Abstract
In this study, CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) nanoparticles were synthesized directly in toluene solvents at high temperature. This approach results in small-size nanoparticles, which can be used in photoelectric components without adding a filtration process to eliminate high-temperature solvents such as octadecene. The high content Mn2+-incorporated CsPb(Cl/Br)3 host is observed by strongly wideband emission at 592 nm of manganese d-states spin and orbital forbidden transition. After infiltration into mesoporous TiO2, the Mn2+ ion receives energy from the CsPb(Cl/Br)3 host (irradiated 405 nm light-emitting diode source) and effectively transfers to the TiO2 scaffold layer. As a result, a metal/semiconductor/metal planar structure photodetector with m-TiO2/CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) composite showed several figures of merit compared to bare m-TiO2 and m-TiO2/CsPbBr3 such as on/off ratio of 104 times, responsivity of 1.67 A·W−1, and detectivity of 4.42 × 109 Jones. The key factors contributing to the growth of the on/off ratio include the decreasing dark current and enhancing exciton energy and transportation due to the infiltration of CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) perovskite quantum dots into mesopores of the m-TiO2 scaffold layer.
1 Introduction
All inorganic perovskite semiconductor materials recently have attracted much attention as active materials for optoelectronic devices such as solar cells [1–3], light-emitting diode (LED) [4,5], photodetector [6–9], etc. Typically, the bulk perovskite crystals exhibit poor phase stability due to the weak ion bonding nature easily leading to phase separation [10–12]. By reducing the size of crystals, the performance of nanosize semiconductor was largely improved due to reduced crystal strain resulting in their good phase stability, narrow photoluminescence (PL) emission, tunable light absorption spectrum, and high PL quantum yield in comparison with bulk counterparts. As a consequence, nanosemiconductors have shown great potential for applications not only in optoelectronic fields but also not restricted in spectral decoy for protecting space assets, photocatalyst, sensor, biosensor, etc. [13–16].
Besides reducing the particle size, replacing Pb2+ ions such as Mn2+ not only minimizes toxic lead elements but also improves optoelectronic performance. Xu et al. have shown that the doping of manganese in CsPbCl3 improves the perovskite UV photodetector performance due to fast exciton to manganese energy transfer, long charge carrier lifetime, and the excited electrons effectively transfer to the TiO2 scaffold layer. As a result, the self-powered UV photodetector of carbon/CsPbCl3:Mn/FTO with photocurrent density has enhancement from 0.08 to 0.14 mA·cm−2 and responsivity of 7.3 mA·cm−2 at 340 nm excitation light source [17]. It requires a dissolve and recrystallization process to ensure that the nanocrystal penetrates into the bottom of the devices. He et al. also fabricated an FTO/TiO2/CsPbBr3:Mn/Spiro/Au, which showed a remarkable improvement in the photocurrent density of components compared to the non-doped CsPbBr3 counterpart [18].
In this study, we introduced a one-step heating process directly in iso-octane solvent for fabrication of CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) nanoparticles (NCs) with a small size. These particles can easily infiltrate into the mesopores of the TiO2 scaffold layer. A metal–semiconductor–metal planar structure of UV photodetector based on TiO2/perovskite composite presented several significant figure-of-merit parameters for evaluating, such as on–off ratio, responsivity (R), and detectivity (D*). Although the primary parameter to be considered may be different depending on the application, this result shows the potential for the UV photodetector application.
2 Experimental section
2.1 Materials
Cesium carbonate (Cs2CO3, Macklin, 99.9%), lead bromide (PbBr2, Macklin, 99.9%), 1-octadecene (ODE, Macklin, >90%), oleic acid (OA, 90%), oleylamine (OAm, 80–90%), iso-octane, ethanol, toluene, and ethyl acetate (EA, >99%) were purchased from Fisher, and TiO2 paste (Dyesol 18NR-T). All chemicals were used without any further purification. Manganese chloride tetrahydrate (MnCl2·4H2O, Aladdin, 99%) was dried by heating in a vacuum at 100°C.
2.2 Fabrication of CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) quantum dots (QDs)
CsPbBr3 and CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) perovskite nanocrystals were fabricated via a one-pot strategy by directly heating perovskite precursors in toluene in air. First, Cs precursors were obtained by mixing 0.6 mmol of Cs2CO3 powder, 30 mL of octane, and 1.5 mL of OA together in a 100 mL stand-up flask and heating the mixture to 90°C in a heating agitator until all the Cs2CO3 powder was dissolved. The MnCl2/PbBr2 with different mol feed ratios were mixed with 30 mL iso-octane, 1.5 mL OA, and 3 mL OAm together in a 100 mL stand-up flask. The mixture was then heated to 110°C in a heating agitator until all the powder was dissolved. The initial solution was centrifuged at 6,000 rpm for 5 min to precipitate the large particles, and the supernatant was retained for subsequent use.
2.3 Photodetector device fabrication
The planar integrated Ti/Pt (50/200 nm) electrode is fabricated using standard photolithography to pattern the photoresist for subsequent metallization and lift-off. The electrode included 40 fingers with the channel width and length of 20 μm and 1.60 mm, respectively. The total active area was 0.128 mm2.
To obtain the 2 μm-thick mesoporous-TiO2 layers on the Ti/Pt electrode, the substrate is deep-coated with TiO2 paste diluted in an ethanol solvent with a mass ratio of 1:3. Then, the layer was sintered under an air atmosphere at 500°C for 30 min. Finally, 1 μL of 1 mg·mL−1 CsPbBr3 or CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) QDs solution was drop-cast directly on the m-TiO2 layer with pre-patterned Pt electrodes and annealed at 80°C for 30 min in a vacuum tube for the solvent evaporation.
2.4 Characterization techniques
The surface morphology and chemical composition of obtained samples were characterized by transmission electron microscopy (TEM, JEOL-JEM 1010, Japan), field emission scanning electron microscopy (FE-SEM) coupled with Energy-dispersive X-ray spectroscopy (EDS), (JSM-7600F, Jeol), respectively. The crystalline structure of samples was analyzed by powder X-ray diffraction (XRD, Rigaku D/MAX-2500/PC) using CuKα radiation (λ = 0.154 nm) operated at 40 mA tube current. PL properties of all samples were investigated by using a PL spectrophotometer (Nanolog, Horiba Jobin Yvon) equipped with a 450 W xenon discharge lamp as the excitation source. The photoresponse was detected by Keithley 4200-SCS, America, coupled with a 405 nm UV LED source.
3 Results and discussion
CsPbBr3 and CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) perovskite nanocrystals have been fabricated simply by mixing the precursors Cs2CO3, PbBr2, MnCl2, and OLA, OA in iso-octane solvents and heating. Although there is no direct evidence of the replacement of Mn2+ with Pb2+ ions in crystals, PL, X-ray, EDS, and PL spectral measurements show their presence in the crystal structure. As shown in Figure 1a, by increasing the MnCl2 to PbBr2 mol feed ratio from 0:1 to 1:1, the narrow emission of CsPb(Cl, Br)3 host crystals was strongly blue-shifted from 520 to 440 nm due to the replacement of Cl– to Br– ions in the host crystals. Besides, a broadband emission centered at 592 nm is assigned to the Mn2+ ions doped in the CsPb(Cl/Br)3 host NCs, which can be attributed to the transition from 4T1 to 6A1 energy levels of the Mn2+ 3d states [17]. With the decrease of CsPb(Cl, Br)3 emission, the strong 592 nm emission at high Mn content indicated that Mn2+ can act as an effective energy acceptor of the excited CsPb(Cl/Br)3 host. Figure 1b and c shows the optical absorption and PL of CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) with 0.6:1 and 1:1 MnCl2/PbBr2 mol feed ratios of precursors, respectively. The optical absorption curve revealed that CsPb(Cl/Br)3:Mn2+ had a large absorbance in the UV, and visible spectral region with band edges started at the first narrow emission band of CsPb(Cl/Br)3 host.
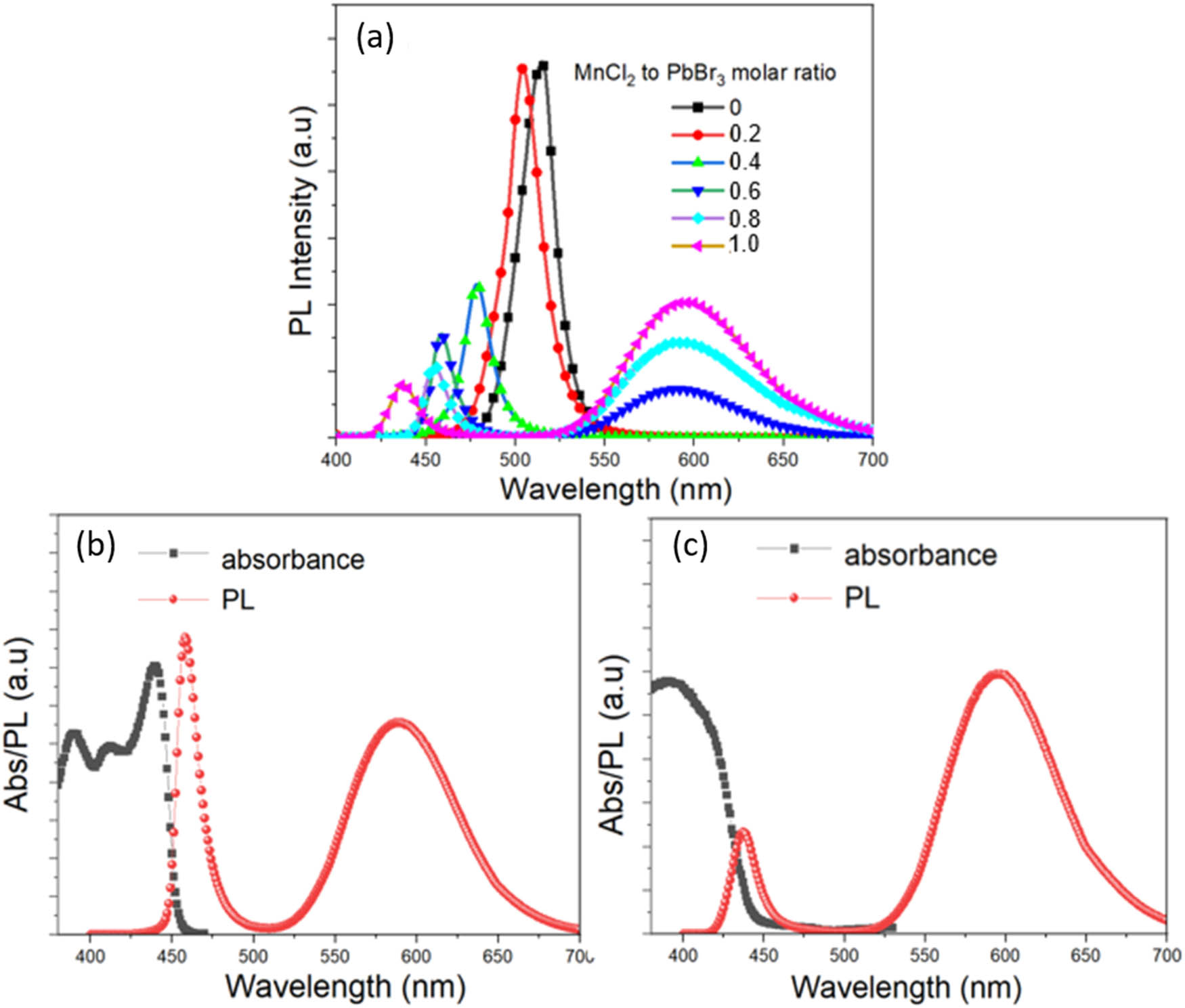
(a) The PL spectra of CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) nanocrystals synthesized directly in iso-octane solvent at 110°C with different MnCl2 to PbBr2 mol feed ratios. (b and c) The optical absorption and PL of CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) with 0.6:1 and 1:1 MnCl2/PbBr2 mol feed ratios of precursors, respectively.
The XRD patterns of CsPbBr3 and CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) NCs are shown in Figure 2. It can be seen that the as-synthesized CsPbBr3 NCs have a cubic structure (JCPDS No. 54-0752) with the 2θ peaks at 21.1, 30.2, 34.2, 36.1, and 44.9°, corresponding to (110), (200), (211), (210), and (202) planes, respectively. A detailed view of the (211) and (220) peak position showed that a peak shifted toward the larger theta angle as MnCl2 content increases. The difference in ion radius between [Pb2+ (1.19 A°), Br− (0.196 A°)] and [Mn2+ (0.8 A°), Cl− (0.175 A°)] is the original of the peak shift. Consequently, the shift indicated the incorporation of MnCl2 in the CsPbBr3 host. However, the main perovskite peaks disappear, and several unknown peaks exist with the further increased MnCl2 content (MnCl2/PbBr2 mol feed ratio up to 1.2).

The XRD pattern of CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) NCs with different MnCl2 to PbBr2 mol feed ratios.
It is well known that temperature and reaction time are important parameters for controlling the size of nanoparticles [19]. Generally, low temperature and fast reaction time can make small size of nanocrystals. Figure 3 shows the TEM image of the CsPbBr3 and CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) nanocrystals after fabrication at 100°C and reaction time at 30 s. The nanocrystals are in the cubic shape with a size of about ∼7 and ∼5 nm, respectively (Figure 3a and c). It is much smaller than the mean size of the mesopores in the m-TiO2 layer (Figure A1), ensuring them deep infiltration into the mesoporous layer. The insets of Figure 3a and c show the size distribution and photos of dried nanocrystal films with strongly bright green and orange-red color in ambient daylight. Samples analyzed by EDX measurements showed a Cs:Pb:Br ratio of 1:1:3 for CsPbBr3 NCs and the Mn2+/Pb2+ ratio of CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) ∼0.9:1 for the MnCl2/PbBr2 ∼1:1 mol feed ratio (Figure 3b and d). Further increasing MnCl2 content sharply decreased the PL intensity (not shown here) due to the changing of the crystal structure as described in the above XRD results.

SEM images and EDX spectra of (a and b) CsPbBr3 and (c and d) CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) nanocrystals.
The temperature-dependent PL study provides information about the physical properties of the nanocrystals. Figure 4a and c shows the temperature-dependent PL spectra of CsPbBr3 and CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) NCs as prepared. Photos of two samples placed in a cooling chamber irradiated with a 405 nm laser source show a strong green light of CsPbBr3 and a bright red light of CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) nanocrystals, respectively. As expected, the peaks are blue shifted due to the thermal expansion of the crystal leading to the forbidden gap increase, which are consistent with the previous reported results [20]. For the CsPbBr3 QDs, the PL spectrum separates into two peaks: a main peak (from 515 to 505 nm according to temperatures between 10 and 300 K) and a small peak at longer wavelengths due to heavy hole–electron and light hole–electron transitions [20]. The PL intensity quickly decreases with increasing temperature. For the CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) QDs, the PL spectra show only one peak, and the peak shifted from 610 to 590 nm when the ambient temperature increased from 10 to 300 K. In particular, the optical intensity of the sample is maintained above 80% when the temperature rises to 300 K. The activation energy is extracted by plotting the relationship between the integral PL intensity and the measured temperature, as shown in Figure 4b and d. The activation energy of samples can be deduced with the following equation [21]:
where I 0 and I(T) represent the intensity of the NCs at the 0 K temperature and the testing temperature, respectively. A is a constant and k is Boltzmann’s constant (8.62 × 10−5 eV·K−1). According to the Arrhenius equation, the activated energy values reached 40 and 120 meV for the CsPbBr3 and CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5), respectively. The high E B value of CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) ensures that the excited state of Mn2+ luminescent energy levels can be able to maintain above room temperature for their potential applications in optoelectronic applications.
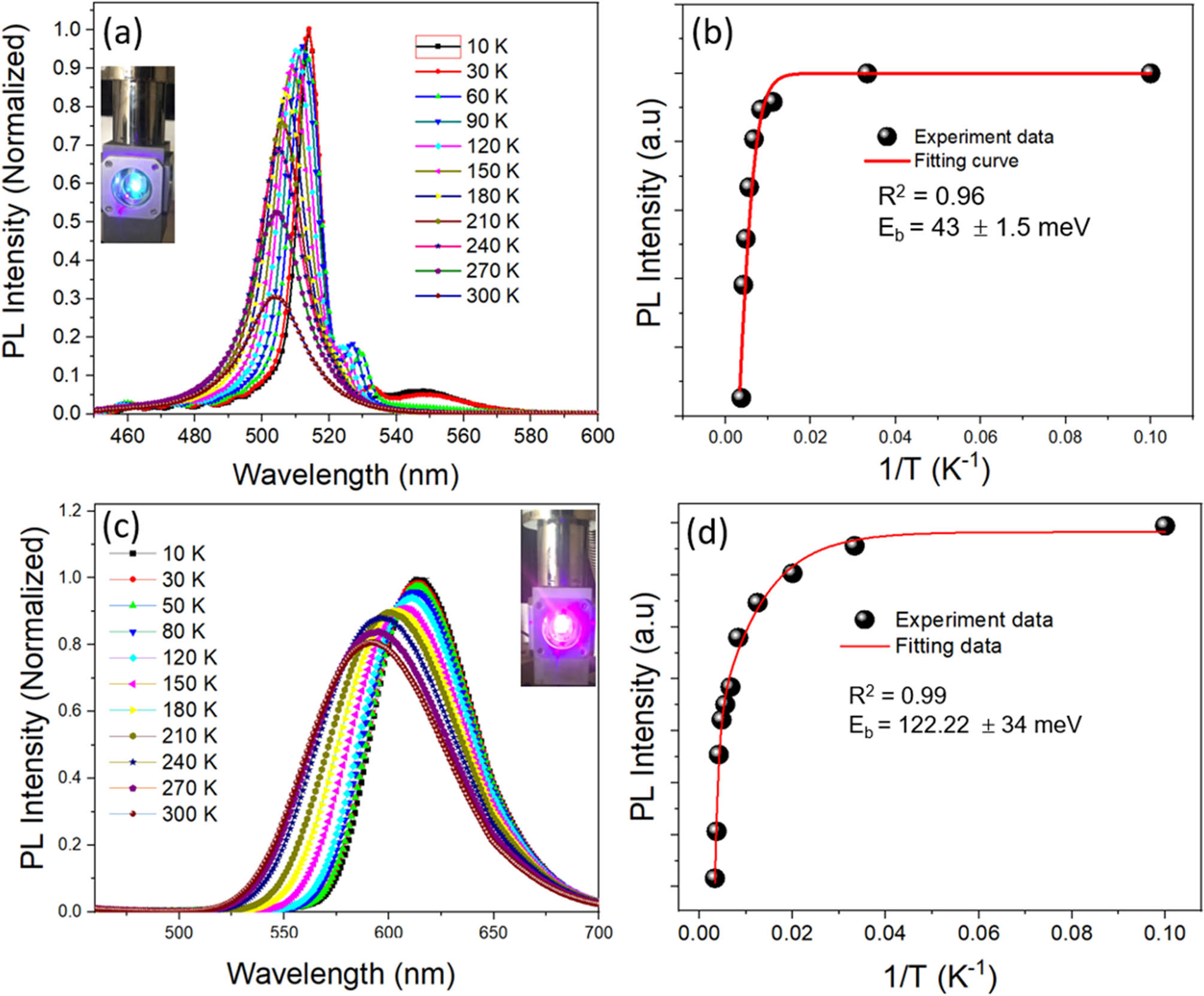
Temperature-dependent PL spectra and PL intensity versus inverse temperature and Arrhenius fit of CsPbBr3 NCs (a and b) and CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) (c and d).
In order to demonstrate the carriers charge transfer between Mn2+ in perovskite NCs and mp-TiO2 scaffold layer, the PL spectrum and decay curve of QDs on mp-TiO2 and aluminum foil substrate were measured (Figure 5). As shown in Figure 5a, the PL intensity of CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) QDs/mp-TiO2 was reduced by a 3.5-time magnitude compared to that of the QDs on aluminum foil substrate. Moreover, the radiative lifetime decreases from 1.54 ms to 80 µs for the CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) in m-TiO2 (Figure 5b). It strongly indicates that mp-TiO2 can extract charge carriers from the CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) QDs.
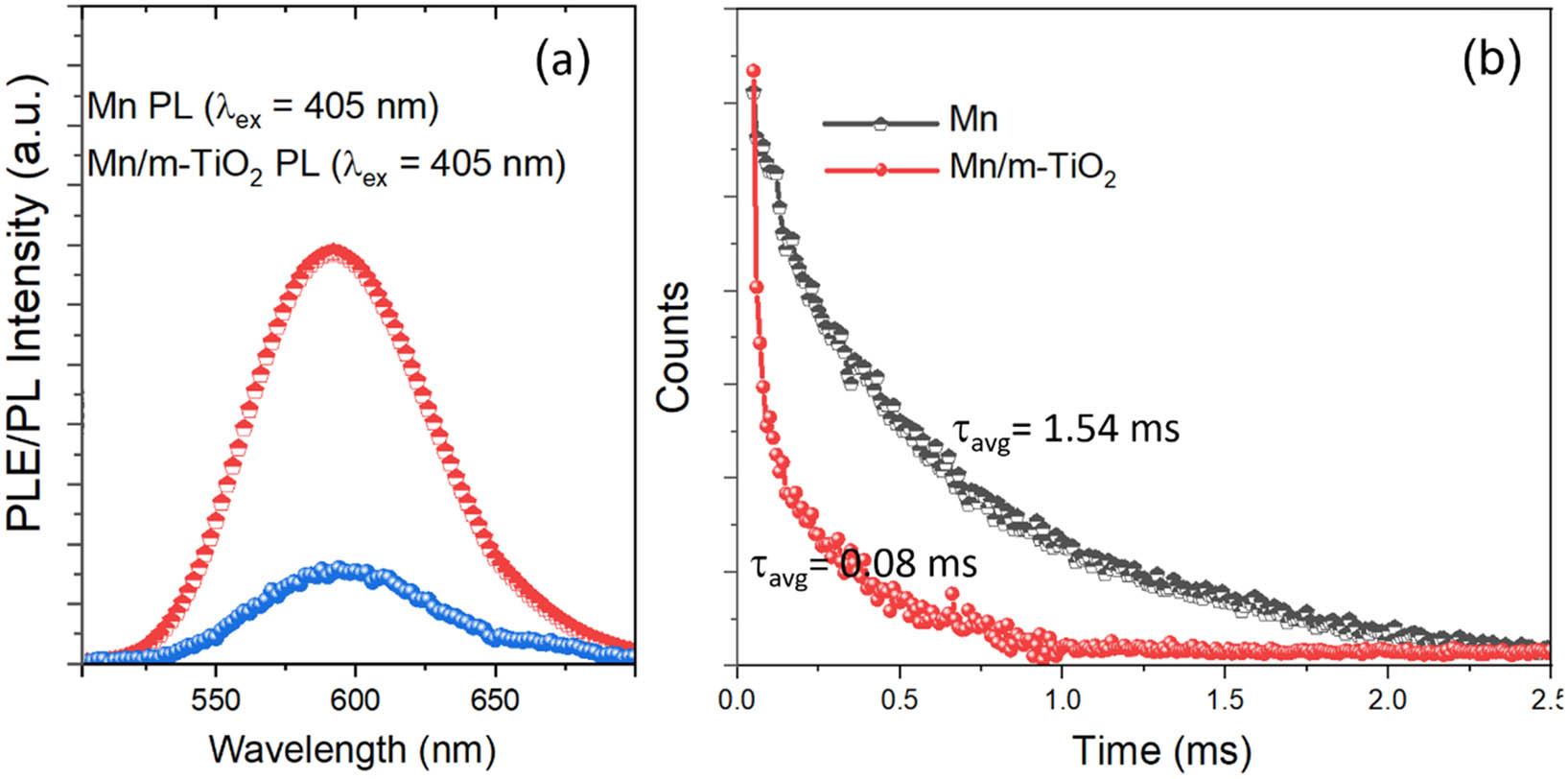
(a) PLE/PL spectra and (b) PL decay curves of CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) NCs.
For the UV photodetector measurement, three samples of m-TiO2, m-TiO2/CsPbBr3, and m-TiO2/CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) on the pre-patterned Pt electrodes were tested as shown in Figure A2. Two probes I–V were measured in the dark and under illumination by using a 405 nm UV-LED chip (3 mW·cm−2). Figure 6a and b shows the I–V curves of three samples under dark and near UV irradiation. For the m-TiO2 sample, the dark current was high with hysteresis due to H2O, O2 absorption, and catalyst activity of TiO2 or Pt electrode materials for water-splitter reaction, as well as a different work function between TiO2 semiconductor and Pt metal electrode [22]. By introducing UV light to their layer, the excited electron of the TiO2 semiconductor was dominant leading to the increase of the current and the hysteresis disappeared. In particular, by infiltration of perovskite QDs into the mesopore of m-TiO2, dark currents have plummeted, while photocurrents have increased markedly. Figure 6c shows the corresponding current on/off ratios of three devices at 1.5 V bias voltage, and it is stable at values of ∼3.5, 102, and 104 times for m-TiO2, m-TiO2/CsPbBr3, and m-TiO2/CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5), respectively. Two important parameters of the device, the sensitivity I and detection index (D*), can be obtained from the following equations [23]:
where I ph is the photocurrent, P light is the light power intensity (3 mW·cm−2), I dark is the dark current, and q is the elementary charge (Coulomb). The highest values of R and D* are 1.67 A·W−1 and 4.42 × 109 Jones, respectively, for the m-TiO2/CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) sample. This result shows the potential for the UV photodetector application.
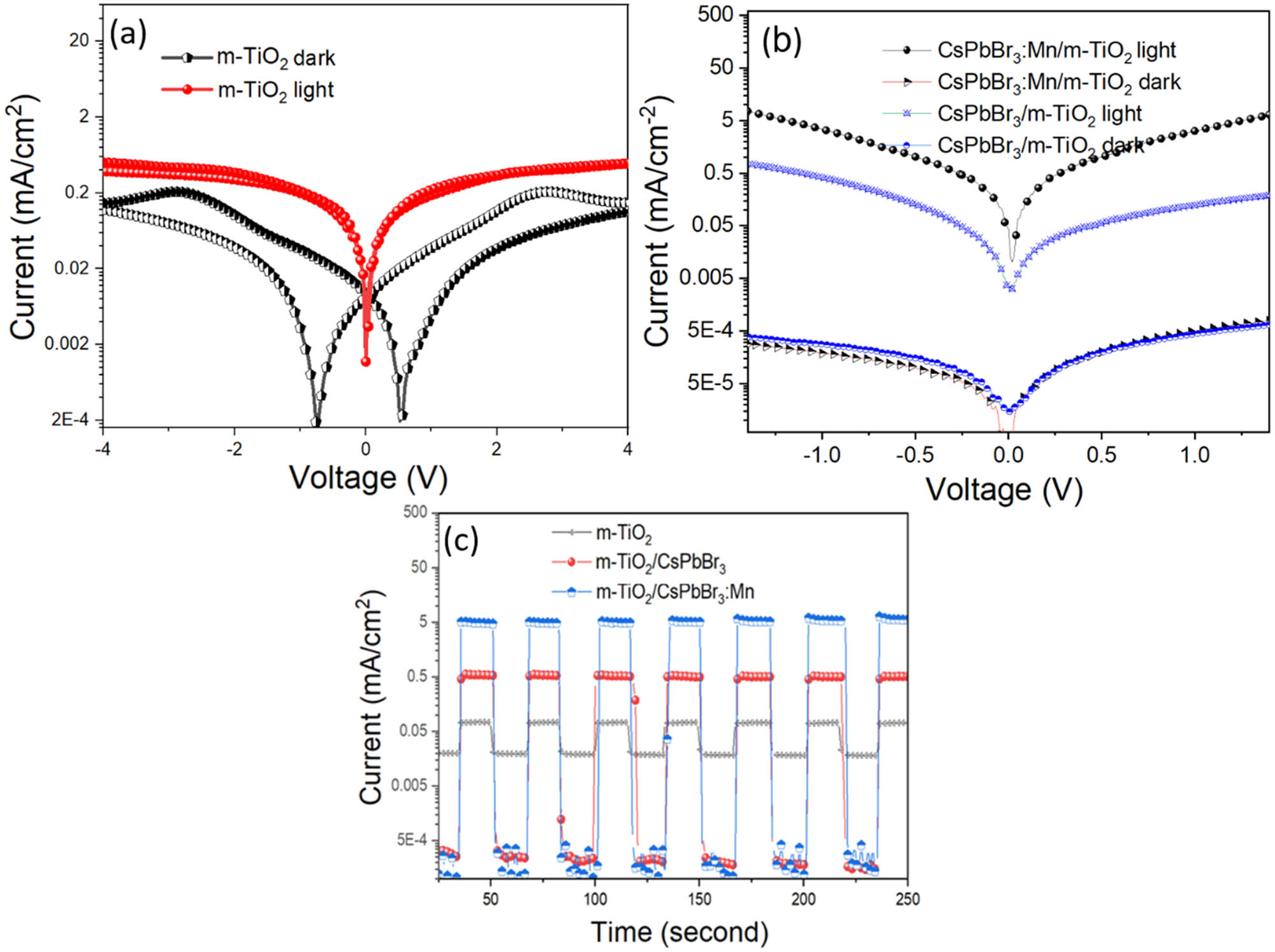
Logarithmic I–V curves of planar structure photodetector based on (a) m-TiO2, (b) m-TiO2/CsPbBr3 and m-TiO2/CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) composite films in the dark and NUV light irradiation. (c) Photocurrent–time response measured in the dark and under illumination under 1.5 V bias.
4 Conclusions
In conclusion, CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) nanocrystals have been fabricated by the one-pot heating directly in toluene solvent method. The small crystals were directly infiltrated into m-TiO2 scaffold layer without further washing process. The Mn2+ effectively received energy from CsPb(Cl/Br)3 host and transferred to m-TiO2 scaffold layer resulting in enhancement of UV photodetector performance. As a consequence, a metal/semiconductor/metal planar structure photodetector with m-TiO2/CsPb1–x Mn x Br3–2x Cl2x (x = 0–0.5) composite showed several figures of merit compared to bare m-TiO2 and m-TiO2/CsPbBr3 such as on/off ratio of 104 times, responsivity of 1.67 A·W−1, and detectivity of 4.42 × 109 Jones. This result shows the potential for the UV photodetector application.
-
Funding information: This research is funded by the Hanoi University of Science and Technology (HUST) under project number T2020-SAHEP-037.
-
Author contributions: Ba-Duc Tran: methodology, investigation; Phuong-Nam Tran: writing – original draft; Xuan-Thanh Bui: methodology; Duy-Hung Nguyen: writing – review and editing; Thanh-Tung Duong: conceptualization, writing – editing.
-
Conflict of interest: Authors state no conflict of interest.
Appendix
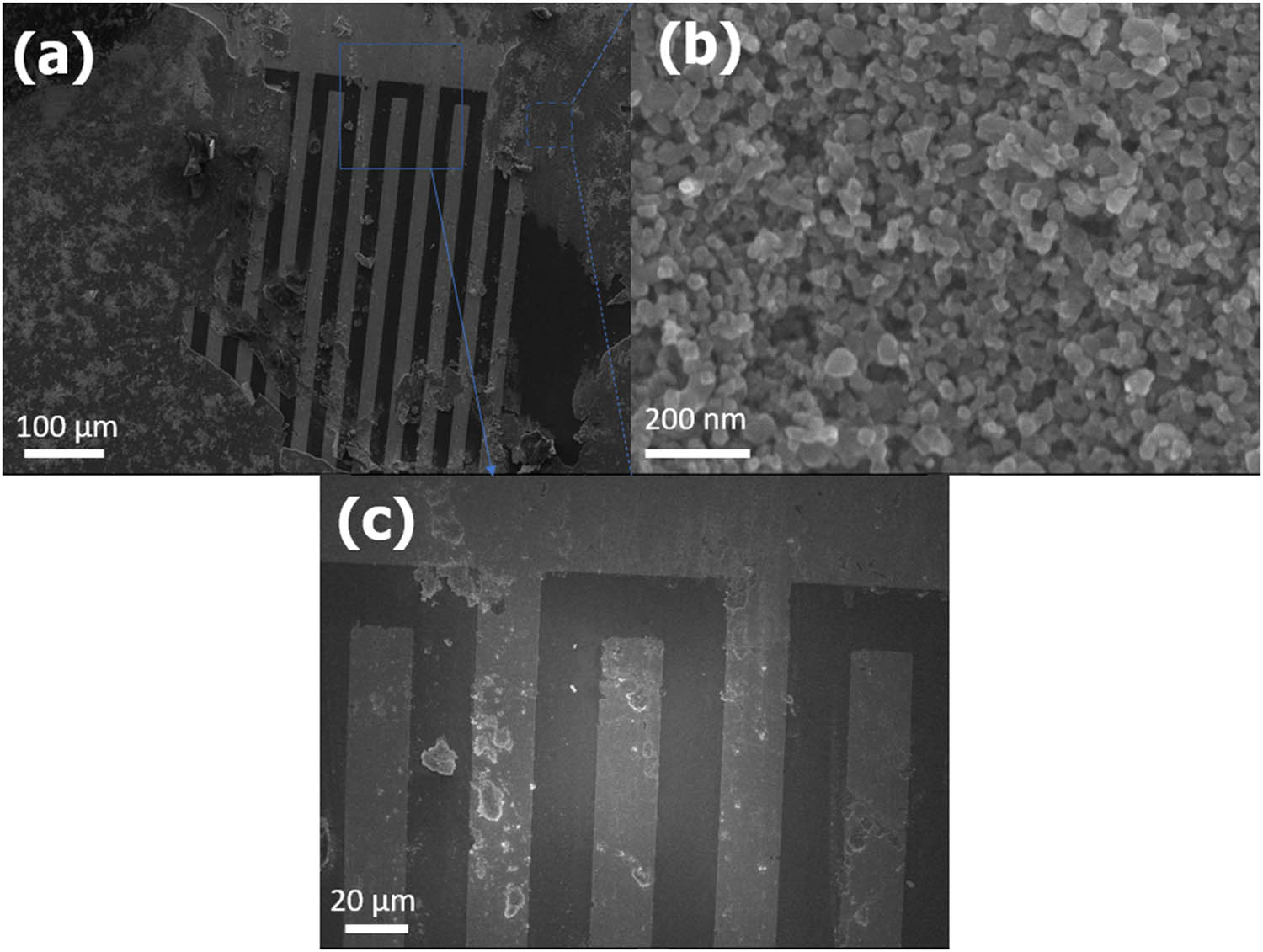
(a–c) The SEM images of patterned Pt electrode-coated mp-TiO2.
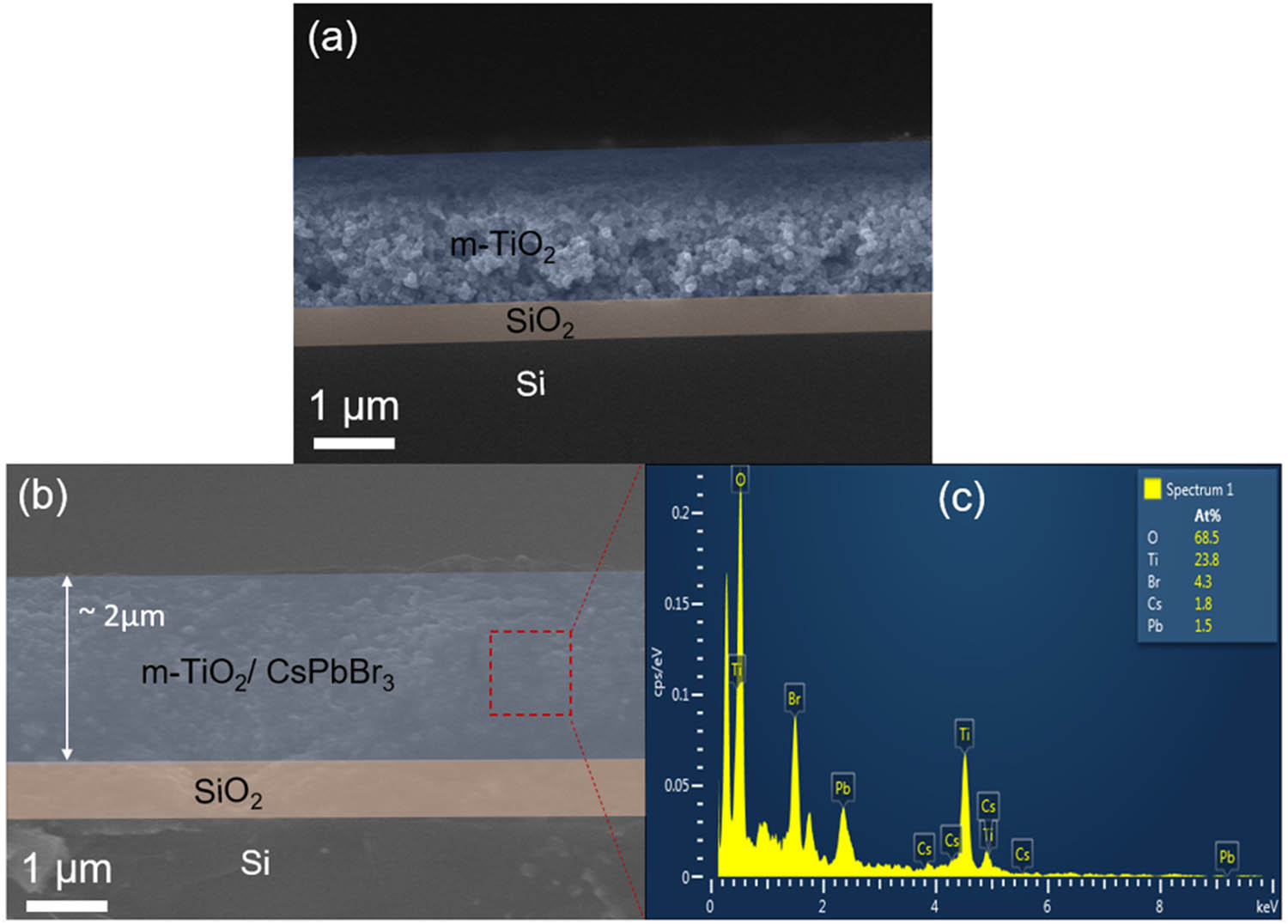
(a and b) The SEM cross-section images of mp-TiO2 before and after perovskite QD infiltration. (c) EDS spectrum of CsPb1–x Mn x Br3–2x Cl2x (x = 0.5)-coated m-TiO2 sample.
References
[1] Liang J, Wang C, Wang Y, Xu Z, Lu Z, Ma Y, et al. All-inorganic perovskite solar cells. J Am Chem Soc. 2016;138(49):15829–32.10.1021/jacs.6b10227Search in Google Scholar PubMed
[2] Feng W, Lin K, Li W, Xiao X, Lu J, Yan C, et al. Efficient all-inorganic perovskite light-emitting diodes enabled by manipulating the crystal orientation. J Mater Chem A. 2021;9:11064–72.10.1039/D1TA00093DSearch in Google Scholar
[3] Fang B, Zhang F, Chen J, Yang S, Xia X, Pullerits T, et al. Ultrasensitive and fast all-inorganic perovskite-based photodetector via fast carrier diffusion. Adv Mater. 2017;29:1703758.10.1002/adma.201703758Search in Google Scholar PubMed
[4] Ling Y, Tian Y, Wang J, Knox J, Perez-Orive F, Du Y, et al. Enhanced optical and electrical properties of polymer-assisted all-inorganic perovskites for light-emitting diodes. Adv Mater. 2016;28(40):8983–9.10.1002/adma.201602513Search in Google Scholar PubMed
[5] Ramasamy P, Lim D, Kim B, Lee S, Lee M, Lee J. All-inorganic cesium lead halide perovskite nanocrystals for photodetector applications. Chem Commun. 2016;52:2067–70.10.1039/C5CC08643DSearch in Google Scholar
[6] Ou Z, Yi Y, Hu Z, Hu J, Zhu J, Wang W, et al. Improvement of CsPbBr3 photodetector performance by tuning the morphology with PMMA additive. J All Comp. 2020;821:153344.10.1016/j.jallcom.2019.153344Search in Google Scholar
[7] Zhou L, Yu K, Yang F, Cong H, Wang N, Zheng J, et al. Insight into the effect of ligand-exchange on colloidal CsPbBr3 perovskite quantum dot/mesoporous-TiO2 composite-based photodetectors: much faster electron injection. J Mater Chem C. 2017;5:6224–33.10.1039/C7TC01611ESearch in Google Scholar
[8] Yang Z, Dou J, Wang M, Li J, Huang J, Shao J. Flexible all-inorganic photoconductor detectors based on perovskite/hole-conducting layer heterostructures. J Mater Chem C. 2018;6:6739–46.10.1039/C8TC02093KSearch in Google Scholar
[9] Deng J, Li J, Yang Z, Wang M. All-inorganic lead halide perovskites: a promising choice for photovoltaics and detectors. J Mater Chem C. 2019;7:12415–40.10.1039/C9TC04164HSearch in Google Scholar
[10] Niu T, Lu J, Munir R, Li L, Barrit D, Zhang X, et al. Stable high-performance perovskite solar cells via grain boundary passivation. Adv Mater. 2018;30(16):1706576.10.1002/adma.201706576Search in Google Scholar PubMed
[11] Knight A, Herz L. Preventing phase segregation in mixed-halide perovskites: a perspective. Energy Env Sci. 2020;13:2024–46.10.1039/D0EE00788ASearch in Google Scholar
[12] Motti SG, Patel JB, Oliver RDJ, Snaith HJ, Johnston MB. Phase segregation in mixed-halide perovskites affects charge-carrier dynamics while preserving mobility. Nat Commun. 2021;12:6955.10.1038/s41467-021-26930-4Search in Google Scholar PubMed PubMed Central
[13] Nijhuis J, Tran Q, Tran N, Dinh T, Phan H, Nguyen N, et al. Toward on-board microchip synthesis of CdSe vs. PbSe nanocrystalline quantum dots as a spectral decoy for protecting space assets. React Chem Eng. 2021;6:471–85.10.1039/D0RE00327ASearch in Google Scholar
[14] Pirsaheb M, Asadi A, Sillanpaa M, Farhadian N. Application of carbon quantum dots to increase the activity of conventional photocatalysts: a systematic review. J Mol Liq. 2018;271:857–71.10.1016/j.molliq.2018.09.064Search in Google Scholar
[15] Molaei M. Principles, mechanisms, and application of carbon quantum dots in sensors: a review. Anal Methods. 2020;12:1266–87.10.1039/C9AY02696GSearch in Google Scholar
[16] Xu A, Wang G, Li Y, Dong H, Yang S, He P, et al. Carbon-based quantum dots with solid-state photoluminescent: mechanism, implementation, and application. Small. 2020;16(2004621):2004621.10.1002/smll.202004621Search in Google Scholar PubMed
[17] Xu S, Huang G, Wang C, Shao H, Cui Y. Carbon-based fully printable self-powered ultraviolet perovskite photodetector: manganese-assisted electron transfer and enhanced photocurrent. Nanomater Nanotechnol. 2020;10:1–8.10.1177/1847980420925674Search in Google Scholar
[18] He W, Zhang Q, Qi Y, Xiong J, Ray P, Pradhan N, et al. Luminescence properties of CsPbBr3:Mn nanocrystals. J Nanopart Res. 2021;23:80–5664.10.1007/s11051-021-05184-7Search in Google Scholar
[19] Zhihai W, Jiao W, Yanni S, Jun W, Yafei H, Pan W, et al. Air-stable all-inorganic perovskite quantum dot inks for multicolor patterns and white LEDs. J Mater Sci. 2019;54:6917–29.10.1007/s10853-019-03382-2Search in Google Scholar
[20] Shinde A, Gahlaut R, Mahamuni S. Low-temperature photoluminescence studies of CsPbBr3 quantum dots. J Phys Chem C. 2017;121(27):14872–78.10.1021/acs.jpcc.7b02982Search in Google Scholar
[21] Pham X-V, Tran B-D, Nguyen D-C, Nguyen T, Nguyen M-V, Nguyen C-N-H, et al. Low-dimensional CsPbBr3@CoBr2 super-nanowire structure for perovskite/PMMA composite with highly blue emissive performance. Crystals. 2021;11:1564.10.3390/cryst11121564Search in Google Scholar
[22] Zhou L, Yua K, Yang F, Zheng J, Zuo Y, Li C, et al. All-inorganic perovskite quantum dot/mesoporous TiO2 composite-based photodetectors with enhanced performance. Dalton Trans. 2017;46:1766–9.10.1039/C6DT04758KSearch in Google Scholar
[23] Nguyen T, Patel M, Kim S, Mir R, Yi J, Dao V, et al. Transparent photovoltaic cells and self-powered photodetectors by TiO2/NiO heterojunction. J Power Sources. 2021;481:228865.10.1016/j.jpowsour.2020.228865Search in Google Scholar
© 2022 Ba-Duc Tran et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

