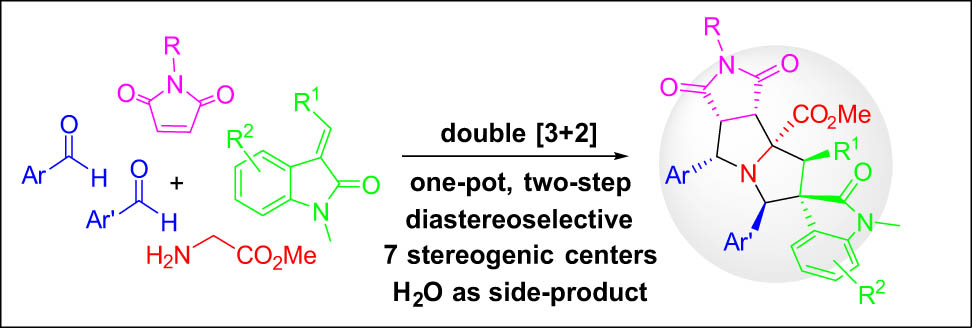
Abstract
One-pot two sequential [3 + 2] cycloadditions of azomethine ylides with different dipolarophiles for diastereoselective synthesis of spirooxindole pyrrolizidines are introduced. This one-pot synthesis involving five components generates a highly condensed ring system bearing seven stereocenters diastereoselectively. The new method has high pot, atom, and step economy. Only two equivalents of water are released as a side product.
Graphical abstract
1 Introduction
Pyrrolidine, pyrrolizidine, and spirooxindole pyrrolidines are privileged heterocycles found in natural products and synthetic drugs [1,2,3,4] such as supinidine [5], slaframine [6], (−)-isoretronecanol [7,8], hyacinthacine A1 [9], pteropodine [10], spirotryprostatin A [11], and an antibacterial agent [12] (Figure 1). In addition to traditional cyclization [13] and carboamination [14] reactions, [3 + 2] cycloadditions [15,16,17] of stabilized [18,19,20,21], metalated [22,23,24,25], and non-stabilized azomethine ylides are an important approach for the synthesis of pyrrolidine derivatives [26,27,28,29,30,31,32]. We have developed a number of azomethine ylide-based double [3 + 2] cycloadditions for making polycyclic scaffolds (Scheme 1) [33,34,35]. Using different dipolarophiles, such as maleimides or olefinic oxindoles, for inter- and intramolecular [3 + 2] cycloadditions could afford products with fused- and spiro-ring skeletons (Scheme 1a and c) [36,37]. Introduced in this article is a double cycloaddition of stabilized azomethine ylides derived from amino esters and maleimides with olefinic oxindoles to form a spirooxindole-pyrrolizidine scaffold (Scheme 1b).
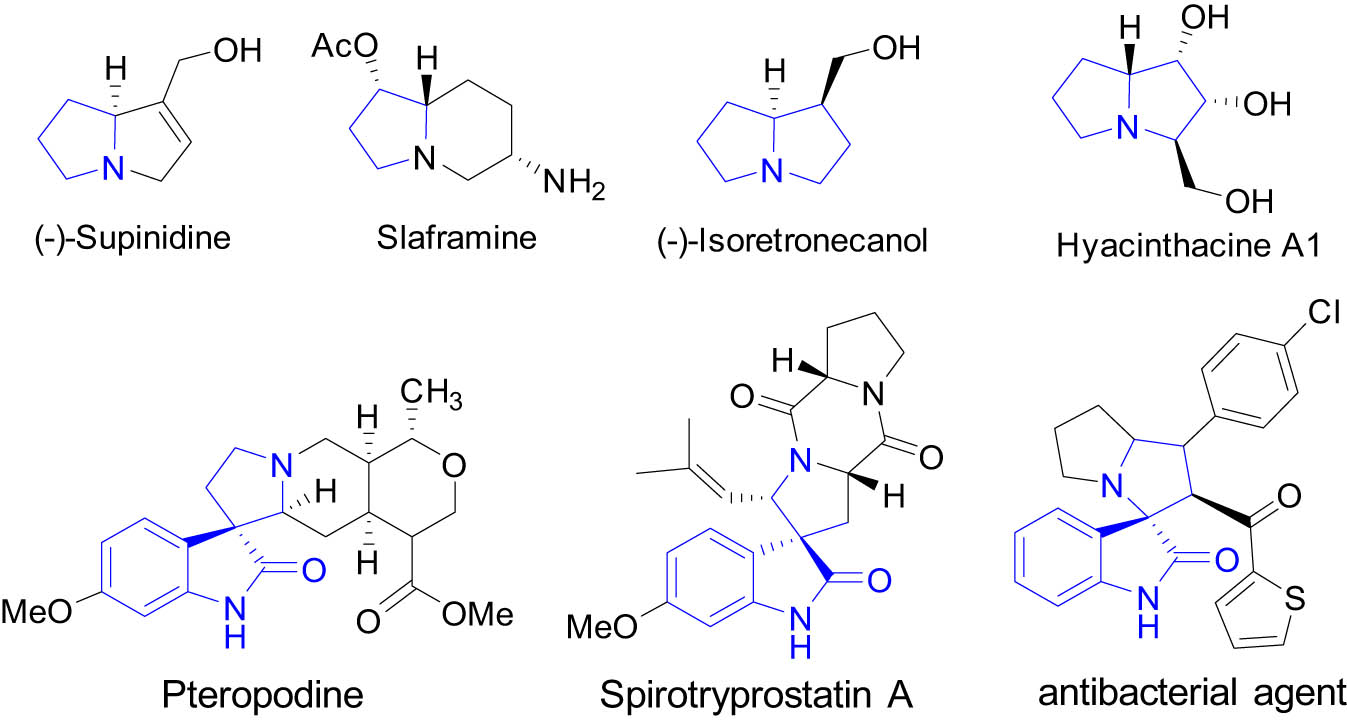
Bioactive pyrrolidine, pyrrolizidine, and spirooxindole-pyrrolidine compounds.
2 Materials and methods
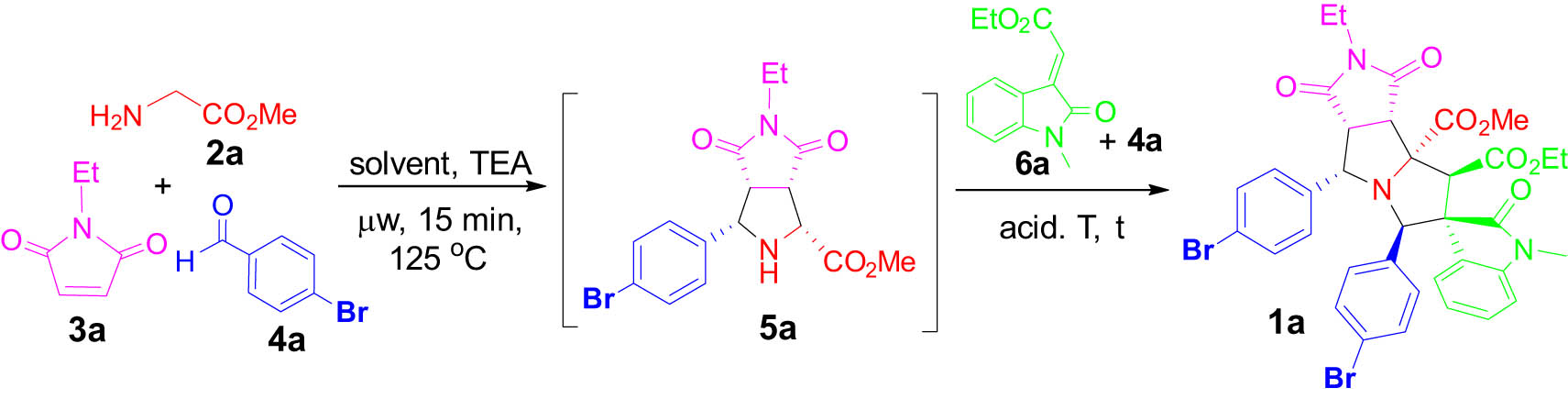
Amino ester-involved diastereoselective [3 + 2] cycloaddition for making compound I shown in Scheme 1 has been well established [29,31,32,36]. Following the literature procedures [36], the three-component [3 + 2] cycloaddition of glycine methyl ester 2a, N-ethylmaleimide 3a, and 4-bromobenzaldehyde 4a was performed in MeCN under microwave heating at 125°C for 15 min. Without purification, the reaction mixture of adduct 5a was used for the second intermolecular [3 + 2] cycloaddition with 4-bromobenzaldehyde 4a and olefinic oxindole 6a for making product 1a. The reaction conditions including solvents, catalysts, and temperature were screened and optimized. As it has been reported that acids could facilitate the cycloaddition of azomethine ylides [38,39,40]. In this work, the addition of TFA increased the LC yield of 1a from 23% to 73% (Table 1, entries 1–6). Other solvents, such as toluene and EtOH, did not improve the yield (Table 1, entries 7 and 8). Microwave heating at 125°C or 150°C for 1h gave 1a in 37% and 26% LC yields, respectively (Table 1, entries 9 and 10). Replacing TFA with AcOH or BzOH as an acid catalyst did not improve the yield. Thus, the optimized condition for one-pot synthesis was to use 1.1:1.0:2.3:1.0 of 2a:3a:4a:6a in MeCN under microwave heating at 125°C for 15 min for making 5a. The reaction mixture was then reacted with 6a and another equivalent of 4a at 125°C for 18 h in the presence of TFA catalyst to give 1a in 62% of isolated yield as a single diastereomer (Table 1, entry 5). The double [3 + 2] cycloaddition reactions using maleimide and then olefinic oxindole as dipolarophiles effectively assembled spirooxindole-pyrrolizidine 1a with two new rings, six bonds, and seven stereocenters.
Double [3 + 2] cycloaddition conditions for 1a
|
|||||
|---|---|---|---|---|---|
| Entry | Solvent | Acid | T (°C) | t (h) | 1a (%)a |
| 1 | MeCN | — | 90 | 72 | Trace |
| 2 | MeCN | TFA | 90 | 36 | 23 |
| 3 | MeCN | TFA | 125 | 36 | 70 |
| 4 | MeCN | TFA | 150 | 36 | 67 |
| 5 | MeCN | TFA | 125 | 18 | 73 (62) |
| 6 | MeCN | TFA | 125 | 12 | 61 |
| 7 | Toluene | TFA | 125 | 18 | 11 |
| 8 | EtOH | TFA | 125 | 18 | 39 |
| 9 | MeCN | TFA | 125b | 1 | 37 |
| 10 | MeCN | TFA | 150b | 1 | 26 |
| 11 | MeCN | AcOH | 125 | 18 | 49 |
| 12 | MeCN | BzOH | 125 | 18 | 31 |
aLC yield, isolated yield in parenthesis and conventional heating. bMicrowave irradiation.
3 Results
With the optimized reaction conditions in hand, double [3 + 2] cycloaddition of amino esters 2 with two equivalents of aldehyde 4 and one equivalent each of dipolarophiles 3 and 6 was carried out to afford spirooxindole-pyrrolizidine analogs 1a–g in 53–66% as single diastereomers (Figure 2). However, product 1f was not obtained due to the olefinic oxindole with the Ph instead of CO2R as R1, which may decrease the reactivity of the second cycloaddition.
![Figure 2
Double [3 + 2] cycloadditions using two equivalents of same aldehyde 4. Isolated yield: reaction conditions are same as in Table 1, entry 5.](/document/doi/10.1515/gps-2022-0088/asset/graphic/j_gps-2022-0088_fig_002.jpg)
Double [3 + 2] cycloadditions using two equivalents of same aldehyde 4. Isolated yield: reaction conditions are same as in Table 1, entry 5.
Different dipolarophiles 4 and 4′ could be used for the first and second cycloaddition reactions, respectively, to give product 7 with two substitution variations Ar and Ar′. Same procedures for making product 1 shown in Figure 2 were used for the synthesis to afford 7a–h in 49–69% yields (Figure 3). The reaction of thiophene-2-carbaldehyde for the first cycloaddition gave product 7h in 49% yield. However, the reaction of heteroaromatic aldehydes, such as isonicotinaldehyde and furan-2-carbaldehyde, for the second cycloaddition failed to produce 7i and 7j. Product 7k was also not obtained in which the R1 is −COMe instead of −CO2Me.
![Figure 3
Double [3 + 2] cycloadditions using different aldehydes 4 and 4′. Isolated yield: reaction conditions are same as in Table 1, entry 5.](/document/doi/10.1515/gps-2022-0088/asset/graphic/j_gps-2022-0088_fig_003.jpg)
Double [3 + 2] cycloadditions using different aldehydes 4 and 4′. Isolated yield: reaction conditions are same as in Table 1, entry 5.
4 Discussion
The one-pot double cycloadditions are diastereoselective to afford products 1 or 7 as single diastereomers. Control experiments were conducted to compare the diastereoselectivity difference of the double cycloaddition reactions with or without intermediate isolation. The cycloaddition with ethyl maleimide as a dipolarophile afforded 5a in 89% yield and 11:1 dr (Scheme 2a), while the reaction with an olefinic oxindole as dipolarophile afforded 8a in 81% yield and 5:1 dr (Scheme 2b). The isolated 5a was then reacted with an olefinic oxindole for the second cycloaddition to afford 1a in 64% and 8:1, but the reaction of 8a with ethyl maleimide failed to give product 9a. The proton at the β-ester group is more acidic, which could lead to the formation of more stable intermediate II, and prevents the cycloaddition of ylide I to form expected product 9a. Compared to the one-pot synthesis of 1a (62% as a single diastereomer) shown in Table 2, the synthesis involving the isolation of intermediate 5a gave 1a in 57% (two steps, dr 8:1). Lower yields and diastereoselectivity for the double cycloadditions involving intermediate isolation were also observed in our previous work [35,37].

Control reactions. (i) TEA, MeCN, μW 125°C, 15 min, (ii) MeCN, TFA (1.0 equivalent), 125°C, 18 h conventional heating. (a) Pathway for 1a and (b) Pathway for 9a.
The reaction mechanism for the one-pot double cycloadditions and the stereochemistry of the products are proposed in Scheme 3. The first cycloaddition of an acyclic W-shape azomethine ylide with a maleimide is in a suprafacial fashion affords 5- endo diastereoselectively. The stereochemistry of the related intermediates 5 has been confirmed by X-ray structure in our reported double 1,3-dipolar cycloaddition of stabilized azomethine ylides [33,36] to give the final products, which have Ar′ trans with Ar and CO2Me. The second cycloaddition of a cyclic S-shaped azomethine ylide with olefinic oxindole 6 affords product 1 or 7. The diastereoselectivity of the final products generated from the second 1,3-dipolar cycloaddition is similar to the compounds reported in the literature, which have X-ray crystal analysis [41,42,43,44]. The cycloaddition of E-olefinic oxindole and S-shaped azomethine ylide is in an endo fashion (for the C(O)NMe group) to afford the product with CO2Me and CO2Et in trans positions.
![Scheme 3
Proposed mechanism for diastereoselective double [3 + 2] cycloadditions.](/document/doi/10.1515/gps-2022-0088/asset/graphic/j_gps-2022-0088_fig_006.jpg)
Proposed mechanism for diastereoselective double [3 + 2] cycloadditions.
5 Conclusions
In summary, one-pot double [3 + 2] cycloadditions of stabilized azomethine ylides using maleimides and olefinic oxindoles as two different dipolarophiles for diastereoselective synthesis of spirooxindole-pyrrolidine compounds are accomplished. This is a highly efficient reaction process to assemble a polycyclic scaffold with six new bonds and seven stereogenic centers, and only two equivalents of water were generated as a side product. It is a new example of green synthesis that has a good pot, atom, and step economy.
Acknowledgement
The authors thank Jason Evans for performing HRMS analysis.
-
Funding information: This work was partly supported by the NIH grant T32 CA 236754-1 (Xiaofeng Zhang).
-
Author contributions: Xiaofeng Zhang: writing – original draft, writing – review and editing, methodology, formal analysis, project administration, data collection, and investigation; Xiaoming Ma and Weiqi Qiu: methodology and formal analysis; JohnMark Awad: writing – review and editing; Wei Zhang: supervision, data investigation, writing – review, and editing.
-
Conflict of interest: One of the corresponding authors (Wei Zhang) is a member of the Editorial Board of Green Processing and Synthesis.
References
[1] Fu PP. Pyrrolizidine alkaloids: metabolic activation pathways leading to liver tumor initiation. Chem Res Toxicol. 2017;30(1):81–93. 10.1021/acs.chemrestox.6b00297.Search in Google Scholar PubMed
[2] Wesseling AM, Demetrowitsch TJ, Schwarz K, Ober D. Variability of pyrrolizidine alkaloid occurrence in species of the grass subfamily pooideae (poaceae. Front Plant Sci. 2017;8:2046. 10.3389/fpls.2017.02046.Search in Google Scholar PubMed PubMed Central
[3] Petri GL, Raimondi MV, Spanò V, Holl R, Barraja P, Montalbano A. Pyrrolidine in drug discovery: A versatile scaffold for novel biologically active compounds. Top Curr Chem. 2021;379:34. 10.1007/s41061-021-00347-5.Search in Google Scholar PubMed PubMed Central
[4] Fang X, Wang CJ. Catalytic asymmetric construction of spiropyrrolidines via 1,3-dipolar cycloaddition of azomethine ylides. Org Biomol Chem. 2018;16:2591–601. 10.1039/C7OB02686B.Search in Google Scholar PubMed
[5] Chogii I, Njardarson JT. Asymmetric [3 + 2] annulation approach to 3-pyrrolines: concise total syntheses of (−)-Supinidine, (−)-Isoretronecanol, and (+)-Elacomine. Angew Chem Int Ed. 2015;127(46):13910–4. 10.1002/ange.201506559.Search in Google Scholar
[6] Cossy J, Willis C, Bellosta V, Saint-Jalmes L. Enantioselective allyltitanation synthesis of (-)-Slaframine. Synthesis. 2002;7:951–7. 10.1055/s-2002-28505.Search in Google Scholar
[7] Li J, Zhao H, Jiang X, Wang X, Hu H, Yu L, et al. The cyano group as a traceless activation group for the intermolecular [3 + 2] cycloaddition of azomethine ylides: a five-step synthesis of (±)-Isoretronecanol. Angew Chem Int Ed. 2015;54(21):6306–10. 10.1002/anie.201500961.Search in Google Scholar PubMed
[8] Liang Y, Chung CC, Huang MW, Uang BJ. Formal syntheses of (−)-isoretronecanol, (+)-laburnine, and a concise enantioselective synthesis of (+)-turneforcidine. J Antibiotics. 2019;72:397–406. 10.1038/s41429-019-0169-9.Search in Google Scholar PubMed
[9] Parmar K, Haghshenas P, Gravel M. Total Synthesis of (+)-Hyacinthacine A1 using a chemoselective cross-benzoin reaction and a furan photooxygenation–amine cyclization strategy. Org Lett. 2021;23(4):1416–21. 10.1021/acs.orglett.1c00090.Search in Google Scholar PubMed
[10] Martin SF, Mortimore M. New methods for the synthesis of oxindole alkaloids. total syntheses of isopteropodine and pteropodine. Tetrahedron Lett. 1990;31(32):4557–60. 10.1016/S0040-4039(00)97675.Search in Google Scholar
[11] Onishi T, Sebahar PR, Williams RM. Concise, Asymmetric total synthesis of Spirotryprostatin A. Org Lett. 2003;5(17):3135–7. 10.1021/ol0351910.Search in Google Scholar PubMed
[12] Thangamani A. Regiospecific synthesis and biological evaluation of spirooxindolopyrrolizidines via [3 + 2] cycloaddition of azomethine ylide. Eur J Med Chem. 2010;45(12):6120–6. 10.1016/j.ejmech.2010.09.051.Search in Google Scholar PubMed
[13] Munnuri S, Adebesin AM, Paudyal MP, Yousufuddin M, Dalipe A, Falck JR. Catalyst-controlled diastereoselective synthesis of cyclic amines via C–H functionalization. J Am Chem Soc. 2017;139(50):18288–94. 10.1021/jacs.7b09901.Search in Google Scholar PubMed
[14] Zhang G, Cui L, Wang Y, Zhang L. Homogeneous gold-catalyzed oxidative carboheterofunctionalization of alkenes. J Am Chem Soc. 2010;132(5):1474–5. 10.1021/ja909555d.Search in Google Scholar PubMed
[15] Coldham I, Hufton R. Intramolecular dipolar cycloaddition reactions of azomethine ylides. Chem Rev. 2005;105(7):2765–810. 10.1021/cr040004c.Search in Google Scholar PubMed
[16] Pandey G, Banerjee P, Gadre SR. Construction of enantiopure pyrrolidine ring system via asymmetric [3 + 2]-cycloaddition of azomethine ylides. Chem Rev. 2006;106(11):4484–517. 10.1021/cr050011g.Search in Google Scholar PubMed
[17] Hashimoto T, Maruoka K. Recent advances of catalytic asymmetric 1,3-dipolar cycloadditions. Chem Rev. 2015;115(11):5366–412. 10.1021/cr5007182.Search in Google Scholar PubMed
[18] Narayan R, Potowski M, Jia ZJ, Antonchick AP, Waldmann H. Catalytic enantioselective 1,3-dipolar cycloadditions of azomethine ylides for biology-oriented synthesis. Acc Chem Res. 2014;47(4):1296–310. 10.1021/ar400286b.Search in Google Scholar PubMed PubMed Central
[19] Selva V, Selva E, Merino P, Najera C, Sansano MJ. Sequential metal-free thermal 1,3-dipolar cycloaddition of unactivated azomethine ylides. Org Lett. 2018;20(12):3522–6. 10.1021/acs.orglett.8b01292.Search in Google Scholar PubMed
[20] Potowski M, Bauer JO, Strohmann C, Antonchick AP, Waldmann H. Highly enantioselective catalytic [6 + 3] cycloadditions of azomethine ylides. Angew Chem Int Ed. 2012;51(38):9512–6. 10.1002/anie.201204394.Search in Google Scholar PubMed
[21] Henke BR, Kouklis AJ, Heathcock CH. Intramolecular 1,3-dipolar cycloaddition of stabilized azomethine ylides to unactivated dipolarophiles. J Org Chem. 1992;57(26):7056–66. 10.1021/jo00052a015.Search in Google Scholar
[22] Wei L, Chang X, Wang CJ. Catalytic asymmetric reactions with N-metallated azomethine ylides. Acc Chem Res. 2020;53(5):1084–100. 10.1021/acs.accounts.0c00113.Search in Google Scholar PubMed
[23] Tang S, Zhang X, Sun J, Niu D, Chruma JJ. 2-Azaallyl anions, 2-azaallyl cations, 2-azaallyl radicals, and azomethine ylides. Chem Rev. 2018;118(20):10393–457. 10.1021/acs.chemrev.8b00349.Search in Google Scholar PubMed
[24] Domingo LR, Ríos-Gutiérrez M, Pérez P. A molecular electron density theory study of the role of the copper metalation of azomethine ylides in [3 + 2] cycloaddition reactions. J Org Chem. 2018;83(18):10959–73. 10.1021/acs.joc.8b01605.Search in Google Scholar PubMed
[25] López-Pérez A, Adrio J, Carretero JC. The phenylsulfonyl group as a temporal regiochemical controller in the catalytic asymmetric 1,3-dipolar cycloaddition of azomethine ylides. Angew Chem Int Ed. 2009;48(2):340–3. org/10.1002/anie.200805063.Search in Google Scholar
[26] Buev EM, Moshkin VS, Sosnovskikh VY. Reagents for storage and regeneration of nonstabilized azomethine ylides: spiroanthraceneoxazolidines. Org Lett. 2016;18(8):1764–7. 10.1021/acs.orglett.6b00475.Search in Google Scholar PubMed
[27] Wang K, Xie Y, Li Y, Chen R, Wang Z. Dearomative [3 + 2] cycloaddition reaction of nitrobenzothiophenes with nonstabilized azomethine ylides. RSC Adv. 2020;10:28720–4. 10.1039/D0RA05687A.Search in Google Scholar PubMed PubMed Central
[28] Buev EM, Moshkin VS, Sosnovskikh VY. Nonstabilized azomethine ylides in the mannich reaction: synthesis of 3,3-disubstituted pyrrolidines, including oxindole alkaloids. J Org Chem. 2017;82(23):12827–33. 10.1021/acs.joc.7b02193.Search in Google Scholar PubMed
[29] Zhang XF, Zhi S, Wang W, Liu S, Jasinski JP, Zhang W. A pot-economical and diastereoselective synthesis involving catalyst-free click reaction for fused-triazolobenzodiazepines. Green Chem. 2016;18:2642–6. 10.1039/C6GC00497K.Search in Google Scholar
[30] Ma XM, Zhang XF, Award J, Xie G, Qiu W, Zhang W. One-pot synthesis of tetrahydro-pyrrolobenzodiazepines and tetrahydro-pyrrolobenzodiazepinones through sequential 1,3-dipolar cycloaddition/N-alkylation (N-acylation)/Staudinger/aza-Wittig reactions. Green Chem. 2019;21:4489–94. 10.1039/C9GC01642B.Search in Google Scholar
[31] Muthengi A, Zhang XF, Dhawan G, Zhang WS, Corsinia F, Zhang W. Sequential (3 + 2) cycloaddition and (5 + n) annulation for modular synthesis of dihydrobenzoxazines, tetrahydrobenzoxazepines and tetrahydrobenzoxazocines. Green Chem. 2018;20:3134–9. 10.1039/C8GC01099D.Search in Google Scholar
[32] Zhang XF, Pham K, Liu S, Legris M, Muthengi A, Jasinski JP, et al. Stereoselective synthesis of fused tetrahydroquinazolines through one-pot double [3 + 2] dipolar cycloadditions followed by [5 + 1] annulation. Beilstein J Org Chem. 2016;12:2204–10. 10.3762/bjoc.12.211.Search in Google Scholar PubMed PubMed Central
[33] Lu Q, Song G, Jasinski JP, Keeley AC, Zhang W. One-pot double [3 + 2] cycloaddition for diastereoselective synthesis of tetracyclic pyrrolidine compounds. Green Chem. 2012;14:3010–2. 10.1039/C2GC36066G.Search in Google Scholar
[34] Zhang W, Lu YM, Geib S. Synthesis of Fluorous and Nonfluorous Polycyclic Systems by One-Pot, Double Intramolecular 1,3-Dipolar Cycloaddition of Azomethine Ylides. Org Lett. 2005;7(11):2269–2. 10.1021/ol0507773.Search in Google Scholar PubMed
[35] Zhang XF, Qiu WQ, Evans J, Kaur M, Jasinski JP, Zhang W. Double 1,3-dipolar cycloadditions of two nonstabilized azomethine ylides for polycyclic pyrrolidines. Org Lett. 2019;21(7):2176–9. 10.1021/acs.orglett.9b00487.Search in Google Scholar PubMed
[36] Zhang XF, Qiu WQ, Ma XM, Evans J, Kaur M, Jasinski JP, et al. One-pot double [3 + 2] cycloadditions for diastereoselective synthesis of pyrrolidine-based polycyclic systems. J Org Chem. 2018;83(21):13536–42. 10.1021/acs.joc.8b02046.Search in Google Scholar PubMed
[37] Zhang XF, Qiu WQ, Murray SA, Zhan D, Kaur M, Jasinski JP, et al. Pseudo-five-component reaction for diastereoselective synthesis of butterfly shaped bispiro[oxindole-pyrrolidine]s. J Org Chem. 2021;86(23):17395–403. 10.1021/acs.joc.1c01797.Search in Google Scholar PubMed
[38] Mantelingu K, Lin YF, Seidel D. Intramolecular [3 + 2]-cycloadditions of azomethine ylides derived from secondary amines via redox-neutral C–H functionalization. Org Lett. 2014;16(22):5910–3. 10.1021/ol502918g.Search in Google Scholar PubMed PubMed Central
[39] Galvis CEP, Kouznetsov VV. Regio- and stereoselective synthesis of spirooxindole 1′-nitro pyrrolizidines with five concurrent stereocenters under aqueous medium and their bioprospection using the zebrafish (Danio rerio) embryo model. Org Biomol Chem. 2013;11:7372–86. 10.1039/C3OB41302K.Search in Google Scholar PubMed
[40] Peng C, Ren J, Xiao JA, Zhang H, Yang H, Luo Y. Additive-assisted regioselective 1, 3-dipolar cycloaddition of azomethine ylides with benzylideneacetone. Beilstein J Org Chem. 2014;10:352–60. 10.3762/bjoc.10.33.Search in Google Scholar PubMed PubMed Central
[41] Sebahar PR, Williams RM. The synthesis of spirooxindole pyrrolidines via an asymmetric azomethine ylide [1,3]-dipolar cycloaddition reaction. Heterocycles. 2002;58:563–75. 10.3987/COM-02-S(M)51.Search in Google Scholar
[42] Lo MM, Neumann CS, Nagayama S, Perlstein EO, Schreiber SL. A library of spirooxindoles based on a stereoselective three-component coupling reaction. J Am Chem Soc. 2004;126(49):16077–86. 10.1021/ja045089d.Search in Google Scholar PubMed
[43] Zhang JX, Wang HY, Jin QW, Zheng CW, Zhao G, Shang YJ. Thiourea–quaternary ammonium salt catalyzed asymmetric 1, 3-dipolar cycloaddition of imino esters to construct spiro[pyrrolidin-3,3′-oxindoles]. Org Lett. 2016;18(19):4774–7. 10.1021/acs.orglett.6b02098.Search in Google Scholar PubMed
[44] Wang L, Shi XM, Dong WP, Zhu LP, Wang R. Efficient construction of highly functionalized spiro[γ-butyrolactone-pyrrolidin-3,3′-oxindole] tricyclic skeletons via an organocatalytic 1,3-dipolar cycloaddition. Chem Commun. 2013;49:3458–60. 10.1039/C3CC40669E.Search in Google Scholar PubMed
© 2022 Xiaofeng Zhang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

![Scheme 1
Double [3 + 2] cycloadditions with different dipolarophiles: (a) reported synthesis in ref. [35], (b) this work, and (c) reported synthesis in ref. [36].](/document/doi/10.1515/gps-2022-0088/asset/graphic/j_gps-2022-0088_fig_004.jpg)
