Abstract
Asthma, the respiratory disorder associated with bronchial hyper-responsiveness, affected 300 million people across the globe, with a prevalence of 4.05% in Saudi Arabia and causing 61.6% of hospital emergency room annual visits. Increased side effects of conventional drugs demand the necessity for the development of natural drugs. In this study, an herbal composite from Bacopa monnieri and Euphorbia hirta was prepared and characterized by Fourier transform infrared spectroscopy (FT-IR) and gas chromatography-mass spectrometry (GC-MS) analysis. In vitro bacterial inhibition and anti-asthmatic activity were evaluated using animal models. Ethanolic herbal composite (EHC) showed significant anti-pathogenic activities. GC-MS analysis identified potential bioactive compounds and FT-IR analysis revealed functional groups corresponding to plant composites. The EHC increased the preconvulsive time against 1% histamine aerosol compared to control animals. In sensitized + EHC-treated animals, total leukocyte, eosinophil, lymphocyte, neutrophil, and monocyte counts were found to be reduced as compared to sensitized and control groups. EHC decreased malondialdehyde and bicarbonate levels denoting the reduced oxidative burden and increased the antioxidant activity by increased intracellular glutathione (GSH) level. The EHC-treated group showed decreased inflammatory cell infiltration compared to the sensitized. A significant anti-asthmatic effect was observed in the EHC-treated group (P < 0.05). Thus, herbal composites are used in the treatment of asthma and can be used as an alternative to commercially available pharmaceutical drugs.
1 Introduction
Asthma is a life-threatening enduring respiratory disorder, resulting in the inflammation and narrowing of airways. Exacerbation includes bronchospasm, edema, mucus secretion, cellular infiltration, and damage of the airway epithelium [1]. Asthma is associated with wheezing, breathlessness, chest tightness, and coughing brought about by inflammation, bronchial constriction, and excessive mucus secretion due to bronchial hyper-responsiveness [2]. The occurrence of asthma worldwide is found to be around 300 million people [3]. An asthma attack is estimated to be around 11% among children and 6% among adults worldwide. In Saudi Arabia, asthma is the 19th disability-adjusted life years and 26th in causing mortality [4]. Different researchers have found that the prevalence of asthma in Saudi Arabia is more than 4% with a higher rate of asthma attack [5,6]. The children in Saudi Arabia (with a prevalence rate ranging from 8 to 25% in various parts of the Kingdom, reported by different researchers) are at a comparatively higher risk [7], but the ground reality is that asthma still has a high prevalence with an increasing pattern and many of the chemical drugs used in the treatment of asthma in Saudi Arabia have been found to have side effects [7,8].
Pathologically, the development of asthma is characterized by the liberation of Th2 or Th1 cells against specific allergens leading to the production of cytokines, such as IL-4, IL-5, IL-9, and IL-13, with an accumulation of collagen deep in the epithelium, resulting in the hyperplasia of the structural elements of all the cells. Interleukins are responsible for the production of immunoglobulin E which binds to mast cells in the airway mucosa and stimulates the release mediators such as histamine, tumor necrotic factor-α, chymase, prostaglandins, leukotrienes C4 and D4, prostaglandin D2, tryptase and amphiregulin [9]. These mediators diffuse through the airway mucosa triggering the bronchial smooth muscle spasm, increased mucus secretion, and vasodilation causing obstruction in the airways. Edema, scarring, and fibrosis buildup lead to thickened basement membranes, which tend to be irreversible [5].
Even though the effect of asthma on Covid-19 patients is not fully understood [10], several scientific investigations are on the way. Some of these studies have concluded that the incidences of Covid-19 have slightly increased in asthma patients [11] and asthma does not create a remarkable burden on Covid-19 patients as some virus infections do [12]. At the same time, an investigation [13] conducted in the Kingdom of Saudi Arabia found that asthmatic patients with Covid-19 infections showed a higher ICU admission rate and oxygen supply requirement compared to other Covid-19 patients.
Pharmaceutical drugs contribute to the major treatment for asthma given to patients worldwide. The increased number of asthma patients in every country led to drug unavailability, expensive drug prices, and exorbitant [14]. The commonly used medications for asthma are inhaled corticosteroids, short- and long-acting beta antagonists, oral steroids, leukotriene modifiers, mast cell stabilizers, and immunomodulators. Though they possess many side effects on treatment, there is a need for a search for an alternative to conventional drugs in the treatment of asthma [15]. Ayurveda, a traditional practice of medicine from India, has described medicinal plants in the treatment of asthma. Using medicinal plants in the treatment of asthma is considered to be natural and safe [16]. Many plant species, such as Rehmannia glutinosa Libosch, Guiera senegalensis, Alisma orientale (Sam.) Juzepcz, Atractylodes ovate, Euphorbia thymifolia L., Paeonia suffruticosa Andr, Glycyrrhiza glabra, Zingiber officinale, Euphorbia thymifolia, Lablab purpureus, Coix lacryma-jobi, Psoralea corylifolia, Schisandra chinensis, Ziziphus jujube Mill, Ophiopogon japonicus tuber, Citrus aurantifolia, and many other plant species, are used in the formulation of drugs against asthma [17]. Such medicinal plants possess various phytochemical compounds, which could result in the treatment of various diseases and infections [18].
Euphorbia hirta a pantropical weed belonging to the plant family Euphorbiaceae is a hairy herb found on roadsides and pathways in India. The bioactive compounds present in E. hirta are found to be quercitrin, afzelin, kaempferol, protocatechuic acid, and gallic acid. The other phytochemicals present are flavonoids, quercitrin, sterols, 24-methylene-cycloartenol, β-sitosterol, triterpene, β-amyrin, and myricitrin, which are responsible for the anti-inflammatory effect. Tannins and tannic acid present in plant extracts are considered to possess antiseptic effects. The triterpenoids, taraxerone, and 11α,12α oxidotaraxerol also exhibit anti-microbial activity against various bacterial and fungal species [19]. Bacopa monnieri (water hyssop) is a perennial herb used in traditional Ayurveda for various ailments. Bioactive compounds present in B. monnieri are tannin, saponin, flavonoid, phenol, alkaloid, and phlobatannin, which are responsible for antifungal and antibacterial activities. Recent studies have described that B. monnieri also possesses anti-inflammatory activity, inhibits mast cell degradation, and thus can be used in the treatment of asthma [20]. Several studies investigated the anti-inflammatory and anti-asthmatic activities of E. hirta [21,22] and B. monnieri [23,24]. They significantly reduced the pro-inflammatory cytokine expressions compared with the commercial drugs. Thus, the present study concentrates on the synergistic activity of the E. hirta and B. monnieri extracts against bronchoconstriction and bronchospasm. Based on the literature review, this is the first reported work on the anti-asthmatic activity of the E. hirta and B. monnieri composite extracts in guinea pigs. The herbal composite was prepared by the ethanolic extracts of E. hirta and B. monnieri. Its bacterial resistance, histamine-induced bronchoconstriction, eosinophil and macrophage counts in the bronchoalveolar fluid, serum bicarbonate level, malondialdehyde (MDA) level, intracellular glutathione level, total protein level, and histopathology studies were evaluated.
2 Materials and methods
2.1 Collection of plants and extraction and preparation of herbal composite
The two selected herbs (E. hirta and B. monnieri) were collected from the territory desert of Riyadh. The ethanolic herbal composite (EHC) was prepared by extracting 50 g of each medicinal herb (leaves) with 125 mL of ethanol using the Soxhlet extraction apparatus for 8 h. The extract was evaporated to dry using a hot air oven kept at 50°C. The obtained powder was stored in a cool dry place. For the preparation of the composite: two selected herbal extracts were mixed in equal ratios (1:1) and used for further studies.
2.2 Gas chromatography-mass spectrometry (GC-MS) analysis of plant extracts
GC-MS analysis of ethanol extracts of B. monnieri and E. hirta was performed using Agilent GC 7890A/MS5975C. The capillary column used was Agilent DB5MS. The column length used for the analysis was 30 m/0.25 mm internal diameter, 0.25 μm film thickness. One microliter of extracts were injected into the instrument and the temperature was raised as follows: 60°C for 2 min; followed by 300°C at the rate of 10°C·min−1; and 300°C, for 6 min. Mass spectra were taken at a scanning interval of 0.5 s. The spectrum with the retention time, percentage composition, and peak area was given. The components were identified by using GC-MS NIST (2008) library.
2.3 Fourier transform infrared spectroscopy (FT-IR) analysis
FT-IR analysis was utilized to identify the functional groups corresponding to the plant extracts. The analysis was carried out in a KBr disk medium interconnected to a spectrometer with a resolution of 4 cm−1 between the range of 400 and 4,000 cm−1. The plant extracts and composites were analyzed. The emission spectrum was recorded under a fluorescence spectrophotometer, and the corresponding functional groups identified were reported.
2.4 Microbial resistivity of EHC
From the resultant herbal composite extract, three different concentrations (1×, 2×, 3×) were prepared to evaluate the antibacterial activity. The antibacterial activity of different herbal composite ratios was evaluated against the significant organisms (Staphylococcus aureus, Escherichia coli, Enterobacter aerogenes, Acinetobacter spp., and Klebsiella pneumoniae) by well diffusion method. About 50 µL of each prepared composite in 5% dimethyl sulfoxide (Sigma-Aldrich) was loaded into the well. The plates were incubated at 37°C for 24–48 h. The antibacterial activity was estimated with respect to the zone of inhibition around the wells of each extract in all the inoculated nutrient agar plates. The inhibition zones were measured and recorded in millimeters.
2.5 Anti-asthmatic activity
2.5.1 Experimental animals
Adult Cavia porcellus (guinea pigs) weighing from 400 to 450 g was obtained from a local bird and animal market at Aziziya in Riyadh. The animals were housed in polypropylene cages at 25 ± 2°C with an 8 h light/8 h dark condition. The animals were fed with standard diet and water (carrot with additional vitamin C). The work was carried out according to the ethical guidelines of the Department of Pharmacology and Toxicology, College of Pharmacy, Prince Sattam bin Abdulaziz University, Al-Kharj, Kingdom of Saudi Arabia (Bioethics Committee, Prince Sattam Bin Abdulaziz University; No: 20/2009/002). About 21 guinea pigs were used for the present study and they were divided into seven groups (three guinea pigs in a group) randomly. Groups I, II, III, and IV were used for the analysis of histamine-induced bronchoconstriction, and Groups V, VI, and VII were used for the ovalbumin (OVA)-induced bronchospasm studies.
2.5.2 Histamine-induced bronchoconstriction
The in vivo anti-histaminic activity of EHC was studied by inducing bronchospasm using histamine aerosols in guinea pigs [25]. Guinea pigs (Groups I, II, III, and IV) selected for this study were fasted for 24 h. Then, all the test animals were exposed to 1% histamine (Sigma-Aldrich) aerosol at a pressure of 300 mmHg using a nebulizer (Kent Scientific Corporation, Torrington, CT, USA) in an airtight chamber of dimension 24 cm × 14 cm × 24 cm. On exposure to 1% histamine, animals showed preconvulsive dyspnea (PCD). The duration of onset of PCD is considered preconvulsive time (PCT). After the onset of PCD, the animals were transferred to fresh air. This PCT was recorded as a 0th-day value. After 15 days, Group I was given 2 mL·kg−1 of normal saline, Group II was given 2 mg·kg−1 of chlorpheniramine (Sigma-Aldrich), Group III was given 100 mg·kg−1 of EHC, and Group IV was given 200 mg·kg−1 of EHC by oral administration. PCD was recorded after 2 h of administration using the following formula:
where T 1 is the duration for PCD on the 0th day and T 2 is the duration for PCD after the 15th day.
2.5.3 OVA-induced bronchospasm
Guinea pigs of Groups V, VI, and VII were used for the induction of bronchospasm. Group V was given normal water (control group), Group VI was given OVA (sensitized group), and Group VII was given EHC (200 mg·kg−1). The guinea pigs of other groups (Groups VI and VII) were sensitized orally with OVA (Sigma-Aldrich) (1 mL, 10% w/v). The animals of Group VII were given ethanolic herbal extract (200 mg·kg−1) orally for up to 15 days along with OVA. On the 15th day, all the test animals were given OVA orally (0.5 mL, 2% w/v) except Group I animals. After 3 h of the OVA administration, the animals were anesthetized with sodium pentobarbital (Sigma-Aldrich) (50 mg·kg−1) ip [26]. The animals were sacrificed, and the bronchia was removed from the animals and washed with 15 mL of saline. The washed fluid is collected in a sterile container and centrifuged (Fisherbrand™ centrific model 225, Waltham, MA, USA) at 2,000 rpm for 5 min. Pellet was separated and dissolved in 0.5 mL of saline. Giemsa stain (0.2 mL) in buffered saline was added to the resuspended pellets.
2.6 Measurement of eosinophil, macrophages, serum bicarbonate, MDA, GSH, and protein levels
About 0.5 mL of the obtained fluid was observed under a microscope for leucocyte count (450× magnification). The results were recorded and compared with the other two groups (Groups V and VI). The specimen required for the estimation of bicarbonate level is serum. The complete blood of the animals was collected and should be tested within 1 h as they remain stable only for 1 h after collection. Bicarbonate level of serum was performed as described by Zilva and Pannall [27]. About 1 mL of lung tissue was added to 10 mL of ice-cold phosphate buffer and homogenized. MDA level of lung tissue was described by Buege and Aust. The amount of MDA was estimated and recorded [28]. GSH level and total protein level were calculated by the procedures described by Prajapati et al. [29]. Results are recorded in GSH mg−1 protein for GSH level and mg·mL−1 for proteins.
2.7 Histopathology of lung
The lungs of the test animals (Groups V, VI, and VII) were isolated after the collection of BALF and placed in 10% formaldehyde (HCHO). A microtome is used to cut the samples (paraffin-embedded lungs) into thin sections of 5 mm thickness. Later, the sections were stained using hematoxylin and eosin dyes and the sections were observed under a microscope for histopathological changes [30]. The histopathological slides were examined under a light microscope (Olympus BX51, Tokyo, Japan) by a pathologist who was unaware about the treatment that each of the animals had received. The pathological slides were graded histopathologically according to the severity of lung damage by using a modified scoring system [31]. In this scoring system, a leukocyte infiltration score was given between 0 and 4 (Table 1).
Histopathological lung leucocyte infiltration according to modified scoring system
| No. | Leucocyte infiltration | Score |
|---|---|---|
| 1 | Less than 10 cells | 0 |
| 2 | At least 10 cells | 1 |
| 3 | At least 25 cells | 2 |
| 4 | At least 50 cells | 3 |
| 5 | Over 75 cells | 4 |
2.8 Statistical analysis to determine the anti-asthmatic effect of EHC on animal models
As all the tests were performed with three different animals of the same group, results were recorded and expressed in terms of mean ± standard deviation. A Chi-square non-parametric test using SPSS-9 for Windows 7 was used as a statistical tool to determine the anti-asthmatic effect of EHC on animal models. The hypothesis selected (H0) was that “there is a significant anti-asthmatic effect on EHC treated group.” The difference in the eosinophil and macrophage counts, serum bicarbonate level, protein, MDA, and GSH levels between the sensitized group and the EHC treated group was statistically calculated with P < 0.05 considered significant.
3 Results
3.1 GC-MS analysis of plant extracts: bioactive compounds present in the plant extracts
E. hirta and B. monnieri were identified by using GC-MS analysis. Figures 1 and 2 show the GC-MS spectrum of the E. hirta and B. monnieri ethanolic extract. The compounds present in the plant extracts with their retention time are shown in Tables 2 and 3. About 26 bioactive compounds were identified from the GC-MS analysis of E. hirta and 19 from B. monnieri.

GC-MS spectrum of E. hirta ethanolic extracts.

GC-MS spectrum of B. monnieri ethanolic extracts.
GC-MS analysis of E. hirta
| No. | Compound name | Retention time |
|---|---|---|
| 1 | 4H-pyran-4-one,5-hydroxy-2-methyl | 6.209 |
| 2 | 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 7.220 |
| 3 | 2-Furancarboxaldehyde, 5-(hydroxymethyl)- | 8.320 |
| 4 | Phthalic anhydride | 9.497 |
| 5 | 1,2,3-Benzenetriol | 10.130 |
| 6 | α-[Di-n-butylamino methyl]-2-ethoxy-4-quinoline methanol | 10.453 |
| 7 | 5-Fluoro-6-methyl-5-hepten-2-one | 10.975 |
| 8 | Pyrazole-5-carboxylic acid, 3-methyl | 11.608 |
| 9 | 2(4H)-benzofuranone,5,6,7,7a-tetrahydro-4,4,7a-trimethyl- | 11.919 |
| 10 | 3-Deoxy-d-mannoic lactone | 12.641 |
| 11 | 1,2,3,5-Cyclohexanetetrol,(1. α.,2. β.,3. α.,5. β.) | 12.819 |
| 12 | 4-O-β-d-Galactopyranosyl-d-glucopyranose | 12.963 |
| 13 | 2-Amino-4-hydroxypyrrolo[2,3-d]pyrimidine | 13.063 |
| 14 | 1,2,3-Thiadiazole-4-carboxylic acid, hydrazide | 13.186 |
| 15 | 2,2-Diethoxytetrahydrofuran | 13.430 |
| 16 | 5-Ethylcyclopent-1-ene-1-carboxylic acid | 14.186 |
| 17 | 2-Pentadecanone, 6,10,14-trimethyl | 14.719 |
| 18 | d-chiro-inositol, 3-O-(2-amino-4-((carboxyiminomethyl)amino)-2,3,4,6-tetradeoxy- α-d-arabino-hexo | 15.308 |
| 19 | Inositol | 15.430 |
| 20 | Neo-inositol | 15.552 |
| 21 | 5-Eicosene, (E)- | 15.996 |
| 22 | Methyl 7,12-octadecadienoate | 16.785 |
| 23 | 10-Octadecenoic acid, methyl ester | 16.830 |
| 24 | Phytol | 16.907 |
| 25 | Cis-vaccenic acid | 17.207 |
| 26 | 3-(3,4-Dimethoxyphenyl)propylamine, PFP | 22.151 |
GC-MS analysis of B. monnieri
| No. | Compound name | Retention time |
|---|---|---|
| 1 | 3-Acetoxy-4-cyano-2,5-dimethylpyri dine | 10.753 |
| 2 | Phenylethyne | 12.341 |
| 3 | 2-Tetradecene, (E)- | 12.419 |
| 4 | Octane, 1,1′-oxybis- | 12.641 |
| 5 | Trichloroacetic acid, tetradecyl ester | 14.297 |
| 6 | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | 15.308 |
| 7 | 9,12-Octadecadienoic acid (Z,Z)- | 17.296 |
| 8 | Cholest-2-ene-2-methanol, (5.α)- | 19.218 |
| 9 | 1,E-6,Z-11-hexadecatriene | 19.485 |
| 10 | 1-Octadecanesulphonyl chloride | 19.674 |
| 11 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 19.763 |
| 12 | Behenyl chloride | 20.307 |
| 13 | 2-Myristynoyl-glycinamide | 20.829 |
| 14 | γ-Sitosterol | 20.974 |
| 15 | 17-OH-pregnenolone 17-α-hydroxypregnenolon | 21.340 |
| 16 | 2,6,10,14,18,22-Tetracosahexaene | 21.618 |
| 17 | 9,19-Cyclolanost-24-en-3-ol, ta.)- | 22.140 |
| 18 | Lupeol | 22.240 |
| 19 | 1,2-Bis(trimethylsilyl)benzene | 22.818 |
3.2 FT-IR of the plant composite
FT-IR analysis of plant composites revealed the functional groups corresponding to the plant extracts. Figure 3 shows the FT-IR spectrum of the composites. The medium band at 3309.85 cm−1 represents the N–H symmetric and asymmetric stretching of the amide functional groups. Weak bands at 2947.23, 2831.50, and 1651.07 cm−1 correspond to C–H stretch alkane compounds, O–H stretch carboxylic acids, and C═C stretch alkenes, respectively. Sharp absorption bands at 1450.47 and 1111.00 cm−1 correspond to C═C stretch aromatic compounds and C–O stretch alcohols, respectively. A very strong band at 1018.41 cm−1 shows the C–F stretch alkyl fluorides. The medium peak at 671.23 cm−1 represents alkynes of ≡C–H bend. The weak band at 555.50 cm−1 is due to the presence of alkyl bromide (C–Br) stretching. The observation of two alkyl halides is due to the composite formation of plant extracts (Table 4).
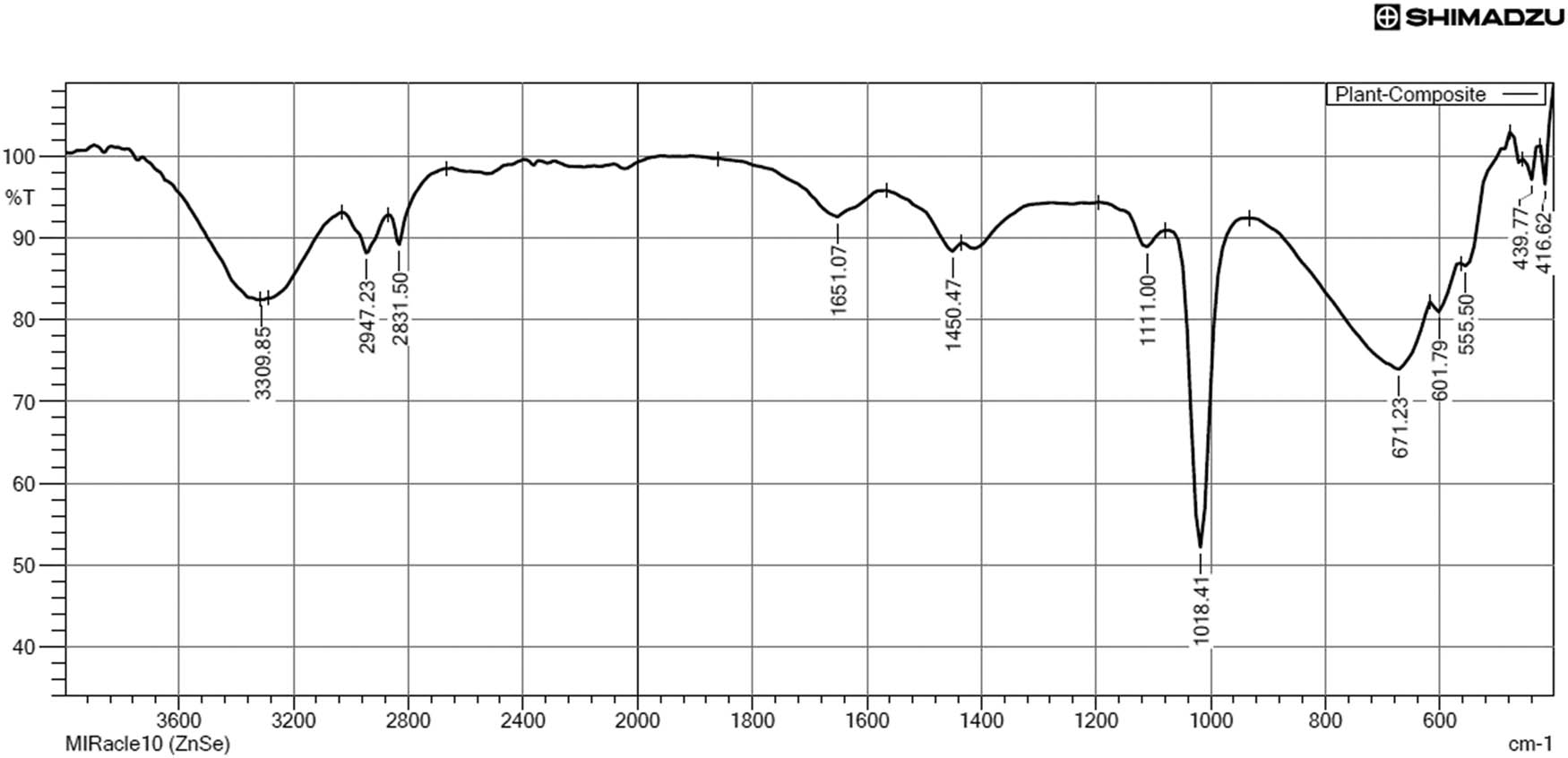
FT-IR spectrum of the plant composites.
FT-IR characterization of the plant composite
| No. | Absorption peaks | Functional group |
|---|---|---|
| 1 | 3,309.85 | N–H symmetric and asym. stretch |
| 2 | 2,947.23 | C–H stretch |
| 3 | 2,831.50 | O–H stretch |
| 4 | 1,651.07 | C═C stretch |
| 5 | 1,450.47 | C═C stretch |
| 6 | 1,111.00 | C–O stretch |
| 7 | 1,018.41 | C–F stretch |
| 8 | 671.23 | ≡C–H bend |
| 9 | 601.79 | ═C–H bend |
| 10 | 555.50 | C–Br |
3.3 Microbial resistance of EHC
Among the given concentration, the 3× concentrates showed more significant antibacterial activity than the other two concentrates. A maximum zone of inhibition was observed against E. coli and S. aureus (31 and 30 mm, respectively). About 28 mm was observed against E. aerogenes and Acinetobacter sp. Herbal composites showed 27 mm of inhibition zone against K. pneumoniae. The zone of inhibition of three concentrates of EHC is presented in Table 5.
Zone of inhibition of EHC against test organisms
| No. | Test organism | Zone of inhibition (mm) | ||
|---|---|---|---|---|
| 1× | 2× | 3× | ||
| 1 | S. aureus | 22 | 25 | 30 |
| 2 | E. coli | 24 | 26 | 31 |
| 3 | E. aerogenes | 19 | 23 | 28 |
| 4 | Acinetobacter sp. | 21 | 25 | 28 |
| 5 | K. pneumoniae | 18 | 23 | 27 |
3.4 Histamine-induced bronchoconstriction
Treatment with EHC showed a significant delay in PCT on exposure to histamine compared to control normal animals. The percentage increase in PCT after treatment with 100–200 mg·kg−1 of EHC was observed as 47.36 ± 4.38% and 64.59 ± 2.94%, respectively, whereas the control group showed only 3.61 ± 2.50% in PCD on histamine aerosol (Table 6).
Histamine-induced bronchoconstriction
| No. | Treatment group | % Increase in time of PCD* |
|---|---|---|
| 1 | Control | 3.61 ± 2.50 |
| 2 | Chlorpheniramine | 82.87 ± 1.63 |
| 3 | 100 mg·kg−1 | 41.36 ± 4.38 |
| 4 | 200 mg·kg−1 | 64.59 ± 2. 94 |
*All values are calculated using mean ± SD.
3.5 Eosinophil and macrophage counts
Significant differences in white blood cells were observed in three groups. There are increased total leukocyte, eosinophil, monocyte, polymorph, and lymphocyte counts in sensitized guinea pigs compared to the control group. EHC-treated animals showed a decrease in levels of total leukocyte, polymorph, eosinophil, monocyte, and lymphocyte counts compared to sensitized animals (Table 7).
Eosinophil and macrophage count in the bronchoalveolar fluid
| No. | Cell count | Normal group* | Pathological score# | Sensitized group* | Pathological score*,# | EHC-treated group* (200 mg·kg−1) | Pathological score# |
|---|---|---|---|---|---|---|---|
| 1 | TLC/cmm | 6,912.17 ± 207.86 | NA | 16813.42 ± 467.33 | NA | 9348.12 ± 362.02a | NA |
| 2 | Polymorph count/cmm | 22.25 ± 1.85 | 1 ± 0.0 | 40.74 ± 0.36 | 2 ± 0.0 | 33.2 ± 0.79a | 2 ± 0.0 |
| 3 | Lymphocyte count/cmm | 46.18 ± 0.43 | 2 ± 0.0 | 54.36 ± 1.75 | 3 ± 0.0 | 49.26 ± 0.66a | 2 ± 0.0 |
| 4 | Eosinophil count/cmm | 8.05 ± 0.16 | 0 ± 0.0 | 18.11 ± 0.47 | 1 ± 0.0 | 11.49 ± 0.27a | 1 ± 0.0 |
| 5 | Monocyte count/cmm | 5.5 ± 0.72 | 0 ± 0.0 | 15.52 ± 0.44 | 1 ± 0.0 | 9.32 ± 0.76a | 0.5 ± 0.3 |
cmm – cells per cubic millimeter.
*All values are calculated using mean ± SD.
#Pathological score described by Yamanel et al. [33].
a P < 0.05 – significant difference in comparison with the sensitized group.
3.6 Serum bicarbonate level, protein, MDA, and GSH levels
Sensitized animals showed an increase in serum bicarbonate level (62.21 ± 0.2 mEq·L−1) compared to the control group (38.36 ± 0.68 mEq·L−1). The group treated with EHC showed a significant reduction in the serum bicarbonate level (35.73 ± 1.06 mEq·L−1). Guinea pigs treated with EHC (0.072 ± 0.0026 mEq·L−1) showed a decrease in the MDA level compared to the sensitized group (0.29 ± 0.0007). Similarly, the EHC-treated groups showed a decrease in levels of protein (7.55 ± 0.072) and GSH (16.87 ± 0.008) compared to protein (6.24 ± 0.041) and GSH (18.43 ± 0.002) levels in the sensitized group (Tables 8 and 9).
Serum bicarbonate level and protein levels
| No. | Treatment group | Serum bicarbonate level* (mEq·L−1) | Protein* (mg·mL−1) |
|---|---|---|---|
| 1 | Normal control group | 38.36 ± 0.68 | 6.24 ± 0.041 |
| 2 | Sensitized group | 62.21 ± 0.2 | 10.08 ± 0.026 |
| 3 | EHC treated (200 mg·kg−1) | 35.73 ± 1.06 | 7.55 ± 0.072 |
*All values are calculated using mean ± SD.
MDA and GSH levels
| No. | Treatment group | MDA level* (μg·mg−1/protein) | GSH level* (μg·mg−1/protein) |
|---|---|---|---|
| 1 | Normal control group | 0.089 ± 0.0013 | 18.43 ± 0.002 |
| 2 | Sensitized group | 0.29 ± 0.0007 | 5.7 ± 0.032 |
| 3 | EHC treated (200 mg·kg−1) | 0.072 ± 0.0026 | 16.87 ± 0.008 |
*All values are calculated using mean ± SD.
3.7 Histopathological study
No changes were observed in the normal control group (Figure 4). Massive infiltration of inflammatory cells in the interstitial space was observed indicating the airway inflammation in the sensitized group (Figure 5). The sensitized group also showed emphysema, hyperplasia, and necrosis. The EHC-treated group showed decreased inflammatory cell infiltration compared to the sensitized group (Figure 6). The treatment with the EHC prevented hyperplasia and bronchoconstriction in sensitized + EHC-treated animals (Group III). Score values were given for the leucocyte counts. Based on the score values, the EHC-treated group showed a significant decrease in leucocytes, which are found to be active in inflammation. Detailed pathological scores and comparisons between groups are shown in Table 7.
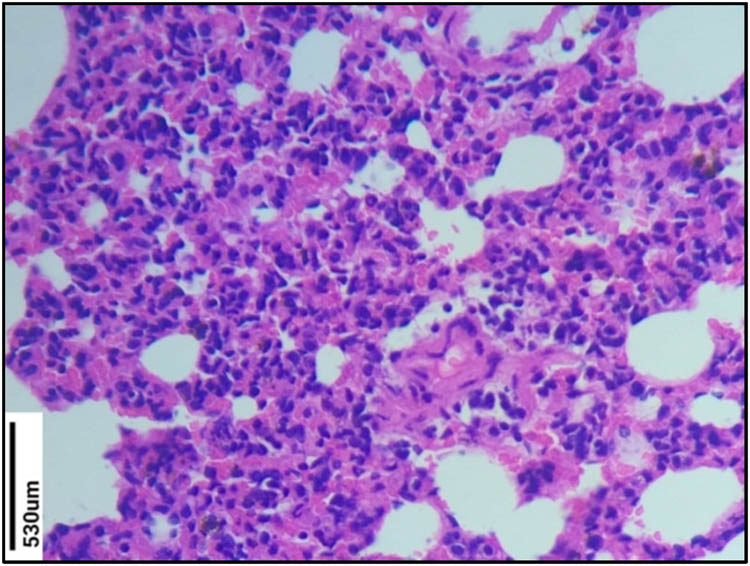
Histopathological study of Group V lung section (control group). No changes were observed in the normal control group.
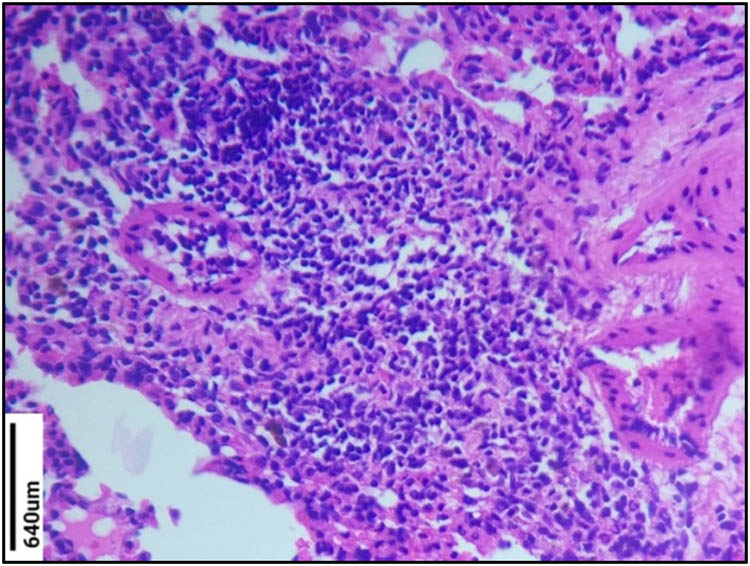
Histopathological study of Group VI lung section (sensitized group). Massive infiltration of inflammatory cells in the interstitial space was observed in the sensitized group. The sensitized group also showed emphysema, hyperplasia, and necrosis.

Histopathological study of Group VII lung section (EHC-treated group). The EHC-treated group showed decreased inflammatory cell infiltration compared to sensitized group. The treatment with the EHC prevented hyperplasia and bronchoconstriction in sensitized + EHC-treated animals.
3.8 Statistical analysis to determine the anti-asthmatic effect of EHC on animal models
The difference in the eosinophil and macrophage counts, serum bicarbonate level, protein, MDA, and GSH levels between the sensitized group and the EHC treated group was taken as the statistical experimental design. The hypothesis selected was “there is a significant anti-asthmatic effect on EHC treated group.” The difference in the eosinophil and macrophage counts, serum bicarbonate level, protein, MDA, and GSH levels between the sensitized group and the EHC-treated group was statistically calculated with P < 0.05 considered significant. For all the data, the values of the EHC treated group (Tables 7–9) were less than that of the sensitized group, which confirmed the anti-asthmatic effects of EHC on the treated group of animals. Hence, the assigned hypothesis was accepted (P < 0.05).
4 Discussion
This study attempts to establish the anti-asthmatic property of an ethanol leaf extract of E. hirta and B. monnieri composite. As the anatomy of the airways, anaphylactic bronchoconstriction on antigen challenge, and inflammatory mediator responses are similar to humans, Guinea pigs are used for histopathological works [32]. The process of inflammation in asthma is classified into seven phases: sensitization, stimulation, cell signaling, migration, cell activation, tissue damage, and resolution. In humans, eosinophil counts are increased in the asthmatic airways and these cells release proteins and growth factors that may damage airway epithelium and cause airway remodeling. Moreover, neutrophil numbers are usually found to be elevated in the airways and in the sputum of patients with severe asthma. Hence, it is useful to evaluate these cell counts to determine if an anti-asthmatic product (EHC) is effective [29].
In this study, histamine is used as a spasmogenic agent to induce PCD. Histamine increases sensitivity by stimulating H1 receptors in airways leading to bronchoconstriction. The EHC increased the PCT against histamine aerosol when compared to control animals; thus, it tends to possess the broncho-dilating activity and an antihistaminic effect. EHC and chlorpheniramine (H1 receptor antagonist) prevented the histamine-induced bronchoconstriction by delaying the onset of PCD. Induction of bronchial hyper-responsiveness by OVA in an animal model is the most commonly used method of allergic asthma model worldwide. Sensitization is carried out by intranasal or intra-tracheal administration. This helps in studying mechanisms and treatments of bronchial hyper-responsiveness as most of the pathophysiological mechanisms resemble those seen in asthma patients [34]. OVA has the ability to induce asthma in humans and causes chronic airway inflammation [33]. Also, OVA is a T-cell-dependent antigen that is non-toxic and inert [35], thus proving suitable for the study. The total leukocyte, eosinophil, polymorph, monocyte, and lymphocytes counts were found to be increased in the sensitized group (Group VI), thus indicating the inflammatory response. In the sensitized + EHC-treated animals (Group VII), the herbal composites reduced the total leukocyte, neutrophil, lymphocyte, eosinophil, and monocyte counts. Thus, the herbal composite acts as a protective agent preventing the release of mediators resulting in bronchoconstriction. An increase in MDA level increases the oxidative stress and lowered glutathione decreases the antioxidant. This effect is seen in a sensitized group, while in the MHC-treated group, the MDA level is reduced and the GSH level is increased. Increased CO2 concentration in the lungs resulted in increased bicarbonate levels. Decreased tissue inflammation was observed in the EHC-treated group. Massive infiltration of inflammatory cells in the interstitial space was observed indicating that airway inflammation was observed in the sensitized group. The EHC-treated group showed decreased inflammatory cell infiltration compared to the sensitized group. The treatment with the EHC prevented hyperplasia and bronchoconstriction in sensitized + EHC-treated animals (Group VII).
Similar work on the anti-asthmatic activity of E. hirta was carried out by Pretorius and Smit [25] on mice. They reported that the treatment with hydrocortisone and the plant extracts decreased the white blood counts such as neutrophils, eosinophils and basophils which were found to be active during inflammation. Platelets and fibrin networks also play a fundamental role in asthma. Pretorius and Smit [25] observed that untreated mice possess major thick fibers and minor thin fibers as well as normal tight round platelet aggregates. Though the hydrocortisone drug made the fibrin more fragile, the E. hirta extracts do not affect the fragility of the fibrin proteins and that it prevents the minor fibers from forming the dense layer over the major fibers, as observed in untreated control asthmatic mice [36]. The morphology of the platelets also remained unaltered on E. hirta extract-treated mice. Hence, E. hirta extracts reduced the inflammatory cells’ level equal to hydrocortisone, indicating that the plant, indeed, has anti-inflammatory properties [37]. These results well correspond with the present work as the developed herbal composite reduced the inflammatory cells to a significant extent.
Upadhyay et al. [26] investigated the anti-inflammatory activity of E. hirta on rats. The anti-inflammatory activity was determined by the carrageenan-induced paw edema experiment. E. hirta extracts decreased the inflammation in a dose-dependent manner. Reduction in inflammation was found to be effective at 500 mg·kg−1 b.w. concentration. Moreover, E. hirta plant extracts decreased the production of PGE-2 and pro-inflammatory cytokines. Similarly, several works were also investigated on extracts of B. monnieri. Hossain et al. [27] studied the anti-inflammatory activity of B. monnieri by the carrageenan-induced paw edema method. Methanolic extracts of B. monnieri showed significant anti-inflammatory activity. The increased reduction in the paw volume at a dose of 400 mg·kg−1 body weight was 62.73%, which was comparable to that of the standard drug indomethacin (67.08%) at 5 h. B. monnieri extracts showed higher anti-inflammatory activity, i.e., 92% (100 mg·kg−1) when compared to standard anti-inflammatory drug aspirin, i.e., 68.62% (25 mg·kg−1). The study provides evidence that extracts of B. monnieri act as a potent anti-inflammatory agent in rats in an acute inflammation model. Phytochemical studies of B. monnieri examined by Jain Paras et al. [24] revealed the presence of several bioactive compounds, such as tannin, saponin, flavonoid, phenol, alkaloid, and phlobatannin. Quantitative analysis revealed the presence of 12.5 mg of tannin, 110 mg of alkaloid, 1.5 mg of saponin, and 24.75 mg·g−1 of phenol in dry plant. Thus, they exert antifungal and antibacterial activities. Recent studies have described that they also possess anti-inflammatory activity, inhibit mast cell degradation, and thus can be used in the treatment of asthma [25].
From the GC-MS analysis, several bioactive compounds responsible for the anti-asthmatic effect were identified. 4H-Pyran-4-one,2,3-dihydro-3,5-dihydroxy-6-methyl identified from E. hirta showed excellent anti-inflammatory potential equal to standard drug diclofenac (10 mg·kg−1) [36]. Other bioactive compounds from E. hirta, such as 2-furancarboxaldehyde [37], 5-(hydroxymethyl) [38], phthalic anhydride [39], 1,2,3 benzenetriol [40], 1,2,3-thiadiazole-4-carboxylic acid hydrazide [41], inositol [42], phytol [43], cis-vaccenic acid [44], and 3-(3,4-dimethoxyphenyl) propylamine [45], possess significant anti-inflammatory potential. Similarly, few bioactive compounds from B. monnieri, i.e., 1,E-6,Z-11-hexadecatriene [46], 1-octadecanesulphonyl chloride [47], gamma-sitosterol [48], and lupeol [49], exert anti-inflammatory potential. The enhanced anti-asthmatic activity is due to the presence of all the bioactive compounds exhibiting synergistic mode of action.
Thus, based on the aforementioned studies, the present work focused on the synergistic anti-asthmatic activity of E. hirta and B. monnieri. A significant reduction in inflammatory cells was observed in EHC extracts when compared to control groups. Moreover, based on the literature review, this is the first reported work on the anti-asthmatic activity of E. hirta and B. monnieri extracts on guinea pigs. As plant extracts are used in this study, the stability and the consistency of the plants showing anti-asthmatic activity need to be determined. This limitation could be evaluated in further studies.
5 Conclusion
EHC was prepared using E. hirta and B. monnieri. A maximum zone of inhibition was observed against E. coli and S. aureus. The EHC increased the PCT against 1% histamine aerosol compared to control animals; thus, it tends to possess broncho-dilating activity and an antihistaminic effect. In the sensitized + EHC-treated animals (Group VII), the herbal composites of E. hirta and B. monnieri significantly reduced the total leukocyte, neutrophil, lymphocyte, eosinophil, and monocyte counts. EHC decreased the MDA levels denoting the reduced oxidative burden and increased the antioxidant activity by increasing the GSH level. There is a significant anti-asthmatic effect on the EHC-treated group (P < 0.05). The difference in the eosinophil and macrophage counts, serum bicarbonate level, protein, MDA, and GSH levels between the sensitized group and the EHC treated group was statistically calculated with P < 0.05 considered significant. The EHC-treated group showed decreased inflammatory cell infiltration compared to the sensitized group, indicating the anti-asthmatic property of the developed herbal composite. GC-MS analysis identified several potential bioactive compounds, and FT-IR analysis revealed the functional groups corresponding to plant extracts. Thus, from the present research, it is evident that composites of E. hirta and B. monnieri (both of which are seen in different parts of the Kingdom) extracts are effective in the treatment of asthma and can also be used as an alternative to commercially available pharmaceutical drugs.
Acknowledgement
The authors are grateful to the Deanship of Scientific Research, Prince Sattam bin Abdulaziz University, Al-Kharj, Saudi Arabia, for its support and encouragement in conducting the research and publishing this report.
-
Funding information: We thank the Deanship of Scientific Research, Prince Sattam bin Abdulaziz University, Al-Kharj, Saudi Arabia, for its support, and this work was funded by the Deanship of Scientific Research (Project No: 2020/03/17302).
-
Author contributions: Muhammad Musthafa Poyil: concept, methodology – collection of samples like the herbs, bacterial isolates, and experimental animals, microbiological analyses, writing – original draft, writing – review and editing; Mohammed H. Karrar Alsharif: methodology – histopathological analyses, statistical analyses, formal analysis, resources; Vidya Devanathadesikan Seshadri: methodology – biochemical analyses, funding acquisition, project administration, writing – review and editing.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Shen Y, Huang S, Kang J, Lin J, Lai K, Sun Y, et al. Management of airway mucus hypersecretion in chronic airway inflammatory disease: Chinese expert consensus (English edition). Int J Chron Obstruct Pulmon Dis. 2018;13:399–407.10.2147/COPD.S144312Search in Google Scholar
[2] Rai S, Patil A, Vardhan V, Marwah V, Pethe M, Pandey I. Best Treatment Guidelines for Bronchial Asthma. Med J Armed Forces India. 2007;63:264–8.10.1016/S0377-1237(07)80151-1Search in Google Scholar
[3] Global Initiative for Asthma (GINA). Pocket Guide Asthma Manag Prev. 2015;P-3.Search in Google Scholar
[4] Alansari D, Mirza TA. Assessment of asthma control among asthmatic patients at primary healthcare centers in Makkah, Saudi Arabia. Cureus. 2020;12(10):e11103. 10.7759/cureus.11103.Search in Google Scholar PubMed PubMed Central
[5] Mohammed O, Ghobain A, Algazlan SS, Talal MO. Asthma prevalence among adults in Saudi Arabia. Saudi Med J. 2018;39(2):179–84.10.15537/smj.2018.2.20974Search in Google Scholar PubMed PubMed Central
[6] Alahmadi TS, Banjari MA, Alharbi AS. The prevalence of childhood asthma in Saudi Arabia. Int J Pediat Adolescent Med. 2019:6(2):74–7. ISSN 2352-6467. 10.1016/j.ijpam.2019.02.004.Search in Google Scholar PubMed PubMed Central
[7] Moradi-Lakeh M, El Bcheraoui C, Daoud F, Tuffaha M, Kravitz H, Al Saeedi M, et al. Prevalence of asthma in Saudi adults: Findings from a national household survey, 2013. BMC Pulmonary Med. 2015;15:77. 10.1186/s12890-015-0080-5.Search in Google Scholar PubMed PubMed Central
[8] Hamam Fayez EA, Albarraq Ahmad KM, Kaabi Yahya AA, Al Faifi Yahya AS, Al Harbi A. The prevalence of asthma and its related risk factors among the children in Taif area, Kingdom of Saudi Arabia. Saudi J Health Sci. 2015;4(3):179–84. 10.4103/2278-0521.171436.Search in Google Scholar
[9] Al-Ghamdi BR, Mahfouz AA, Abdelmoneim, Khan MY, Daffallah AA. Altitude and bronchial asthma in south-western Saudi Arabia. East Mediterr Health J. 2008;2008(14):17–23.Search in Google Scholar
[10] Wil Lieberman-Cribbin JR, Naomi A, Stephanie T, Emanuela T. The Impact of Asthma on Mortality in Patients with COVID-19. Asthma. 2020;158(6):2290–1. 10.1016/j.chest.2020.05.575.Search in Google Scholar PubMed PubMed Central
[11] Eger K, Bel EH. Asthma and COVID-19: do we finally have answers? Eur Respir J. 2021;4(57(3)):2004451. 10.1183/13993003.04451-2020. PMID: 33380511; PMCID: PMC7778875.Search in Google Scholar PubMed PubMed Central
[12] Izquierdo JL, Almonacid C, González Y, Del Rio-Bermudez C, Ancochea J, Cárdenas R, et al. The impact of COVID-19 on patients with asthma. Eur Respir J. 2021;4;57(3):2003142. 10.1183/13993003.03142-2020.Search in Google Scholar PubMed PubMed Central
[13] Alghamdi A, Albaqami A, Areej M, Doha A, Hamad A, Baabbad A. Impact of Covid-19 on asthmatic patients in Western region in Saudi Arabia. Middle East J Family Med. 2021;19(11):58–67.10.5742/MEWFM.2021.94161Search in Google Scholar
[14] Al-Moamary MS, Alhaider SA, Alangari AA, Al Ghobain MO, Zeitouni MO, Idrees MM, et al. The Saudi Initiative for Asthma - 2019 Update: Guidelines for the diagnosis and management of asthma in adults and children. Ann Thorac Med. 2019b;14:3–48.10.4103/atm.ATM_327_18Search in Google Scholar PubMed PubMed Central
[15] Maziar Moradi-Lakeh CharbelElBcheraoui, Daoud1 Farah, Tuffaha Marwa, Kravitz Hannah. Mohammad Al Saeedi, Mohammed Basulaiman, et al. Mokdad. Prevalence of asthma in Saudi adults: findings from a national household survey. BMC Pulmonary Med. 2013;15(77):2–7.10.1186/s12890-015-0080-5Search in Google Scholar
[16] Brunton LL, Parker KL, Blumenthal DK, Buxton ILO. Goodman and gilman’s manual of pharmacology and therapeutics. 12th edn. New York: The McGraw-Hill Company; 2008.Search in Google Scholar
[17] Doeing DC, Solway J. Airway smooth muscle in the pathophysiology and treatment of asthma. J Appl Physiol. 2013;114(7):834–43.10.1152/japplphysiol.00950.2012Search in Google Scholar PubMed PubMed Central
[18] Ademola EF, Cajetan CO, Olumide MS, Ajayi AO, Fasae AJ. Drug prescription pattern for asthma among nigerian doctors in general practice: A cross-sectional survey. Ann Thorac Med. 2012;7(2):78–83.10.4103/1817-1737.94524Search in Google Scholar PubMed PubMed Central
[19] Nelson HS. Is there a problem with inhaled long-acting beta-adrenergic agonists? J Allergy Clin Immunol. 2006;117:3–16.10.1016/j.jaci.2005.10.013Search in Google Scholar PubMed
[20] Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, et al. Trends in alternative medicine use in the United States, 1990–1997: Results of a follow-up national survey. JAMA. 1998;280:1569–75.10.1001/jama.280.18.1569Search in Google Scholar PubMed
[21] Clark CE, Arnold E, Lasserson TJ, Wu T. Herbal interventions for chronic asthma in adults and children: A systematic review and meta-analysis. Prim Care Respi J. 2010;19:307–14.10.4104/pcrj.2010.00041Search in Google Scholar PubMed PubMed Central
[22] Debalke D, Birhan M, Kinubeh A, Yayeh M. Assessments of antibacterial effects of aqueous-ethanolic extracts of Sida rhombifolia aerial part. Sci World J. 2018;2018:1–8.10.1155/2018/8429809Search in Google Scholar PubMed PubMed Central
[23] Patil SB, Nilofar, Naikwade S, Chandrakant S. Review on phytochemistry and pharmacological aspects of Euphorbia hirta linn. Indian J Pharmacol. 2009;1(1):113–33.Search in Google Scholar
[24] Jain Paras P, Prasad JH, Prasad SH, Basri SF, Singh P. Phytochemical analysis of Bacopa monnieri (L.) Wettst. and their anti-fungal activities. Indian J Trad Knowl. 2017;16(2):310–8.Search in Google Scholar
[25] Pretorius Ekpo OE, Smit E. Comparative ultrastructural analyses of platelets and fibrin networks using the murine model of asthma. Expl Toxicol Pathol. 2007;59(2):105–14.10.1016/j.etp.2007.02.011Search in Google Scholar PubMed
[26] Upadhyay A, Chattopadhyay P, Goyary D, Mazumder PM, Anti-inflammatory VV. Effect of Euphorbia hirta leaf extract in rats and modulation of inflammation-associated prostaglandins (PGE-2) and nitric oxide (NO) expression in RAW264.7 macrophage. J Pharma Sci Pharmacol. 2014;1:68–73.10.1166/jpsp.2014.1004Search in Google Scholar
[27] Zilva JF, Pannall PR. Hydrogen ion Homeostasis: Blood gas level in clinical chemistry in diagnosis and treatment. Lloyd-lake Lond. 1979:78–113. Chapter IV.Search in Google Scholar
[28] Mathur A, Verma S, Purohit R, Singh S, Mathur D, Prasad GBKS, et al. Pharmacological investigation of Bacopa monnieri on the basis of antioxidant, antimicrobial and anti-inflammatory properties. J Chem Pharma Res. 2010;2(6):191–8.Search in Google Scholar
[29] Prajapati MS, Shah MS, Saluja AK, Shah UD, Shah SK. Antiasthmatic activity of methanolic extract of Sphaeranthus indicus. Int J Pharma Phytochem Res. 2010;2(3):15–9.Search in Google Scholar
[30] Gijón E, García X, Contreras-Barrios MA, Valencia J, Magos G, Lorenzana-Jiménez M. Effects of Struthanthus venetus methanol extract in the guinea pig heart. Proc West Pharmacol Soc. 2010;53:26–8.Search in Google Scholar
[31] Hossain H, Al-Mansur A, Akter S, Sara U, Ahmed R, Jahangir AA. Evaluation of anti-inflammatory activity and total tannin content from the leaves of Bacopa monnieri (Linn.). Intl J Pharma Sci Res. 2014;5(4):1246–52.Search in Google Scholar
[32] Buege JA, Aust SD. Microsomal lipid peroxidation method. Enzymol. 1978;52:302–10.10.1016/S0076-6879(78)52032-6Search in Google Scholar
[33] Yamanel L, Kaldirim U, Oztas Y, Coskun O, Poyrazoglu Y, Durusu M, et al. Ozone therapy and hyperbaric oxygen treatment in lung injury in septic rats. Int J Med Sci. 2011;8(1):48–55.10.7150/ijms.8.48Search in Google Scholar PubMed PubMed Central
[34] Ricciardolo FL, Nijkamp F, De Rose V, Folkerts G. The guinea pig as an animal model for asthma. Cur Drug Targets. 2008;9:452–65.10.2174/138945008784533534Search in Google Scholar PubMed
[35] Smith N, Broadley KJ. Optimisation of the sensitization conditions for an ovalbumin challenge model of asthma. Int Immunopharmacol. 2007;7:183–90.10.1016/j.intimp.2006.09.007Search in Google Scholar PubMed
[36] Wu Z, Zhou D, Chen G, Lee L. Airway hyperresponsiveness to cigarette smoke in ovalbumin-sensitized guinea pigs. Am J Res Crit Care Med. 2000;161:73–80.10.1164/ajrccm.161.1.9809121Search in Google Scholar PubMed
[37] Kay AB, Phipps S, Robinson DS. A role for eosinophils in airway remodelling in asthma. Trends Immunol. 2004;25:477–82.10.1016/j.it.2004.07.006Search in Google Scholar PubMed
[38] Ashwathanarayana R, Naika R. Anti-inflammatory properties of Pavetta Crassicaulis bremek. leaf and flower crude extracts and its pure compounds collected from Western Ghats, Karnataka, India. Asian J Pharm Clin Res. 2018;11(9):72–90.10.22159/ajpcr.2018.v11i9.21885Search in Google Scholar
[39] Zhao L, Chen J, Su J, Lin L, Hu S-Q, Li B, et al. In vitro antioxidant and antiproliferative activities of 5-hydroxymethyl furfural. J Agric food Chem. 2013;61. 10.1021/jf403098y.Search in Google Scholar PubMed
[40] Kajal A, Bala S, Kamboj S, Saini V. Synthesis, characterization, and computational studies on phthalic anhydride-based benzylidene-hydrazide derivatives as novel, potential anti-inflammatory agents. Med Chem Res. 2014;23:23–2689. 10.1007/s00044-013-0848-1.Search in Google Scholar
[41] Nicolis E, Lampronti I, Dechecchi M, Borgatti M, Tamanini A, Bianchi N, et al. Pyrogallol, an active compound from the medicinal plant Emblica officinalis, regulates expression of pro-inflammatory genes in bronchial epithelial cells. Int Immunopharma. 2008;8:1672–80. 10.1016/j.intimp.2008.08.001 Search in Google Scholar PubMed
[42] Paruch K, Popiołek Ł, Biernasiuk A, Berecka-Rycerz A, Malm A, Gumieniczek A, et al. Novel derivatives of 4-methyl-1,2,3-hiadiazole-5-carboxylic acid hydrazide: synthesis, lipophilicity, and in vitro antimicrobial activity screening. Appl Sci. 2021;11:1180. 10.3390/app 11031180.Search in Google Scholar
[43] Wee Y, Yang C-H, Chen S-K, Yen Y-C, Wang C-S. Inositol hexaphosphate modulates the behavior of macrophages through alteration of gene expression involved in pathways of pro- and anti-inflammatory responses, and resolution of inflammation pathways. Food Sci Nutr. 2021;9:3240–9. 10.1002/fsn3.2286.Search in Google Scholar
[44] Islam MT, Ayatollahi SA, Zihad S, Sifat N, Khan MR, Paul A, et al. Phytol anti-inflammatory activity: Pre-clinical assessment and possible mechanism of action elucidation. Cell Mol Biol (Noisy-le-Grand, Fr). 2020;66(4):264–9.10.14715/cmb/2020.66.4.31Search in Google Scholar
[45] Jacome-Sosa M, Vacca C, Mangat R, Diane A, Nelson RC, Reaney MJ, et al. Vaccenic acid suppresses intestinal inflammation by increasing anandamide and related N-acylethanolamines in the JCR:LA-cp rat. J Lipid Res. 2016;57(4):638–49. 10.1194/jlr.M066308.Search in Google Scholar
[46] Panthong A, Kanjanapothi D, Niwatananant W, Tuntiwachwuttikul P, Reutrakul V. Anti-inflammatory activity of compound D {(E)-4-(3′,4′-dimethoxyphenyl) but-3-en-2-ol} isolated from Zingiber cassumunar Roxb. Phytomed: Int J Phytotherapy Phytopharma. 1997;4(3):207–12. 10.1016/S0944-7113(97)80069-4.Search in Google Scholar
[47] Wu S-Q, Xu N-Y, Zhang J, Yao S, Chu C-J. Three new acyclic diterpenoids from Eupatorium lindleyanum DC. J Asian Nat Products Res. 2012;14:652–6. 10.1080/10286020.2012.684682.Search in Google Scholar PubMed
[48] Balkrishna A, Thakur P, Varshney A. Phytochemical profile, pharmacological attributes and medicinal properties of convolvulus prostratus – A cognitive enhancer herb for the management of neurodegenerative etiologies. Front Pharmacol. 2020;11:171. 10.3389/fphar.2020.00171.Search in Google Scholar PubMed PubMed Central
[49] Rangra NK, Samanta S, Pradhan KK. In vivo anti-inflammatory potential of leaf extracts of Acacia auriculiformis benth. Indian J Pharm Sci. 2019;81(4):709–19.10.36468/pharmaceutical-sciences.562Search in Google Scholar
© 2022 Muhammad Musthafa Poyil et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

