HSiW, (b) Ce-BDC, (c) HSiW@Ce-BDC, (d) UiO-66(Zr), and (e) Ce-BDC@HSiW@UiO-66 hybrids.

TEM images of Ce-BDC@HSiW@UiO-66 hybrids.
The textural properties of UiO-66 and Ce-BDC@HSiW@UiO-66 were studied by nitrogen physisorption, and the isotherms and corresponding pore size distribution profiles are depicted in Figure 5. The isotherm curve of UiO-66(Zr) shows typical type I adsorption isotherms, implying the existence of micro-pore structure [33]. By contrast, the isotherm curve of Ce-BDC@HSiW@UiO-66 hybrids displays a type IV curve with a hysteresis loop at P/P 0 = 0.45–0.95, demonstrating that it is a typical mesoporous material [34], and that the introduction of HSiW@Ce-BDC has a significant effect on the micro-pore structure of the UiO-66(Zr). Remarkably, the calculated BET surface areas and pore volume decreased from 667.2 m2·g−1 and 0.431 cm3·g−1 of UiO-66 to 251.2 m2·g−1 and 0.215 cm3·g−1 of Ce-BDC@HSiW@UiO-66, respectively, it is due to the pore blockage by HSiW@Ce-BDC guest embedded into UiO-66(Zr) host. From Figure 5b, compared to UiO-66(Zr) sample, the main peaks located at 3.9 nm are observed in Ce-BDC@HSiW@UiO-66 sample, demonstrating that the pore size is amplified to mesoporous scale. Meanwhile, the average pore size increased from 2.58 nm of UiO-66 to 3.42 nm of Ce-BDC@HSiW@UiO-66 because of the blockage of micropores and the presence of some mesoporous in the original Ce-BDC sample [35], which further indicates that the Ce-BDC@HSiW@UiO-66 hybrid has been successfully synthesized, similar to the result obtained in SEM and TEM. Moreover, the mesoporous Ce-BDC@HSiW@UiO-66 material is also in favor of reducing mass transfer resistance during catalytic esterification.

Nitrogen physisorption curves (a) and pore diameter distributions (b) of UiO-66(Zr) and Ce-BDC@HSiW@UiO-66 hybrids.
The XPS analysis of the synthesized hybrids is conducted to measure the element composition information. Figure 6a shows the survey XPS spectrum of as-prepared catalyst, implying the existence of O, Ce, Zr, C, and W in the Ce-BDC@HSiW@UiO-66 hybrids. Moreover, NH3-TPD experiments were also carried out for HSiW@Ce-BDC and Ce-BDC@HSiW@UiO-66 samples. As reported in Figure 6b, the HSiW@Ce-BDC showed the formation of weak acidity with a strong acidity desorption peak at 113°C and 570°C, respectively, and this is due to the desorption of NH3 adsorbed on weak acid sites, and the strong acidic nature of HSiW. Note that the Ce-BDC@HSiW@UiO-66 sample displayed a sharper peak at about 570°C with a shoulder. The peak at 579°C is due to the high Brønsted acid of HSiW. In addition, the slight decrease in desorption temperature at 545°C is due to the introduction of UiO-66(Zr), which led to the existence of the strong interaction between HSiW@Ce-BDC and UiO-66(Zr) and might be created due to the Lewis acidity (Zr4+) in the sample surface. Thus, it is expected that the Ce-BDC@HSiW@UiO-66 hybrids will be effective for green fatty acid esterification.

(a) XPS survey spectrum of Ce-BDC@HSiW@UiO-66 hybrids; (b) NH3-TPD curves of HSiW@Ce-BDC and Ce-BDC@HSiW@UiO-66 hybrids.
3.2 Catalytic activity evaluation
3.2.1 Effect of reaction temperature and time
The effects of reaction temperature and time on oleic acid were evaluated at the conditions of 0.2 g of Ce-BDC@HSiW@UiO-66 catalyst and 1:30 oleic acid to methanol mole ratio. Generally, there is an important effect of temperature on the endothermic reaction, and the conversion of oleic acid increased with elevated reaction temperature. As shown in Figure 7, the catalytic activity is gradually improved with the increase in reaction temperature from 120 to 140°C. For instance, the conversion of oleic acid jumped up from 21.8 to 53.7% at 2 h as the reaction temperature raised from 120 to 140°C. Likewise, when reaction temperature was 120°C, 130°C, and 140°C after 4 h of reaction, the oleic acid conversion was 53.1%, 81.5%, and 85.1%, respectively, indicating that the increase in temperature from 130°C to 140°C had no considerable alteration in the conversion of oleic acid, as well the conversions significantly increased with increasing reaction time. However, with progression in reaction time from 4 to 5 h at a temperature of 130°C, the conversion increased from 81.5% to 85.2%, respectively, indicating that no significant change was observed with prolonged reaction time. Taking this into account, 4 h and 130°C was decided as the optimum reaction time and temperature.
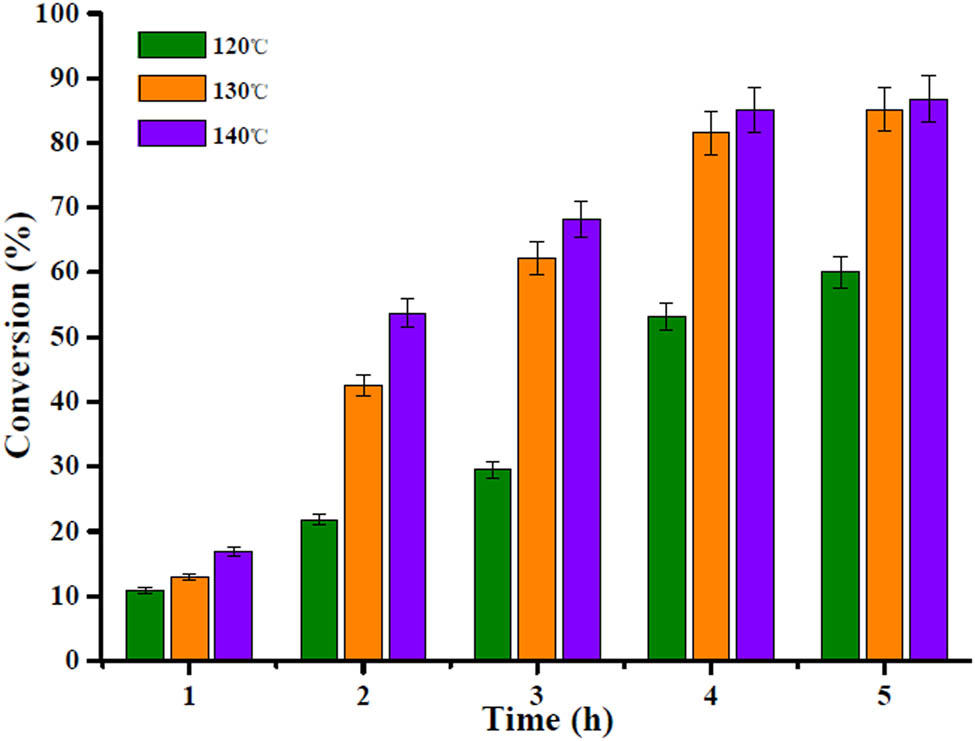
Evaluation of effects of reaction temperature and time on catalyst for the conversion of oleic acid.
3.2.2 Effect of catalyst amount
The influence of catalyst amount on the conversion has been studied for Ce-BDC@HSiW@UiO-66 catalyst at the conditions of 1:30 oleic acid to methanol mole ratio, and 130°C of reaction temperature. In Figure 8a, the conversion increased with increase in the amount of Ce-BDC@HSiW@UiO-66 catalyst from 0.15 to 0.20 g, this is owing directly to the increase in catalytic active sites available for the esterification reaction system. However, when the amount of catalyst was 0.25 g, a slight decrease in conversion was observed. It may be due to the decrease in mass transfer between reactants and catalyst with excessive catalyst amount, which led to reduction in interactions between them [36]. According to these results, 0.2 g was selected as the optimum catalyst amount.

Study of effects of (a) amount of Ce-BDC@HSiW@UiO-66 and (b) mole ratio on the conversion of oleic acid.
3.2.3 Effect of the mole ratio of the reactants
Generally, the mole ratio of the reactants was another important factor for esterification. As esterification is reversible, the excess of methanol is required to shift the reaction in a forward direction. Hence, the effect of amount of methanol on the reaction was investigated at the conditions of 0.2 g of Ce-BDC@HSiW@UiO-66 catalyst and 130°C of reaction temperature. As shown in Figure 8b, as the ratio increases from 1:20 to 1:30, the conversion of oleic acid is significantly improved. And the conversion could reach 81.5% as the mole ratio of oleic acid to methanol was 1:30. Further enhancing the mole ratio to 1:40 resulted in the oleic acid conversion tending to decrease slightly, similar results were found in the literature [37]. Therefore, the mole ratio of oleic acid/methanol of 1:30 is considered to be the optimum condition for the reaction.
3.3 The reusability and heterogeneity of catalyst
The reusability of HSiW@Ce-BDC and Ce-BDC@HSiW@UiO-66 were studied by six recycling experiments and the results are provided in Figure 9a. After each reaction, the used catalyst was removed by centrifugation and then used for the next experiment. According to Figure 9a, the oleic acid conversion obtained by Ce-BDC@HSiW@UiO-66 (81.5%) is higher than that of HSiW@Ce-BDC (21.9%) in the first used cycle. Afterward, the conversion of oleic acid is still around 20% in the third cycle by HSiW@Ce-BDC catalyst. In contrast, after the 6th usage cycle, the conversion of oleic acid decreased only by 4.6% compared to the conversion in the first used cycle, indicating that the Ce-BDC@HSiW@UiO-66 hybrids are an efficient catalyst and can be recycled. This is maybe due to the strong interaction between HSiW@Ce-BDC and UiO-66(Zr), resulting in its synergistic effect enhancing the catalytic activity and stability, similar results were also found by Pithadia and Patel [38]. FTIR and XRD analyses of fresh and recycled Ce-BDC@HSiW@UiO-66 are shown in Figures A1 and A2 (in Appendix), it revealed that there were no discernible differences in the XRD patterns and FT-IR spectra between them, confirming its stability. However, the observed decline in catalytic activity might be due to the loss of catalyst occurred during the recycling process [39].

Recycling (a) and leaching (b) test results for Ce-BDC@HSiW@UiO-66 catalyst.
Leaching test for the Ce-BDC@HSiW@UiO-66 catalyst was also performed by removing the catalyst from the system after 2 h, and the reaction was continued for further 3 h. As displayed in Figure 9b, it is observed that no substantial increase in the oleic acid conversion was observed after the removal of the catalyst, confirming the heterogeneous nature of the Ce-BDC@HSiW@UiO-66 catalyst. Hence, it could be clearly seen that the developed Ce-BDC@HSiW@UiO-66 solid catalyst had a long-term catalytic esterification activity.
3.4 Catalytic performance of Ce-BDC@HSiW@UiO-66 for esterification of other fatty acids
Interestingly, the different fatty acid carbon chain lengths in the reaction with methanol was also investigated for Ce-BDC@HSiW@UiO-66 hybrids, and the results are presented in Table 1. It was found that the Ce-BDC@HSiW@UiO-66 catalyst showed good catalytic performance in these reactions, suggesting that Ce-BDC@HSiW@UiO-66 hybrids could be used as an efficient solid catalyst for other esterification or pre-esterification of low-quality oils.
Reactivity of different fatty acids and methanol by Ce-BDC@HSiW@UiO-66 catalyst
| Free fatty acids (FFAs) | Reaction conditions | Activity (%) | |||
|---|---|---|---|---|---|
| Temperature (°C) | FFA:Methanol molar ratio | Amount of catalyst (g) | Time (h) | ||
| Lauric acid | 130 | 1:30 | 0.2 | 4 | 56.8 |
| Myristic acid | 130 | 1:30 | 0.2 | 4 | 79.8 |
| Palmitic acid | 130 | 1:30 | 0.2 | 4 | 96.3 |
| Stearic acid | 130 | 1:30 | 0.2 | 4 | 77.3 |
| Oleic acid | 130 | 1:30 | 0.2 | 4 | 81.5 |
4 Conclusion
In this research, we have successfully fabricated silicotungstic acid immobilized on Ce-BDC and embedded in UiO-66(Zr) forming hybrids (Ce-BDC@HSiW@UiO-66) via a simple solvothermal strategy. The existence of the UiO-66(Zr) led to an interaction between HSiW@Ce-BDC and UiO-66(Zr), endowing the prepared hybrids with mesoporous structure, large BET surface areas, and high acidity. Meanwhile, the prepared Ce-BDC@HSiW@UiO-66 hybrids showed better catalytic activity in comparison to HSiW@Ce-BDC for esterification of oleic acid. The conversion of 81.5% was attained under mild reaction conditions, catalyst amount of 0.2 g, 1:30 oleic acid to methanol molar ratio at 130°C for 4 h, and the Ce-BDC@HSiW@UiO-66 hybrids could be reused 6 times with a decrease in catalytic activity only by 4.6% in the reaction, indicating higher catalytic activity and better stability. Therefore, the reasonable design of the MOF-based catalyst in this work results in outstanding properties, which may provide a way for the synthesis of other multifunctional hybrids for green biofuel production in the future.
-
Funding information: This work was supported by the Guizhou Science and Technology Foundation ([2020]1Y054), the 2018 Thousand Level Innovative Talents Training Program of Guizhou Province, the Creative Research Groups Support Program of Guizhou Education Department (KY [2017]049), the Project of Anshun University supporting Doctors Research ([2021]asxybsjj01), the Industry-University-Research Cooperative Education Project of Anshun University (asxycxy201802), and the Innovative Entrepreneurship Training Program for Undergraduates of the Guizhou Education Department (202110667006, 202110667073).
-
Author contributions: Qiuyun Zhang: writing – original draft, writing – review and editing, and methodology; Binbin Yang: methodology and formal analysis; Yuanyuan Tian: methodology; Xianju Yang: methodology; Rongfei Yu: methodology; Jialu Wang: visualization; Taoli Deng: visualization; Yutao Zhang: project administration.
-
Conflicts of interest: Authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
Appendix

FTIR spectrum of fresh and used Ce-BDC@HSiW@UiO-66 catalysts.
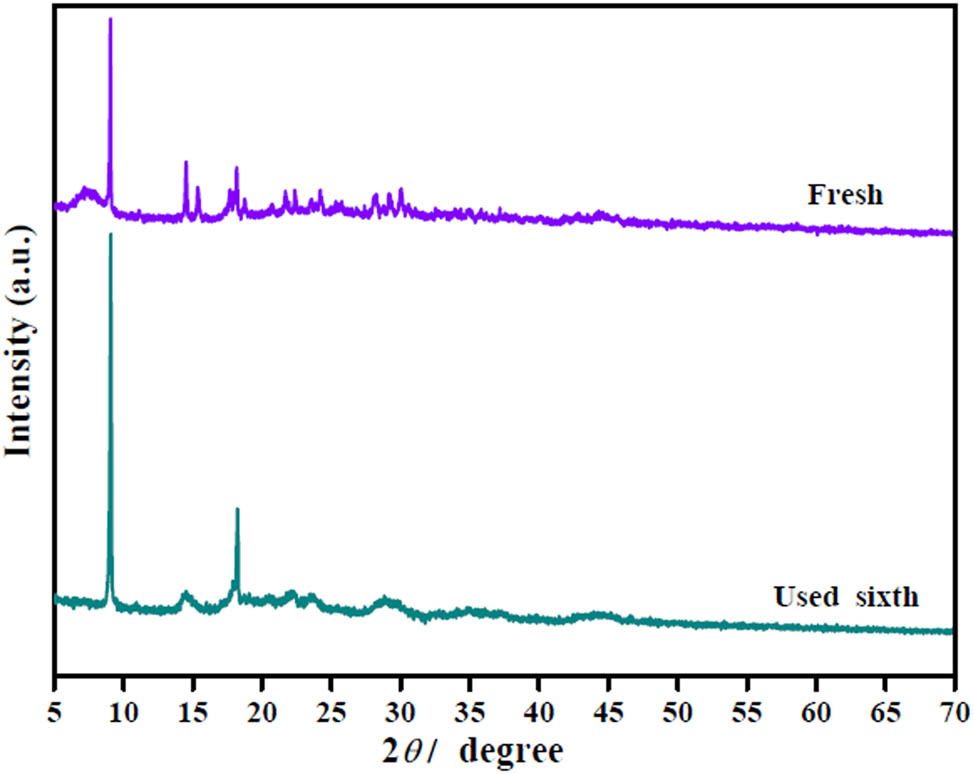
XRD patterns of fresh and used Ce-BDC@HSiW@UiO-66 catalysts.
References
[1] Li H, Fang Z, Smith Jr RL, Yang S. Efficient valorization of biomass to biofuels with bifunctional solid catalytic materials. Prog Energ Combust Sci. 2016;55:98–194.10.1016/j.pecs.2016.04.004Search in Google Scholar
[2] Wu HG, Li H, Fang Z. Hydrothermal amination of biomass to nitrogenous chemicals. Green Chem. 2021;23:6675–97.10.1039/D1GC02505HSearch in Google Scholar
[3] Vahid BR, Haghighi M, Alaei S, Toghiani J. Reusability enhancement of combustion synthesized MgO/MgAl2O4 nanocatalyst in biodiesel production by glow discharge plasma treatment. Energ Conver Manage. 2017;143:23–32.10.1016/j.enconman.2017.03.075Search in Google Scholar
[4] Nezhad MK, Aghaei H. Tosylated cloisite as a new heterofunctional carrier for covalent immobilization of lipase and its utilization for production of biodiesel from waste frying oil. Renew Energy. 2021;164:876–88.10.1016/j.renene.2020.09.117Search in Google Scholar
[5] Wang S, Pu JL, Wu JQ, Liu HJ, Xu HY, Li X, et al. SO42−/ZrO2 as a solid acid for the esterification of palmitic acid with methanol: Effects of the calcination time and recycle method. ACS Omega. 2020;5:30139–47.10.1021/acsomega.0c04586Search in Google Scholar PubMed PubMed Central
[6] Sun N, Zhang MH, Dong XQ, Wang LT. Preparation of sulfonated ordered mesoporous carbon catalyst and its catalytic performance for esterification of free fatty acids in waste cooking oils. RSC Adv. 2019;9:15941–8.10.1039/C9RA02546DSearch in Google Scholar
[7] Zhang QY, Luo QZ, Yang XJ, Wu YP, Yang BB, Wang JL, et al. Metal-organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification. Green Process Synth. 2021;10:284–94.10.1515/gps-2021-0029Search in Google Scholar
[8] Fernandes SA, Cardoso AL, Silva MJA. Novel kinetic study of H3PW12O40-catalyzed oleic acid esterification with methanol via 1H NMR spectroscopy. Fuel Process Technol. 2012;96:98–103.10.1016/j.fuproc.2011.12.025Search in Google Scholar
[9] Helmi M, Tahvildari K, Hemmati A, Azar PA, Safekordi A. Converting waste cooking oil into biodiesel using phosphomolybdic acid/clinoptilolite as an innovative green catalyst via electrolysis procedure; optimization by response surface methodology (RSM). Fuel Process Technol. 2022;225:107062.10.1016/j.fuproc.2021.107062Search in Google Scholar
[10] Joorasty M, Hemmati A, Rahbar-Kelishami A. NaOH/clinoptilolite-Fe3O4 as a novel magnetic catalyst for producing biodiesel from Amygdalus scoparia oil: optimization and kinetic study. Fuel. 2021;303:121305.10.1016/j.fuel.2021.121305Search in Google Scholar
[11] Zhang QY, Yang XJ, Yao JL, Cheng JS. Bimetallic MOF-derived synthesis of cobalt-cerium oxide supported phosphotungstic acid composites for the oleic acid esterification. J Chem. 2021;2021:1–9.10.1155/2021/2131960Search in Google Scholar
[12] Xie WL, Wang H. Immobilized polymeric sulfonated ionic liquid on core-shell structured Fe3O4/SiO2 composites: a magnetically recyclable catalyst for simultaneous transesterification and esterifications of low-cost oils to biodiesel. Renew Energy. 2020;145:1709–19.10.1016/j.renene.2019.07.092Search in Google Scholar
[13] Xie WL, Wang H. Grafting copolymerization of dual acidic ionic liquid on core-shell structured magnetic silica: a magnetically recyclable Brönsted acid catalyst for biodiesel production by one-pot transformation of low-quality oils. Fuel. 2021;283:118893.10.1016/j.fuel.2020.118893Search in Google Scholar
[14] Nata IF, Putra MD, Irawan C, Lee CK. Catalytic performance of sulfonated carbon-based solid acid catalyst on esterification of waste cooking oil for biodiesel production. J Env Chem Eng. 2017;5:2171–5.10.1016/j.jece.2017.04.029Search in Google Scholar
[15] Krishnan SG, Pua FL, Zhang F. A review of magnetic solid catalyst development for sustainable biodiesel production. Biomass Bioenergy. 2021;149:106099.10.1016/j.biombioe.2021.106099Search in Google Scholar
[16] Zhang QY, Ling D, Lei DD, Wang JL, Liu XF, Zhang YT, et al. Green and facile synthesis of metal-organic framework Cu-BTC supported Sn (II)-substituted Keggin heteropoly composites as an esterification nanocatalyst for biodiesel production. Front Chem. 2020;8:129.10.3389/fchem.2020.00129Search in Google Scholar PubMed PubMed Central
[17] Zhang P, Yang BL, Ma HP, Wu ZQ. Graphene modified porous organic polymer supported phosphotungstic acid catalyst for alkylation desulfurization. Fuel. 2021;293:120438.10.1016/j.fuel.2021.120438Search in Google Scholar
[18] da Conceiçao LRV, Carneiro LM, Giordani DS, de Castro HF. Synthesis of biodiesel from macaw palm oil using mesoporous solid catalyst comprising 12-molybdophosphoric acid and niobia. Renew Energy. 2017;113:119–28.10.1016/j.renene.2017.05.080Search in Google Scholar
[19] Kojima K, Osuga R, Yasuda S, Yokoi T, Hosogi Y, Itagaki S, et al. Characterization of H4SiW12O40 supported on mesoporous silica (SBA-15), non-structured amorphous silica and γ-alumina. J Catal. 2021;395:387–98.10.1016/j.jcat.2021.01.011Search in Google Scholar
[20] Cong WJ, Nanda S, Li H, Fang Z, Dalai AK, Kozinski JA. Metal-organic framework-based functional catalytic materials for biodiesel production: a review. Green Chem. 2021;23:2595–618.10.1039/D1GC00233CSearch in Google Scholar
[21] Zhang SW, Ou FX, Ning S, Cheng P. Polyoxometalate-based metal-organic frameworks for heterogeneous catalysis. Inorg Chem Front. 2021;8:1865–99.10.1039/D0QI01407ASearch in Google Scholar
[22] Xie WL, Wan F. Biodiesel production from acidic oils using polyoxometalate‑based sulfonated ionic liquids functionalized metal-organic frameworks. Catal Lett. 2019;149:2916–29.10.1007/s10562-019-02800-zSearch in Google Scholar
[23] Zhang QY, Luo QZ, Wu YP, Yu RF, Cheng JS, Zhang YT. Construction of a Keggin heteropolyacid/Ni-MOF catalyst for esterification of fatty acids. RSC Adv. 2021;11:33416–24.10.1039/D1RA06023FSearch in Google Scholar
[24] Ma YL, Li AR, Wang C, Ge XB. Preparation of HPW@UiO-66 catalyst with defects and its application in oxidative desulfurization. Chem Eng J. 2021;404:127062.10.1016/j.cej.2020.127062Search in Google Scholar
[25] Zhang J, Li XY, He BQ, Song YF, Ji YH, Cui ZY, et al. Biodiesel production through heterogeneous catalysis using a novel poly(phenylene sulfide) catalytic membrane. Energy Fuels. 2020;34:7422–9.10.1021/acs.energyfuels.0c00522Search in Google Scholar
[26] Wei RP, Qu XM, Xiao Y, Fan JD, Geng GL, Gao LJ, et al. Hydrogenolysis of glycerol to propanediols over silicotungstic acid catalysts intercalated with CuZnFe hydrotalcite-like compounds. Catal Today. 2021;368:224–31.10.1016/j.cattod.2020.11.028Search in Google Scholar
[27] Wang MH, Wang CB, Zhu L, Rong FL, He LH, Lou YF, et al. Bimetallic NiCo metal-organic frameworks for efficient non-Pt methanol electrocatalytic oxidation. Appl Catal A, Gen. 2021;619:118159.10.1016/j.apcata.2021.118159Search in Google Scholar
[28] Al-Enizi AM, Ubaidullah M, Ahmed J, Ahamad T, Ahmad T, Shaikh SF, et al. Synthesis of NiOx@NPC composite for high-performance supercapacitor via waste PET plastic-derived Ni-MOF. Compos Part B. 2020;183:107655.10.1016/j.compositesb.2019.107655Search in Google Scholar
[29] Tang J, Dong WJ, Wang G, Yao YZ, Cai LM, Liu Y, et al. Efficient molybdenum (VI) modified Zr-MOF catalysts for epoxidation of olefins. RSC Adv. 2014;4:42977–82.10.1039/C4RA07133FSearch in Google Scholar
[30] Zhao DX, Cai C. Cerium-based UiO-66 metal-organic framework for synergistic dye adsorption and photodegradation: a discussion of the mechanism. Dye Pigment. 2021;185:108957.10.1016/j.dyepig.2020.108957Search in Google Scholar
[31] Meshram AA, Sontakke SM. Synthesis, characterization and stability of Ni-Ce-Zr trimetallic metal organic framework. Mater Today: Proceed. 2021;46:6201–6.10.1016/j.matpr.2020.04.521Search in Google Scholar
[32] Wang QX, Luo X, Liu L, Tao SH, Xu S. Study on heteropolyacid-based catalysts with high activity and reusability for isoprene synthesis from formaldehyde and isobutene. N J Chem. 2021;45:5371–81.10.1039/D0NJ05305HSearch in Google Scholar
[33] Zhang QY, Ling D, Lei DD, Deng TL, Zhang YT, Ma PH. Synthesis and catalytic properties of nickel salts of Keggin-type heteropolyacids embedded metal-organic framework hybrid nanocatalyst. Green Process Synth. 2020;9:131–8.10.1515/gps-2020-0014Search in Google Scholar
[34] Huo Q, Liu GQ, Sun HH, Fu YF, Ning Y, Zhang BY, et al. CeO2-modified MIL-101(Fe) for photocatalysis extraction oxidation desulfurization of model oil under visible light irradiation. Chem Eng J. 2021;422:130036.10.1016/j.cej.2021.130036Search in Google Scholar
[35] Gao GF, Zhao Q, Yang C, Jiang TS. p-Toluenesulfonic acid functionalized imidazole ionic liquids encapsulated into bismuth SBA-16 as high-efficiency catalysts for Friedel-Crafts acylation reaction. Dalton Trans. 2021;50:5871–82.10.1039/D1DT00355KSearch in Google Scholar
[36] Olutoye MA, Wong SW, Chin LH, Amani H, Asif M, Hameed BH. Synthesis of fatty acid methyl esters via the transesterification of waste cooking oil by methanol with a barium-modified montmorillonite K10 catalyst. Renew Energy. 2016;86:392–8.10.1016/j.renene.2015.08.016Search in Google Scholar
[37] Li ZB, Miao ZC, Wang X, Zhao JP, Zhou J, Si WJ, et al. One-pot synthesis of ZrMo-KIT-6 solid acid catalyst for solvent-free conversion of glycerol to solketal. Fuel. 2018;233:377–87.10.1016/j.fuel.2018.06.081Search in Google Scholar
[38] Pithadia D, Patel A. Conversion of bioplatform molecule, succinic acid to value-added products via esterification over 12-tungstosilicic acid anchored to MCM-22. Biomass Bioenerg. 2021;151:106178.10.1016/j.biombioe.2021.106178Search in Google Scholar
[39] Ding J, Zhou CW, Wu ZW, Chen C, Feng NJ, Wang L, et al. Core-shell magnetic nanomaterial grafted spongy-structured poly (ionic liquid): a recyclable brönsted acid catalyst for biodiesel production. Appl Catal A, Gen. 2021;616:118080.10.1016/j.apcata.2021.118080Search in Google Scholar
© 2022 Qiuyun Zhang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

