Abstract
At present, the research on fluorescent molecular probe has become a hot topic in the field of environmental science, chemical materials, medicine, and other fields. Therefore, the detection of industrial mercury-containing wastewater (Hg2+) is of great significance. In this article, the fluorescent probe is used to detect mercury ions, and when compared with the traditional detection method, the fluorescent probe has the advantage of operation such as the effect of simplicity is evident. The experiments first synthesized rhodamine B derivatives and then the synthesized rhodamine B derivative fluorescent molecular probes were constructed and used to detect the mercury ions in water sample and oil paints. It was demonstrated that rhodamine B-derived probes have been constructed by UV and fluorescence spectroscopy. The different metal ions and rhodamine B-derived fluorescent molecular probes were compounded, resulting in the appearance of fluorescence peak centered at 583 nm only after the addition of metallic mercury ions with almost no response from other ions. The mercury ion rhodamine B derivative is more responsive to metallic mercury ions.
1 Introduction
Compared to other metal detection methods, fluorescent molecular type probes are more sensitive because of their good selectivity, no reference term is required, and they have a higher sensitivity, relatively simple operation, low cost of preparation, not easily affected by magnetic and electric fields, and obvious reaction to other characteristics. Therefore, a new fluorescent molecular probe is synthesized [1–4]. Rhodamine has good fluorescence properties, such as large molar absorbance, long excitation and emission wavelengths, strong light stability, and high fluorescence quantum yield. So, this experiment chose to use rhodamine derivatives as probes for detection of mercury ions [5–7]. The main sources of mercury ions are natural environmental and industrial wastewater discharges, which can enter the body by accumulating in bacteria or the food chain and converting to organic mercury. In humans, it can cause irreversible damage to the kidneys, nervous system, and brain function [8–10]. Therefore, it is of great interest to develop new fluorescent probes that respond to mercury ions [11]. There are strict standards for the detection of mercury ions around the world, such as the U.S. EPA’s limit of Hg2+ in drinking water is 2 μg·L−1. Limit standard for Hg2+ in drinking water in China is 1 μg·L−1, and the limit standard for industrial wastewater is 0.25 mmol·L−1. Therefore, high sensitivity is required for mercury ion fluorescence probe [12–15].
Dhaka et al. developed a novel thioether spiro rhodamine B fluorescent probe [16]. By using a simple thioether spirocycle instead of typical spirolactam as the Hg2+ recognition unit, the proposed probe is sensitive to Hg2+ and exhibited an independent ultrasensitive response, and showed that the free probe maintained its spirocyclic form in living cells. The probes are responsive and highly selective for Hg2+ [17]. In the presence of other metal ions, the fluorescence changes of the probe are very pronounced and are particularly suitable for the rapid response of Hg2+ [18,19]. Tian et al. from Hainan Normal University combined rhodamine 6G with hydrazine hydrate and synthesized fluorescent probes. When there was no Hg2+ in solution, the R6GH was non-fluorescent and mercury ions were gradually added to 10 μM-R6GH in ethanol/HEPES buffer solution at room temperature. The excitation wavelength of R6GH is 500 nm and an emission peak appears at 554 nm. In ethanol/water buffer solution, R6GH can specifically recognize mercury ions and emit yellow-green fluorescence, and mercury ions can also make R6GH fluorescence. The solution changes from colorless to pink, so R6GH can also be used for colorimetric detection of mercury ions. Mercury ion is the only ion that causes an increase in R6GH fluorescence, and the addition of other metal ions has no effect on the fluorescence spectrum of R6GH. This fluorescent probe is highly selective and can be successfully used for fluorescence imaging of mercury ions in the living cells [20–22]. These experiments have contributed to the development of rhodamine-derived probe technology, thus making rhodamine an ideal material for fluorescent probes to be used for mercury ions.
Large amount of mercury containing wastewater is produced in non-ferrous metal smelting, chlor-alkali industry, electronic industry production, and other processes. The detection of industrial mercury containing wastewater (Hg2+) is of great significance. If the wastewater containing mercury (Hg2+) is directly discharged into the water, mercury and its compounds can form various forms of mercury through physical, chemical, and biological interactions, and even convert into highly toxic methyl compounds, thus seriously polluting the water environment.
According to literature reports, the methods that can be used for mercury ion monitoring include: high performance liquid chromatography, atomic absorption spectrometry, atomic emission spectrometry, and inductively coupled plasma mass spectrum. However, there are problems in these methods. The operation and maintenance cost of the testing instrument is high, and the instrument is cumbersome which cannot meet the needs of a wide range of applications. Fluorescent molecular probe technology, which has the advantages of high selectivity, high sensitivity, simple operation, and wide application range, has been favored in the detection of mercury ions. According to the different response mechanisms of fluorescent probes to Hg2+, the fluorescent probes can be divided into three types: ratio type, “off-on” type, and “on-off” type. Many fluorescent probes for the detection of mercury ions are designed based on these principles.
In addition, the existence of harmful substances in oil paints, such as mercury and lead metal ions, is very harmful to the human body. In this article, rhodamine B RH-DCP probe was designed for the determination of mercury ions in titanium dioxide and zinc white of oil paints. The probe can be used to detect mercury ions in titanium dioxide and zinc white of oil paints in environmental water samples.
2 Materials and methods
2.1 The white of oil paint is common titanium white, zinc white, and lead white
Titanium dioxide (PW6): Currently, all pigment brands divide the raw materials used to make their colours into two types, R-type and anatase, and the performance of these two materials differs considerably. With strong coverage, it is generally used as the main auxiliary color of oil painting harmonic color. The chemical composition of titanium dioxide is mainly titanium dioxide (TiO2), white powder.
Zinc white (PW4): Transparent white pigment, poor coverage, poor firmness after oxidation, easy to powder. Chemical composition of zinc white is mainly zinc oxide (ZnO), pure zinc white is cold, with slightly blue tendency, is a good oil painting to brighten the auxiliary color. Generally mixed with titanium dioxide to form mixed white, and titanium dioxide ratio is different, mixed white can reflect different physical properties.
Titanium dioxide (PW6) manufacturer, Langfang Lanke Chemical Co., LTD. Zinc white (PW4) manufacturer, Changzhou Yumeng Chemical Co., LTD.
2.2 Construction of mercury ion fluorescence system for rhodamine B derivatives
Add rhodamine, methanol, hydrazine hydrate, and 2,4-dichloroacetophenone to a beaker in turns, stirred, refluxed, and cooled, and then carried out vacuum rotary evaporation, followed by rinsing, vacuum filtration, and drying to obtain new colorless transparent glassy rhodamine B derivatives. The newly obtained rhodamine B derivatives were dissolved in ethanol/water (1:2) and formulated into 0.2 × 10−5 mol·L−1 rhodamine B derivative solution, HgCl2 standard formulated into 5 × 10−4 mol·L−1 Hg2+ standard solution. A certain volume of rhodamine B-derivative solution and a certain volume of Hg2+ reserve solution were added to a 10 mL colorimetric tube. Dilute 10 mL with distilled water, stir, and let stand for 50 min to obtain a mercury ion fluorescent probe system for rhodamine B derivatives.
2.3 Fluorescence spectroscopy detection methods
A mercury ion fluorescent probe system formulated with rhodamine B derivatives was added as a test sample to a 1 cm quartz colorimetric cell. A separate colorimetric tube is taken and under the same conditions, only the fluorescent probe solution of rhodamine class B derivatives is added without metal Hg2+. These solutions were used as blank samples. Use 530 nm as the excitation wavelength. Set the fluorescence parameters with sensitivity set to 2 and slit width set to 5 nm. Fluorescence emission spectrum in the 500–700 nm was scanned by a fluorescence spectrophotometer to determine the fluorescence emission intensity.
To measure the strength of the selectivity of the probe to mercury ions, 14 common metal ions such as Pb2+, Zn2+, Ag+, Ca2+, Mn2+, Cu2+, Al3+, and Cr3+ were added to the rhodamine class B derivative probe solution, respectively.
3 Results
3.1 Spectra of mercury ion fluorescence systems for rhodamine B derivatives
Figure 1a shows the UV-Vis absorption spectra of the mercury ion fluorescence system of rhodamine B derivatives in the wavelength range of 400–700 nm. As shown in Figure 1, the rhodamine B-derivative probe solution of the blank sample without the addition of metallic mercury ions had no UV absorption in the range of 500–650 nm. The addition of metallic mercury ions to the system resulted in peak centered at 558 nm. It can be inferred that mercury ion class B derivatives have been constructed based on the changes in the UV spectrum.
![Figure 1
(a) UV-Vis absorption spectra of rhodamine class B derivatives and Hg2+ fluorescence systems (CH3CH2OH/H2O [v/v,1/2], probe concentration 10 × 10−5 mol·L−1, Hg2+ concentration 10 × 10−5 mol·L−1). (b) IR spectrum of Rh-DCP.](/document/doi/10.1515/gps-2022-0085/asset/graphic/j_gps-2022-0085_fig_001.jpg)
(a) UV-Vis absorption spectra of rhodamine class B derivatives and Hg2+ fluorescence systems (CH3CH2OH/H2O [v/v,1/2], probe concentration 10 × 10−5 mol·L−1, Hg2+ concentration 10 × 10−5 mol·L−1). (b) IR spectrum of Rh-DCP.
Figure 1b shows the infrared spectrum of Rh-DCP, a derivative of rhodamine B. Rh-DCP contains absorption peaks of characteristic functional groups of intermediates, and there is an absorption peak at 1,614 cm−1, which is the stretching vibration peak of C═N. Moreover, rh-DCP has a strong absorption peak at this position, indicating that the –NH2 structure of rhodamine B-hydrazide forms a new (C═N) bond with the C═O structure, which can be judged as the successful synthesis of Rh-DCP.
Figure 2 shows the fluorescence spectra of the mercury ion fluorescence system obtained at a fixed excitation wavelength of 530 nm with a slit width setting of 5.0 nm. The variation of the fluorescence intensity in 530–680 nm was also recorded. The results are shown in Figure 2. There was no peak in the range of 560–620 nm in the rhodamine class B derivative probe solution. After adding Hg2+ to the system and mixing the solution for 50 min before performing the assay, a strong peak centered at 583 nm appeared. It can be inferred that fluorescence spectra has been constructed in this experiment.
![Figure 2
Fluorescence spectrum of rhodamine class B derivatives and Hg2+ fluorescence systems (CH3CH2OH/H2O [v/v, 1/2], λ
ex = 530 nm, probe concentration 10 × 10−5 mol·L−1, Hg2+ concentration 10 × 10−5 mol·L−1).](/document/doi/10.1515/gps-2022-0085/asset/graphic/j_gps-2022-0085_fig_002.jpg)
Fluorescence spectrum of rhodamine class B derivatives and Hg2+ fluorescence systems (CH3CH2OH/H2O [v/v, 1/2], λ ex = 530 nm, probe concentration 10 × 10−5 mol·L−1, Hg2+ concentration 10 × 10−5 mol·L−1).
Figure 3 shows the fluorescence spectra of the binding ratio of rhodamine class B derivatives to the mercury ion fluorescence system. In order to confirm the binding pattern of rhodamine class B derivative probe and mercury ion, the binding ratio of rhodamine B-derived probes to Hg2+ was determined by an iso-molar continuous change method (Job-Plot). The molar ratio of Hg2+ concentration in the system is from 0.1 to 0.9, and the solution at different ratios was recorded. It can be seen from the graph that the maximum fluorescence intensity is reached when the molar concentration ratio of metal ions to the probe is 1:1, which means that the ratio of mercury ions to the probe is 1:1. The optimal binding ratio of the probe also indicates a 1:1 binding ratio between the mercury ion and the probe.
![Figure 3
Determination of the binding ratio (CH3CH2OH/H2O [v/v, 1/2], λ
ex = 530 nm); the fluorescent system by the equimolar method.](/document/doi/10.1515/gps-2022-0085/asset/graphic/j_gps-2022-0085_fig_003.jpg)
Determination of the binding ratio (CH3CH2OH/H2O [v/v, 1/2], λ ex = 530 nm); the fluorescent system by the equimolar method.
3.2 Response of rhodamine B derivatives to metal ion fluorescent probes for mercury ions
Figure 4 shows the effect of rhodamine class B derivative probes on Pb2+, Zn2+, Ag+, Ca2+, Mn2+, and Cu2+ and the fluorescence spectra of the selective responses of 14 common metal ions. From Figure 4, it can be seen that the only significant response to the fluorescence intensity is the metal mercury ion solution. In the range of 400–600 nm, the fluorescence emission spectra are shown by the absence of significant fluorescence before the addition of metal. After metallic mercury ions, a peak centered at 583 nm appeared. Except for Hg2+, the fluorescence intensity of Al3+ is also slightly enhanced, while the other ions have almost no response and do not affect the detection of mercury ions. The results showed that the mercury ion rhodamine B derivative probe molecule was selective only for metallic mercury ions. In order to ensure the safety of RH-DCP in daily application, the in vitro toxicity of RH-DCP was measured by MTT cell survival test. The cell survival rate was higher than 76% at all concentrations, indicating that RH-DCP is safe enough for routine use.
![Figure 4
Fluorescence spectrum of different metal ions and rhodamine class B derivatives response (CH3CH2OH/H2O [v/v, 1/2], λ
ex = 530 nm, probe concentration 10 × 10−5 mol·L−1, metal ions concentration 10 × 10−5 mol·L−1).](/document/doi/10.1515/gps-2022-0085/asset/graphic/j_gps-2022-0085_fig_004.jpg)
Fluorescence spectrum of different metal ions and rhodamine class B derivatives response (CH3CH2OH/H2O [v/v, 1/2], λ ex = 530 nm, probe concentration 10 × 10−5 mol·L−1, metal ions concentration 10 × 10−5 mol·L−1).
The synthesis of rhodamine B derivative Rh-DCP is shown in Figure 5. Figure 6 shows possible binding mechanism of probe Rh-DCP to Hg2+. According to the above experiment, the binding ratio of probe RH-DCP to Hg2+ was 1:1. When the RH-DCP fluorescent probe was alone, the spirolactam ring in the structure was in a closed loop state and hardly produced fluorescence. After the addition of Hg2+ ions, Hg2+ coordinates with oxygen and nitrogen on amide, and electron rearrangement occurs in the molecule. Therefore, the spirolactam ring of the probe molecule breaks, and rH-DCP in the ring-open state will cause changes in fluorescence intensity and color.
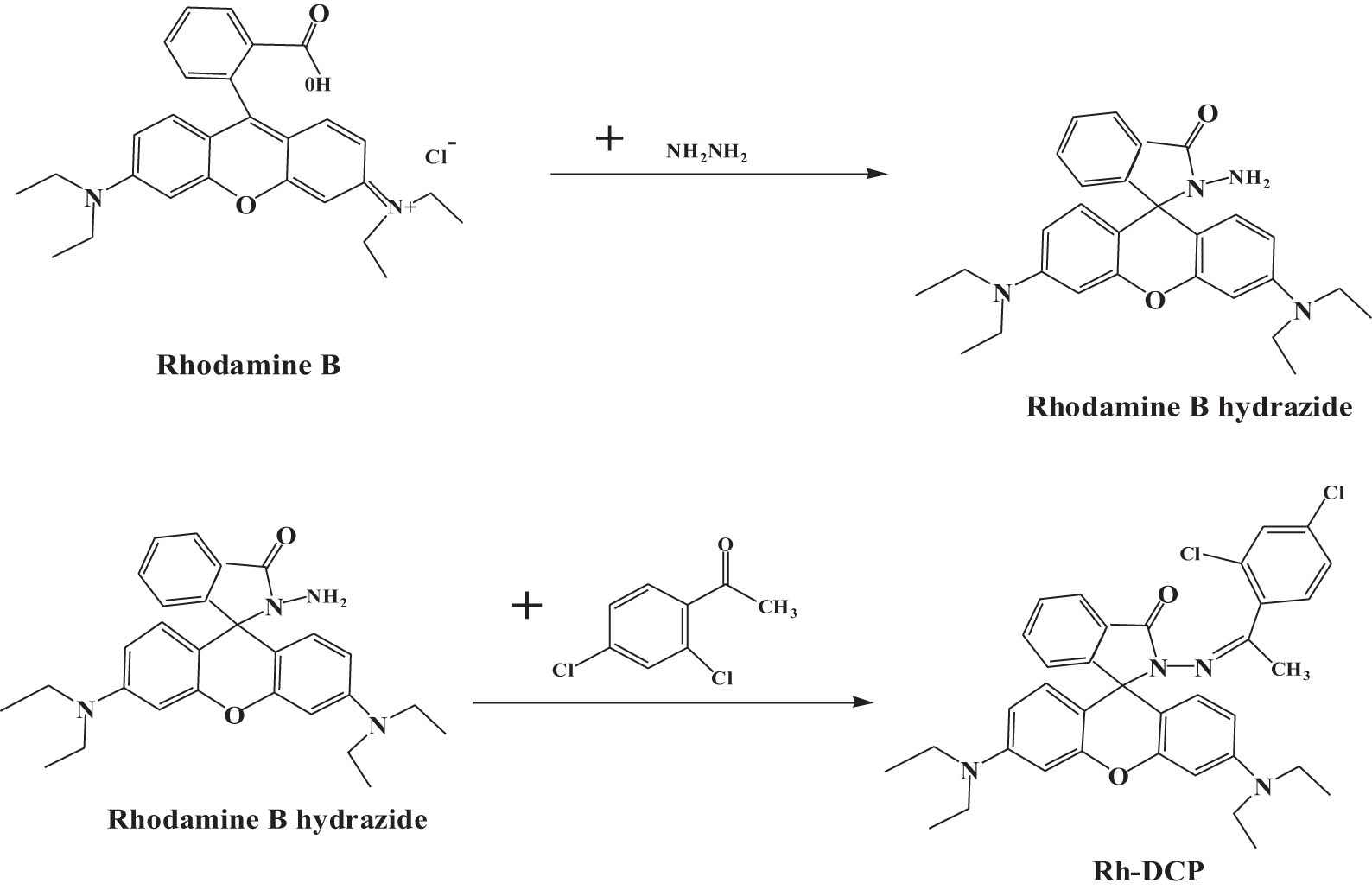
Synthesis of rhodamine B derivative Rh-DCP.

Possible binding mechanism of probe Rh-DCP to Hg2+.
Compared with the reported methods, the fluorescence molecular probe technology has the advantages of high selectivity, high sensitivity, simple operation, and wide application range, and has been favored in the study and detection of mercury ions.
3.3 Determination of the sample
3.3.1 Determination of Hg(ii) in water samples
Table 1 is the water sample determination experiment. The river water and tap water are filtered through the filter membrane to remove impurities in the water. Mercury ion content was measured by fluorescence probe, and no response indicated that there was no Hg2+. The addition scalar of Hg2+ at high and low levels is consistent with the measured data. The experimental results show that the synthesized probe can be applied to the analysis and determination of Hg2+ in tap water and lake water and has high practical value.
Sample determination experiment (n = 6)
| Sample | Detection volume (mol·L−1) | Add amount (mol·L−1) | Recovery rate (%) | RSD (%) |
|---|---|---|---|---|
| 10 × 10−6 | 99.6 | 1.88 | ||
| Tap water | — | 20 × 10−6 | 100.7 | 2.03 |
| 40 × 10−6 | 101.2 | 1.95 | ||
| 10 × 10−6 | 98.5 | 2.11 | ||
| Songhua river water | — | 20 × 10−6 | 100.6 | 1.95 |
| 40 × 10−6 | 101.6 | 1.83 |
3.3.2 Determination of Hg(ii) in titanium dioxide and zinc white samples
Table 2 shows the determination experiment of titanium dioxide and zinc white samples of oil paint. First, the sample solution of titanium dioxide and zinc white of oil paint was prepared. An appropriate amount of mercury ions was added to the prepared fluorescent probe, and the mercury content in the two samples was measured by fluorescence analysis. The reaction showed no response, indicating that there was no Hg2+. As can be seen from Table 1, three different high and low levels of Hg2+ were added to the markers. The results show that the probe RH-DCP can be effectively applied to the determination of Hg2+ in zinc white and titanium white solution of oil paint samples. Avoid painting workers, contact with paint containing toxic metals, has high practical application value.
Sample determination experiment (n = 6)
| Sample | Detection volume | Add amount (mol·L−1) | Recover amount (mol·L−1) | Recovery rate (%) | RSD (%) |
|---|---|---|---|---|---|
| 50 × 10−6 | 49.81 × 10−6 | 99.7 | 1.86 | ||
| Titanium white | — | 80 × 10−6 | 81.02 × 10−6 | 101.1 | 2.21 |
| 100 × 10−6 | 100.8 × 10−6 | 100.8 | 1.95 | ||
| 50 × 10−6 | 50.86 × 10−6 | 101.7 | 2.12 | ||
| Chinese white | — | 80 × 10−6 | 80.61 × 10−6 | 100.8 | 2.02 |
| 100 × 10−6 | 102.5 × 10−6 | 102.3 | 1.78 |
3.4 Standard curve drawing
Figure 7 shows the standard curve of RH-DCP for mercury ion detection. According to the analysis of detection system, the fluorescence intensity of the fluorescence system increased with the increase of the concentration of Hg2+ added. In a certain range, the relative fluorescence intensity of the system has a good linear relationship with the concentration, and the regression curve is y = 8.059X + 23.986, R 2 = 0.9989. Therefore, the fluorescence system can be used as a probe to determine the content of Hg2+ in water.

Standard curve of Rh-DCP detection of Hg2+.
3.5 Linear detection range and detection limit
Figure 8 shows the fluorescence spectra of blank samples. By measuring the blank solution 11 times in parallel and then calculating the three times standard deviation, δ = 0.525 was calculated. The detection limit of the probe molecule detected by this method was calculated to be 0.2 × 10−6 mol·L−1. When Hg2+ was in the range of 10.0 × 10−6−12.8 × 10−5 mol·L−1, the relative fluorescence intensity of the system had a good linear relationship with the concentration, and the linear correlation coefficient was 0.9993.

Spectral detection limit of blank sample (n = 11).
3.6 The graph results of the MTT cytotoxicity test
The results of MTT cytotoxicity test were as follows (Figure 9): the fluorescence of rH-DCP-treated cells was weak, and the HeLa cells showed strong green fluorescence when Hg2+ and probe were added, indicating that the RH-DCP probe had good membrane permeability and could be used for the detection of Hg2+ in HeLa cells. In the follow-up experiments, our research group will carry out relevant work according to the experimental ideas of literature [23,24].
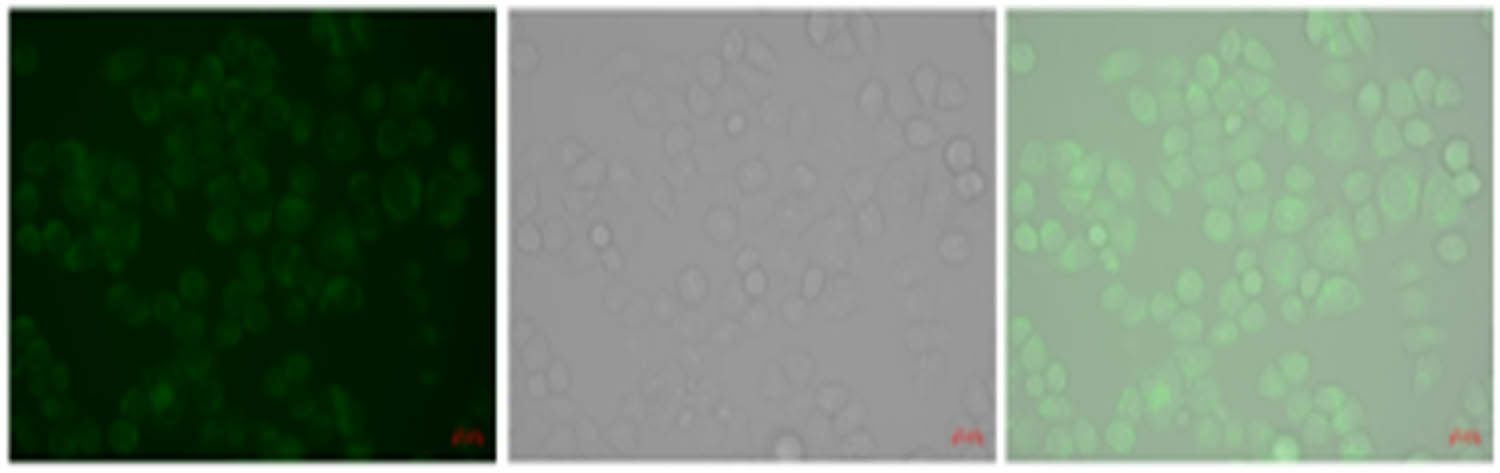
Cell fluorescence imaging of the probe.
4 Discussion
In recent years, fluorescent molecular probes have attracted extensive attention because of their advantages such as good selectivity, no reference term, high sensitivity, relatively simple operation, low preparation cost, and not easily affected by magnetic and electric fields. In the design of fluorescent probes, fluorophores commonly used are rhodamine, coumarin, and so on. In the selection of these types of materials, rhodamine has the highest fluorescence quantum yield, which is cheap and easy to obtain, and can even be seen by the naked eye, and has unique advantages such as obvious color change of the target detection object. Therefore, rhodamine was selected as fluorophore in this article to prepare the fluorescence probe for detecting metal ions in the environment. In this article, the mercury ion fluorescence system of rhodamine B derivatives was first constructed and then based on its UV and fluorescence absorption in the addition of metallic mercury. An absorption peak centered at 558 nm is obtained in the UV-Vis absorption spectrum. Peak centered at 583 nm is obtained in the fluorescence spectrum, and all of the above indicate that the mercury ion fluorescence system of rhodamine B derivatives has been constructed. Detection of the response of rhodamine B derivatives to mercury and other metal ions by fluorescence spectroscopy resulted at 583 nm, while other ions showed little response and did not affect the detection of mercury ions. By changing the concentration of Hg2+, the whole UV-Vis and fluorescence titration spectra of the ligand were changed. It was found that the peak position of the spectra did not change, but the peak intensity changed. The results show that the rhodamine B derivative probe molecule has a good selectivity only for metallic mercury ions and that metallic mercury ions have a good selectivity. The maximum fluorescence intensity is reached at the molar concentration ratio of 1:1, which is the best binding ratio of mercury ions to the probe.
Therefore, the treatment methods of mercury-containing wastewater mainly include chemical precipitation, adsorption, ion exchange, and ultrafiltration. At present, chemical precipitation method is mostly used to treat mercury-containing wastewater in industry, while other methods are still in the stage of experimental research, or the treatment cost is high, so it is difficult to popularize and apply. Therefore, adsorption, ion exchange, and ultrafiltration methods should be used.
5 Conclusions
In addition, the existence of harmful substances in oil paints, such as mercury and lead metal ions, is very harmful to the human body. In this article, rhodamine B RH-DCP probe was designed for the determination of mercury ions in titanium dioxide and zinc white of oil paints. The probe can be used to detect mercury ions in titanium dioxide and zinc white of oil paints in environmental water samples.
Acknowledgements
The authors thank Yanqiu Hu, Xiaoxuan Zhou, and Yuguang Lv (College of Pharmacy, Jiamusi University) for their assistance in rhodamine B derivative fluorescence probe research.
-
Funding information: This work was financially supported by the Department of Scientific Research project in Heilongjiang province (No. LH2022049), Basic Scientific Research Project of Heilongjiang Provincial Colleges and Universities (No. 2021-KYYWF-0589), and the Innovation and Entrepreneurship Project for College students in Heilongjiang Province (S202210222018).
-
Author contributions: Zhankun Wang: writing – original draft, writing – review and editing, methodology, formal analysis; Yanqiu Hu: writing – original draft, formal analysis, visualization, project administration; Xiaoxuan Zhou: data analysis; and Yuguang Lv: resources.
-
Conflict of interest: Authors state no conflict of interest
-
Data availability statement: The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Li JL, Ding GH, Liu YP, Feng HJ, Niu YY, He MX, et al. Spectroscopic properties of 5-phenyl-2-(4-methoxy)-2H-1,2,3-triazole-4-carboxylic acid ethyl ester and its rhodamine-derived chromogenic mechanism on Hg2+ and cellular imaging. Chin J Lumin. 2019;40(8):969–78. 10.3788/fgxb20194008.0969.Search in Google Scholar
[2] Yang B, Wu WH. Synthesis of rhodamine B derivatives with fluorescence-enhancing properties and their detection of mercury ions. Imag Sci Photochem. 2013;31(6):421–7. 10.7517/j.issn.1674-0475.2013.06.421.Search in Google Scholar
[3] Sivaraman G, Iniya M, Anand T, Kotla NG, Sunnapu O, Singaravadivel S, et al. Chemically diverse small molecule fluorescent chemosensors for copper ion. Coord Chem Rev. 2018;357:50–104. 10.1016/j.ccr.2017.11.020.Search in Google Scholar
[4] Martens P, Verbrugge F, Nijst P, Dupont M, Tang WHW, Mullens W. Impact of iron deficiency on response to and remodeling after cardiac resynchronization therapy. J Am Coll Cardiol. 2017;119(1):65–70. 10.1016/j.amjcard.2016.09.017.Search in Google Scholar PubMed
[5] Zhang B, Huang WT, Wang XC, Wu HS, Yang C, Yang YN, et al. Research progress on fluorescent probes for rhodamine B derivatives. J Jilin Med Coll. 2019;40(4):288–90. 10.13845/j.cnki.issn1673-2995.2019.04.019.Search in Google Scholar
[6] Dong T, Lei L, Zhang B. Progress in the preparation and application of rhodamine-like mercury ion fluorescent probes. Mod Salt Chem. 2017;44(5):18–21. 10.19465/j.cnki.2095-9710.2017.05.008.Search in Google Scholar
[7] Zhang JY, Wan ZT, Wei YX, Li LQ. Research progress of mercury ion fluorescent probe. Shandong Chem Ind. 2018;47(11):48–50. 10.19319/j.cnki.issn.1008-021x.2018.11.021.Search in Google Scholar
[8] Shi L, Lu Z, Gong YJ, Bi JJ, Ma NN, Xu JJ, et al. Recognition of Hg2+ by new Rhodamine amide thiourea fluorescent probe in aqueous solution. Anal Lab. 2017;36(12):1365–6. 10.13595/j.cnki.issn1000-0720.2017.0292.Search in Google Scholar
[9] Zhang D, Li M, Wang M, Wang JH, Yang X, Ye Y, et al. A rhodamine-phosphonate off-on fluorescent sensor for Hg2+ in natural water and its application in live cell imaging. Sens Actuat B-Chem. 2013;177:997–1002. 10.1016/j.snb.2012.11.080.Search in Google Scholar
[10] Luo AL, Gong YJ, Yuan Y, Zhang J, Zhang CC, Zhang XB, et al. A simple and pH-independent and ultrasensitive fluorescent probe for the rapid detection of Hg2+. Talanta. 2013;117:326–32. 10.1016/j.talanta.2013.09.033.Search in Google Scholar PubMed
[11] Liu HX, Liu Q, Shen YF. Research progress based on rhodamine-based fluorescent probes. Mod Chem Eng. 2017;37(4):197–204. 10.16606/j.cnki.issn.0253-4320.2017.04.049.Search in Google Scholar
[12] Guo ZL, Yang Y, Liu JL, Xu L. Synthesis of rhodamine Schiff base fluorescent probe and its application in Cu2+ and Fe3+ detection. Petrochemical. 2019;48(9):963–7. 10.3969/j.issn.1000-8144.2019.09.014.Search in Google Scholar
[13] Huang Y, Yang MP, Zhang WH, Wu S. Specific recognition of Fe3+ by rhodamine-like Schiff base fluorescent probe. Chem Res Appl. 2018;30(1):88–94.Search in Google Scholar
[14] Liu JF, Qian Y. A novel pyridylvinyl naphthalimide-rhodamine dye: synthesis, naked-eye visible and ratiometric chemo do simeter for Hg2+/Fe3+. J Lumin. 2017;187:33–9. 10.1016/j.jlumin.2017.02.058.Search in Google Scholar
[15] Han WQ, Tian MZ, Feng F, Bai YF, Chen ZZ. Application of double-armed rhodamine-type fluorescent probes in the identification of Hg2+. Phys Chem Test (Chem Subser). 2018;54(3):255–9. 10.11973/lhjy-hx201803002.Search in Google Scholar
[16] Dhaka G, Kaur N, Singh J. Luminescent benzothiazole-based fluorophore of anisidine scaffoldings: a “Turn-On” fluorescent probe for Al3+ and Hg2+ ions. J Fluoresc. 2017;27(6):1943–8. 10.1007/s10895-017-2148-5.Search in Google Scholar PubMed
[17] Tian F. Application of rhodamine-type fluorescent probes in the detection of heavy and transition metal ions. Petrochem Technol. 2019;26(12):364–5. 10.3969/j.issn.1006-0235.2019.12.228.Search in Google Scholar
[18] Wang JQ, Lei L, Dong T. Preparation and properties of a mercury ion fluorescent probe with rhodamine structure. Energy Chem. 2017;38(6):48–53. 10.3969/j.issn.1006-7906.2017.06.012.Search in Google Scholar
[19] Qiao R, Xiong WZ, Bai CB, Liao JX, Zhang L. A highly selective fluorescent chemosensor for Fe(iii) based on rhodamine 6G dyes derivative. Supramol Chem. 2018;30(11):911–7. 10.1080/10610278.2018.1467016.Search in Google Scholar
[20] Jiang HE, Li ZJ, Kang YF, Ding LP, Qiao S, Jia ST, et al. A two-photon fluorescent probe for Cu2+ based on dansyl moiety and its application in bioimaging. Sens Actuators B Chem. 2017;242:112–7. 10.1016/j.snb.2016.11.033.Search in Google Scholar
[21] Qian J, Wang K, Wang CQ, Ren CC, Liu Q, Hao N, et al. Ratiometric fluorescence nanosensor for selective and visual detection of cadmium ions using quencher displacement-induced fluorescence recovery of CdTe quantum dots-based hybrid probe. Sens Actuators B Chem. 2017;241:1153–60. 10.1016/j.snb.2016.10.020.Search in Google Scholar
[22] Lv T, Xu YQ, Li HJ, Liu FY, Sun SG. A rhodamine B-based fluorescent probe for imaging Cu2+ in maize roots. Bioorg Med Chem. 2018;26(8):1448–52. 10.1016/j.bmc.2017.09.026.Search in Google Scholar PubMed
[23] Wang Y, Gao M, Liao CY, Yu FB, Chen LX. A sulfydryl-based near-infrared ratiometic fluorescent probe for assessment of acute/chronic mercury exposure via associated determination of superoxide anion and mercury ion in cells and in vivo. Actuators B Chem. 2019;301:127038. 10.1016/j.snb.2019.127038.Search in Google Scholar
[24] Wang Y, Gao M, Chen GG, Yu FB, Jiang GB, Chen LX. Associated detection of O2•- and Hg2+ under chronic mercury exposure in cells and mice models via a three-channel fluorescent probe. Anal Chem. 2018;20:2–9. 10.1021/acs.analchem.8b01442.Search in Google Scholar PubMed
© 2022 Zhankun Wang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

