Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
-
Gani Purwiandono
, Imam Sahroni
Abstract
Water pollution has become one of the most serious environmental issues recently, especially in relation to chemical-containing wastewater. Uncontrolled industrial waste, including large amounts of dye-containing wastewater from textile industries, needs intensive attention. In this work, the synthesis of Fe3O4@SiO2 nanocomposite biogenic silica from Salacca zalacca leaf ash was conducted for the photo-Fenton-like degradation of dye waste. The use of Salacca zalacca leaf ash and the nanoflake form is the novelty of this work. The physicochemical characterization of the material was conducted using X-ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscope (TEM), and diffuse reflectance UV-visible spectroscopy (UV-DRS) analyses, and photocatalytic activity of material was investigated in wet peroxidation of rhodamine B and batik wastewater. The results showed homogeneously dispersed Fe3O4 in SiO2 support with a nanoflake form, and a crystallite size of 44.9 nm was obtained. XRD investigation revealed the single phase of Fe3O4, which is consistent with the TEM analysis. The bandgap energy of 2.21 eV was reported from UV-DRS measurements, which influenced the increasing photocatalytic activity and reusability of the nanocomposite compared to pure Fe3O4. The photocatalyst showed the maximum degradation efficiency (DE) of 99.9% after 60 min, and the reusability feature was expressed, as there was an insignificant change in the DE over the fifth cycle of use. The material exhibited photocatalytic oxidation of batik wastewater as the removal of total suspended solids, chemical oxygen demand, and color reached 95.55%, 89.59%, and 90.00%, respectively.
1 Introduction
Water pollution has become one of the most serious environmental issues recently, especially in relation to chemical-containing wastewater. Uncontrolled industrial waste, including large amounts of dye-containing wastewater from textile industries, needs intensive attention [1,2]. Persistent chemicals and their toxicity in wastewater are known to cause serious negative effects on the aquatic environment and human life, such as carcinogenic and mutagenic effects [3,4]. Within the classification of dye-utilizing industries, batik is a popular traditional dying industry in Indonesia, and it reaches 50,000 small home industries. Considering that each company uses dyes and that the home industries discharge dye-containing wastewater in large amounts during batik production, simple, economic, and efficient techniques for wastewater treatment are required for environmental and business sustainability [5].
Many techniques have been reported for the degradation of dye contaminants, such as Fenton and photo-Fenton oxidation procedures, including photocatalytic peroxidation (PCPO) [1]. These procedures have been noted to be more effective compared with adsorption or other methods due to their low cost and ease of use. Complete oxidation of organic compounds producing harmless CO2 and H2O is possible in these methods. The photoactive materials for these applications are mainly iron oxide-based materials, and in order to enhance their photocatalytic activity, structures supporting iron oxide within solid supports have been developed [6,7,8]. Within this scope, some papers have notified that Fe3O4 dispersed into silica gives higher stability and photocatalytic activity enhancement due to the nanostructure formation [9]. Previous investigations have reported the immobilization of an Fe2O3–Fe3O4 mixture in biogenic silica obtained from bamboo leaf ash and the immobilization of Fe2O3 in SiO2 extracted from rice husk ash [10,11].
From the perspective of the development of magnetite nanostructures and the utilization of agricultural waste, this research aimed to evaluate the capability of Fe3O4 dispersed in biogenic silica (Fe3O4@SiO2) obtained from snake fruit (Salacca zalacca) leaf ash (SLA) as a photocatalyst. Snake fruit is widely cultivated in Indonesia, and until now, its leaves have not been utilized. Previous studies have extracted silica from snake fruit and reported the high yield of silica with high specific surface area, and these inspired to create the innovation of the use of SLA as the raw material of the photocatalyst. The evolution of the physicochemical character of these materials consists of the formation of a porous structure, bandgap energy, and chemical stability, which are advantageous for such adsorption and photocatalytic applications [12].
In referring to the physicochemical performance of the extracted silica, a potential development for supporting Fe3O4 was conclusively obtained, and to our knowledge, it has not yet been reported. A physicochemical study of the Fe3O4@SiO2 synthesized using SLA and a kinetics study in the photocatalytic oxidation reaction were conducted in this research. In particular, rhodamine B (RhB) was chosen based on its widespread use in many industries, including the small-scale coloring industry (the batik industry), which potentially discharges RhB into its wastewater. RhB-contaminated water and the batik industry’s wastewater must be treated due to the carcinogenicity and damage imposed on the aquatic environment. Based on these backgrounds, this research aimed to study the physicochemical characterization of Fe3O4@SiO2 synthesized using SLA and the mechanistic insight into its activity as a photocatalyst in photocatalytic wet peroxidation and adsorption of rhodamine B and contaminants in batik wastewater.
2 Materials and methods
2.1 Materials
Salacca zalacca leaves were obtained from the agro-industrial area in Sleman, Yogyakarta Province, Indonesia. Chemicals consisting of NaOH, HCl, cetyl trimethyl ammonium bromide (CTMA), FeCl2·4H2O, and FeCl3·6H2O in the analytical grade were purchased from Merck-Millipore (Darmstadt, Germany).
2.2 Preparation of materials
SiO2 twas extracted from SLA using a procedure previously published [12]. About 10 g of SLA was refluxed with a 1 M NaOH solution for 1 h. The resulting mixture was then filtered, and the filtrate was titrated with 1 M HCl until a white gel was produced and a pH of 7.4 was reached. The precipitate was then kept in a hot air oven at 80°C overnight before sintering at 500°C to obtain a dry powder.
Fe3O4@SiO2 was prepared by the dispersion of an iron oxide precursor into a SiO2 slurry at an Fe content set up at 15 wt%. The SiO2 slurry was prepared by dispersing the SiO2 powder in double-distilled water, and then, a solution of CTMA of 2% was added. The precursor solution was prepared by mixing FeCl2·4H2O and FeCl3·6H2O in an Fe(ii):Fe(iii) molar ratio of 1:4, followed by the addition of 0.1 M NaOH at an Fe:−OH molar ratio of 1:1. The mixture was stirred for 4 h and then hydrothermally treated in an autoclave overnight at 150°C. The resulting sample from these steps was cooled at −2°C before being spray-dried, and the powder was then calcined at 500°C for 4 h. For comparison purposes, Fe3O4 nanoparticles were also prepared by a similar method but without dispersion into a SiO2 slurry.
2.3 Characterization of materials
The XRD spectra of the materials were obtained with a Rigaku XRD instrument. A Ni-filtered Cu Kα radiation source (λ = 1.54 Å) was utilized as the radiation source, and the measurement was taken at the 2θ range from 10° to 90° at a scanning rate of 4°·min−1 and a step-size increase of 0.02°. A JASCO V760 spectrophotometer was employed for diffuse reflectance UV-visible analysis (UV-DRS) and photoluminescence (PL) spectroscopy analysis. The surface morphology of the material was determined using a scanning electron microscope-energy dispersive X-ray spectrophotometer (SEM-EDX) Phenom X, and the particle form and size were identified using a TEM on JEOL JEM 2100, which was operated with an acceleration voltage of 200 kV with a resolution of 0.1 nm. Surface profiles of the materials consisting of specific surface area, pore distribution, and pore radius parameters were recorded using gas sorption analysis on a porosimeter Nova1200 (Quantachrome) with nitrogen gas. The sample outgassing was performed at 95°C for 3 h prior to the analysis.
2.4 Photocatalytic activity evaluation
The photocatalytic activity of Fe3O4@SiO2 was examined using photocatalytic degradation (PC) and PCPO of RhB and batik wastewater. The reactions on RhB were performed in a water-jacketed batch reactor equipped with a lamp in the center of the reactor. For the PC treatment, about 500 mL of an RhB 20 mg·L−1 solution was added with 0.25 g of photocatalyst and 0.5 mL of H2O2 30%. Light illumination was conducted using a UV lamp (40 W, 295 nm) and a xenon lamp (40 W). The difference between photocatalysis and photocatalytic oxidation is in the addition of oxidant in photocatalytic oxidation, while the photocatalysis process is without oxidant addition. For RhB solution as the tested dye, the progress of the reaction was monitored by ultraviolet visible spectroscopy using a colorimetric analytical method. The degradation efficiency (DE) of the treatment to RhB was calculated using the following formula:
where C 0 and C t are the parameters at the initial time and at the time t, which are the initial concentration and the concentration at the sampling time, respectively, determined using UV-visible (UV-vis) spectrophotometric analysis.
The photocatalytic activity of Fe3O4@SiO2 for batik wastewater treatment was performed similar to the treatment of RhB, but the photocatalyst dosage was varied at 5, 10, 20, and 25 g·L−1, and the evaluation was based on decreasing the total suspended solid (TSS), chemical oxygen demand (COD), and color. The TSS assays were conducted using the gravimetric analysis method, while COD was determined by applying chromate digestion followed by spectrophotometric analysis. The color removal efficiency was determined by comparing the absorbance values of the treated solutions at 500 nm, which is the wavelength having maximum absorbance for the wastewater sample. The parameters of the batik wastewater quality are listed in Table 1.
The parameters of batik’s wastewater quality
| Parameter | Value |
|---|---|
| COD (mg·L−1) | 658 |
| TSS (mg·L−1) | 2,400 |
| Color (absorbance at 500 nm) | 0.987 |
2.5 Adsorption study
The adsorption study of Fe3O4@SiO2 was evaluated for RhB as the control treatment, under similar conditions, but without light illumination.
3 Results and discussion
3.1 Physicochemical characterization of Fe3O4@SiO2
XRD analysis is the most important analysis for phase identification in material synthesis; therefore, XRD analysis was performed before other characterization techniques. Figure 1 demonstrates the XRD pattern of Fe3O4@SiO2 in comparison with those of SiO2 and Fe3O4. The diffraction peaks of Fe3O4 appear at 2θ ∼ 30.3, 34.9, 43.1, 53.4, and 56.2, which are matched with the crystal planes of (2 2 0), (3 1 1), (4 0 0), (4 4 2), and (5 1 1), according to JCPDS card number 00-019-0629 [13,14,15]. Similar reflections are found in Fe3O4@SiO2, except for the (4 4 2) peak, indicating the dispersion of Fe3O4 in the silica support. By using the corresponding peaks, the crystallite size of Fe3O4 was calculated based on the Scherer equation:
where d is the mean crystalline size of the nanoparticles, λ is the wavelength of the radiation (1.5406 Å), θ is the angle of the selected reflection, and B is the intensity of full width at half maximum (FWHM) of the selected reflection.
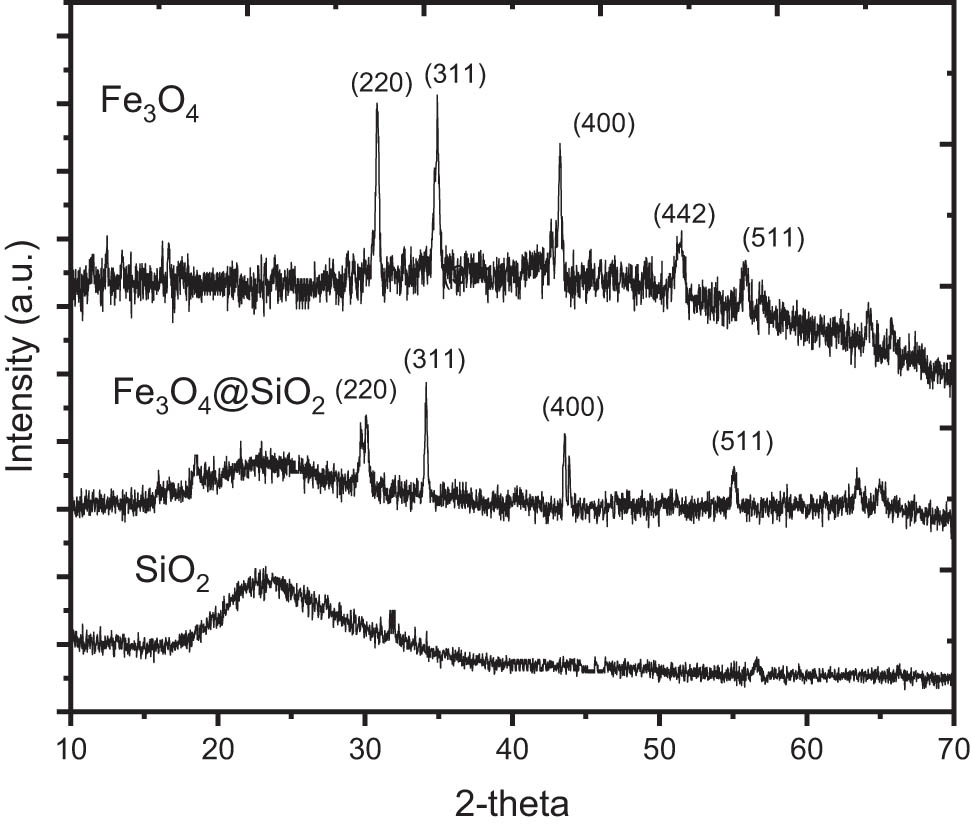
XRD patterns of Fe3O4@SiO2 in comparison with Fe3O4 and SiO2.
The calculated data are presented in Table 2, and from the calculations, it can be concluded that the mean particle size of Fe3O4 nanoparticles in Fe3O4@SiO2 is 44.9 nm.
Calculated crystallite size from XRD measurement
| 2θ | Fe3O4 | Fe3O4@SiO2 | ||
|---|---|---|---|---|
| FWHM | Crystallite size | FWHM | Crystallite size | |
| 30.3 | 0.143 | 63.8 | 0.128 | 59.8 |
| 34.9 | 0.145 | 67.6 | 0.149 | 50.0 |
| 43.1 | 0.227 | 39.9 | 0.179 | 38.8 |
| 56.2 | 0.158 | 57.2 | 0.235 | 31.3 |
| Crystallite size (nm) | 57.1 | 44.9 | ||
Interestingly, SEM studies (Figure 2) revealed that Fe3O4@SiO2 composite is formed in the shape of nanoflakes. Referring to previous studies on the synthesis of Fe3O4, the nanoflake formation can be attributed to the formation of the goethite phase as an intermediate in the crystallite growth, in reference to the following reaction:
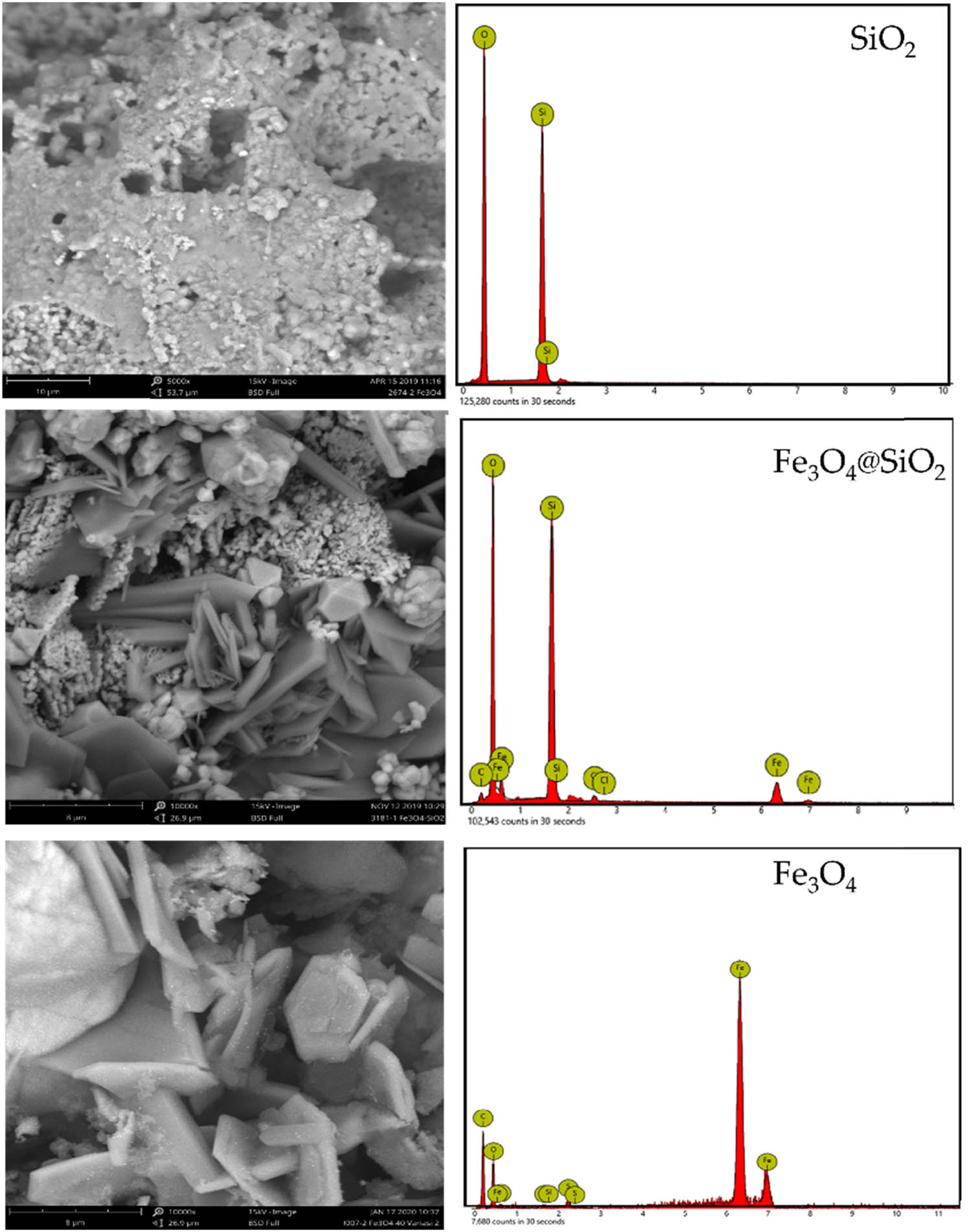
SEM images of Fe3O4@SiO2, Fe3O4, and SiO2.
This condition is probably facilitated by the hydrothermal condition, and in addition, the presence of support in the dispersion system contributed to generating overpressure for directing particle growth [16]. Moreover, the addition of CTMA as a template governed well-distributed nanoparticles for creating the flaky structure. This nanoflake structure also appeared for pure Fe3O4 as a comparison in the synthesis. A similar morphology has appeared in the preparation of Fe3O4 under the hydrothermal method [17] and in the dispersed Fe3O4 in carbon support [18]. Further EDS analyses gave the composition of the nanocomposite, as listed in Table 3. The Fe and O elements predominately existed in the Fe3O4@SiO2 and Fe3O4 samples, and in particular, the Fe content was 16.9%, which is slightly higher than the set-up amount (15%).
Elemental analysis of materials
| Element | SiO2 | Fe3O4 | Fe3O4@SiO2 |
|---|---|---|---|
| O | 67.97 | 31.81 | 50.28 |
| Si | 32.03 | n.d. | 26.05 |
| Fe | n.d | 68.19 | 16.90 |
n.d., not detected.
A detailed analysis of the Fe3O4 nanoparticles dispersed in the SiO2 support was performed by TEM analysis with the results presented in Figure 3. The irregular forms distributed on the silica material appeared in the Fe3O4@SiO2 image as identified from the darker spots. Referring to the pattern, the dispersed particle sizes are within the range of 20–50 nm, which is in confirmation with the crystallite size calculated using XRD measurements mentioning the mean particle size of 44.9 nm. The range is smaller compared to the particle size distribution of pure Fe3O4 nanoparticles.
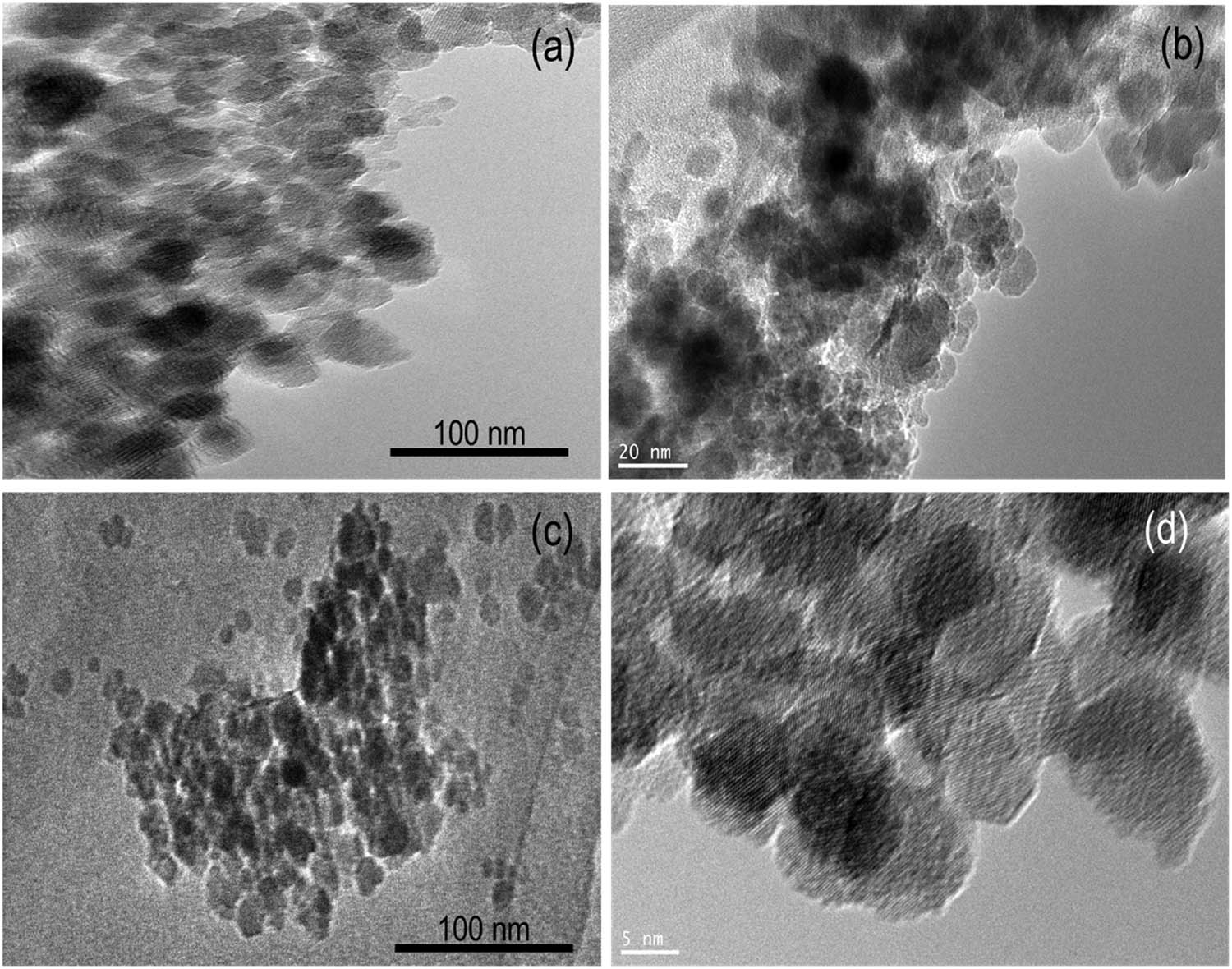
TEM images of (a and b) Fe3O4@SiO2 and (c and d) Fe3O4 in different magnifications.
3.2 Optical properties of Fe3O4@SiO2
Optical properties of Fe3O4@SiO2 were studied using UV-DRS analysis. Figure 4 shows the UV-DRS, Tauc plot, and PL spectra of the prepared photocatalyst and Fe3O4. Obviously, Fe3O4 and Fe3O4@SiO2 exhibit absorption in the 200–550 nm region. Moreover, Fe3O4@SiO2 demonstrates an absorption region similar to that of Fe3O4. The bandgap energy values were determined using a Tauc plot with the following equation:
where α, h, ν, E g, and A are the absorption coefficient, Planck’s constant, the light frequency, the bandgap energy, and a constant, respectively. The extrapolation of the linear portion of the (α h ν)2 curve versus hν to zero was utilized for bandgap energy estimation, and it was found that the bandgap energy of Fe3O4@SiO2 and Fe3O4 materials is 2.21 and 2.19 eV, respectively. This suggests that the dispersion of magnetite into silica support slightly increases the bandgap energy. A similar pattern has also been reported from Fe3O4 incorporated into mesoporous silica [19,20,21], in that the bandgap energy is inversely proportional to the particle size. Theoretically, this increasing energy value is attributed to the decreasing particle size of the material, which is consistent with the smaller dispersed particle size in Fe3O4@SiO2 identified using TEM analysis. The PL spectrum in Figure 4c exhibits UV emission peaks in the range of λ = 200–400 nm and a peak in the visible region of λ = 470.50 nm. These positions correspond to the emission pattern of Fe3O4-containing nanocomposites in the literature [22]. The lower intensity of the peak in the visible range relative to the UV range indicates diminished electron-hole recombination [23].
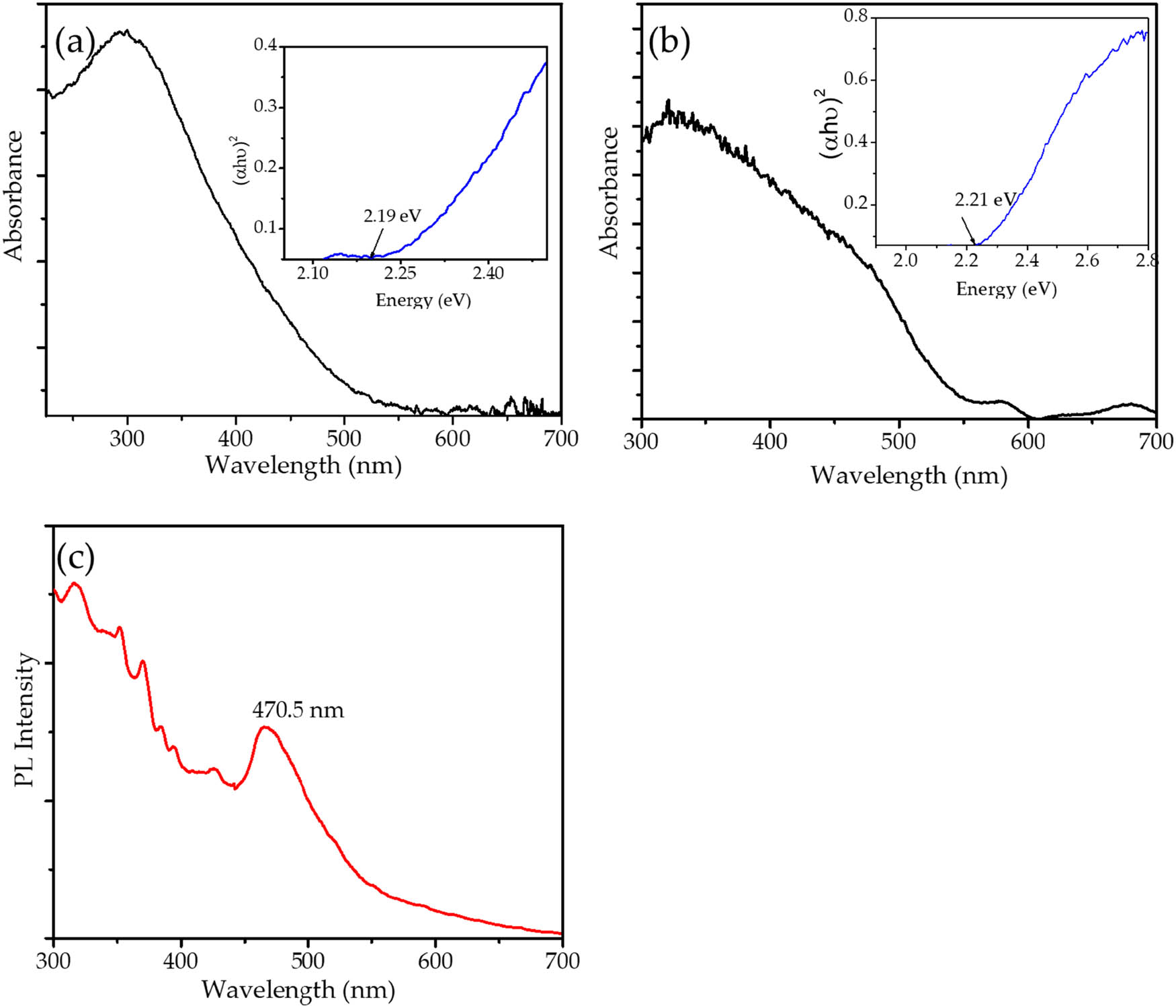
(a) UV-DRS spectrum of Fe3O4; (b) UV-DRS spectrum of Fe3O4@SiO2; and (c) PL spectrum of Fe3O4@SiO2.
Figure 5 shows the SiO2 and Fe3O4@SiO2 adsorption–desorption isotherms and their pore distribution profile, and the calculated parameters are presented in Table 4. Enhancement of adsorption capacity appeared, as shown by a higher adsorbed volume by Fe3O4@SiO2 compared to pure SiO2 at all P/P o ranges. This is confirmed by the higher specific surface area and pore volume of Fe3O4@SiO2, and moreover, the pore distribution reveals that the higher BET-specific surface area, external surface area, and pore volume parameters are related to the formation of modal pores at around 6.45 and 40 Å. The pore distribution suggests the change of porosity from the microporous material into the combination of microporous and mesoporous classification.

(a) Adsorption–desorption profile; (b) pore distribution of Fe3O4@SiO2 in comparison with SiO2.
Surface parameters of Fe3O4@SiO2 in comparison with SiO2
| Parameter | SiO2 | Fe3O4@SiO2 |
|---|---|---|
| BET-specific surface area (m2·g−1) | 95.62 | 79.56 |
| External surface area (m2·g−1) | 44.69 | 20.84 |
| Pore volume (cc·g−1) | 0.77 | 0.45 |
| Pore radius | 18.29 | 13.34 |
3.3 Photocatalytic activity
The comparisons of RhB removal over varied treatments – adsorption, PC, and PCPO over Fe3O4@SiO2 – are presented by the kinetics of RhB removal in Figure 6. As expected, the removal by the photocatalytic process gave significant enhancement in RhB removal compared to the adsorption process. In more detail, the kinetics plots revealed that the photocatalytic activity of the RhB removal over PC and PCPO is higher compared to the adsorption process, in the following order: PCPO + UV > PCPO + Vis > PCPO + Vis > PC + Vis > adsorption.

Kinetics plot of RhB removal utilizing various methods on (a) Fe3O4@SiO2 and (b) Fe3O4.
Moreover, kinetics analysis of RhB removal over PC and PCPO shows that the processes obey second-order kinetics by the following equation:
where C t and C 0 are the concentrations of RhB at the time t and at the initial time, respectively, and k obs is the observed kinetics constant [24,25]. The kinetics plots in Figure 7 show that the highest kinetics constant is observed from the PCPO process under UV and visible light over Fe3O4@SiO2 and Fe3O4, and in more detail, the calculated parameters are presented in Table 5.

Second-order plots of RhB PCPO processes over Fe3O4@SiO2 and Fe3O4.
Calculated parameters from kinetics studies
| Material | Process | Kinetics constant (mg·min·L−1) | Kinetics equation | R 2 | DE (%) |
|---|---|---|---|---|---|
| Fe3O4@SiO2 | Adsorption | 1.978 × 10−3 |
|
0.987 | 18.1 |
| Fe3O4@SiO2 | PC + UV | 2.630 × 10−2 |
|
0.973 | 75.9 |
| Fe3O4@SiO2 | PCPO + UV | 6.525 |
|
0.991 | 99.9 |
| Fe3O4@SiO2 | PC + Vis | 0.251 |
|
0.990 | 70.2 |
| Fe3O4@SiO2 | PCPO + Vis | 3.385 × 10−3 |
|
0.991 | 96.8 |
| Fe3O4 | Adsorption | 9.926 × 10−4 |
|
0.987 | 10.1 |
| Fe3O4 | PC + UV | 2.023 × 10−2 |
|
0.982 | 67.9 |
| Fe3O4 | PCPO + UV | 3.425 × 10−2 |
|
0.989 | 78.5 |
| Fe3O4 | PC + Vis | 1.018 × 10−2 |
|
0.993 | 56.9 |
| Fe3O4 | PCPO + Vis | 2.228 × 10−2 |
|
0.979 | 68.5 |
| SiO2 | Adsorption | 1.297 × 10−3 |
|
0.978 | 12.8 |
From the parameters presented in Table 5, it is seen that the kinetics constant and the DE of the processes utilizing Fe3O4@SiO2 demonstrated higher activities compared to the use of Fe3O4 alone for all mechanisms. In addition, from the DE values, it is seen that the nearly complete removal of RhB was attained by PCPO using Fe3O4@SiO2, which shows a higher efficiency compared to the DE achieved by Fe3O4 alone. This result is due to the immobilized Fe3O4 on the SiO2 support. For both Fe3O4@SiO2 and Fe3O4, the PCPO processes gave higher kinetics constants and DE values, suggesting the role of H2O2 as an oxidant in the photocatalytic system.
The presence of Fe3O4 as a photoactive material leads to the formation of radicals and oxidizing agents via its interaction with photons by the following mechanism [26,27]:
The production of radicals is enhanced by the addition of an oxidant, producing faster propagation steps within the organic molecule degradation. The comparison between the use of UV light and visible light implied the higher feasibility of UV light exposure for accelerating the reaction mainly at the initial step of radical formation, and by the additional time of treatment, it is seen that the removal reached similar results for both PC and PCPO. Based on the DE, the photocatalytic activity of Fe3O4@SiO2 in comparison with other materials is presented in Table 6 [28,29,30,31,32,33].
Photocatalytic activity of Fe3O4@SiO2 in comparison with other materials
| Material | Remark | DE (%) | Reference |
|---|---|---|---|
| SiO2@TiO2 nanospheres | Maximum DE was achieved at 4 h of treatment | ∼99.9 | [28] |
| ZnO ceramic | Maximum DE was achieved at 2 h | 40 | [29] |
| BiMnO3 nanoparticles | Photocatalyst dosage of 0.4 g·L−1, treatment for 2 h using 0.5 mL H2O2 | 90 | [30] |
| W(N x S1−x )2 nanoflowers | Photocatalyst dosage of 0.2 g·L−1, treatment for 1 h using 0.5 mL H2O2 under visible light | 50 | [31] |
| Co3O4-Bi2O3 | Photocatalyst dosage of 5.0 g·L−1, treatment for 2 h under visible light | 92 | [32] |
| α-Fe2O3 | Maximum DE was achieved at pH 10, photocatalyst dosage of 0.8 g·L−1 for 40 min under UV light | 90.13 | [33] |
| Fe2O3/SiO2 | SiO2 was extracted from Bamboo leaf ash, [RhB] = 20 mg·L−1, catalyst dosage = 0.2 g/100 mL, H2O2 = 1 mL·L−1 for 2 h | 99.00 | [10] |
| Fe3O4@SiO2 | Maximum DE was achieved at pH 7, photocatalyst dosage of 0.5 g·L−1 for 60 min under UV light, [RhB] = 20 mg·L−1, H2O2 = 1 mL·L−1 | 99.9 | This work |
Conclusively, the photocatalytic activity of Fe3O4@SiO2 in this work is comparable to other photocatalyst materials. The renewable resource of biogenic silica extracted from SLA becomes competitive in this degradation application.
3.4 Role of support
From the data given in Table 4, it is seen that the adsorption rate of RhB is in the following order: Fe3O4@SiO2 > SiO2 > Fe3O4. Fe3O4@SiO2 represents the higher capability of the composite form to adsorb RhB.
The kinetics data were evaluated according to Lagergren’s pseudo-first order, Ho and McKay’s pseudo-second, and Weber and Morris’s intraparticle diffusion models based on the following equations:
where q e (mg·g−1) is the adsorption capacity, q t (mg·g−1) is the amount of adsorbed metal ions at the time t, k (min−1) is the first-order rate constant, k 2 [g·(mg·min)−1] is the second-order rate constant of adsorption (min−1), and k i (mg·min1/2·g−1) and C are constants, the kinetics constant and the constant of the intra-particle diffusion model, respectively [34,35].
The kinetic parameters are listed in Table 7. Referring to the R 2 values, the adsorption by all samples represents the best fit with the intra-particle diffusion model, and the plots from these kinetics calculations are presented in Figure 8.
Calculated parameters from adsorption kinetics studies
| Material | R 2 | q e (mg·g−1) | ||
|---|---|---|---|---|
| Lagergren’s pseudo-first order | Ho and McKay’s pseudo-second | Weber and Morris’ intraparticle diffusion | ||
| Fe3O4@SiO2 | 0.977 | 0.985 | 0.992 | 5.6 |
| Fe3O4 | 0.949 | 0.545 | 0.995 | 4.3 |
| SiO2 | 0.990 | 0.245 | 0.995 | 8.6 |

Intra-particle diffusion plots of RhB adsorption by Fe3O4@SiO2, SiO2, and Fe3O4.
The fitness of the kinetics data with the pseudo-first-order kinetics suggests that the adsorption by Fe3O4@SiO2 is largely controlled by the internal diffusion of the molecules, and so, this is the rate-controlling step during the adsorption process. The diffusion itself is influenced by the surface area, pore structure, and reactivity of the surface. In general, the small particle sizes of the adsorbent lead to the higher affinity of the adsorbate. Considering that the specific surface area of Fe3O4@SiO2 is less than the specific surface area of SiO2, it can be noted that the presence of the dispersed Fe3O4 increased the affinity by possible chemisorption. In addition, the higher adsorption capability of Fe3O4@SiO2 compared to Fe3O4 alone indicates that the porous structure available in Fe3O4@SiO2 provides the synergistic effect of both physisorption and chemisorption. This is also confirmed by the constant (intercept) values as representative of the boundary layer effect of which Fe3O4@SiO2 has the highest value [36,37]. From this analysis, it can be concluded that photocatalysis is supported by an adsorption mechanism. The capability of Fe3O4 for conducting chemisorption is presented in the schematic diagram in Figure 9.
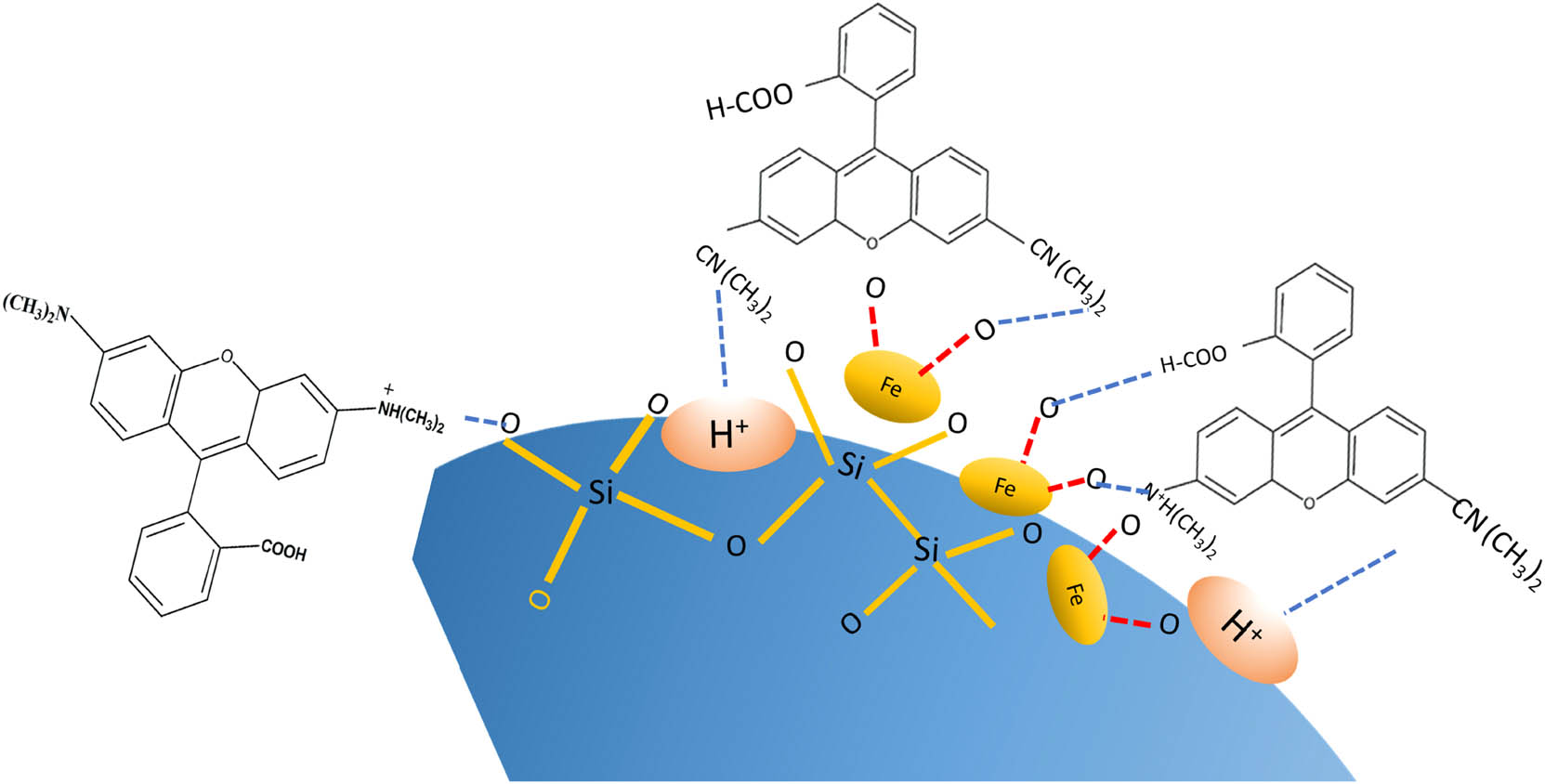
Schematic representation of adsorption mechanism of RhB using Fe3O4@SiO2.
Besides the availability of pores in the composite, the adsorption of RhB occurs due to the available hydrogen bonding and electrostatic interaction among hydrophobic and hydrophilic parts [38,39]. This assumption aligns with the kinetics and adsorption model used in studies that have reported that Fe3O4@C nanoparticles and Fe3O4@SiO2 are functionalized by polypropylene [39].
3.5 Degradation mechanism
In order to identify the mechanism of removal, UV-Vis spectrophotometry and LCMS analyses were performed. The UV-Vis spectra of PCPO-treated solution with UV light illumination are depicted in Figure 10. The pattern suggests the evolution of the RhB spectrum along with the time of treatment has not only reduced absorbance, which serves as proof of reduced concentration, but also the redshift of the characteristic peak of 556 nm, which indicates the de-ethylation and decarboxylation of the RhB structure. These possible structural changes are confirmed by the LCMS analysis presented in Figure 11.

UV-Vis spectra of initial and treated solutions.
![Figure 11
LCMS analysis of initial RhB and treated solution for 30 min: [Rhb]0 = 20 mg·L−1, time of treatment = 30 min, [H2O2] = 1 mL, light = UV, and photocatalyst dose = 0.5 g·L−1.](/document/doi/10.1515/gps-2022-0034/asset/graphic/j_gps-2022-0034_fig_011.jpg)
LCMS analysis of initial RhB and treated solution for 30 min: [Rhb]0 = 20 mg·L−1, time of treatment = 30 min, [H2O2] = 1 mL, light = UV, and photocatalyst dose = 0.5 g·L−1.
The comparison of the chromatograms of RhB for the initial and treated solutions showed proof of the degradation products. The presence of RhB is identified by the peak at a retention time of 6.3 min. As can be seen in the treated solution, some of the other peaks appeared along with the reduced peak associated with the presence of RhB. MS analyses revealed that the fraction of m/z with values of 359, 331, 181, 168, 146, and 128 is identified in the spectra. These spectra elucidate the possible mechanism of degradation, which can be described by the scheme in Figure 12 [40,41].
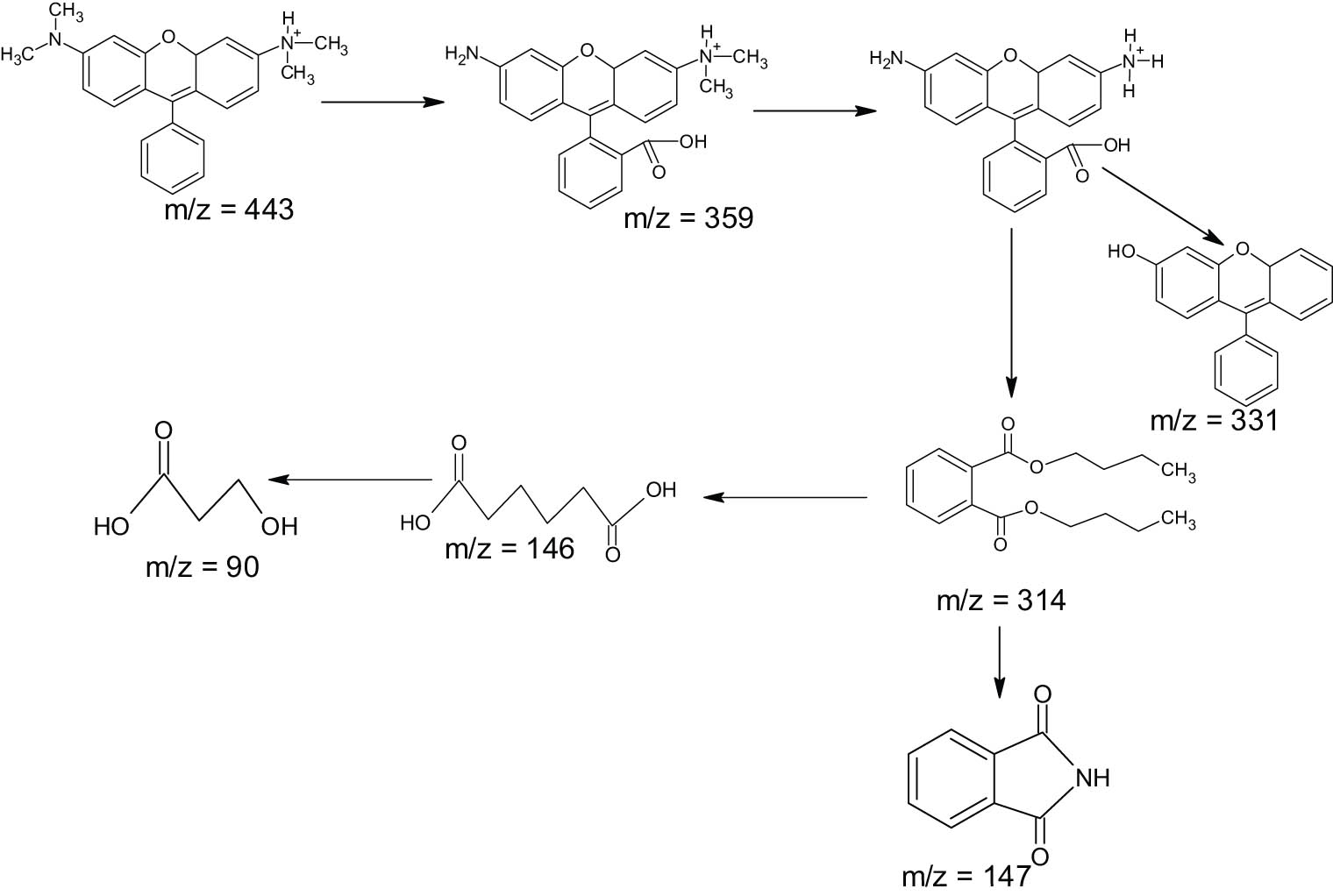
Possible degradation mechanism of RhB.
3.6 Effect of pH
In order to examine the effect of pH, PCPO treatments using Fe3O4@SiO2 were conducted at varied pH: 4, 7, 9, and 11. The kinetics of RhB removal at varied pH is presented in Figure 13.
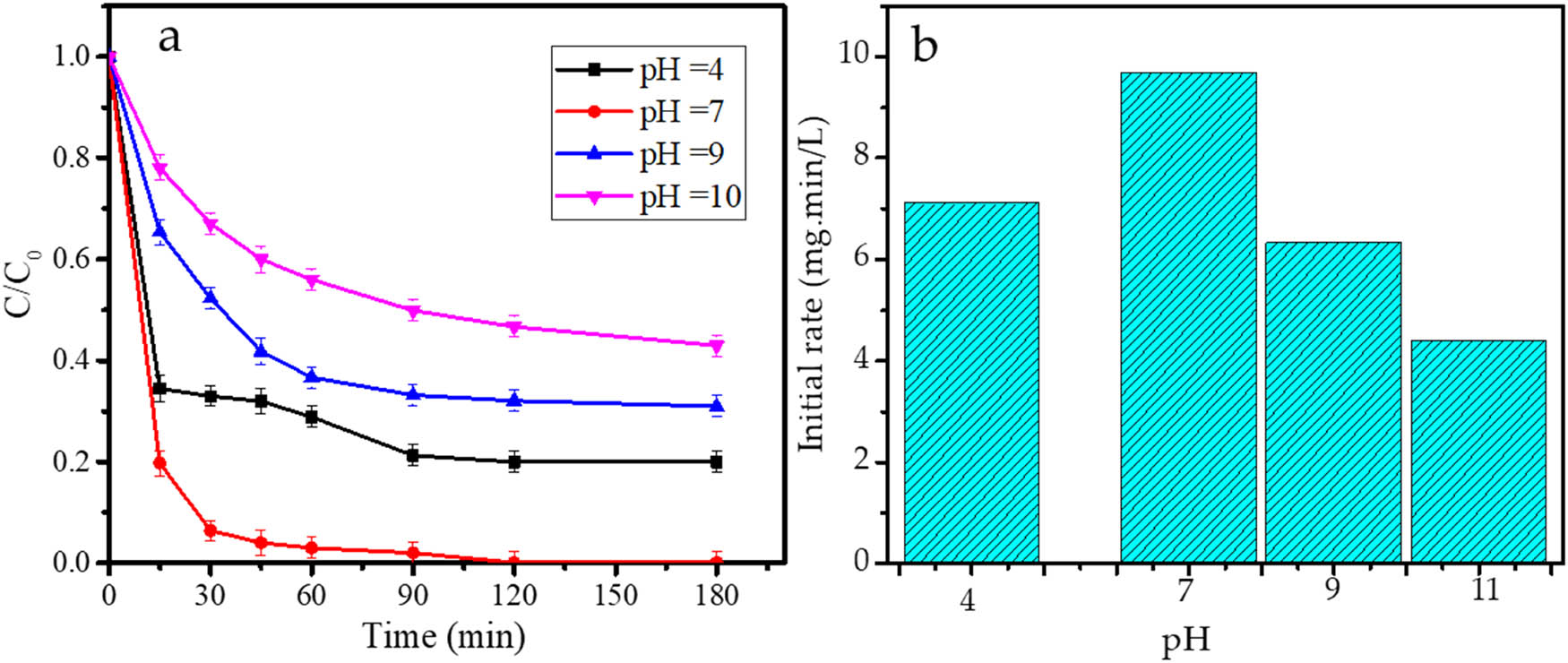
(a) Kinetics plots; (b) initial rate of PCPO treatments by Fe3O4@SiO2 at varied pH.
The kinetics plots and initial rate data showed the optimum pH at 7, and the photodegradation rate reduced under acidic and basic conditions. The trend suggests that the surface charge properties of the photocatalyst influence the interaction between the photocatalyst surface and RhB as adsorbate. Under acidic conditions, the photocatalyst, which mainly consists of an oxide structure, will be covered with protons that have a higher affinity to the surface compared with the RhB. On the other hand, basic conditions will inhibit RhB adsorption due to the electrostatic interaction between RhB with its positive charge and the hydroxyl group. Moreover, the decomposition of H2O2 is retarded by the presence of OH¯, so the propagation steps occur at a slower rate [42,43]. A similar phenomenon is also presented in the photocatalytic oxidation by TiO2 [44,45], Fe2O3 [44], and Fe3O4@SiO2@ZnO [46].
3.7 Effect of radical scavengers
To determine the reactive species controlling the degradation mechanism, a PCPO kinetics study with the addition of the hydroxy radical and hole to the photocatalytic system was conducted. Isopropanol (IPR) and ethylenediaminetetraacetic acid (EDTA) were employed as the hydroxy radical and hole scavenger, respectively. The kinetics plots of PCPO in the presence and absence of the scavenger are depicted in Figure 14.

The kinetics of PCPO in the presence and absence of the scavengers.
It can be seen from Figure 14 that the photodegradation rate decreased with the addition of IPR and increased with the addition of EDTA. IPR in the solution has the capability of trapping the hydroxy radicals or other radical forms produced from the interaction between the holes and the solution at the initiation step. The trapping inhibited propagation steps owing to their need for further RhB oxidation. In contrast, an increasing oxidation rate was attained through the hole-blocking by EDTA, as the excited electron–hole recombination was suppressed. More electrons can migrate on the surface to further produce more radicals through interaction with the solvent or O2. These results imply not only that the radicals were the dominant reactive species for the photocatalytic oxidation mechanism but also that the electron–hole recombination influenced the rate of reaction. A similar effect was also reported for the photocatalytic activity of Fe2O3 nanoparticles [47] and SnO2 [40].
3.8 Reusability of photocatalyst
The investigation of reusability is one of the most required studies of photocatalysts for applicability on the industrial scale. The examinations were based on the DE evaluation of the fresh and recycled Fe3O4@SiO2, which underwent five cycles. Recycling was conducted by filtering the powder, washing with ethanol, and recalcination at 200°C after the completion of each cycle. From the bar chart depicted in Figure 15, it can be seen that DE values were maintained with insignificant changes, as the DE reductions were no more than 10%. Thus, the prepared Fe3O4@SiO2 exhibited stability, so it is noted to be potentially developed for upscaling.

DE of PCPO process using Fe3O4@SiO2 at first–fifth cycles.
Generally speaking, the prepared Fe3O4@SiO2 exhibited excellent physicochemical properties as a photocatalyst in the PCPO process toward dye removal. As the nanocomposite is prepared by using biogenic silica extracted from SLA, the material has a high potential for being developed as a low-cost photocatalyst.
3.9 Photocatalytic activity in PCPO of batik wastewater
In order to evaluate the applicability of Fe3O4@SiO2 for industrial wastewater, the PCPO of batik wastewater under UV light was investigated. The activity was determined based on the removal of TSS, COD, and color at varied photocatalyst doses of 5, 10, 20, and 25 g·L−1. The selected doses were determined by prior trials, and similar to the treatment for RhB, the treatment without light illumination was also performed. The graphs presented in Figure 16 demonstrate the effectiveness of Fe3O4@SiO2 as a photocatalyst, as the TSS, COD, and color removals over (a) PCPO are higher compared to the adsorption process (b). It is also noted that increased removal values resulted from increased photocatalyst doses. As can be seen from the removal values, the TSS removal reached 95.55%, while the removal of COD and color is about 89.59% and 90.00%, respectively.
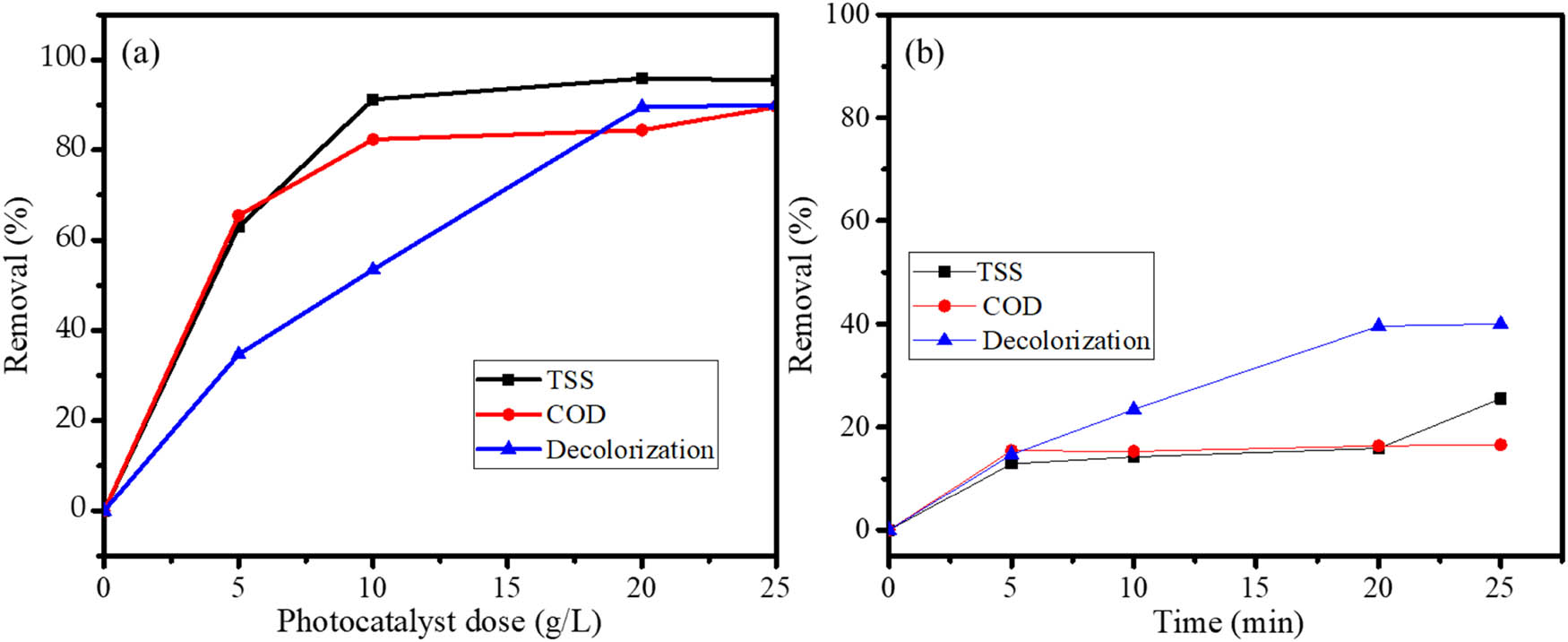
The removals of TSS, COD, and decolorization of Batik wastewater by (a) PCPO and (b) adsorption of Fe3O4@SiO2.
Investigation of reusability is one of the most required studies of photocatalysts for applicability on the industrial scale. The examinations were based on the DE evaluation of the fresh and recycled Fe3O4@SiO2, which underwent five cycles. Recycling was conducted by filtering powder, washing with ethanol, and recalcination at 200°C after the completion of each cycle. From the bar chart depicted in Figure 15, it can be seen that the DE values were maintained with insignificant changes, as the DE reductions were no more than 10%. Quantitatively, the removals are less than that of the treatment with RhB solution. In this case, batik wastewater is composed of multicomponent, being a mixture of dyes and additional preservatives in colorization. However, the effectiveness of the treatment in this work is higher compared to the photocatalytic activity of plastic-coated TiO2 [48] and titanium dioxide (TiO2)-coated aluminum plates [49], where the removal achieved was about 95% with the treatment for 5 days and 4 h, respectively.
Generally speaking, the finding in this work suggests the potency of SLA as the raw material of effective and low-cost photocatalyst for industrial application. Based on that, the results were obtained on a laboratory scale; of course, intensive studies on a larger scale including cost-effectiveness calculations are required.
4 Conclusion
The synthesis of Fe3O4@SiO2 nanoflakes has been conducted by using SiO2 derived from SLA. Physicochemical characterization of the material revealed the nanoflake form of the dispersed single-phase Fe3O4 onto silica support having a particle size of about 44.9 nm. Optical studies of the nanoflakes showed that the bandgap energy of 2.21 eV is higher compared to the bandgap energy of Fe3O4 (2.19 eV). The kinetics study of RhB removal over various methods suggested a significant increase in the photocatalytic activity of the nanoflakes compared to Fe3O4 in either the photocatalytic or photocatalytic oxidation processes. The highest DE was achieved by the photocatalytic oxidation process under UV light, in which about 99.9% of the RhB was removed. The support of Fe3O4 in SiO2 contributed to the material reusability since it gave an insignificant change in the DE until after the fifth cycle of usage.
Acknowledgement
The authors would like to express their appreciation for the support from the Ministry of Education, Culture, Research, and Technology through the World Class Professor Program in 2021.
-
Funding information: Research was funded by the Ministry of Education, Culture, Research, and Technology via World Class Research 2021.
-
Author contributions: Gani Purwiandono: formal analysis, visualization; Is Fatimah: conceptualization, methodology; Imam Sahroni: visualization, data curation; Putwi Widya Citradewi: data curation, formal analysis; Azlan Kamari: writing – review and editing; Suresh Sagadevan: supervising, project administration; Won-Chun Oh: writing – review and editing; Ruey-an Doong: methodology, funding acquisition.
-
Conflict of interest: One of the authors (Suresh Sagadevan) is a member of the Editorial Board of Green Processing and Synthesis.
References
[1] Deng Y, Zhao R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr Pollut Reports. 2015;1:167–76. 10.1007/s40726-015-0015-z.Search in Google Scholar
[2] Javaid R, Qazi UY. Catalytic oxidation process for the degradation of synthetic dyes: an overview. Int J Environ Res Public Health. 2019;16:1–27. 10.3390/ijerph16112066.Search in Google Scholar PubMed PubMed Central
[3] Gita S, Shukla SP, Saharan N, Prakash C, Deshmukhe G. Toxic effects of selected textile dyes on elemental composition, photosynthetic pigments, protein content and growth of a freshwater chlorophycean alga chlorella vulgaris. Bull Environ Contam Toxicol. 2019;102:795–801. 10.1007/s00128-019-02599-w.Search in Google Scholar PubMed
[4] Lellis B, Fávaro-Polonio CZ, Pamphile JA, Polonio JC. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol Res Innov. 2019;3:275–90. 10.1016/j.biori.2019.09.001.Search in Google Scholar
[5] Giwa A, Yusuf A, Balogun HA, Sambudi NS, Bilad MR, Adeyemi I, et al. Recent advances in advanced oxidation processes for removal of contaminants from water: a comprehensive review. Process Saf Environ Prot. 2021;146:220–56. 10.1016/j.psep.2020.08.015.Search in Google Scholar
[6] Badmapriya D, Asharani IV. Dye degradation studies catalysed by green synthesized Iron oxide nanoparticles. Int J Chemtech Res. 2016;9:409–16.Search in Google Scholar
[7] Meng L, Chan Y, Wang H, Dai Y, Wang X, Zou J. Recycling of iron and silicon from drinking water treatment sludge for synthesis of magnetic iron oxide@SiO2 composites. Environ Sci Pollut Res. 2016;23:5122–33. 10.1007/s11356-015-5742-6.Search in Google Scholar PubMed
[8] Mehrdad A, Massoumi B, Hashemzadeh R. Kinetic study of degradation of Rhodamine B in the presence of hydrogen peroxide and some metal oxide. Chem Eng J. 2011;168:1073–8. 10.1016/j.cej.2011.01.087.Search in Google Scholar
[9] Pereira MC, Oliveira LCA, Murad E. Iron oxide catalysts: Fenton and Fenton-like reactions – a review. Clays Clay Miner. 2012;47:285–302.10.1180/claymin.2012.047.3.01Search in Google Scholar
[10] Fatimah I, Amaliah SN, Andrian MF, Handayani TP, Nurillahi R, Prakoso NI, et al. Iron oxide nanoparticles supported on biogenic silica derived from bamboo leaf ash for rhodamine B photodegradation. Sustain Chem Pharm. 2019;13:100149. 10.1016/j.scp.2019.100149.Search in Google Scholar
[11] Fatimah I, Fadhilah S, Mawardani SA. γ-Fe2O3nanoparticles immobilized in SiO2 aerogel synthesized from rice husk ash for photofenton like degradation of rhodamine B. Rasayan J Chem. 2018;11:544–53. 10.7324/RJC.2018.1122067.Search in Google Scholar
[12] Fatimah I, Zaenuri FU, Doewandono LN, Yahya A, Citradewi PW, Sagadevan S. Biogenic silica extracted from salacca leaf ash for salicylic acid adsorption. Sci Technol Indones. 2021;6:296–302.10.26554/sti.2021.6.4.296-302Search in Google Scholar
[13] Alfredo Reyes Villegas V, Isaías De León Ramírez J, Hernandez Guevara E, Perez Sicairos S, Angelica Hurtado Ayala L, Landeros Sanchez B. Synthesis and characterization of magnetite nanoparticles for photocatalysis of nitrobenzene. J Saudi Chem Soc. 2020;24:223–35. 10.1016/j.jscs.2019.12.004.Search in Google Scholar
[14] Sarwar A, Wang J, Khan MS, Farooq U, Riaz N, Nazir A, et al. Iron oxide (Fe3O4)-supported SiO2 magnetic nanocomposites for efficient adsorption of fluoride from drinking water: synthesis, characterization, and adsorption isotherm analysis. Water (Switzerland). 2021;13. 10.3390/w13111514.Search in Google Scholar
[15] Zhang X, Wang G, Yang M, Luan Y, Dong W, Dang R, et al. Synthesis of a Fe3O4-CuO@meso-SiO2 nanostructure as a magnetically recyclable and efficient catalyst for styrene epoxidation. Catal Sci Technol. 2014;4:3082–9. 10.1039/c4cy00430b.Search in Google Scholar
[16] Šutka A, Lagzdina S, Juhnevica I, Jakovlevs D, Maiorov M. Precipitation synthesis of magnetite Fe3O4 nanoflakes. Ceram Int. 2014;40:11437–40. 10.1016/j.ceramint.2014.03.140.Search in Google Scholar
[17] Rudra S, Nayak AK, Koley S, Chakraborty R, Maji PK, Pradhan M. Redox-mediated shape transformation of Fe3O4 nanoflakes to chemically stable Au–Fe2O3 composite nanorods for a high-performance asymmetric solid-state supercapacitor device. ACS Sustain Chem Eng. 2019;7:724–33. 10.1021/acssuschemeng.8b04300.Search in Google Scholar
[18] Chen L, Xu X, Wan L, Zhu G, Li Y, Lu T, et al. Carbon-incorporated Fe3O4 nanoflakes: high-performance faradaic materials for hybrid capacitive deionization and supercapacitors. Mater Chem Front. 2021;5:3480–8. 10.1039/d0qm00946f.Search in Google Scholar
[19] Nikmah A, Taufiq A, Hidayat A. Synthesis and characterization of Fe3O4/SiO2 nanocomposites. IOP Conf Ser Earth Environ Sci. 2019;276. 10.1088/1755-1315/276/1/012046.Search in Google Scholar
[20] Liu T, Liu L, Liu J, Liu S, Qiao SZ. Fe3O4 encapsulated mesoporous silica nanospheres with tunable size and large void pore. Front Chem Sci Eng. 2014;8:114–22. 10.1007/s11705-014-1413-2.Search in Google Scholar
[21] Hussein EA, Kareem SH. Magnetic mesoporous silica material (Fe3O4@mSiO2) as adsorbent and delivery system for ciprofloxacin drug. IOP Conf Ser Mater Sci Eng. 2020;871. 10.1088/1757-899X/871/1/012020.Search in Google Scholar
[22] Karunakaran C, Senthilvelan S. Fe2O3-photocatalysis with sunlight and UV light: Oxidation of aniline. Electrochem Commun. 2006;8:95–101. 10.1016/j.elecom.2005.10.034.Search in Google Scholar
[23] Kurien U, Hu Z, Lee H, Dastoor AP, Ariya PA. Radiation enhanced uptake of Hg0(g) on iron (oxyhydr)oxide nanoparticles. RSC Adv. 2017;7:45010–21. 10.1039/c7ra07401h.Search in Google Scholar
[24] Shokoohi R, Dargahi A, Azami Gilan R, Zolghadr Nasab H, Zeynalzadeh D, Molla Mahmoudi M. Magnetic multi-walled carbon nanotube as effective adsorbent for ciprofloxacin (CIP) removal from aqueous solutions: isotherm and kinetics studies. Int J Chem React Eng. 2020;18:1–14. 10.1515/ijcre-2019-0130.Search in Google Scholar
[25] Samarghandi MR, Asgari G, Shokoohi R, Dargahi A, Arabkouhsar A. Removing amoxicillin antibiotic from aqueous solutions by Saccharomyces cerevisiae bioadsorbent: kinetic, thermodynamic and isotherm studies. Desalin Water Treat. 2019;152:306–15. 10.5004/dwt.2019.23941.Search in Google Scholar
[26] Zuorro A, Lavecchia R, Monaco MM, Iervolino G, Vaiano V. Photocatalytic degradation of azo dye reactive. Catalysts. 2019;9:645–61.10.3390/catal9080645Search in Google Scholar
[27] Leonard K. Green synthesis of mesoporous hematite (α – Fe2O3) nanoparticles and their photocatalytic activity. Adv Powder Technol. 2015;24:160–7. 10.1016/j.apt.2012.04.005.Search in Google Scholar
[28] Wilhelm P, Stephan D. Photodegradation of rhodamine B in aqueous solution via SiO2@TiO2 nano-spheres. J Photochem Photobiol A Chem. 2007;185:19–25. 10.1016/j.jphotochem.2006.05.003.Search in Google Scholar
[29] Ruellas TMO, Domingos GHS, Peçanha LOO, Maestrelli SC, Giraldi TR. Photodegradation of Rhodamine B catalyzed by ZnO pellets. Ceramica. 2019;65:47–53. 10.1590/0366-6913201965S12609.Search in Google Scholar
[30] Revathi B, Balakrishnan L, Pichaimuthu S, Nirmala Grace A, Krishna Chandar N. Photocatalytic degradation of rhodamine B using BiMnO3 nanoparticles under UV and visible light irradiation. J Mater Sci Mater Electron. 2020;31:22487–97. 10.1007/s10854-020-04750-4.Search in Google Scholar
[31] Liu P, Zhang J, Gao D, Ye W. Efficient visible light-induced degradation of rhodamine B by W(NxS1-x)2 nanoflowers. Sci Rep. 2017;7:70784.10.1038/srep40784Search in Google Scholar PubMed PubMed Central
[32] Saeed M, Jamal MA. Co3O4–Bi2O3 heterojunction: an effective photocatalyst for photodegradation of rhodamine B dye. Arab J Chem. 2021;15(4):103732.10.1016/j.arabjc.2022.103732Search in Google Scholar
[33] Jahagirdar A, Ahmed Z, Donappa N, Nagabhushana H, Nagabhushana BM. Photocatalytic degradation of Rhodamine B using nanocrystalline α-Fe2O3. J Mater Environ Sci. 2014;5:1426–33.Search in Google Scholar
[34] Zou C, Liang J, Jiang W, Guan Y, Zhang Y. Adsorption behavior of magnetic bentonite for removing Hg(ii) from aqueous solutions. RSC Adv. 2018;8:27587–95. 10.1039/c8ra05247f.Search in Google Scholar PubMed PubMed Central
[35] Lee W, Yoon S, Choe JK, Lee M, Choi Y. Anionic surfactant modification of activated carbon for enhancing adsorption of ammonium ion from aqueous solution. Sci Total Environ. 2018;639:1432–9. 10.1016/j.scitotenv.2018.05.250.Search in Google Scholar PubMed
[36] Plazinski W, Rudzinski W. Kinetics of adsorption at solid/Solution interfaces controlled by intraparticle diffusion: a theoretical analysis. J Phys Chem C. 2009;113:12495–501. 10.1021/jp902914z.Search in Google Scholar
[37] Pholosi A, Naidoo EB, Ofomaja AE. Intraparticle diffusion of Cr(vi) through biomass and magnetite coated biomass: a comparative kinetic and diffusion study. South African J Chem Eng. 2020;32:39–55. 10.1016/j.sajce.2020.01.005.Search in Google Scholar
[38] Chatterjee S, Guha N, Krishnan S, Singh AK, Mathur P, Rai DK. Selective and recyclable congo red dye adsorption by spherical Fe3O4 nanoparticles functionalized with 1,2,4,5-benzenetetracarboxylic acid. Sci Rep. 2020;10:1–11. 10.1038/s41598-019-57017-2.Search in Google Scholar PubMed PubMed Central
[39] Xiang H, Ren G, Zhong Y, Xu D, Zhang Z, Wang X, et al. Fe3O4@C nanoparticles synthesized by in situ solid-phase method for removal of methylene blue. Nanomaterials. 2021;11:330.10.3390/nano11020330Search in Google Scholar PubMed PubMed Central
[40] Fatimah I, Rubiyanto D, Sahroni I, Putra RS, Nurillahi R, Nugraha J. Physicochemical characteristics and photocatalytic performance of Tin oxide/montmorillonite nanocomposites at various Sn/montmorillonite molar to mass ratios. Appl Clay Sci. 2020;193:105671. 10.1016/j.clay.2020.105671.Search in Google Scholar
[41] Akbari A, Sabouri Z, Hosseini HA, Hashemzadeh A, Khatami M, Darroudi M. Effect of nickel oxide nanoparticles as a photocatalyst in dyes degradation and evaluation of effective parameters in their removal from aqueous environments. Inorg Chem Commun. 2020;115:107867. 10.1016/j.inoche.2020.107867.Search in Google Scholar
[42] Mahajan R, Suriyanarayanan S, Nicholls IA. Improved solvothermal synthesis of γ-Fe2O3 magnetic nanoparticles for SiO2 coating. Nanomaterials. 2021;11. 10.3390/nano11081889.Search in Google Scholar PubMed PubMed Central
[43] Hazarika M, Saikia I, Das J, Tamuly C, Das MR. Biosynthesis of Fe2O3@SiO2 nanoparticles and its photocatalytic activity. Mater Lett. 2016;164:480–3. 10.1016/j.matlet.2015.11.042.Search in Google Scholar
[44] Liu Y, Sun N, Hu J, Li S, Qin G. Photocatalytic degradation properties of a-Fe2O3 nanoparticles for dibutyl phtalate in aqueous solution system. R Soc Open Sci. 2018;5:172196.10.1098/rsos.172196Search in Google Scholar PubMed PubMed Central
[45] Steplin Paul Selvin S, Ganesh Kumar A, Sarala L, Rajaram R, Sathiyan A, Princy Merlin J, et al. Photocatalytic degradation of Rhodamine B using zinc oxide activated charcoal polyaniline nanocomposite and its survival assessment using aquatic animal model. ACS Sustain Chem Eng. 2018;6:258–67. 10.1021/acssuschemeng.7b02335.Search in Google Scholar
[46] Qin Y, Zhang H, Tong Z, Song Z, Chen N. A facile synthesis of Fe3O4@SiO2@ZnO with superior photocatalytic performance of 4-nitrophenol. J Environ Chem Eng. 2017;5:2207–13. 10.1016/j.jece.2017.04.036.Search in Google Scholar
[47] Kusior A, Michalec K, Jelen P, Radecka M. Shaped Fe2O3 nanoparticles – synthesis and enhanced photocatalytic degradation towards RhB. Appl Surf Sci. 2019;476:342–52. 10.1016/j.apsusc.2018.12.113.Search in Google Scholar
[48] Sutisna S, Wibowo E, Rokhmat M, Rahman DY, Murniati R, Khairurrijal K, et al. Batik wastewater treatment using TiO2 nanoparticles coated on the surface of plastic sheet. Procedia Eng. 2017;170:78–83. 10.1016/j.proeng.2017.03.015.Search in Google Scholar
[49] Sharfan N, Shobri A, Anindria FA, Mauricio R, Tafsili MAB, Slamet S. Treatment of batik industry waste with a combination of electrocoagulation and photocatalysis. Int J Technol. 2018;5:936–43.10.14716/ijtech.v9i5.618Search in Google Scholar
© 2022 Gani Purwiandono et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

