Abstract
Microfluidic plasma is a novel process intensification strategy that integrates microfluidic and plasma together and uses their synergistic effects to provide new pathways for chemistry and chemical engineering. In this work, the unique properties and synergistic advantages of microfluidic plasma are introduced. According to the reactor configuration, three types of microfluidic plasmas are elaborated, including chip-based microfluidic plasma, tubular-based microfluidic plasma, and jet-based microfluidic plasma. Selected examples in nanofabrication, chemical synthesis, water treatment, etc., are provided to show their applications in diverse fields. Finally, the existing challenges of this technique have prospected.
1 Introduction
Process intensification (PI) is defined as any chemical engineering development that leads to substantially smaller, cleaner, safer, and more energy-efficient technology [1]. It can be divided into two sub-domains: equipment and methods. The former deals with PI from the reactor engineering view, such as static mixers, membrane distillation, spinning disc reactors, and microreactors, while the latter handles PI from the process view, like hybrid separations, integrated reaction and separation, heat exchange, phase transition, and techniques using alternative energy sources (e.g., microwave, ultrasound, electric field, centrifugal field) [2]. As yet, many disruptive strategies or devices have been designed and implemented in the chemical industry to improve process efficiency and economic sustainability. The cardinal principle governing PI can be improved by utilizing molecular interactions at optimized conditions of driving forces and synergistic effects of different unit operations [3]. Indeed, most chemical reactions are mass or heat-transfer-controlled rather than kinetically controlled [4]. There is an increasing trend of applying microreactors to realize PI by maximizing the specific surface areas as well as the effectiveness of intra- and intermolecular events. Microfluidics, featuring fluid manipulation at submillimeter level, has attracted great attention over the past decade. Thanks to the specificities of small-scale physics, microfluidic allows the rapid mixing of reagents, the precise controls over flows, the accurate regulation of reaction conditions, and the handling of hazardous chemicals [5,6,7]. These features along with the continuous operation mode make microfluidic a powerful platform for chemical synthesis or nanofabrication with good stability and reproducibility [8].
In addition to overcoming mass and heat transfer limitations from the reactor engineering view, the concept of novel process windows was also proposed to speed up kinetics through the use of harsh conditions from the process view [4,9]. In this manner, reaction times can be further reduced from an hour or minute to the second level. Plasma is perceived as an exciting PI technique that uses diverse energetic species (e.g., electrons, ions, photons, free radicals, excited atoms, or molecules) to intensify reactions [10,11]. In plasma media, traditional solvents are not necessitated; thus, it not only avoids chemical residues or diffusion pathways caused by solvents but also gets rid of additional steps for the removal of solvents. Besides, since different gases (e.g., Ar, He, O2, N2, air, NH3, CO, CO2, H2, CH4, fluorine, and chlorine) or their mixtures can be incorporated as working gas, with diverse precursors as reagents, plasma offers great flexibility in reactions ranging from oxidation, nitration, carbonation, hydrogenation, to sulfuration, chlorination, and polymerization.
Inspired by the encouraging achievements, novel ideas naturally come out: By merging microfluidics with plasma technology, is it possible to create an efficient and completely new PI strategy? If so, can we use their synergistic advantages to open new pathways in chemistry and chemical engineering fields? In the constant pursuit of fascinating ideas, the integration of microfluidic and plasma (i.e., microfluidic plasma) has been explored, aiming to use their synergistic advantages to solve formidable challenges that cannot be solved by each alone or by conventional technology [12]. According to Paschen’s law, the breakdown voltage of plasma is a function of the ambient pressure and the gap between two electrodes [13,14]. Thus, miniaturization of plasma reactors not only has practical potential for low power operation at high pressures but also leads to small portable devices with fascinating synergistic effects. Figure 1 summarizes the advantages of microfluidic, plasma, and microfluidic plasma. Typically, microfluidic plasma is strongly nonequilibrium, where gas temperature is much lower than electron temperature. This is particularly favored by nanomaterial synthesis due to the much-reduced particle agglomeration and growth [15]. The high-pressure chemistry contributes to enhanced radical collisions and densities, which further accelerates chemical reactions and intensifies processes. Another important feature of microfluidic plasma is the great flexibility in precursor choice, configuration design, and process control. The wide operational spaces may open up new possibilities for chemistry and chemical engineering.
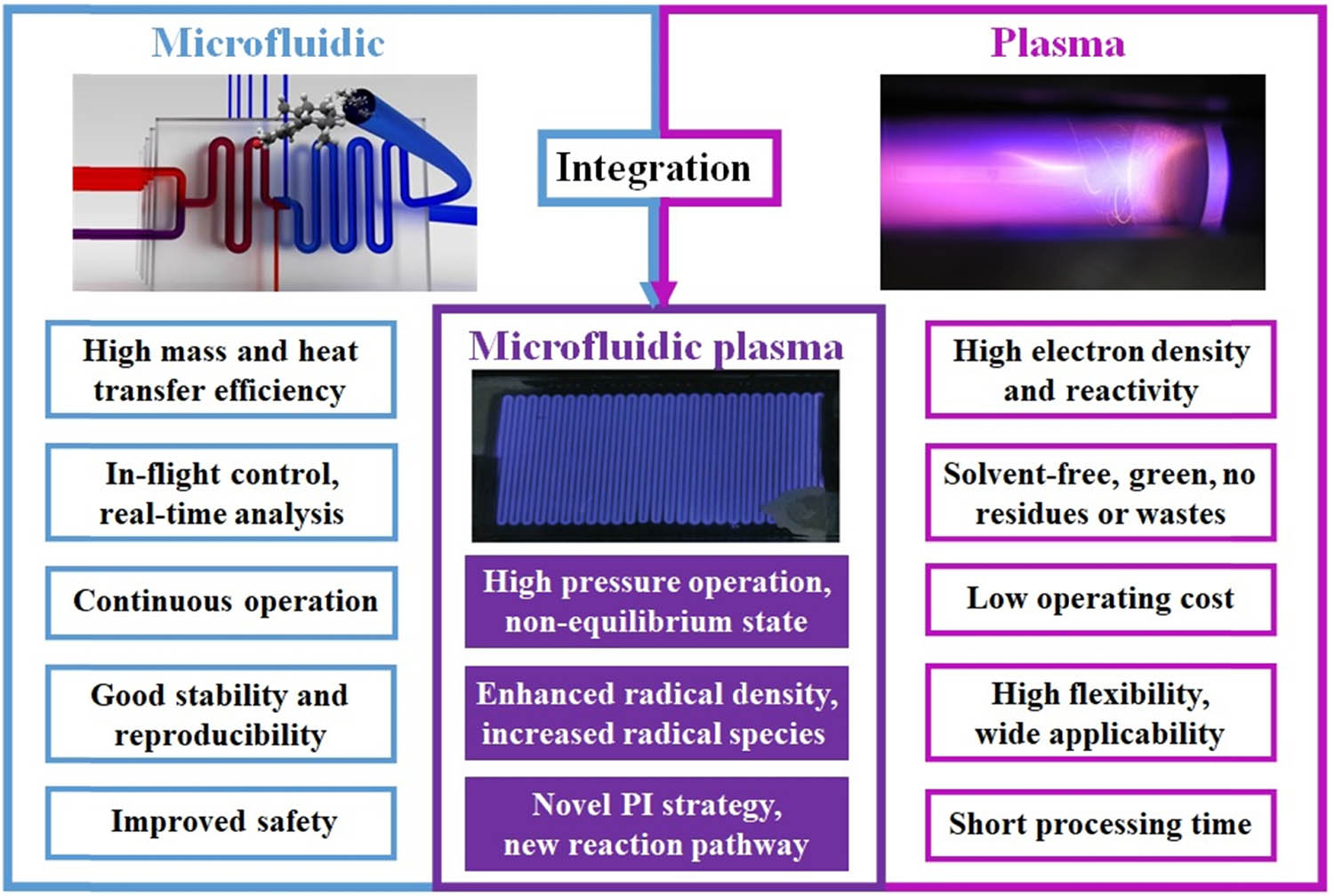
The advantages of microfluidic, plasma, and microfluidic plasma.
In this timely Mini-Review, we discussed microfluidic plasma in terms of synergistic effects, configurations, applications, and challenges, aiming to bring this new PI strategy to academic and industrial communities and to offer innovative solutions to approach intractable challenges that cannot be solved by conventional techniques.
2 Representative microfluidic plasma configurations
Driven by their fascinating properties, different types of microfluidic plasmas have been designed and developed, with materials ranging from glass, quartz, silicon, ceramics, metals, polymers, or composite materials. Due to the high degree of flexibility, they come in many shapes and sizes. Based on the microreactor configuration, they can be roughly classified into three categories: chip-based microfluidic plasma, tubular-based microfluidic plasma, and jet-based microfluidic plasma. Selected configurations are shown in Figure 2.
![Figure 2
Representative microfluidic plasma configurations: (a) chip-based microfluidic plasma, (b) tubular-based microfluidic plasma, and (c) jet-based microfluidic plasma. (a) Reprinted with permission from [26,27,28], Copyright 2018 The Royal Society of Chemistry, Copyright 2018 Springer Nature, Copyright 2022 The Royal Society of Chemistry. (b) Reprinted with permission from [16,29], Copyright 2022 American Chemical Society, Copyright 2018 Springer Nature. (c) Reprinted with permission from [30,31,32], Copyright 2022 American Chemical Society, Copyright 2018 PCCP Owner Societies, and Copyright 2020 IOP Publishing.](/document/doi/10.1515/gps-2022-0092/asset/graphic/j_gps-2022-0092_fig_002.jpg)
Representative microfluidic plasma configurations: (a) chip-based microfluidic plasma, (b) tubular-based microfluidic plasma, and (c) jet-based microfluidic plasma. (a) Reprinted with permission from [26,27,28], Copyright 2018 The Royal Society of Chemistry, Copyright 2018 Springer Nature, Copyright 2022 The Royal Society of Chemistry. (b) Reprinted with permission from [16,29], Copyright 2022 American Chemical Society, Copyright 2018 Springer Nature. (c) Reprinted with permission from [30,31,32], Copyright 2022 American Chemical Society, Copyright 2018 PCCP Owner Societies, and Copyright 2020 IOP Publishing.
2.1 Chip-based microfluidic plasma
Chip-based microfluidic plasmas refer to the configuration where plasmas are generated and confined in chip-form microreactors. They are typically manufactured using glass, quartz, silicon, or certain polymers like polydimethylsiloxane, Teflon, polymethylmarthacrylate (PMMA), polyetheretherketone (PEEK), perfluoroalkoxy (PFA), and polycarbonate by micromachining, etching, lithography, and photolithography methods. Glass or indium tin oxides (ITO) are the most commonly used materials, since they are transparent and chemically inert, allowing visual observation of microchannels. These devices are characteristically comprised of two parts, on which the surfaces are engraved with fluidic channels of various widths and depths, before being fused by thermal bonding to form a compact vessel (Figure 2a). Capillaries are connected to the reactor via fluidic connections, so that gas or liquid precursors can be delivered into the reactor. The electrical connection between the electrodes and high voltage power supply can be realized with the assistance of metal strips or paints. Upon imposing high voltage on the electrodes, plasma discharges are generated within the chip microreactor.
Chip-based configuration gives a very high degree of control over the chemical reactions and can integrate multiple processes into a single device while maintaining a small overall size. It also provides great flexibility in the design of channel patterns without dead volume. Furthermore, due to the high surface-to-volume ratio, it offers several advantages including rapid heat and mass transfer, high reaction rates, low reagent consumption, and high efficiency. On the other hand, the complicated fabrication process along with the expensive fabrication facilities may limit their industrial use.
2.2 Tubular-based microfluidic plasma
Tubular-based microfluidic plasmas refer to the configuration that employs capillary tubes as the reactors, and plasma discharges are generated inside the capillary tubes. They are commonly made by commercially available micrometer-sized capillaries such as metal tubing (e.g., stainless steel, nickel, and copper), fused-silica tubing, quartz tubing, Teflon tubing, PFA tubing, and PEEK tubing. As a consequence, this configuration is often longer in one dimension. A representative design is shown in Figure 2b, where a PFA capillary tube (O.D. = 1/16 inch, I.D. = 0.03 inch) is wrapped around a stainless steel grounded electrode [16]. A copper or an ITO foil serves as the high-voltage electrode and is wrapped outside the coiled PFA capillary. Both electrodes are connected to a sinusoidal alternate current power supply. A continuous helium flow is incorporated into the PFA capillary tube as the plasma gas. Once the high voltage is applied to the electrodes, plasma discharges are formed inside the capillary. By varying the length of the outer electrode, the discharge region can be tuned, making it possible to adjust the residence time.
The tubular-based configuration is modular and adaptable by extending the length of the tube capillary and electrodes in one dimension to control the residence time in the plasma region. Complex and expensive processing (e.g., micromachining, lithography, etching, electroplating, and molding) is not required for manufacturing microreactors, since the capillary tubes are commercially available. Besides, reactors are easily connected with other microfluidic devices or in-situ characterization techniques (optical emission spectroscopy, chromatography, mass spectrometry, or electrophoresis) for real-time process monitoring and product analysis [17,18]. In comparison to the chip-based reactors, this configuration is much cheaper to be fabricated, easier to be scaled up, and can produce a larger quantity of products.
2.3 Jet-based microfluidic plasma
Jet-based microfluidic plasmas are the most intensively studied and frequently used configurations till now. They typically operate as dielectric barrier discharges (DBD) with a central needle as the inner electrode and an outer ring electrode, or a single electrode with capacitive coupling [19]. Each of the electrodes is either grounded or connected to high power supply, with the distance ranging from tens of microns to several millimeters. Another common configuration consists of two concentric tubes with the same diameter as the electrodes, which are inserted in a larger tube and separated by a certain distance. Each electrode composes of a copper ring that connects to the high power supply. A continuous gas is coupled in the electrodes as working gas and flows into the ambient to ensure low-temperature character. In general, noble gases such as Ar or He are used, while the admixture of reactive gases like O2, N2, H2, and CH4 offers the opportunity to tune the plasma chemistry. Therefore, the jet-based configuration is capable of generating diverse short-living reactive species depending on the working gas and precursor. With the supply of high voltage to electrodes, it launches a stable plasma plume to the ambient air. According to the electrode arrangement, jet-based plasmas can be further categorized as dielectric-free electrode jets, single electrode jets, DBD jets, and DBD-like jets [20]. Representative configurations are provided in Figure 2c.
Compared with the chip-based or tubular-based configuration, jet-based microfluidic plasmas have a higher degree of flexibility in configuration. This type of microfluidic plasma can be driven by direct current, pulsed direct, or high-frequency alternating currents like radio frequency (RF) or microwave frequency power supplies, offering more alternative ways to couple the power supply. Another important feature is that plasma jets can directly contact objects, which open up many possibilities for practical applications, like plasma additive manufacturing [21,22], nanomaterials deposition [23], plasma medicine [24], or portable plasma devices [25].
The selection of microfluidic plasma configuration mainly depends on applications. Chip-based configuration allows precise control over process parameters, which is suitable for fundamental studies like kinetics, dynamics, or mechanisms. Tubular-based configuration is more adaptable to diverse processes, with the ability to continuously produce a larger quantity of products. Thus, it is favored by chemical synthesis or nanofabrication purposes. As to the jet-based configuration, it has a high degree of design flexibility, rendering it possible to be applied for surface modification, water treatment, and biomedical science.
3 Applications of microfluidic plasmas
Owing to the synergistic effects of microfluidics and plasma technology, microfluidic plasma has demonstrated itself as an efficient PT strategy, with rapidly expanding applications in chemistry and chemical engineering fields. In this section, we highlight several promising areas in which this technique may pave.
The strong nonequilibrium state of microfluidic plasmas makes them particularly suitable for nanomaterials synthesis and processing since low background temperature would suppress particle nucleation and growth. In some cases, particles are also charged in plasma, which further reduces aggregation. Another benefit of nanomaterials synthesis is electrons can replace chemical reductants to avoid contaminations (e.g.,
Compared with conventional batch reactions, microfluidic plasmas have better control and versatility both in terms of reactor configuration and operational space. The combination of physical (heat, light, electrical field) and chemical (electrons, ions, photons, metastable atoms, or excited radicals) effects provides a highly reactive environment at mild conditions, leading to new chemical routes without catalysts. Thus, they were also explored for chemical synthesis. A smart Biflow 2.7 chip-based microfluidic plasma reactor was designed by Lepoetre et al. and applied for various chemical reactions, such as cyclohexane amination [37], CO2 carbonylation [38], amine N-acylation [28], and 1-hexene oligomerization [39]. Results showed the reactor has a high degree of flexibility and versatility and can trigger reactions at rather low temperatures (close to room temperature) and atmospheric pressure. Furthermore, the substantially intensified heat and mass transfer enabled the reactor to produce target products in good to excellent yields. Another salient example was reported by Cameli et al. [16], where a tubular-based microfluidic plasma was developed for intensified H2O2 production. A water stream together with a helium gas flow was delivered in a tubular capillary. When high voltage was supplied to the electrodes around the microreactor, plasma discharges were generated within the capillary, leading to the formation of H2O2 through the below reactions:
With rapid industrial development, the continued production of pollution remains inevitable. Environmental monitoring has become increasingly important to minimize the adverse impacts on human health and the environment. Nitrosamines are a class of strong carcinogens that widely exist in cosmetics, like N-nitrosodiethanolamine, N-nitrosodimethylamine (NDMA), N-nitrosodiethylamine (NDEA), N-nitrosodibutylamine, N-nitrosodiphenylamine, and N-nitrosodicyclohexylamine. In the study of Lin et al. [40], the ion beam technique was utilized to fabricate 1,2-ethanedithiol-modified gold nanorods for surface-enhanced Raman scattering detection of NDMA and NDEA. Characteristic peaks of NDMA/NDEA were observed, a detection limit of 10−8 M for both molecules. In a recent study, a jet-based microfluidic plasma system was developed for the continuous synthesis of glucose-functionalized gold nanoparticles (G-AuNPs) and their use for real-time Pb2+ detection [41]. Results showed the whole process took less than 30 s, including the G-AuNPs synthesis and colorimetric Pb2+ detection. This reveals the high efficiency of the microfluidic plasma system and is very promising for the detection of hazardous materials like nitrosamines and heavy metal ions.
Microfluidic plasmas also have been applied for water treatment to remove microbial and chemical contaminants. This is because high energy electrons (1–10 eV) and reactive species in plasma have the potential to oxidize organic pollutants to their less toxic forms and enhance their potential for degradation. Meanwhile, plasma also supplies the water with ultraviolet light and reactive radicals, which further penetrate and interact with the microorganism to repel or kill bacteria. The integration of microfluidics for plasma water treatment provides additional benefits like continuous operation, enhanced treatment efficiency, portable devices, and shorter distances for the transfer of plasma species from a gas into a liquid. This may overcome limitations such as mass transfer in bulk reactors. Sun et al. [29] reported a jet-based microfluidic plasma device for the control and removal of biofilms in drinking water. A 9 × 9 array of microchannels with an overall area of 125.4 mm2 were adopted to conduct experiments. Results showed biofilms vanished rapidly after plasma exposure, which was attributed to the generation of oxygen-bearing species like hydroxyl radical (OH), singlet oxygen (1O2), hydrogen peroxide (H2O2), and ozone (O3). In addition to the jet-based configuration, chip-based microfluidic plasma was also used for water treatment. Specifically, a DBD chip microreactor with 100 or 50 µm depths and 390 or 330 µm channel widths was fabricated by Patinglag et al. [27] for the degradation of methylene blue. They found the degradation rates were related to the residence time of the sample in the plasma zone, the plasma working gas, the channel depth, and flow rate. At the optimum condition, the degradation level of methylene blue was greater than 97%.
Apart from the aforementioned applications, there are studies exploiting microfluidic plasmas for surface modification of materials or biomedical science applications. For instance, by incorporating suitable precursors in microchannels and igniting plasma discharges inside the chip microreactor, Wu et al. [42] successfully modified the inner surface of microfluidic platforms with hydrophobicity or hydrophilicity. The developed strategy is surface-material-independent, including sapphire, PET, PMMA, and silicon wafers. A similar method was reported by Escobedo and Sinton [43] to actuate liquids in microchannels by tuning the surface energy of microchannels by plasma impacts. In the study of Neretti et al. [44], jet-based microfluidic plasma arrays were developed for biomedical applications. Results revealed the existence of nitrogen species as well as helium metastable excited states, which exhibited a remarkable disinfectant effect against bacteria and fungi. To show the wide applications of microfluidic plasma in diverse fields, Table 1 summarizes selected examples reported in recent years.
Typical examples of microfluidic plasma in diverse fields
| Applications | Examples | Configurations | References |
|---|---|---|---|
| Nanofabrication | Au nanoparticles | Chip-based reactor | [34] |
| Nanodiamonds | Chip-based reactor | [45] | |
| Ag–Au nanoalloys | Jet-based reactor | [30] | |
| Bimetallic Fe–Ni nanoparticles | Tubular-based reactor | [46] | |
| Chemical synthesis | Amination of cyclohexane | Chip-based reactor | [37] |
| Carbonylation of CO2 | Chip-based reactor | [38] | |
| N-Acylation of amines | Chip-based reactor | [28] | |
| H2O2 production | Tubular-based reactor | [16] | |
| Water treatment | Methylene blue degradation | Chip-based reactor | [27] |
| Biological compounds degradation | Chip-based reactor | [47] | |
| Biofilm control and removal | Jet-based reactor | [29] | |
| Surface modification | Corrosion resistance | Jet-based reactor | 10.1016/j.jtice.2022.104467 |
| Liquid actuation | Chip-based reactor | [43] | |
| Wettability effects | Jet-based reactor | [48] | |
| Biomedical science | Wound healing | Chip-based reactor | [49] |
| Microbiological inactivation | Jet-based reactor | [50] | |
| Sterilization of human biofilm | Jet-based reactor | [44] |
4 Conclusion and outlook
PI through microfluidic plasma technology is an emerging field, paving an efficient way for chemical reactions and processes at mild conditions (low temperature, atmospheric pressure, without catalysts). Many researchers are exploiting the synergistic effects of both techniques to address challenges that cannot be solved by conventional approaches. Despite the indisputable advantages, microfluidic plasmas still face several problems. Currently, they are only suitable for fundamental research rather than large-scale industrial use due to the low throughput. We believe this technique has promising commercial applications in the synthesis of nanomaterials and chemicals, as long as the throughput can be enhanced. Adding devices in parallel or enhancing the productivity of each device may be an answer. The influence of the electrical field and plasma species on fluids and their transportation in microchannels is not clear yet, requiring both modeling and experiments to reveal their interactions. Also, microfluidic plasma is a multidisciplinary field of research at the intersection of physics, chemistry, engineering, and materials, but has been applied in rather limited areas. Active collaborations among diverse fields are needed to further expand their applications.
-
Funding information: This work was supported by the Natural Science Foundation of Jiangsu Province (BK20190605), National Natural Science Foundation of China (22078125, 52004102), China Postdoctoral Science Foundation (2021M690068), Fundamental Research Funds for the Central Universities (JUSRP221018, JUSRP622038), and NMPA Key Laboratory of Cosmetic Safety Assessment, Guangdong Institute for Drug Control (KF2021014).
-
Author contributions: Liangliang Lin: conceptualization, formal analysis, writing – original draft, writing – review and editing; Ziyi Zhang: review and writing; Yuanping Min: review and writing.
-
Conflict of interest: The corresponding author (Liangliang Lin) is a member of the Editorial Board of Green Processing and Synthesis.
References
[1] Baldea M. From process integration to process intensification. Comput Chem Eng. 2015;81:104–14. 10.1016/j.compchemeng.2015.03.011.Suche in Google Scholar
[2] Stankiewicz AI, Moulijn JA. Process intensification: transforming chemical engineering. Chem Eng Prog. 2000;96:22–34.Suche in Google Scholar
[3] Moorthy RK, Baksi S, Biswas S. Process intensification - an insight. Chem Eng World. 2016;51:41–7. 10.35543/osf.io/5nghs.Suche in Google Scholar
[4] Hessel V, Cortese B, de Croon MHJM. Novel process windows - Concept, proposition and evaluation methodology, and intensified superheated processing. Chem Eng Sci. 2011;66:1426–48. 10.1016/j.ces.2010.08.018.Suche in Google Scholar
[5] Lin L, Yin YJ, Starostin SA, Xu HJ, Li CD, Wu K, et al. Microfluidic fabrication of fluorescent nanomaterials: A review. Chem Eng J. 2021;425:131511.10.1016/j.cej.2021.131511Suche in Google Scholar
[6] Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature. 2014;507:181–9. 10.1038/nature13118.Suche in Google Scholar PubMed
[7] Stone HA, Kim S. Microfluidics: Basic issues, applications, and challenges. AIChE J. 2001;47:1250–4. 10.1002/aic.690470602.Suche in Google Scholar
[8] Epps RW, Bowen MS, Volk AA, Kameel AL, Han SY, Reyes KG, et al. Artificial chemist: An autonomous quantum dot synthesis bot. Adv Mater. 2020;32:2001626. 10.1002/adma.202001626.Suche in Google Scholar PubMed
[9] Hessel V. Novel process windows - gate to maximizing process intensification via flow chemistry. Chem Eng Technol. 2009;32:1655–81. 10.1002/ceat.200900474.Suche in Google Scholar
[10] Lin L, Li X, Zhou J, Zou JL, Lai JH, Chen ZH, et al. Plasma-aided green and controllable synthesis of silver nanoparticles and their compounding with gemini surfactant. J Taiwan Inst Chem Eng. 2021;122:311–9. 10.1016/j.jtice.2021.04.061.Suche in Google Scholar
[11] Xu H, He C, Lin L, Shen J, Shang SM. Direct formation of carbon supported Pt nanoparticles by plasma-based technique. Mater Lett. 2019;255:126532. 10.1016/j.matlet.2019.126532.Suche in Google Scholar
[12] Lin L, Quoc Pho H, Zong L, Li SR, Pourali N, Rebrov E, et al. Microfluidic plasmas: Novel technique for chemistry and chemical engineering. Chem Eng J. 2021;417:129355. 10.1016/j.cej.2021.129355.Suche in Google Scholar
[13] Long NV, Al-Bared M, Lin L, Davey K, Tran NN, Pourali N, et al. Understanding plasma-assisted ammonia synthesis via crossing discipline borders of literature: A critical review. Chem Eng Sci. 2022;263:118097. 10.1016/j.ces.2022.118097.Suche in Google Scholar
[14] Lozano-Parada JH, Zimmerman WB. The role of kinetics in the design of plasma microreactors. Chem Eng Sci. 2010;65:4925–30. 10.1016/j.ces.2010.03.056.Suche in Google Scholar
[15] Mariotti D, Sankaran RM. Microplasmas for nanomaterials synthesis. J Phys D Appl Phys. 2010;43:323001. 10.1088/0022-3727/43/32/323001.Suche in Google Scholar
[16] Cameli F, Dimitrakellis P, Chen TY, Vlachos DG. Modular plasma microreactor for intensified hydrogen peroxide production. ACS Sustain Chem Eng. 2022;10:1829–38. 10.1021/acssuschemeng.1c06973.Suche in Google Scholar
[17] Yue J. Multiphase flow processing in microreactors combined with heterogeneous catalysis for efficient and sustainable chemical synthesis. Catal Today. 2018;308:3–19. 10.1016/j.cattod.2017.09.041.Suche in Google Scholar
[18] Pattanayak P, Singh SK, Gulati M, Vishwas S, Kapoor B, Chellappan DK, et al. Microfluidic chips: recent advances, critical strategies in design, applications and future perspectives. Microfluid Nanofluidics. 2021;25:1–28. 10.1007/s10404-021-02502-2.Suche in Google Scholar PubMed PubMed Central
[19] Khlyustova A, Labay C, Machala Z, Ginebra MP, Canal C. Important parameters in plasma jets for the production of RONS in liquids for plasma medicine: A brief review. Front Chem Sci Eng. 2019;13:238–52. 10.1007/s11705-019-1801-8.Suche in Google Scholar
[20] Lin L, Wang Q. Microplasma: A new generation of technology for functional nanomaterial synthesis. Plasma Chem Plasma Process. 2015;35:925–62. 10.1007/s11090-015-9640-y.Suche in Google Scholar
[21] Sui Y, Zorman CA, Sankaran RM. Plasmas for additive manufacturing. Plasma Process Polym. 2020;17:1–25. 10.1002/ppap.202000009.Suche in Google Scholar
[22] Hong J, Murphy AB, Ashford B, Cullen PJ, Belmonte T, Ostrikov K. Plasma-digital nexus: plasma nanotechnology for the digital manufacturing age. Rev Mod Plasma Phys. 2020;4:1.10.1007/s41614-019-0039-8Suche in Google Scholar
[23] Shimizu Y, Sasaki T, Chandra Bose A, Terashimab K, Koshizaki N. Development of wire spraying for direct micro-patterning via an atmospheric-pressure UHF inductively coupled microplasma jet. Surf Coat Technol. 2006;200:4251–6. 10.1016/j.surfcoat.2005.01.113.Suche in Google Scholar
[24] Kubinova S, Zaviskova K, Uherkova L, Zablotskii V, Churpita O, Lunov O, et al. Non-thermal air plasma promotes the healing of acute skin wounds in rats. Sci Rep. 2017;7:1–11. 10.1038/srep45183.Suche in Google Scholar PubMed PubMed Central
[25] Dzimitrowicz A, Bielawska-Pohl A, Jamroz P, Dora J, Krawczenko A, Busco G, et al. Activation of the normal human skin cells by a portable dielectric barrier discharge-based reaction-discharge system of a defined gas temperature. Plasma Chem Plasma Process. 2020;40:79–97. 10.1007/s11090-019-10039-0.Suche in Google Scholar
[26] Li DE, Lin CH. Microfluidic chip for droplet-based AuNP synthesis with dielectric barrier discharge plasma and on-chip mercury ion detection. RSC Adv. 2018;8:16139–45. 10.1039/c8ra02468e.Suche in Google Scholar PubMed PubMed Central
[27] Patinglag L, Sawtell D, Iles A, Melling LM, Shaw KJ. A microfluidic atmospheric‑pressure plasma reactor for water treatment. Plasma Chem Plasma Process. 2019;39:561–75.10.1007/s11090-019-09970-zSuche in Google Scholar
[28] Abedelnour E, Ognier S, Zhang M, Schio L, Venier O, Cossy J, et al. Plasma flow chemistry for direct N-acylation of amines by esters. Chem Commun. 2022;58:7281–4. 10.1039/D2CC01940J.Suche in Google Scholar PubMed
[29] Sun PP, Araud EM, Huang C, Shen Y, Monroy GL, Zhong SY, et al. Disintegration of simulated drinking water biofilms with arrays of microchannel plasma jets. npj Biofilms Microbiomes. 2018;4:24. 10.1038/s41522-018-0063-4.Suche in Google Scholar PubMed PubMed Central
[30] Lin L, Li X, Gao H, Xu HJ, Starostin SA, Ostrikov K, et al. Microfluidic plasma-based continuous and tunable synthesis of Ag−Au nanoparticles and their SERS properties. Ind Eng Chem Res. 2021;61(5):2183–94. 10.1021/acs.iecr.1c04048.Suche in Google Scholar
[31] Benedikt J, Mokhtar Hefny M, Shaw A, Buckley BR, Iza F, Schäkermann S, et al. The fate of plasma-generated oxygen atoms in aqueous solutions: Non-equilibrium atmospheric pressure plasmas as an efficient source of atomic O(aq). Phys Chem Chem Phys. 2018;20:12037–42. 10.1039/c8cp00197a.Suche in Google Scholar PubMed
[32] Tschang CYT, Bergert R, Mitic S, Thoma M. Effect of external axial magnetic field on a helium atmospheric pressure plasma jet and plasma-treated water. J Phys D Appl Phys. 2020;53:215202. 10.1088/1361-6463/ab78d6.Suche in Google Scholar
[33] Lin L, Xu H, Gao H, Zhu XM, Hessel V. Plasma-assisted nitrogen fixation in nanomaterials: Fabrication, characterization, and application. J Phys D Appl Phys. 2020;53(13):133001. 10.1088/1361-6463/ab5f1f.Suche in Google Scholar
[34] Maguire P, Rutherford D, Macias-Montero M, Mahony C, Kelsey C, Tweedie M, et al. Continuous in-flight synthesis for on-demand delivery of ligand-free colloidal gold nanoparticles. Nano Lett. 2017;17:1336–43. 10.1021/acs.nanolett.6b03440.Suche in Google Scholar PubMed
[35] Pho QH, Lin LL, Tran NN, Tran TT, Nguyen AH, Losic D, et al. Rational design for the microplasma synthesis from vitamin B9 to N-doped carbon quantum dots towards selected applications. Carbon. 2022;198:22–33. 10.1016/j.carbon.2022.07.004.Suche in Google Scholar
[36] Pho QH, Lin LL, Rebrov EV, Sarafraz MM, Tran TT, Tran NN, et al. Process intensification for gram-scale synthesis of N-doped carbon quantum dots immersing a microplasma jet in a gas-liquid reactor. Chem Eng J. 2023;452:139164. 10.1016/j.cej.2022.139164.Suche in Google Scholar
[37] Lepoetre A, Ognier S, Zhang M, Wengler J, Ayoubi SA, Ollivier C, et al. Amination of cyclohexane by dielectric barrier discharge processing in a continuous flow microreactor: Experimental and simulation studies. Plasma Chem Plasma Process. 2021;41:351–68. 10.1007/s11090-020-10140-9.Suche in Google Scholar
[38] Gaudeau M, Zhang M, Tatoulian M, Lescotb C, Ognier S. Fast carbonylation reaction from CO2 using plasma gas/liquid microreactors for radiolabeling applications. React. Chem Eng. 2020;5:1981–91. 10.1039/D0RE00289E.Suche in Google Scholar
[39] Royoux PA, Ognier S, Zhang M, Thomas CM, Tatoulian M. Plasma activated 1-hexene oligomerization in a gas-liquid microreactor. React Chem Eng. 2022;7:1115–25. 10.1039/d1re00464f.Suche in Google Scholar
[40] Lin CH, Wang PH, Wang TH, Yang LJ, Wen TC. The surface-enhanced Raman scattering detection of N -nitrosodimethylamine and N-nitrosodiethylamine via gold nanorod arrays with a chemical linkage of zwitterionic copolymer. Nanoscale. 2020;12:1075–82. 10.1039/c9nr09404k.Suche in Google Scholar PubMed
[41] Li X, Zhao C, Lin L. Plasma-based instant synthesis of functionalized gold nanoparticles for colorimetric detection of lead ions. Chem Eng Sci. 2022;260:117849. 10.1016/j.ces.2022.117849.Suche in Google Scholar
[42] Wu ST, Huang CY, Weng CC, Chang CC, Li BR, Hsu CS. Rapid prototyping of an open-surface microfluidic platform using wettability-patterned surfaces prepared by an atmospheric-pressure plasma jet. ACS Omega. 2019;4:16292–99. 10.1021/acsomega.9b01317.Suche in Google Scholar PubMed PubMed Central
[43] Escobedo C, Sinton D. Microfluidic liquid actuation through ground-directed electric discharge. Microfluid Nanofluidics. 2011;11:653–62. 10.1007/s10404-011-0831-4.Suche in Google Scholar
[44] Neretti G, Tampieri F, Borghi CA, Brun P, Cavazzana R, Cordaro L, et al. Characterization of a plasma source for biomedical applications by electrical, optical, and chemical measurements. Plasma Process Polym. 2018;15:1–12. 10.1002/ppap.201800105.Suche in Google Scholar
[45] Ishii C, Stauss S, Kuribara K, Urabe K, Sasaki T, Terashima K, et al. Atmospheric pressure synthesis of diamondoids by plasmas generated inside a microfluidic reactor. Diam Relat Mater. 2015;59:40–6. 10.1016/j.diamond.2015.08.017.Suche in Google Scholar
[46] Chiang W-H, Sankaran RM. Synergistic effects in bimetallic nanoparticles for low temperature carbon nanotube growth. Adv Mater. 2008;20:4857–61. 10.1002/adma.200801006.Suche in Google Scholar
[47] Christianson R, You S, Wang Y. Degradation of contaminants in plasma technology: An overview. J Hazard Mater. 2022;424:127390.10.1016/j.jhazmat.2021.127390Suche in Google Scholar PubMed PubMed Central
[48] Lin L, Rui L, Li C, Liu QS, Li SR, Xia Y, et al. Study on CO2-based plasmas for surface modification of polytetrafluoroethylene and the wettability effects. J CO2 Util. 2021;53:101752. 10.1016/j.jcou.2021.101752.Suche in Google Scholar
[49] Shao P, Liao J, Wu S, Chen YH, Wong TW. Microplasma treatment versus negative pressure therapy for promoting wound healing in diabetic mice. Int J Mol Sci. 2021;22:10266.10.3390/ijms221910266Suche in Google Scholar PubMed PubMed Central
[50] Misra NN, Jo C. Applications of cold plasma technology for microbiological safety in meat industry. Trends Food Sci Technol. 2017;64:74–86. 10.1016/j.tifs.2017.04.005.Suche in Google Scholar
© 2022 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Artikel in diesem Heft
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

